Abstract
Background
Electroconvulsive therapy (ECT) is a rapid and effective treatment for major depressive disorder. Chronic stress-induced depression causes dendrite atrophy and deficiencies in brain-derived neurotrophic factor (BDNF), which are reversed by anti-depressant drugs. Electroconvulsive seizures (ECS), an animal model of ECT, robustly increase BDNF expression and stimulate dendritic outgrowth.
Objective
The present study aims to understand cellular and molecular plasticity mechanisms contributing to the efficacy of ECS following chronic stress-induced depression.
Methods
We quantify Bdnf transcript levels and dendritic spine density and morphology on cortical pyramidal neurons in mice exposed to vehicle or corticosterone and receiving either Sham or ECS treatment.
Results
ECS rescues corticosterone-induced defects in spine morphology and elevates Bdnf exon 1 and exon 4-containing transcripts in cortex.
Conclusions
Dendritic spine remodeling and induction of activity-induced BDNF in the cortex represent important cellular and molecular plasticity mechanisms underlying the efficacy of ECS for treatment of chronic stress-induced depression.
Keywords: Brain-derived neurotrophic factor (BDNF), Electroconvulsive seizures, ECT, Dendritic spine, Cingulate cortex, Morphology
Introduction
Electroconvulsive therapy (ECT) is one of the most rapid and effective treatments for major depressive disorder (1, 2) and is associated with normalization of hypothalamic-pituitary-adrenal (HPA) axis abnormalities (3, 4). In rodent models, elevated glucocorticoids resulting from HPA axis dysregulation during stress cause dendritic atrophy and decreased spine density in the hippocampus and cortex (5–10), which can be reversed with anti-depressant drugs (11–13). This reorganization of morphology is associated with improved behavioral outcomes (14). While ECS can also prevent stress-induced dendrite retraction in the hippocampus (15, 16), its effects on cortical neurons, which are key players in mediating the antidepressant response (17), has not yet been determined.
Successful ECT treatment is linked to increases in the activity-dependent neurotrophin, brain-derived neurotrophic factor (BDNF) (18–21), which is a robust modulator of neuronal morphology, especially dendritic spine density and structure (22–24). Indeed, a large body of literature suggests that BDNF and glucocorticoid activities are calibrated such that BDNF levels influence susceptibility and recovery from chronic stress-induced depression (25, 26). Furthermore, a genetic variation G196A (Val66Met polymorphism) that alters levels of activity-dependent BDNF (27) may influence responsiveness to ECT and cognitive outcome (28), further suggesting that BDNF may be a potential biomarker for ECT efficacy. Importantly, Bdnf has a unique genomic structure with nine promoters that drive expression of multiple transcripts encoding an identical protein (29, 30). Methylation studies suggest that patients remitting under ECT have lower methylation at specific Bdnf promoters (31).
Here we investigate the ability of ECS to remodel cortical dendritic spines and induce activity-dependent BDNF from specific promoters in a neuroendocrine model of depression. This model employs chronic administration of corticosterone (Cort) in the drinking water, which recapitulates the HPA axis dysfunction and dendritic atrophy observed in chronic stress-induced depression (14, 32–34).
Materials and Methods
Treatment conditions
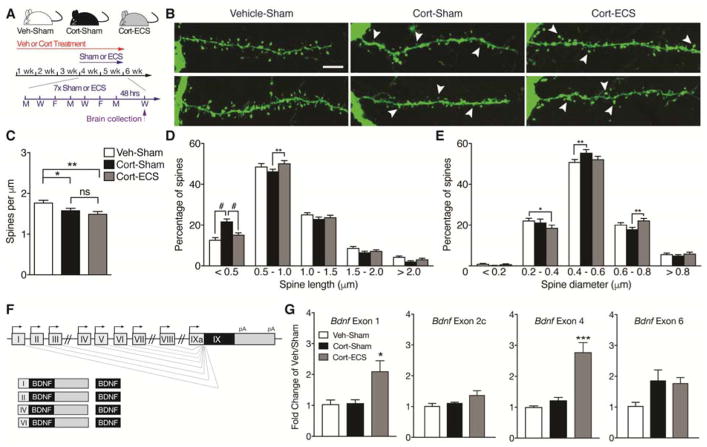
Adult male mice expressing yellow fluorescent protein (YFP) in cortical layer 5 pyramidal neurons under control of the Thy1 promoter (Thy1 “H” line; Jackson Laboratory Stock #003782(35)) were administered either vehicle (0.45% hydrox-ypropyl-β-cyclodextrin, β –CD, Sigma-Aldrich) or corticosterone (35 μg/ml/d, Sigma) dissolved in vehicle via the animal’s drinking water. Seven sessions of Sham or ECS were administered as previously described (34). Briefly, ECS was delivered with an Ugo Basile pulse generator using a corneal electrode fork placed over the frontal cortex (model #57800-001, shock parameters: 100 pulse/s frequency, 0.3 ms pulse width, 1s shock duration and 50 mA current). The stimulation parameters were chosen because they reliably induced tonic-clonic convulsions. Mice were administered inhaled isoflurane anesthesia prior to ECS sessions, and remained anesthetized for the procedure. Figure 1A displays a timeline of the study and conditions. All animal experiments were approved by the SoBran Institutional Animal Care and Use Committee.
Figure 1.
ECS rescues corticosterone-induced defects in dendritic spine morphology and modulates BDNF expression through distinct promoters. (A) Schematic of experimental timeline in which mice received vehicle (Veh) or corticosterone (Cort) in the drinking water for 3 weeks followed by administration of 7 sessions of Sham or ECS over 2 weeks (Veh or Cort treatment continued during ECS weeks). Brains were collected 48 hours after the last ECS session. (B) Representative confocal micrographs of cortical layer 5 oblique apical dendrites from mice exposed to Veh-Sham, Cort-Sham, and Cort-ECS. While corticosterone treatment decreases spine density and size compared to vehicle, chronic administration of ECS partially rescues spine morphology, but not spine density. (C) Quantification of spine density (spines per μm) on cortical layer 5 oblique apical dendrites of mice treated with Veh-Sham, Cort-Sham, and Cort-ECS (n= 61 to 67 branches per group; n = 4 mice per group). Corticosterone decreases spine density and is not rescued by ECS. (D) Frequency histogram of spine height size on cortical layer 5 oblique apical dendrites of mice treated with Veh-Sham, Cort-Sham, and Cort-ECS. Corticosterone causes a decrease in spine length that is rescued by ECS. (E) Frequency histogram of spine head width on cortical layer 5 oblique apical dendrites of mice treated with Veh-Sham, Cort-Sham, and Cort-ECS. Corticosterone causes a decrease in spine diameter that is reversed by ECS. (F) Schematic of Bdnf gene transcription. Transcription is initiated from promoters upstream of unique 5′-untranslated regions (UTRs) and spliced to the common coding exon IX. Each transcript produces the same BDNF protein. (G) Quantitative PCR for Bdnf exon 1, exon 2c, exon 4, and exon 6-containing transcripts in cingulate cortex of mice exposed to Veh-Sham, Cort-Sham, and Cort-ECS (n = 4 to 5 mice per group; brains collected 90 min after final ECS session). ECS robustly increases expression of Bdnf exon 1 and 4-containing transcripts. Data are presented as the mean ± SEM. *p<0.05, **p<0.01, ***p<0.001, #p<0.0001. Scale bar 5μm.
Confocal Imaging and Neuronal reconstruction
Animals were anesthetized with isoflurane and perfused transcardially with 4% paraformaldehyde (PFA) in phosphate-buffered saline. Brains were extracted, post-fixed in 4% PFA, cryoprotected in 30% sucrose, cut coronally at 50μm on a microtome, and stained in 0.05% Sudan Black (Sigma) to decrease autofluorescence. Isolated oblique apical dendrites (between 100 and 400um from the cell body) on layer 5 cortical neurons in somatosensory cortex were imaged on a Zeiss LSM 510 confocal microscope according to previously described criteria with experimenter blinded to genotype (23). Dendritic spine structure was reconstructed using Neurolucida (MicroBrightField Biosciences). Spine number, width, and height were quantified and statistically analyzed in GraphPad Prism using one- or two-way ANOVA and Bonferroni post hoc tests where appropriate (*p<0.05, **p<0.01, ***p<0.001, #p<0.0001).
Quantitative PCR (qPCR)
Following cervical dislocation, cingulate cortex was collected and RNA was extracted, quantified, normalized in concentration, and reverse-transcribed into cDNA as previously described (36). qPCR was performed using a Realplex thermocycler (Eppendorf) using GEMM mastermix (Life Technologies) with 40ng of synthesized DNA as previously described(36). Individual mRNA levels were normalized for each well to Gapdh mRNA levels.
Results
To investigate cellular plasticity associated with the anti-depressant response mediated by ECS, we quantified spine density and morphology on cortical pyramidal neurons of mice treated with vehicle or Cort and receiving either Sham or ECS treatment (Fig. 1A). Cort significantly reduced spine density, which was not effectively rescued by ECS (one-way ANOVA, F2, 189 = 6.829, p=0.0014, Fig. 1B and 1C). We next measured both the height and width of spine heads on cortical oblique apical dendritic branches, and spines were classified by size and binned to generate frequency histograms depicting the percentage of differently sized spine populations along a defined branch (37). Cort-Sham branches showed a significant increase in the percentage of shorter spines (<0.5μm) compared to Veh-Sham branches, which was reversed following ECS (two-way ANOVA, treatment x bin interaction, F8, 945 = 8.302, p<0.0001, Fig. 1D). Similarly, Cort-Sham branches showed a significant increase in the percentage of smaller spine heads (0.4 – 0.6μm in diameter) compared to Veh-Sham branches, which was rescued following ECS (two-way ANOVA, treatment x bin interaction, F8, 945 = 3.277, p=0.0011, Fig. 1E). To investigate molecular pathways potentially contributing to this plasticity, we examined expression of Bdnf transcripts derived from promoters I, II, IV and VI (Bdnf exon 1, 2, 4, and 6-containing transcripts, respectively; Fig. 1F) using qPCR. While Cort exposure did not alter expression of distinct Bdnf transcripts, ECS treatment significantly elevated Bdnf exon 1 and 4-containing transcripts in cingulate cortex (one-way ANOVAs; F2, 11 = 6.953, p=0.0112 and F2, 11 = 22.95, p=0.0001, respectively; Fig. 1G). Taken together, these results provide novel insights into cellular and molecular mechanisms underlying the efficacy of ECS for treatment of chronic stress-induced depression.
Discussion
In the present study, we demonstrate that ECS reverses glucocorticoid-induced defects in spine morphology on cortical pyramidal neurons and augments BDNF expression. Extensive work has linked spine size and shape to synapse stability and function where larger spine heads represent more mature spines likely to contain postsynaptic densities and glutamate receptors (38, 39). Chronic stress and glucocorticoid administration has been shown to impair spine density and morphology with detectable effects after 10 to 21 days (40, 41). Previous studies show that ECS is able to prevent dendritic atrophy in the hippocampus following chronic stress (15, 16); however, these studies did not explore other brain regions associated with the anti-depressant response, including the cerebral cortex (17). Furthermore, the impact of ECS on dendritic spine morphology and BDNF levels was not investigated. Here we provide evidence that remodeling of cortical dendritic spines and induction of Bdnf from promoters I and IV, which are highly regulated by neural activity (29), may represent critical plasticity mechanisms underlying the anti-depressant efficacy of ECS.
Our data show that ECS does not reverse cortical spine loss, but may instead stabilize existing spines to promote network connectivity (42). As Cort-induced changes in spine density and morphology are likely underway before administration of ECS (40), our results suggest that ECS can reverse these changes to baseline levels. This is consistent with other reports showing that ECS directly modulates spine density and morphology (43). However, because Cort treatment is continued during ECS administration as a more realistic model of stress-induced depression, it is also possible that ECS has a protective effect to block further Cort-induced changes in spine morphology. Future studies should parse out the contribution of ECS to these distinct aspects of the anti-depressant response.
Our results demonstrate that Cort does not decrease Bdnf transcripts, suggesting that loss of BDNF may not drive stress-induced changes in spine density and morphology. However, our findings are consistent with previous reports showing that ECS induces BDNF and extensive work implicating BDNF in the antidepressant response (19, 44, 45). Acute elevations in Bdnf Ex1 and Ex4 transcripts support the notion of activity-induced BDNF as a potential biomarker to predict and monitor response to ECT. Increases in promoter I-derived BDNF following ECS are consistent with studies in patients showing reduced methylation at BDNF promoter I for ECT remitters (31). Importantly, we measure Bdnf transcript levels acutely after 7 sessions of ECS, but experiments in the hippocampus suggest that promoter I-derived BDNF is dynamically elevated after each ECS session and that this elevation persists for up to 24 hours following the final ECS session (data not shown).
Future studies should directly address the relationship between ECS, BDNF, and spine remodeling by examining spine morphology in BDNF deficient mice subjected to the same paradigm. While construct validity of rodent models of the antidepressant response to ECS may be limited (46), such experiments could help establish a stronger direct link between BDNF-dependent molecular plasticity mechanisms and the antidepressant action of ECT. These experiments can be used to inform further studies in humans exploring BDNF induction and the relationship between brain structure and function in the context of ECT.
Highlights.
Stress-induced depression causes dendritic spine atrophy in the cortex.
We hypothesize that electroconvulsive seizures (ECS) reverse spine defects.
The hypothesis is tested in a neuroendocrine model of depression in mice.
ECS rescues stress-induced spine morphology changes on pyramidal neurons.
ECS elevates specific isoforms of brain-derived neurotrophic factor (BDNF).
Acknowledgments
Funding for these studies was provided by the Lieber Institute for Brain Development and the National Institute for Mental Health (T32MH01533037 to KRM and RO1MH105592 to KM).
Footnotes
Conflict: All authors declare no conflict of interest. We further confirm that all aspects of the work covered in the manuscript using experimental animals has been conducted with the necessary ethical approvals.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Janicak PG, Davis JM, Gibbons RD, Ericksen S, Chang S, Gallagher P. Efficacy of ECT: a meta-analysis. Am J Psychiatry. 1985;142(3):297–302. doi: 10.1176/ajp.142.3.297. [DOI] [PubMed] [Google Scholar]
- 2.Group UER. Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet. 2003;361(9360):799–808. doi: 10.1016/S0140-6736(03)12705-5. [DOI] [PubMed] [Google Scholar]
- 3.McKay MS, Zakzanis KK. The impact of treatment on HPA axis activity in unipolar major depression. J Psychiatr Res. 2010;44(3):183–92. doi: 10.1016/j.jpsychires.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Yuuki N, Ida I, Oshima A, Kumano H, Takahashi K, Fukuda M, et al. HPA axis normalization, estimated by DEX/CRH test, but less alteration on cerebral glucose metabolism in depressed patients receiving ECT after medication treatment failures. Acta Psychiatr Scand. 2005;112(4):257–65. doi: 10.1111/j.1600-0447.2005.00625.x. [DOI] [PubMed] [Google Scholar]
- 5.McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–22. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 6.Goldwater DS, Pavlides C, Hunter RG, Bloss EB, Hof PR, McEwen BS, et al. Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery. Neuroscience. 2009;164(2):798–808. doi: 10.1016/j.neuroscience.2009.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS, et al. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol. 2008;507(1):1141–50. doi: 10.1002/cne.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alfarez DN, De Simoni A, Velzing EH, Bracey E, Joels M, Edwards FA, et al. Corticosterone reduces dendritic complexity in developing hippocampal CA1 neurons. Hippocampus. 2009;19(9):828–36. doi: 10.1002/hipo.20566. [DOI] [PubMed] [Google Scholar]
- 9.Duman CH, Duman RS. Spine synapse remodeling in the pathophysiology and treatment of depression. Neurosci Lett. 2015;601:20–9. doi: 10.1016/j.neulet.2015.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morales-Medina JC, Sanchez F, Flores G, Dumont Y, Quirion R. Morphological reorganization after repeated corticosterone administration in the hippocampus, nucleus accumbens and amygdala in the rat. J Chem Neuroanat. 2009;38(4):266–72. doi: 10.1016/j.jchemneu.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe Y, Gould E, Daniels DC, Cameron H, McEwen BS. Tianeptine attenuates stress-induced morphological changes in the hippocampus. Eur J Pharmacol. 1992;222(1):157–62. doi: 10.1016/0014-2999(92)90830-w. [DOI] [PubMed] [Google Scholar]
- 12.Magarinos AM, Deslandes A, McEwen BS. Effects of antidepressants and benzodiazepine treatments on the dendritic structure of CA3 pyramidal neurons after chronic stress. Eur J Pharmacol. 1999;371(2–3):113–22. doi: 10.1016/s0014-2999(99)00163-6. [DOI] [PubMed] [Google Scholar]
- 13.Wood GE, Young LT, Reagan LP, Chen B, McEwen BS. Stress-induced structural remodeling in hippocampus: prevention by lithium treatment. Proc Natl Acad Sci U S A. 2004;101(11):3973–8. doi: 10.1073/pnas.0400208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sousa N, Lukoyanov NV, Madeira MD, Almeida OF, Paula-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97(2):253–66. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- 15.Hageman I, Nielsen M, Wortwein G, Diemer NH, Jorgensen MB. Electroconvulsive stimulations prevent stress-induced morphological changes in the hippocampus. Stress. 2008;11(4):282–9. doi: 10.1080/10253890701783794. [DOI] [PubMed] [Google Scholar]
- 16.Kaastrup Muller H, Orlowski D, Reidies Bjarkam C, Wegener G, Elfving B. Potential roles for Homer1 and Spinophilin in the preventive effect of electroconvulsive seizures on stress-induced CA3c dendritic retraction in the hippocampus. Eur Neuropsychopharmacol. 2015;25(8):1324–31. doi: 10.1016/j.euroneuro.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt EF, Warner-Schmidt JL, Otopalik BG, Pickett SB, Greengard P, Heintz N. Identification of the cortical neurons that mediate antidepressant responses. Cell. 2012;149(5):1152–63. doi: 10.1016/j.cell.2012.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altar CA, Laeng P, Jurata LW, Brockman JA, Lemire A, Bullard J, et al. Electroconvulsive seizures regulate gene expression of distinct neurotrophic signaling pathways. J Neurosci. 2004;24(11):2667–77. doi: 10.1523/JNEUROSCI.5377-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altar CA, Whitehead RE, Chen R, Wortwein G, Madsen TM. Effects of electroconvulsive seizures and antidepressant drugs on brain-derived neurotrophic factor protein in rat brain. Biol Psychiatry. 2003;54(7):703–9. doi: 10.1016/s0006-3223(03)00073-8. [DOI] [PubMed] [Google Scholar]
- 20.Kim J, Gale K, Kondratyev A. Effects of repeated minimal electroshock seizures on NGF, BDNF and FGF-2 protein in the rat brain during postnatal development. Int J Dev Neurosci. 2010;28(3):227–32. doi: 10.1016/j.ijdevneu.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rocha RB, Dondossola ER, Grande AJ, Colonetti T, Ceretta LB, Passos IC, et al. Increased BDNF levels after electroconvulsive therapy in patients with major depressive disorder: A meta-analysis study. J Psychiatr Res. 2016;83:47–53. doi: 10.1016/j.jpsychires.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Cohen-Cory S, Kidane AH, Shirkey NJ, Marshak S. Brain-derived neurotrophic factor and the development of structural neuronal connectivity. Dev Neurobiol. 2010;70(5):271–88. doi: 10.1002/dneu.20774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maynard KR, Hobbs JW, Sukumar M, Kardian AS, Jimenez DV, Schloesser RJ, et al. Bdnf mRNA splice variants differentially impact CA1 and CA3 dendrite complexity and spine morphology in the hippocampus. Brain Struct Funct. 2017 doi: 10.1007/s00429-017-1405-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vigers AJ, Amin DS, Talley-Farnham T, Gorski JA, Xu B, Jones KR. Sustained expression of brain-derived neurotrophic factor is required for maintenance of dendritic spines and normal behavior. Neuroscience. 2012;212:1–18. doi: 10.1016/j.neuroscience.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeanneteau F, Chao MV. Are BDNF and glucocorticoid activities calibrated? Neuroscience. 2013;239:173–95. doi: 10.1016/j.neuroscience.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castren E, Rantamaki T. The role of BDNF and its receptors in depression and antidepressant drug action: Reactivation of developmental plasticity. Dev Neurobiol. 2010;70(5):289–97. doi: 10.1002/dneu.20758. [DOI] [PubMed] [Google Scholar]
- 27.Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–69. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 28.Pinna M, Manchia M, Oppo R, Scano F, Pillai G, Loche AP, et al. Clinical and biological predictors of response to electroconvulsive therapy (ECT): a review. Neurosci Lett. 2016 doi: 10.1016/j.neulet.2016.10.047. [DOI] [PubMed] [Google Scholar]
- 29.Timmusk T, Palm K, Metsis M, Reintam T, Paalme V, Saarma M, et al. Multiple promoters direct tissue-specific expression of the rat BDNF gene. Neuron. 1993;10(3):475–89. doi: 10.1016/0896-6273(93)90335-o. [DOI] [PubMed] [Google Scholar]
- 30.Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;85(3):525–35. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kleimann A, Kotsiari A, Sperling W, Groschl M, Heberlein A, Kahl KG, et al. BDNF serum levels and promoter methylation of BDNF exon I, IV and VI in depressed patients receiving electroconvulsive therapy. J Neural Transm (Vienna) 2015;122(6):925–8. doi: 10.1007/s00702-014-1336-6. [DOI] [PubMed] [Google Scholar]
- 32.David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62(4):479–93. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Darcet F, Mendez-David I, Tritschler L, Gardier AM, Guilloux JP, David DJ. Learning and memory impairments in a neuroendocrine mouse model of anxiety/depression. Front Behav Neurosci. 2014;8:136. doi: 10.3389/fnbeh.2014.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schloesser RJ, Orvoen S, Jimenez DV, Hardy NF, Maynard KR, Sukumar M, et al. Antidepressant-like Effects of Electroconvulsive Seizures Require Adult Neurogenesis in a Neuroendocrine Model of Depression. Brain Stimul. 2015;8(5):862–7. doi: 10.1016/j.brs.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28(1):41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 36.Maynard KR, Hill JL, Calcaterra NE, Palko ME, Kardian A, Paredes D, et al. Functional Role of BDNF Production from Unique Promoters in Aggression and Serotonin Signaling. Neuropsychopharmacology. 2016;41(8):1943–55. doi: 10.1038/npp.2015.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belichenko NP, Belichenko PV, Kleschevnikov AM, Salehi A, Reeves RH, Mobley WC. The “Down syndrome critical region” is sufficient in the mouse model to confer behavioral, neurophysiological, and synaptic phenotypes characteristic of Down syndrome. J Neurosci. 2009;29(18):5938–48. doi: 10.1523/JNEUROSCI.1547-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hering H, Sheng M. Dendritic spines: structure, dynamics and regulation. Nat Rev Neurosci. 2001;2(12):880–8. doi: 10.1038/35104061. [DOI] [PubMed] [Google Scholar]
- 39.Arellano JI, Benavides-Piccione R, Defelipe J, Yuste R. Ultrastructure of dendritic spines: correlation between synaptic and spine morphologies. Front Neurosci. 2007;1(1):131–43. doi: 10.3389/neuro.01.1.1.010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liston C, Gan WB. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc Natl Acad Sci U S A. 2011;108(38):16074–9. doi: 10.1073/pnas.1110444108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson RM, Glanz RM, Johnson SB, Miller MM, Romig-Martin SA, Radley JJ. Prolonged corticosterone exposure induces dendritic spine remodeling and attrition in the rat medial prefrontal cortex. J Comp Neurol. 2016;524(18):3729–46. doi: 10.1002/cne.24027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heikman P, Salmelin R, Makela JP, Hari R, Katila H, Kuoppasalmi K. Relation between frontal 3–7 Hz MEG activity and the efficacy of ECT in major depression. J ECT. 2001;17(2):136–40. doi: 10.1097/00124509-200106000-00009. [DOI] [PubMed] [Google Scholar]
- 43.Zhao C, Warner-Schmidt J, Duman RS, Gage FH. Electroconvulsive seizure promotes spine maturation in newborn dentate granule cells in adult rat. Dev Neurobiol. 2012;72(6):937–42. doi: 10.1002/dneu.20986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15(11):7539–47. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bjorkholm C, Monteggia LM. BDNF - a key transducer of antidepressant effects. Neuropharmacology. 2016;102:72–9. doi: 10.1016/j.neuropharm.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Theilmann W, Loscher W, Socala K, Frieling H, Bleich S, Brandt C. A new method to model electroconvulsive therapy in rats with increased construct validity and enhanced translational value. J Psychiatr Res. 2014;53:94–8. doi: 10.1016/j.jpsychires.2014.02.007. [DOI] [PubMed] [Google Scholar]