Abstract
Cryptococcus neoformans (Cn) is a fungal pathogen with worldwide distribution. Cn resides within mature phagolysosomes where it often evades killing and replicates. Cn induces phagolysosomal membrane permeabilization (PMP), but the mechanism for this phenomenon and its consequences for macrophage viability are unknown. In this study, we used flow cytometry methodology in combination with cell viability markers and LysoTracker to measure PMP in J774.16 and murine bone marrow derived macrophage infected with Cn. Our results showed that cells manifesting PMP were positive for apoptotic markers, indicating an association between PMP and apoptosis. We investigated the role of phospholipase B1 (PLB1) in Cn-induction of PMP. Macrophages infected with a Cn Δplb1 mutant had reduced PMP compared to those infected with wild-type and PLB1-complemented strains, suggesting a mechanism of action for this virulence factor. Capsular enlargement inside macrophages was identified as an additional likely mechanism for phagolysosomal membrane damage. Macrophages undergoing apoptosis did not maintain an acidic phagolysosomal pH. Induction of PMP with ciprofloxacin enhanced macrophages to trigger lytic exocytosis while non-lytic exocytosis was common in those without PMP. Our results suggest that modulation of PMP is a critical event in determining the outcome of the Cn-macrophage interaction.
Introduction
Cryptococcus neoformans (Cn) is an important fungal pathogen with a worldwide distribution causing around 600,000 deaths annually, mostly in patients with advanced HIV infection [1]. Cn infection occurs following the inhalation of spores or desiccated cells from the environment. Although most infections are controlled in the lung, dissemination can occur in the setting of impaired immunity. The most common clinical manifestation of cryptococcosis is meningoencephalitis, which is fatal if not treated. Cn expresses a variety of virulence factors that contribute to its pathogenesis including a polysaccharide capsule, melanin deposition in the cell wall, the capacity to growth at 37°C and the production of extracellular enzymes like laccases and urease [2, 3].
Cn is a facultative intracellular pathogen in vitro and in vivo is often found residing in macrophages in infected tissues [4, 5]. Macrophages play a crucial role in determining the outcome of infection [6–13] and presumably serve as the first line of defense in the alveolar space. Control of Cn infection is efficient when classical macrophage activation concomitant with CD4+ Th1-type response develops. In contrast, alternative macrophage activation associated with a CD4+ Th2-type response favors Cn growth and establishment of latent infection [14]. After Cn is ingested by macrophages it resides within a mature phagosome, where it survives and replicates despite the acidic pH and the presence of antimicrobial compounds such as proteases (reviewed in [15]). One mechanism by which Cn survives in mature phagosomes involves the expression of powerful antioxidant systems that can absorb the oxidative burst, i.e. the capsule, melanin and enzymes [16–18]. There is also evidence that Cn delays phagolysosome maturation, which has been previously described as an antimicrobial mechanism [19]. There are three general outcomes to the Cn-macrophage interaction: fungal cell death, macrophage cell death, or non-lytic exocytosis resulting in the survival of both cells. The mechanisms and factors that contribute to each outcome remain poorly understood.
Prior studies have shown that Cn intracellular residence is associated with phagolysosomal membrane permeabilization (PMP) [5, 20]. More recent studies suggest that the integrity of the phagolysosomal membrane is a critically important variable in determining the ability of macrophages to control Cn [21]. Furthermore, Cn intracellular residence in macrophages is associated with damage to numerous cellular systems including mitochondrial depolarization and activation of cell death pathways [22]. Studies in other cell models have shown that PMP can either be complete or partial and lead to cell death [23]. Despite PMP occurring in macrophages infected with Cn the mechanism for this phenomenon or its effect on the macrophage cell remain unknown. PLB1 is an enzyme that hydrolyzes one or more ester linkages in glycerophospholipids, which can result in the destabilization of membranes [24]. Cn PLB1 was suggested to have a role in inducing PMP but this effect has not been experimentally established.
PMP in macrophages infected with Cn can lead to several disastrous consequences for the host cell. First, leakage of phagolysosomal contents means loss of antimicrobial compounds used to control intracellular infection, which could facilitate fungal survival. Second, PMP allows the fungal cells to gain access to cytoplasmic nutrients. Third, leakage of phagolysosomal contents into the cytoplasm can cause cellular damage that could trigger programmed cell death (PCD) [23]. Cathepsins (CTS) are the most studied phagolysosomal proteins involved in activating PCD upon leakage into the cytoplam [25]. CTS are lysosomal proteases that belong to the papain family and these are synthesized as inactive zymogens, with processing to its active form occurring in lysosomes [25]. CTS are primarily cysteine proteases with the exception of cathepsin D (CTSD) and cathepsin E (CTSE), which are aspartic proteases, and cathepsin A (CTSA) and cathepsin G (CTSG), which are serine proteases. CTS activity is highly regulated and is more active in acidic pH and reducing environments, which are both conditions that exist in Cn-containing phagolysosomes. Immunofluorescence analysis of J774.16 macrophage-like cells after Cn phagocytosis shows localization of CTSD within the Cn-containing vacuole [26], and CTSB isolated from lysosomal extracts of dendritic cells has an anti-fungal effect on Cn [27].
Given the potential importance of phagosomal membrane integrity in the control of intracellular Cn infection as well as its contribution to host cell damage (acquisition of an apoptotic phenotype), we evaluated the integrity of Cn-containing phagolysosomes and its effect on various host cell parameters including phagolysosomal pH and non-lytic exocytosis. Our results indicate that the outcome of the Cn-macrophage interactions, including the type of exocytosis and the fate of the host cell, depend on phagolysosomal membrane integrity.
Materials and methods
Cryptococcus neoformans
C. neoformans (Cn) serotype A strain H99 cells (ATCC 208821, Manassas, VA) were cultured in Sabouraud dextrose broth (Difco, Carlsbad, CA) for 2 d at 30°C with constant agitation. A 1 ml volume of culture was added to a 1.5 ml tube and centrifuged at 350 g. Cells were washed twice and resuspended using 1X phosphate buffered saline (PBS)(Corning cellgro, Manassas, VA). Cells were counted using a hemacytometer (Hausser scientific, Horsham, PA) and used at the indicated effector cell to target cell ratio (E:T) in the methods of each experiment. Extracellular phospholipase B1 mutant (Δplb1) and the reconstituted strain (Δplb1::PLB1) were obtained from the John Perfect laboratory [24]. Cn was heat-killed by incubation at 60°C for 1 h.
Macrophage cells
Two types of macrophage-like cells were used in these studies: J774.16 macrophage-like cells (J774.16 cells) [28] and bone marrow derived macrophages (BMDM) obtained from 6 week old C57BL/6 female mice acquired from the Jackson laboratory (Bar Harbor, ME). In general, the approach was to work out the methodology with J774.16 cells and subsequently confirm significant findings in BMDM. J774.16 cells were cultured in Dulbecco’s Modification of Eagle’s Medium with 4.5 g/L glucose, L-glutamine and sodium pyruvate (DMEM) (Corning cellgro), 10% Medium NCTC-109 (Gibco, Grand Island, NY), 10% heat-inactivated (56°C for 30 min) fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA), 1% MEM Nonessential Amino Acids 100× solution (Corning cellgro) and 1% Penicillin–Streptomycin Solution (Corning cellgro). BMDM were isolated from BALB/c, CBA/J and C57BL/6 as described previously [22]. Both cells lines were incubated at 37°C with 10% CO2.
Cn phagocytosis
Phagocytosis assays were performed as previously described [20]. J774.16 cells and BMDM were distributed in the plates and activated by incubating overnight using 0.5 μg/ml of LPS (Sigma-Aldrich, St. Louis, MO) and 100 U/ml of IFN-γ (Roche, Indianapolis, IN). Briefly, Cn was washed twice with PBS and added to macrophages at an E:T ratio of 5:1 unless otherwise indicated. Opsonization was done by adding IgG1 monoclonal antibody (MAb) 18B7 at 10 μg/ml [29]. Following incubation, the media was removed from the wells and 100 μl of ice-cold methanol was added to each well which was removed after 30 min of incubation followed by washing with PBS. A volume of 50 μl per well of a 1:20 Giemsa solution (Sigma-Aldrich) was then added. Phagocytosis percentage was determined at 2 h by counting the number of macrophages with intracellular Cn and dividing it by the total number of macrophages.
LysoTracker and Dextran Comparison Flow Cytommetry
BMDM cells (1 × 106) were seeded in a 6-well dish and activated overnight. The next day, BMDMs were stained with Dextran-FITC 40,000 Da (Invitrogen, Carlsbad, CA) (1 mg/mL) for 1 h. Afterwards, BMDMs were infected using Uvitex 2B (Polysciences, Warrington, PA) stained Cn at an E:T ratio of 1:1 for 2 h. One hour prior to incubation end-time, LysoTracker Deep Red (ThermoFisher Scientific, Waltham, MA) was added (50 nM). After incubation, the medium was collected and washed twice with 1 ml of Hank’s balanced salt solution (HBSS)(Corning cellgro). A 1 ml of Cellstripper (Corning cellgro) was added and incubated for 5 min at 37°C followed by the collection of cells and centrifugation at 350 g for 10 min at 0 deceleration. All washes and media were collected in the same tube to ensure collection of floating cells. After centrifugation, we discarded the supernatant and resuspended the pellet in HBSS. Cells were then immunostained with CD11b-PE (clone M1/70, 12-0112-82) (Thermo Scientific) at a dilution of 1:1000 and incubated for 7 min. This was followed by centrifugation for 10 min at 350 g and the pellet was washed twice in HBSS buffered to its respective pH. Pellets were then resuspended in 1 mL HBSS supplemented with or without nigericin (Invitrogen) (5 μg/uL). Analysis was performed by flow cytometry with the Becton Dickinson LSRII instrument (BD Biosciences, Franklin Lakes, New Jersey).
Apoptosis and PMP flow cytometry analysis
J774.16 cells and BMDM cells (1 × 106) were seeded in a 6-well dish and activated overnight. The next day, J774.16 cells were infected using Uvitex 2B (Polysciences) stained Cn at an E:T ratio of 2:1 for the indicated time period (the Cn strain used is indicated within individual experiments). One hour prior to incubation time, LysoTracker Deep Red (ThermoFisher Scientific) was added (50 nM). After incubation, the medium was collected and washed with 1 ml of Hank’s balanced salt solution (HBSS)(Corning cellgro). A 1 ml of Cellstripper (Corning cellgro) was added and incubated for 5 min at 37°C followed by the collection of cells and centrifugation at 150 g for 5 min. All washes and media were collected in the same tube to ensure collection of floating cells. After centrifugation, we discarded the supernatant and resuspended the pellet in HBSS. Cells were then immunostained with CD11b-PE (clone M1/70, 12-0112-82) (Thermo Scientific) at a dilution of 1:1000 and incubated for 7 min. This was followed by centrifugation for 5 min at 150 g and the pellet was resuspended in HBSS. To measure early and late apoptosis, two dyes were used respectively: 4′-N,N-diethylamino-6-(N,N,N-dodecyl-methylamino-sulfopropyl)-methyl-3-hydroxyflavone (F2N12S) and SYTOX-PE-Cy5.5 AADvanced dead cells (SYTOX) (ThermoFisher Scientific). F2N12S was used to detect membrane asymmetry changes during apoptosis and SYTOX would stain dead cells. Both dyes were added to label the cells 5 min prior to analysis by flow cytometry with the Becton Dickinson LSRII instrument (BD Biosciences, Franklin Lakes, New Jersey).
Immunofluorescence analysis of PMP
J774.16 cells (5 × 104) were seeded in a circular microscope coverslips and activated overnight. The next day, J774.16 cells were infected with heat-killed Cn and viable Cn, both stained with Uvitex 2B, at an E:T ratio of 2:1 for 2 h. One hour before the completion of the experiment LysoTracker Deep Red (50 nM) was added. After 2 h cells were washed with PBS three times. Coverslips were mounted using ProLong antifade mounting solution (Invitrogen) and visualized using a Zeiss AxioImager M2 fluorescence microscope equipped with an oil-immersion Zeiss plan Apo 100×/NA 1.4 objective and a Hamamatsu ORCA-R2 camera (Hamamatsu Photonics, Hamamatsu, Japan). Optical z-sections with 0.2 μm spacing were acquired using Volocity acquisition software (Perkin Elmer, Waltham, MA). Analysis was performed as described previously [30].
Non-lytic exocytosis assay
BMDM (5 × 104) were seeded in 35 mm glass bottom culture dishes (MakTek, Ashland, MA), activated and treated with 12 μg/ml of Pepstatin A (PepsA) (Peptides international, Louisville, KY) overnight. The following day, the cells were washed with PBS and fresh media was added, with Cn at an E:T of 1:3 and incubated for 2 h. After incubation, the cells were washed 6 times with PBS to remove extracellular Cn and then 2 mL of fresh media was added to the plate. Yeast cell extrusion was analyzed using an Axiovert 200M inverted microscope (Carl Zeiss MicroImaging, NY) used in conjunction with an AxiocamMR camera. Images of macrophage-Cn interactions were collected every 4 min for 24 h to produce time-lapse movies. Cells were incubated within a temperature controlled microscopy chamber at 37°C with 10% CO2.
β-hexosaminidase activity assay
The activity of β-hexosaminidase was measured using an optimized fluorescence-based assay in a 96-well plate format. Rat liver lysosomes were isolated and centrifuged immediately after isolation at 25,000 g for 5 min [31] in a Beckman Allegra 64R centrifuge (F2020 rotor). Lysosomal fractions were incubated with ciprofloxacin (350 μl/ml) (Sigma-Aldrich), helenalin (1 μM) (Santa Cruz Biotechnology, Dallas, TX) and Leu-Leu-O-Me (10 mM) (Santa Cruz Biotechnology) with 0.25 M sucrose to a final volume of 25 μl. 100 μl of the reaction buffer containing the substrate (4-methylumbelliferyl-N-acetyl-β-D-glucopyranosidase) was added to each well and the plate was incubated at 37°C for 30 min. After incubation, 75 μl of glycine/carbonate was added to stop the reaction and the fluorescence was measured in a FLUOstar Optima plate reader (BMG Lab technologies, Offenburg, Germany) at excitation and emission wavelengths of 370 nm and 450 nm respectively. The extent of broken lysosomes was calculated by the activity in the supernatant as a percentage of the total activity in the lysosomal fraction, which represented the capacity of the drugs to damage the lysosomal membrane.
Measurement of Capsule Growth inside macrophages
BMDM cells were seeded to a 24-well plate at 2.5 × 104 cells per well; wells were prepared in triplicate for each time measurement. Cn cells were suspended in 200 uL of BMDM media and opsonized with 10 ug/uL mAb 18B7 for 5 minutes. Media was then aspirated from the BMDM culture and replaced with the suspension containing opsonized Cn at a fungal:macrophage ratio of 3:1 and incubated for one hour at 37°C and 9.5% CO2. The extracellular cryptococcal cells were removed from the well by washing three times using 500 uL BMDM media. BMDM were then incubated for 0 or 24 h after which extracellular crypto was again removed by three washes of 500 uL BMDM media. Cells were then lysed by replacing media with ice cold distilled water and incubating for 10 minutes. Cells were pipetted ten times to ensure lysis, and freed Cryptococcus cells were pelleted by centrifugation at 13k rpm for 5 minutes. Cryptococcus were then resuspended in 40 uL PBS for capsule measurement. India Ink exclusion slides were prepared by mixing 10 uL India Ink with 7 uL of this suspension. Slide images were acquired at 100 × magnification with 2 × 2 binning. Capsule and body radius measurements were acquired manually with ImageJ.
Phagolysosomal pH measurements
BMDM were seeded (4 × 105 cells per well) and activated overnight in 24-well plates with 12 mm circular coverslip coated with poly-D-lysine (100 μg/ml). Cn were added at an E:T of 1:3, with the yeast opsonized with 10 μg/ml pH-sensitive Oregon green-conjugated 18B7 Ab (life technologies, Carlsbad, CA). The coverslip was immediately centrifuged at 270 g at 4° C for 1 min to maximize contact of macrophages and yeast cells and then incubated at 37°C for 1 min. The extracellular Cn was then washed using 37°C pre-heated HBSS, and cells were incubated for 24 h. Annexin V (Invitrogen) staining was performed following the manufacturer’s instructions. The coverslip was then placed on the microwell of a MatTek dish (35 mm; 10 mm diameter microwell) with the annexin V binding buffer. Bright field and fluorescence images were acquired (Excitation wavelengths of 440 nm, 488 nm and 555 nm) using an Olympus AX70 at the 40X objective lens. Image acquisition and analysis was done using the MetaMorph software (Molecular devices, Sunnyvale CA). The pH calibration curve was acquired in cells in which its intracellular pH was equilibrated by adding 10μM nigericin and incubated with buffers containing 140 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 5 mM glucose and buffered to different pH values (≤ pH 5.0; acetate-acetic acid; pH 5.5–6.5; MES; ≥ pH 7.0: HEPES). The pH was adjusted using KOH or HCl.
Quantitative Real-Time PCR
J774.16 cells (5 × 106) were seeded in 10 mm plates and activated overnight. The next day, fresh media was added containing Cn to achieve an E:T ratio of 1:5 and infection allowed to proceed for 2 and 4 h. RNA extraction was performed using Trizol following the manufacturer’s instructions (Invitrogen). cDNA synthesis was generated from total RNA using Superscript III reverse-transcriptase (Invitrogen) as per the manufacturer’s protocol. Real-time PCR was performed using Power SYBR green PCR master mix (ThermoFisher Scientific) using CTSB, CTSD, CTSH, CTSL, CTSS and β-actin specific primers (Table 1). The amplification was performed by: Stage 1, 2 min incubation at 50°C; Stage 2, 10 min incubation at 95°C; Stage 3, 15 sec incubation at 95°C and 1 min incubation at 62°C for 40 cycles; Stage 4, 15 sec incubation at 95°C, followed by 15 sec incubation at 60°C and 15 sec incubation at 95°C. Fold changes were calculated using ΔΔCt values.
Table 1.
Sequences of the primer used for qRT-PCR analysis of CTS
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| Cathepsin B | CATGCACTGGAGAAGGAGATAC | CTGTTAGACACGCTGTAGGAAG |
| Cathepsin D | CCTGGGCGATGTCTTCATT | GTGGAGAAGGAGCAAGTTAGAG |
| Cathepsin H | AAAGCTGTTGCATTCGTCAAG | CGAAGCTCACAGGGTTGTATAG |
| Cathepsin L | GAACAGCCTTAGCTACTCCAAA | CTTCCTCATTCGTGCCATACA |
| Cathepsin S | GGGAGACATGACCAATGAAGAA | TGTCAGGCAATGTCCGATTAG |
Western Blotting
J774.16 cells (5 × 105) were seeded in 6-well dishes and activated overnight. The cells were then challenged with Cn and incubated for 0, 2 and 4 h. Following each incubation, cells were washed with ice cold PBS, lysed with RIPA buffer (Sigma-Aldrich), and centrifuged at 150 g (Eppendorf centrifuge 5417c) for 5 min. Supernatant was collected, and the protein concentration was determined using the DC Protein assay (Bio-Rad, Hercules, CA). Protein samples were boiled for 7 min in sample buffer (150 mM Tris-Cl (pH 6.8), 300 mM DTT, 6% SDS, 0.03% bromophenol blue and 30% glycerol). An amount of 30 μg total protein was loaded into 10% Mini-PROTEAN TGX PRE CAST GEL (Bio-Rad) and then transferred onto a PVDF membrane (Millipore, Bedford, MA). Membranes were blocked with 5% nonfat milk/1X Tris-buffered saline and 0.1 % Tween-20 (TBST) for 1 h at room temperature, followed by three washes with TBST. Primary antibodies (1:200) were incubated overnight at 4°C in 5% nonfat milk/TBST. The primary antibodies used were anti-rabbit CTSB polyclonal antibody, anti-goat CTSD polyclonal antibody, anti-goat CTSH polyclonal antibody, anti-mouse CTSL polyclonal antibody, anti-goat CTSS polyclonal antibody (Santa Cruz Biotechnology) and for loading control we used β-actin antibody (1:3000; Santa Cruz Biotechnology). Membranes were washed three times with TBST and the corresponding secondary antibody (1:5000) was added for 1 h at room temperature in 5% nonfat milk/TBST. Secondary antibodies used were goat anti-rabbit Ig-HRP, rabbit anti-goat Ig-HRP (SouthernBiotech, Birmingham, AL) and goat anti-mouse IgG-HRP (Santa Cruz Biotechnology). The signal was developed using Supersignal West Pico Chemiluminescent substrate (ThermoFisher Scientific).
Cathepsins Activity assays
J774.16 cells (5 × 105) were seeded in a 6-well dish, activated with LPS and IFN-γ, and pre-treated with or without 8.7 μM PepsA over-night. The next day, the cells were washed with PBS and Cn was added in fresh media at an E:T ratio of 1:5. After 0, 2 and 4 h, the cells were washed with PBS and the CTS activity assay was performed following the manufacturer’s instructions (Abcam, Cambridge, MA). Briefly, cells were lysed using the cell lysis buffer and incubated on ice for 10 min, followed by centrifugation at 248 g for 5 min. The supernatant was collected and 50 μl of the cell lysate was added to a 96 well Optiplate (Perkin Elmer, Waltham, MA), plus 50 μl of the reaction buffer and 2 μl of the corresponding substrate. After 1 h of incubation, the fluorescence was read using the manufacturer recommended excitation and emission filters in a fluorimeter.
Cn killing by macrophages
J774.16 cells (2 × 104 per well) were seeded in 96-wells plate, activated and pre-treated with 8.7 μM PepsA overnight. The following day, the cells were washed with PBS and fresh media containing Cn was added to achieve an E:T ratio of 1:10, and the infected cells were then incubated for 24 h. The media from each well was then transferred to a separate tube containing 600 μl of PBS. A volume of 100 μl of sterile H2O was then added to each well and incubated at room temperature for 30 min to lyse the macrophages, which was hastened by pipetting the contents up and down several times. Each well was then rinsed with an additional 100 μl of PBS and the fractions collected into a single tube for CFU determination. A dilution of 1:1000 of fungal cells in each tube was plated on Saboraud’s dextrose agar and incubated at 30°C for two days and the colonies on each plate was counted.
Caspase 3 activity assay
J774.16 cells (2 × 106) were seeded in 6-well dishes, activated and pre-treated with the corresponding amount of 8.7 μM PepsA (Sigma-Aldrich) overnight. The cells were washed with PBS and fresh media was added with Cn at an E:T ratio of 1:2. After 24 and 48 h of incubation, cells were washed with PBS. The caspase enzymatic activity assay was performed following the manufacturer’s directions (R&D systems, Minneapolis, MN). Briefly, cells were collected after incubation and centrifuged at 152 g for 10 min, the supernatant discarded, and the pellet resuspended in the lysis buffer and followed by incubation on ice for 10 min. The samples were then centrifuged at 150 g for 2 min and the supernatants were transferred to new tubes. The cell lysate was transferred to a 96-well plate and mixed with 50 μl of 2X Reaction Buffer 3 and 5 μl of Caspase-3 colorimetric substrate. After 1 h of incubation, samples were read in a microplate reader (Labsystems Inc., Franklyn, MA) using a wavelength of 405 nm.
Statistical analysis
All our experiments were done at least three times, with the exception of some of the flow cytometry experiments, which were done only twice due to cost and because the result of the replicate was consistent. For cell viability and PMP analysis, unpaired t-test and one-way ANOVA was done relative to WT. The experiments were done three times or twice in triplicates with comparable results, data are mean ± SEM. The numbers inside each flow cytometry scatter plot represent the percentage of events on each quadrant and those numbers were used to make the cell viability graphs, subsequently we determine the percentage of cells with low LysoTracker signal (PMP), and reported as number of cells in the graph. Data are presented as the number of cells, which reflect actual events, rather than percentages since the former allows greater appreciation of difference between experimental groups. Chi-square was used for the analysis of exocytosis results.
Results
Cn-mediated PMP is associated with macrophage apoptosis
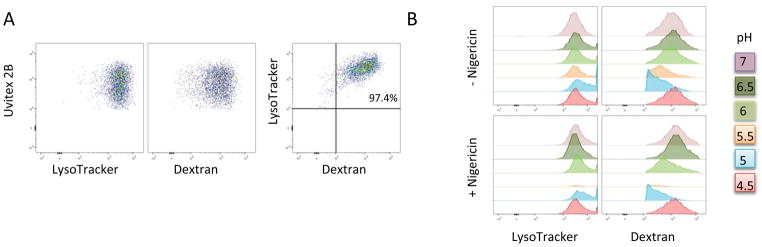
One of the consequences of PMP is cell death. Whereas partial permeabilization may activate different apoptotic signaling pathways, complete permeabilization bypasses PCD completely and instead leads to necrosis. Consequently, the ensuing cell death type is a function of the extent of membrane damage [32]. PMP is known to occur in Cn-infected macrophages [5, 20] and the damage progressed as the same rate as Cn replication [21]. Furthermore, induction of lysosomal damage enhanced Cn replication. Taken together, these observations suggest that Cn-induction of phagolysosomal damage may be beneficial for Cn survival [21]. To study the effect of Cn-mediated PMP on macrophage viability, we devised a flow cytometry methodology to measure Cn-mediated PMP in macrophages. With this method, we discriminated live population from apoptotic and dead cell populations using the dyes F2N12S and SYTOX (Fig 1). In a fashion similar to the one used in other studies [33, 34] we examined phagolysosomal permeabilization via measuring the intensity of LysoTracker DeepRed (LysoTracker): a strong staining indicates an intact phagolysosomal membrane while a dim signal is characteristic of PMP (Fig 1). We carried out control studies to determine whether LysoTracker signal correlated with the signal from FITC-dextran dyes and ascertained if these signals intensity were affected by pH. We tested by flow cytometry whether LysoTracker and FITC-dextran accumulates in the same cell population of macrophages infected with Cn. Our results showed that cells either are double positive (LysoTracker and FITC-dextran positive (97.4%)) or double negatives (LysoTracker and FITC-dextran negative (2.71%)) with discrepant staining not accounting for more than 1% (Fig 2A). Additionally we infected macrophages with Cn, and subjected these cells to a range of pH from 4–7 and measured the LysoTracker and FITC-dextran signal intensity by flow cytometry. Our results showed that LysoTracker intensity is not affected by pH, while FITC-dextran is (Fig 2B). These results are consistent with data showed by another other group reporting that LysoTracker staining occurs independently of poor acidification [35] and supports our use of this method.
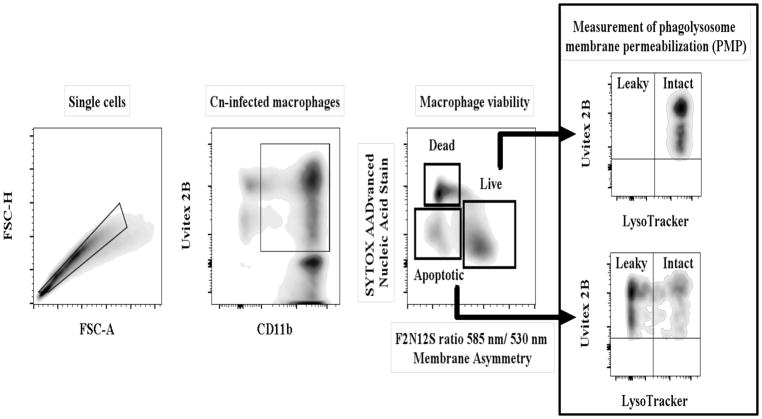
Fig 1. Schematic representation of the flow cytometry protocol used to analyze cell viability and PMP.
Macrophage-like cells were stained with CD11b and infected with Cn pre-stained with Uvitex 2B. LysoTracker was added to the cells 1 h before completion of the experiment to allow for accumulation in the phagolyososomes. Infected macrophages were selected by choosing double positive for CD11b and Uvitex 2B. Viability analysis in this population was selected by staining with F2N12S-ratiometry of membrane potential and SYTOX-live/dead exclusion. We collected the same number of events in the CD11b+ gate (50,000 per sample in triplicates). Live and apoptotic populations were plotted for LysoTracker intensity (vs Uvitex 2B) to measure PMP. Cells presenting a strong LysoTracker signal contained an intact phagolysosomal membrane while a weak LysoTracker signal indicated inability to retain LysoTracker in the phagolysosome, due to damage of the phagolysosomal membrane. The dead population was excluded from the analysis, because cell membrane damage causes unspecific staining with LysoTracker.
Fig 2. LysoTracker and dextran co-localize and Lysotracker intensity signal is independent of pH.
BMDM were infected with Cn for 24h and stained with LysoTracker and 40KDa Dextran-FITC. (A) CD11b+ Uvitex + cells stained for both LysoTracker and Dextran. (B) Cn-infected BMDM were incubated with LysoTracker and Dextran in a range of different pH (4.5–7.0) with and without nigericin. Our results showed that LysoTracker signal intensity was more stable than dextran. Experiments were repeated 2 times in triplicates. Shown are results of a representative experiment.
To test our system we compared uninfected macrophages, macrophages that ingested heat-killed Cn and macrophages infected with live Cn (S1 Fig). Our results showed that there was no significant difference in the percentage of cells between the live populations (P = 0.83) and the apoptotic populations (P = 0.99) of uninfected macrophages and macrophages infected with heat-killed Cn. There was a significant decrease in the percentage of cells in the live populations (P = 0.03) and in the apoptotic populations (P = 0.0003) of uninfected macrophage compared to macrophage infected with live Cn (S1 Fig). When comparing macrophages infected with heat-killed and live Cn, the difference in the percentage of live cells (P = 0.08) does not reach significance levels, but there was a significant difference comparing the apoptotic populations (P = 0.0003). Unfortunately, this methodology is not sensitive enough to identify all the dead macrophages (S1 Fig). Some dead macrophage end up as debris, and excluded from the analysis. Dead macrophages are also rapidly phagocytosed by adjacent macrophages. Hence, even though we report the percentage of dead macrophages it is important to note that this percentage is probably an underestimation of the real amount of dead macrophages in the experiment. Subsequently, we analyzed PMP in each of the populations (S1 Fig). Again there was no significant difference in the number of PMP-positive cells in the live populations (P = 0.99) or apoptotic (P = 0.95) populations of uninfected macrophages and macrophages infected with heat-killed Cn (S1 Fig). There was a significant increase in the amount of PMP-positive cells, both the live (P = 0.01) and apoptotic (P < 0.0001) populations, in macrophages infected with live Cn versus uninfected macrophages (S1 Fig). This increase was also observed when comparing PMP-positive cells, live (P = 0.01), as well as apoptotic (P < 0.0001), population of macrophages infected with live Cn versus heat-killed Cn (S1 Fig). For this experiment we performed our analysis in all the cells CD11b+ because our control group was uninfected macrophages. For subsequent experiments we wished to focus on infected macrophages only, and subsequently we performed our analysis in CD11b+, Uvitex2B+ cells (Fig 1).
Ingestion of live Cn, as compared to heat-killed Cn, by J774.16 cells was associated with a lower percentage of live J774.16 cell populations (30.9% ± 2.15%) and a higher percentage of apoptotic J774.16 cell populations (10.77% ± 0.41%), the difference in the number of cells between both populations was statistically significant (Fig 3A–C). More detailed analyses revealed that there was a significant increase (P = 0.006) between the PMP-positive cells of the live population of J774.16 cells infected with Cn (10.67 ± 1.45) compared to heat-killed Cn (1.66 ± 0.88). There was also a significant increase (P < 0.0001) between the cells PMP positive in the apoptotic population of the J774.16 cells infected with Cn (1834 ± 60.53) compared to J774.16 cells infected with heat-killed Cn (19 ± 2.30) (Fig 3D–I). PMP was observed more frequently in the cells from the apoptotic populations, than in cells within the live population. Live Cn ingestion by BMDM showed a significant increase (74.2% ± 0.94%) in the percentage of the number of cells in the live BMDM cell population compared to macrophages infected with heat-killed Cn (59.9% ± 3.90%) (S2 A–C Fig). There was also a significant increase between the number of cells in the apoptotic populations of BMDM infected with live Cn (11.67% ± 0.44%) and heat-killed Cn. (2.06% ± 0.64%) (S2 A–C Fig). PMP analysis of the BMDM in the live and apoptotic population also showed that the majority of cells with PMP had an apoptotic phenotype. Because the majority of J774.16 and BMDM cells that were positive for PMP were apoptotic (Fig 3D–I and S2 D–I) (low membrane potential but not permeable to SYTOX), we hypothesized that cells with PMP in the live population are still at an early stage, and will eventually transition into the apoptotic population. Interestingly, we observed a subset of apoptotic cells without PMP (Fig 3H), implying that in the Cn-macrophage interaction apoptosis activation also occurs independently of PMP. Imaging of Lysotracker-stained J774.16 cells confirmed a diffuse pattern of LysoTracker staining in cells infected with live Cn for 2 h, which is characteristic of PMP (Fig 3J–M). This is in contrast with heat-killed Cn-infected J774.16 cells, where LysoTracker aggregates appeared to co-localize with Cn (as seen via Uvitex cell wall labelling), consistent with an intact and non-leaky phagolysosome (Fig 3J–M).
Fig 3. Cn-induced PMP is associated with apoptosis in J774.16 cells. Cell viability of J774.16 cells infected with Cn and heat-killed Cn measured by flow cytometry.
(A–C) Macrophages infected with Cn showed an increase in the apoptotic population when compared to macrophages infected with heat-killed Cn. (D–I) PMP was induced in macrophages infected with Cn but not in macrophages infected with heat-killed Cn. The majority of macrophages that manifested PMP were positive for apoptotic markers. (J–M) Confirmation of flow cytometry analysis by immunofluorescence microscopy. J774.16 cells infected for 2 h with heat-killed Cn (J–K) showed an aggregation of LysoTracker (Red) that co-localizes with Cn (stained with Uvitex2B in blue). In macrophages infected with Cn (L–M), the LysoTracker signal co-localized with Cn, and showed a diffused pattern, characteristic of PMP. Shown is representative data from 3 experiments mean and SD. Unpaired t-test *p > 0.0332, **p < 0.0021, ***p < 0.0002 and #p < 0.0001.
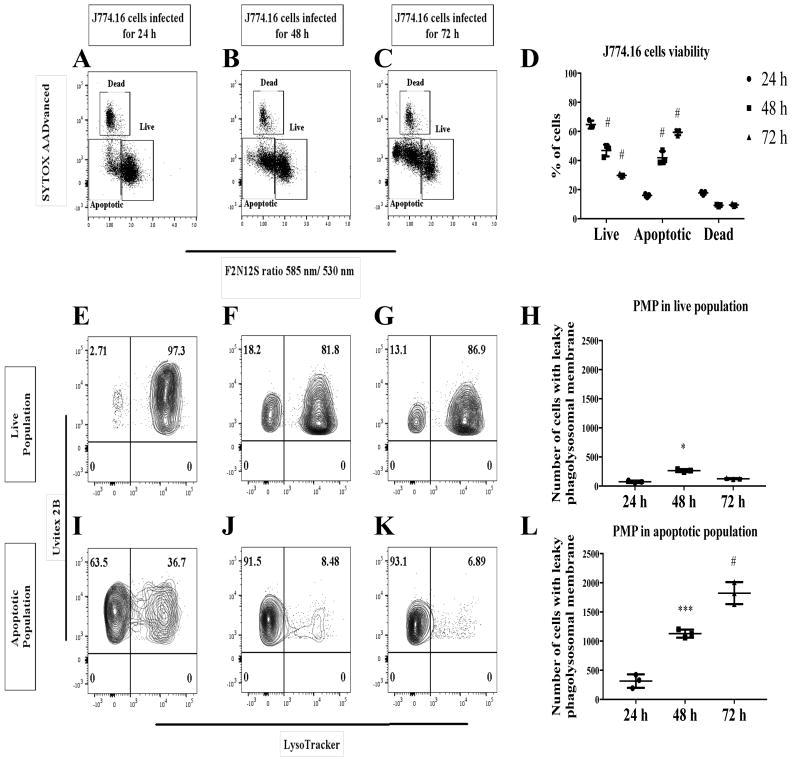
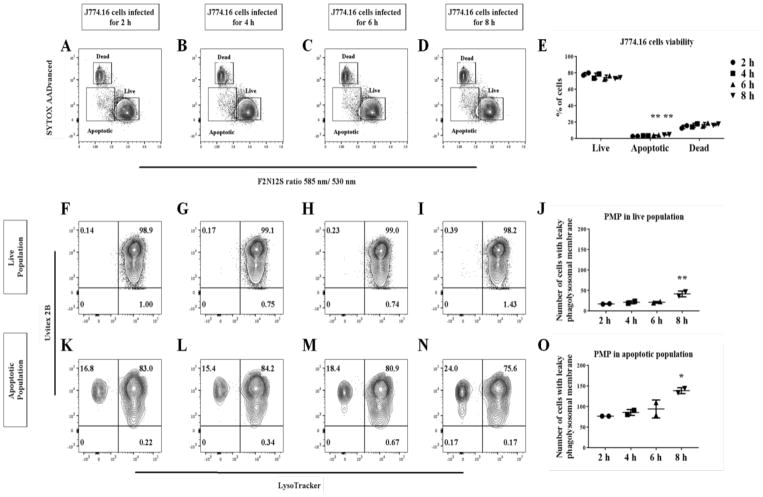
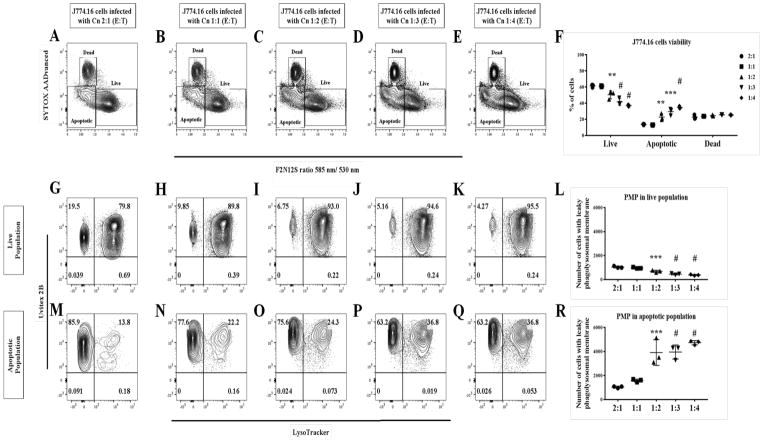
To test the notion that PMP is an early event, we performed an infection time course to monitor the host cell apoptotic population. Cn-infected, apoptotic J774.16 cells populations significantly increased (P < 0.0001) over the period of 72 h (Fig 4A–D), as did cells with PMP (Fig 4E–L). PMP occurs as early as 2 h post infection with a significant increase (P = 0.0017) in the percent of apoptotic cells at 8 h post infection (Fig 5A–E). There was also a significant increase (P = 0.023) in the number of J774.16 cells PMP-positive in the apoptotic population (Fig 5F–O). Further analysis showed that increasing the infection burden led to a significant increase (P = 0.0003) in apoptotic population of J774.16 cells (Fig 6A–F) and cells displaying PMP (P = 0.0078) (Fig 6G–R), particularly significant at the E:T (Macrophage:Cn) ratio above 2.
Fig 4. Cn-induction of PMP increases over infection time. Cell viability and PMP analysis of Cn-infected macrophages measured at 24, 48 and 72 h.
(A–D) Our analysis showed that there is a significant increase in the apoptotic population over the 72 h period. (E–L) PMP seems to increase only in the apoptotic population. Shown is representative data from 2 experiments mean and SD. One-way ANOVA with Dunnett’s multiple comparison test *p > 0.0332, **p < 0.0021, ***p < 0.0002 and #p < 0.0001.
Fig 5. Cn-induction of PMP occurs early during infection.
Cell viability and PMP analysis of Cn-infected macrophages was measured every 2 h for a period of 8 hrs. (A–E) There is a significant increase in the apoptotic populations. (F–O) PMP seems to be an event that happens quickly after Cn infection and remains constant for the first 8 h. Shown is representative data from 2 experiments mean and SD One-way ANOVA with Dunnett’s multiple comparison test *p > 0.0332, **p < 0.0021, ***p < 0.0002 and #p < 0.0001.
Fig 6. Increasing the number of Cn per macrophage is associated with increased PMP occurrence.
Macrophages infected with a higher E:T showed an increase in the apoptotic population (A–F). Increasing E:T resulted in an significantly increase in PMP in the macrophage apoptotic population, but not in the live population (G–R). Shown is representative data from 2 experiments mean and SD. One-way ANOVA with Dunnett’s multiple comparison test *p > 0.0332, **p < 0.0021, ***p < 0.0002 and #p < 0.0001.
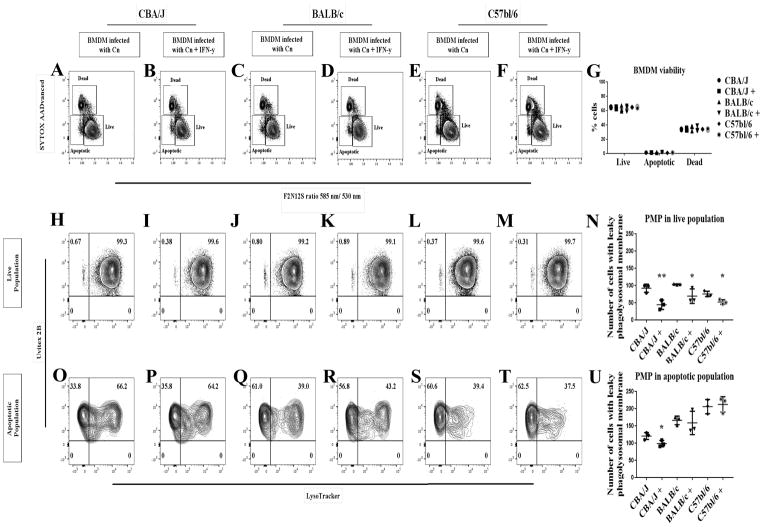
Modulation of PMP by different mouse strains
The cellular response to PMP, i.e. the activation of various apoptotic pathways, depends on the original stimuli that trigger the apoptotic pathway and is cell type dependent [23]. The varying degree of susceptibility that different mouse strains exhibit to Cn pulmonary infection has been linked to macrophage permissiveness to intracellular replication [36]. Additionally, there is growing evidence of a link between PMP occurrence and increased Cn intracellular replication, in particular as IFN-γ treatment not only negatively affects Cn replication, but reduces PMP too [21]. To explore whether PMP was potentially correlated with the outcome of the Cn-macrophage interaction in addition to mouse strain susceptibility, we measured PMP in BMDM isolated from three different mouse strains BALB/c, CBA/J and C57/BL6 (Fig 7). Concomitantly, we analyzed the effect of IFN-γ on BMDM derived from each stain. Our results showed that BMDM isolated from BALB/c, CBA/J and C57BL/6 mice were similarly susceptible to Cn-infection in vitro, showing no significant difference in the number of cells in the apoptotic population (P = 0.19) (Fig 7A–G). Treatment with IFN-γ significantly reduced (P < 0.05) the number of macrophages positive for PMP in the live population of Cn-infected BMDM isolated from the three mouse strains (Fig 7H–N). Interestingly, all the strains showed a different rate of occurrence of Cn-induced PMP in the apoptotic population, with CBA/J being the strain with the lower percentage of cells displaying PMP and the only one in which treatment with IFN-γ resulted in a significant decrease (P < 0.05) in the number of cells with PMP (Fig 7O–U). Our data imply that IFN-γ treatment reduces the number of cells with PMP in the live population, but does not completely block PMP in Cn-infected macrophages, like in Davis et al. study. Like the Davis et al. study we also stimulated the macrophages overnight with 100 ng/mL of IFN-γ. The main differences between Davis et al. and our study are: 1) their use of F12D2 IgG and 20% normal mouse serum for opsonization method while we used an IgG monoclonal antibody (18B7), 2) their use of FITC-Dextran to track PMP while we used LysoTracker; and 3) their use of 0.05–0.1 MOI, which is much lower than the 0.5 MOI (equivalent to E:T 1:2 for most experiments) used in our study. Furthermore, a major difference between our study and that of David et al. is that their group did not evaluate the cell viability of the macrophages (they used SYTOX to exclude dead cells from their analysis, but did not used apoptotic markers) and focused only on the lysosomal damage and Cn viability. In addition, they employed microscopy to analyze the cells, which is more time consuming and produces a n < 100 while our flow cytometry method in which we analyzed 50,000 cells and thus we could analyze the population of relatively rare apoptotic cells. Therefore, the discrepancy can be ascribed to different experimental focus: we focused on PMP correlation to macrophage death while Davis et al. focused on the mechanism of IFN-γ influencing the phagolysosome. Further kinetic analysis is required to understand whether IFN-γ is delaying the onset of PMP or only partially blocking it.
Fig 7. IFN-γ treatment delayed PMP in BMDM from three different mice strains.
(A–G) BMDM isolated from three different mice strains showed no significant difference in cell viability after 24 h infection with Cn. (H–N) Overnight treatment of macrophage with 100 ng/ml IFN-γ showed a significant reduction in Cn-induction of PMP in macrophage in the live population isolated from the three mice strains. (O–U) BMDM isolated from CBA/J mouse strain were less susceptible to Cn-induction of PMP than BMDM isolated from BALB/c and C57BL/6 mice. Shown is representative data from 2 experiments mean and SD. Unpaired t-test *p > 0.0332, **p < 0.0021, ***p < 0.0002 and #p < 0.0001.
Chemical manipulation of PMP affects the outcome of Cn-macrophage interactions
During lytic exocytosis Cn is released to the extracellular environment due to complete lysis of the host macrophage, regardless of which cell death pathway induced host cell lysis. In contrast, non-lytic exocytosis results in the release of viable Cn to the extracellular environment with the survival of the host cell, via a still poorly understood mechanism. Previous studies have shown that this phenomenon, non-lytic exocytosis, was reduced in macrophages treated with concanamycin A and bafilomycin A two compounds involved in the inhibition of the vacuolar ATPase (V-ATPase), while macrophages treated with the lysosomal inhibitors ammonium chloride and chloroquine showed a significantly increase in non-lytic exocytosis [26, 37, 38]. We induced PMP chemically to address the question of whether the phenomenon of non-lytic exocytosis is influenced by PMP. To induce PMP we tested several drugs previously shown to induced PMP [39]. Ciprofloxacin, Leu-Leu-O-Me and helenalin were tested in isolated rat lysosomes in vitro for their capacity to induce leakage of lysosomes. All three compounds showed a significant increase (P < 0.0001) in β-hexosaminidase activity, characteristic of an increase in broken lysosomes (S3 A Fig). We decided to proceed with ciprofloxacin due to its lower toxicity in macrophages and Cn (S3 B–E), compared to the other compounds (data not shown). Treatment of Cn-infected J774 cells with ciprofloxacin showed no significant difference (P = 0.10) in the percentage of cells in the live population, but there was a significant reduction in the apoptotic population in cells treated with 10 μM of ciprofloxacin but no significant reduction in the cells treated with 100 μM of ciprofloxacin (S3 F–H Fig). Surprisingly we did not see an increase in the amount of cells with PMP in neither of the experimental groups (S3 D–E, G–H Fig). Interestingly, when we tested the effect of ciprofloxacin in BMDM our results showed a significant increase (P = 0.002) in the live cell population (Fig 8A–D). Treatment with 100 μM of ciprofloxacin caused a significant increase (P < 0.05) in the number of PMP-positive cells in the apoptotic population (Fig 8A–L). To further study the effect of PMP in the different types of non-lytic exocytosis, we treated BMDM with 15 μM of ciprofloxacin, a non-toxic dose to the host cell, and evaluated the number of lytic and non-lytic exocytosis events over 24 h (Fig 8M). Analysis of Cn escape from macrophages over this time course revealed that ciprofloxacin caused a significant increase (P < 0.05) in lytic exocytosis events and a non significant decrease in non-lytic exocytosis (Fig 8M). These results imply that J774.16 cells have a different dose-response profile than BMDM, however, it appears that ciprofloxacin treatment affects PMP, possibly inducing complete PMP, and therefore we observed ciprofloxacin affected non-lytic exocytosis versus lytic exocytosis balance. Further, these results suggest that substantial damage to the phagolysosomal membrane is linked to lytic exocytosis, and implies that an intact phagolysosomal membrane is required for non-lytic exocytosis.
Fig 8. Chemical manipulation of PMP affects the outcome of Cn and BMDM interaction.
(A–D) Ciprofloxacin treatment of BMDM infected with Cn resulted in a statistically significant increase in apoptosis. (E–L) Further analysis revealed an increase in the number of cells with PMP in the apoptotic population, but not in the live population. (M) Exocytosis analysis of macrophages (n ± 700) treated with 15 μM ciprofloxacin revealed a shift in Cn-macrophage interactions, as chemical induction of PMP results in an increase in cells undergoing lytic exocytosis when compared to non-treated cells. Shown is representative data from 2 experiments mean and SD. One-way ANOVA with Dunnett’s multiple comparison test *p > 0.0332, **p < 0.0021, ***p < 0.0002 and #p < 0.0001.
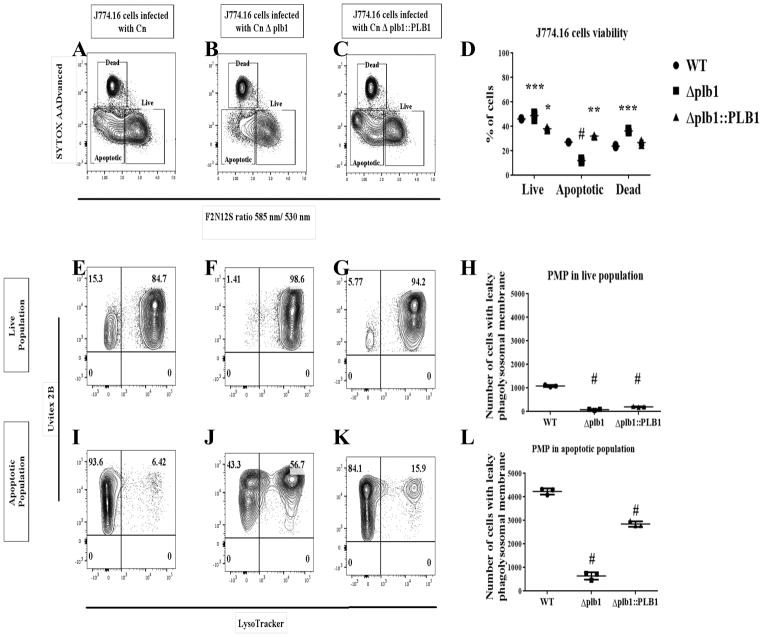
Cn PLB1 contributes to PMP
To date no mechanism has been reported for Cn-mediated PMP. PLB1-deficient strains are less virulent in a nasal inhalation mouse model and given the known biochemical activity of this enzyme the authors hypothesized that PLB1 to play a role in inducing PMP [24]. To test this association, we infected macrophages with WT, Δplb1, and Δplb1::PLB1 Cn and analyzed the contribution of PLB1 to PMP (Fig 9). J774.16 cells infected with Δplb1 showed a significant decrease (P < 0.0001) in the apoptotic population and a significant increase (P < 0.05) in the live population relative to J774.16 cells infected with WT and Δplb1::PLB Cn (Fig 9A–D). Accordingly, J774.16 cells infected with Δplb1 showed a significant decrease (P < 0.0001) in the number of cells with PMP (both in live and apoptotic populations) when compared to J774.16 cells infected with WT and Δplb1::PLB Cn (Fig 9E–L). Similar results were obtained in BMDM infected with WT, Δplb1, and Δplb1::PLB1 Cn strains (S4 Fig). These results suggest that Cn PLB1 contributes to PMP. Interestingly, non-lytic exocytosis was reduced in macrophages infected with Δplb1 [40], providing further evidence that an interplay may exist between the integrity of the phagolysosomal membrane and the outcome of Cn-macrophage interactions.
Fig 9. PLB1 contributes to Cn-mediated PMP.
(A–D) J774.16 cells infected with Δplb1 showed a decrease in the apoptotic population and an increase in the live population when compared to WT and Δplb1::PLB. (E–L) Macrophages-like cells infected with Δplb1 showed a significant decrease in the amount of cells with PMP when compared to macrophages infected with WT and Δplb1::PLB. Shown is representative data from 3 experiments mean and SD One-way ANOVA with Dunnett’s multiple comparison test and unpaired t-test, *p < 0.0332, **p < 0.0021, ***p < 0.0002 and #p < 0.0001.
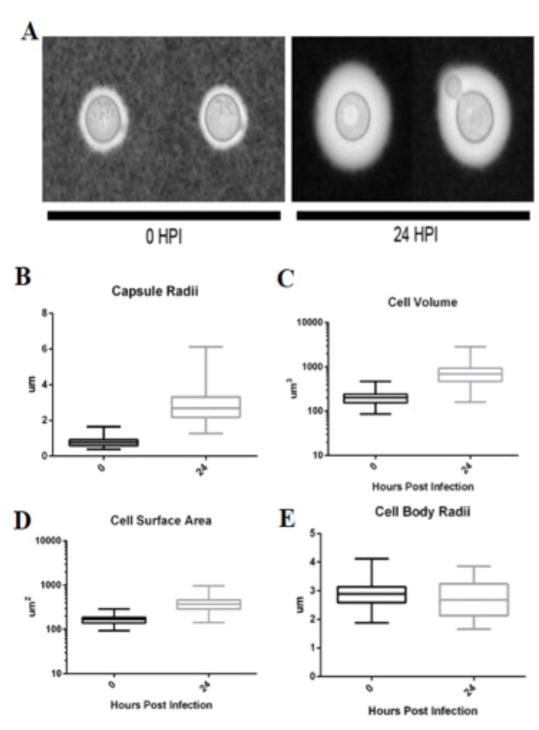
Cn capsular dynamics in infected macrophages
Analysis of cryptococcal cells immediately after phagocytosis and at 24 h revealed large increases in capsule size (Fig 10A). The average capsule radius (Fig 10B), volume (Fig 10C) and cell surface area (Fig 10D) for cryptococcal cells at 0 and 24 h were 0.6511 ± 0.1793 μm, 215.9 ± 79.50 μm3, 171.8 ± 40.41 μm2, and 2.263 ± 0.5073 μm, 589.4 ± 306.5 μm3, 330.4 ± 114.4 μm2, respectively. Consequently, the area calculated for the surface of encapsulated cells increased by 92.32 % (Fig 10D). This increase occurred despite the average radii of the cell body been similar (Fig 10E). Cells recovered from inside macrophages after 24 h manifested greater heterogeneity in cellular and capsular dimensions that cells recovered shortly after phagocytosis (t = 0).
Fig 10. Cn polysacharride capsule increases after pahgocytosis.
Analysis of Cn cells shortly after phagocytosis (t = 0) and after 24 of residence inside macrophages (t = 24). Results are representative of two biological replicates of three technical replicates each. (A) Representative images of cryptococcal cells at t = 0 and 24 h. Images were acquired via bright field microscopy of India Ink exclusion slides. (B) Capsule Radii of cryptococcal cells at t = 0 (0.7864 μm ± 0.2687) and 24 h (2.802 μm ± 0.8592), with numbers being means and standard deviations of 81 and 131 cells, respectively. P value of 2.57E-58 according to two-tailed, unpaired t test; (C) Cell volumes of cryptococcal cells at t = 0 (215.2 μm 3 ± 82.94) and 24 h (765.2 μm 3 ± 422.5) with numbers being means and standard deviations of 81 and 131 cells, respectively. P value of 5.98E-30 according to two-tailed, unpaired t test; (D) Cell surface area of cryptococcal cells at t = 0 (171.1 μm 2 ± 42.69) and 24 h (392.2 μm 2 ± 142.0), with numbers being means and standard deviations of 81 and 131 cells, respectively. P value of 3.58E–37 according to two-tailed, unpaired t test. (E) Cell Body Radii of cryptococcal cells at t = 0 (2.877 μm ± 0.3899) and 24 h (2.696 μm ± 0.5943), with numbers representing mean and standard deviations of 81 and 131 cells, respectively. P value of 0.0159 according to two-tailed, unpaired t test.
Cn-infected macrophages are not able to maintain acidification
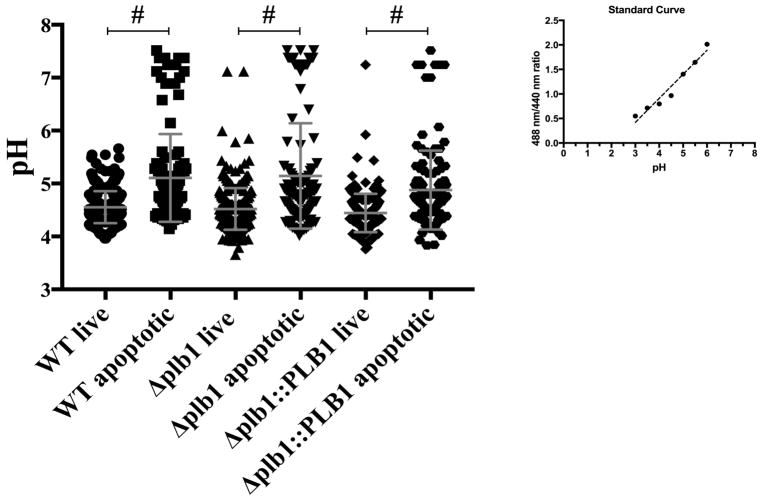
Phagocytosis of Cn results in localization of Cn into a mature phagolysosome [4, 5, 26]. Phagolysosomal membrane integrity is presumably required for maintaining an acidic environment inside the phagolysosome [41] and different studies have reported discrepancies on whether Cn can block phagolysosomal acidification [4, 19, 42]. To test the hypothesis that PLB1 modulates the phagosomal environment and causes an alteration in pH in the phagolysosome, we studied phagolysosomal pH changes in cells undergoing early apoptosis, by staining with the early apoptotic marker, annexin V. To assess whether PLB1 affected the pH of the phagolysosome, we measured the phagolysosomal pH of BMDM infected with WT, Δplb1, and Δplb1::PLB1 Cn strains (Fig 11). Predictably, apoptotic Cn-infected macrophages were not able to maintain acidification to the same extent as non-apoptotic Cn-infected macrophages after 24h of infection. Cn-infected macrophages positive for annexin V had phagolysosomes with significantly (P < 0.0001) higher pH (pH=4.55) than Cn-infected macrophages negative for annexin V (pH=5.10) for all three strains tested (Fig 11). We found no significant difference (P = 0.39) between the phagolysosomal pH of cells infected with WT and Δplb1 strain within Annexin V negative cells (P = 0.82), but a significant difference (P = 0.001) between cells infected with WT and Δplb1::PLB1 Cn strains (Fig 11). In the apoptotic population we observed no significant difference (P = 0.8929) between the phagolysosomal pH of cells infected with either Cn strain (Fig 11). Our results showed that despite PLB1 been associated with PMP, this is not reflected in alterations in phagolysosomal pH. Interestingly, these results show that Cn phagosome is maintained at a less acidic value than the ones reported for fully functional phagolysosomes suggesting that Cn is able to modulate the phagolysosomal pH.
Fig 11. Ph measurment of Cn-containing phagolysosomes.
BMDM infected with WT, Δplb1 and Δplb1::PLB showed no significant difference between the different strains, but there was a significant difference when we compare live versus apoptotic BMDM between the BMDM infected with the different strains. The pH of Cn-containing phagolysosomes was less acidic in BMDM positive for annexin V in comparison to BMDM negative for annexin V. Determination of pH by using a standard curve of buffers with different pH in the presence of nigericin to equilibrate the extracellular and intracellular pH. One-way ANOVA with Dunnett’s multiple comparison test *p > 0.0332, **p < 0.0021, ***p < 0.0002 and #p < 0.0001.
CTS B, D, H, L and S expression in Cn-infected macrophages
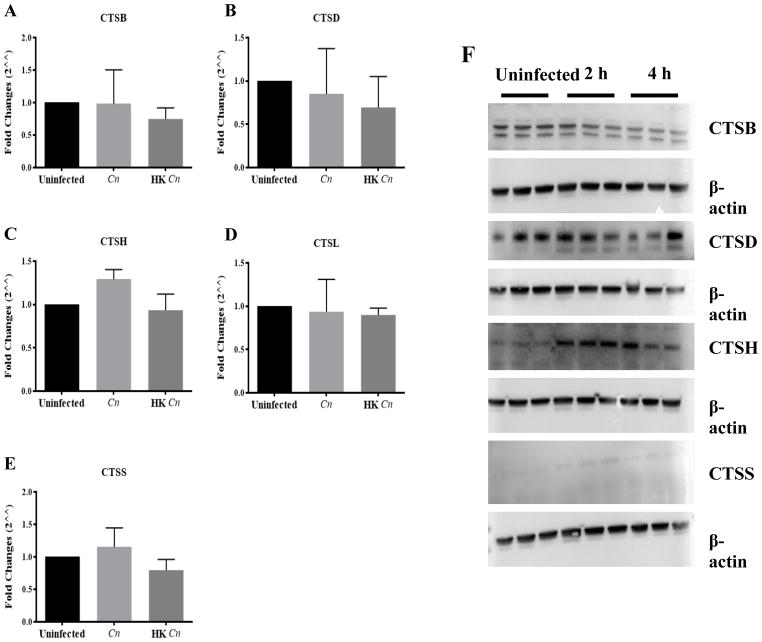
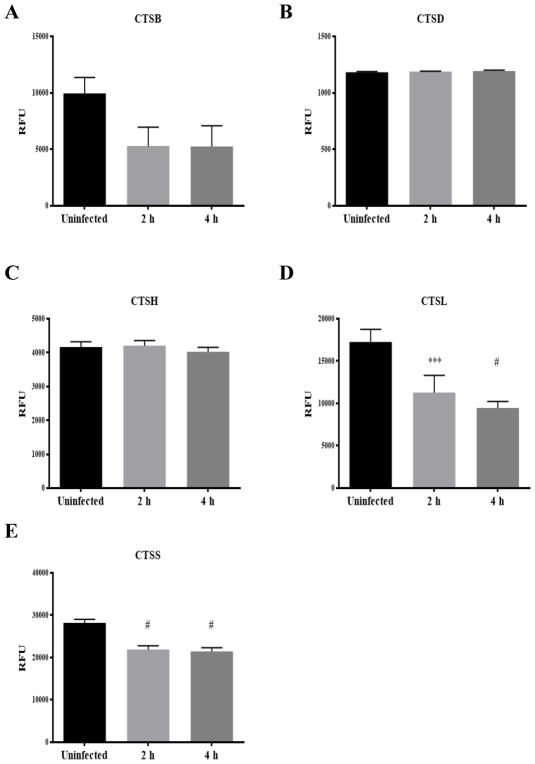
Since PMP causes the release of phagolysosomal contents into the cytosol, including proteases of the CTS family, we hypothesized that release of CTS may contribute to the macrophage’s demise. We sought to profile CTS activity in infected cells via studies of gene expression, protein expression, and enzymatic activity measurements of CTS in Cn-infected macrophages. CTS mRNA expression was measured at 2 and 4 h after live and heat-killed Cn macrophage infection and revealed no significant differences (Fig 12A–E). Western blot analysis showed that CTSD and CTSH protein expression increased 4 h after phagocytosis whereas CTSB protein expression decreases, while CTSS detection was too low to reliably interpret protein levels (Fig 12F). Subsequently, we analyzed CTS protein activity to examine if Cn phagocytosis may inhibit its enzymatic activity (Fig 13). Our results showed that CTSB, CTSL and CTSS protein activity decrease after phagocytosis, although only CTSL CTSS reached a statistical significance difference (P = 0.05). CTSD and CTSH enzymatic activity was maintained during this period of time (Fig 13A–E). Our results suggest CTSB, CTSL and CTSS protein expression are modulated during Cn infection implying that these proteins may play a role in host cell defense.
Fig 12. Chracterization of CTS mRNA and protein expression in Cn-infected J774.16 macrophage-like cells.
(A–E) CTSB, CTSD, CTSH, CTSL and CTSS mRNA expression was measured by RT-qPCR. In macrophages infected with Cn and heat-killed Cn, no significant difference between the groups was observed. (F) CTSB, CTSD, CTSH, and CTSS protein expression was measured by western blot 2 and 4 hours after infection. Western blot analysis showed that CTSD and CTSH protein expression increased 4 h after phagocytosis whereas CTSB protein expression decreases, while CTSS detection was too low to reliably interpret protein levels. The three triplicates lanes represent cell lysate from three different experiments.
Fig 13. Chracterization of CTS mRNA and protein expression in Cn-infected J774.16 macrophage-like cells.
(A–E) CTSB, CTSD, CTSH, CTSL and CTSS enzymatic activity was measured 2 and 4 hours after infection. CTSD and CTSH showed no change in enzymatic activity but CTSB, CTSH and CTSS showed a decrease in enzymatic activity after Cn. One-way ANOVA with Dunnett’s multiple comparison test *p > 0.0332, **p < 0.0021, ***p < 0.0002 and #p < 0.0001.
Inhibition of CTS and caspases in Cn infected macrophages
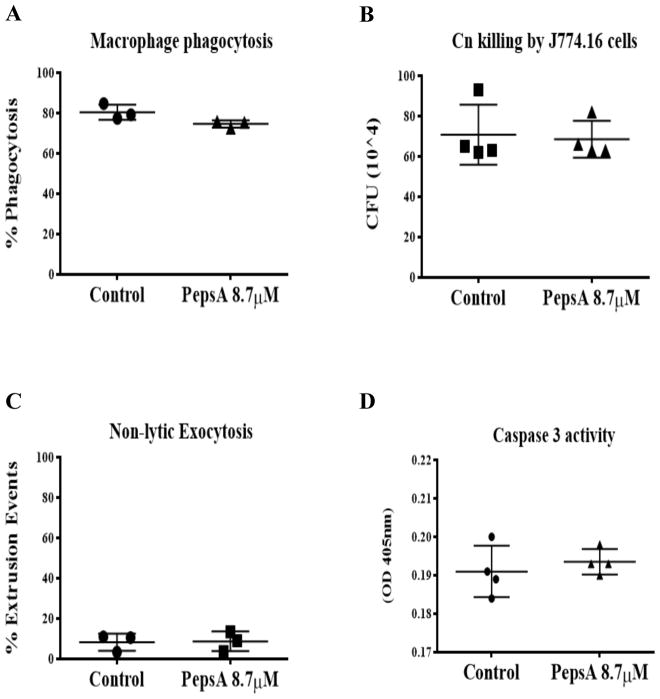
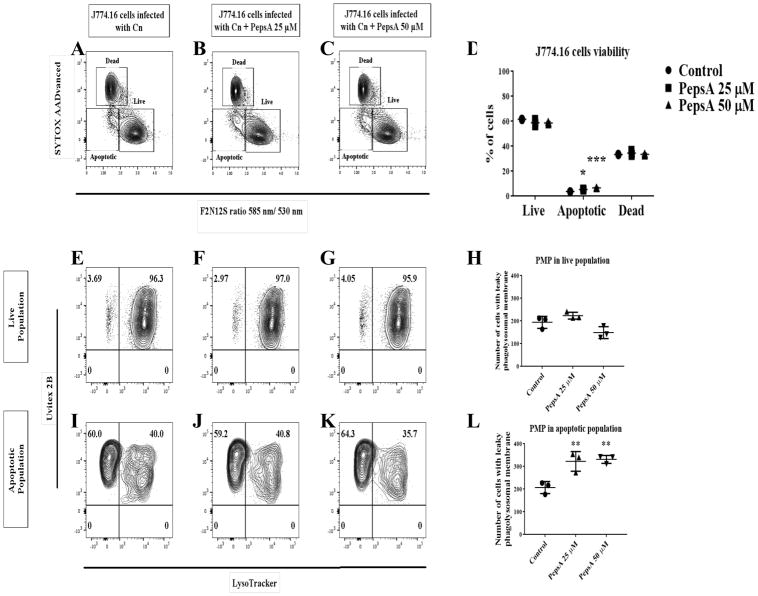
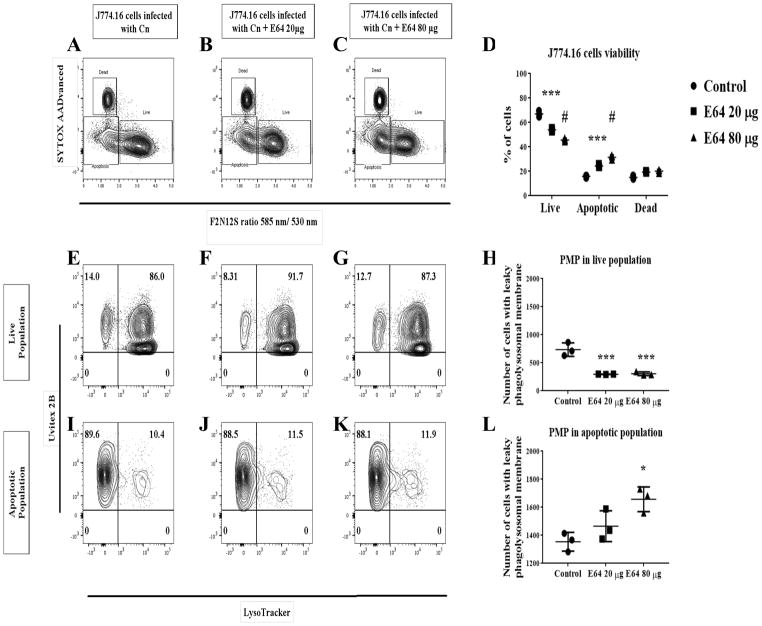
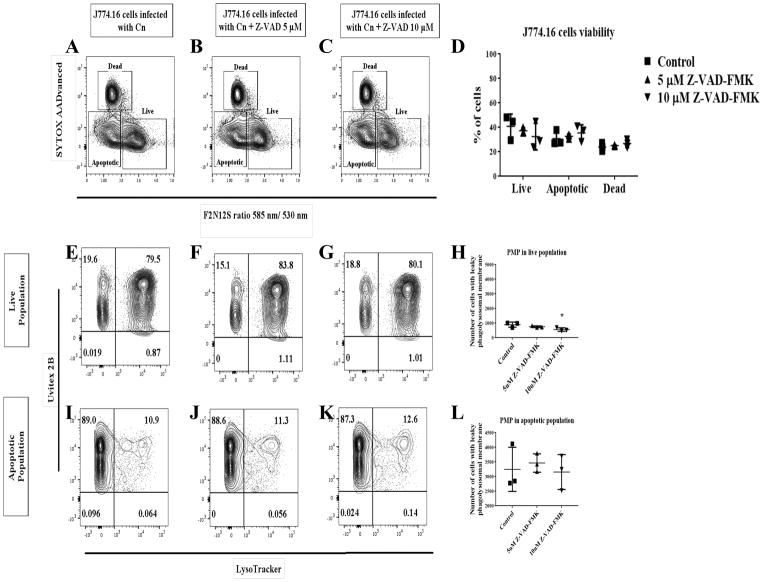
To further explore the role of proteases during Cn infection in macrophages and its potential to affect non-lytic exocytosis, we treated macrophages with PepsA, an inhibitor of aspartic proteases including CTSD. Preliminary studies showed that PepsA inhibited Cn growth in liquid cultures, which is not surprising as Cn contains aspartic proteases which are inhibited by PepsA [43]. To overcome this problem, we pre-treated macrophages overnight with PepsA, and then removed PepsA for the duration of Cn infection in order to leave the Cn proteases intact. This method was sufficient to inhibit CTSD enzymatic activity in J774.16 cells and BMDM (data not shown). Using this approach, we analyzed the effect of CTSD inhibition on phagocytosis and Cn killing by macrophages. Our data showed that there are no significant differences in both Cn phagocytosis between the PepsA treated macrophages and our controls (Fig 14A), nor in macrophage killing of Cn (Fig 14B). Analysis of the effect of inhibition of CTSD in non-lytic exocytosis (Fig 14C) showed no significant difference between the PepsA treated cells and the controls. To examine the role of aspartic and cysteine CTS as well as caspases in cell viability and PMP, we treated macrophages with PepsA (Fig 15A–L), E64 (Fig 16A–L), and Z-VAD-FMK (Fig 17A–L). Inhibition of aspartic CTS (Fig 15A–L) and cysteine CTS (Fig 16A–L) increased apoptosis of infected macrophages and increased PMP rates in the apoptotic population. For aspartic CTS, the live population showed a decrease in PMP which could be interpreted as a faster progression to apoptosis. However, pre-treatment of macrophages with PepsA did not alter Caspase 3 activation after 24 h of infection (Fig 14D). These data imply that CTS effect on Cn could favor macrophage viability. In contrast, inhibition of caspases did not alter macrophage viability or PMP (Fig 17A–L), suggesting a caspase- independent mechanism of PCD.
Fig 14. Effect of aspartic cathepsin inhibition on the outcome of the Cn and macrophage interaction.
(A) Inhibition of aspartic CTS does not affect phagocytosis potential or (B) the killing capability of J774.16 macrophage-like cells. (C) Inhibition of aspartic CTS does not have an effect on non-lytic exocytosis or (D) on the activation of caspase 3 activity. Unpaired t-test, ns = 0.1234.
Fig 15. Effect of inhibition of aspartic CTS in J774.16 cells viability and PMP.
(A–D) PepsA treatment showed a significant increase in the apoptotic population. (E–L) PMP analysis shows an increase in cells with PMP only in the apoptotic population. Shown is representative data from 2 experiments mean and SD. One-way ANOVA with Dunnett’s multiple comparison test *p > 0.0332, **p < 0.0021, ***p < 0.0002 and #p < 0.0001.
Fig 16. Effect of inhibition of cysteine CTS in J774.16 cells viability and PMP.
(A–D) E64 treatment showed a significant increase in the apoptotic population. (E–L) PMP analysis shows an increase in cells with PMP only in the apoptotic population. Shown is representative data from 2 experiments mean and SD. One-way ANOVA with Dunnett’s multiple comparison test *p > 0.0332, **p < 0.0021, ***p < 0.0002 and #p < 0.0001.
Fig 17. Effect of inhibition of caspases on J774.16 cells viability and PMP.
(A–D) Z-VAD-FMK treatment did not affect Cn-induction of apoptosis or PMP (E–L). Shown is representative data from 2 experiments mean and SD. One-way ANOVA with Dunnett’s multiple comparison test *p < 0.0332, **p < 0.0021, ***p < 0.0002 and #p < 0.0001.
Discussion
There is considerable evidence that the Cn-induction of PMP may be a critical event in the intracellular pathogenic strategy of this fungus [5, 20, 21], but the consequences of Cn-mediated PMP for macrophage viability are unknown. Electron microscopy studies have shown a discontinuous phagolysosomal membrane in alveolar macrophages infected with Cn in vivo [5]. Subsequent studies by our group showed that Cn-containing phagolysosomes become permeable to macromolecules implying release of phagolysosomal content to the cytosol [20]. Release of phagolysosomal contents to the cytosol may activate different cell death pathways depending on both the cell type and initial stimuli; [23] partial PMP results in activation of apoptosis while massive PMP results in necrosis [23]. Additionally, phagolysosomal destabilization results in necrosis and induction of an inflammatory response, which could be driven by either pyroptosis (caspase-1 mediated necrosis) or necroptosis (RIP-1-mediated necrosis) [44]. Our study demonstrates an association between PMP and the emergence of an apoptotic phenotype in Cn-infected macrophage-like cells. Our time interval analyses suggest that roughly one percent of Cn-infected macrophages become apoptotic every hour. Studies done in bone marrow derived dendritic cells showed that Cn-induction of PMP results in activation of the canonical NLPR3-ASC-caspase 1 inflammasome and secretion of IL-β1 [45]. Previous work by our group has shown an increase in the protein expression of RIP, AIF and cytochrome c release in Cn-infected macrophage-like cells [22]. Cn-induction of PMP and subsequent release of CTS into the cytosol strongly suggests this is an upstream event culminating in cytochrome c release. Further, studies are required to determine which PCD pathways are activated after PMP.
Different methods have been developed to study PMP in the context of different pathogen infection mostly by using several LysoTracker dyes or Acridine-Orange [33, 34, 46–48]. In this study we used flow cytometry and combined the use of LysoTracker DeepRed with cell viability markers, which allowed us the opportunity to identify whether cells displaying PMP correlated with the live or apoptotic cell populations. This technique has the advantage of being able to discriminate between infected and uninfected cells. More importantly, this method allows us to simultaneously ascertain the viability state of Cn-infected macrophages and the stability of the phagolysosomal membrane. However, this method is limited in that it is not precise enough to measure partial PMP, or to discriminate between the different cell death pathways that could be activated in those cells. In the future, this technique can be used in combination with other flow cytometry assays such as caspase activation measurement or mitochondrial membrane potential assays to further characterize the effect of PMP in Cn-infected cells.
CTS are released into the cytosol following PMP, which could then trigger PCD [49]. Previously, purified CTSB and CTSL (cysteine CTS) from bone marrow-derived dendritic cells were shown to inhibit Cn growth and disrupt the Cn cell wall in vitro [27]. However, CTSB and CTSL showed little enzymatic activity in Cn-infection of J774A.1 macrophage-like cells (Smith 2015). Our analysis demonstrates a reduction in the enzymatic activity of cysteine CTS (CTSB, CTSL and CTSS) and no effect on the aspartic CTS (CTSD and CTSH) after Cn-infection. Here, inhibition of aspartic and cysteine CTS led to an increase in the apoptotic populations with concomitant increases in PMP occurrences in these cells, which may imply an accumulation of early apoptotic cells which are delayed in their progression towards later stages of apoptosis. Therefore, this may suggest that CTS are important to control Cn and would influence host cell damage. Besides having a direct effect on Cn, CTS could also be a major player in the induction of PCD, but their inhibition in Cn-infected cells is not sufficient to prevent host cell death presumably because of activation of other PCD pathways [50]. This scenario was described in other pathogens like Salmonella enterica, where infection of macrophages, deficient in caspase-1, still resulted in host cell death but with features reminiscent of autophagy [51]. Interestingly, a similar scenario is seen following the inhibition of caspases, which are key downstream targets of cytosolic CTS. In our study, PMP resulted in cell death, independent of caspases activation since caspases inhibition did not modulate the viability of infected cells, suggesting a caspase-independent mechanism of PCD. These data implies that Cn-induction of PCD manifests great complexity and may involve multiple cell death pathways. Consequently, once PMP occurs it may not be possible to prevent PCD. Hence, identifying strategies to reduce or block Cn-induction of PMP, upstream of PCD, may be important for the control of Cn infection. We have focused our study on host molecules that can be released from the phagolysosome due to PMP. However, PMP has other consequences such as Cn access to the nutrient-rich host cytosol whereby Cn may manipulate the host cell, a scenario that remains to be explored.
Modulation of PMP is a common and successful strategy used by a variety of intracellular pathogens. Some, like Vibrio parahaemolyticus [52], Streptococcus pneumoniae [53], Pseudomonas aeruginosa [54] and Bacillus anthracis [35] have been shown to induce PMP that favor their intracellular survival. However, for others such as Mycobacterium tuberculosis, PMP leads to the opposite: host control of infection [55]. Cn intracellular proliferation and exocytosis can be reduced by treating macrophages with IFN-γ and TNF-α [56]. Interestingly, macrophages treated with IFN-γ also showed a reduction in PMP and an increase in Cn growth inhibition [21]. Our study reinforces the view that the phagosome is a key battleground in determining the outcome of the Cn-macrophage interaction and by extension, the host-pathogen interaction.
The three major outcomes of the Cn-macrophage interaction are: 1) killing or restriction of Cn growth by the macrophage; 2) Cn release following macrophage lysis; and 3) non-lytic exocytosis that results in the survival of both the macrophage and Cn [15]. Non-lytic exocytosis occurs with a frequency of 10–30%, and can be altered by neutralization of phagosomal acidity with ammonium chloride and chloroquine [38]. Interestingly, these two compounds raise the phagolysosomal pH by creating a proton sponge effect, which can also build osmotic pressure across the phagolysosomal membrane, thus altering its permeability [57]. We showed that chemical induction of PMP in Cn-infected macrophages leads to a decrease of non-lytic exocytosis events, to mainly lytic exocytosis. In contrast, our results suggest that non-lytic exocytosis appears to occur primarily in macrophages without PMP. This suggestion is inferred from the observation that the frequency of non-lytic exocytosis peaks before 4 h [58], when the majority of macrophages still have intact phagolysosomal membranes. Together these observations imply that modulation of the phagolysosomal membrane can drive Cn-macrophage interaction outcomes and that non-lytic exocytosis occurs in the majority of cells with intact phagolysosomal membranes.
PLB1 is a virulence factor for both Cn and C. gattii [59]. PLB1 cleaves phospholipids and consequently its mechanism of action in virulence was inferred to be associated with host cell damage, but this inference has not been experimentally validated. An earlier study showed that PLB1-deficient Cn exhibited delayed intracellular replication in macrophages [24]. From our analysis we cannot distinguish whether PMP induces or amplifies apoptosis. In this study we show that macrophages containing PLB1-deficient strains have significantly less PMP. We noted that in the Δplb1::PLB1 strain did not complete restore the phenotype to the same levels of the WT strain. In the Cn field, transformations are generated by random ectopic integration using biolistic delivery [60] Given the caveats of genetic reconstitution in Cryptococcus the plb1 rec does show partial reconstitution of the phenotype, which gave us, confidence that the Plb1 plays a role in PMP.
Given that PMP must reflect phagosomal membrane damage and that PLB degrades phospholipids we infer that PLB contributes to PMP through its enzymatic activity. Since Cn-mediated PMP was associated with macrophage apoptosis, this result suggests a potential mechanism by which PLB1 contributes to fungal virulence. However, we note that even though PLB1-deficient strains were less virulent than WT cells in a mouse infection model, PLB1-deficient Cn still caused host death [24] and that in our studies macrophages infected with PLB1-deficient Cn still manifested PMP. We attribute this to the likely existence of redundancy in the mechanisms for Cn-mediated damage to phagolysosomal membranes. To explore other mechanisms for phagosomal membrane damage we investigated the state of cryptococcal cells inside macrophages. Residence of Cn cells within macrophages was associated with large increases in capsule radius, volume and cell surface area and a slight reduction in the average cell body radius. The phenomenon of capsule growth during murine infection [5] and in response to macrophage lipids [61] are known but capsule enlargement during intracellular residence in macrophage has not been quantitated previously. Electron microscopy images show that Cn inside macrophages the capsule surface and phagosomal membranes are in contact [5], which means that the phagosomal membrane surface area can be closely approximated by the capsule surface area. Capsule enlargement in a phagolysosome can be expected to place major stress on the surrounding lipid membrane since any increase in capsule radius would necessitate a disproportionately large increase membrane area, which is 4πr2. Consequently, any increase in capsule radius would require an increase of in membrane lipids to insure phagosome membrane integrity. In our experiment we measure a 1.92-fold increase in average surface, which would necessitate a commensurate increase in lipid membrane area. Although the mechanism, by which a phagolysosome would compensate for an enlarging microbe to maintain membrane integrity, if any, is not known, this physical stress combined with enzymatic damage from fungal phospholipases can be expected to produce tremendous physical stress on phagosomal membrane and lead to failure of containment of vacuolar contents. Capsule enlargement would also favor intracellular fungal cell survival since the capsule react with and deactivates nitrogen- and oxygen-derived oxidants that are produced in phagosomes by the respiratory burst [16]. Cn mutants defective in capsule enlargement are attenuated in virulence (reviewed in [62]). Capsule enlargement inside macrophage could promote fungal cell survival through reduced susceptibility to microbicidal compounds and damage to the phagolysosomal membrane, which in turn allows leakage of contents and damages the macrophage. The greater heterogeneity in cellular and capsule dimensions observed for Cn cells recovered from macrophages after 24 h could reflect differences in the conditions experienced inside individual phagosomes, which manifested differences in pH and would differ in size depending on the dimension of cryptococcal cells.
Phagolysosomal acidification is a key process for the activation of proteases in this compartment, and thus several pathogens have evolved strategies by which to avoid this acidification to circumvent their own demise. PMP results in destabilization of the proton gradient that is required for maintaining phagolysosomal pH, and this could result in deacidification. Our study provides new insight may help to explain the difference reported by other groups regarding the acidification of Cn-containing phagolysosomes (reviewed in [15]). To determine the pH of Cn-containing phagolysosomes we stained Cn with the monoclonal antibody 18B7- conjugated to Oregon green and detected apoptotic macrophages using annexin V labeling. This method allowed us to determine the specific pH value of Cn-containing phagolysosomes in macrophages with and without an apoptotic phenotype. Interestingly, our results showed that Cn-infected macrophages that become apoptotic (annexin V positive) contain phagolysosomes with a less acidic pH than Cn-infected macrophages that were not apoptotic (annexin V negative). Taken together with our earlier findings, a correlation between Cn-infected macrophages and PMP, these results together suggest that the reason the pH is less acidic in the Cn-containing phagolysosomes of apoptotic macrophages is due to PMP. Although apoptotic cells had higher phagolysosomal pH that even in some cases were no longer acidic, PMP was not associated with complete loss of acidity. We attribute this effect to the buffering capacity of the capsule, which contains glucuronic acid residues that can modulate pH.
The role of macrophage apoptosis in host defense varies for individual pathogenic microbes [63]. For some pathogens, induction of apoptosis is part of the pathogenic strategy since it causes the death of an important host immune cell [64]. For others, induction of host cell apoptosis is a host defense mechanism that enables control of the infection. In this study we show that PMP temporally precedes the induction of apoptosis, an event that is also causally related by the fact that phagosome contents can induce death pathways [49]. Our results identify two potential mechanisms for PMP in C. neoformans-infected macrophages: PLB1 and capsular enlargement. PMP resulting from capsular enlargement and/or other mechanisms could explain why PLB1-deficient Cn manifested only a lag in intracellular growth [21]. Hence, the role of PLB1 in the pathogenesis of intracellular Cn appears to be that of a contributor to PMP, such that its presence hastens this process. PMP was also associated with lytic exocytosis, which results in the release of viable cryptococcal cells and death of the host cell. Given that the macrophage is a critical host cell for the control of cryptococcal infection and that PMP leads to macrophage apoptosis we interpret these findings as an indication that induction of apoptosis during cryptococcal infection favors the microbe and is part of its pathogenic strategy.
Supplementary Material
Acknowledgments
AC is supported by 5R01HL059842, 5R01AI033774, 5R37AI033142, and 5R01AI052733. DCPR was supported by FAPESP 2015/129099.
References
- 1.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23(4):525–30. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 2.Kronstad JW, Attarian R, Cadieux B, Choi J, D’Souza CA, Griffiths EJ, Geddes JM, Hu G, Jung WH, Kretschmer M, Saikia S, Wang J. Expanding fungal pathogenesis: Cryptococcus breaks out of the opportunistic box. Nat Rev Microbiol. 2011;9(3):193–203. doi: 10.1038/nrmicro2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Almeida F, Wolf JM, Casadevall A. Virulence-Associated Enzymes of Cryptococcus neoformans. Eukaryot Cell. 2015;14(12):1173–85. doi: 10.1128/EC.00103-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levitz SM, Nong SH, Seetoo KF, Harrison TS, Speizer RA, Simons ER. Cryptococcus neoformans resides in an acidic phagolysosome of human macrophages. Infect Immun. 1999;67(2):885–90. doi: 10.1128/iai.67.2.885-890.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feldmesser M, Kress Y, Novikoff P, Casadevall A. Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect Immun. 2000;68(7):4225–37. doi: 10.1128/iai.68.7.4225-4237.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shao X, Mednick A, Alvarez M, van Rooijen N, Casadevall A, Goldman DL. An innate immune system cell is a major determinant of species-related susceptibility differences to fungal pneumonia. J Immunol. 2005;175(5):3244–51. doi: 10.4049/jimmunol.175.5.3244. [DOI] [PubMed] [Google Scholar]
- 7.Tenor JL, Oehlers SH, Yang JL, Tobin DM, Perfect JR. Live Imaging of Host-Parasite Interactions in a Zebrafish Infection Model Reveals Cryptococcal Determinants of Virulence and Central Nervous System Invasion. MBio. 2015;6(5):e01425–15. doi: 10.1128/mBio.01425-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leopold Wager CM, Hole CR, Wozniak KL, Olszewski MA, Mueller M, Wormley FL., Jr STAT1 signaling within macrophages is required for antifungal activity against Cryptococcus neoformans. Infect Immun. 2015;83(12):4513–27. doi: 10.1128/IAI.00935-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnston SA, Voelz K, May RC. Cryptococcus neoformans Thermotolerance to Avian Body Temperature Is Sufficient For Extracellular Growth But Not Intracellular Survival In Macrophages. Sci Rep. 2016;6:20977. doi: 10.1038/srep20977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kechichian TB, Shea J, Del Poeta M. Depletion of alveolar macrophages decreases the dissemination of a glucosylceramide-deficient mutant of Cryptococcus neoformans in immunodeficient mice. Infect Immun. 2007;75(10):4792–8. doi: 10.1128/IAI.00587-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alanio A, Desnos-Ollivier M, Dromer F. Dynamics of Cryptococcus neoformans-macrophage interactions reveal that fungal background influences outcome during cryptococcal meningoencephalitis in humans. MBio. 2011;2(4) doi: 10.1128/mBio.00158-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mansour MK, Vyas JM, Levitz SM. Dynamic virulence: real-time assessment of intracellular pathogenesis links Cryptococcus neoformans phenotype with clinical outcome. MBio. 2011;2(5) doi: 10.1128/mBio.00217-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabiiti W, Robertson E, Beale MA, Johnston SA, Brouwer AE, Loyse A, Jarvis JN, Gilbert AS, Fisher MC, Harrison TS, May RC, Bicanic T. Efficient phagocytosis and laccase activity affect the outcome of HIV-associated cryptococcosis. J Clin Invest. 2014;124(5):2000–8. doi: 10.1172/JCI72950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olszewski MA, Zhang Y, Huffnagle GB. Mechanisms of cryptococcal virulence and persistence. Future Microbiol. 2010;5(8):1269–88. doi: 10.2217/fmb.10.93. [DOI] [PubMed] [Google Scholar]
- 15.DeLeon-Rodriguez CM, Casadevall A. Cryptococcus neoformans: Tripping on Acid in the Phagolysosome. Front Microbiol. 2016;7:164. doi: 10.3389/fmicb.2016.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaragoza O, Chrisman CJ, Castelli MV, Frases S, Cuenca-Estrella M, Rodriguez-Tudela JL, Casadevall A. Capsule enlargement in Cryptococcus neoformans confers resistance to oxidative stress suggesting a mechanism for intracellular survival. Cell Microbiol. 2008;10(10):2043–57. doi: 10.1111/j.1462-5822.2008.01186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox GM, Harrison TS, McDade HC, Taborda CP, Heinrich G, Casadevall A, Perfect JR. Superoxide dismutase influences the virulence of Cryptococcus neoformans by affecting growth within macrophages. Infect Immun. 2003;71(1):173–80. doi: 10.1128/IAI.71.1.173-180.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Aisen P, Casadevall A. Cryptococcus neoformans melanin and virulence: mechanism of action. Infect Immun. 1995;63(8):3131–6. doi: 10.1128/iai.63.8.3131-3136.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith LM, Dixon EF, May RC. The fungal pathogen Cryptococcus neoformans manipulates macrophage phagosome maturation. Cell Microbiol. 2015;17(5):702–13. doi: 10.1111/cmi.12394. [DOI] [PubMed] [Google Scholar]
- 20.Tucker SC, Casadevall A. Replication of Cryptococcus neoformans in macrophages is accompanied by phagosomal permeabilization and accumulation of vesicles containing polysaccharide in the cytoplasm. Proc Natl Acad Sci U S A. 2002;99(5):3165–70. doi: 10.1073/pnas.052702799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis MJ, Eastman AJ, Qiu Y, Gregorka B, Kozel TR, Osterholzer JJ, Curtis JL, Swanson JA, Olszewski MA. Cryptococcus neoformans-induced macrophage lysosome damage crucially contributes to fungal virulence. J Immunol. 2015;194(5):2219–31. doi: 10.4049/jimmunol.1402376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coelho C, Souza AC, da Derengowski SL, de Leon-Rodriguez C, Wang B, Leon-Rivera R, Bocca AL, Goncalves T, Casadevall A. Macrophage mitochondrial and stress response to ingestion of Cryptococcus neoformans. J Immunol. 2015;194(5):2345–57. doi: 10.4049/jimmunol.1402350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boya P, Kroemer G. Lysosomal membrane permeabilization in cell death. Oncogene. 2008;27(50):6434–51. doi: 10.1038/onc.2008.310. [DOI] [PubMed] [Google Scholar]
- 24.Cox GM, McDade HC, Chen SC, Tucker SC, Gottfredsson M, Wright LC, Sorrell TC, Leidich SD, Casadevall A, Ghannoum MA, Perfect JR. Extracellular phospholipase activity is a virulence factor for Cryptococcus neoformans. Mol Microbiol. 2001;39(1):166–75. doi: 10.1046/j.1365-2958.2001.02236.x. [DOI] [PubMed] [Google Scholar]
- 25.Conus S, Simon HU. Cathepsins and their involvement in immune responses. Swiss Med Wkly. 2010;140:w13042. doi: 10.4414/smw.2010.13042. [DOI] [PubMed] [Google Scholar]
- 26.Qin QM, Luo J, Lin X, Pei J, Li L, Ficht TA, de Figueiredo P. Functional analysis of host factors that mediate the intracellular lifestyle of Cryptococcus neoformans. PLoS Pathog. 2011;7(6):e1002078. doi: 10.1371/journal.ppat.1002078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hole CR, Bui H, Wormley FL, Jr, Wozniak KL. Mechanisms of dendritic cell lysosomal killing of Cryptococcus. Sci Rep. 2012;2:739. doi: 10.1038/srep00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Damiani G, Kiyotaki C, Soeller W, Sasada M, Peisach J, Bloom BR. Macrophage variants in oxygen metabolism. J Exp Med. 1980;152(4):808–22. doi: 10.1084/jem.152.4.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casadevall A, Cleare W, Feldmesser M, Glatman-Freedman A, Goldman DL, Kozel TR, Lendvai N, Mukherjee J, Pirofski LA, Rivera J, Rosas AL, Scharff MD, Valadon P, Westin K, Zhong Z. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob Agents Chemother. 1998;42(6):1437–46. doi: 10.1128/aac.42.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nolan SJ, Romano JD, Luechtefeld T, Coppens I. Neospora caninum Recruits Host Cell Structures to Its Parasitophorous Vacuole and Salvages Lipids from Organelles. Eukaryot Cell. 2015;14(5):454–73. doi: 10.1128/EC.00262-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Storrie B, Madden EA. Isolation of subcellular organelles. Methods Enzymol. 1990;182:203–25. doi: 10.1016/0076-6879(90)82018-w. [DOI] [PubMed] [Google Scholar]
- 32.Appelqvist H, Waster P, Kagedal K, Ollinger K. The lysosome: from waste bag to potential therapeutic target. J Mol Cell Biol. 2013;5(4):214–26. doi: 10.1093/jmcb/mjt022. [DOI] [PubMed] [Google Scholar]
- 33.Lima H, Jr, Jacobson LS, Goldberg MF, Chandran K, Diaz-Griffero F, Lisanti MP, Brojatsch J. Role of lysosome rupture in controlling Nlrp3 signaling and necrotic cell death. Cell Cycle. 2013;12(12):1868–78. doi: 10.4161/cc.24903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alduaij W, Ivanov A, Honeychurch J, Cheadle EJ, Potluri S, Lim SH, Shimada K, Chan CH, Tutt A, Beers SA, Glennie MJ, Cragg MS, Illidge TM. Novel type II anti-CD20 monoclonal antibody (GA101) evokes homotypic adhesion and actin-dependent, lysosome-mediated cell death in B-cell malignancies. Blood. 2011;117(17):4519–29. doi: 10.1182/blood-2010-07-296913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Averette KM, Pratt MR, Yang Y, Bassilian S, Whitelegge JP, Loo JA, Muir TW, Bradley KA. Anthrax lethal toxin induced lysosomal membrane permeabilization and cytosolic cathepsin release is Nlrp1b/Nalp1b-dependent. PLoS One. 2009;4(11):e7913. doi: 10.1371/journal.pone.0007913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zaragoza O, Alvarez M, Telzak A, Rivera J, Casadevall A. The relative susceptibility of mouse strains to pulmonary Cryptococcus neoformans infection is associated with pleiotropic differences in the immune response. Infect Immun. 2007;75(6):2729–39. doi: 10.1128/IAI.00094-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma H, Croudace JE, Lammas DA, May RC. Expulsion of live pathogenic yeast by macrophages. Curr Biol. 2006;16(21):2156–60. doi: 10.1016/j.cub.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 38.Nicola AM, Robertson EJ, Albuquerque P, da Derengowski SL, Casadevall A. Nonlytic exocytosis of Cryptococcus neoformans from macrophages occurs in vivo and is influenced by phagosomal pH. MBio. 2011;2(4) doi: 10.1128/mBio.00167-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erdal H, Berndtsson M, Castro J, Brunk U, Shoshan MC, Linder S. Induction of lysosomal membrane permeabilization by compounds that activate p53-independent apoptosis. Proc Natl Acad Sci U S A. 2005;102(1):192–7. doi: 10.1073/pnas.0408592102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chayakulkeeree M, Johnston SA, Oei JB, Lev S, Williamson PR, Wilson CF, Zuo X, Leal AL, Vainstein MH, Meyer W, Sorrell TC, May RC, Djordjevic JT. SEC14 is a specific requirement for secretion of phospholipase B1 and pathogenicity of Cryptococcus neoformans. Mol Microbiol. 2011;80(4):1088–101. doi: 10.1111/j.1365-2958.2011.07632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mindell JA. Lysosomal acidification mechanisms. Annu Rev Physiol. 2012;74:69–86. doi: 10.1146/annurev-physiol-012110-142317. [DOI] [PubMed] [Google Scholar]
- 42.Nessa K, Gross NT, Jarstrand C, Johansson A, Camner P. In vivo interaction between alveolar macrophages and Cryptococcus neoformans. Mycopathologia. 1997;139(1):1–7. doi: 10.1023/a:1006843202124. [DOI] [PubMed] [Google Scholar]
- 43.Pinti M, Orsi CF, Gibellini L, Esposito R, Cossarizza A, Blasi E, Peppoloni S, Mussini C. Identification and characterization of an aspartyl protease from Cryptococcus neoformans. FEBS Lett. 2007;581(20):3882–6. doi: 10.1016/j.febslet.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 44.Jacobson LS, Lima H, Jr, Goldberg MF, Gocheva V, Tsiperson V, Sutterwala FS, Joyce JA, Gapp BV, Blomen VA, Chandran K, Brummelkamp TR, Diaz-Griffero F, Brojatsch J. Cathepsin-mediated necrosis controls the adaptive immune response by Th2 (T helper type 2)-associated adjuvants. J Biol Chem. 2013;288(11):7481–91. doi: 10.1074/jbc.M112.400655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen M, Xing Y, Lu A, Fang W, Sun B, Chen C, Liao W, Meng G. Internalized Cryptococcus neoformans Activates the Canonical Caspase-1 and the Noncanonical Caspase-8 Inflammasomes. J Immunol. 2015;195(10):4962–72. doi: 10.4049/jimmunol.1500865. [DOI] [PubMed] [Google Scholar]
- 46.De Milito A, Iessi E, Logozzi M, Lozupone F, Spada M, Marino ML, Federici C, Perdicchio M, Matarrese P, Lugini L, Nilsson A, Fais S. Proton pump inhibitors induce apoptosis of human B-cell tumors through a caspase-independent mechanism involving reactive oxygen species. Cancer Res. 2007;67(11):5408–17. doi: 10.1158/0008-5472.CAN-06-4095. [DOI] [PubMed] [Google Scholar]
- 47.Hornick JR, Vangveravong S, Spitzer D, Abate C, Berardi F, Goedegebuure P, Mach RH, Hawkins WG. Lysosomal membrane permeabilization is an early event in Sigma-2 receptor ligand mediated cell death in pancreatic cancer. J Exp Clin Cancer Res. 2012;31:41. doi: 10.1186/1756-9966-31-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu Y, Hao BX, Graeff R, Wong CW, Wu WT, Yue J. Two pore channel 2 (TPC2) inhibits autophagosomal-lysosomal fusion by alkalinizing lysosomal pH. J Biol Chem. 2013;288(33):24247–63. doi: 10.1074/jbc.M113.484253. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Kirkegaard T, Jaattela M. Lysosomal involvement in cell death and cancer. Biochim Biophys Acta. 2009;1793(4):746–54. doi: 10.1016/j.bbamcr.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 50.Kroemer G, Jaattela M. Lysosomes and autophagy in cell death control. Nat Rev Cancer. 2005;5(11):886–97. doi: 10.1038/nrc1738. [DOI] [PubMed] [Google Scholar]
- 51.Hernandez LD, Pypaert M, Flavell RA, Galan JE. A Salmonella protein causes macrophage cell death by inducing autophagy. J Cell Biol. 2003;163(5):1123–31. doi: 10.1083/jcb.200309161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsuda S, Okada N, Kodama T, Honda T, Iida T. A cytotoxic type III secretion effector of Vibrio parahaemolyticus targets vacuolar H+-ATPase subunit c and ruptures host cell lysosomes. PLoS Pathog. 2012;8(7):e1002803. doi: 10.1371/journal.ppat.1002803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bewley MA, Naughton M, Preston J, Mitchell A, Holmes A, Marriott HM, Read RC, Mitchell TJ, Whyte MK, Dockrell DH. Pneumolysin activates macrophage lysosomal membrane permeabilization and executes apoptosis by distinct mechanisms without membrane pore formation. MBio. 2014;5(5):e01710–14. doi: 10.1128/mBio.01710-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prince LR, Bianchi SM, Vaughan KM, Bewley MA, Marriott HM, Walmsley SR, Taylor GW, Buttle DJ, Sabroe I, Dockrell DH, Whyte MK. Subversion of a lysosomal pathway regulating neutrophil apoptosis by a major bacterial toxin, pyocyanin. J Immunol. 2008;180(5):3502–11. doi: 10.4049/jimmunol.180.5.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watson RO, Manzanillo PS, Cox JS. Extracellular M. tuberculosis DNA targets bacteria for autophagy by activating the host DNA-sensing pathway. Cell. 2012;150(4):803–15. doi: 10.1016/j.cell.2012.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Voelz K, Lammas DA, May RC. Cytokine signaling regulates the outcome of intracellular macrophage parasitism by Cryptococcus neoformans. Infect Immun. 2009;77(8):3450–7. doi: 10.1128/IAI.00297-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boya P, Andreau K, Poncet D, Zamzami N, Perfettini JL, Metivier D, Ojcius DM, Jaattela M, Kroemer G. Lysosomal membrane permeabilization induces cell death in a mitochondrion-dependent fashion. J Exp Med. 2003;197(10):1323–34. doi: 10.1084/jem.20021952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stukes SA, Cohen HW, Casadevall A. Temporal kinetics and quantitative analysis of Cryptococcus neoformans nonlytic exocytosis. Infect Immun. 2014;82(5):2059–67. doi: 10.1128/IAI.01503-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen SC, Muller M, Zhou JZ, Wright LC, Sorrell TC. Phospholipase activity in Cryptococcus neoformans: a new virulence factor? J Infect Dis. 1997;175(2):414–20. doi: 10.1093/infdis/175.2.414. [DOI] [PubMed] [Google Scholar]
- 60.Toffaletti DL, Rude TH, Johnston SA, Durack DT, Perfect JR. Gene transfer in Cryptococcus neoformans by use of biolistic delivery of DNA. J Bacteriol. 1993;175(5):1405–11. doi: 10.1128/jb.175.5.1405-1411.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chrisman CJ, Albuquerque P, Guimaraes AJ, Nieves E, Casadevall A. Phospholipids trigger Cryptococcus neoformans capsular enlargement during interactions with amoebae and macrophages. PLoS Pathog. 2011;7(5):e1002047. doi: 10.1371/journal.ppat.1002047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O’Meara TR, Alspaugh JA. The Cryptococcus neoformans capsule: a sword and a shield. Clin Microbiol Rev. 2012;25(3):387–408. doi: 10.1128/CMR.00001-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ashida H, Mimuro H, Ogawa M, Kobayashi T, Sanada T, Kim M, Sasakawa C. Cell death and infection: a double-edged sword for host and pathogen survival. J Cell Biol. 2011;195(6):931–42. doi: 10.1083/jcb.201108081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73(4):1907–16. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.