Abstract
Purpose:
To characterize practice patterns, including temporal trends, in fractionation schedules among patients in the United States undergoing definitive radiotherapy for early-stage glottic cancer and to compare overall survival outcomes between fractionation schedules.
Methods and Materials:
We queried the National Cancer Database for patients with TisN0M0, T1N0M0, or T2N0M0 squamous cell carcinoma of the glottic larynx diagnosed between 2004 and 2012 and undergoing definitive radiotherapy. Dose per fraction was calculated to define cohorts undergoing conventional fractionation (CFxn) and hypofractionation (HFxn). Logistic regression was performed to identify predictors of receiving HFxn, and Cox regression was used to determine predictors of mortality. One-to-one propensity-score matching (PSM) was then employed to compare survival between fractionation schedules.
Results:
10,539 patients were included, with 6,576 undergoing CFxn and 3,963 undergoing HFxn. T1 patients comprised a majority of each cohort. Use of HFxn increased significantly over the period studied (p<0.001), but even in the final year nearly one half of patients continued to receive CFxn. Receipt of HFxn was also independently associated with higher income and facility types other than community cancer program on logistic regression. On multivariate Cox regression, HFxn was associated with improved survival (hazard ratio [HR] for death 0.90, 95% confidence interval [95%CI] 0.83–0.97, p=0.008), a finding redemonstrated on univariate Cox regression among a well-matched PSM cohort (HR 0.88, 95%CI 0.80–0.96, p=0.003). Subgroup Cox multivariate analysis demonstrated a significant survival advantage with HFxn among T1 patients (HR 0.90, 95%CI 0.81–0.99; p=0.042), but a nonsignificant benefit among those with Tis (HR 0.86, 95%CI 0.57–1.30; p=0.472) or T2 disease (HR 0.88, 95%CI 0.76–1.02; p=0.099).
Conclusions:
Utilization of HFxn is increasing and is associated with improved survival over CFxn. Our findings support the broadened use of HFxn for patients with early-stage glottic cancer undergoing definitive radiotherapy.
Keywords: hypofractionation, conventional fractionation, laryngeal cancer
Introduction:
Over 13,000 laryngeal squamous cell carcinoma (SCC) diagnoses are anticipated in the United States in 2016, comprising approximately one in five newly diagnosed cancers of the head and neck [1]. For the large portion of patients presenting with early stage disease, radiotherapy offers an effective definitive treatment modality with the promise of anatomic and functional preservation [2–6].
Ten years ago, Yamazaki et al. published the results of a randomized trial comparing conventionally-fractionated (2.00 Gy per fraction) and hypofractionated (2.25 Gy per fraction) schedules of definitive radiotherapy in 180 patients with T1N0M0 glottic SCC [7]. At a median follow-up of just over 5 years, the hypofractionated arm experienced significantly improved local control (92% vs 77%), but this did not translate into a significant advantage in cancer-specific (100% vs 98%) or overall survival (88% vs 87%). However, the primary endpoint for this study was local control rather than survival, likely limiting its power to detect a significant survival difference.
Perhaps as a result of these comparable survival outcomes between conventional fractionation and hypofractionation, national guidelines for the radiotherapeutic management of early-stage glottic SCC are inconsistent, with the American College of Radiology’s Appropriateness Criteria favoring hypofractionation [8] and the National Comprehensive Cancer Network guidelines deeming either schedule suitable [9].
We therefore sought to describe patterns of care, particularly temporal trends with respect to the publication of the Japanese trial, in the application of fractionation schedules among American patients undergoing definitive radiotherapy for early-stage glottic SCC using the National Cancer Database (NCDB). We also sought to compare the efficacy of these schedules in terms of overall survival.
Methods and Materials:
The NCDB, a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society, is a hospital-based registry capturing approximately 70% of incident cancer cases in the United States and drawing data from more than 1,500 commission-accredited cancer programs. The NCDB contains detailed information on demographic, clinical, and treatment-related factors. The American College of Surgeons and the CoC have not verified and are not responsible for the analytic or statistical methodology employed or for the conclusions drawn from these data by the investigators. The CoC mandates follow-up rates of 90% per year for surviving subjects diagnosed in the preceding five years and 80% per year for others, with registrars reporting follow-up on an annual basis. The present analysis was performed with the approval of our local institutional review board.
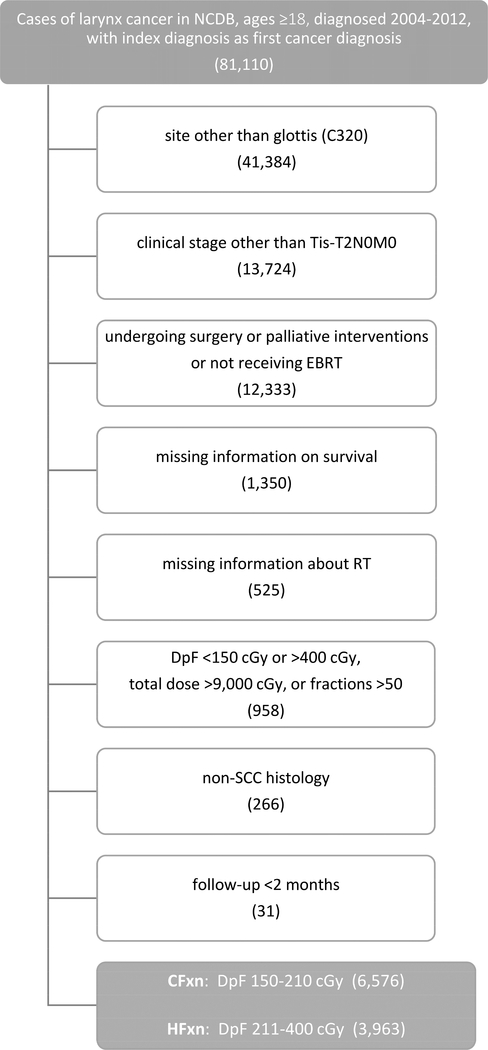
We initially queried the NCDB for patients with a first oncologic diagnosis of larynx cancer diagnosed from 2004–2012 and aged ≥18 years (n=81,110). We excluded patients with cancer arising from sites other than the glottis (n=41,384) and those with clinical stage other than TisN0M0, T1N0M0, T1aN0M0, T1bN0M0, or T2N0M0 (n=13,724). We then removed patients coded as receiving surgery or palliative interventions and those not receiving EBRT (n=12,333). Next, we excluded patients with missing survival information (n=1,350) or incomplete information regarding their regional radiation dose, boost radiation dose, and number of fractions (n=525). We then divided each subject’s total radiation dose in centigray (cGy) by the number of fractions to calculate a dose per fraction and then removed patients with dose-per-fraction <150 cGy or >400 cGy, total RT dose >9,000 cGy, or >50 fractions (n=958). We then excluded those with non-SCC histology codes (n=266) and fewer than 2 months of follow-up (n=31). To account for potential inaccuracies in recorded dose and fraction number, we broadly defined two fractionation cohorts: a conventional fractionation (CFxn) group receiving 150–210 cGy per fraction and a hypofractionation group receiving 211–400 cGy per fraction.
Sociodemographic covariates incorporated into our analysis include age, year of diagnosis, gender, race, insurance status, income quartile, facility type, and distance from facility. We also analyzed patient- and tumor-specific factors including Charlson-Deyo comorbidity and T-classification.
Next, using a logistic regression model with fractionation schedule as the outcome, propensity scores using the aforementioned covariates were estimated. The data were then stratified into five equal strata based on the value of the propensity score, and samples in both fractionation schedules were randomly one-to-one matched.
Statistical analyses were performed using SPSS V24.0 (SPSS Inc., Chicago, IL). Covariates were selected a priori. Pearson chi-square tests were used to assess associations between variables and fractionation schedule. Multiple binary logistic regression models were used to assess the association between fractionation and patient characteristics, with the results reported as odds ratios (OR) for receipt of HFxn. Follow-up time was defined as the amount of time in months between date of diagnosis and date of either last contact or death. Censoring occurred if and only if patients were coded as alive at time of last follow-up, according to the Kaplan-Meier method. Overall survival (OS), defined as time from diagnosis to death, was first examined using the Kaplan-Meier method. Univariate analysis (UVA) for survival was performed among both the overall population and the propensity-score-matched cohort with the log-rank test, and unadjusted Cox proportional hazards regression was used to determine hazard ratios (HR), with HR>1 corresponding to worse OS. A frailty model was employed for matched data. Multivariate Cox regression analysis was performed with OS as the outcome. Subgroup Cox regression analysis was then performed for each T-classification, with a separate HR, confidence interval, and p-value reported for patients with Tis, T1, and T2 disease. All tests were two-sided with a 0.05 level of significance. The Hosmer-Lemeshow test was used to check for the goodness-of-fit of regression models.
Results:
10,539 patients met our inclusion criteria, with 6,576 receiving CFxn and 3,963 undergoing HFxn (Figure 1). T1 patients comprised a majority of both groups with 4,372 (66.5%) in CFxn and 2,891 (72.9%) in HFxn. Median follow-up for the overall cohort was 45.5 months (range 2.0–103.3).
Figure 1.
Cohort derivation. NCDB, National Cancer Database; EBRT, external beam radiotherapy; RT, radiotherapy; cGy, centigray; SCC, squamous cell carcinoma; CFxn, conventional fractionation; HFxn, hypofractionation
There were significant baseline differences between the fractionation groups (Table 1), with HFxn patients more likely to be diagnosed in later years, to reside in higher-income areas, to undergo care at academic/research facilities, to live more than 5 miles from a treatment facility, and to have T1 disease. They were also less likely to be white or to have Medicare insurance (all p≤0.019). The median CFxn patient received 6,600 cGy (interquartile range [IQR] 6,600–7,000) at 200 cGy per fraction (IQR 200–200) over 49 days (IQR 46–51) starting 29 days after diagnosis (IQR 21–40), while the median HFxn patient received 6,300 cGy (IQR 6,300–6,525) at 225 cGy per fraction (IQR 225–225) over 41 days (IQR 39–43) starting 30 days after diagnosis (IQR 22–41). CFxn patients were more likely to be coded as receiving IMRT, although most patients in both groups were coded as receiving non-IMRT techniques.
Table 1.
Demographic, Clinical, and Treatment Characteristics
| Patients (N=10,539) |
|||||
|---|---|---|---|---|---|
| CFxn (n=6,576) |
HFxn (n==3,963) |
||||
| # | % | # | % | p | |
| Ag(yr) | 0.398 | ||||
| <60 | 2,253 | 34.3 | 1,406 | 35.5 | |
| 60–70 | 2,135 | 32.5 | 1,278 | 32.2 | |
| >70 | 2,188 | 33.3 | 1,279 | 32.3 | |
| Gender | 0.448 | ||||
| male | 5,689 | 86.5 | 3,449 | 87.0 | |
| female | 887 | 13.5 | 514 | 13.0 | |
| Race | <0.001 | ||||
| white | 5,699 | 86.7 | 3,367 | 85.0 | |
| black | 714 | 10.9 | 441 | 11.1 | |
| other | 112 | 1.7 | 94 | 2.4 | |
| unknown | 51 | 0.8 | 61 | 1.5 | |
| Comorbidity | 0.494 | ||||
| 0 | 5,362 | 81.5 | 3,256 | 82.2 | |
| 1 | 953 | 14.5 | 567 | 14.3 | |
| 2+ | 261 | 4.0 | 140 | 3.5 | |
| Yr of Dx | <0.001 | ||||
| 2004–2006 | 2,542 | 38.7 | 963 | 24.3 | |
| 2007–2009 | 2,202 | 33.5 | 1,263 | 31.9 | |
| 2010–2012 | 1,832 | 27.9 | 1,737 | 43.8 | |
| Insurance | 0.005 | ||||
| uninsured | 241 | 3.7 | 140 | 3.5 | |
| Medicaid | 314 | 4.8 | 229 | 5.8 | |
| Medicare | 3,215 | 48.9 | 1,831 | 46.2 | |
| private insurance | 2,541 | 38.6 | 1,576 | 39.8 | |
| other government | 142 | 2.2 | 119 | 3.0 | |
| unknown | 123 | 1.9 | 68 | 1.7 | |
| Income | <0.001 | ||||
| 1st quartile | 1,417 | 21.5 | 735 | 18.5 | |
| 2nd quartile | 1,778 | 27.0 | 995 | 25.1 | |
| 3rd quartile | 1,626 | 24.7 | 1,087 | 27.4 | |
| 4th quartile | 1,632 | 24.8 | 1,094 | 27.6 | |
| unknown | 123 | 1.9 | 52 | 1.3 | |
| Facility Type | <0.001 | ||||
| CCP | 1,037 | 15.8 | 403 | 10.2 | |
| comprehensive CCP | 3,423 | 52.1 | 1,908 | 48.1 | |
| academic/research | 1,619 | 24.6 | 1,241 | 31.3 | |
| other | 423 | 6.4 | 372 | 9.4 | |
| unknown | 74 | 1.1 | 39 | 1.0 | |
| Distance (mi) | 0.019 | ||||
| ≤5 | 2,201 | 33.5 | 1,253 | 31.6 | |
| >5 & ≤15 | 2,312 | 35.2 | 1,434 | 36.2 | |
| >15 | 1,952 | 29.7 | 1,231 | ||
| unknown | 111 | 1.7 | 1.1 | ||
| T Classification | <0.001 | ||||
| Tis | 394 | 6.0 | 266 | 6.7 | |
| T1 | 4,372 | 66.5 | 2,891 | 72.9 | |
| T2 | 1,810 | 27.5 | 806 | 20.3 | |
| RT Technique | <0.001 | ||||
| 3D-CRT | 712 | 10.8 | 568 | 14.3 | |
| IMRT | 630 | 9.6 | 289 | .73 | |
| other | 5,234 | 79.6 | 3,106 | 78.4 | |
| RT Total Dose (cGy) | |||||
| Median | 6600 | 6300 | |||
| IQR | 6600–7000 | 6300–6525 | |||
| RT Dose per Fxn (cGy) | |||||
| Median | 200 | 225 | |||
| IQR | 200–200 | 225–225 | |||
| Time from Dx to RT (days) | |||||
| Median | 29 | 30 | |||
| IQR | 21–40 | 22–41 | |||
| RT Duration (days) | |||||
| Median | 49 | 41 | |||
| IQR | 46–51 | 39–43 | |||
| Survival (mo) | <0.001 | ||||
| Median | 106.0 | 116.1 | |||
| Range | 2.0–129.3 | 2.1–130.3 | |||
Abbreviations: CFxn, conventional fractionation; HFxn, hypofractionation; yr, year; Dx, diagnosis; CCP, community cancer program; mi, miles; RT, radiation therapy; 3DCRT, 3D conformal radiation therapy; IMRT, intensity-modulated radiation therapy; cGy, centigray; IQR, interquartile range; Fxn, fraction; mo, months
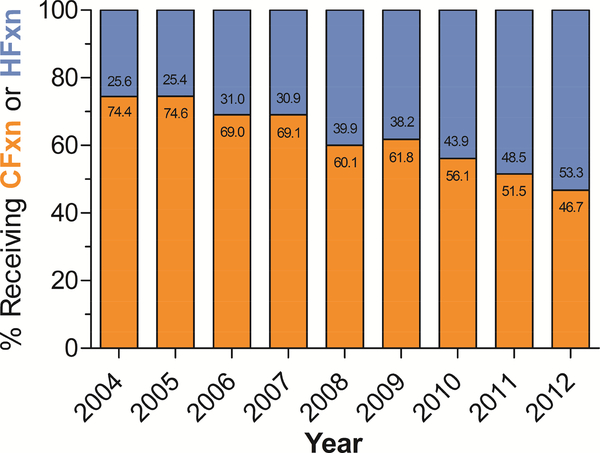
Analyzing year as a continuous variable demonstrated significantly increased use of HFxn over time, from just over 25% of patients receiving it prior to 2006 to over 50% of patients receiving it in 2012 (p<0.001) (Figure 2).
Figure 2.
Percentage of early-stage larynx cancer patients receiving indicated fractionation schedule over time
Controlling for baseline differences on multivariate analysis (MVA), HFxn patients remained more likely to be diagnosed after 2006 (OR 1.55, 95% confidence interval [95%CI] 1.39–1.73, p<0.001 for 2007–2009; OR 2.68, 95%CI 2.42–3.00, p<0.001 for 2010–2012) and to reside in higher-income areas (Table 2). They were also significantly more likely to receive care at facilities other than community cancer programs (OR 1.43, 95%CI 1.25–1.64, p<0.001 for comprehensive cancer programs; OR 1.95, 95%CI 1.69–2.25, p<0.001 for academic/research facilities). As compared to patients with Tis disease, T1 patients had similar odds of receiving HFxn (OR 1.03, 95%CI 0.87–1.22, p=0.738), whereas T2 patients were significantly less likely to receive it (OR 0.66, 95%CI 0.55–0.80, p<0.001).
Table 2.
Predictors of Receipt of HFxn (Multivariate Logistic Regression)
| Univariate Analysis |
Multivariate Analysis |
|||||
|---|---|---|---|---|---|---|
| OR | 95%CI | P | OR | 95%CI | P | |
| Age (v ≤60) | ||||||
| 61–70 | 0.96 | 0.87–1.06 | 0.396 | 0.99 | 0.88–1.11 | 0.812 |
| >70 | 0.94 | 0.85–1.03 | 0.181 | 0.99 | 0.87–1.13 | 0.900 |
| Gender (v male) | ||||||
| female | 0.96 | 0.85–1.07 | 0.448 | 0.94 | 0.83–1.06 | 0.307 |
| Race (v white) | ||||||
| black | 1.05 | 0.92–1.19 | 0.490 | 1.08 | 0.93–1.24 | 0.315 |
| other | 1.42 | 1.08–1.88 | 0.013 | 1.17 | 0.87–1.58 | 0.309 |
| Comorbidity (v 0) | ||||||
| 1 | 0.98 | 0.88–1.10 | 0.723 | 0.95 | 0.84–1.07 | 0.414 |
| 2+ | 0.88 | 0.72–1.09 | 0.247 | 0.88 | 0.70–1.09 | 0.239 |
| Yr of Dx (v 2004–2006) | ||||||
| 2007–2009 | 1.51 | 1.37–1.68 | <0.001 | 1.55 | 1.39–1.73 | <0.001 |
| 2010–2012 | 2.50 | 2.27–2.76 | <0.001 | 2.68 | 2.42–3.00 | <0.001 |
| Insurance (v uninsured) | ||||||
| Medicaid | 1.26 | 0.96–1.64 | 0.097 | 1.22 | 0.91–1.62 | 0.178 |
| Medicare | 0.98 | 0.79–1.22 | 0.857 | 0.96 | 0.77–1.25 | 0.845 |
| private insurance | 1.07 | 0.86–1.33 | 0.555 | 1.03 | 0.82–1.30 | 0.776 |
| other government | 1.44 | 1.05–1.99 | 0.025 | 1.32 | 0.93–1.86 | 0.118 |
| Income (v 1st quartile) | ||||||
| 2nd quartile | 1.08 | 0.96–1.21 | 0.208 | 1.11 | 0.98–1.27 | 0.095 |
| 3rd quartile | 1.29 | 1.14–1.45 | <0.001 | 1.30 | 1.15–1.48 | <0.001 |
| 4th quartile | 1.29 | 1.15–1.45 | <0.001 | 1.25 | 1.10–1.43 | <0.001 |
| Facility Type (v CCP) | ||||||
| comprehensive CCP | 1.43 | 1.26–1.63 | <0.001 | 1.43 | 1.25–1.64 | <0.001 |
| academic/research | 1.97 | 1.72–2.26 | <0.001 | 1.95 | 1.69–2.25 | <0.001 |
| other | 2.26 | 1.89–2.71 | <0.001 | 2.22 | 1.83–2.68 | <0.001 |
| Distance (v ≤5mi) | ||||||
| >5 & ≤15mi | 1.09 | 0.99–1.20 | 0.079 | 1.02 | 0.92–1.13 | 0.754 |
| >15mi | 1.11 | 1.00–1.22 | 0.044 | 1.10 | 0.99–1.23 | 0.079 |
| T Classification (v Tis) | ||||||
| T1 | 0.98 | 0.83–1.15 | 0.802 | 1.03 | 0.87–1.22 | 0.738 |
| T2 | 0.66 | 0.55–0.79 | <0.001 | 0.66 | 0.55–0.80 | <0.001 |
Abbreviations: OR, odds ratio; 95%CI, 95% confidence interval; yr, year; Dx, diagnosis; CCP, community cancer program; mi, miles
Unadjusted median OS was significantly longer for HFxn than for CFxn (116.1 months vs 106.0 months, respectively, p<0.001) (Supplementary Figure 1, Table 1). Median OS was not reached for Tis patients, 113.5 months for T1 patients, and 84.1 months for T2 patients.
Controlling for covariates on Cox MVA, older age, comorbidity score ≥1, and Medicaid insurance were associated with worse OS (Table 3). Conversely, female gender, private insurance, higher income, and receipt of care at an academic/research facility were independent predictors of improved OS. With respect to T-classification, worse survival was seen among those with T1 (HR 1.50, 95%CI 1.24–1.82, p<0.001) or T2 (HR 2.42, 95%CI 1.99–2.94, p<0.001) than with Tis disease. HFxn was associated with improved OS as compared to CFxn (HR 0.90, 95%CI 0.83–0.97, p=0.008).
Table 3.
Predictors of Mortality (Cox Regression)
| Univariate Analysis |
Multivariate Analysis |
|||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | |
| Age (v ≤60) | ||||||
| 61–70 | 1.52 | 1.37–1.68 | <0.001 | 1.35 | 1.20–1.52 | <0.001 |
| >70 | 2.76 | 2.52–3.03 | <0.001 | 2.29 | 2.02–2.59 | <0.001 |
| Gender (v male) | ||||||
| female | 0.78 | 0.70–0.87 | <0.001 | 0.80 | 0.71–0.90 | <0.001 |
| Race (v white) | ||||||
| black | 1.12 | 1.00–1.25 | 0.050 | 1.09 | 0.96–1.23 | 0.189 |
| other | 0.78 | 0.58–1.06 | 0.110 | 0.75 | 0.54–1.04 | 0.082 |
| Comorbidity (v 0) | ||||||
| 1 | 1.48 | 1.34–1.63 | <0.001 | 1.27 | 1.15–1.40 | <0.001 |
| 2+ | 2.49 | 2.14–2.89 | <0.001 | 1.96 | 1.68–2.28 | <0.001 |
| Yr of Dx (v 2004–2006) | ||||||
| 2007–2009 | 1.06 | 0.97–1.15 | 0.179 | 1.02 | 0.93–1.11 | 0.736 |
| 2010–2012 | 1.14 | 1.02–1.27 | 0.019 | 1.06 | 0.95–1.18 | 0.325 |
| Insurance (v uninsured) | ||||||
| Medicaid | 1.69 | 1.29–2.21 | <0.001 | 1.53 | 1.16–2.02 | 0.003 |
| Medicare | 1.83 | 1.45–2.30 | <0.001 | 1.09 | 0.85–1.40 | 0.508 |
| private insurance | 0.81 | 0.64–1.03 | 0.089 | 0.77 | 0.61–0.99 | 0.039 |
| other government | 1.79 | 1.31–2.44 | <0.001 | 1.38 | 1.00–1.90 | 0.053 |
| Income (v 1st quartile) | ||||||
| 2nd quartile | 0.91 | 0.82–1.01 | 0.072 | 0.90 | 0.81–1.00 | 0.004 |
| 3rd quartile | 0.79 | 0.72–0.88 | <0.001 | 0.84 | 0.75–0.94 | 0.002 |
| 4th quartile | 0.74 | 0.66–0.82 | <0.001 | 0.79 | 0.70–0.88 | <0.001 |
| Facility Type (v CCP) | ||||||
| comprehensive CCP | 0.92 | 0.82–1.02 | 0.096 | 0.96 | 0.87–1.08 | 0.507 |
| academic/research | 0.79 | 0.71–0.89 | <0.001 | 0.85 | 0.75–0.96 | 0.008 |
| other | 0.78 | 0.66–0.93 | 0.004 | 0.91 | 0.77–1.08 | 0.287 |
| Distance (v ≤5mi) | ||||||
| >5 & ≤15mi | 0.89 | 0.82–0.97 | 0.011 | 0.96 | 0.87–1.04 | 0.317 |
| >15mi | 0.90 | 0.83–0.99 | 0.029 | 0.94 | 0.86–1.03 | 0.193 |
| T Classification (v Tis) | ||||||
| T1 | 1.52 | 1.26–1.83 | <0.001 | 1.50 | 1.24–1.82 | <0.001 |
| T2 | 2.44 | 2.01–2.95 | <0.001 | 2.42 | 1.99–2.94 | <0.001 |
| Radiation (v CFxn) | ||||||
| HFxn | 0.84 | 0.77–0.91 | <0.001 | 0.90 | 0.83–0.97 | 0.008 |
Abbreviations: HR, hazard ratio; 95%CI, 95% confidence interval; yr, year; Dx, diagnosis; CCP, community cancer program; mi, miles; CFxn, conventional fractionation; HFxn, hypofractionation
Propensity-score matching yielded 7,918 well-matched patients, with 3,959 receiving each fractionation schedule (Supplementary Table 1). Both Kaplan-Meier analysis (Supplementary Figure 2) and Cox UVA of these matched cohorts demonstrated an association between HFxn and improved OS (HR 0.88, 95%CI 0.80–0.96, p=0.003).
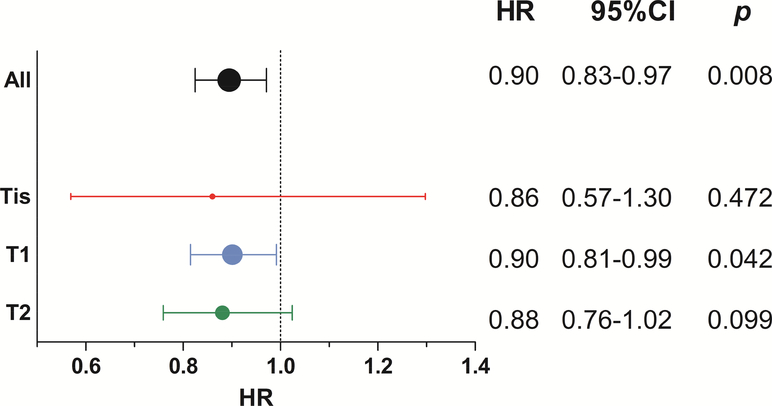
Subgroup Cox regression was then performed on each T-classification group (Figure 3). While HFxn remained associated with improved OS among T1 patients (HR 0.90, 95%CI 0.81–0.99, p=0.042), the improvement was not significant among patients with Tis (HR 0.86, 95%CI 0.57–1.30, p=0.472) or T2 (HR 0.88, 95%CI 0.76–1.02, p=0.099) disease.
Figure 3.
Forest plot of OS benefit of HFxn (v CFxn), segmented by T-classification
Discussion:
In the largest study to date of early-stage glottic SCC managed with radiotherapy, we identified increased use of HFxn since the publication of the Japanese trial in 2006, and we found a survival advantage of HFxn over CFxn.
Our study joins other recent NCDB-based analyses of nationwide patterns of radiotherapy fractionation. Consistent with earlier studies of radiation delivered in the adjuvant setting for early-stage breast cancer [10] and in the palliative setting for bone metastases [11], we identified greater administration of shorter courses at academic facilities and with the passage of time. These commonalities suggest that the observed variations in fractionation may be related to similar factors.
It is remarkable that as late as 2012, six years after the Japanese trial demonstrating superiority of HFxn was published, roughly one-half of early-stage glottic SCC patients undergoing definitive radiotherapy continue to receive CFxn. This points to a dramatic underutilization of HFxn in this patient population at the national level and emphasizes the importance of identifying clinical and nonclinical factors associated with various fractionation schedules. One such factor emerging from the present analysis is facility type, with patients undergoing care at academic facilities significantly more likely to receive HFxn than their counterparts treated at comprehensive community cancer programs, and nearly twice as likely as those treated at community cancer programs. This finding raises a few possibilities that could explain the national patterns we identified. First, community providers may not be as familiar with the randomized evidence demonstrating the superiority of HFxn over CFxn. Growing awareness could therefore explain the observed growth in HFxn utilization over time. A more concerning possibility is the differential reimbursement between HFxn and CFxn, with the latter entailing more fractions over a longer duration of time and therefore more lucrative on a fee-forservice basis. To the extent that academic providers are less sensitive to this financial incentive, they may be more likely to offer HFxn. An additional factor outside the scope of this analysis that may nevertheless underlie the persistent prevalence of CFxn is the ambiguity in clinical guidelines available to oncologists treating head and neck cancer. While radiotherapy-focused guidelines describe the Yamazaki trial in detail and explicitly rate HFxn as “usually appropriate” and CFxn as “usually not appropriate” [8], more general multidisciplinary cancer guidelines do not mention the local control advantage conferred by HFxn and permit either CFxn or HFxn for Tis-T2N0 glottic cancer [9]. Broadening utilization of HFxn will ultimately require heightened efforts to educate radiation oncologists and consistency among clinical guidelines in uniformly advocating HFxn for the radiotherapeutic management of early-stage glottic cancer.
While questions regarding comparative effectiveness are ideally addressed in a prospective clinical trial, not all topics are well-suited for a randomized comparison. In the case of fractionation for early-stage glottic cancer, patients have a generally favorable prognosis, which results in a low mortality event rate and increases the amount of subjects necessary for an adequately-powered survival analysis. Moreover, the availability of randomized data [7] demonstrating superior local control with one intervention over another raises ethical issues around the allocation of patients to the intervention known to be inferior. A retrospective analysis of existing data from a large database such as NCDB is therefore ideally suited to investigate the question of OS among various fractionation schedules for this disease. Our finding on Cox MVA of an OS benefit with HFxn over CFxn is supported by concordant findings in a PSM cohort.
Our results complement the findings of two randomized trials investigating HFxn for early-stage glottic cancer [7,12]. In addition to the now decade-old Japanese trial demonstrating a local control benefit with HFxn over CFxn [7], a similarly-designed Korean trial comparing the same fractionation schedules among 156 patients with T1N0M0 or T2N0M0 glottic SCC identified a nonsignificant improvement in local progression-free survival with HFxn [12]. Importantly, this latter study was designed as a non-inferiority trial but fell short of its targeted enrollment of 282 patients, rendering it underpowered and raising the possibility that a significant difference in favor of HFxn may have been observed had this trial accrued as intended. Together, the Japanese and Korean randomized trials point to HFxn improving local disease control over CFxn in early-stage glottic SCC.
With our NCDB analysis, we can now add a survival advantage to this local control benefit. In light of existing retrospective data demonstrating poor survival in patients with recurrence [13,14], it would be reasonable to assume that the improved local control with HFxn identified in the trials translates to the prolonged survival found in the present study.
In addition to the T1 early-stage glottic cancer patients examined in Japanese trial, our analysis also included patients with Tis and T2 disease. While subgroup analysis did not demonstrate a significant benefit among these two smaller groups, it should be noted that the HR’s for each are directionally consistent with that for T1 disease (i.e. favoring HFxn), raising the possibility that with additional patient-years of follow-up a significant advantage may accrue to these groups. In the case of T2 disease, it is also possible that many patients are clinically understaged, as involvement of the paraglottic space and thyroid cartilage can be challenging to identify. This underascertainment of true T3 disease may underlie both the markedly worse OS outcomes among our T2 patients as compared to their Tis and T1 counterparts and the absence of a significant survival benefit of HFxn over CFxn.
The excellent survival outcomes we observed in the NCDB for Tis disease are consistent with those reported in case series from the University of Florida [5,15,16] and Princess Margaret [17], both of which utilized a hypofractionated radiotherapy approach. This favorable prognosis is not unexpected for a pre-invasive cancerous lesion.
A variety of altered fractionation schema have proven superior to CFxn in the management of T2N0M0 glottic SCC. A retrospective analysis of patients treated at MD Anderson demonstrated a trend toward improved local control with hyperfractionated (twice-daily) over once-daily radiation [18]. RTOG 9512 redemonstrated this nonsignificant trend in a randomized setting [19]. At the other end of the spectrum, retrospective data from UCSF identified improved local control with hypofractionation and shorter overall treatment time [20]. Similarly, a retrospective analysis of patients treated in New Zealand demonstrated numerically but not significantly superior laryngectomy-free survival with an accelerated hypofractionated course over a conventionally-fractionated one [21]. Our analysis also identified a trend in favor of HFxn in our T2 subset with respect to OS.
Overall, our findings contribute to the wealth of evidence supporting broader use of HFxn for early-stage glottic SCC. The treatment course is inherently shorter and, therefore, more convenient for patients and less demanding on health care resources. It has a similar toxicity profile to CFxn [7,12], offers comparable if not superior local control [7,12], and is now associated with improved OS.
As a database analysis, our study has inherent limitations including the potential for miscoding of variables and selection bias that is not accounted for by variables available in the database. For example, NCDB also does not capture information related to tumor extent (e.g. involvement of vocal fold and/or anterior commissure) and treatment specifics (e.g. field size, use of bolus). While we identified a survival advantage for HFxn over CFxn on both Cox MVA and a PSM analysis, neither technique can entirely mitigate the effects of selection bias. Moreover, outcome measurements in NCDB are limited to OS, as data on locoregional control, distant metastases, salvage therapy, cause of death, toxicity, and quality of life are unavailable.
In conclusion, HFxn for early-stage glottic SCC is increasingly utilized and more likely to be delivered at academic facilities. As it is associated with improved local control and now a survival benefit, it appears underutilized in the United States.
Supplementary Material
Footnotes
Conflict of interest: none.
While a 2006 Japanese trial demonstrated that patients undergoing definitive radiotherapy for early-stage glottic larynx cancer derive a local control benefit from hypofractionation, it is unclear how its publication has influenced fractionation patterns in the US or whether hypofractionation translates to a survival advantage. In an analysis of care patterns and outcomes in the National Cancer Database, we identified both increased use of hypofractionation after 2006 through 2012 and improved overall survival with this approach.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: a cancer journal for clinicians 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Mendenhall WM, Amdur RJ, Morris CG, Hinerman RW. T1-T2N0 squamous cell carcinoma of the glottic larynx treated with radiation therapy. Journal of Clinical Oncology 2001;19:4029–4036. [DOI] [PubMed] [Google Scholar]
- 3.Chera BS, Amdur RJ, Morris CG, Kirwan JM, Mendenhall WM. T1N0 to T2N0 squamous cell carcinoma of the glottic larynx treated with definitive radiotherapy. International Journal of Radiation Oncology*Biology*Physics 2010;78:461–466. [DOI] [PubMed] [Google Scholar]
- 4.Cellai E, Frata P, Magrini SM, Paiar F, Barca R, Fondelli S, Polli C, Livi L, Bonetti B, Vitali E, De Stefani A, Buglione M, Biti G. Radical radiotherapy for early glottic cancer: Results in a series of 1087 patients from two Italian radiation oncology centers. I. The case of T1N0 disease. International journal of radiation oncology, biology, physics 2005;63:1378–1386. [DOI] [PubMed] [Google Scholar]
- 5.Sengupta N, Morris CG, Kirwan J, Amdur RJ, Mendenhall WM. Definitive radiotherapy for carcinoma in situ of the true vocal cords. American journal of clinical oncology 2010;33:94–95. [DOI] [PubMed] [Google Scholar]
- 6.Aaltonen LM, Rautiainen N, Sellman J, Saarilahti K, Makitie A, Rihkanen H, Laranne J, Kleemola L, Wigren T, Sala E, Lindholm P, Grenman R, Joensuu H. Voice quality after treatment of early vocal cord cancer: A randomized trial comparing laser surgery with radiation therapy. International journal of radiation oncology, biology, physics 2014;90:255–260. [DOI] [PubMed] [Google Scholar]
- 7.Yamazaki H, Nishiyama K, Tanaka E, Koizumi M, Chatani M. Radiotherapy for early glottic carcinoma (T1N0M0): Results of prospective randomized study of radiation fraction size and overall treatment time. International Journal of Radiation Oncology* Biology* Physics 2006;64:77–82. [DOI] [PubMed] [Google Scholar]
- 8.Ridge JA, Lawson J, Yom SS, Garg MK, McDonald MW, Quon H, Saba N, Salama JK, Smith RV, Worden F, Yeung AR, Beitler JJ. American college of radiology appropriateness criteria: treatment of stage I T1 glottic cancer. Head & neck 2014;36:3–8. [DOI] [PubMed] [Google Scholar]
- 9.Pfister DG, Spencer S, Brizel DM, Burtness B, Busse PM, Caudell JJ, Cmelak AJ, Colevas AD, Dunphy F, Eisele DW, Foote RL, Gilbert J, Gillison ML, Haddad RI, Haughey BH, Hicks WL Jr, Hitchcock YJ, Jimeno A, Kies MS, Lydiatt WM, Maghami E, McCaffrey T, Mell LK, Mittal BB, Pinto HA, Ridge JA, Rodriguez CP, Samant S, Shah JP, Weber RS, Wolf GT, Worden F, Yom SS, McMillian N, Hughes M. Head and neck cancers, version 1.2015. Journal of the National Comprehensive Cancer Network : JNCCN 2015;13:847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang EH, Mougalian SS, Soulos PR, Rutter CE, Evans SB, Haffty BG, Gross CP, James BY. Adoption of hypofractionated whole-breast irradiation for early-stage breast cancer: A national cancer data base analysis. International Journal of Radiation Oncology* Biology* Physics 2014;90:993–1000. [DOI] [PubMed] [Google Scholar]
- 11.Rutter CE, James BY, Wilson LD, Park HS. Assessment of national practice for palliative radiation therapy for bone metastases suggests marked underutilization of single-fraction regimens in the united states. International Journal of Radiation Oncology* Biology* Physics 2015;91:548–555. [DOI] [PubMed] [Google Scholar]
- 12.Moon SH, Cho KH, Chung EJ, Lee CG, Lee KC, Chai GY, Kang KM, Lee JY, Chung WK, Park WY, Kim JH. A prospective randomized trial comparing hypofractionation with conventional fractionation radiotherapy for T1–2 glottic squamous cell carcinomas: Results of a korean radiation oncology group (krog-0201) study. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 2014;110:98–103. [DOI] [PubMed] [Google Scholar]
- 13.McLaughlin MP, Parsons JT, Fein DA, Stringer SP, Cassisi NJ, Mendenhall WM, Million RR. Salvage surgery after radiotherapy failure in T1-T2 squamous cell carcinoma of the glottic larynx. Head & neck 1996;18:229–235. [DOI] [PubMed] [Google Scholar]
- 14.Ganly I, Patel SG, Matsuo J, Singh B, Kraus DH, Boyle JO, Wong RJ, Shaha AR, Lee N, Shah JP. Results of surgical salvage after failure of definitive radiation therapy for earlystage squamous cell carcinoma of the glottic larynx. Archives of otolaryngology--head & neck surgery 2006;132:59–66. [DOI] [PubMed] [Google Scholar]
- 15.Fein DA, Mendenhall WM, Parsons JT, Stringer SP, Cassisi NJ, Million RR. Carcinoma in situ of the glottic larynx: The role of radiotherapy. International journal of radiation oncology, biology, physics 1993;27:379–384. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Serra A, Hinerman RW, Amdur RJ, Morris CG, Mendenhall WM. Radiotherapy for carcinoma in situ of the true vocal cords. Head & neck 2002;24:390–394. [DOI] [PubMed] [Google Scholar]
- 17.Spayne JA, Warde P, O’Sullivan B, Payne D, Liu FF, Waldron J, Gullane PJ, Cummings BJ. Carcinoma-in-situ of the glottic larynx: Results of treatment with radiation therapy. International journal of radiation oncology, biology, physics 2001;49:1235–1238. [DOI] [PubMed] [Google Scholar]
- 18.Garden AS, Forster K, Wong PF, Morrison WH, Schechter NR, Ang KK. Results of radiotherapy for T2N0 glottic carcinoma: Does the “2” stand for twice-daily treatment? International journal of radiation oncology, biology, physics 2003;55:322–328. [DOI] [PubMed] [Google Scholar]
- 19.Trotti A 3rd, Zhang Q, Bentzen SM, Emami B, Hammond ME, Jones CU, Morrison WH, Sagar SM, Ridge JA, Fu KK, Ang KK. Randomized trial of hyperfractionation versus conventional fractionation in T2 squamous cell carcinoma of the vocal cord (rtog 9512). International journal of radiation oncology, biology, physics 2014;89:958–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le QT, Fu KK, Kroll S, Ryu JK, Quivey JM, Meyler TS, Krieg RM, Phillips TL. Influence of fraction size, total dose, and overall time on local control of T1-T2 glottic carcinoma. International journal of radiation oncology, biology, physics 1997;39:115–126. [DOI] [PubMed] [Google Scholar]
- 21.Short S, Krawitz H, Macann A, West T, Morton RP, McIvor NP, Chaplin J, Simcock P, Gathercole J, Dorman B, Hindley A. T1N0/T2N0 glottic carcinoma: A comparison of two fractionation schedules. Australasian radiology 2006;50:152–157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.