Abstract
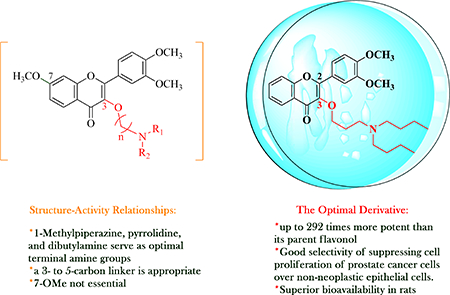
Thirty-eight 3-O-substituted-3′,4′-dimethoxyflavonols and twenty-five 3-O-substituted-3′,4′,7-trimethoxyflavonols have been synthesized for systematic investigation on the structure-activity relationships of 3-O-substituted-3′,4′-dimethoxyflavonols in three human prostate cancer cell models. Our findings indicate that incorporation of an appropriate amino group to 3-OH of 3′,4′dimethoxyflavonol and 3′,4′,7-trimethoxyflavonol through a 3- to 5-carbon linker can substantially improve the in vitro antiproliferative potency in three human prostate cancer cell models, but not in two non-neoplastic human epithelial cell models (MCF 10A and PWR-1E). 1-Methylpiperazine, pyrrolidine, and dibutylamine are optimal terminal amine groups that, in combination with a 3- to 5carbon linker, are notably beneficial to the anti-proliferative potency of 3-O-substituted-3′,4′dimethoxyflavonols. It is worth noting that 3-O-(4-methylpiperazin-1-yl)propyl-3′,4′,7-trimethoxyflavonol (76) induces PC-3 cell death in a completely different way from 3-O-pyrrolidinopentyl-3′,4′,7-trimethoxyflavonol (81) even though they belong to 3-O-substituted-3′,4′,7-trimethoxyflavonols and exhibit similar potency in inhibiting PC-3 cell proliferation, suggesting that the mechanism of action for each specific 3-O-substitutedflavonol varies with different amino moiety. 3-O-(N,N-Dibutylamino)propyl-3′,4′-dimethoxyflavonol (42) emerged as the most promising derivative due to its substantially improved potency in cell models, superior bioavailability in rats, and good selectivity of inhibiting prostate cancer cell proliferation over non-neoplastic human epithelial cell proliferation.
Keywords: Flavonol, Prostate Cancer, Structure-activity relationship, Pharmacokinetic study, Cell apoptosis
Graphical Abstract
1. INTRODUCTION
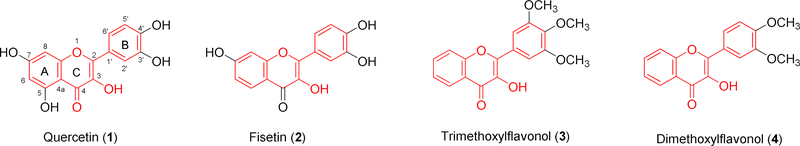
Flavonoids are structurally characteristic of two aromatic rings and a central heterocyclic ring. They are widely distributed in a variety of dietary plants, displaying various health and medicinal benefits. The prime attention of researchers to the potential of flavonoids in preventing and treating prostate cancer is mainly due to an inverse association between the intake of flavonoid-enriched diet and the incidence of prostate cancer [1–8]. Flavonols are classified as one of the largest subgroups of flavonoids, structurally featuring a hydroxyl group at C-3 [9–10].
Both naturally occurring and synthetic flavonols, as exemplified by quercetin (1), fisetin (2), and 3′,4′,5′-trimethoxyflavonol (3) (Fig. 1), have been demonstrated by in vitro cell-based and in vivo animal experiments to possess potential in treating prostate cancer [1,11–16]. However, the reported in vitro anti-prostate cancer potency is moderate, typically with IC50 values in the range of 15–64 μM. The synthetic 3′,4′,5′-trimethoxyflavonol (3) exhibits 5- to 16-fold greater potency than quercetin (1) and fisetin (2), but only possesses very moderate in vivo antitumor efficacy even at the highest dose (0.73 mmol/kg) in two prostate cancer xenografts in nude mice [16] and poor bioavailability [17]. The moderate potency and poor bioavailability have hindered the further advancement of these flavonols as chemotherapeutic agents. Poor bioavailability of naturally occurring flavonols is mainly due to their polyphenolic structures that are highly susceptible to first-pass metabolism of glucuronidation and sulfation [18]. We envision that modifications on the phenolic groups of flavonols may lead to simultaneous improvement of potency and bioavailability. Specifically, we are interested in the chemical manipulations on 3-OH, the unique hydroxyl group for all flavonols, while converting all other phenolic hydroxyl groups into methoxyl groups.
Figure 1.
Structures of representative flavonols
Our earlier WST-1 cell proliferation assay in three prostate cancer cell models suggests that the antiproliferative potency of 3′,4′-dimethoxyflavonol (4) and 3′,4′,5′-trimethoxyflavonol (3) could be appreciably enhanced through chemical modifications on its 3-OH group [19–20]. 3′,4′Dimethoxyflavonol (4) and 3′,4′,5′-trimethoxyflavonol (3) were chosen as our first two lead flavonols because they contain two or three methoxyl groups instead of phenolic hydroxyl groups on ring B. These lead flavonols were expected to overcome, to some degree, pharmacokinetic limitations caused by the phenolic hydroxyl groups and to provide an efficient approach to incorporation of a basic nitrogen-containing group to the only hydroxyl group at C-3. Encouraged by these preliminary data, the present study aims to further investigate the in-depth structure-activity relationships of 3-O-substituted3′,4′-dimethoxyflavonols and to explore the pharmacokinetic profiles for the optimal derivatives. The reason that 3′,4′,5′-trimethoxyflavonols were not selected for in-depth investigation is because 3-O-substituted-3′,4′,5′-trimethoxyflavonols did not display significantly improved potency as compared with the corresponding 3-O-substituted-3′,4′-dimethoxyflavonols.
2. RESULTS AND DISCUSSION
2.1. Chemistry.
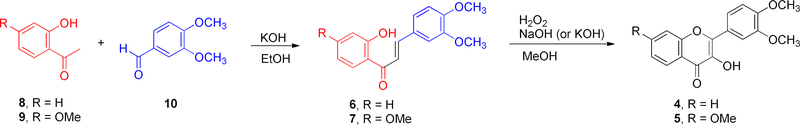
3′,4′-Dimethoxyflavonol (4) and 3′,4′,7-trimethoxyflavonol (5) were synthesized through a well-known two-step procedure, including Claisen-Schmidt condensation and Algar-Flynn-Oyamada (AFO) reactions as illustrated in Scheme 1, that is the general approach to the synthesis of flavonols [21–23]. Specifically, the intermediates 3′,4′-dimethoxychalcone (6) and 3′,4′,7-trimethoxychalcone (7) were prepared by Claisen-Schmidt condensation of 2′-hydroxyacetophenone (8) or 2′-hydroxy-4′-methoxyacetophenone (9) with 3,4-dimethoxybenzaldehyde (10), which were directly subjected to the AFO reaction without further purification to furnish 3′,4′-dimethoxyflavonol (4) and 3′,4′,7-trimethoxyflavonol (5) in 34% and 64% overall yields for two steps, respectively.
Scheme 1.
Synthesis of 3′,4′-dimethoxyflavonol (4) and 3′,4′,7-trimethoxyflavonol (5)
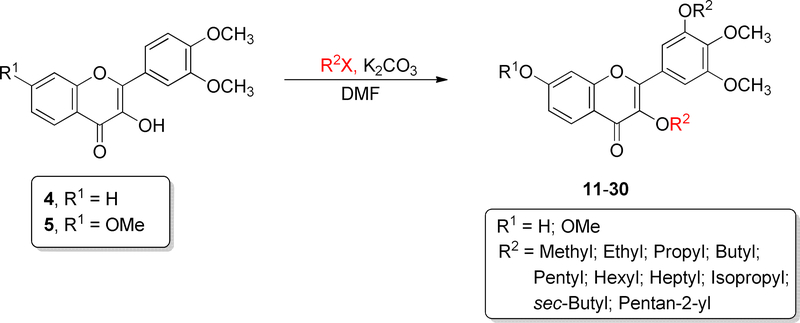
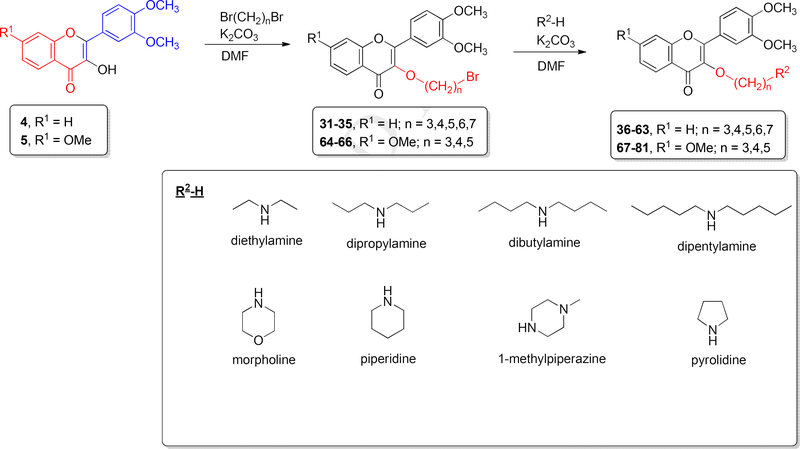
As shown in Scheme 2, ten 3-O-alkyl-3′,4′-dimethoxyflavonols (11-20) and ten 3-O-alkyl-3′,4′,7-trimethoxyflavonols (21-30) have been synthesized in 11–99% yields by O-alkylating 3′,4′dimethoxyflavonol (4) and 3′,4′,7-trimethoxyflavonol (5) with the appropriate alkyl halide using potassium carbonate as base and DMF as an aprotic solvent. Twenty eight 3-O-aminoalkyl-3′,4′dimethoxyflavonols (36-63) and fifteen 3-O-aminoalkyl-3′,4′,7-trimethoxyflavonols (67-81) have been synthesized from 3′,4′-dimethoxyflavonol (4) and 3′,4′,7-trimethoxyflavonol (5) in 27–94% yields through intermediates 31-35 and 64-66 via two sequential alkylation reactions as illustrated in Scheme 3. Potassium carbonate was used as the base and DMF as the polar aprotic solvent in both O-alkylation and N-alkylation reactions. Each crude 3-O-bromoalkylflavonol (31-35 or 64-66) was directly used, without further purification, for the follow-up N-alkylation. All synthesized 3-O-substitutedflavonols were purified by preparative thin layer chromatography (PTLC) over silica gel and the nitrogen-containing final compounds were retrieved by washing the PTLC silica gel with dichloromethane-diethylamine (100:3, v/v).
Scheme 2.
Synthesis of 3-O-alkyl-3′,4′-dimethoxyflavonols (11-20) and 3-O-alkyl-3′,4′,7-trimethoxyflavonols (21-30). For the specific structure for each of 11-30, see Tables 1 and 2.
Scheme 3.
Synthesis of 3-O-aminoalkyl-3′,4′-dimethoxyflavonols (36-63) and 3-O-aminoalkyl-3′,4′,7-trimethoxyflavonols (67-81). For the specific structure for each of 36-63, see Table 1. For the specific structure for each of 67-81, see Table 2.
2.2. Antiproliferative activity and structure-activity relationships in three prostate cancer cell models.
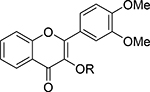
The in vitro antiproliferative activity of thirty-eight 3-O-substituted-3′,4′-dimethoxyflavonols (11-20, 36-63) and twenty-five 3-O-substituted-3′,4′,7-trimethoxyflavonols (21-30, 67-81) against a panel of prostate cancer cell lines (PC-3, DU145, and LNCaP) was assessed using WST-1 cell proliferation assay according to the procedure described in the Experimental Section. The PC-3, DU145, and LNCaP cell lines are the most common cell-based models for in vitro assessment of potency and efficacy of anti-prostate cancer agents. The androgen-insensitive PC-3 and DU145 cell lines are derived from bone and brain metastasis of prostate tumor, respectively, which cannot express prostate-specific antigen [24–25]. The androgen-sensitive LNCaP cell line is derived from lymph node metastasis of prostate tumor and capable of expressing prostate-specific antigen. Two naturally occurring flavonols, fisetin and quercetin, and two parent flavonols, 3′,4′-dimethoxyflavonol (4) and 3′,4′,7-trimethoxyflavonol (5), were used as positive controls. DMSO was used as negative control. The IC50 values, as summarized in Tables 1 and 2, were calculated from the dose-response curves based on at least five dosages for each compound. Our findings indicated that 3′,4′-dimethoxyflavonol (4) is notably less potent than fisetin and quercetin; in contrast, 3′,4′,7-trimethoxyflavonol (5) is appreciably more potent than fisetin and quercetin. This suggests that the overall size of a flavonol may be critical to the antiproliferative potency. Among sixty-three 3-O-substitutedflavonols tested, forty-three displayed markedly improved ability in suppressing the human prostate cancer cell proliferation, as compared with two naturally occurring flavonols (quercetin and fisetin).
Table 1.
Anti-Proliferative Activity of 3-O-Substituted-3′,4′-dimethoxyflavononols (11-20; 36-63)
 | ||||
|---|---|---|---|---|
| Compd | IC50 (μM)a | |||
| R | PC-3b | DU145c | LNCaPd | |
| 4 | H | 409.0 ± 1.8 | 174.6 ± 31.6 | 202.5 ± 32.2 |
| 11 | Methyl | 30.0 ± 15.0 | 46.1 ± 28.5 | 16.2 ± 9.4 |
| 12 | Ethyl | 13.7 ± 9.3 | 39.2 ± 11.4 | 9.8 ± 0.6 |
| 13 | Propyl | 10.1± 3.6 | 14.6 ± 0.3 | 6.4 ± 2.2 |
| 14 | Butyl | 16.8 ± 6.7 | 26.7 ± 7.6 | 8.1 ± 3.1 |
| 15 | Pentyl | 16.8 ± 4.6 | 38.9 ± 6.1 | 14.2 ± 7.6 |
| 16 | Hexyl | 14.0 ± 6.5 | 19.4 ± 5.7 | 12.6 ± 5.8 |
| 17 | Heptyl | 86.3 ± 21.2 | 111.9 ± 71.1 | 43.9 ± 3.3 |
| 18 | Isopropyl | 33.0 ± 7.7 | 38.0 ± 1.6 | 10.1 ± 4.3 |
| 19 | Sec-Butyl | 44.2 ± 19.6 | > 50 | 10.9 ± 2.9 |
| 20 | Pentan-2-yl | 28.1 ± 5.7 | > 50 | 13.1 ± 5.3 |
| 36 | (N,N-Diethylamino)propyl | 57.2 ± 5.0 | 49.8 ± 1.2 | 13.2 ± 6.4 |
| 37 | (N,N-Diethylamino)butyl | 60.7 ± 5.6 | > 50 | 40.5 ± 16.1 |
| 38 | (N,N-Diethylamino)pentyl | 32.0 ± 2.4 | 48.1 ± 10.2 | 18.9 ± 7.4 |
| 39 | (N,N-Dipropylamino)propyl | 26.0 ± 7.5 | 38.6 ± 6.7 | 13.5 ± 6.3 |
| 40 | (N,N-Dipropylamino)butyl | 25.7 ± 6.1 | 24.3 ± 11.4 | 13.2 ± 6.5 |
| 41 | (N,N-Dipropylamino)pentyl | 13.1 ± 4.0 | 14.1 ± 6.5 | 2.7 ± 2.1 |
| 42 | (N,N-Dibutylamino)propyl | 1.4 ± 0.2 | 7.6 ± 1.7 | 2.4 ± 1.5 |
| 43 | (N,N-Dibutylamino)butyl | 2.7 ± 0.8 | 16.3 ± 5.5 | 4.3 ± 1.3 |
| 44 | (N,N-Dibutylamino)pentyl | 4.0 ± 1.0 | 11.9 ± 3.4 | 5.0 ± 1.3 |
| 45 | (N,N-Dipentylamino)propyl | 2.4 ± 0.8 | 12.8 ± 3.0 | 4.8 ± 1.3 |
| 46 | (N,N-Dipentylamino)butyl | 7.7 ± 2.2 | 26.9 ± 1.6 | 10.2 ± 2.7 |
| 47 | (N,N-Dipentylamino)pentyl | 4.9 ± 1.5 | 14.4 ± 4.3 | 7.8 ± 2.1 |
| 48 | Morpholinopropyl | 10.9 ± 4.7 | 38.6 ± 4.3 | 14.9 ± 12.1 |
| 49 | Morpholinobutyl | 9.1 ± 1.8 | 44.0 ± 1.3 | 8.5 ± 6.2 |
| 50 | Morpholinopentyl | 4.5 ± 1.0 | 25.9 ± 4.1 | 7.7 ± 3.5 |
| 51 | Piperidinopropyl | > 50 | > 50 | 36.0 ± 14.4 |
| 52 | Piperidinobutyl | 13.4 ± 3.6 | > 50 | 24.1 ± 8.9 |
| 53 | Piperidinopentyl | 38.6 ± 6.5 | > 50 | 26.5 ± 4.4 |
| 54 | (4-Methylpiperazin-1-yl)propyl | 3.3 ± 0.5 | > 50 | 12.2 ± 0.1 |
| 55 | (4-Methylpiperazin-1-yl)butyl | 1.1 ± 0.8 | 31.6 ± 0.7 | 6.4 ± 1.0 |
| 56 | (4-Methylpiperazin-1-yl)pentyl | 0.5 ± 0.4 | 20.3 ± 8.4 | 3.9 ± 0.7 |
| 57 | (4-Methylpiperazin-1-yl)hexyl | 19.8 ± 3.1 | 24.6 ± 0.6 | 12.2 ± 3.5 |
| 58 | (4-Methylpiperazin-1-yl)heptyl | 6.2 ± 0.6 | 8.3 ± 3.2 | 4.0 ± 2.4 |
| 59 | Pyrrolidinopropyl | 2.0 ± 1.9 | 27.1 ± 6.7 | 10.6 ± 2.1 |
| 60 | Pyrrolidinobutyl | 1.5 ± 0.6 | 13.0 ± 7.1 | 4.5 ± 1.6 |
| 61 | Pyrrolidinopentyl | 0.7 ± 0.5 | 29.2 ± 2.2 | 4.9 ± 1.0 |
| 62 | Pyrrolidinohexyl | 17.4 ± 3.5 | 17.3 ± 5.5 | 10.3 ± 2.6 |
| 63 | Pyrrolidinoheptyl | 13.5 ± 2.2 | 8.5 ± 4.3 | 7.4 ± 3.7 |
| Quercetin | - | >100 | >100 | 45.5 ± 1.3 |
| Fisetin | - | > 50 | > 50 | 34.1 ± 7.7 |
IC50 is the drug concentration effective in inhibiting 50% of the cell viability measured by WST-1 cell proliferation assay after 3 days of exposure. The data were presented as the mean ± SD from n = 3.
Human androgen-insensitive prostate cancer cell line derived from bone metastasis of prostate tumor
Human androgen-insensitive prostate cancer cell line derived from brain metastasis of prostate tumor
Human androgen-sensitive prostate cancer cell line derived from lymph node metastasis of prostate tumor
Table 2.
Anti-Proliferative Activity of 3-O-Substituted-3′,4′,7-trimethoxyflavononols (21-30; 67-81)
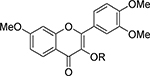 | ||||
|---|---|---|---|---|
| Compd | IC50 (μM)a | |||
| R | PC-3b | DU145c | LNCaPd | |
| 5 | H | 19.9 ± 3.4 | 24.9 ± 12.0 | 24.5 ± 4.5 |
| 21 | Methyl | 14.9 ± 4.1 | 30.8 ± 17.1 | 15.6 ± 4.6 |
| 22 | Ethyl | 8.5 ± 1.7 | 59.8 ± 5.2 | 30.2 ± 12.2 |
| 23 | Propyl | 10.0 ± 7.0 | 24.3 ± 8.9 | 6.2 ± 2.9 |
| 24 | Butyl | 14.1 ± 6.6 | 26.3 ± 6.5 | 9.4 ± 2.4 |
| 25 | Pentyl | 20.3 ± 1.7 | 33.2 ± 1.0 | 17.7 ± 1.2 |
| 26 | Hexyl | 42.3 ± 4.5 | 64.4 ± 12.3 | 19.6 ± 3.4 |
| 27 | Heptyl | 25.7 ± 13.0 | 127.2 ± 45.6 | 22.9 ± 5.7 |
| 28 | Isopropyl | 38.0 ± 8.0 | 103.1 ± 13.0 | 27.1 ± 2.2 |
| 29 | Sec-Butyl | 22.0 ± 3.0 | > 50 | 13.5 ± 3.2 |
| 30 | Pentan-2-yl | 39.0 ± 9.1 | > 50 | 12.2 ± 1.8 |
| 67 | (N,N-Dibutylamino)propyl | 1.9 ± 0.4 | 11.8 ± 1.7 | 5.5 ± 1.1 |
| 68 | (N,N-Dibutylamino)butyl | 3.2 ± 0.2 | 16.5 ± 1.0 | 7.1 ± 1.3 |
| 69 | (N,N-Dibutylamino)pentyl | 5.2 ± 1.8 | 26.0 ± 2.8 | 12.4 ± 0.6 |
| 70 | Morpholinopropyl | 12.4 ± 5.9 | 41.3 ± 13.6 | 32.2 ± 12.3 |
| 71 | Morpholinobutyl | 9.1 ± 3.3 | 119.9 ± 5.5 | 10.0 ± 4.5 |
| 72 | Morpholinopentyl | 9.5 ± 1.3 | 34.6 ± 4.6 | 11.1 ± 2.8 |
| 73 | Piperidinopropyl | 24.5 ± 5.6 | > 50 | 30.7 ± 10.7 |
| 74 | Piperidinobutyl | 16.3 ± 4.4 | > 50 | 16.1 ± 6.3 |
| 75 | Piperidinopentyl | 8.9 ± 1.2 | 27.1 ± 7.2 | 11.0 ± 3.2 |
| 76 | (4-Methylpiperazin-1-yl)propyl | 0.79 ± 0.17 | 20.1 ± 6.1 | 6.0 ± 0.4 |
| 77 | (4-Methylpiperazin-1-yl)butyl | 0.62 ± 0.10 | 34.7 ± 9.7 | 4.6 ± 0.7 |
| 78 | (4-Methylpiperazin-1-yl)pentyl | 1.6 ± 0.8 | 22.1 ± 3.1 | 5.7 ± 1.3 |
| 79 | Pyrrolidinopropyl | 0.82 ± 0.10 | 21.3 ± 2.1 | 4.5 ± 2.2 |
| 80 | Pyrrolidinobutyl | 4.2 ± 0.5 | 25.0 ± 2.6 | 7.4 ± 1.3 |
| 81 | Pyrrolidinopentyl | 0.54 ± 0.31 | 15.4 ± 5.8 | 6.3 ± 1.4 |
| Quercetin | - | >100 | >100 | 45.5 ± 1.3 |
| Fisetin | - | > 50 | > 50 | 34. 1 ± 7.7 |
IC50 is the drug concentration effective in inhibiting 50% of the cell viability measured by WST-1 cell proliferation assay after 3 days of exposure. The data were presented as the mean ± SD from n = 3.
Human androgen-insensitive prostate cancer cell line derived from bone metastasis of prostate tumor
Human androgen-insensitive prostate cancer cell line derived from brain metastasis of prostate tumor
Human androgen-sensitive prostate cancer cell line derived from lymph node metastasis of prostate tumor
As illustrated in Table 1, twenty-eight out of thirty-eight 3-O-substituted-3′,4′-dimethoxylflavonols (1120, 36-63) are significantly more potent than the parent 3′,4′-dimethoxylflavonol (4), quercetin (1), and fisetin (2). Alkylation of 3-OH in parent 3′,4′-dimethoxylflavonol (4) with an appropriate alkyl group leads to a significant increase in the inhibitory activity against prostate cancer cell proliferation. The optimal alkyl groups include ethyl, propyl, butyl, pentyl, and hexyl. The potency of 3-O-alkyl-3′,4′dimethoxylflavonols is however dramatically decreased with increasing length or branching of the alkyl group (e.g. 17, 18, 19, 20), especially towards androgen-insensitive PC-3 and DU145 prostate cancer cell lines. Introduction of a suitable amino moiety (i.e. N,N-dibutylamino, N,N-dipentylamino morpolinopentyl, 4-methylpiperazin-1-yl, and pyrrolidinopropyl) to 3-OH of 3′,4′-dimethoxylflavonol through a three- to five-carbon linker bestows up to 818-fold enhancement in antiproliferative effect in prostate cancer cell models. The potency of 3-O-aminoalkyl-3′,4′-dimethoxylflavonol is seriously diminished if the three- to five-carbon linker is substituted by a six- or seven-carbon linker (e.g. 57, 58, 62, 63).
As shown in Table 2, 3′,4′,7-trimethoxylflavonol (5) is more potent than 3′,4′-dimethoxylflavonol (4), but 3-O-substituted-3′,4′-dimethoxylflavonols (11-20, 36-63) exhibit similar potency as the corresponding 3-O-substituted-3′,4′,7-trimethoxylflavonols (21-30, 67-81). Eighteen out of twenty-five 3-O-substituted-3′,4′,7-trimethoxylflavonols possess improved potency towards the PC-3 prostate cancer cell line, as compared with the parent 3′,4′,7-trimethoxylflavonol (5). Twenty-one out of twenty-five 3-O-substituted-3′,4′,7-trimethoxylflavonols have enhanced potency against LNCaP prostate cancer cells, as compared with the parent flavonol 5. The common structure-activity relationship of 3O-substituted-3′,4′-dimethoxylflavonols and 3-O-substituted-3′,4′,7-trimethoxylflavonols can be concluded as below:
The in vitro antiproliferative potency of 3′,4′-dimethoxylflavonol and 3′,4′,7-trimethoxylflavonol can be substantially ameliorated by an appropriate chemical modification on 3-OH.
N,N-dibutylamino, N,N-dipentylamino morpolinopentyl, 4-methylpiperazin-1-yl, and pyrrolidinopropyl serve as the optimal amino groups for the activity against prostate cancer cells.
Three- to five-carbon are the optimal length for the linker, between 7-OH of flavonoid and the amino moiety, that is beneficial to the potency.
The PC-3 and LNCaP prostate cancer cells are generally more susceptible than the DU145 cells to the 3-O-substituted-flavonols.
Several optimal derivatives (56, 61, 76, 77, 79, and 81) reveal submicromolar IC50 values against PC-3 prostate cancer cells.
2.3. Anti-proliferative activity towards MCF 10A and PWR-1E non-neoplastic human epithelial cell lines
One parent flavonol, 5, and three 3-O-substitutedflavonols, 42, 76, and 81, together with two naturally occurring flavonols, quercetin (1) and fisetin (2), were selected for further evaluation against MCF 10A non-neoplastic human mammary epithelial cell line and PWR-1E non-neoplastic human prostate epithelial cells. It is arguable whether the MCF 10A cells can truly represent normal human mammary cells [26] and it was believed by Rhim and co-workers [27] that PWR-1E immortalized human prostate epithelial cell model stands for an early stage in tumor progression. They are therefore called non-neoplastic cell models rather than normal cell models. Four synthetic flavonols (5, 42, 76, and 81) were chosen based on the following grounds: 5 represents the more potent parent flavonol; 42 is the most potent 3-O-substituted-3′,4′-dimethoxyflavonol considering its overall potency towards three prostate cancer cell models; 76 and 81 are the most promising 3-O-substituted-3′,4′,7-trimethoxyflavonols. As shown in Table 3, two natural flavonols, fisetin (2) and quercetin (1), demonstrate significantly higher capability of suppressing non-neoplastic cell (MCF 10A and PWR-1E) proliferation than prostate cancer cell proliferation. The parent flavonol, 3-O-3′,4′,7-trimethoxyflavonol (5), did not exhibit significantly differential responses to prostate cancer cells and to non-neoplastic MCF 10A cells. However, it doesn’t demonstrate apparent antiproliferative activity towards PWR-1E non-neoplastic epithelial cells up to 50 μM concentration. Interestingly, the 3-O-substituted-3′,4′-dimethoxyflavonol 42 and the 3-O-substituted-3′,4′,7-trimethoxyflavonols 76 and 81 illustrate good selectivity of inhibiting prostate cancer cell proliferation over non-neoplastic MCF 10A and PWR-1E human epithelial cell proliferation (Table 3), suggesting that modification at 3-OH of 3′,4′-dimethoxyflavonol and 3′,4′,7-trimethoxyflavonol improves the antiproliferative potency only towards prostate cancer cells but not non-neoplastic epithelial cells.
Table 3.
Antiproliferative activity of selected derivatives against MCF-10A and PWR-1E
| compound | IC50 (μM)a | ||||
|---|---|---|---|---|---|
| PC-3 | DU145 | LNCaP | MCF 10A | PWR-1E | |
| Fisetin | > 50 | > 50 | 34.1 ± 7.7 | 2.44 ± 0.27 | 5.39 ± 1.16 |
| Quercetin | > 100 | > 100 | 45.5 ± 1.3 | 11.97 ± 1.49 | 15.08 ± 1.41 |
| 5 | 19.9 ± 3.4 | 24.9 ± 12.0 | 24.5 ± 4.5 | 23.95 ± 5.05 | > 50 |
| 81 | 0.54 ± 0.3 | 15.4 ± 5.8 | 6.3 ± 1.4 | 35.69 ± 7.69 | > 50 |
| 76 | 0.79 ± 0.2 | 20.1 ± 6.1 | 6.0 ± 0.4 | 33.75 ± 14.22 | > 50 |
| 42 | 1.4 ± 0.2 | 7.6 ± 1.7 | 2.4 ± 1.5 | 20.93 ± 0.81 | > 50 |
IC50 is the drug concentration effective in inhibiting 50% of the cell viability measured by WST-1 cell proliferation assay after 3 days of exposure. The data were presented as the mean ± SD from n = 3.
2.4. Cell Cycle Regulation and Cell Apoptosis Induction:
It has been demonstrated that quercetin and fisetin, as two naturally occurring flavonols, possess cell cycle perturbation properties in the androgen-insensitive PC-3 human prostate cancer cell line by arresting cell cycle at G2/M phase [12,15]. 3-O-Substitued-3′,4′-dimethoxyflavonol 42 can induce PC-3 cell cycle arrest at the G2/M phase as well, as reported in our earlier study [19]. The effect of 3′,4′,7-trimethoxyflavonol 5 and two 3-O-substituted-3′,4′,7-trimethoxyflavonols 76 and 81 at 10 μM and 20 μM on the PC-3 cell cycle was assessed using flow cytometric analysis with propidium iodide DNA staining. 3′,4′,7-trimethoxyflavonol (5) arrests PC-3 cell cycle at the G0/G1 phase in 16 hours by increasing PC-3 cell population. In contrast, two 3-O-substituted-3′,4′,7-trimethoxyflavonols 76 and 81 induce PC-3 cell cycle at the S phase in 16 hours. The above-mentioned data imply that modification of 3-OH of 3′,4′,7-trimethoxyflavonol can alter its regulatory effect on PC-3 cell cycle.
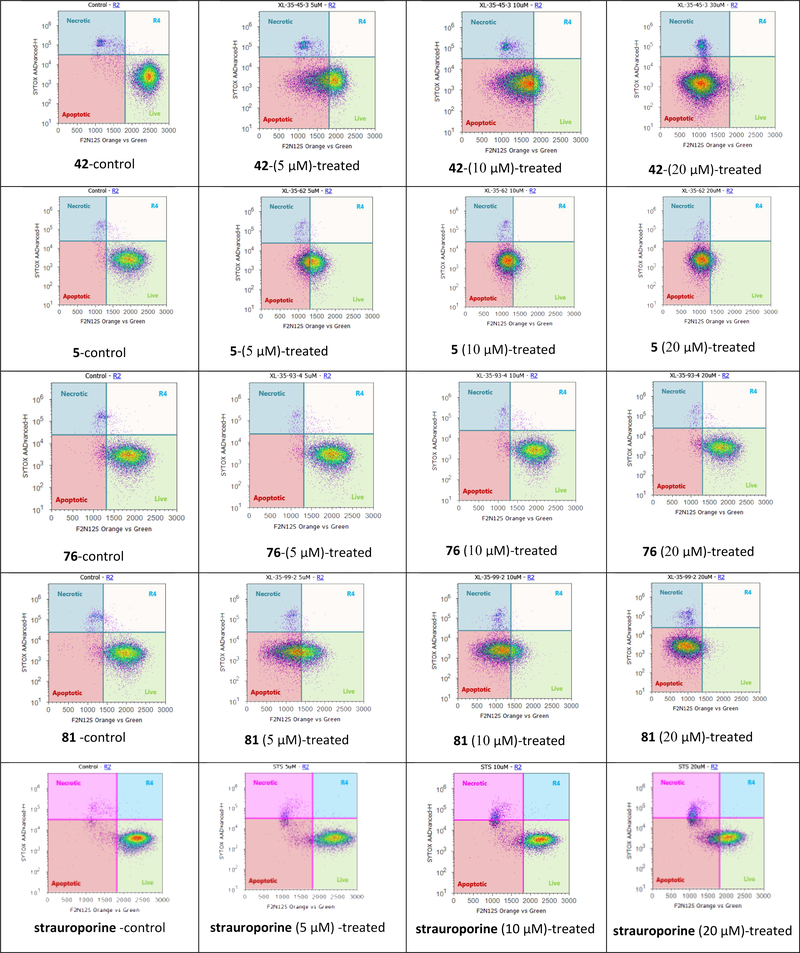
The PC-3 cell proliferation inhibition of quercetin and fisetin, two naturally occurring flavonols, has been revealed to be associated with cell apoptosis promotion [28–30]. The F2N12S and SYTOX AADVanced double staining assay in a flow cytometer was used for the discrimination between early apoptotic PC-3 cells and late apoptotic/necrotic PC-3 cells when treated with one parent flavonol 5 and three 3-O-substitutedflavonols (42, 76, and 81) at 0, 5, 10 and 20 μM concentrations for 16 h. The known apoptotic inducer staurosporine was used as the positive control in apoptotic experiments. As illustrated in Figure 2, 3-O-substituted-3′,4′-dimethoxyflavonol 42, 3′,4′,7-trimethoxyflavonol (5), and 3-O-pyrrolidinopentyl-3′,4′,7-trimethoxyflavonol (81) can significantly activate apoptotic cell death in the androgen-insensitive PC-3 prostate cancer cell line in a dose-dependent manner after a 16-hour treatment. Specifically, treatment of 42, 5, and 81 at 5 μM can lead to 45%, 37%, and 59% of PC-3 cells in early phase of apoptosis as compared with control cells; exposure of PC-3 cells to 42, 5, and 81 at 10 μM induces 80%, 79%, and 77% early apoptotic cells together with 5%, 2%, and 3% late apoptotic/necrotic cells; 20 μM of 42, 5, and 81 activates notable apoptosis as well, with 93%, 90%, and 92% early apoptotic cells and 4%, 2%, and 4% late apoptotic/necrotic cells. All these three flavonols (42, 5, and 81) promote androgen-insensitive PC-3 cell apoptosis much more effectively than the known apoptotic inducer staurosporine (Figure 2). In contrast, treatment of the PC-3 cells with 3-O-(4-methylpiperazin-1-yl)propyl-3′,4′,7-trimethoxyflavonol (76) up to 100 μM high concentration only led to 7% early apoptotic cells. It is worth noting that 3-O-(4-methylpiperazin-1-yl)propyl-3′,4′,7-trimethoxyflavonol (76) induces PC-3 cell death in a completely different way from 3-O-pyrrolidinopentyl-3′,4′,7-trimethoxyflavonol (81) even though they both belong to 3-O-substituted3′,4′,7-trimethoxyflavonols and exhibit similar potency in inhibiting PC-3 cell proliferation, suggesting that the action of mechanism for each specific 3-O-substitutedflavonol varies with different amino moiety.
Figure 2.
Apoptosis in PC-3 cells treated with 42, 5, 76, and 81 at 5 μM, 10 μM, and 20 μM (by F2N12S and SYTOX AADvanced double staining)
2.5. In vivo pharmacokinetic studies
To evaluate if the 3-O-substituted-flavonols with significantly improved in vitro anticancer activities could also possess greater bioavailability, we selected three of the most promising derivatives, 42, 76, and 81, together with one naturally occurring flavonol (fisetin), for pharmacokinetic studies in rats. Sprague Dawley rats, a species that are more suited for bioavailability study, were chosen in this study as the animal models for the pharmacokinetic studies. The animals administered with fisetin, 42, 76, or 81, via oral gavage at a single dose of 10 mg/kg, and blood samples were collected at 1, 3, 6, 8, and 24 h after oral administration. Plasma was prepared from the blood samples and was analyzed by HPLCMS/MS for determination of drug concentration as described in the Experimental Section. The PK parameters for fisetin cannot be calculated because its plasma concentrations can barely be achieved at 1h time point, implying the poor pharmacokinetic profile of fisetin. Summarized in Table 4 are the pharmacokinetic parameters for three 3-O-substituted flavonols (42, 76, or 81), which lead to the conclusion that they gain the obvious improvement in their bioavailability with the peak concentration at 5773 ng/mL (12.36 μM), 894 ng/mL (1.92 μM), and 172 ng/mL (0.37 μM), respectively. Among the three 3-O-substituted-flavonols, 42 gains the greatest improvement in its bioavailability; its peak concentration at 5773 ng/mL (12.36 μM) is far exceeding its IC50 values ranging from 1.4–7.6 μM in three human prostate cancer cell lines. It is thus logical to conclude that the excellent bioavailability of 42, as demonstrated by its high peak plasma concentration and AUC value, will provide the therapeutic efficacy necessary to suppress in vivo tumor growth.
Table 4.
Pharmacokinetic parameters for 42, 76, and 81
| Parameter | Unit | 42 | 76 | 81 |
|---|---|---|---|---|
| Lambda_z | 1/h | 0.18 | 0.079 | 0.074 |
| t1/2 | h | 3.87 | 8.83 | 9.39 |
| Tmax | h | 1 | 8 | 3 |
| Cmax | ng/ml | 5773.00 | 893.99 | 171.81 |
| Tlag | h | 0 | 0 | 0 |
| Clast_obs/Cmax | 0.00044 | 0.044 | 0.036 | |
| AUC 0-t | ng/ml*h | 51805.98 | 16599.23 | 2947.61 |
| AUC 0-inf obs | ng/ml*h | 51820.33 | 17101.39 | 3032.42 |
| AUC 0-t/0-inf_obs | 1.00 | 0.97 | 0.97 | |
| AUMC 0-inf obs | ng/ml*h^2 | 343077.41 | 270875.20 | 39656.44 |
| MRT 0-inf_obs | h | 6.62 | 15.84 | 13.08 |
| Vz/F_obs | (mg/kg)/(ng/ml) | 0.0011 | 0.0074 | 0.045 |
| Cl/F_obs | (mg/kg)/(ng/ml)/ h | 0.00019 | 0.00058 | 0.0033 |
3. CONCLUSION
To further investigate the in-depth structure-activity relationships of 3-O-substitutedflavonols, sixty three 3-O-substituted-3′,4′-dimethoxyflavonols and 3-O-substituted-3′,4′,7-trimethoxyflavonols have been synthesized for anti-proliferative evaluation in both androgen-sensitive (LNCaP) and androgen-insensitive (PC-3 and DU145) prostate cancer cell lines. Forty-six out of sixty-three 3-O- substitutedflavonols are significantly more potent than naturally occurring flavonols quercetin and fisetin, with the optimal 3-O-substitutedflavonol being up to 808-fold more potent than fisetin and quercetin. The acquired structure-activity relationship data indicated that i) 1-methylpiperazine, pyrrolidine, and dibutylamine serve as optimal amino groups for notably improved in vitro potency; and ii) a 3-carbon to 5-carbon linker contributes to substantially improved antiproliferative effect in prostate cancer cell models. Interestingly, incorporation of an appropriate amino group to 3-OH of 3′,4′dimethoxyflavonol and 3′,4′,7-trimethoxyflavonol through a 3- to 5-carbon linker can substantially improve the in vitro antiproliferative potency in three human prostate cancer cell models, but not in two non-neoplastic human epithelial cell models (MCF 10A and PWR-1E). It is worth noting that 3-O-(4methylpiperazin-1-yl)propyl-3′,4′,7-trimethoxyflavonol (76) induces PC-3 cell death in a completely different way from 3-O-pyrrolidinopentyl-3′,4′,7-trimethoxyflavonol (81) even though they belong to 3-O-substituted-3′,4′,7-trimethoxyflavonols and exhibit similar potency in inhibiting PC-3 cell proliferation, suggesting that the action of mechanism for each specific 3-O-substitutedflavonol varies with different amino moiety. Pharmacokinetic studies in rat model identified compound 42 possesses the excellent bioavailability, as demonstrated by its high peak plasma concentration and AUC value. 3O-(N,N-Dibutylamino)-propyl-3′,4′-dimethoxyflavonol (42) has been established as the most promising derivative at this point because of its substantially improved potency in cell models, superior bioavailability in rats, and good antiproliferative selectivity of prostate cancer cells over non-neoplastic human epithelial cells.
4. EXPERIMENTAL SECTION
4.1. General Procedures.
HRMS were obtained on an Orbitrap mass spectrometer with electrospray ionization (ESI). IR spectra were recorded on a Nicolet Nexus 470 FTIR spectrophotometer. NMR spectra were obtained on a Bruker Fourier 300 spectrometer in CDCl3. The chemical shifts are given in ppm referenced to the respective solvent peak, and coupling constants are reported in Hz. All reagents and solvents were purchased from commercial sources and were used without further purification. Silica gel column chromatography was performed using silica gel (32–63 μm). Preparative thin-layer chromatography (PTLC) separations were carried out on thin layer chromatography plates loaded with silica gel 60 GF254 (EMD Millipore Corporation, MA, USA). The purities of biologically tested compounds are ≥ 95% as determined by HPLC. Specifically, the major peak accounted for ≥ 95% of the combined total peak area when monitored by a Diode Array Detector (DAD) at 325 ± 100 nm. The HPLC analyses were performed on an Agilent Hewlett Packard 1100 Series HPLC DAD system using a 5 μM C18 reversed phase column (4.6 mm × 250 mm) and a Diode Array Detector.
4.2. The general procedure for the synthesis of 3-O-alkyl-3′,4′-dimethoxyflavonols (11–20).
The solution of 3′,4′-dimethoxyflavonol (50 mg, 0.17 mmol) and potassium carbonate (70 mg, 0.51 mmol, 3.0 equiv.) in DMF (0.5 mL) was stirred for 10 min at R.T.. To this solution was added an appropriate alkyl iodide or alkyl bromide (0.51 mmol, 3.0 equiv.) through syringe, the reaction was kept at room temperature over 12 h until the starting material was consumed as detected by TLC (dichloromethane/methanol, 100/3, v/v). The mixture was poured into ice water (3.0 mL), and the solution was extracted with ethyl acetate three times. The combined extracts were sequentially rinsed with saturated sodium bicarbonate and brine, dried over anhydrous sodium sulfate, and concentrated in vacuo to give a crude mass, which was purified by PTLC eluting with dichloromethane/methanol (100/3, v/v) to furnish the respective 3-O-alkyl-3′,4′-dimethoxyflavonols. The yields and spectral data for compounds 11, 12, 13, 14, 15, 16, 17, and 18 have been reported in the supporting information of our previous Letter report [19]. HPLC purity for 4 97.5% (30 min run of 45–80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min). HPLC purity for 11 is 95.7% (30 min run of 45–80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min). HPLC purity for 12 is 99.5% (30 min run of 45–80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min). HPLC purity for 13 is 96.7% (30 min run of 45–80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min). HPLC purity for 14 is 98.8% (30 min run of 45–80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min). HPLC purity for 15 is 95.1% (30 min run of 45–80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min). HPLC purity for 16 is 95.1% (30 min run of 45–80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min). HPLC purity for 17 is 99.7% (30 min run of 45–80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min). HPLC purity for 18 is 97.6% (30 min run of 45–80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
4.2.1. 3-O-Sec-butyl-3′,4′-dimethoxyflavonol (19)
This derivative was achieved in 67% yield as a syrup. IR (film) νmax: 2965, 2931, 1634, 1614, 1614, 1598, 1557, 1509 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.25 (dd, J = 8.0, 1.4 Hz, 1H), 7.84 (d, J = 1.9 Hz, 1H), 7.77 (dd, J = 8.5, 2.0 Hz, 1H), 7.66 (ddd, J = 8.4, 6.9, 1.5 Hz, 1H), 7.53 (d, J = 8.3 Hz, 1H), 7.39 (t, J = 7.2 Hz, 1H), 6.99 (d, J = 8.6 Hz, 1H), 4.58 (sextet, J = 6.3 Hz, 1H), 3.971 (s, 3H), 3.965 (s, 3H), 1.80 – 1.65 (m, 1H), 1.57 – 1.45 (m, 1H), 1.12 (d, J = 6.2 Hz, 3H), 0.91 (t, J = 7.5 Hz, 3H). 13CNMR (75 MHz, CDCl3) δ 175.5, 156.2, 155.3, 151.0, 148.4, 138.8, 133.3, 125.9, 124.6, 124.2, 124.2, 122.5, 118.0, 112.5, 110.6, 79.3, 56.1, 56.1, 29.7, 19.4, 9.9. HR-MS (ESI) m/z: calcd for C21H22O5 [M+H]+: 355.1545; found 355.1540. HPLC purity 96.9% (30 min run of 45–80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
4.2.2. 3-O-(Pentan-2-yl)-3′,4′-dimethoxyflavonol (20)
This derivative was achieved in 20% yield as a syrup. IR (film) νmax: 2957, 2930, 2870, 1634, 1614, 1598, 1558, 1509 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.24 (dd, J = 8.0, 1.6 Hz, 1H), 7.84 (d, J = 2.0 Hz, 1H), 7.77 (dd, J = 8.5, 2.1 Hz, 1H), 7.66 (ddd, J = 8.6, 7.1, 1.7 Hz, 1H), 7.53 (d, J = 8.0 Hz, 1H), 7.38 (ddd, J = 8.0, 7.1, 1.0 Hz, 1H), 6.98 (d, J = 8.7 Hz, 1H), 4.67 (sextet, J = 6.1 Hz, 1H), 3.97 (s, 3H), 3.96 (s, 3H), 1.77 – 1.60 (m, 1H), 1.53 – 1.34 (m, 3H), 1.10 (d, J = 6.2 Hz, 3H), 0.88 (t, J = 7.2 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 175.5, 156.1, 155.3, 151.0, 148.4, 138.8, 133.2, 125.9, 124.6, 124.3, 124.2, 122.4, 118.0, 112.5, 110.7, 77.8, 56.1, 56.0, 39.2, 19.91, 18.87, 14.3. HR-MS (ESI) m/z: calcd for C22H24O5 [M+H]+: 369.1702; found 369.1695. HPLC purity 95.0% (30 min run of 45–80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
4.3. The general procedure for the synthesis of 3-O-aminoalkyl-3′,4′-dimethoxyflavonols (36–63).
The solution of 3′,4′-dimethoxyflavonol (4, 50 mg, 0.17 mmol) and potassium carbonate (70 mg, 0.51 mmol, 3 equiv.) in DMF (0.5 mL) was stirred at room temperature for 10 min before an appropriate alkyl dibromide (0.51 mmol, 3 equiv.) was added through a syringe. The reaction was allowed to proceed at room temperature over 12 h until the starting material was fully consumed as indicated by TLC (dichloromethane/methanol, 100/3, v/v). The reaction mixture was poured into ice water (3.0 mL), and the subsequent mixture was extracted with ethyl acetate three times. The combined organic extracts were sequentially rinsed with saturated sodium bicarbonate and brine, dried over anhydrous sodium sulfate, and concentrated in vacuo to generate a crude product. To the solution of this crude product in DMF (0.5 ml) was added potassium carbonate (0.51 mmol, 3 equiv.), the mixture was stirred for 10 min. The appropriate amine (3 equiv.) was added to the reaction mixture through a syringe, and the subsequent reaction mixture was stirred at room temperature over 12 h until no starting material was detected by TLC (dichloromethane/methanol, 100/3, v/v). The mixture was poured into ice water (3.0 mL), and the mixture was extracted with ethyl acetate three times. The combined extracts were sequentially rinsed with saturated sodium bicarbonate and brine, dried over anhydrous sodium sulfate, and concentrated in vacuo to give a crude product, which was subjected to PTLC purification using dichloromethane/methanol (100/3, v/v) as eluent. The respective 3-O-dialkylaminoalkyl-3′,4′,5′trimethoxyflavonol was retrieved from PTLC silica gel by washing with dichloromethane/diethylamine (100/3, v/v). The yields and spectral data for compounds 42, 43, 44, 48, 49, and 50 have been reported in the supporting information of our previous Letter [19]. HPLC purity for 42 is 95.3% (30 min run of 15–75% CH3CN in aqueous solution of ammonium formate (1.26 g ammonium formate in 1000 mL water), with 15 min gradient, 1.0 mL/min). HPLC purity for 43 is 95.8% [30 min run of 15–75% CH3CN in aqueous ammonium formate solution (1.26 g per 1000 mL water), with 15 min gradient, 1.0 mL/min]. HPLC purity for 44 is 97.9% (30 min run of 45–80% CH3CN in in aqueous solution of ammonium formate (1.26 g ammonium formate in 1000 mL water), with 15 min gradient, 1.0 mL/min). HPLC purity for 48 is 95.6% (30 min run of 15–75% CH3CN in aqueous solution of ammonium formate (1.26 g ammonium formate in 1000 mL water), with 15 min gradient, 1.0 mL/min). HPLC purity for 50 is 97.0% (30 min run of 45–80% CH3CN in aqueous solution of ammonium formate (1.26 g ammonium formate in 1000 mL water), with 15 min gradient, 1.0 mL/min). HPLC purity for 3-O-Mopholinobutyl-3′,4′-dimethoxyflavonol (49) is 96.5% (30 min run of 45–80% CH3CN in aqueous solution of ammonium formate (1.26 g ammonium formate in 1000 mL water), with 15 min gradient, 1.0 mL/min).
4.3.1. 3-O-(N,N-Diethylamino)propyl-3′,4′-dimethoxyflavonol (36)
This derivative was achieved in 88% overall yield for two steps as a syrup. IR (film) νmax: 2964, 2835, 1635, 1614, 1599, 1559, 1510 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.22 (dd, J = 8.0, 1.5 Hz, 1H), 7.74 (dd, J = 8.5, 2.0 Hz, 1H), 7.70 (d, J = 1.9 Hz, 1H), 7.64 (ddd, J = 8.6, 7.1, 1.6 Hz, 1H), 7.50 (d, J = 8.2 Hz, 1H), 7.36 (br.d, J = 7.2 Hz, 1H), 6.97 (d, J = 8.5 Hz, 1H), 4.07 (t, J = 6.5 Hz, 2H), 3.94 (s, 6H), 2.54 (t, J = 7.5 Hz, 2H), 2.47 (q, J = 7.2 Hz, 4H), 1.87 (quin, J = 7.2 Hz, 2H), 0.96 (t, J = 7.1 Hz, 6H). 13C NMR (75 MHz, CDCl3) δ 175.1, 155.8, 155.2, 151.2, 148.7, 140.1, 133.3, 125.8, 124.6, 124.2, 123.6, 122.4, 118.0, 111.7, 110.8, 71.4, 56.1, 56.0, 49.8, 46.9, 28.0, 11.6. HR-MS (ESI) m/z: calcd for C24H29NO5 [M+H]+: 412.2124; found 412.2118. HPLC purity 95.3% (30 min run of 45–80% CH3CN in aqueous solution of ammonium formate (1.26 g ammonium formate in 1000 mL water), with 15 min gradient, 1.0 mL/min).
4.3.2. 3-O-(N,N-Diethylamino)butyl-3′,4′-dimethoxyflavonol (37)
This derivative was achieved in 90% overall yield for two steps as a syrup. IR (film) νmax: 2963, 2797, 1635, 1614, 1599, 1560, 1511 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.22 (d, J = 8.0 Hz, 1H), 7.757.73 (overlapped, 2H), 7.70 – 7.61 (m, 1H), 7.51 (d, J = 8.4 Hz, 1H), 7.37 (t, J = 7.5 Hz, 1H), 6.98 (d, J = 8.3 Hz, 1H), 4.04 (t, J = 6.6 Hz, 2H), 3.95 (s, 6H), 2.49 (q, J = 7.2 Hz,4H), 2.43 (t, J = 7.8 Hz, 2H), 1.72 (quin, J = 7.2 Hz, 2H), 1.53 (quin, J = 7.2 Hz, 2H), 0.98 (t, J = 7.2Hz, 6H). 13C NMR (75 MHz, CDCl3) δ 175.1, 155.8, 155.2, 151.2, 148.6, 140.2, 133.3, 125.9, 124.7, 124.2, 123.6, 122.4, 118.0, 111.7, 110.8, 72.6, 56.1, 56.1, 52.5, 46.8, 28.4, 23.2, 11.6. HR-MS (ESI) m/z: calcd for C25H31NO5 [M+H]+: 426.2280; found 426.2273. HPLC purity 95.1% [30 min run of 45–80% acetonitrile in ammonium formate solution (1.26 g ammonium formate in 1000 mL water), with 15 min gradient, 1.0 mL/min].
4.3.3. 3-O-(N,N-Diethylamino)pentyl-3′,4′-dimethoxyflavonol (38)
This derivative was achieved in 85% overall yield for two steps as a syrup. IR (film) νmax: 2933, 1636, 1614, 1599, 1559, 1511 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.23 (dd, J = 8.0, 1.2 Hz, 1H), 7.80 – 7.70 (m, 2H), 7.64 (ddd, J = 8.7, 6.9, 1.5 Hz, 1H), 7.51 (d, J = 8.3 Hz, 1H), 7.37 (t, J = 7.2 Hz, 1H),6.98 (d, J = 9.1 Hz, 1H), 4.01 (t, J = 6.7 Hz, 2H), 3.95 (s, 3H), 3.94 (s, 3H), 2.50 (q, J = 7.2 Hz, 4H), 2.38 (t, J = 8.1 Hz,2H), 1.74 (quin, J = 7.2 Hz, 2H), 1.47–1.34(overlapped, 4H), 0.99 (t, J = 7.2 Hz, 6H). 13C NMR (75 MHz, CDCl3) δ 175.1, 155.8, 155.2, 151.2, 148.6, 140.2, 133.3, 125.9, 124.7, 124.3, 123.7, 122.3, 118.0, 111.8, 110.8, 72.7, 56.1, 56.1, 52.7, 46.9, 30.3, 26.6, 24.1, 11.5. HR-MS (ESI) m/z: calcd for C26H33NO5 [M+H]+: 440.2437; found 440.2428. HPLC purity 97.2% (30 min run of 45–80% CH3CN in aqueous solution of ammonium formate (1.26 g ammonium formate in 1000 mL water), with 15 min gradient, 1.0 mL/min).
4.3.4. 3-O-(N,N-Dipropylamino)propyl-3′,4′-dimethoxyflavonol (39)
This derivative was achieved in 44% overall yield for two steps as a syrup. IR (film) νmax: 2955, 2871, 1636, 1614, 1599, 1559, 1511 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.24 (dd, J = 8.0, 1.4 Hz, 1H), 7.75 (dd, J = 8.5, 2.0 Hz, 1H), 7.71 (d, J = 1.9 Hz, 1H), 7.66 (ddd, J = 8.6, 7.1, 1.6 Hz, 1H), 7.52 (d, J = 8.0 Hz, 1H), 7.39 (ddd, J = 7.8, 7.2, 0.9 Hz, 1H), 6.99 (d, J = 8.5 Hz, 1H), 4.07 (t, J = 6.4 Hz, 2H), 3.96 (s, 6H), 2.63 (br.s, 2H), 2.40 (t, J = 6.3 Hz, 4H),1.93 (quin, J = 7.2 Hz, 2H), 1.44 (sextet, J = 7.5 Hz, 4H), 0.83 (t, J = 7.3 Hz, 6H). 13C NMR (75 MHz, CDCl3) δ 175.1, 155.9, 155.3, 151.3, 148.8, 140.1, 133.4, 125.9, 124.7, 124.3, 123.6, 122.5, 118.0, 111.7, 110.9, 71.3, 56.2, 56.1, 56.0, 51.1, 27.8, 19.8, 11.9. HR-MS (ESI) m/z: calcd for C26H33NO5 [M+H]+: 440.2437; found 440.2429. HPLC purity 97.8% (30 min run of 15–70% CH3CN in aqueous solution of ammonium formate (1.26 g ammonium formate in 1000 mL water), with 15 min gradient, 1.0 mL/min).
4.3.5. 3-O-(N,N-Dipropylamino)butyl-3′,4′-dimethoxyflavonol (40)
This derivative was achieved in 64% overall yield for two steps as a syrup. IR (film) νmax: 2954, 2870, 1636, 1614, 1599, 1560, 1511 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.24 (dd, J = 8.0, 1.4 Hz, 1H), 7.78–7.74 (overlapped, 1H), 7.74 (s, 1H), 7.66 (ddd, J = 8.7, 6.9, 1.5 Hz, 1H), 7.52 (d, J = 8.2 Hz, 1H), 7.38 (t, J = 7.5 Hz, 1H), 6.99 (d, J = 8.3 Hz, 1H), 4.04 (t, J = 6.6 Hz, 2H), 3.962 (s, 3H), 3.957 (s, 3H), 2.49 (t, J = 7.6 Hz, 2H), 2.39 (t, J = 8.3 Hz, 4H), 1.74 (quin, J = 7.3 Hz, 2H), 1.62–1.57 (overlapped, 2H), 1.44 (sextet, J = 7.4 Hz, 4H), 0.84 (t, J = 7.3 Hz, 6H). 13C NMR (75 MHz, CDCl3) δ 175.2, 155.8, 155.3, 151.2, 148.7, 140.2, 133.4, 125.9, 124.7, 124.3, 123.7, 122.4, 118.0, 111.8, 110.9, 72.6, 56.2, 56.1, 56.0, 53.7, 28.3, 23.2, 19.9, 12.0. HR-MS (ESI) m/z: calcd for C27H35NO5 [M+H]+: 454.2593; found 454.2581. HPLC purity 96.7% (30 min run of 45–80% CH3CN in aqueous solution of ammonium formate (1.26 g ammonium formate in 1000 mL water), with 15 min gradient, 1.0 mL/min).
4.3.6. 3-O-(N,N-Dipropylamino)pentyl-3′,4′-dimethoxyflavonol (41)
This derivative was achieved in 69% overall yield for two steps as a syrup. IR (film) νmax: 2932, 2869, 2797, 1636, 1614, 1599, 1560, 1511 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.24 (dd, J = 8.0, 1.4 Hz, 1H), 7.78 – 7.73 (overlapped, 2H), 7.65 (ddd, J = 8.6, 7.2, 1.6 Hz, 1H), 7.52 (d, J = 8.2 Hz, 1H), 7.38 (ddd, J = 8.6, 6.9, 0.9 Hz, 1H), 6.99 (d, J = 9.0Hz, 1H), 4.03 (t, J = 6.7 Hz, 2H), 3.96 (s, 3H), 3.95 (s, 3H), 2.37 (q, J = 7.5 Hz, 6H), 1.74 (quin, J = 6.9 Hz, 2H), 1.50 – 1.33 (overlapped, 8H), 0.85 (t, J = 7.4 Hz, 6H). 13C NMR (75 MHz, CDCl3) δ 175.1, 155.8, 155.3, 151.2, 148.7, 140.2, 133.3, 125.9, 124.7, 124.3, 123.7, 122.4, 118.0, 111.8, 110.8, 72.7, 56.2, 56.14, 56.08, 54.1, 30.3, 26.6, 24.0, 20.1, 12.0. HR-MS (ESI) m/z: calcd for C28H37NO5 [M+H]+: 468.2750; found 468.2739. HPLC purity 97.3% (30 min run of 45–80% CH3CN in aqueous solution of ammonium formate (1.26 g ammonium formate in 1000 mL water), with 15 min gradient, 1.0 mL/min).
4.3.7. 3-O-(N,N-Diamylamino)propyl-3′,4′-dimethoxyflavonol (45)
This derivative was achieved in 46% overall yield for two steps as a syrup. IR (film) νmax: 2952, 2929, 2858, 1637, 1615, 1600, 1560, 1511 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.24 (dd, J = 8.0, 1.4 Hz, 1H), 7.76 (dd,J = 8.7, 1.8 Hz,1H), 7.72 (d, J = 1.8 Hz), 7.65 (ddd, J = 8.4, 7.2, 1.8 Hz, 1H), 7.52 (d, J = 8.2 Hz, 1H), 7.38 (t, J = 7.1 Hz, 1H), 6.98 (d, J = 8.5 Hz, 1H), 4.08 (t, J = 6.5 Hz, 2H), 3.96 (s, 6H), 2.56 (t, J = 6.6 Hz. 2H), 2.37 (t, J = 8.1 Hz, 4H), 1.89 (quin, J = 6.9 Hz, 2H), 1.56 – 1.12 (m, 12H), 0.85 (t, J = 6.8 Hz, 6H). 13C NMR (75 MHz, CDCl3) δ 175.1, 155.8, 155.2, 151.2, 148.7, 140.1, 133.3, 125.9, 124.7, 124.3, 123.6, 122.4, 118.0, 111.7, 110.8, 71.4, 56.1, 56.1, 54.1, 52.2, 51.1, 35.6, 29.8, 28.0, 26.6, 26.5, 22.8, 22.7, 14.2. HR-MS (ESI) m/z: calcd for C30H41NO5 [M+H]+: 496.3063; found 496.3055. HPLC purity 95.3% (30 min run of 15–75% CH3CN in aqueous solution of ammonium formate (1.26 g ammonium formate in 1000 mL water), with 15 min gradient, 1.0 mL/min).
4.3.8. 3-O-(N,N-Diamylamino)butyl-3′,4′-dimethoxyflavonol (46)
This derivative was achieved in 55% overall yield for two steps as a syrup. IR (film) νmax: 2929, 2859, 1636, 1615, 1599, 1560, 1511 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.24 (dd, J = 8.0, 1.4 Hz, 1H), 7.77 (dd, J = 7.5, 2.1 Hz,1H), 7.75 (s, 1H),7.65 (ddd, J = 8.4, 6.9, 1.8 Hz, 1H), 7.52 (d, J = 8.2 Hz, 1H), 7.38 (t, J = 7.1 Hz, 1H), 6.98 (d, J = 8.5 Hz, 1H), 4.04(t, J = 6.5 Hz, 2H), 3.95(s, 6H), 2.42 (t, J = 8.4 Hz, 2H), 2.36 (t, J = 7.2 Hz, 4H), 1.73 (quin, J = 7.5 Hz, 2H), 1.58– 1.12 (m, 12H), 0.85 (t, J = 6.8 Hz, 7H). 13C NMR (75 MHz, CDCl3) δ 175.1, 155.8, 155.2, 151.2, 148.7, 140.1, 133.3, 125.9, 124.7, 124.3, 123.6, 122.4, 118.0, 111.7, 110.8, 71.4, 56.1, 56.1, 54.1, 52.2, 51.1, 35.6, 29.8, 28.0, 26.6, 26.5, 22.8, 22.7, 14.2. HR-MS (ESI) m/z: calcd for C31H43NO5 [M+H]+: 510.3219; found 510.3213. HPLC purity 95.0% (30 min run of 45–80% CH3CN in aqueous solution of ammonium formate (1.26 g ammonium formate in 1000 mL water), with 15 min gradient, 1.0 mL/min).
4.3.9. 3-O-(N,N-Diamylamino)pentyl-3′,4′-dimethoxyflavonol (47)
This derivative was achieved in 46% overall yield for two steps as a syrup. IR (film) νmax: 2929, 2859, 1636, 1615, 1599, 1560, 1511 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.23 (dd, J = 8.0, 1.3 Hz, 1H),7.75 (dd, J = 4.3, 2.5 Hz, 2H), 7.65 (ddd, J = 8.6, 7.1, 1.6 Hz, 1H), 7.51 (d, J = 8.3 Hz, 1H), 7.37 (ddd, J = 7.8, 7.2, 0.9 Hz, 1H), 6.98 (d, J = 9.1 Hz, 1H), 4.02 (t, J = 6.7 Hz, 2H), 3.96 (s, 3H), 3.95 (s, 3H), 2.38 (t, J = 7.8 Hz, 6H), 1.75 (quin, J = 6.9 Hz, 2H), 1.60 – 1.12 (m, 16H), 0.91 – 0.81 (m, 6H). 13C NMR (75 MHz, CDCl3) δ 175.1, 155.7, 155.2, 151.2, 148.6, 140.2, 133.3, 125.9, 124.7, 124.3, 123.7, 122.3, 118.0, 111.8, 110.8, 72.7, 56.1, 56.1, 54.1, 54.0, 52.2, 35.6, 30.3, 29.9, 26.7, 26.6, 26.5, 24.1, 22.9, 22.7, 14.2. HR-MS (ESI) m/z: calcd for C33H47NO5 [M+H]+: 538.3532; found 538.3529. HPLC purity 95.0% (30 min run of 15–75% CH3CN in aqueous solution of ammonium formate (1.26 g ammonium formate in 1000 mL water), with 15 min gradient, 1.0 mL/min).
4.3.10. 3-O-Piperidinopropyl-3′,4′-dimethoxyflavonol (51)
This derivative was achieved in 75% overall yield for two steps as a syrup. IR (film) νmax: 2937, 2847, 2779, 1635, 1611, 1598, 1558, 1513 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.22 (dd, J = 8.0, 1.3 Hz, 1H), 7.73 (dd, J = 8.4, 2.1 Hz, 1H), 7.69 (d, J = 1.8 Hz, 1H), 7.64 (ddd, J = 8.7, 7.2, 1.5 Hz, 1H),7.51 (d, J = 8.2 Hz, 1H), 7.37 (t, J = 6.9 Hz, 1H), 6.97 (d, J = 8.5 Hz, 1H), 4.06 (t, J = 6.4 Hz, 2H), 3.95 (s, 6H), 2.45 (t, J = 7.5 Hz, 2H), 2.36 (br.s, 3H), 1.93 (quin, J = 6.6 Hz, 2H), 1.55 (quin, J = 5.7 Hz, 4H), 1.43–1.36 (m, 2H). 13C NMR (75 MHz, CDCl3) δ 175.1, 155.9, 155.2, 151.2, 148.7, 140.1, 133.4, 125.8, 124.7, 124.2, 123.6, 122.5, 118.0, 111.7, 110.8, 71.3, 56.2, 56.1, 54.6, 27.6, 25.8, 24.3. HR-MS (ESI) m/z: calcd for C25H29NO5 [M+H]+: 424.2124; found 424.2119. HPLC purity 99.0% (30 min run of 45–80% CH3CN in aqueous solution of ammonium formate (1.26 g ammonium formate in 1000 mL water), with 15 min gradient, 1.0 mL/min).
4.3.11. 3-O-Piperidinobutyl-3′,4′-dimethoxyflavonol (52)
This derivative was achieved in 84% overall yield for two steps as a syrup. IR (film) νmax: 2931, 1634, 1614, 1599, 1558, 1511 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.24 (dd, J = 8.0, 1.4 Hz, 1H), 7.74 (overlapped, 2H), 7.66 (ddd, J = 8.6, 7.1, 1.7 Hz, 1H), 7.52 (dd, J = 8.4, 0.5 Hz, 1H), 7.38 (ddd, J = 8.1, 7.1, 1.1 Hz, 1H), 6.99 (d, J = 8.4 Hz, 1H), 4.04 (t, J = 6.4 Hz, 2H), 3.963 (s, 3H), 3.957 (s, 3H), 2.302.45 (overlapped, 6H), 1.78–1.56 (overlapped, 8H), 1.48– 1.37 (m, 2H). 13C NMR (75 MHz, CDCl3) δ175.1, 155.9, 155.3, 151.3, 148.7, 140.1, 133.4, 125.9, 124.7, 124.3, 123.6, 122.5, 118.0, 111.7, 110.9, 72.2, 58.5, 56.2, 56.1, 54.2, 28.2, 25.1, 23.9, 22.6. HR-MS (ESI) m/z: calcd for C26H31NO5 [M+H]+: 438.2280; found 438.2272. HPLC purity 95.2% (30 min run of 45–80% CH3CN in aqueous solution of ammonium formate (1.26 g ammonium formate in 1000 mL water), with 15 min gradient, 1.0 mL/min).
4.3.12. 3-O-Piperidinopentyl-3′,4′-dimethoxyflavonol (53)
This derivative was achieved in 91% overall yield for two steps as a syrup. IR (film) νmax: 2931, 2852, 2763, 1635, 1614, 1599, 1560, 1510 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.23 (dd, J = 8.0, 1.4 Hz, 1H), 7.44 (dd, J = 7.2, 1.8 Hz, 1H), 7.73 (s, 1H), 7.65 (ddd, J = 8.4, 7.2, 1.5 Hz, 1H), 7.51 (d, J = 8.2 Hz, 1H), 7.38 (t, J = 7.2 Hz, 1H), 6.99 (d, J = 9.1 Hz, 1H), 4.01 (t, J = 6.6 Hz, 2H), 3.96 (s, 3H), 3.95 (s, 3H), 2.42 (s, 4H), 2.31 (t, J = 7.8 Hz, 2H), 1.74 (quin, J = 7.0 Hz, 2H), 1.61 (quin, J = 5.4 Hz, 4H), 1.56 – 1.47 (m, 2H), 1.44–1.33 (overlapped, 4H). 13C NMR (75 MHz, CDCl3) δ 175.1, 155.8, 155.3, 151.2, 148.7, 140.2, 133.4, 125.9, 124.7, 124.3, 123.7, 122.4, 118.0, 111.8, 110.8, 72.6, 59.1, 56.2, 56.1, 54.4, 30.2, 26.1, 25.5, 24.2, 24.1. HR-MS (ESI) m/z: calcd for C27H33NO5 [M+H]+: 452.2437; found 452.2428. HPLC purity 96.0% (30 min run of 45–80% CH3CN in aqueous solution of ammonium formate (1.26 g ammonium formate in 1000 mL water), with 15 min gradient, 1.0 mL/min).
4.3.13. 3-O-(4-Methylpiperazin-1-yl)propyl-3′,4′-dimethoxyflavonol (54)
This derivative was achieved in 77% overall yield for two steps as a syrup. IR (film) νmax: 2931, 2852, 2763, 1635, 1614, 1599, 1560, 1510 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.20 (dd, J = 8.0, 1.4 Hz, 1H), 7.70 (dd, J = 8.7, 1.8 Hz,1H), 7.65 (d, J = 1.5 Hz, 1H), 7.62 (ddd, J = 8.4, 7.2, 2.1 Hz, 1H), 7.48 (d, J = 8.3 Hz, 1H), 7.34 (t, J = 7.5 Hz, 1H), 6.95 (d, J = 8.5 Hz, 1H), 4.05 (t, J = 6.4 Hz, 2H), 3.92 (s, 6H), 2.69 – 2.27 (overlapped, 10H), 2.23 (s, 3H), 1.88 (quin, J = 6.0 Hz, 2H). 13C NMR (75 MHz, CDCl3) δ 175.0, 155.8, 155.2, 151.2, 148.7, 140.0, 133.3, 125.8, 124.7, 124.2, 123.6, 122.4, 117.9, 111.8, 110.8, 71.1, 56.1, 56.0, 55.2, 55.0, 53.0, 46.0, 27.7. HR-MS (ESI) m/z: calcd for C25H30N2O5 [M+H]+: 439.2233; found 439.2218. HPLC purity 98.8% (30 min run of 45–80% CH3CN in aqueous solution of ammonium formate (1.26 g ammonium formate in 1000 mL water), with 15 min gradient, 1.0 mL/min).
4.3.14. 3-O-(4-Methylpiperazin-1-yl)butyl-3′,4′-dimethoxyflavonol (55)
This derivative was achieved in 80% overall yield for two steps as a syrup. IR (film) νmax: 2931, 2852, 2763, 1635, 1614, 1599, 1560, 1510 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.21 (dd, J = 8.0, 1.4 Hz, 1H), 7.72 (dd, J = 8.1, 1.8 Hz,1H), 7.70 (s,1H), 7.63 (ddd, J = 8.6, 7.1, 1.6 Hz, 1H), 7.49 (d, J = 8.0 Hz, 1H), 7.35 (ddd,7.70 (dd, J = 8.1, 6.9, 1.2 Hz,1H), 6.96 (d, J = 8.5 Hz, 1H), 4.02 (t, J = 6.5 Hz, 2H), 3.94(s, 3H), 3.93 (s, 3H), 2.91 (br.s, 2H), 2.41 (br.s, 6H), 2.29 (t, J = 7.5 Hz,2H), 2.24 (s, 3H), 1.71 (quin, J = 6.9 Hz, 2H), 1.64 – 1.49 (m, 2H). 13C NMR (75 MHz, CDCl3) δ 175.1, 155.8, 155.2, 151.2, 148.6, 140.1, 133.3, 125.8, 124.7, 124.2, 123.6, 122.4, 117.9, 111.8, 110.8, 72.5, 58.2, 56.1, 56.0, 55.0, 53.0, 46.0, 28.2, 23.3. HR-MS (ESI) m/z: calcd for C26H32N2O5 [M+H]+: 453.2389; found 453.2383. HPLC purity 95.5% (30 min run of 15–75% CH3CN in aqueous solution of ammonium formate (1.26 g ammonium formate in 1000 mL water), with 15 min gradient, 1.0 mL/min).
4.3.15. 3-O-(4-Methylpiperazin-1-yl)pentyl-3′,4′-dimethoxyflavonol (56)
This derivative was achieved in 63% overall yield for two steps as a syrup. IR (film) νmax: 2931, 2852, 2763, 1635, 1614, 1599, 1560, 1510 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.21 (d, J = 8.0 Hz, 1H), 7.73–7.71 (overlapped, 2H), 7.67 – 7.58 (m, 1H), 7.49 (d, J = 8.3 Hz, 1H), 7.35 (t, J = 7.5 Hz, 1H), 6.96 (dd, J = 9.0, 3.6 Hz, 1H), 4.00 (t, J = 6.6 Hz, 2H), 3.94 (s, 3H), 3.93 (s, 3H), 2.44 (br.s, 7H), 2.32 – 2.11 (overlapped, 3H), 2.25 (s, 3 H), 1.72 (quin, J = 7.2 Hz, 2H), 1.53 – 1.29 (m, 4H). 13C NMR (75 MHz, CDCl3) δ 175.1, 155.7, 155.2, 151.2, 148.6, 140.2, 133.3, 125.9, 124.6, 124.3, 123.7, 122.3, 118.0, 111.8, 110.8, 72.6, 58.5, 56.1, 56.1, 55.0, 53.1, 46.0, 30.2, 26.6, 24.0. HR-MS (ESI) m/z: calcd for C27H34N2O5 [M+H]+: 467.2546; found 467.2546. HPLC purity 98.4% (30 min run of 45–80% CH3CN in aqueous solution of ammonium formate (1.26 g ammonium formate in 1000 mL water), with 15 min gradient, 1.0 mL/min).
4.3.16. 3-O-(4-Methylpiperazin-1-yl)hexyl-3′,4′-dimethoxyflavonol (57)
This derivative was achieved in 58% overall yield for two steps as a syrup. IR (film) νmax: 2932, 2792, 1636, 1599, 1559, 1511 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.23 (dd, J = 8.0, 1.4 Hz, 1H), 7.767.74 (overlapped, 2H), 7.65 (ddd, J = 8.7, 7.2, 1.5 Hz, 1H), 7.51 (d, J = 8.3 Hz, 1H), 7.37 (t, J = 7.5 Hz, 1H), 6.98 (d, J = 9.1 Hz, 1H), 4.01 (t, J = 6.7 Hz, 2H), 3.96 (s, 3H), 3.94 (s, 3H), 2.81 (br.s, 2H), 2.48 (br.s, 6H), 2.36 – 2.18 (overlapped, 2H), 2.28 (s, 3H), 1.72 (quin, J = 7.5 Hz, 2H), 1.52 – 1.32 (m, 4H), 1.27 (m, 2H). 13C NMR (75 MHz, CDCl3) δ 175.1, 155.7, 155.2, 151.2, 148.6, 140.2, 133.3, 125.9, 124.7, 124.3, 123.7, 122.3, 118.0, 111.9, 110.8, 72.7, 58.6, 56.1, 56.1, 55.0, 53.1, 46.0, 30.3, 27.4, 26.8, 26.0. HR-MS (ESI) m/z: calcd for C28H36N2O5 [M+H]+: 481.2702; found 481.2694. HPLC purity 95.6% (30 min run of 45–80% CH3CN in aqueous solution of ammonium formate (1.26 g ammonium formate in 1000 mL water), with 15 min gradient, 1.0 mL/min).
4.3.17. 3-O-(4-Methylpiperazin-1-yl)heptyl-3′,4′-dimethoxyflavonol (58)
This derivative was achieved in 62% overall yield for two steps as a syrup. IR (film) νmax: 2931, 2854, 2792, 1636, 1614, 1599, 1559, 1511 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.24 (dd, J = 8.0, 1.4 Hz, 1H), 7.75–7.72 (overlapped, 2H), 7.65 (ddd, J = 8.6, 7.1, 1.6 Hz, 1H), 7.51 (d, J = 8.1 Hz, 1H), 7.38 (ddd, J = 7.8, 7.2, 0.9 Hz, 1H), 6.98 (d, J = 9.1 Hz, 1H), 4.01 (t, J = 6.8 Hz, 2H), 3.96 (s, 3H), 3.95 (s, 3H), 2.96 (br.s, 2H), 2.52 (br.s, 6H), 2.36–2.30 (overlapped, 2H), 2.30 (s, 3H), 1.71 (quin, J = 7.5 Hz, 2H), 1.51–1.46 (m, 2H), 1.37–1.32 (overlapped, 2H), 1.30–1.24 (overlapped, 4H). 13C NMR (75 MHz, CDCl3) δ 175.2, 155.8, 155.3, 151.2, 148.6, 140.3, 133.3, 125.9, 124.7, 124.3, 123.7, 122.3, 118.0, 111.9, 110.8, 72.8, 58.6, 56.1, 56.1, 54.8, 52.9, 45.9, 30.3, 29.4, 27.6, 26.7, 26.0. HR-MS (ESI) m/z: calcd for C29H38N2O5 [M+H]+: 495.2859; found 495.2848. HPLC purity 96.4% (30 min run of 45–80% CH3CN in aqueous solution of ammonium formate (1.26 g ammonium formate in 1000 mL water), with 15 min gradient, 1.0 mL/min).
4.3.18. 3-O-Pyrrolidinopropyl-3′,4′-dimethoxyflavonol (59)
This derivative was achieved in 94% overall yield for two steps as a syrup. IR (film) νmax: 2956, 2823, 2789, 1611, 1597, 1558, 1512 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.22 (dd, J = 8.0, 1.4 Hz, 1H), 7.74 (dd, J = 8.4,2.1 Hz, 1H), 7.70 (d, J = 1.8 Hz, 1H), 7.64 (ddd, J = 8.6, 7.1, 1.6 Hz, 1H), 7.51 (d, J = 8.0 Hz, 1H), 7.37 (ddd, J = 8.1, 6.9, 0.9 Hz, 1H), 6.97 (d, J = 8.5 Hz, 1H), 4.08 (t, J = 6.5 Hz, 2H), 3.95 (s, 6H), 2.58 (t, J = 7.5 Hz, 2H), 2.47 (br.s, 4H), 1.95 (quin, J = 7.2 Hz, 2H), 1.74 (quin, J = 6.6 Hz, 4H). 13C NMR (75 MHz, CDCl3) δ 175.1, 155.9, 155.2, 151.2, 148.7, 140.1, 133.4, 125.9, 124.7, 124.3, 123.6, 122.4, 118.0, 111.8, 110.8, 71.2, 56.2, 56.1, 54.2, 53.3, 29.7, 23.5. HR-MS (ESI) m/z: calcd for C24H27NO5 [M+H]+: 410.1967; found 410.1964. HPLC purity 97.7% (30 min run of 45–80% CH3CN in aqueous solution of ammonium formate (1.26 g ammonium formate in 1000 mL water), with 15 min gradient, 1.0 mL/min).
4.3.19. 3-O-Pyrrolidinobutyl-3′,4′-dimethoxyflavonol (60)
This derivative was achieved in 42% overall yield for two steps as a syrup. IR (film) νmax: 2931, 1633, 1614, 1598, 1558, 1511 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.22 (dd, J = 8.0, 1.4 Hz, 1H), 7.75 (dd, J = 8.5, 2.1 Hz, 1H), 7.71(d, J = 2.1 Hz, 1H), 7.66 (ddd, J = 8.4, 7.2, 1.8 Hz, 1H), 7.52 (d, J = 8.0 Hz, 1H), 7.38 (ddd, J = 7.8, 7.2, 0.9 Hz, 1H), 4.02 (t, J = 5.9 Hz, 2H), 3.96 (s, 3H), 3.95 (s, 3H), 3.503.15 (br.s, 1H), 2.75–2.64 (overlapped, 5H), 1.91 – 1.81 (m, 4H), 1.81 – 1.51 (m, 4H). 13C NMR (75 MHz, CDCl3) δ 175.1, 155.9, 155.3, 151.3, 148.8, 140.2, 133.4, 125.9, 124.7, 124.3, 123.7, 122.5, 118.0, 111.8, 110.9, 72.3, 56.2, 56.1, 56.0, 54.1, 28.3, 25.0, 23.5. HR-MS (ESI) m/z: calcd for C25H29NO5 [M+H]+: 424.2124; found 424.2115. HPLC purity 95.0% (30 min run of 15–75% CH3CN in aqueous solution of ammonium formate (1.26 g ammonium formate in 1000 mL water), with 15 min gradient, 1.0 mL/min).
4.3.20. 3-O-Pyrrolidinopentyl-3′,4′-dimethoxyflavonol (61)
This derivative was achieved in 93% overall yield for two steps as a syrup. IR (film) νmax: 2933, 2872, 2785, 1635, 1614, 1599, 1559, 1510 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.18 (dd, J = 8.0, 1.4 Hz, 1H), 7.72 – 7.66 (overlapped, 2H), 7.59 (ddd, J = 8.5, 7.1, 1.6 Hz, 1H), 7.46 (d, J = 8.3 Hz, 1H), 7.32 (t, J = 7.5 Hz, 1H), 6.93 (d, J = 9.1 Hz, 1H), 3.98 (t, J = 6.7 Hz, 2H), 3.91 (s, 6H), 2.41 (t, J = 5.3 Hz, 4H), 2.34(t, J = 7.2 Hz, 2H), 1.77 – 1.62 (m, 6H), 1.53 – 1.29 (m, 4H). 13C NMR (75 MHz, CDCl3) δ 175.0, 155.6, 155.1, 151.1, 148.5, 140.1, 133.2, 125.7, 124.5, 124.2, 123.6, 122.2, 117.9, 111.7, 110.7, 72.5, 56.4, 56.0, 55.9, 54.1, 30.1, 28.7, 24.0, 23.4. HR-MS (ESI) m/z: calcd for C26H32NO5 [M+H]+: 438.2281; found 438.2276. HPLC purity 96.3% (30 min run of 45–80% CH3CN in aqueous solution of ammonium formate (1.26 g ammonium formate in 1000 mL water), with 15 min gradient, 1.0 mL/min).
4.3.21. 3-O-Pyrrolidinohexyl-3′,4′-dimethoxyflavonol (62)
This derivative was achieved in 75% overall yield for two steps as a syrup. IR (film) νmax: 2931, 1634, 1599, 1558, 1510 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.24 (dd, J = 8.0, 1.3 Hz, 1H), 7.75 (dd, J = 6.6, 2.1 Hz,1H), 7.74 (s, 1H),7.66 (ddd, J = 8.7, 6.9, 1.5 Hz, 1H), 7.52 (d, J = 8.3 Hz, 1H), 7.38 (t, J = 7.5 Hz, 1H), 6.99 (d, J = 9.1 Hz, 1H), 4.02 (t, J = 6.7 Hz, 2H), 3.96 (s, 3H), 3.95 (s, 3H), 2.58 (br.s, 4H), 2.47 (t, J = 8.1 Hz, 2H), 1.81 (br.s, 4H), 1.72 (quin, J = 6.9 Hz, 2H), 1.53 (quin, J = 7.5 Hz, 2H), 1.46 – 1.36 (m, 2H), 1.36 – 1.25 (m, 2H). 13C NMR (75 MHz, CDCl3) δ 175.2, 155.8, 155.3, 151.2, 148.7, 140.2, 133.4, 125.9, 124.7, 124.3, 123.7, 122.4, 118.0, 111.8, 110.8, 72.7, 56.5, 56.2, 56.1, 54.2, 30.3, 28.5, 27.5, 25.9, 23.5. HR-MS (ESI) m/z: calcd for C27H33NO5 [M+H]+: 452.2437; found 452.2424. HPLC purity 95.8% (30 min run of 45–80% CH3CN in aqueous solution of ammonium formate (1.26 g ammonium formate in 1000 mL water), with 15 min gradient, 1.0 mL/min).
4.3.22. 3-O-Pyrrolidinoheptyl-3′,4′-dimethoxyflavonol (63).
This derivative was achieved in 68% overall yield for two steps as a syrup. IR (film) νmax: 2931, 2854, 2792, 1636, 1614, 1599, 1559, 1511 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.24 (dd, J = 8.0, 1.4 Hz, 1H), 7.75–7.72 (overlapped, 2H), 7.65 (ddd, J = 8.6, 7.1, 1.6 Hz, 1H), 7.51 (d, J = 8.1 Hz, 1H), 7.38 (ddd, J = 7.8, 7.2, 0.9 Hz, 1H), 6.98 (d, J = 9.1 Hz, 1H), 4.01 (t, J = 6.8 Hz, 2H), 3.96 (s, 3H), 3.95 (s, 3H), 2.96 (br.s, 2H), 2.52 (br.s, 6H), 2.36–2.30 (overlapped, 2H), 2.30 (s, 3H), 1.71 (quin, J = 7.5 Hz, 2H), 1.51–1.46 (m, 2H), 1.37–1.32 (overlapped, 2H), 1.30–1.24 (overlapped, 4H). 13C NMR (75 MHz, CDCl3) δ 175.2, 155.8, 155.3, 151.2, 148.6, 140.3, 133.3, 125.9, 124.7, 124.3, 123.7, 122.3, 118.0, 111.9, 110.8, 72.8, 58.6, 56.1, 56.1, 54.8, 52.9, 45.9, 30.3, 29.4, 27.6, 26.7, 26.0. HR-MS (ESI) m/z: calcd for C28H35NO5 [M+H]+: 466.2593; found 466.2583. HPLC purity 95.9% (30 min run of 45–80% CH3CN in aqueous solution of ammonium formate (1.26 g ammonium formate in 1000 mL water), with 15 min gradient, 1.0 mL/min).
4.4. The general procedure for the synthesis of 3-O-alkyl-3′,4′,7-trimethoxyflavonols (21–30).
To the solution of 3′,4′,7-trimethoxyflavonol (50 mg, 0.15 mmol) in DMF (0.5 mL) was added potassium carbonate (62 mg, 0.45 mmol, 3.0 equiv.) was stirred for 10 min at R.T.. To this solution was added an appropriate alkyl iodide or alkyl bromide (0.45 mmol, 3.0 equiv.) through syringe, the reaction was kept at room temperature over 12 h until the starting material was consumed as detected by TLC (dichloromethane/methanol, 100/3, v/v). The mixture was poured into ice water (3.0 mL), and the solution was extracted with ethyl acetate three times. The combined extracts were sequentially rinsed with saturated sodium bicarbonate and brine, dried over anhydrous sodium sulfate, and concentrated in vacuo to give a crude mass, which was purified by PTLC eluting with dichloromethane/methanol (100/3, v/v) to furnish the respective 3-O-alkyl-3′,4′,7-trimethoxyflavonol. HPLC purity for 5 is 95.5% (30 min run of 45–80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
4.4.1. 3-O-Methyl-3′,4′,7-trimethoxyflavonol (21)
This derivative was achieved in 95% yield as a yellow solid. m.p. 148–150oC. IR (film) νmax: 2930, 2827, 1633, 1618, 1598, 1565, 1502 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.12 (d, J = 8.9 Hz, 1H), 7.71 (dd, J = 8.1, 1.8 Hz, 1H), 7.70 (s, 1H), 6.97 (d, J = 8.4 Hz, 1H), 6.94 (dd, J = 9.0, 2.1 Hz, 1H), 6.88 (d, J = 2.2 Hz, 1H), 3.95 (s, 6H), 3.90 (s, 3H), 3.86 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 174.5, 164.0, 157.0, 155.1, 151.1, 148.8, 140.8, 127.2, 123.6, 122.0, 118.1, 114.3, 111.4, 110.9, 100.0, 60.1, 56.1, 56.1, 55.9. HR-MS (ESI) m/z: calcd for C19H18O6 [M+H]+: 343.1181; found 343.1175. HPLC purity 96.2% (30 min run of 45–80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
4.4.2. 3-O-Ethyl-3′,4′,7-trimethoxyflavonol (22)
This derivative was achieved in 95% yield as a yellow solid. m.p. 115–117oC. IR (film) νmax: 2971, 2923, 2839, 1616, 1597, 1558, 1512 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.11 (d, J = 8.8 Hz, 1H), 7.76 (s, 1H), 7.71 (d, J = 8.6 Hz, 1H), 6.96 (d, J = 9.3 Hz, 1H), 6.93 (d, J = 9.0 Hz, 1H), 6.87 (s, 1H), 4.09 (q, J = 6.9 Hz, 2H), 3.94 (s, 6H), 3.89 (s, 3H), 1.32 (t, J = 6.9 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 174.6, 164.0, 156.9, 155.2, 151.0, 148.6, 139.8, 127.2, 123.8, 121.9, 118.1, 114.3, 111.7, 110.8, 100.0, 68.4, 56.1, 56.0, 55.9, 15.8. HR-MS (ESI) m/z: calcd for C20H20O6 [M+H]+: 357.1338; found 357.1338. HPLC purity 97.7% (30 min run of 45–80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
4.4.3. 3-O-Propyl-3′,4′,7-trimethoxyflavonol (23)
This derivative was achieved in 82% yield as a yellow solid. m.p. 104–106 oC. IR (film) νmax: 2966, 2936, 2842, 1633, 1602, 1515, 1503 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.11 (d, J = 8.9 Hz, 1H), 7.73 (d, J = 1.8 Hz, 1H), 7.70 (dd, J = 8.4, 1.8 Hz, 1H), 6.96 (d, J = 8.4 Hz, 1H), 6.93 (dd, J = 9.0, 2.1 Hz, 1H), 6.87 (d, J = 2.2 Hz, 1H), 3.98 (overlapped, J = 6.9 Hz, 2H), 3.94 (s, 6H), 3.89 (s, 3H), 1.73 (sextet, J = 7.2 Hz, 2H), 0.92 (t, J = 7.4 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 174.6, 164.0, 156.9, 155.2, 151.0, 148.6, 140.0, 127.1, 123.8, 122.0, 118.2, 114.3, 111.7, 110.7, 100.0, 74.4, 56.1, 56.0, 55.9, 23.6, 10.5. HR-MS (ESI) m/z: calcd for C21H22O6 [M+H]+: 371.1494; found 371.1486. HPLC purity 97.8% (30 min run of 45–80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
4.4.4. 3-O-Butyl-3′,4′,7-trimethoxyflavonol (24)
This derivative was achieved in 59% yield as a yellow solid. m.p. 81–83oC. IR (film) νmax: 2953, 2930, 2869, 2833, 1637, 1622, 1601, 1567, 1515, 1505 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.13 (d, J = 8.9 Hz, 1H), 7.74 (d, J = 1.8 Hz, 1H), 7.71 (dd, J = 8.4, 2.1 Hz, 1H), 6.97 (d, J = 8.7 Hz, 1H), 6.94 (dd, J 9.6, 2.4 Hz, 1H), 6.89 (d, J = 2.2 Hz, 1H), 4.02 (t, J = 6.8 Hz, 2H), 3.96 (s, 6H), 3.90 (s, 3H), 1.70 (quin, J = 7.1 Hz, 2H), 1.40 (sextet, J = 7.4 Hz, 2H), 0.88 (t, J = 7.4 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 174.7, 164.0, 157.0, 155.3, 151.0, 148.6, 140.1, 127.2, 123.9, 122.1, 118.2, 114.3, 111.8, 110.8, 100.0, 72.6, 56.1, 56.1, 55.9, 32.4, 19.2, 13.9. HR-MS (ESI) m/z: calcd for C22H24O6 [M+H]+: 385.1651; found 385.1641. HPLC purity 96.6% (30 min run of 45–80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
4.4.5. 3-O-Pentyl-3′,4′,7-trimethoxyflavonol (25)
This derivative was achieved in 58% yield as a yellow solid. m.p. 79–81 oC. IR (film) νmax: 2957, 2930, 2861, 2834, 1636, 1621, 1598, 1566, 1515, 1505 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.13 (d, J = 8.9 Hz, 1H), 7.74 (d, J = 1.8 Hz, 1H), 7.71 (dd, J = 8.4, 2.1 Hz, 1H), 6.98 (d, J = 8.7 Hz, 1H), 6.95 (dd, J = 9.0, 2.1 Hz, 1H), 6.89 (d, J = 2.2 Hz, 1H), 4.01 (t, J = 6.8 Hz, 2H), 3.96 (s, 6H), 3.91 (s, 3H), 1.72 (quin, J = 6.9 Hz, 2H),1.40 – 1.22 (m, 4H), 0.85 (t, J = 7.0 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 174.7, 164.0, 157.0, 155.3, 151.0, 148.6, 140.1, 127.2, 123.9, 122.1, 118.2, 114.3, 111.8, 110.8, 100.0, 72.9, 56.14, 56.08, 56.0, 30.1, 28.2, 22.6, 14.1. HR-MS (ESI) m/z: calcd for C23H26O6 [M+H]+: 399.1807; found 399.1799. HPLC purity 98.1% (30 min run of 45–80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
4.4.6. 3-O-Hexyl-3′,4′,7-trimethoxyflavonol (26)
This derivative was achieved in 72% yield as a yellow solid. m.p. 57–59oC. IR (film) νmax: 2957, 2929, 2862, 1635, 1621, 1600, 1567, 1515, 1505 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.12 (d, J = 8.9 Hz, 1H), 7.72 (d, J = 2.1 Hz, 1H), 7.70 (dd, J = 8.4, 2.1 Hz, 1H), 6.96 (d, J = 8.4 Hz, 1H), 6.94 (dd, J = 8.7, 2.1 Hz, 1H), 6.88 (d, J = 2.2 Hz, 1H), 4.01 (t, J = 6.8 Hz, 2H), 3.95 (s, 6H), 3.90 (s, 3H), 1.70 (quin, J = 7.1 Hz, 2H), 1.42 – 1.28 (m, 2H), 1.23 (overlapped, 4H), 0.83 (t, J = 6.8 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 174.6, 164.0, 157.0, 155.3, 151.0, 148.6, 140.0, 127.2, 123.8, 122.1, 118.2, 114.3, 111.8, 110.7, 100.0, 72.9, 56.1, 56.0, 55.9, 31.7, 30.3, 25.7, 22.7, 14.1. HR-MS (ESI) m/z: calcd for C24H28O6 [M+H]+: 413.1964; found 413.1955. HPLC purity 98.9% (30 min run of 45–80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
4.4.7. 3-O-Heptyl-3′,4′,7-trimethoxyflavonol (27)
This derivative was achieved in 50% yield as a syrup. IR (film) νmax: 2928, 2855, 1619, 1598, 1512 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.13 (d, J = 8.9 Hz, 1H), 7.73 (d, J = 2.1 Hz, 1H), 7.71 (dd, J = 9.3, 1.8 Hz, 1H), 6.97 (d, J = 8.1 Hz, 1H), 6.95 (dd, J = 9.0, 2.1 Hz, 1H), 6.89 (d, J = 2.2 Hz, 1H), 4.02 (t, J = 6.8 Hz, 2H), 3.96 (s, 6H), 3.91 (s, 3H), 1.71 (quin, J = 7.0 Hz, 2H), 1.40 – 1.29 (m, 2H), 1.24 (overlapped, 6H), 0.85 (t, J = 6.7 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 174.7, 164.0, 157.0, 155.3, 151.0, 148.6, 140.1, 127.3, 123.9, 122.1, 118.2, 114.3, 111.8, 110.7, 100.1, 73.0, 56.2, 56.1, 56.0, 31.9, 30.4, 29.2, 26.1, 22.7, 14.2. HR-MS (ESI) m/z: calcd for C25H30O6 [M+H]+: 427.2120; found 427.2111. HPLC purity 95.1% (30 min run of 45–80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
4.4.8. 3-O-Isopropyl-3′,4′,7-trimethoxyflavonol (28)
This derivative was achieved in 43% yield as a syrup. IR (film) νmax: 2929, 2838, 1617, 1597, 1511 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.13 (d, J = 8.9 Hz, 1H), 7.82 (d, J = 2.0 Hz, 1H), 7.73 (dd, J = 8.5, 2.0 Hz, 1H), 6.97 (d, J =8.4 Hz, 1H), 6.95 (dd, J = 9.0, 2.1, 1H), 6.90 (d, J = 2.3 Hz, 1H), 4.70 (septet, J = 6.2 Hz, 1H), 3.96 (s, 6H), 3.91 (s, 3H), 1.19 (d, J = 6.2 Hz, 6H). 13C NMR (75 MHz, CDCl3) δ 175.0, 164.0, 157.0, 155.8, 150.8, 148.4, 138.5, 127.2, 124.3, 122.1, 118.1, 114.3, 112.3, 110.6, 100.0, 74.6, 56.1, 56.0, 56.0, 22.6. HR-MS (ESI) m/z: calcd for C21H22O6 [M+H] +: 371.1494; found 371.1485. HPLC purity 97.1% (30 min run of 45–80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
4.4.9. 3-O-sec-Butyl-3′,4′,7-trimethoxyflavonol (29)
This derivative was achieved in 34% yield as a syrup. IR (film) νmax: 2964, 2930, 2839, 1615, 1597, 1511 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.13 (d, J = 8.9 Hz, 1H), 7.82 (d, J = 2.0 Hz, 1H), 7.73 (dd, J = 8.5, 2.0 Hz, 1H), 6.99 (d, J = 8.4 Hz, 1H), 6.97 (dd, J = 8.7, 2.1 Hz, 1H), 6.90 (d, J = 2.3 Hz, 1H), 4.70 (sextet, J = 6.2 Hz, 1H), 3.96 (s, 6H), 3.91 (s, 3H), 1.79–1.65 (m, 1H), 1.57–1.43 (m, 1H), 1.10 (d, J = 6.3 Hz, 3H), 0.90 (t, J = 7.5 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 175.0, 164.0, 157.0, 155.8, 150.8, 148.4, 138.6, 127.3, 124.3, 122.2, 118.1, 114.3, 112.4, 110.6, 100.0, 79.3, 56.1, 56.07, 55.98, 29.7, 19.4, 9.9. HR-MS (ESI) m/z: calcd for C22H24O6 [M+H] +: 385.1651; found 385.1641. HPLC purity 98.0% (30 min run of 45–80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
4.4.10. 3-O-(Pentan-2-yl)-3′,4′,7-trimethoxyflavonol (30)
This derivative was achieved in 11% yield as a syrup. IR (film) νmax: 2957, 2930, 2838, 1616, 1597, 1510 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.13 (d, J = 8.9 Hz, 1H), 7.81 (d, J = 2.0 Hz, 1H), 7.72 (dd, J = 8.5, 2.1 Hz, 1H), 6.97 (d, J = 8.7 Hz, 1H), 6.96 (dd, J = 9.0, 2.1 Hz, 1H), 6.90 (d, J = 2.3 Hz, 1H), 4.65 (sextet, J = 6.2 Hz, 1H), 3.96 (s, 6H), 3.92 (s, 3H),1.75 – 1.63 (m, 1H), 1.49 – 1.35 (m, 3H), 1.08 (d, J = 6.2 Hz, 3H), 0.87 (t, J = 7.2 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 174.9, 164.0, 157.0, 155.7, 150.8, 148.4, 138.5, 127.3, 124.4, 122.2, 118.2, 114.3, 112.4, 110.6, 100.0, 77.8, 56.1, 56.1, 56.0, 39.2, 19.9, 18.9, 14.3. HR-MS (ESI) m/z: calcd for C23H26O6 [M+H]+: 399.1807; found 399.1800. HPLC purity 95.0% (30 min run of 45–80% CH3CN in H2O, with 15 min gradient, 1.0 mL/min).
4.5. The general procedure for the synthesis of 3-O-aminoalkyl-3′,4′,7-trimethoxyflavonols (67–81).
The solution of 3′,4′,7-trimethoxyflavonol (5, 50 mg, 0.15 mmol and potassium carbonate (62 mg, 0.45 mmol, 3 equiv.) in DMF (0.5 mL) was stirred at room temperature for 10 min before an appropriate alkyl dibromide (0.45 mmol, 3 equiv.) was added through a syringe. The reaction was allowed to proceed at room temperature over 12 h until the starting material was fully consumed as indicated by TLC (dichloromethane/methanol, 100/3, v/v). The reaction mixture was poured into ice water (3.0 mL), and the subsequent mixture was extracted with ethyl acetate three times. The combined organic extracts were sequentially rinsed with saturated sodium bicarbonate and brine, dried over anhydrous sodium sulfate, and concentrated in vacuo to generate a crude product. To the solution of this crude product in DMF (0.5 ml) was added potassium carbonate (3 equiv.), the mixture was stirred for 10 min. The appropriate amine (0.45 mmol, 3 equiv.) was added to the reaction mixture through a syringe, and the subsequent reaction mixture was stirred at room temperature over 12 h until no starting material was detected by TLC (dichloromethane/methanol, 100/3, v/v). The mixture was poured into ice water (3.0 mL), and the mixture was extracted with ethyl acetate three times. The combined extracts were sequentially rinsed with saturated sodium bicarbonate and brine, dried over anhydrous sodium sulfate, and concentrated in vacuo to give a crude product, which was subjected to PTLC purification using dichloromethane/methanol (100/3, v/v) as eluent. The respective 3-O-aminoalkyl-3′,4′,7-trimethoxyflavonol was retrieved from PTLC silica gel by washing with dichloromethane/diethylamine (100/3, v/v).
4.5.1. 3-O-(N,N-Dibutylamino)propyl-3′,4′,7-trimethoxyflavonol (67)
This derivative was achieved in 33% overall yield for two steps as a syrup. IR (film) νmax: 2929, 2838, 1617, 1597,1511 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.12 (d, J = 6.8 Hz, 1H), 7.71 (d, J = 9.2 Hz, 1H), 7.69 (s, 1H), 6.97 (d, J = 7.8 Hz, 1H), 6.95 (dd, J = 7.8, 1.5 Hz, 1H), 6.95 (dd, J = 11.0, 4.7 Hz, 2H), 6.89 (d, J = 1.6 Hz, 1H), 4.06 (t, J = 6.4 Hz, 2H), 3.95 (s, 6H), 3.91 (s, 3H), 2.55 (t, J = 7.1 Hz, 2H), 2.37 (t, J = 7.3 Hz, 4H), 1.88 (quin, J = 6.8 Hz, 2H), 1.36 (quin, J = 7.8 Hz, 4H), 1.23 (sextet, J = 6.9 Hz, 4H), 0.86 (t, J = 7.1 Hz, 6H). 13C NMR (75 MHz, CDCl3) δ 174.6, 164.0, 157.0, 155.4, 151.1, 148.7, 139.9, 127.2, 123.8, 122.2, 118.2, 114.4, 111.7, 110.8, 100.0, 71.4, 56.2, 56.1, 56.0, 53.8, 51.1, 28.9, 27.9, 20.8, 14.1. HR-MS (ESI) m/z: calcd for C29H39NO6 [M+H]+: 498.2855; found 498.2845. HPLC purity 96.5% [30 min run of 15–75% and 75–85% acetonitrile in ammonium formate solution (1.26 g ammonium formate in 1000 mL water), with 15 min and 10 min gradient, respectively, 1.0 mL/min].
4.5.2. 3-O-(N,N-Dibutylamino)butyl-3′,4′,7-trimethoxyflavonol (68)
This derivative was achieved in 38% overall yield for two steps as a syrup. IR (film) νmax: 2931, 2861, 1618, 1598, 1512 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.13 (d, J = 8.9 Hz, 1H), 7.73 (br.s, 2H), 6.98(d, J = 8.4 Hz, 1H), 6.95 (d, J = 8.4 Hz, 1H), 6.90 (s, 1H), 4.03 (t, J = 6.7 Hz, 2H), 3.96 (s, 6H), 3.92 (s, 3H), 2.37 (t, J = 6.6 Hz, 6H), 1.73 (quin, J = 7.0 Hz, 2H), 1.58–1.45 (m, 2H), 1.45 – 1.30 (m, 4H), 1.26 (sextet, J = 7.2 Hz, 4H), 0.88 (t, J = 7.1 Hz, 6H). 13C NMR (75 MHz, CDCl3) δ 174.6, 164.0, 157.0, 155.3, 151.0, 148.7, 140.0, 127.2, 123.8, 122.1, 118.2, 114.3, 111.7, 110.8, 100.0, 72.7, 56.2, 56.1, 56.0, 53.8, 29.2, 28.4, 23.4, 20.8, 14.2. HR-MS (ESI) m/z: calcd for C30H41NO6 [M+H]+: 512.3012; found 512.2996. HPLC purity 95.6% [30 min run of 15–75% and 75–85% acetonitrile in ammonium formate solution (1.26 g ammonium formate in 1000 mL water), with 15 min and 10 min gradient, respectively, 1.0 mL/min].
4.5.3. 3-O-(N,N-Dibutylamino)pentyl-3′,4′,7-trimethoxyflavonol (69)
This derivative was achieved in 27% overall yield for two steps as a syrup. IR (film) νmax: 2930, 2859, 1619, 1599, 1562, 1512 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.13 (d, J = 8.9 Hz, 1H), 7.73 (s, 1H), 7.71 (d, J = 7.2 Hz, 1H), 6.98 (d, J = 8.4 Hz, 1H), 6.95 (dd, J = 8.7, 2.1 Hz, 1H), 6.89 (d, J = 1.9 Hz, 1H), 4.01 (t, J = 6.7 Hz, 2H), 3.96 (s, 6H), 3.91 (s, 3H), 2.38(t, J = 6.6 Hz, 6H), 1.73 (quin, J = 7.0 Hz, 2H), 1.44–1.30 (m, 8H), 1.26 (sextet, J = 7.5 Hz, 4H), 0.89 (t, J = 7.2 Hz, 6H). 13C NMR (75 MHz, CDCl3) δ 174.6, 164.0, 157.0, 155.3, 151.0, 148.7, 140.0, 127.2, 123.9, 122.1, 118.2, 114.3, 111.8, 110.8, 100.1, 72.8, 56.2, 56.1, 56.0, 54.1, 53.9, 30.3, 29.1, 26.7, 24.1, 20.9, 14.2. HR-MS (ESI) m/z: calcd for C31H43NO6 [M+H]+: 526.3168; found 526.3162. HPLC purity 100.0% (30 min run of 45–80% CH3CN in aqueous solution of ammonium formate (1.26 g ammonium formate in 1000 mL water), with 15 min gradient, 1.0 mL/min).
4.5.4. 3-O-Morpholinopropyl-3′,4′,7-trimethoxyflavonol (70)
This derivative was achieved in 48% overall yield for two steps as a yellow solid. m.p. 89–91oC. IR (film) νmax: 2943, 2899, 2839, 1637, 1625, 1603, 1516, 1505 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.13 (d, J = 8.9 Hz, 1H), 7.70 (dd, J = 8.4, 1.8 Hz, 1H), 7.67 (d, J = 1.8 Hz, 1H), 6.98 (d, J = 8.4 Hz, 1H), 6.97 (dd, J = 9.0, 2.1 Hz, 1H), 6.90 (d, J = 2.2 Hz, 1H), 4.07 (t, J = 6.3 Hz, 2H), 3.96 (s, 6H), 3.92 (s, 3H), 3.70 (t, J = 4.5 Hz, 4H), 2.53 (t, J = 7.4 Hz, 2H), 2.44 (br.s, 4H), 1.93 (quin, J = 6.9 Hz, 2H). 13C NMR (75 MHz, CDCl3) δ 174.6, 164.1, 157.1, 155.5, 151.1, 148.8, 139.9, 127.2, 123.7, 122.3, 118.2, 114.4, 111.8, 110.9, 100.1, 71.0, 66.8, 56.3, 56.1, 56.0, 55.8, 53.7, 27.2. HR-MS (ESI) m/z: calcd for C25H29NO7 [M+H]+: 456.2022; found 456.2013. HPLC purity 98.8% (30 min run of 45–80% CH3CN in aqueous solution of ammonium formate (1.26 g ammonium formate in 1000 mL water), with 15 min gradient, 1.0 mL/min).
4.5.5. 3-O-Morpholinobutyl-3′,4′,7-trimethoxyflavonol (71)
This derivative was achieved in 39% overall yield for two steps as a syrup. IR (film) νmax: 2934, 2840, 1616, 1597, 1511cm−1. 1H NMR (300 MHz, CDCl3) δ 8.12 (d, J = 8.9 Hz, 1H), 7.72–7.67 (overlapped, 2H), 6.97 (d, J = 9.0 Hz, 1H), 6.95 (dd, J = 9.0, 2.1 Hz, 1H), 6.89 (d, J = 2.1 Hz, 1H), 4.03 (t, J = 6.5 Hz, 2H), 3.95 (s, 6H), 3.91 (s, 3H), 3.67 (t, J = 4.5 Hz, 4H), 2.38 (br.s, 4H), 2.33 (t, J = 7.5 Hz, 4H), 1.73 (quin, J = 6.9 Hz, 2H), 1.57 (quin, J = 7.3 Hz, 2H). 13C NMR (75 MHz, CDCl3) δ 174.6, 164.0, 157.0, 155.3, 151.0, 148.7, 139.9, 127.2, 123.8, 122.1, 118.2, 114.4, 111.8, 110.8, 100.1, 72.5, 67.0, 58.7, 56.2, 56.1, 56.0, 53.7, 28.2, 23.0. HR-MS (ESI) m/z: calcd for C26H31NO7 [M+H]+: 470.2179; found 470.2175. HPLC purity 95.7% (30 min run of 45–80% CH3CN in aqueous solution of ammonium formate (1.26 g ammonium formate in 1000 mL water), with 15 min gradient, 1.0 mL/min).
4.5.6. 3-O-Morpholinopentyl-3′,4′,7-trimethoxyflavonol (72)
This derivative was achieved in 37% overall yield for two steps as a syrup. IR (film) νmax: 2935, 2854, 1617, 1597, 1512 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.12 (d, J = 8.9 Hz, 1H), 7.71 (s, 1H), 7.70 (d, J = 7.6 Hz, 1H), 6.98 (d, J = 7.5 Hz, 1H), 6.95 (dd, J = 8.7, 2.1 Hz, 1H), 6.89 (d, J = 1.9 Hz, 1H), 4.01 (t, J = 6.6 Hz, 2H), 3.96 (s, 3H), 3.95 (s, 3H), 3.91 (s, 3H), 3.69 (t, J = 4.5 Hz, 4H), 2.40 (br.s, 4H), 2.28 (t, J = 7.4 Hz, 2H), 1.73 (quin, J = 6.9 Hz, 2H), 1.52–1.38 (overlapped, 4H). 13C NMR (75 MHz, CDCl3) δ 174.6, 164.0, 157.0, 155.3, 151.0, 148.6, 140.0, 127.2, 123.8, 122.1, 118.2, 114.3, 111.8, 110.8, 100.1, 72.6, 67.0, 59.1, 56.2, 56.1, 56.0, 53.8, 30.2, 26.3, 24.0. HR-MS (ESI) m/z: calcd for C27H37NO7 [M+H]+: 484.2335; found 484.2332. HPLC purity 95.7% (30 min run of 45–80% CH3CN in in aqueous solution of ammonium formate (1.26 g ammonium formate in 1000 mL water), with 15 min gradient, 1.0 mL/min).
4.5.7. 3-O-Piperidinopropyl-3′,4′,7-trimethoxyflavonol (73)
This derivative was achieved in 39% overall yield for two steps as a syrup. IR (film) νmax: 2935, 2851, 2758, 1637, 1621, 1596, 1564, 1510 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.11 (d, J = 8.9 Hz, 1H), 7.69 (dd, J = 8.5, 2.1 Hz, 1H), 7.65 (d, J = 2.0 Hz, 1H), 6.97 (d, J = 8.4 Hz, 1H), 6.94 (dd, J = 8.7, 2.4 Hz, 1H), 6.88 (d, J = 2.3 Hz, 1H), 4.04 (t, J = 6.4 Hz, 2H), 3.95 (s, 6H), 3.90 (s, 3H), 2.52 (t, J = 7.4 Hz, 2H), 2.42 (br.s, 4H), 1.95 (quin, J = 7.0 Hz, 2H), 1.59 (quin, J = 5.4 Hz, 4H), 1.47 – 1.36 (m, 2H). 13C NMR (75 MHz, CDCl3) δ 174.5, 164.0, 156.9, 155.4, 151.0, 148.6, 139.7, 127.1, 123.6, 122.2, 118.0, 114.3, 111.5, 110.8, 100.0, 71.1, 56.1, 56.0, 55.8, 54.4, 27.3, 25.5, 24.1. HR-MS (ESI) m/z: calcd for C26H31NO6 [M+H]+: 454.2229; found 454.2220. HPLC purity 95.9% (30 min run of 45–80% CH3CN in in aqueous solution of ammonium formate (1.26 g ammonium formate in 1000 mL water), with 15 min gradient, 1.0 mL/min).
4.5.8. 3-O-Piperidinobutyl-3′,4′,7-trimethoxyflavonol (74)
This derivative was achieved in 51% overall yield for two steps as a syrup. IR (film) νmax: 2931, 2840, 1616, 1597, 1511 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.11 (d, J = 8.9 Hz, 1H), 7.70 (dd, J = 7.2, 2.1 Hz, 1H), 7.69 (s, 1H), 6.97 (d, J = 9.3 Hz, 1H), 6.94 (dd, J = 8.7, 2.4 Hz, 1H), 6.88 (d, J = 2.2 Hz, 1H), 4.01 (t, J = 6.5 Hz, 2H), 3.95 (s, 6H), 3.90 (s, 3H), 2.30 (t, J = 7.8 Hz, 6H), 1.70 (quin, J = 6.8 Hz, 2H), 1.62–1.52 (m, 6H), 1.46 – 1.34 (m, 2H). 13C NMR (75 MHz, CDCl3) δ 174.6, 164.0, 157.0, 155.3, 151.0, 148.6, 139.9, 127.2, 123.8, 122.2, 118.2, 114.3, 111.7, 110.8, 100.0, 72.6, 59.0, 56.2, 56.0, 55.9, 54.5, 28.4, 25.8, 24.4, 23.2. HR-MS (ESI) m/z: calcd for C28H35NO6 [M+H]+: 468.2386; found 468.2376. HPLC purity 95.1% (30 min run of 45–80% CH3CN in aqueous solution of ammonium formate (1.26 g ammonium formate in 1000 mL water), with 15 min gradient, 1.0 mL/min).
4.5.9. 3-O-Piperidinopentyl-3′,4′,7-trimethoxyflavonol (75)
This derivative was achieved in 34% overall yield for two steps as a syrup. IR (film) νmax: 2932, 2853, 1617, 1597, 1511 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.11 (d, J = 8.7 Hz, 1H), 7.22–7.68 (overlapped, 2H), 6.97 (d, J = 9.3 Hz, 1H), 6.95 (dd, J = 9.0, 2.1 Hz, 1H), 6.89 (d, J = 2.2 Hz, 1H), 4.00 (t, J = 6.7 Hz, 2H), 3.96 (s, 3H), 3.95 (s, 3H), 3.90 (s, 3H), 2.42 (br.s, 4H), 2.31 (t, J = 8.0 Hz, 2H), 1.72 (quin, J = 7.1 Hz, 2H), 1.61 (quin, J = 5.6 Hz, 4H), 1.56 – 1.47 (m, 2H), 1.44–1.32 (m, 4H). 13C NMR (75 MHz, CDCl3) δ 174.6, 164.0, 157.0, 155.4, 151.0, 148.6, 140.0, 127.2, 123.8, 122.1, 118.2, 114.3, 111.7, 110.8, 100.0, 72.6, 59.2, 56.2, 56.1, 56.0, 54.4, 30.2, 26.2, 25.5, 24.2, 24.1. HR-MS (ESI) m/z: calcd for C28H35NO6 [M+H]+: 482.2542; found 482.2532. HPLC purity 99.6% (30 min run of 4580% CH3CN in aqueous solution of ammonium formate (1.26 g ammonium formate in 1000 mL water), with 15 min gradient, 1.0 mL/min).
4.5.10. 3-O-(4-Methylpiperazin-1-yl)propyl-3′,4′,7-trimethoxyflavonol (76)
This derivative was achieved in 42% overall yield for two steps as a yellow solid. m.p. 99–101oC. IR (film) νmax: 2938, 2837, 2790, 1634, 1621, 1596, 1563, 1514 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.11 (d, J = 8.9 Hz, 1H), 7.70–7.66 (overlapped, 1H), 7.66 (s, 1H), 6.95 (d, J = 8.4 Hz, 1H), 6.94 (dd, J = 8.7, 2.4 Hz, 1H), 6.87 (d, J = 2.0 Hz, 1H), 4.05 (t, J = 6.4 Hz, 2H), 3.94 (s, 6H), 3.89 (s, 3H), 2.43 (t, J = 7.5Hz, 10H), 2.24 (s, 3H), 1.88 (quin, J = 7.0 Hz, 2H). 13C NMR (75 MHz, CDCl3) δ 174.5, 164.0, 157.0, 155.4, 151.0, 148.7, 139.9, 127.2, 123.7, 122.2, 118.2, 114.3, 111.7, 110.8, 100.0, 71.2, 56.2, 56.1, 55.9, 55.3, 55.1, 53.2, 46.1, 27.7. HR-MS (ESI) m/z: calcd for C26H32N2O6 [M+H]+: 469.2338; found 469.2335. HPLC purity 95.0% (30 min run of 45–80% CH3CN in aqueous solution of ammonium formate (1.26 g ammonium formate in 1000 mL water), with 15 min gradient, 1.0 mL/min).
4.5.11. 3-O-(4-Methylpiperazin-1-yl)butyl-3′,4′,7-trimethoxyflavonol (77)
This derivative was achieved in 40% overall yield for two steps as a syrup. IR (film) νmax: 2934, 2836, 2793, 1616, 1597, 1511 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.12 (d, J = 8.9 Hz, 1H), 7.70 (d, J = 6.2 Hz, 1H), 7.69 (s, 1H), 6.97 (d, J = 8.1 Hz, 1H), 6.95 (dd, J = 9.0, 2.1 Hz, 1H), 6.88 (d, J = 2.0 Hz, 1H), 4.03 (t, J = 6.5 Hz, 2H), 3.95 (s, 6H), 3.90 (s, 3H), 2.43 (br.s, 8H), 2.33 (t, J = 7.8 Hz, 2H), 2.27 (s, 3H), 1.72 (quin, J = 7.0 Hz, 2H), 1.56 (quin, J = 7.4 Hz, 2H). 13C NMR (75 MHz, CDCl3) δ 174.6, 164.0, 157.0, 155.3, 151.0, 148.7, 140.0, 127.2, 123.8, 122.2, 118.2, 114.3, 111.7, 110.8, 100.0, 72.6, 58.2, 56.2, 56.1, 55.9, 55.1, 53.1, 46.1, 28.3, 23.4. HR-MS (ESI) m/z: calcd for C27H34N2O6 [M+H]+: 483.2495; found 483.2488. HPLC purity 95.5% (30 min run of 45–80% CH3CN in aqueous solution of ammonium formate (1.26 g ammonium formate in 1000 mL water), with 15 min gradient, 1.0 mL/min).
4.5.12. 3-O-(4-Methylpiperazin-1-yl)pentyl-3′,4′,7-trimethoxyflavonol (78)
This derivative was achieved in 37% overall yield for two steps as a syrup. IR (film) νmax: 2932, 2837, 2794, 1634, 1620, 1597, 1558, 1515 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.12 (d, J = 8.9 Hz, 1H), 7.70 (s, 1H), 7.69 (d, J = 6.9 Hz, 1H), 6.96 (d, J = 8.4 Hz, 1H), 6.94 (dd, J = 8.7, 2.1 Hz, 1H), 6.88 (d, J = 2.1 Hz, 1H), 3.99 (t, J = 6.6 Hz, 2H), 3.95 (s, 3H), 3.94 (s, 3H), 3.90 (s, 3H), 2.46 (br.s, 8H), 2.31–2.25 (overlapped, 2H), 2.18 (s, 3H), 1.72 (quin, J = 7.0 Hz, 2H), 1.53–1.41 (m, 2H), 1.41–1.34 (m, 2H). 13C NMR (75 MHz, CDCl3) δ 174.6, 164.0, 157.0, 155.3, 151.0, 148.6, 140.0, 127.2, 123.8, 122.1, 118.2, 114.3, 111.8, 110.8, 100.0, 72.6, 58.5, 56.2, 56.1, 55.9, 55.0, 53.1, 46.0, 30.2, 26.6, 24.0. HRMS (ESI) m/z: calcd for C28H36N2O6 [M+H]+: 497.2651; found 497.2640. HPLC purity 95.0% (30 min run of 15–55% CH3CN in aqueous solution of ammonium formate (1.26 g ammonium formate in 1000 mL water), with 15 min gradient, 1.0 mL/min).
4.5.13. 3-O-Pyrrolidinopropyl-3′,4′,7-trimethoxyflavonol (79)
This derivative was achieved in 50% overall yield for two steps as a syrup. IR (film) νmax: 2933, 2837, 2790, 1616, 1597, 1511 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.11 (d, J = 8.9 Hz, 1H), 7.71–7.66 (overlapped, 2H), 6.96 (d, J = 8.1 Hz, 1H), 6.94 (dd, J = 9.0, 2.4 Hz, 1H), 6.88 (d, J = 2.2 Hz, 1H), 4.06 (t, J = 6.4 Hz, 2H), 3.95 (s, 6H), 3.90 (s, 3H), 2.60 (t, J = 7.7 Hz, 2H), 2.50 (br.s, 4H), 1.95 (quin, J = 7.1 Hz, 2H), 1.78–1.72 (m, 4H). 13C NMR (75 MHz, CDCl3) δ 174.6, 164.0, 157.0, 155.5, 151.0, 148.7, 139.8, 127.2, 123.7, 122.2, 118.1, 114.4, 111.7, 110.8, 100.0, 71.2, 56.2, 56.1, 55.9, 54.2, 53.3, 29.6, 23.5. HR-MS (ESI) m/z: calcd for C25H29NO6 [M+H]+: 440.2073; found 440.2068. HPLC purity 96.4% [30 min run of 45–80% acetonitrile in ammonium formate solution (1.26 g ammonium formate in 1000 mL water), with 15 min gradient, 1.0 mL/min].
4.5.14. 3-O-Pyrrolidinobutyl-3′,4′,7-trimethoxyflavonol (80)
This derivative was achieved in 40% overall yield for two steps as a syrup. IR (film) νmax: 2935, 2838, 1610, 1596, 1511 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.12 (d, J = 8.9 Hz, 1H), 7.72 (d, J = 8.4, 2.1 Hz, 1H), 7.69 (d, J = 1.8 Hz, 1H), 6.99 (d, J = 8.4 Hz, 1H), 6.95 (dd, J = 8.7, 2.1 Hz, 1H), 6.90 (d, J = 2.2 Hz, 1H), 4.01 (t, J = 6.1 Hz, 2H), 3.96 (s, 3H), 3.95 (s, 3H), 3.91 (s, 3H), 2.63 (br.s, J = 5.6 Hz, 6H), 1.83–1.70 (overlapped, 8H). 13C NMR (75 MHz, CDCl3) δ 174.6, 164.1, 157.0, 155.4, 151.1, 148.7, 139.9, 127.2, 123.7, 122.2, 118.2, 114.4, 111.6, 110.8, 100.0, 72.2, 56.2, 56.1, 56.0, 54.0, 28.2, 24.9, 23.5. HR-MS (ESI) m/z: calcd for C26H31NO6 [M+H]+: 454.2229; found 454.2217. HPLC purity 95.5% (30 min run of 45–80% CH3CN in aqueous solution of ammonium formate (1.26 g ammonium formate in 1000 mL water), with 15 min gradient, 1.0 mL/min).
4.5.15. 3-O-Pyrrolidinopentyl-3′,4′,7-trimethoxyflavonol (81)
This derivative was achieved in 35% yield for two steps as a syrup. IR (film) νmax: 2932, 1615, 1597, 1558, 1512 cm−1. 1H NMR (300 MHz, CDCl3) δ 8.11 (d, J = 8.9 Hz, 1H), 7.72 (d, J = 8.4, 2.1 Hz, 1H), 7.69 (d, J = 1.8 Hz, 1H), 6.95 (overlapped, 2H), 6.89 (d, J = 2.1 Hz, 1H), 3.99 (t, J = 6.6 Hz, 2H), 3.96(s, 3H), 3.95 (s, 3H), 3.90 (s, 4H), 2.66 (br.s, 4H), 2.53 (t, J = 7.5 Hz, 2H), 1.84 (br.s, 4H), 1.73 (quin, J = 7.1 Hz, 2H), 1.61 (quin, J = 7.7 Hz, 2H), 1.42 (quin, J = 7.3 Hz, 2H). 13C NMR (75 MHz, CDCl3) δ 174.6, 164.0, 157.0, 155.4, 151.1, 148.7, 140.0, 127.2, 123.8, 122.2, 118.2, 114.4, 111.8, 110.9, 100.1, 72.4, 56.3, 56.2, 56.1, 56. 0, 54.1, 30.0, 27.8, 24.0, 23.5. HR-MS (ESI) m/z: calcd for C27H33NO6 [M+H]+: 468.2386; found 468.2379. HPLC purity 97.3% (30 min run of 45–80% CH3CN in aqueous solution of ammonium formate (1.26 g ammonium formate in 1000 mL water), with 15 min gradient, 1.0 mL/min).
4.6. Cell culture
All cell lines were initially purchased from American Type Culture Collection (ATCC). The PC-3 and LNCaP prostate cancer cell lines were routinely cultured in RPMI-1640 medium supplemented with 10% FBS and 1% penicillin/streptomycin. The DU145 prostate cancer cells were routinely cultured in Eagle’s Minimum Essential Medium (EMEM) supplemented with 10% FBS and 1% penicillin/streptomycin. The MCF 10A mammary epithelial cells were routinely cultured in MEGM Bulletkit growth medium (Lonza). The PWR-1E non-neoplastic prostate epithelial cells were routinely cultured in Keratinocyte serum free medium (K-SFM) supplemented with bovine pituitary extract and human recombinant epidermal growth factor. Cultures were maintained in a high humidity environment supplemented with 5% carbon dioxide at a temperature of 37oC. The DU-145 prostate cancer cells were routinely cultured in Eagle’s Minimum Essential Medium (EMEM) supplemented with 10% FBS and 1% penicillin/ streptomycin.
4.7. WST-1 cell proliferation assay
PC-3, DU145, LNCaP, MCF 10A, or PWR-1E cells were plated in 96-well plates at a density of 3,200 each well in 200 μL of culture medium. The cells were then treated with quercetin, fisetin, or synthesized flavonols separately at different doses (3.125 μM, 6.25 μM, 12.5 μM, 25 μM, and 50 μM for the compounds with IC50 values greater than 5 μM; 0.1 μM, 0.25 μM, 0.5 μM, 1 μM, and 5 μM for the compounds with IC50 values less than 5 μM) for 3 days, while equal treatment volumes (200 μL) of DMSO (0.25%) in medium were used as vehicle control. The cells were cultured in a CO2 incubator at 37 oC for three days. 10 μL of the premixed WST-1 cell proliferation reagent (Clontech) was added to each well. After mixing gently for one minute on an orbital shaker, the cells were incubated for additional 3 hours at 37 oC. To ensure homogeneous distribution of color, it is important to mix gently on an orbital shaker for one minute. The absorbance of each well was measured using a microplate-reader (Synergy HT, BioTek) at a wavelength of 430 nm. The IC50 value is the concentration of each compound that inhibits cell proliferation by 50% under the experimental conditions and is the average from triplicate determinations that were reproducible and statistically significant. For calculating the IC50 values, a linear proliferative inhibition was made based on at least five dosages for each compound.
4.8. Cell cycle analysis
PC-3 cells were plated in 24-well plates at a density of 200,000 each well in 400 μL of culture medium. After 3 h of cell attachment, the cells were then treated with test compound at 10 μM and 20 μM, while equal treatment volumes (400 μL) of DMSO (0.25%) in medium were used as vehicle control. The cells were cultured in CO2 incubator at 37 oC for 16 h. Both attached and floating cells were collected in a centrifuge tube by centrifugation at rcf value of 450 g for 5 min. After discarding the supernatant, the collected cells were re-suspended with 500 μL 80% cold ethanol to fix for 30 min in 4 oC. The fixed cells could be stored at -20 oC for one week. After fixation, the ethanol was removed after centrifuging and the cells were washed with PBS. The cells were then re-suspended with 100 μL of 100 mg/mL ribonuclease and were cultured at 37 oC for 30 min to degrade all RNA. The cells were stained with 200 μL of 50 μg/mL propidium iodide (PI) stock solution for 30 min at -20 oC, and then the fluorescence intensity of PI was detected in individual PC-3 cells using an Attune flow cytometer (Life Technologies) within 0.5–1 h after staining.
4.9. F2N12S and SYTOX AADvanced double staining assay
PC-3 cells were plated in 24-well plates at a density of 200,000 each well in 400 μL of culture medium. After 3 h of cell attachment, the cells were then treated with the test compound at different concentrations (5 μM, 10 μM, 20 μM) and cultured in CO2 incubator at 37 oC for 16 h, while equal treatment volumes (400 μL) of DMSO (0.25%) in medium were used as vehicle control. Both attached and floating cells were collected in a centrifuge tube by centrifugation at rcf value of 450 g for 5 min. The collected cells were re-suspended with 500 μL HBSS to remove proteins which may affect flow signal and centrifuged again. After discarding the supernatant, the collected cells were re-suspended with 300 μL HBSS and stained with 0.3 μL of F2N12S for 3–5 min followed by 0.3 μL SYTOX AADvanced for an additional 5 min. The fluorescence intensity of the two probes was further measured in individual PC-3 cells using an Attune flow cytometer (Life Technologies) within 0.5–1 h after staining.
4.10. Pharmacokinetic Study (Sampling and Analysis) [31].
Male Sprague-Dawley rats, weighing between 250 and 300 g (Charles River Laboratories, Portage, MI) were used for the pharmacokinetic study of fisetin, 3-O-substituted-3′,4′-dimethoxyflavonol (42) and two 3-O-substituted-3′,4′,7-trimethoxyflavonols (76 and 81). Rats (n = 4) were given oral gavage containing 5% dimethyl sulfoxide (DMSO), 40% polyethylene glycol 400, 55% saline-dissolved fisetin, 42, 76, and 81 at a single dose of 10 mg/kg. After oral administration, blood samples were collected from the lateral tail vein of the rats at 1, 3, 6, 8, and 24 h. Rat blood was collected with a capillary into 1.5 mL microcentrifuge tubes containing 0.01 mL of 10% EDTA anticoagulant. Plasma was then separated from red cells by centrifugation in a refrigerated centrifuge at 4 oC and transferred to a separate tube. The plasma samples were frozen at -80 oC until analysis. All procedures involving these animals were conducted in compliance with state and federal laws, standards of the U.S. Department of Health and Human Services, and guidelines established by Xavier University Animal Care and Use Committee. The facilities and laboratory animals program of Xavier University are accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care.
4.11. High Performance Liquid Chromatography-Tandem Mass Spectrometry (HPLC-MS/MS) for Drug Analysis in Plasma Samples.
Plasma samples were extracted with chloroform/methanol (2:1) using traditional Folch method for lipid extraction. Methanol (1 mL) and chloroform (2 mL) were added to each plasma sample followed by addition of 5 ng of trans-tamoxifen-13C2, 15N to each sample as the internal standard. The mixtures were stored at -20 oC overnight. Next, the samples were sonicated for 5 min and centrifuged with a Thermo Scientific Heraeus Megafuge 16 centrifuge. The top layer was transferred to another test tube. The bottom layer was washed with 1 mL of chloroform/methanol (2:1), centrifuged, and the top layer was transferred and combined with the previous top layer. Eight-tenth of a milliliter of HPLC grade water was added to the extracts. After vortexing, the mixture was centrifuged. The bottom layer was dried out with nitrogen and re-suspended in 100 μL of HPLC grade acetonitrile. An aliquot of 10 μL of sample was injected onto a Hypersil Gold column (50 mm × 2.1 mm; particle size 1.9 μM, Thermo Scientific) on a Dionex Ultimate 3000 UPLC system equipped with a TSQ Vantage triple quadrupole mass spectrometer for analysis. A binary mobile phase (A, water with 0.05% formic acid; B, acetonitrile with 0.05% formic acid) was used to achieve the gradient of initial 30% B for 1 min and then to 80% B at 8 min, to 100% B at 9 min, and returned to 30% B for 4 min. The flow rate was controlled at 0.6 mL/min. The settings of Heated Electrospray Ionization source were as follows: spray voltage (3200 V); vaporizer temperature (365 oC); sheath gas pressure (45 psi); auxiliary gas pressure (10 psi); capillary temperature (330 oC). Nitrogen was used as the sheath gas and auxiliary gas. Argon was used as the collision gas.
Supplementary Material
Highlights:
63 3-O-substituted flavonols were evaluated in prostate epithelial cell models.
An appropriate amino group to 3-OH of flavonols did improve the potency.
The optimal carbon linkers and terminal amino groups were identified.
One promising compound exhibit improved potency and bioavailability.
ACKNOWLEDGMENT
This work was financially supported by California State University (CSU)-Fresno. Pharmacokinetic Studies and HRMS were supported by NIH RCMI program at Xavier University of Louisiana through Grant 2G12MD007595 (G. Wang). We are also grateful to the ACS Project SEED Program for the support in 2016 summer (to A Gonzalez), to the Division of Graduate Studies at CSU-Fresno for a Graduate Student and Creative Activities Support Award (to X. Li), and to the Undergraduate Studies at CSU-Fresno for Undergraduate Research Grants (to P. Rajaram and M. Lee). We also thank Mrs. Katie Heredia (CSU-Fresno) and Mr. Erik R. Range (CSU-Fresno) for assistance in working out the HPLC analysis method.
Footnotes
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ejmech
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Vue B, Zhang S, Chen Q-H, Flavonoids with therapeutic potential in prostate cancer, Anti-Cancer Agents Med. Chem 16 (2016) 1205–1229. [DOI] [PubMed] [Google Scholar]
- [2].Lee MM, Gomez SL, Chang JS, Wey M, Wang R-T, Hsing AW, Soy and isoflavone consumption in relation to prostate cancer risk in China, Cancer Epidemiol Biomarkers Prev. 12 (2003) 665–668. [PubMed] [Google Scholar]
- [3].McCann SE, Ambrosone CB, Moysich KB, Brasure J, Marshall JR, Freudenheim JL, Wilkinson GS, Graham S, Intakes of selected nutrients, foods, and phytochemicals and prostate cancer risk in western New York, Nutr. Cancer 53 (2005) 33–41. [DOI] [PubMed] [Google Scholar]
- [4].Bosetti C, Bravi F, Talamini R, Parpinel M, Gnagnarella P, Negri E, Montella M, Lagiou P, Franceschi S, La Vecchia C, Flavonoids and prostate cancer risk: a study in Italy, Nutr. Cancer 56 (2006) 123–127. [DOI] [PubMed] [Google Scholar]
- [5].Geybels MS, Verhage BAJ, Arts ICW, van Schooten FJ, Goldbohm RA, van den Brandt PA, Dietary flavonoid intake, black tea consumption, and risk of overall and advanced stage prostate cancer, Am J. Epidemiol 177 (2013) 1388–1398. [DOI] [PubMed] [Google Scholar]
- [6].Liu HL, Jiang WB, Xie MX, Flavonoids: recent advances as anticancer drugs, Recent Pat. Anticancer Drug Discov 5 (2010) 152–164. [DOI] [PubMed] [Google Scholar]
- [7].Ravishankar D, Rajora AK, Greco F, Osborn HMI, Flavonoids as prospective compounds for anti-cancer therapy, Int. J. Biochem. Cell Biol 45 (2013) 2821–2831. [DOI] [PubMed] [Google Scholar]
- [8].Kelly GE, Husband AJ, in: Culig Z (Ed.), Prostate Cancer Methods and Protocols, Springer, 2003, pp. 377. [Google Scholar]
- [9].Perez-Vizcaino F, Fraga CG, Research trends in flavonoids and health, Arch. Biochem. Biophysics 646 (2018) 107–112. [DOI] [PubMed] [Google Scholar]
- [10].Harborne JB, Williams CA, Advances in flavonoid research since 1992, Phytochemistry 55 (2000) 481–504. [DOI] [PubMed] [Google Scholar]
- [11].Britton RG, Horner-Glister E, Pomenya OA, Smith EE, Denton R, Jenkins PR, Steward WP, Brown K, Gescher A, Sale S, Synthesis and biological evaluation of novel flavonols as potential anti-prostate cancer agents, Eur. J. Med. Chem 54 (2012) 952–958. [DOI] [PubMed] [Google Scholar]
- [12].Haddad AQ, Venkateswaran V, Viswanathan L, Teahan SJ, Fleshner NE, Klotz LH, Novel antiproliferative flavonoids induce cell cycle arrest in human prostate cancer cell lines, Prostate Cancer Prostatic Dis. 9 (2006) 68–76. [DOI] [PubMed] [Google Scholar]
- [13].Poyil P, Budhraja A, Son Y-O, Wang X, Zhang Z, Ding S, Wang L, Hitron A, Lee J-C, Xu M, Chen G, Luo J, Shi X, Quercetin inhibits angiogenesis mediated human prostate tumor growth by targeting VEGFR-2 regulated AKT/mTOR/P70S6K signaling pathways, PLoS One 7 (2012) e47516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Firdous AB, Sharmila G, Balakrishnan S, RajaSingh P, Suganya S, Srinvasan N, Arunakaran J, Quercetin, a natural dietary flavonoid, acts as a chemopreventive agent against prostate cancer in an vivo model by inhibiting the EGFR signaling pathway, Food Function 5 (2014) 2632–2645. [DOI] [PubMed] [Google Scholar]
- [15].Khan N, Asim M, Afaq F, Abu ZM, Mukhtar H, A novel dietary flavonoid fisetin inhibits androgen receptor signaling and tumor growth in athymic nude mice, Cancer Res. 68 (2008) 8555–8563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hill CUFK, Saad SEA, Britton RG, Gescher AJ, Sale S, Brown K, Howells LM, Inhibition of prostate cancer cell growth by 3′,4′,5′-trimethoxyflaconol (TMFol), Cancer Chemother. Pharmacol 76 (2015) 179–185. [DOI] [PubMed] [Google Scholar]
- [17].Saad SEA, Jones DJL, Norris LM, Horner-Glister E, Patel KR, Britton RG, Steward WP, Gescher AJ, Brown K, Sale S, Tissue distribution and metabolism of the putative cancer chemopreventative agent 3′,4′,5′-trimethoxyflavonol (TMFol) in mice, Biomed. Chromatogr 26 (2012) 1559–1566. [DOI] [PubMed] [Google Scholar]
- [18].Wu B, Kulkarni K, Basu S, Zhang S, Hu M, First-pass metabolism via UDP-glucuronosyltransferase: a barrier to oral bioavailability of phenolics, J. Pharm. Sci 100 (2011) 3655–3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Li X, Chen G, Zhang X, Zhang Q, Zheng S, Wang G, Chen Q-H, A new class of flavonol-based anti-prostate cancer agents: Design, synthesis, and evaluation in cell models, Bioorg. Med. Chem. Lett 26 (2016) 4241–4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Li X, Lee M, Chen G, Zhang Q, Zheng S, Wang G, Chen Q-H, 3-O-Substituted-3′,4′,5′trimethoxyflavonols: synthesis and cell-based evaluation as anti-prostate cancer agents, Bioorg. Med. Chem 25 (2017) 4786–4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dias TA, Duarte CL, Lima CF, Proenca MF, Pereira-Wilson C, Superior anticancer activity of halogenated chalcones and flavonols over the natural flavonol quercetin, Eur. J. Med. Chem 65 (2013) 500–510. [DOI] [PubMed] [Google Scholar]
- [22].Bennett M, Burke AJ, O'Sullivan WI, Aspects of the Algar-Flynn-Oyamada (AFO) reaction, Tetrahedron 52 (1996) 7163–7178. [Google Scholar]
- [23].Hasan A, Sadiq A, Abbas A, Mughal E, Khan KM, Ali M, Isolation and synthesis of flavonols and comparison of their antioxidant activity, Nat. Prod. Res 24 (2010) 995–1003. [DOI] [PubMed] [Google Scholar]
- [24].Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW, Establishment and characterization of a human prostatic carcinoma cell line (PC-3), Invest. Urol 17 (1979) 16–23. [PubMed] [Google Scholar]
- [25].Stone KR, Mickey DD, Wunderli H, Michey GH, Paulson DF, Isolation of a human prostate carcinoma cell line (DU145), Int. J. Cancer 21 (1978) 274–281. [DOI] [PubMed] [Google Scholar]
- [26].Qu Y, Han B, Yu Y, Yao W, Bose S, Karlan BY, Giuliano AE, Cui X, Evaluation of MCF10A as a reliable model for normal human mammary epithelial cells, PLoS ONE, 10 (2015) e0131285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rhim JS, Li H, Furusato B, Novel human prostate epithelial cell culture models for the study of carcinogenesis and of normal stem cells and cancer stem cells, Adv. Exp. Med. Biol 720 (2011) 71–80. [DOI] [PubMed] [Google Scholar]
- [28].Meng F-M, Yang J-B, Yang C-H, Jiang Y, Zhou Y-F, Yu B, Yang H, Vitexicarpin induces apoptosis in human prostate carcinoma PC-3 cells through G2/M phase arrest, Asian Pac. J. Cancer Prev 13 (2012) 6369–6374. [DOI] [PubMed] [Google Scholar]
- [29].Vijayababu MR, Kanagaraj P, Arunkumar A, Ilangovan R, Aruldhas MM, Arunakaran J, Quercetin-induced growth inhibition and cell death in prostate carcinoma cells (PC-3) are associated with increase in p21 and hypophosphorylated retinoblastoma proteins expression, J. Cancer Res. Clin. Oncol 131 (2005) 765–771. [DOI] [PubMed] [Google Scholar]
- [30].Haddad AQ, Fleshner N, Nelson C, Saour B, Musquera M, Venkateswaran V, Klotz L, Antiproliferative mechanism of the flavonoids 2,2′-dihydroxychalcone and fisetin in human prostate cancer cells, Nutr. Cancer 62 (2010) 668–681. [DOI] [PubMed] [Google Scholar]
- [31].Lee Y-Z, Yang C-W, Hsu H-Y, Qiu Y-Q, Yeh T-K, Chang H-Y, Chao Y-S, Lee S-J, Synthesis and biological evaluation of tylophorine-derived dibenzoquinolines as orally active agents: exploration of the role of tylophorine E ring on biological activity, J. Med. Chem 55 (2012) 10363–10377. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.