Abstract
Background
Schistosoma mansoni infection has been associated with increased risk of HIV transmission in African women. This association might be causal or mediated through shared socio-behavioural factors and associated co-infections. We tested the latter hypothesis in a cross-sectional pilot study in a cohort of women from a S. mansoni endemic region of Uganda. To validate the immunological effects of S. mansoni in this cohort, we additionally assessed known schistosomiasis biomarkers.
Methods
HIV-uninfected non-pregnant adult women using public health services were tested for schistosomiasis using the urine circulating cathodic antigen test, followed by serology and Schistosoma spp.-specific PCR. Blood was obtained for herpes simplex virus (HSV)-2 serology, eosinophil counts and cytokine analysis. Samples collected from the genitourinary tract were used to test for classical sexually transmitted infections (STI), for bacterial vaginosis and to assess recent sexual activity via prostate-specific antigen testing. Questionnaires were used to capture a range of socio-economic and behavioral characteristics.
Results
Among 58 participants, 33 (57%) had schistosomiasis, which was associated with elevated levels of interleukin (IL)-10 (0.32 vs. 0.19 pg/ml; p = 0.038) and a trend toward increased tumour necrosis factor (TNF) (1.73 vs. 1.42 pg/ml; p = 0.081). Eosinophil counts correlated with levels of both cytokines (r = 0.53, p = 0.001 and r = 0.38, p = 0.019, for IL-10 and TNF, respectively); the association of eosinophilia with schistosomiasis was not significant (OR = 2.538, p = 0.282). Further, schistosomiasis was associated with lower age (per-year OR = 0.910, p = 0.047), being unmarried (OR = 0.263, p = 0.030), less frequent hormonal contraceptive (HC) use (OR = 0.121, p = 0.002, dominated by long acting injectable contraceptives) and a trend to longer time since penile-vaginal sex (OR = 0.350, p = 0.064). All women infected by Chlamydia trachomatis (n = 5), were also positive for schistosomiasis (Fisher’s exact p = 0.064).
Conclusions
Intestinal schistosomiasis in adult women was associated with systemic immune alterations, suggesting that associations with immunological correlates of HIV susceptibility warrant further investigation. S. mansoni associations with socio-behavioral parameters and C. trachomatis, which may alter both genital immunity and HIV exposure and/or acquisition risk, means that future studies should carefully control for potential confounders. These findings have implications for the design and interpretation of clinical studies on the effects of schistosomiasis on HIV acquisition.
Keywords: Schistosoma mansoni, Intestinal schistosomiasis, HIV susceptibility, HIV risk factors, Injectable hormonal contraceptives, Sexually transmitted infections
Background
Schistosomiasis is a neglected tropical disease caused by trematode worms inhabiting the gastrointestinal and/or genitourinary venules. Over 200 million people are infected globally, with a disproportionate burden in Africa, where approximately 90% cases are found alongside significant co-endemicity with HIV-1 (HIV) [1, 2]. Accumulating evidence suggests that schistosomiasis may increase the risk of HIV transmission through complex effects on mucosal immunity and antiviral defenses [2–4]. This association is not only seen for infection by Schistosoma haematobium, the cause of genitourinary schistosomiasis, but also for S. mansoni, which predominantly affects the gut and causes intestinal/hepatic schistosomiasis [5–10]. Specifically, studies performed in Tanzania reported that S. mansoni-infected women were six-fold more likely to be HIV-infected compared to their female peers without schistosomiasis [10]. Subsequently, a prospective study performed in Tanzania found that S.mansoni-infected women had a 2.8-fold increased risk of HIV acquisition [11]. Notably, these effects of S.mansoni on HIV acquisition in the Tanzanian studies were only seen in women, but not men [9, 11], implying that the effects on HIV susceptibility are mediated by either biological or socio-behavioral factors specific to women.
While various systemic and mucosal immune mechanisms have been hypothesized to explain the latter association [2, 11], the exact underlying cause of increased HIV susceptibility in the context of S. mansoni infection remains unclear. Furthermore, studies in this area could be confounded if socio-behavioral factors associated with HIV risk differed between women with and without schistosomiasis.
Active S. mansoni infection results in parasite egg-induced granulomatous inflammation in the colon and surrounding internal organs, and subsequent changes in various immunological processes, such as immune cell trafficking [3]. The extent of immune alteration caused by schistosomiasis can be assessed by measuring the levels of specific immune mediators and immune cells in the blood of infected individuals. For example, circulating cytokines interleukin-10 (IL-10) and tumor necrosis factor (TNF) are two biomarkers that have consistently been shown as elevated in human schistosomiasis [12–16]. Eosinophilia (elevated eosinophil counts) is yet another common diagnostic used to assess the severity of helminth infection [17, 18].
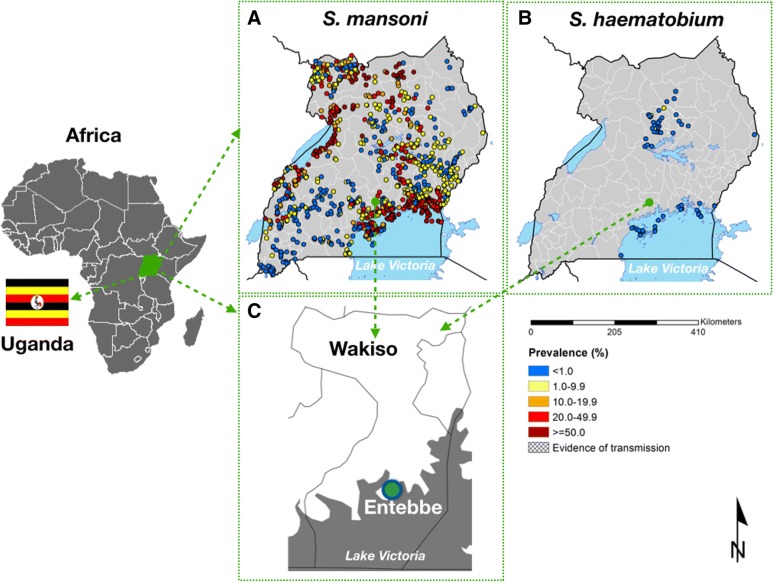
As a precursor to studies on the immune impact of schistosomiasis in adults, we performed a cross-sectional observational study, which examined the relationship between schistosomiasis and behavioral HIV risk factors in adult women from Wakiso district, a region endemic for S. mansoni [19, 20] (Fig. 1). To this end, we recruited HIV-negative non-pregnant women in Entebbe town and collected from the study participants demographic and diagnostic data, including data on the prevalence of schistosomiasis and classical sexually transmitted infection (STI), as well as measured circulating cytokine levels.
Fig. 1.
Distribution of schistosomiasis and the study site location in Uganda. a. Prevalence and distribution of S. mansoni in Uganda; b. Prevalence and distribution of S. haematobium in Uganda; c. Map of Wakiso district and location of the study site (Entebbe). Note that Entebbe is endemic for S. mansoni but not S. haematobium. The maps show the location of schistosomiasis surveys and the reported prevalence of schistosomiasis across Uganda. Scale is given for the maps of Uganda. Map source: The Global Atlas of Helminth Infection [21]
Methods
Study setting and participant recruitment
The study was conducted in Entebbe, a town situated on a peninsula in Lake Victoria (Fig. 1), between September 2015–February 2016. Entebbe has an HIV prevalence of ~ 20% [19] and a schistosomiasis prevalence of ~ 70%, largely due to S. mansoni [19] with much lower (< 1%) rates of S. haematobium [21]. Consenting women aged 18–45 years (n = 58) attending family planning or child vaccination clinics at Entebbe General Hospital or a nearby General Practice clinic were screened for HIV, malaria and pregnancy as previously described [22]; those who tested positive for any of the three conditions were referred for appropriate care according to the Uganda clinical guidelines while those testing negative were eligible for enrolment. This study was designed to provide pilot data for future immune studies, therefore no formal sample size calculations were made.
Sample collection and diagnostic testing
Blood (16 ml) was collected by venipuncture and blood plasma was isolated and stored at -80 °C prior to downstream testing. Eosinophil counts were acquired using an ACT 5diff automated hematology analyzer (Beckman Coulter, USA), and eosinophilia was defined as an eosinophil count > 450 cells per μl of whole blood [17]. Schistosomiasis was diagnosed based on the urine circulating cathodic antigen (CCA), a by-product of adult schistosome metabolism. The CCA test (Rapid Medical Diagnostics, Pretoria, South Africa) is highly sensitive but does not allow schistosome speciation [23]. Therefore, stored plasma was used to extract cell free DNA using QIAamp MinElute extraction kit (Qiagen, Germany) according to the manufacturer’s protocol for subsequent species-specific PCR performed as previously described [24–26]. Testing for Chlamydia trachomatis (Ct) and Neisseria gonorrhoeae (Ng) was performed on urine samples using the Roche Cobas PCR (Roche Diagnostics Corp, Indianapolis, USA). One vaginal swab was tested for Trichomonas vaginalis (Tv) using the OSOM rapid test (Sekisui Diagnostics, Framingham, USA), and a second vaginal swab was smeared onto a glass slide, air-dried and Gram’s stained to diagnose bacterial vaginosis (BV) using Nugent criteria [27]. A SoftCup (EvoFem, San Diego, USA) was used to collect cervico-vaginal secretions for prostate-specific antigen testing (PSA; Seratec PSA Semiquant kit, Göttingen, Germany) according to the PSA kit manufacturer’s instructions. Recent (≤ 3 days) unprotected penile vaginal-sex was defined as a positive PSA test result, since softcup PSA levels increase immediately after unprotected sex and return to baseline levels by approximately 72 h post-exposure. Stored plasma was used to perform serology for the presence of antibody specific to S. mansoni soluble egg antigen (SmSEA; Scimedx, New Jersey, USA) and optic density (OD) values > 0.2 were considered positive. Herpes simplex virus type 2 (Kalon HSV-2 IgG, Kalon Biological Ltd., UK) testing was performed on stored plasma. Measurements of circulating interleukin-10 (IL-10) and tumor necrosis factor (TNF) were performed on a subset of stored plasma samples using the Meso Scale Discovery electrochemiluminescent ELISA (MD, USA) as done previously [28]. All experimental assays were performed by research personnel blinded to the status of participants. Questionnaires capturing specific socio-economic and behavioral characteristics, such as self-reported sex and contraceptive use (Table 1) were administered.
Table 1.
Associations of participant characteristics with schistosome infection
| Participant characteristic | Entire cohort (n = 58) | Schistosoma spp. ag -positive (N = 33) | Schistosoma spp. ag -negative (N = 25) | OR for association with schistosomiasis (95% CI) | P value (α = 0.05) |
|---|---|---|---|---|---|
| Median age (IQR) | 27.5 (23.8–32.0) | 25.0 (22.5–29.5) | 30.0 (25.0–34.0) | 0.910 (0.830–0.999) | 0.047 |
| Married, % | 60.7 (34/56) | 50.0 (16/32) | 79.2 (19/24) | 0.263 (0.079–0.878) | 0.030 |
| Sexual behaviour | |||||
| Hormonal contraceptive use, % | 30.4 (17/56) | 12.5 (4/32) | 54.2 (13/24) | 0.121 (0.032–0.452) | 0.002 |
| DMPAa, % | 19.6 (11/56) | 9.4 (3/32) | 33.3 (8/24) | ||
| NetEna, % | 8.9 (5/56) | 3.1 (1/32) | 16.7 (4/24) | ||
| Oral pill, % | 1.8 (1/56) | 0 (0/32) | 4.2 (1/24) | ||
| Sex in last 3 days | |||||
| PSA+, % | 41.8 (23/55) | 31.3 (10/32) | 56.5 (13/23) | 0.350 (0.115–1.064) | 0.064b |
| Self-reported, % | 29.6 (16/54) | 26.7 (8/30) | 33.3 (8/24) | 0.727 (0.225–2.349) | 0.595 |
| Reported condom use in last sex, % | 19.2 (10/52) | 20.0 (6/30) | 18.2 (4/22) | 1.125 (0.276–4.585) | 1.00 |
| Presence of eosinophiliaa, % | 14.3 (8/56) | 18.8 (6/32) | 8.3 (2/24) | 2.538 (0.465–13.868) | 0.282 |
| HSV-2 seropositive, % | 58.6 (34/58) | 63.6 (21/33) | 52.0 (13/25) | 1.615 (0.561–4.652) | 0.374 |
| Genital conditions | |||||
| Presence of tested STI, % | 12.1 (7/58) | 15.2 (5/33) | 8.0 (2/25) | 2.054 (0.364–11.585) | 0.408 |
| T.vaginalis | 1.7 (1/58) | 0.0 (0/33) | 4.0 (1/25) | ||
| C. trachomatis | 8.6 (5/58) | 15.2 (5/33) b | 0.0 (0/25) | ||
| N. gonorrhoeae | 1.7 (1/58) | 0.0 (0/33) | 4.0 (1/25) | ||
| Self-reporting genital condition in past month, % | 30.9 (17/55) | 38.7 (12/31) | 20.8 (5/24) | 2.4 (0.707–8.144) | 0.160 |
| Presence of bacterial vaginosis, % | 30.6 (11/36) | 20.0 (4/20) | 43.8 (7/16) | 0.321 (0.074–1.405) | 0.159 |
ag antigen, OR odds ratio, DMPA depot-medroxyprogesterone acetate, NET-EN norethisterone enanthate, PSA prostate-specific antigen, STI sexually transmitted infection; aeosinophilia was defined as > 450 eosinophils per ul of blood; b trend. Data were assessed using univariate binomial logistic regression with the Schistosoma spp. ag-free (CCA-negative) group as the reference category. When OR is above 1, there is a positive association of given factor with schistosomiasis; OR value above 1 represents inverse relationship of given factor with schistosomiasis. OR for age is a per year OR
Statistical analysis
To examine associations between each factor and the presence/absence (+/−) of schistosomiasis, we first performed univariate binomial logistic regression with age as a continuous variable and 10 categorical variables (Table 1) and the schistosomiasis-free (CCA-negative) group (n = 25) as the reference category. Then factors found to be significantly associated with schistosomiasis in the binomial regressions (age, marital status, hormonal contraceptive (HC) use (all HC categories combined); recent sex was also included although its association was not significant in the binomial regression) were tested for multicollinearity prior to inclusion in a multivariable binomial regression (Table 2). Cytokine levels between schistosoma +/− groups were compared using a Mann-Whitney U test. Correlations of cytokine levels and eosinophil counts were assessed on log10-transformed values by Pearson’s correlation analysis. All statistical analyses were conducted using IBM SPSS V.23 (NY, US). Graphs were plotted using GraphPad Prism V.6.0. (CA, US).
Table 2.
Associations of age, marital status, hormonal contraceptive use and recent sex with schistosome infection as assessed by multivariable logistic regression
| Participant characteristic | Entire cohort (n = 58) | Schistosoma spp. ag -positive (N = 33) | Schistosoma spp. ag -negative (N = 25) | OR for association with schistosomiasis (95% CI) | P value (α = 0.05) |
|---|---|---|---|---|---|
| Median age (IQR) | 27.5 (23.8–32.0) | 25.0 (22.5–29.5) | 30.0 (25.0–34.0) | 0.934 (0.838–1.041) | 0.216 |
| Married, % | 60.7 (34/56) | 50.0 (16/32) | 79.2 (19/24) | 0.590 (0.138–2.527) | 0.477 |
| Sexual behaviour | |||||
| Hormonal contraceptive use, % | 30.4 (17/56) | 12.5 (4/32) | 54.2 (13/24) | 0.151 (0.037–0.611) | 0.008 |
| DMPA*, % | 19.6 (11/56) | 9.4 (3/32) | 33.3 (8/24) | ||
| NetEn*, % | 8.9 (5/56) | 3.1 (1/32) | 16.7 (4/24) | ||
| Oral pill, % | 1.8 (1/56) | 0 (0/32) | 4.2 (1/24) | ||
| Sex in last 3 days | |||||
| PSA+, % | 41.8 (23/55) | 31.3 (10/32) | 56.5 (13/23) | 0.480 (0.130–1.773) | 0.271 |
ag antigen, OR odds ratio, DMPA depot-medroxyprogesterone acetate, NET-EN norethisterone enanthate, PSA prostate-specific antigen. Data were assessed using multivariable binomial logistic regression with factors that were found to have significant associations in univariate analysis and the Schistosoma spp. ag-free (CCA-negative) group as the reference category. When OR is above 1, there is a positive association of given factor with schistosomiasis; OR value above 1 represents inverse relationship of given factor with schistosomiasis. OR for age is a per year OR
Results
Participant demographics
A total of 58 women met inclusion criteria and were enrolled; socio-behavioural characteristics are shown in Table 1. The median participant age was 27.5 years, and 56.9% (33/58) of women were diagnosed with schistosomiasis based on urine CCA testing. No participant recalled having received antihelminthic or anti-schistosomal treatment in the last 10 years.
Systemic immune biomarkers of schistosomiasis
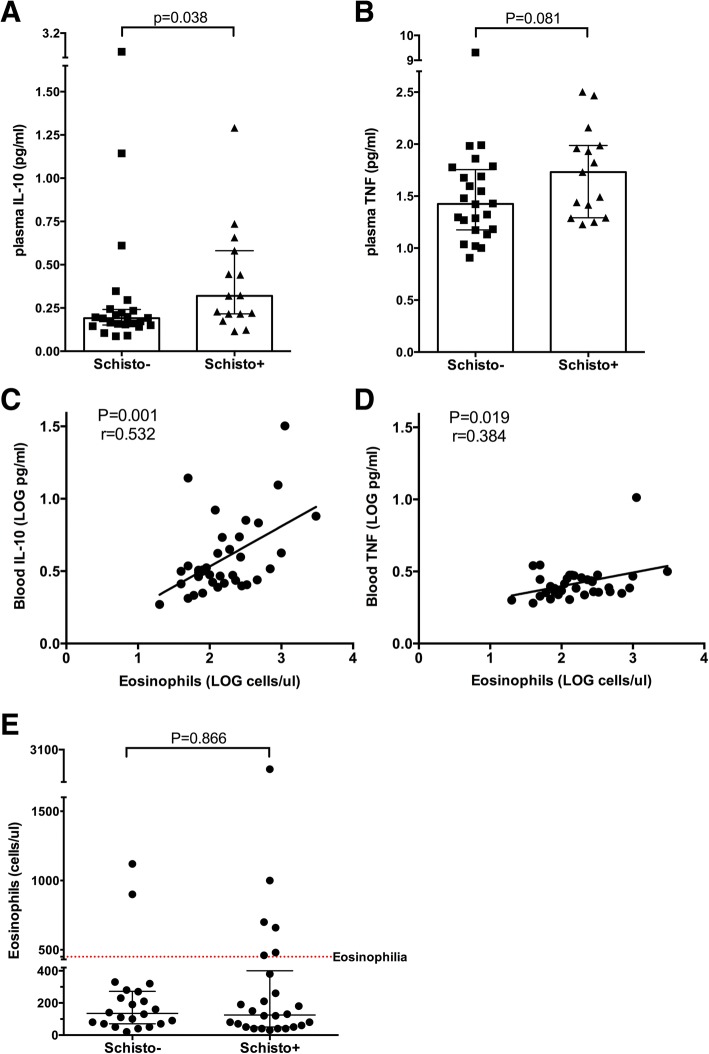
First, we examined levels of blood cytokines IL-10 and TNF and eosinophil counts. Participants diagnosed with schistosomiasis based on CCA positivity had increased levels of IL-10 (median of 0.32 pg/ml vs. 0.19 pg/ml in CCA-negative controls, p = 0.038, ~ 1.70 fold difference), and tended to have elevated levels of TNF (median of 1.73 pg/ml vs. 1.42 pg/ml, p = 0.081, ~ 1.21 fold difference) compared to schistosoma-negative women (Fig. 2a & b). Further, both blood IL-10 and TNF levels were positively correlated with eosinophil counts (r = 0.53, p = 0.001 and r = 0.38, p = 0.019, respectively; Fig. 2c & d), although the associations of eosinophilia and eosinophil counts with schistosomiasis were not significant (OR = 2.538, p = 0.282 for eosinophilia and p = 0.866 for eosinophil counts; Table 1 and Fig. 2e).
Fig. 2.
Systemic immunological differences observed between women with (schisto+) and without schistosomiasis (schisto-). a. Plasma IL-10 levels; b. Plasma TNF levels; c and d. Correlations between eosinophil counts and IL-10 (c) and TNF (d). e. Eosinophil counts, where red dotted line depicts the conventional threshold of eosinophilia (450 cells per μl of whole blood). Multiplex ELISA assays were conducted by a technologist blinded to schistosomiasis status on plasma samples available for 39 women (15 positive and 24 negative for schistosomiasis). Cytokine levels and eosinophil counts were compared by Mann-Whitney test (p = 0.05); plots depict medians and interquartile ranges. Correlations were assessed on LOG-transformed values by Spearman test (p = 0.05)
Socio-behavioural associations of schistosomiasis
Women with schistosomiasis differed from their infection-free peers in several parameters previously linked to both mucosal immunology and HIV risk. Specifically, CCA-positive participants were younger (median age 25 vs. 30 years; per year OR = 0.910, p = 0.047), less likely to be married (50.0% vs. 79.2%; OR = 0.263, p = 0.030) and less likely to be using hormonal contraceptives (HC, 12.5% vs. 54.2%; OR = 0.121, p = 0.002), mainly consisting of long acting injectable depot-medroxyprogesterone acetate (DMPA, 64.7%) and norethisterone enanthate (NetEn, 29.4%). Recent unprotected penile-vaginal sex, defined as the detection of PSA in cervico-vaginal secretions, tended to be less common in women with schistosomiasis (31.3% vs. 56.5%; OR = 0.350, p = 0.064). Although the detection of any classical STI (defined as Ng, Ct or Tv) was not associated with schistosomiasis, all 5 Ct-infected participants were co-infected with schistosomiasis (Fisher’s exact p = 0.064). No associations were apparent between schistosomiasis and condom use, HSV-2 infection, self-reported genital symptoms or BV (Table 1).
To assess whether the associations with age, marital status, HC use and unprotected sex could be driven by a subset of factors, we assessed the correlation and multicollinearity among factors and performed multivariable regression. Age and marital status were significantly correlated (point-biserial correlation, p < 0.001, r = 0.462), but multicollinearity was not detected based on a variance inflation factor threshold of three. Thus, we performed multivariable regression with age, marital status, use of hormonal contraceptives and PSA-positivity as independent variables and determined that only the use of long-acting injectable contraceptives remained significantly associated with schistosomiasis status (OR = 0.151, p = 0.008; Table 2); inclusion of fewer variables in the model did not significantly change the OR for any of the factors under consideration.
Sub-analysis based on schistosome speciation
The CCA test exhibits high sensitivity for active schistosomiasis, but may give false positive results in the context of urinary tract infections [29]. Therefore, we retrospectively performed PCR and serology testing on stored plasma samples to validate the results derived from CCA alone. When analysis was restricted to CCA+ participants who were positive by either S. mansoni-specific PCR and/or serology (n = 10), significant associations of schistosomiasis were again seen with marital status (OR = 0.113, p = 0.011), long-acting contraceptive use (OR = 0.094, p = 0.037) and the presence of blood eosinophilia (OR of 7.33, p = 0.042). One CCA-negative woman was found to have a positive PCR result for S. haematobium (but not for S. mansoni); exclusion of this participant did not have a significant effect on analysis outcomes.
Discussion
In this pilot study, our aim was to examine the relationship between intestinal schistosomiasis and socio-behavioral HIV risk factors in a cohort of adult women from the Wakiso district of Uganda, a region endemic for S. mansoni. In addition, we were interested in validating in this cohort the known associations of S. mansoni with circulating IL-10, TNF and eosinophilia. To this end, we compared the diagnostic and demographic profiles of adult women with and without schistosomiasis. We observed that S. mansoni in this cohort was associated with differences in several socio-behavioral factors, including HC use, and C. trachomatis prevalence, which could influence genital immunity and HIV susceptibility. At the same time, schistosomiasis-infected women exhibited previously described systemic immune alterations, emphasizing the relevance of further studies of immunological correlates of HIV susceptibility.
Previously, S. mansoni infection has been linked to increased HIV acquisition in women in some [10, 11], but not all [19, 20], epidemiological studies. Since the mucosal immune environment is a key determinant of HIV acquisition risk [30], defining the genital immune impact of schistosomiasis could clarify biological mechanism(s) and lead to novel means of HIV prevention. It is widely recognized that S. haematobium infection directly involves the urogenital mucosa, compromising epithelial integrity and causing mucosal inflammation [2, 5]. However, the biologic basis for an association between S. mansoni infection and genital HIV susceptibility is less clear, since the parasite primarily infects the gastrointestinal and portal vasculature [2, 11]. While genital immune studies may help to clarify this question, our study demonstrates that schistosomiasis - and S. mansoni infection in particular - is associated with differences in women’s age, marital status, hormonal contraceptive use, sexual behavior and C. trachomatis prevalence. Since all of these parameters can both modulate HIV risk and alter genital immunology [30–32], they may confound clinical studies of the impact of schistosomiasis on mucosal HIV susceptibility. Potential study designs to overcome this barrier would include a large enough sample size to permit robust multivariable analysis, or longitudinal studies that control for inter-individual confounders by assessing changes in mucosal immunology before and after schistosomiasis treatment.
Consistent with our previous study in men [33] and despite the socio-behavioral differences observed here, we found that S. mansoni infection was associated with distinct systemic immunological signatures. Specifically, elevated levels of IL-10 and TNF indicate the presence of parasite-driven inflammation in schistosoma-infected women, suggesting that associations with immunological correlates of HIV susceptibility warrant further investigation.
Our observation that schistosomiasis-infected women are younger than schistosomiasis-free women is consistent with other reports indicating that both schistosomiasis prevalence and intensity of infection peak at 10–20 years and then decline with age due to a combination of changing behavioural patterns of exposure to schistosome-contaminated water and build-up of anti-schistosomal immunity [8, 34]. Younger women were also found to have a higher prevalence of C. trachomatis [35] and, not surprisingly, were less likely to be married. The latter has important implications for hormonal contraceptive choices and the frequency of sex [32], so that the age association of schistosomiasis could be the primary driver of the observed differences. However, in the multivariable regression, only HC use remained significantly associated with schistosomiasis status after inclusion of age and marital status, implying that the observed associations may not be driven by age alone, and could also involve other latent socio-behavioral characteristics. Notably, no significant multi-collinearity was detected among the factors included in the multivariable model, suggesting that each factor contributes independently to the overall interaction with S. mansoni infection.
To our best knowledge, the current study is the first to report the inverse association of injectable contraceptive use with S. mansoni infection in African women. To date, several studies have assessed the epidemiological association of S. mansoni with HIV, with conflicting results. Tanzanian women (but not men) with S. mansoni infection were more likely to acquire HIV [9–11], but studies in Uganda [19, 20] did not find similar HIV risk associations in either women or men. Interestingly, none of these studies assessed injectable contraceptive use, known to considerably vary across East African countries [36, 37], to be linked with both HIV acquisition [38] and altered genital immunology [31], and was less common in women with schistosomiasis in this study.
Our findings should be interpreted in the light of several limitations. First, the study was designed as a pilot with a small sample size, precluding a more detailed assessment of parameters such as HSV-2 infection, the prevalence of which tended to be increased in schistosomiasis-infected women despite their lower age. Therefore, larger studies will be necessary for a more robust assessment of these parameters. In addition, we screened for schistosomiasis by urine CCA testing; while this test is well adapted for field use and is more sensitive than stool microscopy [39], it is not species-specific and can yield false positive readouts in the presence of urinary tract infections [29]. However, our results remained consistent in participants, who were demonstrated by PCR/serology to have S. mansoni infection (n = 10); this subset would be expected to have a relatively high worm burden (as confirmed by a significant association with eosinophilia), and represent a minority of infected individuals. Lastly, our study recruitment took place at clinics that offered and/or monitored family planning, which might amplify the observed hormonal contraceptive-S.mansoni association. Nevertheless, the overall rate of HC use in our study (~ 30%) was similar to that observed in broader communities from the Lake Victoria region [40].
Conclusions
This study demonstrated that S. mansoni infection in Ugandan women was associated with previously described systemic immune alterations, warranting further investigation of immunological correlates of HIV susceptibility. Schistosomiasis was also associated with differences in age, marital status, hormonal contraceptive use, recent sex and C. trachomatis prevalence. The direction of these associations is complex and would be expected to confound future studies that aim to define the impact of S. mansoni infection on HIV susceptibility. These findings will need to be considered in the design and interpretation of such studies.
Acknowledgements
We thank the participants and members of all research teams involved in the study. We are especially grateful to Dr. Moses Muwanga, Irene Wesonga and Shardiah Namusisi (Entebbe General Hospital), Dr. George Miiro, Obenyu P. Akiteng and David A. Drajole (UVRI clinic), Sanja Huibner (University of Toronto), Rachel Lau (Public Health Ontario Laboratories) and to the staff of the UVRI-IAVI and UVRI-MRC Clinical Laboratories. We thank Dr. Dominic Wichmann (University Hospital Hamburg-Eppendorf) for his expert advice on Schistosoma spp.-specific PCR.
Funding
This study was supported by the Canadian Institutes of Health Research (CIHR) to RK (TMI-138656), CIHR Vanier Canada Graduate Scholarship to SY and the Fogarty HIV Research Training Program of the National Institutes of Health to RMG (4D43TW009578–04).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors’ contributions
SY, RK: conceived, designed and implemented the study, drafted the manuscript. SY, RMG, SVG: data collection and analysis. JM: contributed to study design and supervision at clinical sites. ET, AB: oversaw schistosomiasis PCR/serology testing. NK, BSB, RK: overall study conception, design, implementation and supervision. All authors critically reviewed the draft of the paper and approved the final version of the manuscript.
Ethics approval and consent to participate
All study procedures were approved by the Uganda Virus Research Institute Research and Ethics Committee, the Uganda National Council for Science and Technology, and the Institutional Review Board at the University of Toronto. Written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sergey Yegorov, Email: sergey.yegorov@mail.utoronto.ca.
Ronald M. Galiwango, Email: ronald.galiwango@mail.utoronto.ca
Sara V. Good, Email: sara.good@sickkids.ca
Juliet Mpendo, Email: jmpendo@iavi.or.ug.
Egbert Tannich, Email: tannich@bnitm.de.
Andrea K. Boggild, Email: andrea.boggild@utoronto.ca
Noah Kiwanuka, Email: nkiwanuka@gmail.com.
Bernard S. Bagaya, Email: bbagaya@yahoo.com
Rupert Kaul, Email: rupert.kaul@utoronto.ca.
References
- 1.World Health Organization. Schistosomiasis Fact Sheet [Internet]. 2017. Available from: http://www.who.int/en/news-room/fact-sheets/detail/schistosomiasis. Accessed 22 Feb 2018.
- 2.Secor WE. The effects of schistosomiasis on HIV/AIDS infection, progression and transmission. Curr Opin HIV AIDS. 2012;7(3):254–259. doi: 10.1097/COH.0b013e328351b9e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mouser EE, Pollakis G, Paxton WA. Effects of helminths and Mycobacterium tuberculosis infection on HIV-1: a cellular immunological perspective. Curr Opin HIV AIDS. 2012;7(3):260–267. doi: 10.1097/COH.0b013e3283521144. [DOI] [PubMed] [Google Scholar]
- 4.Hotez PJ, Fenwick A, Kjetland EF. Africa's 32 cents solution for HIV/AIDS. PLoS Negl Trop Dis. 2009;3(5):e430. doi: 10.1371/journal.pntd.0000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mbabazi PS, Andan O, Fitzgerald DW, Chitsulo L, Engels D, Downs JA. Examining the relationship between urogenital schistosomiasis and HIV infection. PLoS Negl Trop Dis. 2011;5(12):e1396. doi: 10.1371/journal.pntd.0001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kjetland EF, Ndhlovu PD, Gomo E, Mduluza T, Midzi N, Gwanzura L, et al. Association between genital schistosomiasis and HIV in rural Zimbabwean women. AIDS. 2006;20(4):593–600. doi: 10.1097/01.aids.0000210614.45212.0a. [DOI] [PubMed] [Google Scholar]
- 7.Mbah MLN, Poolman EM, Drain PK, Coffee MP, van der Werf MJ, Galvani AP. HIV and Schistosoma haematobium prevalences correlate in sub-Saharan Africa. Tropical Med Int Health. 2013;18(10):1174–1179. doi: 10.1111/tmi.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Downs JA, Mguta C, Kaatano GM, Mitchell KB, Bang H, Simplice H, et al. Urogenital schistosomiasis in women of reproductive age in Tanzania's Lake Victoria region. Am J Trop Med Hyg. 2011;84(3):364–369. doi: 10.4269/ajtmh.2011.10-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Downs JA, de Dood CJ, Dee HE, McGeehan M, Khan H, Marenga A, et al. Schistosomiasis and human immunodeficiency virus in men in Tanzania. Am J Trop Med Hyg. 2017;96(4):856–862. doi: 10.4269/ajtmh.16-0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Downs JA, van Dam GJ, Changalucha JM, Corstjens PL, Peck RN, de Dood CJ, et al. Association of Schistosomiasis and HIV infection in Tanzania. Am J Trop Med Hyg. 2012;87(5):868–873. doi: 10.4269/ajtmh.2012.12-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Downs JA, Dupnik KM, van Dam GJ, Urassa M, Lutonja P, Kornelis D, et al. Effects of schistosomiasis on susceptibility to HIV-1 infection and HIV-1 viral load at HIV-1 seroconversion: a nested case-control study. PLoS Negl Trop Dis. 2017;11(9):e0005968. doi: 10.1371/journal.pntd.0005968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Jesus AR, Silva A, Santana LB, Magalhaes A, de Jesus AA, de Almeida RP, et al. Clinical and immunologic evaluation of 31 patients with acute schistosomiasis mansoni. J Infect Dis. 2002;185(1):98–105. doi: 10.1086/324668. [DOI] [PubMed] [Google Scholar]
- 13.Zwingenberger K, Irschick E, Vergetti Siqueira JG, Correia Dacal AR, Feldmeier H. Tumour necrosis factor in hepatosplenic schistosomiasis. Scand J Immunol. 1990;31(2):205–211. doi: 10.1111/j.1365-3083.1990.tb02761.x. [DOI] [PubMed] [Google Scholar]
- 14.Mwatha JK, Kimani G, Kamau T, Mbugua GG, Ouma JH, Mumo J, et al. High levels of TNF, soluble TNF receptors, soluble ICAM-1, and IFN-gamma, but low levels of IL-5, are associated with hepatosplenic disease in human schistosomiasis mansoni. J Immunol. 1998;160(4):1992–1999. [PubMed] [Google Scholar]
- 15.Secor WE. Immunology of human schistosomiasis: off the beaten path. Parasite Immunol. 2005;27(7–8):309–316. doi: 10.1111/j.1365-3024.2005.00778.x. [DOI] [PubMed] [Google Scholar]
- 16.Elfaki TE, Arndts K, Wiszniewsky A, Ritter M, Goreish IA, Atti El Mekki Mel Y, et al. Multivariable regression analysis in Schistosoma mansoni-infected individuals in the Sudan reveals unique Immunoepidemiological profiles in uninfected, egg+ and non-egg+ infected individuals. PLoS Negl Trop Dis. 2016;10(5):e0004629. doi: 10.1371/journal.pntd.0004629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fulkerson PC, Rothenberg ME. Targeting eosinophils in allergy, inflammation and beyond. Nat Rev Drug Discov. 2013;12(2):117–129. doi: 10.1038/nrd3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pardo J, Carranza C, Muro A, Angel-Moreno A, Martin AM, Martin T, et al. Helminth-related eosinophilia in African immigrants, gran Canaria. Emerg Infect Dis. 2006;12(10):1587–1589. doi: 10.3201/eid1210.060102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanya RE, Muhangi L, Nampijja M, Nannozi V, Nakawungu PK, Abayo E, et al. Schistosoma mansoni and HIV infection in a Ugandan population with high HIV and helminth prevalence. Tropical Med Int Health. 2015;20(9):1201–1208. doi: 10.1111/tmi.12545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ssetaala A, Nakiyingi-Miiro J, Asiki G, Kyakuwa N, Mpendo J, Van Dam GJ, et al. Schistosoma mansoni and HIV acquisition in fishing communities of Lake Victoria, Uganda: a nested case-control study. Tropical Med Int Health. 2015;20(9):1190–1195. doi: 10.1111/tmi.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.London Applied & Spatial Epidemiology Reseach Group The Global Atlas of Helminth Infection [Internet]. 2017 [Accessed 26 July 2018]. Available from: http://www.thiswormyworld.org/maps/distribution-of-schistosomiasis-survey-data-in-uganda.
- 22.Yegorov S, Galiwango RM, Ssemaganda A, Muwanga M, Wesonga I, Miiro G, et al. Low prevalence of laboratory-confirmed malaria in clinically diagnosed adult women from the Wakiso district of Uganda. Malar J. 2016;15(1):555. doi: 10.1186/s12936-016-1604-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kildemoes AO, Vennervald BJ, Tukahebwa EM, Kabatereine NB, Magnussen P, de Dood CJ, et al. Rapid clearance of Schistosoma mansoni circulating cathodic antigen after treatment shown by urine strip tests in a Ugandan fishing community - relevance for monitoring treatment efficacy and re-infection. PLoS Negl Trop Dis. 2017;11(11):e0006054. doi: 10.1371/journal.pntd.0006054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cnops L, Soentjens P, Clerinx J, Van Esbroeck M. A Schistosoma haematobium-specific real-time PCR for diagnosis of urogenital schistosomiasis in serum samples of international travelers and migrants. PLoS Negl Trop Dis. 2013;7(8):e2413. doi: 10.1371/journal.pntd.0002413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wichmann D, Poppert S, Von Thien H, Clerinx J, Dieckmann S, Jensenius M, et al. Prospective European-wide multicentre study on a blood based real-time PCR for the diagnosis of acute schistosomiasis. BMC Infect Dis. 2013;13:55. doi: 10.1186/1471-2334-13-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wichmann D, Panning M, Quack T, Kramme S, Burchard GD, Grevelding C, et al. Diagnosing schistosomiasis by detection of cell-free parasite DNA in human plasma. PLoS Negl Trop Dis. 2009;3(4):e422. doi: 10.1371/journal.pntd.0000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29(2):297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shannon B, Yi T J, Perusini S, Gajer P, Ma B, Humphrys M S, Thomas-Pavanel J, Chieza L, Janakiram P, Saunders M, Tharao W, Huibner S, Shahabi K, Ravel J, Rebbapragada A, Kaul R. Association of HPV infection and clearance with cervicovaginal immunology and the vaginal microbiota. Mucosal Immunology. 2017;10(5):1310–1319. doi: 10.1038/mi.2016.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Technical brochure for "Schisto POC-CCA cassette based test". Rapid Medical Diagnostics 2017. http://www.rapid-diagnostics.com/products.html. Accessed 26 July 2018.
- 30.Yi TJ, Shannon B, Prodger J, McKinnon L, Kaul R. Genital immunology and HIV susceptibility in young women. Am J Reprod Immunol. 2013;69 Suppl 1:74–79. doi: 10.1111/aji.12035. [DOI] [PubMed] [Google Scholar]
- 31.Hall OJ, Klein SL. Progesterone-based compounds affect immune responses and susceptibility to infections at diverse mucosal sites. Mucosal Immunol. 2017;10(5):1097–1107. doi: 10.1038/mi.2017.35. [DOI] [PubMed] [Google Scholar]
- 32.Ramjee G, Daniels B. Women and HIV in sub-Saharan Africa. AIDS Res Ther. 2013;10(1):30. doi: 10.1186/1742-6405-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prodger JL, Ssemaganda A, Ssetaala A, Kitandwe PK, Muyanja E, Mpendo J, et al. Schistosoma mansoni infection in Ugandan men is associated with increased abundance and function of HIV target cells in blood, but not the foreskin: a cross-sectional study. PLoS Negl Trop Dis. 2015;9(9):e0004067. doi: 10.1371/journal.pntd.0004067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kabatereine NB, Brooker S, Tukahebwa EM, Kazibwe F, Onapa AW. Epidemiology and geography of Schistosoma mansoni in Uganda: implications for planning control. Tropical Med Int Health. 2004;9(3):372–380. doi: 10.1046/j.1365-3156.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 35.Masese L, Baeten JM, Richardson BA, Deya R, Kabare E, Bukusi E, et al. Incidence and correlates of chlamydia trachomatis infection in a high-risk cohort of Kenyan women. Sex Transm Dis. 2013;40(3):221–225. doi: 10.1097/OLQ.0b013e318272fe45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.United Nations Department of Economic and Social Affairs, Population Division. World Contraceptive Use 2017; 2017 [Accessed 22 Feb 2018]. Available from: http://www.un.org/en/development/desa/population/publications/dataset/contraception/wcu2017.shtml.
- 37.Dennis ML, Radovich E, Wong KLM, Owolabi O, Cavallaro FL, Mbizvo MT, et al. Pathways to increased coverage: an analysis of time trends in contraceptive need and use among adolescents and young women in Kenya, Rwanda, Tanzania, and Uganda. Reprod Health. 2017;14(1):130. doi: 10.1186/s12978-017-0393-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ralph LJ, McCoy SI, Shiu K, Padian NS. Hormonal contraceptive use and women's risk of HIV acquisition: a meta-analysis of observational studies. Lancet Infect Dis. 2015;15(2):181–189. doi: 10.1016/S1473-3099(14)71052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Danso-Appiah A, Minton J, Boamah D, Otchere J, Asmah RH, Rodgers M, et al. Accuracy of point-of-care testing for circulatory cathodic antigen in the detection of schistosome infection: systematic review and meta-analysis. Bull World Health Organ. 2016;94(7):522–33A. doi: 10.2471/BLT.15.158741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nanvubya A, Ssempiira J, Mpendo J, Ssetaala A, Nalutaaya A, Wambuzi M, et al. Use of modern family planning methods in fishing communities of Lake Victoria, Uganda. PLoS One. 2015;10(10):e0141531. doi: 10.1371/journal.pone.0141531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.