Abstract
High-density lipoproteins (HDLs) protect against atherosclerosis by removing excess cholesterol from macrophages through the ATP-binding cassette transporter A1 (ABCA1) and ATP-binding cassette transporter G1 (ABCG1) pathways involved in reverse cholesterol transport. Factors that impair the availability of functional apolipoproteins or the activities of ABCA1 and ABCG1 could, therefore, strongly influence atherogenesis. HDL also inhibits lipid oxidation, restores endothelial function, exerts anti-inflammatory and antiapoptotic actions, and exerts anti-inflammatory actions in animal models. Such properties could contribute considerably to the capacity of HDL to inhibit atherosclerosis. Systemic and vascular inflammation has been proposed to convert HDL to a dysfunctional form that has impaired antiatherogenic effects. A loss of anti-inflammatory and antioxidative proteins, perhaps in combination with a gain of proinflammatory proteins, might be another important component in rendering HDL dysfunctional. The proinflammatory enzyme myeloperoxidase induces both oxidative modification and nitrosylation of specific residues on plasma and arterial apolipoprotein A-I to render HDL dysfunctional, which results in impaired ABCA1 macrophage transport, the activation of inflammatory pathways, and an increased risk of coronary artery disease. Understanding the features of dysfunctional HDL or apolipoprotein A-I in clinical practice might lead to new diagnostic and therapeutic approaches to atherosclerosis.
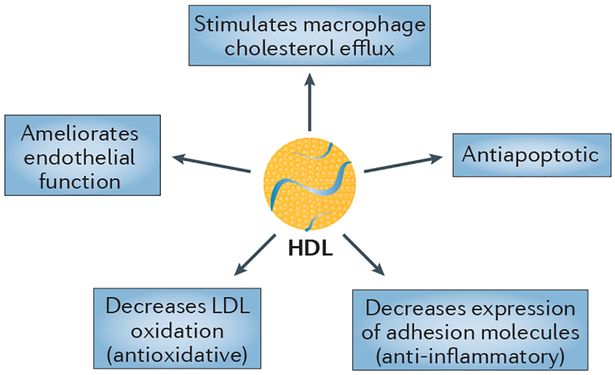
High-density lipoproteins (HDLs) confer protection against atherosclerosis in several ways and, consequently, low levels of HDL cholesterol (HDL-C) are present in many conditions that are associated with an increased risk of cardiovascular disease (CVD). Particles of HDL and/or its main protein constituent, apolipoprotein A-I (apoA-I), have diverse antiatherosclerotic influences1 that are determined by their physicochemical properties (FIG. 1)2. The most established functional property associated with HDL is macrophage cholesterol efflux3. Efflux of cholesterol through the ATP-binding cassette transporter A1 (ABCA1) is mediated most effectively by cholesterol-deficient and phospholipid-deficient apoA-I complexes1 and by very small HDL particles (HDL-VS)4. By enhancing the endothelial synthesis of nitric oxide (NO), a potent vasodilator, HDL can also ameliorate endothelial dysfunction. Furthermore, HDL reduces coronary atherosclerosis by decreasing the expression of adhesion molecules on endothelial cells and thereby reducing inflammation, and by inhibiting the oxidation of low-density lipoprotein (LDL). HDL can also exert an endothelial antiapoptotic function that involves activation of phosphoinositide 3-kinase (PI3K)/Akt and upregulation of the apoptotic protein Bcl-2-like protein 1 (also known as apoptosis regulator Bcl-X)5.
Figure 1 |. Particles of HDL and/or its main protein constituent, apolipoprotein A-I, have diverse anti- atherosclerotic influences.
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein.
In patients who present with acute coronary syndromes (ACS), the myeloperoxidase enzyme oxidizes particular residues on apoA-I, and thereby diminishes the efficiency of ABCA1-mediated macrophage cholesterol efflux6,7. Antioxidative properties of HDL particles are associated with compositional changes in the HDL proteome and lipidome8. However, alterations in HDL/apoA-I proteins and lipid constituents diminish the antioxidant, anti-inflammatory, antiapoptotic, and endothelial functional and reparative properties of HDL. Specifically, dysfunctional HDL results in increased content of oxidized phospholipids, triglycerides9, serum amyloid A (SAA), complement C3, and other inflammatory proteins7. Myeloperoxidase mediates oxidation of lipid-poor apoA-I in the vessel wall that creates a dysfunctional HDL particle, which activates nuclear factor NF-κB and promotes arterial inflammation6.
Under particular circumstances, HDL particles can become dysfunctional independently of the levels of HDL-C. Evidence that HDL and/or apoA-I exert proatherosclerotic functions as well as antiathero-sclerotic functions has increased interest in exploring the contribution of dysfunctional HDL to atherosclerotic CVD. We outline the concept of dysfunctional HDL in the context of available assays that might be clinically relevant1. Substantial efforts are required to improve the reproducibility of functional assays and to determine whether functional biomarkers provide accurate and reliable assessments of atherosclerotic CVD. An important requirement for such assays is the demonstration that they provide clinically useful information that is distinct from static measures of HDL-C and HDL particles, and that these tests provide a better assessment of CVD risk1, as shown with the macrophage cholesterol efflux assay10,11. Compositional features of HDL (the lipidome and proteome) might prove useful as reliable analytical biomarkers to be applied to clinical studies1,2,7. Post-translational modifications of apoA-I in human atherosclerotic lesions by distinct oxidative processes create dysfunctional HDL particles6,12, and prospective studies suggest that quantifying specific oxidized residues on circulating HDL might provide a useful indication of HDL dysfunction and CVD risk6,13.
In this Review, we summarize the accumulating evidence that, under particular circumstances, HDL/apoA-I loses its protective functions and might actually contribute to inflammatory processes that promote CVD in humans with atherosclerosis. We also consider the effect of therapeutic lifestyle changes and pharmacotherapies on HDL function, particularly under circumstances in which increases in HDL-C are not predictive of a reduced risk of atherosclerotic cardiovascular events, as well as considering the potential of dysfunctional HDL as a therapeutic target.
Concept of dysfunctional HDL
Nearly 20 years ago, a team of investigators reported that HDL was converted from an anti-inflammatory particle to a proinflammatory particle during an acute-phase response in humans and in a croton oil model of inflammation in rabbits14. This report developed the concept of an ‘inflammatory index’ for HDL, which was defined as the relative capacity of the ‘test’ HDL to inhibit monocyte chemotaxis induced by oxidized LDL14–16. This monocyte chemotaxis assay has been used to distinguish between patients with coronary heart disease (CHD) and control individuals without vascular disease, with higher accuracy than the measurement of HDL-C levels. Indeed, 26 patients with CHD and normal HDL-C levels (57 ± 13 mg/dl) had an inflammatory index of 1.38 ± 0.91, compared with 0.38 ± 0.14 (P <0.001) for 26 age-matched and sex-matched controls who were free from the disease and who had similar HDL-C levels (64 ± 6 mg/dl; P = 0.008)16.
The proinflammatory HDL particle was characterized by an altered protein composition: compared with its anti-inflammatory counterpart, it contained increased levels of ceruloplasmin and SAA, and decreased amounts of apoA-I, paraoxonase (PON), and platelet-activating factor-acetylhydrolase (PAF-AH)14. Concurrently, on the basis of results from a series of experiments in animal models17–20, investigators at the NIH described HDL as ‘dysfunctional’ with regard to the increased risk of developing atherosclerosis, rather than the decreased risk associated with increased plasma HDL-C levels. Overexpression in mice of human lecithin-cholesterol acyltransferase (LCAT), which catalyses the esterification of free cholesterol to cholesteryl esters in circulating plasma lipoproteins, increased the risk of atherosclerosis despite elevated levels of HDL-C and apoA-I in plasma19. This initial study and subsequent reports demonstrated that, in the absence of cholesteryl ester transfer protein (CETP), the HDL cholesteryl esters that are generated by LCAT overexpression cannot be transferred to triglyceride-rich lipoproteins, resulting in the formation of HDL with altered composition18 and function in the intermediary and terminal aspects of reverse cholesterol transport. Reduced interactions between cholesteryl esters and CETP19, as well as with hepatic lipase20, also resulted in a reduced capacity to deliver the esters to hepatic scavenger receptor class B member 1 (SCARB1).
These early studies outlined the need to distinguish between the loss of HDL function and the gain of dysfunction. Indeed, HDL could completely lose its anti-inflammatory activities and acquire proinflammatory properties with a gain of dysfunction. However, in some circumstances, the capacity of HDL to deliver cholesteryl esters by SCARB1 might be attenuated, demonstrating only a partial loss of function. Subsequent studies have revealed that the degree of loss of normal HDL function and gain of dysfunction might differ with respect to the different biological activities of HDL. In some studies, HDL displayed a complete loss of function and gain of dysfunction in terms of anti-inflammatory activity15–18,20, vasodilatory function21–25, and antiapoptotic activity5, but a deficiency in normal HDL function rather than complete dysfunction with regard to antioxidative activity26–30 and cholesterol efflux capacity31–34.
Genetics studies of HDL cholesterol
Human genome-wide association studies (GWAS) have identified a plethora of genetic loci that influence the levels of HDL-C35. Although some of these loci comprise genes that are already implicated in HDL metabolism from monogenic disorders, many are novel and the relationships between the associated genes and HDL functions are poorly understood. Voight et al. used a Mendelian randomization approach to study whether single-nucleotide polymorphisms (SNPs) associated with increased levels of HDL-C were also linked with changes in the risk of CHD, but found no significant association for the selected SNPs; by contrast, SNPs associated with high levels of LDL-C increased the risk of myocardial infarction36. Although this analysis excluded SNPs in genes with large effect sizes on the levels of HDL-C (such as those encoding CETP and lipoprotein lipase), which are associated with CHD but also affect other lipoprotein traits (such as the levels of triglycerides and LDL-C), SNPs in the gene encoding endothelial lipase, which promotes the turnover of HDL, showed considerable effects on the levels of HDL-C, but no association with CHD36. The investigators concluded that not all interventions that increase the levels of HDL-C, such as inhibition of endothelial lipase, are associated with reductions in the risk of CHD.
Subsequent GWAS have identified additional HDL-associated SNPs and have shown that the magnitude of the effect size of these SNPs on HDL-C levels does not correlate with the magnitude of the effect size on CHD37,38. By contrast, these relationships were positive for SNPs that influenced the levels of LDL-C and triglycerides39. These analyses led to the broad conclusion that HDL does not have a causal association with atherosclerotic CVD, or that a causal role for HDL-C — while possible — remains uncertain40.
However, although these studies benefit from large diverse populations and huge statistical power, some caveats to the broader conclusion might be warranted. First, the SNPs that were included in the analysis account for a minor part of the overall variation in HDL-C levels40 and include many genes with unknown functions that might have pleiotropic effects, thereby affecting atherosclerosis independently of any HDL effects. Second, the distribution of effect sizes included in the analyses differed according to the particular lipoprotein traits. Thus, SNPs affecting LDL-C and triglycerides had larger effect sizes than those affecting HDL37. The analysis of the effect on CHD of SNPs that affect an intermediate phenotype such as HDL-C levels with very small effect sizes could be influenced by a confounding factor41. Triglyceride-associated SNPs with similarly small effect sizes to those of HDL-associated SNPs also seem to have no clear relationship to CHD37. Third, GWAS have also identified many SNPs that are associated with type 2 diabetes mellitus, several of which are associated with triglycerides and HDL-C41. However, this list of SNPs does not overlap with the list of SNPs associated with premature CHD42, further illustrating the complexity of this genetic approach. Finally, the extent to which the SNPs that are associated with increases in HDL-C are also linked to relevant changes in HDL function is uncertain.
With regard to changes in HDL function, SNPs located near the ABCA1 gene, which encodes the ATP-binding cassette transporter A1 involved in cholesterol efflux, were notably associated with small changes in the levels of HDL-C, but not with the risk of CHD in a large meta-analysis37,42. In contrast to these SNPs in ABCA1, which show small effect sizes, missense variants in ABCA1 that are associated with large decreases in cholesterol efflux have been shown to correlate with increased carotid atherosclerosis using advanced carotid MRI43. Other studies of different ABCA1 coding variants with smaller effects on cholesterol efflux have been reported, and show variable relationships to CHD43. However, these functional variants also cause a reduction in LDL-C levels, which could confound the analysis. So, most studies suggest that defective cholesterol efflux from ABCA1 missense variants is associated with a proatherogenic role; however, these studies suffer from the problem of referral bias — that is, the patients who were studied were obtained from clinic populations and, therefore, were more likely to have CHD on that basis alone.
Overall, the results from GWAS cast some doubt on the causal relationship between HDL-C and CHD, and even on macrophage cholesterol efflux, the critical component of reverse cholesterol transport. Although not mutually exclusive with the involvement of HDL in reverse cholesterol transport, the results from these GWAS provide increased evidence for an atherogenic role of triglyceride-rich lipoproteins or their breakdown products, including oxidized phospholipids39. However, in view of the caveats stated above combined with strong evidence from preclinical animal models and limited human data in support of reverse cholesterol transport, we believe that firm conclusions are not yet warranted.
HDL functionality and atherosclerosis
Data from both experimental and human studies support the involvement of HDL in reducing oxidative damage and inflammation, and enhancing endothelial function and macrophage-mediated cholesterol efflux. In this section, we provide an overview of HDL function (and dysfunction) in the context of available clinical measures that have been used to identify loss of HDL function, or HDL dysfunction (TABLE 1). Although these functional measures have previously been extensively reviewed1, a few important high-throughput assays have since been developed, and these advances facilitate the evaluation of dysfunctional HDL in large clinical trials (BOX 1).
Table 1 |.
| Test | Population | Disease association |
|---|---|---|
| Macrophage cholesterol efflux | Patients with stable CHD (case-control) | CAD81 |
| Monocyte chemotaxis assay | Patients with stable CHD (case-control) | CAD16 |
| Cell-free assay | Patients with stable CHD (case-control) | CAD16 |
| Proteomics | ||
| Oxidized tryptophan | Patients with stable CHD or ACS (case-control) | CVD6 |
| Chlorinated tyrosine-192 | Patients with stable CHD or ACS (case-control) | CAD13 |
| Oxidized methionine-148 | Patients with stable CHD or ACS (case-control) | CAD13 |
| Apolipoprotein C-III on HDL | Prospective population | CVD events50 |
Abbreviations: ACS, acute coronary syndrome; CAD, coronary artery disease; CHD, coronary heart disease; CVD, cardiovascular disease; HDL, high-density lipoprotein.
Box 1|. Assays to evaluate dysfunctional HDL.
The capacity of high-density lipoprotein (HDL) to prevent the formation of, or to inactivate, oxidized phospholipids can be assessed in cell-based assays and cell-free assays to measure the effect of HDL on the production of reactive oxygen species; however, this approach has been impeded by the oxidative instability of the fluorogenic probe dichlorodihydro-fluorescein diacetate (DCFH-DA), which is currently used to measure oxidation to fluorescent 2’,7’-dichlorofluorescein (DCF) by cellular reactive oxygen species. An alternative assay can be used to measure the rate of oxidation by cellular reactive oxygen species of the fluorogenic probe dihydrorhodamine-123 (DHR) to fluorescent rhodamine; however, this method does not accurately capture matrix-lipid probe interactions that occur with circulating inflammatory mediators that are characteristic of systemic inflammation.
A new method uses polyethylene glycol precipitation and immunocapture to isolate HDL, and measures HDL lipid peroxidation fluorometrically to assess HDL function. In the presence of horseradish peroxidase, the conversion of the fluorochrome Amplex Red to highly fluorescent resorufin is used specifically to quantify the endogenous lipid hydroperoxides present in the captured HDL-cholesterol sample. This cell-free, fluorometric, high-throughput assay correlated modestly with the currently used cell-based assay (r=0.47, P <0.001) and cell-free assays (r=0.46, P <0.001)131,132
HDL composition and cardiovascular events
The anti-inflammatory and antioxidative activities of HDL can be impaired as a result of the accumulation in HDL of oxidized phospholipids, which possess potent proinflammatory and pro-oxidative properties14–16,44–46. In a case-control series of patients with clinical CHD despite supranormal HDL-C levels (118 ± 24 mg/dl), the monocyte chemotaxis assay inflammatory index of HDL was 1.28 ± 0.29, compared with 0.35 ± 0.11 in the age- matched and sex-matched controls (P <0.001)16. This finding was corroborated by similar results using a cell- free assay to evaluate the effect of phospholipid oxidation on HDL particles, as well as by the results of studies in which both assays were used in animal models15.
In a proteomics study of patients with ACS, the HDL proteome had an increased abundance of SAA, complement C3, and other inflammatory proteins compared with control individuals without ACS47; however, this change in HDL composition did not seem to impair macrophage cholesterol efflux through ABCA1, ABCG1, or SCARB1-mediated pathways. By contrast, in another study, profound alterations in the HDL phospholipidome with an enrichment in lipolytic products (such as lysophosphatidylcholine and phosphatidic acid), as observed in patients with ACS, were linked to an impaired cholesterol efflux capacity from human THP-1 macrophages, which predominantly mediate cholesterol efflux through ABCA1 (REF. 48).
ApoC-III is a proinflammatory protein that resides on the surface of very-low-density lipoprotein (VLDL), LDL, and HDL particles49. In an analysis of the Nurses’ Health and Health Professionals Follow-Up Studies, the presence or absence of apoC-III in HDL subfractions from plasma could be used to identify participants with a risk of future CHD50. The relative risk of CHD per standard deviation of HDL without apoC-III was 0.66 (95% CI 0.53–0.93), whereas it was 1.18 (95% CI 1.03–1.34) for HDL with apoC-III; these findings indicate that HDL subtypes without apoC-III were inversely associated with the risk of CHD, whereas HDL subtypes with apoC-III showed a direct association with the risk of CHD. These data suggest that apoC-III interferes with the atheroprotective function of HDL.
HDL/apoA-I oxidation
Increased oxidative stress is characteristic of established coronary atherosclerosis16,34 and other inflammatory disorders14,15, and frequently leads to damaging oxidative modifications.
Myeloperoxidase-mediated modifications.
Myeloperoxidase, a haem protein that is expressed at high concentrations by macrophages, monocytes, and neutrophils in human atherosclerotic tissue51–54, can use hydrogen peroxide to generate a wide range of reactive intermediates, which can subsequently modify lipids, proteins, nucleic acids, and lipoproteins50. The expression of myeloperoxidase in myeloid cells of mice that lack the LDL receptor promotes atherosclerosis, suggesting that myeloperoxidase exacerbates atherosclerosis in this model of hypercholesterolaemia55,56.
Oxidation of apoA-I by myeloperoxidase occurs mainly within the subendothelial compartment57 and results in the increased oxidation of multiple residues, which have been defined by mass spectrometric analysis58,59. Oxidative damage to HDL-associated apoA-I or lipid-poor apoA-I in the artery wall might limit the capacity of HDL/apoA-I to mediate cholesterol efflux from macrophages and thus promote the development of experimental and human atherosclerosis (FIG. 2)6,12,13,60,61. The distribution and biological function of apoA-I within the artery wall is distinct to that of HDL6,12. In contrast to circulating HDL, the majority of apoA-I in atheroma is not associated with HDL particles6,12,61. Post-translational modifications of lipid-poor apoA-I are common at sites of inflammation in atherosclerotic plaques.
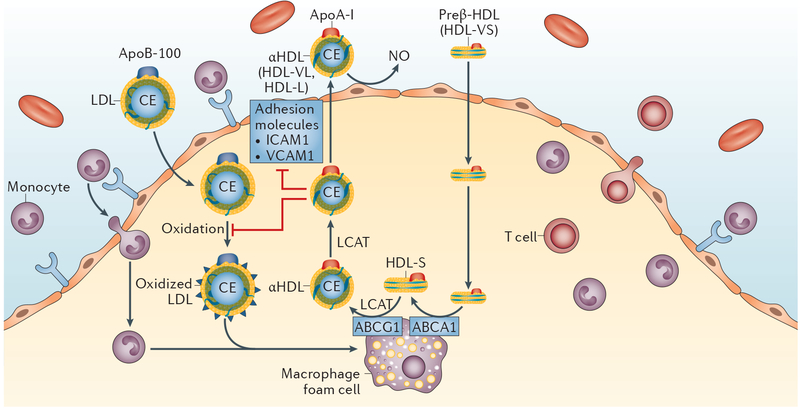
Figure 2 |. Role of HDL in the modulation of coronary atherosclerosis.
HDL protects against atherosclerosis through multiple mechanisms, as illustrated. HDL performs a pivotal role in removing cholesterol from cholesterol-loaded macrophages by binding to ABCA1 and stimulating the process of reverse cholesterol transport. In reverse cholesterol transport, preβ-HDL (HDL-VS) binds to the ABCA1 transporter and initiates cholesterol efflux with the conversion of preβ-HDL (HDL-VS) to HDLα (HDL-S). LCAT catalyses the esterification of cholesterol to CEs and the maturation of HDL to CE-rich mature HDL, which can transport cholesterol back to the liver or exchange CEs for triglycerides with the apolipoprotein B-containing lipoproteins. HDL also enhances the endothelial synthesis of NO, a potent vasodilator, to ameliorate endothelial dysfunction, and might also reduce coronary atherosclerosis by decreasing the expression of adhesion molecules on endothelial cells to reduce inflammation, and by decreasing LDL oxidation. Abbreviations: ABCA1, ATP-binding cassette transporter A1; ABCG1, ATP-binding cassette transporter G1; apoA-I, apolipoprotein A-I; apoB-100, apolipoprotein B-100; CE, cholesteryl ester; HDL, high-density lipoprotein; HDL-L, large HDL particle; HDL-S, small HDL particle; HDL-VL, very large HDL particle; HDL-VS, very small HDL particle; ICAM1, intercellular adhesion molecular 1; LCAT, lecithin-cholesterol acetyltransferase; LDL, low-density lipoprotein; NO, nitric oxide; VCAM1, vascular cell adhesion protein 1.
Several mechanisms have been proposed to explain the impaired capacity of myeloperoxidase-oxidized HDL-associated apoA-I to mediate sterol efflux7,9,51–55,57–65. One mechanism involves methionine6,45,48,49,65 oxidation and site-specific chlorination of Tyr192 on apoA-I, which impairs ABCA1-dependent cholesterol efflux and the activity of LCAT13,46. The levels of chlorinated Tyr192 and oxidized Met148 are higher in patients with stable coronary artery disease (CAD) or ACS than in control individuals, and were reported to be associated with reduced ABCA1-mediated macrophage cholesterol efflux and CAD status13. Another proposed mechanism for oxidative inactivation involves oxidation of tryptophan residues by myeloperoxidase. Substitution of all four tryptophan residues on apoA-I with phenylalanine conferred partial protection from myeloperoxidase-mediated dysfunction. However, substitution of phenylalanine residues for tryptophan residues also greatly increases the a-helical content of lipid-free apoA-I, which raises the possibility that the resistance of the mutant protein to oxidative loss of efflux capacity reflects nonspecific structural changes.
In humans, the levels of protein-bound 3-chloro-tyrosine are markedly higher on circulating HDL from patients with established CHD than on circulating HDL from healthy individuals, and HDL isolated from coronary atherosclerotic lesions contains higher concentrations of 3-chlorotyrosine and 3-nitrotyrosine than plasma HDL52,60.
HDL/apoA-I composition and inflammation
As well as the aforementioned changes that occur in response to increased oxidative stress during inflammation, HDL also undergoes substantial modification during the inflammatory response, which affects both the quantity and composition of HDL particles7,14,34,47. Circulating levels of HDL and apoA-I are markedly decreased during the acute-phase response of inflammation, and HDL particles become enriched in triglycerides and depleted of cholesteryl esters (FIG. 3)45. Several mechanisms are likely to contribute to these reduced levels of HDL-C and apoA-I during the acute-phase response7.
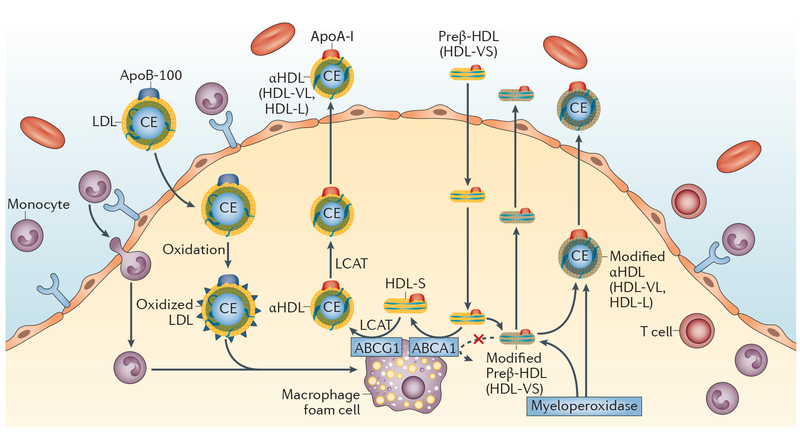
Figure 3 |. Myeloperoxidase-mediated modification of apoA-I and sterol efflux.
In the absence of myeloperoxidase activity, preβ-HDL (HDL-VS) binds to the ABCA1 transporter and initiates the efflux of cholesterol from macrophage foam cells with the concomitant conversion of preβ-HDL (HDL-VS) to HDLα4 (HDL-S). Oxidation of apoA-I on multiple residues by the proinflammatory enzyme myeloperoxidase might limit the capacity of HDL or apoA-I to mediate cholesterol efflux from macrophages and thus promote the development of experimental and human atherosclerosis. Abbreviations: ABCA1, ATP-binding cassette transporter A1; ABCG1, ATP-binding cassette transporter G1; apoA-I, apolipoprotein A-I; apoB-100, apolipoprotein B-100; CE, cholesteryl ester; HDL, high-density lipoprotein; HDL-L, large HDL particle; HDL-S, small HDL particle; HDL-VL, very large HDL particle; HDL-VS, very small HDL particle; LCAT, lecithin-cholesterol acetyltransferase; LDL, low-density lipoprotein.
SAA.
HDL present in the acute phase contains a marked increase in SAA45. SAA secreted by hepatocytes might associate with existing spherical HDL particles through a remodelling process that results in the displacement of apoA-I (FIG. 4)66. Alternatively, in concert with endothelial lipase, SAA might reduce the levels of HDL-C by impeding the formation of nascent HDL67,68. Inflammatory cytokines such as tumour necrosis factor (TNF) and IL-6 increase the expression of hepatic SAA by as much as 1,000-fold. The vast majority of SAA secreted by the liver is found associated with HDL, where it can comprise the major apolipoprotein. However, decreases in the levels of circulating apoA-I often precede the increase in SAA, suggesting that the presence of SAA does not entirely account for reduced levels of HDL-C in inflammation. The levels of SAA and apoA-I seem to be reciprocally and coordinately regulated in the liver by inflammatory cytokines, such that the reduction of apoA-I during the acute phase might be partially attributed to transcriptional downregulation concomitantly with an increase in the expression of SAA68. In healthy humans exposed to endotoxin and in a mouse model of inflammation, enrichment of the HDL proteome with SAA1 and SAA2 reduced cholesterol efflux from J7774 macrophage cells69.
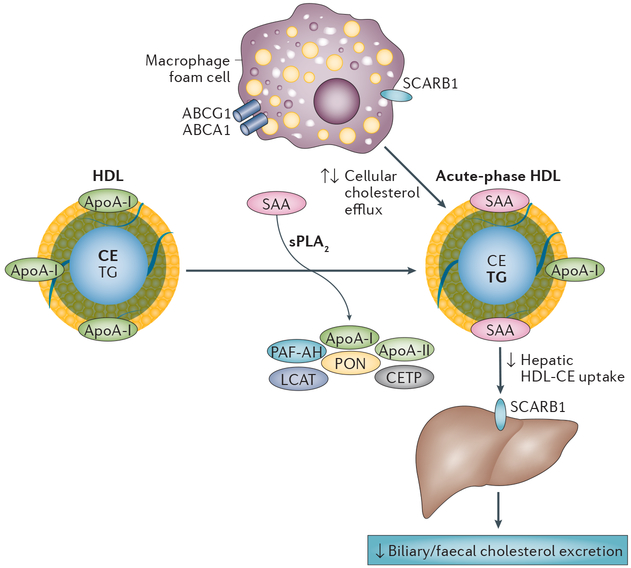
Figure 4 |. Acute-phase HDL.
HDL undergoes substantial modification during an acute-phase response. Inflammatory cytokines induce the hepatic expression of acute-phase SAA and group IIa sPLA2, which leads to the formation of HDL particles that are relatively enriched in SAA and depleted of apoA-I and phospholipid. Increased oxidative stress during inflammation generates HDL that contains oxidatively modified apoA-I. In addition, HDL remodelling during inflammation generates an abundance of triglycerides and a loss of HDL-associated proteins such as apoA-II, CETP, LCAT, PAF-AH, and PON. Results from studies investigating the effect of inflammation on steps in the reverse cholesterol transport pathway have been conflicting. During inflammation, cellular cholesterol efflux by ABCA1 and ABCG1 might be unchanged, decreased, or increased, and the uptake by SCARB1 of CEs from acute-phase HDL into the liver for subsequent excretion is either decreased or unchanged. Abbreviations: ABCA1, ATP-binding cassette transporter A1; ABCG1, ATP-binding cassette transporter G1; apoA, apolipoprotein A; CE, cholesteryl ester; CETP, cholesterol ester transfer protein; HDL, high-density lipoprotein; LCAT, lecithin-cholesterol acyltransferase; PAF-AH, platelet-activating factor acetylhydrolase; PON, paraoxonase; SAA, serum amyloid A; SCARB1, scavenger receptor class B member 1; sPLA2, secretory phospholipase A2; TG, triglyceride.
HDL-associated enzymes.
HDL remodelling during inflammation also leads to the loss of HDL-associated PON and PAF-AH. PON1 and PON3 are calcium-dependent HDL-associated enzymes that impede the peroxidation of LDL particles, so the loss of these enzymes decreases the antioxidative and anti-inflammatory capacity of HDL14,65.
PON1 and PON3 differ in their expression profile, protein localization, and enzymatic activity, but are considered to have similar effects on the hydrolysis of oxidized lipids and promotion of macrophage cholesterol efflux in experimental models70. Although low levels of PON1 activity were associated with a modest inverse risk of CHD in a population-based study, this relationship was abolished after adjustment for HDL-C and apoA-I, suggesting that low PON1 activity is not a causal factor in atherogenesis71. In a cross-sectional analysis of patients with autoimmune disease or type 1 diabetes mellitus with subclinical atherosclerosis, PON3 was depleted in HDL particles and the antioxidant function of HDL was reduced72. We are unaware, however, of prospective studies that have established a role for PON3 in human atherosclerosis. Given that PAF-AH readily hydrolyses proinflammatory, oxidatively fragmented short-chain phospholipids73 and phospholipid hydroperoxides74, the loss of PAF-AH activity from HDL, as observed under inflammatory conditions, can contribute to HDL dysfunction.
Changes in the HDL lipidome.
Changes in the lipid constituents of HDL might contribute to the reduced function of HDL during inflammation. Group IIA secretory phospholipase A2 (sPLA2) is another prominent acute- phase reactant, the expression of which is highly induced in the liver by inflammatory cytokines75. Mice harbouring PLA2G2A, a transgene encoding human group IIA sPLA2, show reduced plasma levels of HDL-C, which are associated with increased rates of apoA-I catabolism76,77. In humans, endotoxaemia is associated with significantly reduced levels of plasma and HDL phospholipids78, which is consistent with enhanced phospholipase activity during inflammation.
Acute-phase HDL is also enriched in triglycerides45, which might lead to impaired HDL stability and enhanced apoA-I catabolism79. Oxidization of the acyl chains of phospholipids in HDL increases polarity of the lipid domains, which allows water to penetrate into the hydrophobic cellular lipid membrane. The increased polarity of the lipid domains of HDL particles, which alters the binding and orientation of apoA-I, results in a reduced capacity to stimulate the activity of PON 1 and LCAT80. Oxidization of phospholipids might, therefore, contribute to the generation of dysfunctional HDL in patients with ACS or other conditions involving high oxidative stress.
Macrophage cholesterol efflux
Measuring the cholesterol efflux activity of HDL obtained by apoB precipitation from macrophage THP-1 cells was found to be superior to measuring the absolute levels of HDL-C in distinguishing between patients with incident coronary disease and control individuals81,82. This finding might, in part, be because the cholesterol efflux capacity of macrophages is predominantly mediated by very small, cholesterol-depleted HDL particles3,4, the circulating levels of which provide only a minor contribution to plasma HDL-C3. Paradoxically, Hazen et al. reported that elevated cholesterol efflux activity was associated with an increased prospective risk of CVD events in patients within the stable angiographic cohort82. These unexpected findings might result from the inclusion of individuals with nonobstructive CAD at the time of arteriography, a low event rate in the overall cohort, and differences in baseline characteristics between patients and controls81. Among patients with ischaemic cardiomyopathy, the macrophage cholesterol efflux capacity of HDL was reduced compared with that of control individuals with preserved left ventricular function and no CHD83.
Macrophage cholesterol efflux capacity measurements have been performed in large, prospective studies using standard labelled cholesterol11 and fluorescent BODIPY-cholesterol10. In a prospective study of 2,924 adults without CVD who were participants in the Dallas Heart Study10, the highest versus lowest quartile of macrophage cholesterol efflux capacity measured using the fluorescent BODIPY-cholesterol showed a 67% reduction in CVD risk in a fully adjusted model that included traditional risk factors as well as the levels of HDL-C and HDL particles. Investigators in the EPIC- Norfolk study11 measured macrophage cholesterol efflux capacity using radiolabelled cholesterol. Although these two methods for assessing macrophage cholesterol efflux activity were not applied to the same population, the fluorescence-based assay11 demonstrated more consistent associations with incident atherosclerotic CVD events than the radiolabelled cholesterol approach10.
With regard to the macrophage cholesterol efflux activity assays, the HDL isolated by ultracentrifugation of plasma samples and the HDL derived from the supernatant obtained after apoB precipitation are not equivalent biochemical entities, primarily owing to the presence in the supernatant of high amounts of albumin, immunoglobulin G, transferrin, and other major plasma proteins, which have multiple biological activities. In addition, apoB precipitation might cause the removal of apoE-containing HDL. These factors might inadvertently introduce changes in HDL composition. However, HDL obtained by ultracentrifugation also has its limitations — for example, ultracentrifugation methods that limit HDL density to 1.21 g/ml will not accurately represent the contribution of pre-β HDL (HDL-VS) that is found in the density range 1.21–1.25 g/ml84. No data are available to suggest that one assay might be more physiologically relevant or better than the other for assessing the cellular cholesterol efflux activity of HDL.
Endothelial function
HDL derived from patients with acute myocardial infarction is deficient in its capacity to stimulate the production of the vasodilator endothelial NO24, and to protect endothelial cells from apoptosis5. Such dysfunction might reflect alterations in the HDL proteome associated with acute myocardial infarction, including enrichment in inflammatory proteins, such as SAA, complement C3 and complement C9, and apoC-III, and/or a depletion of clusterin (apoJ) and apoA-IV5,7.
HDL particles perform an important and beneficial role in endothelial function. A single intravenous infusion of reconstituted HDL into humans with hypercholesterolaemia85, or with low levels of HDL secondary to partial deficiency of ABCA1 (REF. 86), normalizes endothelial function by a mechanism that involves restoration of the production of endothelial NO. Ex vivo studies of endothelial cells incubated with HDL isolated from healthy individuals show that HDL induces the expression of endothelial NO synthase (eNOS), stimulates the production of NO in endothelial cells, and reduces endothelial oxidative stress87. The capacity of HDL to induce the expression of eNOS is partly dependent on SCARB1. Binding of apoA-I-containing HDL to SCARB1 in endothelial cells initiates a signalling cascade, which, through activation of Src family kinases, phosphatidyl- inositol 3-kinase, and Akt, leads to the phosphorylation and subsequent activation of eNOS88. This capacity of HDL to induce eNOS activity and enhance endothelial function is not apparent in preparations of HDL isolated from patients with type 2 diabetes mellitus23 or CAD24.
During the acute phase of ST-segment elevation myocardial infarction (STEMI), the concentration of HDL particles is reduced, and these HDL particles are less protective against LDL oxidation and more susceptible to auto-oxidation; both of these changes are associated with a reduction in brachial artery endothelial vasomotor function89. Whether these HDL functional changes would remain significant after adjustment for reduced HDL particle number, however, is unknown.
Other causes of dysfunctional HDL
Diabetes mellitus
HDL dysfunction has been reported in patients with insulin resistance90,91 or diabetes mellitus46,92. Alterations in HDL metabolism that accompany insulin resistance lead to the formation of small triglyceride-enriched and cholesteryl-ester-depleted HDL particles, glycation of apoA-I and other HDL-associated proteins, and oxidative modification of HDL lipids, apolipoproteins, and enzymes8,9,93. The compositional modification of the HDL lipid core (triglyceride enrichment and depletion of cholesteryl esters) can also alter the conformation of apoA-I, thereby reducing its surface binding affinity. These intrinsically unstable HDL particles are rapidly cleared from the circulation, which results in a reduced total concentration of HDL particles.
Chronic inflammation in patients with type 2 diabetes also contributes to the enhanced clearance of HDL from the plasma through alterations in the HDL proteome. For example, increased levels of SAA in these patients45,94 displace apoA-I and other proteins from the surface of HDL, thereby accelerating clearance.
Enhanced oxidative stress in type 2 diabetes and prediabetes (metabolic syndrome) reduces the antioxidative capacity and antiapoptotic activity of small HDL particles by depleting the HDL-associated proteins apoA-I and PON127,28,30,87,95,96. Altered phospholipid composition of HDL in patients with type 2 diabetes results in an elevated sphingomyelin-to-phosphatidylcholine ratio, which increases the rigidity of the HDL surface96, a major determinant ofthe antioxidative activity of HDL97. Inactivation of oxidized phospholipids requires transfer to HDL, and the rate of such transfer is inversely related to the rigidity of the HDL surface phospholipid monolayer.
Tandem mass spectrometry studies with internal standard peptides demonstrated a strong, negative correlation between the concentration of clusterin in HDL and both insulin sensitivity and BMI in two independent male populations98,99. The clusterin content of HDL was unrelated to elevated blood pressure, another component of the metabolic syndrome. However, levels of HDL clusterin were significantly decreased in patients with metabolic syndrome. These observations suggest that insulin resistance and the metabolic syndrome are associated with low clusterin levels in HDL. Clusterin confers a protective function in mouse models of tissue injury5. Moreover, obesity and insulin resistance are important CVD risk factors100. These observations suggest that depletion of clusterin in HDL might impair its cardioprotective function.
Cigarette smoking
Increasing frequency and intensity of cigarette smoking is associated with lower HDL-C levels, lower HDL particle concentrations in female but not male smokers101, and compositional changes in HDL fractions that include phospholipid depletion in HDL2 (HDL-VL, HDL-L) and HDL3 (HDL-VS, HDL-S, and HDL-M) fractions, and triglyceride enrichment in the HDL2 fraction102. Higher levels of CETP activity in smokers than in nonsmokers provide one explanation for these lipid compositional changes in HDL particles103. Smoking also causes the production of dysfunctional HDL3 particles that are characterized by increased sensitivity to glycation and reduced antioxidative capacity, as measured by ferric-reducing capacity, and HDL2 particles that are less effective at inhibiting the uptake of acetyl-LDL by THP-1 cells104.
Improving HDL functionality
Interventions that have been shown to improve particular aspects of HDL functionality include lifestyle changes, weight loss achieved through bariatric surgery, and the use of statins and CETP inhibitors. Therapies that have been shown to improve or restore the functionality of some components of dysfunctional HDL include statins and niacin105.
Diet and exercise
The effect of a short-term diet and exercise intervention was evaluated using the monocyte chemotaxis assay and HDL isolated from 22 patients with metabolic syndrome98. In contrast to the modest reduction in the levels of HDL-C that is typical of patients undergoing active weight loss, significant decreases in monocyte chemotaxis (from 1.19 to 0.94; P <0.05) were induced by this nonpharmacological intervention98. Conversely, Nicholls et al. showed that expression of intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) in human endothelial cells, as measures of vascular inflammation, persistently increased in response to treatment with HDL collected from humans after a meal containing predominantly saturated fat, in contrast to one consisting largely of polyunsaturated fat106.
Bariatric surgery
The effect of Roux-en-Y gastric bypass surgery on HDL function was investigated in 34 morbidly obese women107. A total of 6 months after surgery, 20% weight loss was accompanied by a 14% increase in the levels of HDL-C (P <0.04), which resulted mainly from a 42% increase in the HDL2 subfraction (HDL-L; P <0.01)107. As expected, this increase in HDL-L increased the cholesterol efflux capacity of macrophages through the SCARB1 pathway (+58%; P <0.001) and the ABCG1 pathway (+26%; P <0.0001).
Statins
Statin therapy further improves the effects of HDL on cholesterol efflux from hepatoma cells, but has a variable effect on efflux from macrophages81,108. In patients with type IIb hyperlipoproteinaemia, treatment with atorvastatin was accompanied by a dose-dependent increase (<10%) in cholesterol efflux from Fu5AH hepatoma cells109. In dyslipidaemic individuals, pitavastatin increased the cholesterol efflux capacity from THP-1 macrophage cells108, but treatment of patients with CHD with statins had no effect on the capacity of J774 cells to mediate cholesterol efflux81.
Statins might interfere with HDL-mediated macrophage cholesterol efflux through ABCA1-induced pathways110. In THP-1 cells, statins increased the expression of miR-33, resulting in inhibited ABCA1 expression, and incubation of J774 cells with statins (in particular, simvastatin) reduced the effect of human HDL on ABCA1-induced cholesterol efflux, suggesting a mechanism for the lack of efficacy of HDL-raising therapies in clinical trials in which all participants received statin therapy. However, miR-33 does not target the expression of ABCG1 or SCARB1 in human cells and, therefore, these pathways for HDL-mediated efflux would still be operative. Moreover, whether the effects of statins observed under cell-culture conditions are relevant in vivo is currently unknown.
Some therapeutic interventions using statins have been reported to increase HDL function — and, in some cases, dysfunction — as assessed by monocyte chemotaxis and other assays16,108,110. Investigators in the Statin HDL Improvement in Function Trial (SHIFT16) reported that, in patients with clinically evident atherosclerosis who had not previously received lipid-lowering medication, treatment with simvastatin improved the inflammatory index (as characterized by the monocyte chemotaxis assay and a cell-free assay) relative to baseline. Although the improvement was significant, the HDL from statin-treated patients retained a tendency towards a proinflammatory phenotype. Furthermore, the correlation between chemotactic activity and the lipid hydroperoxide content of HDL was significant both before (r = 0.214, P = 0.039) and after (r = 0.454, P = 0.031) simvastatin treatment. However, the activity of PON was unchanged after simvastatin treatment, suggesting that the lack of the statin effect on PON1 might have contributed to the incomplete normalization of the inflammatory index of HDL in this study. In patients with dyslipidaemia, pitavastatin increased the HDL phospholipid content and HDL-associated PON1 activity; however, no effect on PAF-AH activity was observed108.
Niacin
Niacin elevates HDL-C levels, and reduces those of triglycerides and apoB-containing lipoproteins. However, the effects of niacin on various HDL functions are variable. For example, niacin treatment moderately enhanced the capacity of serum HDL to promote cholesterol efflux from cholesterol-loaded THP-1 macrophages111, but had no effect on cholesterol efflux from J774 macrophages in statin-treated patients112. The increased macrophage cholesterol efflux in THP-1 macrophages was dependent on the expression of ABCA1 and ABCG1, with ABCG1 carrying out a more important role, consistent with an increase in large HDL particles112.
HDL isolated from niacin-treated individuals also showed a diminished macrophage inflammatory response to the activation of Toll-like receptor 4 by lipid A in THP-1 cells111. This anti-inflammatory effect of niacin was partly dependent upon the expression of ABCA1 and ABCG1. In rodents, niacin also reduces acute vascular inflammation and improves endothelial function99. Niacin therapy (2 g daily) for 2 months restored the capacity of HDL to increase the production of endothelial NO, reduce oxidative stress, and facilitate endothelial repair mediated by endothelial progenitor cells in patients with type 2 diabetes23.
Despite these observations, the results from the prospective, randomized AIM-HIGH trial113 demonstrated that adding niacin to statin therapy had no further effect on the risk of CVD in patients with low HDL-C levels and established atherosclerosis. Data from the HPS2-THRIVE study114 also showed no change in CVD risk after the addition of niacin-laropiprant therapy to statin-treated patients with low LDL-C and non-HDL-C levels and normal HDL-C levels. Unlike with niacin monotherapy, the concentration of HDL particles does not increase in statin-treated patients who receive niacin115,116. Furthermore, HDL from niacin-treated individuals enrolled in the AIM-HIGH trial showed only marginal benefits on cholesterol efflux in THP-1 cells and no effect in J774 cells112, despite inducing higher incremental HDL-C levels than achieved in either the AIM-HIGH113 or HPS2-THRIVE114 trials. Therefore, niacin might not confer the same atheroprotective properties in statin-treated patients as reported for niacin monotherapy. Moreover, it remains to be determined whether pretreatment with a statin actually interferes with the favourable niacin-mediated HDL functions that have been reported for niacin monotherapy.
CETP inhibitors
A great deal of interest has been generated by the availability of CETP inhibitors, which markedly increase the levels of HDL-C. However, the first clinical trial with the CETP inhibitor torcetrapib was terminated owing to off-target toxicity117. Dalcetrapib, a weak CETP inhibitor, increased the levels of HDL-C by 30–35% and of HDL particle number by 9%118, but the phase III dal-OUTCOMES trial119 with this agent in patients with previous ACS was terminated owing to lack of efficacy. However, the patients with ACS enrolled in this trial had compromised HDL functionality7,24. The possibility that these patients had dysfunctional HDL is further supported by the observation that the concentration of HDL-C in the placebo group was unrelated to CVD events120. So, the 30% increase in HDL-C levels induced by dalcetrapib in the dal-OUTCOMES study might have had either a minimal effect on HDL functionality or increased the proportion of dysfunctional HDL that had little capacity to protect. In cell-culture experiments, the enhanced HDL-induced macrophage efflux produced by dalcetrapib was abrogated by co-incubation with a statin110.
SNPs in the adenylate cyclase 9 (ADCY9) gene on chromosome 16 were reported to identify participants in the dal-OUTCOMES trial121 who had lower or higher rates of cardiovascular events. Specifically, participants with the genotype AA at rs1967309 showed a 39% reduction in the composite cardiovascular end point after treatment with dalcetrapib versus placebo, whereas patients with the genotype GG showed a 27% increase in events121. Further prospective trials are needed to validate this pharmacogenomic approach with dalcetrapib.
Researchers in the dal-ACUTE trial122 investigated the effects of dalcetrapib on markers of HDL function within 1 week of an ACS event. After 4 weeks of treatment with dalcetrapib, the total cholesterol efflux capacity had increased by 9.5% compared with placebo, primarily through an increase in non-ABCA1-mediated transport. The increased total efflux capacity correlated with increases in the levels of HDL-C (r = 0.43) and apoA-I (r = 0.46). Anacetrapib and evacetrapib are two newer CETP inhibitors, and both dramatically increase the levels of HDL-C and reduce those of LDL-C120,123. Clinical trials with these potent CETP inhibitors are currently ongoing124,125.
From the available reports, CETP inhibitors have been shown to improve particular aspects of HDL functionality111, although we recognize the need to express these functions on a per HDL particle basis1. Among patients with type IIB hyperlipoproteinaemia treated with atorvastatin (10 mg daily), the addition of torcetrapib (60 mg daily) improved the impaired functional capacity of HDL2 (HDL-VL, HDL-L) and HDL3 (HDL-M, HDL-S, HDL-VS) particles to mediate free cholesterol efflux through the SCARB1 and ABCG1 pathways126. HDL isolated from anacetrapib-treated patients as well as HDL from CETP-deficient patients increased cholesterol efflux when compared with control HDL111. This increase in HDL efflux capacity was attributed to the increase in apoE and LCAT present on HDL particles127. Anacetrapib effectively increased HDL-mediated macrophage cholesterol efflux through the ABCG1 pathway111, and HDL isolated from anacetrapib-treated individuals reduced macrophage inflammatory responses to the activation of Toll-like receptor 4, similar to the results obtained after niacin treatment. Ongoing trials with CETP inhibitors will provide additional information on HDL and CVD events124,125.
Conclusions
The measurement of HDL-C has been useful for assessing CVD risk in epidemiological surveys, and does correlate with macrophage cholesterol efflux. However, the available data suggest that functional measurements might contribute important information on HDL activities that are involved in atherogenesis, inflammation, infection, and immunity1,8. The importance of HDL functionality in cardiovascular events has now been confirmed in the Dallas Heart Study9 and EPIC-Norfolk study11. As previously discussed, the use of reproducible, clinically available, and cost-effective measures of HDL function is an essential step for future exploration of functional and dysfunctional HDL1. These measures require validation in trials of atherosclerosis imaging and cardiovascular events.
Several compelling proof-of-concept studies currently illustrate the heterogeneity of various HDL functions among different HDL subpopulations, provide a correlation between inflammation and HDL dysfunction in atherosclerotic CVD, and offer some promise for existing and emerging therapies aimed at improving or maintaining the anti-inflammatory properties of HDL/apoA-I. However, no consensus exists about optimal testing to characterize HDL function and, although particular post-translationally-modified variants of apoA-I have been identified as risk markers for atherosclerosis, no assays are currently available for routine clinical use6,12.
The development of monoclonal antibodies that identify specific forms of dysfunctional apoA-I is a promising area of investigation for monitoring pathophysiological processes within the arterial wall and plasma, and for the evaluation of HDL/apoA-I therapies that are directed at mitigating the proatherogenic effects of dysfunctional HDL. As further research expands our knowledge in this arena, testing of HDL function could allow for better clinical risk stratification, optimization of individual clinical treatments, and the evaluation of novel therapeutics.
MRI with a gadolinium-based sensor has been used in a rabbit model of atherosclerosis to detect myeloperoxidase-rich areas that contained an abundance of macrophages on histopathological examination128, and a gadolinium-based activatable sensor has been used to detect myeloperoxidase activity in neutrophils and macrophages in ischaemic myocardium in mice after myocardial infarction129. Nanoparticles that detect macrophage activity in atherosclerotic plaques in mice have been developed130, and future advances in nanotechnology methods might enable the identification of specific myeloperoxidase-induced or other chemically-induced apoA-I modifications in the arterial wall that could serve as clinically useful tools in atherosclerosis imaging. These advanced imaging modalities might be used to investigate the effects of potential therapeutic agents designed to inhibit the detrimental effects of dysfunctional HDL. Owing to the diverse contributions of dysfunctional HDL to various stages of atherosclerosis, pending investigations of dysfunctional HDL/apoA-I will require the evaluation of multiple plasma and vascular HDL/apoA-I functions in diverse populations of patients, the results of which will need to be incorporated into randomized, double-blind clinical trials of atherosclerosis and CVD events. Only through these efforts can it be determined whether particular HDL/apoA-I-based measures, in addition to and beyond the measurement of HDL-C levels, might evolve into critical biomarkers of disease and therapeutic targets.
Key points.
HDL protects against atherosclerosis through multiple mechanisms that include amelioration of endothelial dysfunction, removal of excess cholesterol from macrophages, and antioxidative, anti-inflammatory, and antiapoptotic effects
Under particular circumstances, HDL loses its atheroprotective properties, resulting in the formation of dysfunctional HDL particles
Dysfunctional HDL particles increase proinflammatory signalling and reduce the efflux of cholesterol from macrophages by the ATP-binding cassette transporter A1
In prospective studies, myeloperoxidase-mediated oxidation of particular residues on apolipoprotein A-I creates a dysfunctional HDL particle that is associated with an increased incidence of cardiovascular events
Footnotes
Competing interests statement
R.S.R., H.B.B., B.J.A., P.B., M.J.C., J.W.H., A.K., and A.R.T. declare competing interests. Please see the article online for details. N.R.W. declares no competing interests.
References
- 1.Rosenson RS et al. Translation of high-density lipoprotein function into clinical practice: current prospects and future challenges. Circulation 128, 1256–1267 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Rosenson RS et al. HDL measures, particle heterogeneity, proposed nomenclature, and relation to atherosclerotic cardiovascular events. Clin. Chem 57, 392–410 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Rosenson RS et al. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation 125, 1905–1919 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du X et al. HDL particle size is a critical determinant of ABCA1-mediated macrophage cellular cholesterol export. Circ. Res 116, 1133–1142 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Riwanto M et al. Altered activation of endothelial anti- and proapoptotic pathways by high-density lipoprotein from patients with coronary artery disease: role of high-density lipoprotein-proteome remodeling. Circulation 127, 891–904 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Huang Y et al. An abundant dysfunctional apolipoprotein A1 in human atheroma. Nat. Med 20, 193–203 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenson RS, Brewer HB & Rader DJ Lipoproteins as biomarkers and therapeutic targets in the setting of acute coronary syndrome. Circ. Res 114, 1880–1889 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Camont L et al. Small, dense high-density lipoprotein-3 particles are enriched in negatively charged phospholipids: relevance to cellular cholesterol efflux, antioxidative, antithrombotic, anti-inflammatory, and antiapoptotic functionalities. Arterioscler. Thromb. Vasc. Biol 33, 2715–2723 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Patel S et al. Acute hypertriglyceridaemia in humans increases the triglyceride content and decreases the anti-inflammatory capacity of high density lipoproteins. Atherosclerosis 204, 424–428 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Rohatgi A et al. HDL cholesterol efflux capacity and incident cardiovascular events. N. Engl. J. Med 371, 2383–2393 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saleheen D et al. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case-control study. Lancet Diabetes Endocrinol 3, 507–513 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiDonato JA et al. Site-specific nitration of apolipoprotein A-I at tyrosine 166 is both abundant within human atherosclerotic plaque and dysfunctional. J. Biol. Chem 289, 10276–10292 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shao B et al. Humans with atherosclerosis have impaired ABCA1 cholesterol efflux and enhanced high- density lipoprotein oxidation by myeloperoxidase. Circ. Res 114, 1733–1742 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Lenten BJ et al. Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J. Clin. Invest 96, 2758–2767 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navab M et al. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: steps 2 and 3. J. Lipid Res 41, 1495–1508 (2000). [PubMed] [Google Scholar]
- 16.Ansell BJ et al. Inflammatory/antiinflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation 108, 2751–2756 (2003). [DOI] [PubMed] [Google Scholar]
- 17.Berard AM et al. High plasma HDL concentrations associated with enhanced atherosclerosis in transgenic mice overexpressing lecithin-cholesteryl acyltransferase. Nat. Med 3, 744–749 (1997). [DOI] [PubMed] [Google Scholar]
- 18.Vaisman BL et al. Overexpression of human lecithin cholesterol acyltransferase leads to hyperalphalipoproteinemia in transgenic mice. J. Biol. Chem 270, 12269–12275 (1995). [DOI] [PubMed] [Google Scholar]
- 19.Foger B et al. Cholesteryl ester transfer protein corrects dysfunctional high density lipoproteins and reduces aortic atherosclerosis in lecithin cholesterol acyltransferase transgenic mice. J. Biol. Chem 274, 36912–36920 (1999). [DOI] [PubMed] [Google Scholar]
- 20.Dugi KA et al. Adenovirus-mediated expression of hepatic lipase in LCAT transgenic mice. J. Lipid Res 38, 1822–1832 (1997). [PubMed] [Google Scholar]
- 21.Persegol L, Verges B, Foissac M, Gambert P & Duvillard L Inability of HDL from type 2 diabetic patients to counteract the inhibitory effect of oxidised LDL on endothelium-dependent vasorelaxation. Diabetologia 49, 1380–1386 (2006). [DOI] [PubMed] [Google Scholar]
- 22.Persegol L et al. HDL particles from type 1 diabetic patients are unable to reverse the inhibitory effect of oxidised LDL on endothelium-dependent vasorelaxation. Diabetologia 50, 2384–2387 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Sorrentino SA et al. Endothelial-vasoprotective effects of high-density lipoprotein are impaired in patients with type 2 diabetes mellitus but are improved after extended-release niacin therapy. Circulation 121, 110–122 (2010). [DOI] [PubMed] [Google Scholar]
- 24.Besler C et al. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J. Clin. Invest 121, 2693–2708 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Speer T et al. Abnormal high-density lipoprotein induces endothelial dysfunction via activation of Toll-like receptor-2. Immunity 38, 754–768 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Kontush A & Chapman MJ Antiatherogenic function of HDL particle subpopulations: focus on antioxidative activities. Curr. Opin. Lipidol 21, 312–318 (2010). [DOI] [PubMed] [Google Scholar]
- 27.Hansel B et al. Metabolic syndrome is associated with elevated oxidative stress and dysfunctional dense high-density lipoprotein particles displaying impaired antioxidative activity. J. Clin. Endocrinol. Metab 89, 4963–4971 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Nobecourt E et al. Defective antioxidative activity of small dense HDL3 particles in type 2 diabetes: relationship to elevated oxidative stress and hyperglycaemia. Diabetologia 48, 529–538 (2005). [DOI] [PubMed] [Google Scholar]
- 29.Kontush A, de Faria EC, Chantepie S & Chapman MJ Antioxidative activity of HDL particle subspecies is impaired in hyperalphalipoproteinemia: relevance of enzymatic and physicochemical properties. Arterioscler. Thromb. Vasc. Biol 24, 526–533 (2004). [DOI] [PubMed] [Google Scholar]
- 30.Kontush A, de Faria EC, Chantepie S & Chapman MJ A normotriglyceridemic, low HDL- cholesterol phenotype is characterised by elevated oxidative stress and HDL particles with attenuated antioxidative activity. Atherosclerosis 182, 277–285 (2005). [DOI] [PubMed] [Google Scholar]
- 31.Banka CL et al. Serum amyloid A (SAA): influence on HDL-mediated cellular cholesterol efflux. J. Lipid Res 36, 1058–1065 (1995). [PubMed] [Google Scholar]
- 32.Cavallero E et al. Abnormal reverse cholesterol transport in controlled type II diabetic patients. Studies on fasting and postprandial LpA-I particles. Arterioscler. Thromb. Vasc. Biol 15, 2130–2135 (1995). [DOI] [PubMed] [Google Scholar]
- 33.Brites FD et al. Alterations in the main steps of reverse cholesterol transport in male patients with primary hypertriglyceridemia and low HDL-cholesterol levels. Atherosclerosis 152, 181–192 (2000). [DOI] [PubMed] [Google Scholar]
- 34.Pennathur S et al. Human atherosclerotic intima and blood of patients with established coronary artery disease contain high density lipoprotein damaged by reactive nitrogen species. J. Biol. Chem 279, 42977–42983 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Teslovich TM et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature 466, 707–713 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Voight BF et al. Plasma HDL cholesterol and risk of myocardial infarction: a Mendelian randomisation study. Lancet 380, 572–580 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Global Lipids Genetics C et al. Discovery and refinement of loci associated with lipid levels. Nat. Genet 45, 1274–1283 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Do R et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat. Genet 45, 1345–1352 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenson RS, Davidson MH, Hirsh BJ, Kathiresan S & Gaudet D Genetics and causality of triglyceride-rich lipoproteins in atherosclerotic cardiovascular disease. J. Am. Coll. Cardiol 64, 2525–2540 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Holmes MV et al. Mendelian randomization of blood lipids for coronary heart disease. Eur. Heart J 36, 539–550 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harrison SC, Holmes MV & Humphries SE Mendelian randomisation, lipids, and cardiovascular disease. Lancet 380, 543–545 (2012). [DOI] [PubMed] [Google Scholar]
- 42.The CARDIoGRAMplusC4D Consortium. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat. Genet 45, 25–33 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Westerterp M et al. ATP-binding cassette transporters, atherosclerosis, and inflammation. Circ. Res 114, 157–170 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Pirillo A, Uboldi P, Bolego C, Kuhn H & Catapano AL The 15-lipoxygenase-modified high density lipoproteins 3 fail to inhibit the TNF-alpha-induced inflammatory response in human endothelial cells. J. Immunol 181, 2821–2830 (2008). [DOI] [PubMed] [Google Scholar]
- 45.Cabana VG, Lukens JR, Rice KS, Hawkins TJ & Getz GS HDL content and composition in acute phase response in three species: triglyceride enrichment of HDL a factor in its decrease. J. Lipid Res 37, 2662–2674 (1996). [PubMed] [Google Scholar]
- 46.Fisher EA, Feig JE, Hewing B, Hazen SL & Smith JD High-density lipoprotein function, dysfunction, and reverse cholesterol transport. Arterioscler. Thromb. Vasc. Biol 32, 2813–2820 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alwaili K et al. The HDL proteome in acute coronary syndromes shifts to an inflammatory profile. Biochim. Biophys. Acta 1821, 405–415 (2012). [DOI] [PubMed] [Google Scholar]
- 48.Rached F et al. Defective functionality of small, dense HDL3 subpopulations in ST segment elevation myocardial infarction: relevance of enrichment in lysophosphatidylcholine, phosphatidic acid and serum amyloid A. Biochim. Biophys. Acta 1851, 1254–1261 (2015). [DOI] [PubMed] [Google Scholar]
- 49.Kawakami A et al. Apolipoprotein CIII in apolipoprotein B lipoproteins enhances the adhesion of human monocytic cells to endothelial cells. Circulation 113, 691–700 (2006). [DOI] [PubMed] [Google Scholar]
- 50.Jensen MK, Rimm EB, Furtado JD & Sacks FM Apolipoprotein C-III as a potential modulator of the association between HDL-cholesterol and incident coronary heart disease. J. Am. Heart Assoc 1, e000232 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Daugherty A, Dunn JL, Rateri DL & Heinecke JW Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J. Clin. Invest 94, 437–444 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng L et al. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J. Clin. Invest 114, 529–541 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shao B, Oda MN, Oram JF & Heinecke JW Myeloperoxidase: an oxidative pathway for generating dysfunctional high-density lipoprotein. Chem. Res. Toxicol 23, 447–454 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heinecke JW Pathways for oxidation of low density lipoprotein by myeloperoxidase: tyrosyl radical, reactive aldehydes, hypochlorous acid and molecular chlorine. Biofactors 6, 145–155 (1997). [DOI] [PubMed] [Google Scholar]
- 55.Undurti A et al. Modification of high density lipoprotein by myeloperoxidase generates a pro-inflammatory particle. J. Biol. Chem 284, 30825–30835 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takeshita J et al. Myeloperoxidase generates 5-chlorouracil in human atherosclerotic tissue: a potential pathway for somatic mutagenesis by macrophages. J. Biol. Chem 281, 3096–3104 (2006). [DOI] [PubMed] [Google Scholar]
- 57.Baldus S et al. Endothelial transcytosis of myeloperoxidase confers specificity to vascular ECM proteins as targets of tyrosine nitration. J. Clin. Invest 108, 1759–1770 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shao B et al. Tyrosine 192 in apolipoprotein A-I is the major site of nitration and chlorination by myeloperoxidase, but only chlorination markedly impairs ABCA1 -dependent cholesterol transport. J. Biol. Chem 280, 5983–5993 (2005). [DOI] [PubMed] [Google Scholar]
- 59.Bergt C, Fu X, Huq NP, Kao J & Heinecke JW Lysine residues direct the chlorination of tyrosines in YXXK motifs of apolipoprotein A-I when hypochlorous acid oxidizes high density lipoprotein. J. Biol. Chem 279, 7856–7866 (2004). [DOI] [PubMed] [Google Scholar]
- 60.Bergt C et al. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc. Natl Acad. Sci. USA 101, 13032–13037 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hewing B et al. Effects of native and myeloperoxidase- modified apolipoprotein A-I on reverse cholesterol transport and atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol 34, 779–789 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shao B, Tang C, Heinecke JW & Oram JF Oxidation of apolipoprotein A-I by myeloperoxidase impairs the initial interactions with ABCA1 required for signaling and cholesterol export. J. Lipid Res 51, 1849–1858 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shao B, Cavigiolio G, Brot N, Oda MN & Heinecke JW Methionine oxidation impairs reverse cholesterol transport by apolipoprotein A-I. Proc. Natl Acad. Sci. USA 105, 12224–12229 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peng DQ et al. Apolipoprotein A-I tryptophan substitution leads to resistance to myeloperoxidase- mediated loss of function. Arterioscler. Thromb. Vasc. Biol 28, 2063–2070 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van Lenten BJ et al. High-density lipoprotein loses its anti-inflammatory properties during acute influenza A infection. Circulation 103, 2283–2288 (2001). [DOI] [PubMed] [Google Scholar]
- 66.Coetzee GA et al. Serum amyloid A-containing human high density lipoprotein 3. Density, size, and apolipoprotein composition. J. Biol. Chem 261, 9644–9651 (1986). [PubMed] [Google Scholar]
- 67.Wroblewski JM et al. Nascent HDL formation by hepatocytes is reduced by the concerted action of serum amyloid A and endothelial lipase. J. Lipid Res 52, 2255–2261 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Han CY et al. Reciprocal and coordinate regulation of serum amyloid A versus apolipoprotein A-I and paraoxonase-1 by inflammation in murine hepatocytes. Arterioscler. Thromb. Vasc. Biol 26, 1806–1813 (2006). [DOI] [PubMed] [Google Scholar]
- 69.Vaisar T et al. Inflammatory remodeling of the HDL proteome impairs cholesterol efflux capacity. J. Lipid Res 56, 1519–1530 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang C et al. Studies on protective effects of human paraoxonases 1 and 3 on atherosclerosis in apolipoprotein E knockout mice. Gene Ther 17, 626–633 (2010). [DOI] [PubMed] [Google Scholar]
- 71.Birjmohun RS et al. Both paraoxonase-1 genotype and activity do not predict the risk of future coronary artery disease; the EPIC-Norfolk Prospective Population Study. PLoS ONE 4, e6809 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marsillach J et al. Paraoxonase-3 is depleted from the high-density lipoproteins of autoimmune disease patients with subclinical atherosclerosis. J. Proteome Res 14, 2046–2054 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marathe GK, Zimmerman GA & McIntyre TM Platelet-activating factor acetylhydrolase, and not paraoxonase-1, is the oxidized phospholipid hydrolase of high density lipoprotein particles. J. Biol. Chem 278, 3937–3947 (2003). [DOI] [PubMed] [Google Scholar]
- 74.Kriska T, Marathe GK, Schmidt JC, McIntyre TM & Girotti AW Phospholipase action of platelet-activating factor acetylhydrolase, but not paraoxonase-1, on long fatty acyl chain phospholipid hydroperoxides. J. Biol. Chem 282, 100–108 (2007). [DOI] [PubMed] [Google Scholar]
- 75.Rosenson RS & Gelb MH Secretory phospholipase A2: a multifaceted family of proatherogenic enzymes. Curr. Cardiol. Rep 11, 445–451 (2009). [DOI] [PubMed] [Google Scholar]
- 76.de Beer FC et al. HDL modification by secretory phospholipase A2 promotes scavenger receptor class B type I interaction and accelerates HDL catabolism. J. Lipid Res 41, 1849–1857 (2000). [PubMed] [Google Scholar]
- 77.de Beer FC et al. Secretory non-pancreatic phospholipase A2: influence on lipoprotein metabolism. J. Lipid Res 38, 2232–2239 (1997). [PubMed] [Google Scholar]
- 78.Tietge UJ et al. Overexpression of secretory phospholipase A2 causes rapid catabolism and altered tissue uptake of high density lipoprotein cholesteryl ester and apolipoprotein A-I. J. Biol. Chem 275, 10077–10084 (2000). [DOI] [PubMed] [Google Scholar]
- 79.McGillicuddy FC et al. Inflammation impairs reverse cholesterol transport in vivo. Circulation 119, 1135–1145 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kar S et al. Oxidized phospholipid content destabilizes the structure of reconstituted high density lipoprotein particles and changes their function. Biochim. Biophys. Acta 1821, 1200–1210 (2012). [DOI] [PubMed] [Google Scholar]
- 81.Khera AV et al. Cholesterol efflux capacity, high- density lipoprotein function, and atherosclerosis. N. Engl. J. Med 364, 127–135 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li XM et al. Paradoxical association of enhanced cholesterol efflux with increased incident cardiovascular risks. Arterioscler. Thromb. Vasc. Biol 33, 1696–1705 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Patel PJ, Khera AV, Wilensky RL & Rader DJ Anti-oxidative and cholesterol efflux capacities of high-density lipoprotein are reduced in ischaemic cardiomyopathy. Eur. J. Heart Fail 15, 1215–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Miyazaki O, Ogihara J, Fukamachi I & Kasumi T Evidence for the presence of lipid-free monomolecular apolipoprotein A-1 in plasma. J. Lipid Res 55, 214–225 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Spieker LE et al. High-density lipoprotein restores endothelial function in hypercholesterolemic men. Circulation 105, 1399–1402 (2002). [DOI] [PubMed] [Google Scholar]
- 86.Bisoendial RJ et al. Restoration of endothelial function by increasing high-density lipoprotein in subjects with isolated low high-density lipoprotein. Circulation 107, 2944–2948 (2003). [DOI] [PubMed] [Google Scholar]
- 87.Yuhanna IS et al. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat. Med 7, 853–857 (2001). [DOI] [PubMed] [Google Scholar]
- 88.Zhao Y, Sparks DL & Marcel YL Specific phospholipid association with apolipoprotein A-I stimulates cholesterol efflux from human fibroblasts. Studies with reconstituted sonicated lipoproteins.J. Biol. Chem 271, 25145–25151 (1996). [DOI] [PubMed] [Google Scholar]
- 89.Carvalho LS et al. HDL levels and oxidizability during myocardial infarction are associated with reduced endothelial-mediated vasodilation and nitric oxide bioavailability. Atherosclerosis 237, 840–846 [DOI] [PubMed] [Google Scholar]
- 90.McMillen TS, Heinecke JW & LeBoeuf RC Expression of human myeloperoxidase by macrophages promotes atherosclerosis in mice. Circulation 111, 2798–2804 (2005). [DOI] [PubMed] [Google Scholar]
- 91.Kaysen GA Disorders in high-density metabolism with insulin resistance and chronic kidney disease. J. Ren. Nutr 17, 4–8 (2007). [DOI] [PubMed] [Google Scholar]
- 92.Morgantini C et al. Anti-inflammatory and antioxidant properties of HDLs are impaired in type 2 diabetes. Diabetes 60, 2617–2623 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kontush A & Chapman MJ Why is HDL functionally deficient in type 2 diabetes? Curr. Diab. Rep 8, 51–59 (2008). [DOI] [PubMed] [Google Scholar]
- 94.Choudhury RP & Leyva F C-Reactive protein, serum amyloid A protein, and coronary events. Circulation 100, e65–e66 (1999). [DOI] [PubMed] [Google Scholar]
- 95.Bagdade JD, Buchanan WE, Kuusi T & Taskinen MR Persistent abnormalities in lipoprotein composition in noninsulin-dependent diabetes after intensive insulin therapy. Arteriosclerosis 10, 232–239 (1990). [DOI] [PubMed] [Google Scholar]
- 96.de Souza JA et al. Metabolic syndrome features small, apolipoprotein A-I-poor, triglyceride-rich HDL3 particles with defective anti-apoptotic activity. Atherosclerosis 197, 84–94 (2008). [DOI] [PubMed] [Google Scholar]
- 97.Zerrad-Saadi A et al. HDL3-mediated inactivation of LDL-associated phospholipid hydroperoxides is determined by the redox status of apolipoprotein A-l and HDL particle surface lipid rigidity: relevance to inflammation and atherogenesis. Arterioscler. Thromb. Vasc. Biol 29, 2169–2175 (2009). [DOI] [PubMed] [Google Scholar]
- 98.Roberts CK, Ng C, Hama S, Eliseo AJ & Barnard RJ Effect of a short-term diet and exercise intervention on inflammatory/antiinflammatory properties of HDL in overweight/obese men with cardiovascular risk factors. J. Appl. Physiol 101, 1727–1732 (2006). [DOI] [PubMed] [Google Scholar]
- 99.Hoofnagle AN et al. Low clusterin levels in high- density lipoprotein associate with insulin resistance, obesity, and dyslipoproteinemia. Arterioscler. Thromb. Vasc. Biol 30, 2528–2534 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li S et al. Reduction of cold ischemia-reperfusion injury by graft-expressing clusterin in heart transplantation. J. Heart Lung Transplant 30, 819–826 (2011). [DOI] [PubMed] [Google Scholar]
- 101.Beauchamp A et al. Associations among smoking status, lifestyle and lipoprotein subclasses. J. Clin. Lipidol 4, 522–530 (2010). [DOI] [PubMed] [Google Scholar]
- 102.Park KH, Shin DG & Cho KH Dysfunctional lipoproteins from young smokers exacerbate cellular senescence and atherogenesis with smaller particle size and severe oxidation and glycation. Toxicol. Sci 140, 16–25 (2014). [DOI] [PubMed] [Google Scholar]
- 103.He BM, Zhao SP & Peng ZY Effects of cigarette smoking on HDL quantity and function: implications for atherosclerosis. J. Cell. Biochem 114, 2431–2436 (2013). [DOI] [PubMed] [Google Scholar]
- 104.Song W et al. The implication of cigarette smoking and cessation on macrophage cholesterol efflux in coronary artery disease patients. J. Lipid Res 56, 682–691 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Luscher TF, Landmesser U, von Eckardstein A & Fogelman AM High-density lipoprotein: vascular protective effects, dysfunction, and potential as therapeutic target. Circ. Res 114, 171–182 (2014). [DOI] [PubMed] [Google Scholar]
- 106.Nicholls SJ et al. Consumption of saturated fat impairs the anti-inflammatory properties of high- density lipoproteins and endothelial function. J. Am. Coll. Cardiol 48, 715–720 (2006). [DOI] [PubMed] [Google Scholar]
- 107.Aron-Wisnewsky J et al. Effect of bariatric surgery- induced weight loss on SR-BI-, ABCG1 -, and ABCA1-mediated cellular cholesterol efflux in obese women. J. Clin. Endocrinol. Metab 96, 1151–1159 (2011). [DOI] [PubMed] [Google Scholar]
- 108.Miyamoto-Sasaki M et al. Pitavastatin increases HDL particles functionally preserved with cholesterol efflux capacity and antioxidative actions in dyslipidemic patients. J. Atheroscler. Thromb 20, 708–716 (2013). [DOI] [PubMed] [Google Scholar]
- 109.Guerin M et al. Dose-dependent action of atorvastatin in type IIB hyperlipidemia: preferential and progressive reduction of atherogenic apoB- containing lipoprotein subclasses (VLDL-2, IDL, small dense LDL) and stimulation of cellular cholesterol efflux. Atherosclerosis 163, 287–296 (2002). [DOI] [PubMed] [Google Scholar]
- 110.Niesor EJ et al. Statin-induced decrease in ATP- binding cassette transporter A1 expression via microRNA33 induction may counteract cholesterol efflux to high-density lipoprotein. Cardiovasc. Drugs Ther 29, 7–14 (2015). [DOI] [PubMed] [Google Scholar]
- 111.Yvan-Charvet L et al. Cholesterol efflux potential and antiinflammatory properties of high-density lipoprotein after treatment with niacin or anacetrapib. Arterioscler. Thromb. Vasc. Biol 30, 1430–1438 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Khera AV, Patel PJ, Reilly MP & Rader DJ The addition of niacin to statin therapy improves high- density lipoprotein cholesterol levels but not metrics of functionality. J. Am. Coll. Cardiol 62, 1909–1910 (2013). [DOI] [PubMed] [Google Scholar]
- 113.The AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med 365, 2255–2267 (2011). [DOI] [PubMed] [Google Scholar]
- 114.The HPS2-THRIVE Collaborative Group. Effects of extended-release niacin with laropiprant in high-risk patients. N. Engl. J. Med 371, 203–212 (2014). [DOI] [PubMed] [Google Scholar]
- 115.Bays H, Giezek H, McKenney JM, O’Neill EA & Tershakovec AM Extended-release niacin/laropiprant effects on lipoprotein subfractions in patients with type 2 diabetes mellitus. Metab. Syndr. Relat. Disord 10, 260–266 (2012). [DOI] [PubMed] [Google Scholar]
- 116.Airan-Javia SL et al. Atheroprotective lipoprotein effects of a niacin-simvastatin combination compared to low- and high-dose simvastatin monotherapy. Am. Heart J 157, 687, e1–e8 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Barter PJ et al. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med 357, 2109–2122 (2007). [DOI] [PubMed] [Google Scholar]
- 118.Ballantyne CM et al. Effect of dalcetrapib plus pravastatin on lipoprotein metabolism and high- density lipoprotein composition and function in dyslipidemic patients: results of a phase IIb dose- ranging study. Am. Heart J 163, 515–521.e3 (2012). [DOI] [PubMed] [Google Scholar]
- 119.Schwartz GG et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N. Engl. J. Med 367, 2089–2099 (2012). [DOI] [PubMed] [Google Scholar]
- 120.Nicholls SJ et al. Effects of the CETP inhibitor evacetrapib administered as monotherapy or in combination with statins on HDL and LDL cholesterol: a randomized controlled trial. JAMA 306, 2099–2109 (2011). [DOI] [PubMed] [Google Scholar]
- 121.Tardif JC et al. Pharmacogenomic determinants of the cardiovascular effects of dalcetrapib. Circ. Cardiovasc. Genet 8, 372–383 (2015). [DOI] [PubMed] [Google Scholar]
- 122.Ray KK et al. The effect of cholesteryl ester transfer protein inhibition on lipids, lipoproteins, and markers of HDL function after an acute coronary syndrome: the dal-ACUTE randomized trial. Eur. Heart J 35, 1792–1800 (2014). [DOI] [PubMed] [Google Scholar]
- 123.Cannon CP et al. Safety of anacetrapib in patients with or at high risk for coronary heart disease. N. Engl. J. Med 363, 2406–2415 (2010). [DOI] [PubMed] [Google Scholar]
- 124.US National Library of Medicine. ClinicalTrials.gov [online], https://clinicaltrials.gov/ct2/show/NCT01252953 (2015).
- 125.US National Library of Medicine. ClinicalTrials.gov [online], https://clinicaltrials.gov/ct2/show/NCT01687998 (2015).
- 126.Catalano G et al. Torcetrapib differentially modulates the biological activities of HDL2 and HDL3 particles in the reverse cholesterol transport pathway. Arterioscler. Thromb. Vasc. Biol 29, 268–275 (2009). [DOI] [PubMed] [Google Scholar]
- 127.Castro-Perez J et al. Anacetrapib promotes reverse cholesterol transport and bulk cholesterol excretion in Syrian golden hamsters. J. Lipid Res 52, 1965–1973 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nahrendorf M et al. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation 117, 379–387 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nahrendorf M et al. Activatable magnetic resonance imaging agent reports myeloperoxidase activity in healing infarcts and noninvasively detects the antiinflammatory effects of atorvastatin on ischemia-reperfusion injury. Circulation 117, 1153–1160 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ronald JA et al. Enzyme-sensitive magnetic resonance imaging targeting myeloperoxidase identifies active inflammation in experimental rabbit atherosclerotic plaques. Circulation 120, 592–599 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kelesidis T et al. Effects of lipid-probe interactions in biochemical fluorometric methods that assess HDL redox activity. Lipids Health Dis 11, 87 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kelesidis T et al. A high throughput biochemical fluorometric method for measuring lipid peroxidation in HDL. PLoS ONE 9, e111716 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]