Abstract
Mrs. A. is a 73-year-old woman who has developed increasing fatigue and lower back pain over the past year. The pain limits her exercise tolerance such that she can now walk only 1 block. She is a retired schoolteacher who does volunteer efforts in her community but has limited her activities due to fatigue. Karnofsky performance status is 70%. She has a history of chronic hypertension treated with a diuretic, adult-onset diabetes mellitus treated with metformin, and hypothyroidism treated with levothyroxine. Initial evaluation reveals anemia, renal dysfunction, an elevated total protein, and an L2 compression fracture on lumbosacral radiographs. Results of initial and subsequent evaluation are shown below, and she is referred to a hematologist for further evaluation, which revealed the following: calcium 9.0 mg/dL, creatinine 3.2 mg/dL with estimated creatinine clearance using the Modification of Diet in Renal Disease equation of 15 mL/min, hemoglobin 9.6 g/dL, total protein 11 g/dL, albumin 3.2 g/dL, immunoglobulin A (IgA) λ M protein 6.8 g/dL, total IgA 7.2 g/dL, IgG 0.4g/dL, IgM 0.03 g/dL, free κ <0.01 mg/L, free λ 1000 mg/L, serum free light chain ratio <0.01, β-2–microglobulin 4.2, viscosity 3.0, lactate dehydrogenase 200 U/L, urine protein electrophoresis: 125 mg/dL with 30% M protein, and urine immunofixation: λ light chain. Skeletal bone survey showed lytic lesions in femurs and humeri and diffusely in ribs bilaterally as well as compression fractures at T4, T6, and L2. Bone marrow biopsy revealed λ-restricted plasma cells comprising 50% of the bone marrow core. Fluorescence in situ hybridization testing on marrow showed that del 17p was present in 80% of the plasma cells. Mrs. A. is informed of the diagnosis of multiple myeloma and the need for therapy. She requests consultation with 2 of the leading world experts. However, she wants to be treated near her home and does not want treatment on a clinical trial.
Learning Objectives
Appreciate that performance status and comorbidities are not adequate to encompass aging-associated vulnerabilities that impact treatment tolerance and prognosis in older adults with multiple myeloma
Apply measures of frailty to treatment considerations in older adults with multiple myeloma
Consider the intersection of treatment options and geriatric concerns in older adults with multiple myeloma from diagnosis through relapse
Introduction
In approaching initial treatment recommendations for an older patient with newly diagnosed multiple myeloma, 2 tasks should simultaneously be undertaken: staging the malignancy and “staging the aging.” Staging the myeloma will inform selection of the optimal treatment from a disease-focused perspective, assuming typical tolerance of the regimen. Staging the aging will provide insight into the patient’s physiologic aging and vulnerability to toxicity of therapy. With respect to the former, disease-focused prognostic markers can aid in categorizing the myeloma biology and inform treatment approaches. In this case, the patient has an elevated β-2–microglobulin, low albumin, normal lactate dehydrogenase, and high-risk chromosomal abnormalities, yielding Revised International Stating System stage II.1
In parallel with the malignancy staging, we must consider the patient’s physiologic age. Given the aging population and concomitant increase in the number of older adults with cancer, clinicians increasingly recognize the need to categorize the varied health status of older adults and incorporate the geriatric medicine principles into the care of older adults with cancer.2 Numerous studies have shown that Karnofsky or Eastern Cooperative Oncology Group (ECOG) performance status does not fully capture the level of functional limitation in an older adult with cancer and that comorbidities are independent from performance status.3,4 Similarly, frailty cannot be categorically equated with comorbidity or functional dependence, which are interrelated but distinct concepts.5 Recently, the American Society of Clinical Oncology issued guidelines recommending, at minimum, evaluation of function, comorbidity, falls, depression, cognition, and nutrition in older adults with cancer.6
The relevance of these guidelines in the care of older adults with myeloma is supported by the prevalence of geriatric impairments in this population. In a cohort of 869 older adults with myeloma, most of whom had an ECOG performance status of 0 or 1, 14% were dependent in 2 or more activities of daily living, and 18% were dependent in 3 or more instrumental activities of daily living.7 In a cohort of 40 patients who underwent comprehensive geriatric assessment, 62.5% were dependent on 1 or more instrumental activities of daily living. Other geriatric syndromes are extremely common as well. In a cohort of 801 patients with myeloma, common comorbidities included renal impairment (68%), cardiac impairment (45%), and pulmonary impairment (32%). In a cohort of 24 older men with myeloma, 1/2 screened positive for nutritional impairment, 1/2 were frail using the Rockwood clinical frailty scale, 1/4 were impaired in performing the Timed Up and Go Test (an objective measure of physical performance), and 29% screened positive for risk of depression.8 Falls were reported by about 30% of older adults with myeloma in a 400-patient cohort, more than in noncancer controls.9 Geriatric impairments are very common in older adults with myeloma, and their presence may influence an individual’s risk of adverse outcomes.
The International Myeloma Working Group (IMWG) has developed a model of frailty based on geriatric factors associated with adverse outcomes in older adults with myeloma (Table 1). The model was developed in a cohort of 869 older patients with myeloma enrolled in clinical trials. The model was built using factors associated with overall survival, including patient age, comorbidities, and functional status (activities of daily living/instrumental activities of daily living), to categorize patients as fit, intermediate fit, or frail.7 The resultant frailty status was shown to be associated with grade ≥3 nonhematologic adverse events, drug discontinuation, and lower overall survival. The model has since been externally validated in a cohort of 125 patients, with frail patients having a hazard ratio (HR) for mortality of 6.06 (95% confidence interval, 1.35-27.25) after adjusting for International Staging System Stage, cytogenetics, and therapy.10 Of note, when the IMWG frailty model was applied in a younger cohort (69% under age 65), frailty was associated with nonhematologic toxicity but not treatment discontinuation or overall survival.11 In our case presentation, we are unable to ascertain the patient’s IMWG frailty status with the data provided. A Karnofsky performance status of 70% is defined as “[c]ares for self; unable to carry on normal activity or do active work.” This suggests that the patient is independent in activities of daily living (eg, bathing, toileting, and transferring), but we do not know her level of independence in instrumental activities of daily living (eg, meal preparation, medication management, and finances).
Table 1.
Comparison of select risk prediction models relevant to older adults with multiple myeloma
| Factors associated with increased risk | IMWG7 | Revised Myeloma Comorbidity Index13 | Geriatric Assessment in Hematology Scale16,17 | Cancer and Aging Research Group Toxicity Tool*72,73 | CRASH score†74 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Points | Parameter | Points | Parameter | Points | Parameter | Points | Parameter | Points | |
| Age | Age 76-80 | 1 | 60-69 | 1 | — | ≥72 | 2 | — | ||
| Age >80 | 2 | ≥70 | 2 | — | — | |||||
| Performance/functional status | Any ADL dependence | 1 | KPS 80-90 | 2 | Gait speed ≤0.8 m/s | 1 | ≥1 falls in past 6 mo | 3 | ECOG 1-2 | 1 |
| Any IADL dependence | 1 | KPS < 70% | 3 | Any ADL dependence | 1 | Dependent in taking medications | 1 | ECOG 3-4 | 2 | |
| Limited in walking 1 block | 2 | |||||||||
| Comorbidities | Charlson Comorbidity Index ≥2 | 1 | Renal disease: eGFR < 60 | 1 | Diabetes, BMI > 25 kg/m2 | 1 | Creatinine clearance <34 mL/min | 3 | — | |
| Moderate/severe pulmonary disease | 1 | Cancer, lung disease, heart failure, or smoking‡ | ||||||||
| Medications/polypharmacy | — | — | ≥5 medications | 1 | — | |||||
| Nutrition | — | — | ≤8 on MNA-SF | 1 | — | MNA < 28 | 2 | |||
| Cognition | — | — | ≥3 errors on SPMSQ | 1 | — | MMS any errors | 2 | |||
| Psychosocial | — | — | Felt depressed 3-7 d of past week | 1 | Decreased social activities due to physical or emotional health | 1 | ||||
| Other | — | Moderate/severe frailty phenotype | 1 | Self-reported health fair or poor | 1 | Anemia | 3 | |||
| Hearing fair or worse | 2 | |||||||||
| Cytogenetics | — | Unfavorable | 1 | — | — | |||||
| Total score | Fit | 0 | Fit | 0-3 | Range | 0-8 | Range | 0-19 | Range | 0-8 |
| Intermediate fit | 1 | Intermediate | 4-6 | |||||||
| Frail | 2 | Frail | 7-9 | |||||||
ADL, activity of daily living; BMI, body mass index; CRASH, Chemotherapy Risk Assessment for High Age Patients; eGFR, estimated glomerular filtration rate; IADL, instrumental activity of daily living; KPS, Karnofsky Performance Status; MMS, Mini Mental Status Exam; MNA, Mini Nutritional Assessment; MNA-SF, Mini Nutritional Assessment—Short Form; SPMSQ, Short Portable Mental Status Questionnaire.
Developed and validated in solid tumor malignancies; original model includes points for gastrointestinal or genitourinary malignancies and chemotherapy type.
Developed and validated in primarily solid tumor malignancies; original model includes points for chemotherapy type. Variables listed are in the model for nonhematologic toxicity of chemotherapy.
Another frailty model, the Revised Myeloma Comorbidity Index (R-MCI), predicts survival in older adults with myeloma. The R-MCI incorporates specific comorbidities (ie, renal or pulmonary disease), Karnofsky performance status, age, frailty as defined by Fried et al,12 and cytogenetics (Table 1). This model was developed in 552 patients with a median age of 62 and validated in 249 patients with a median age of 63. Using this model, patients categorized as frail had a 9-fold greater risk of death compared with those categorized as fit (HR, 9.57; 95% confidence interval, 6.52-14.03).13 When the R-MCI and IMWG were directly compared in a cohort of 125 patients with myeloma, patients categorized as frail using the IMWG model had an HR for mortality of 6.06, whereas patients categorized as frail by the R-MCI had an adjusted HR for mortality of 8.34 (95% confidence interval, 1.69-41.17).10 In our case, although we do not have data on the patient’s frailty phenotype12 (which requires data on grip strength, weight loss, gait speed, self-reported exhaustion, and low physical activity), she would already be categorized as frail with the R-MCI based on her renal impairment, age, performance status, and cytogenetics, and she would be expected to have a greater risk for mortality.
Additional models incorporating components of geriatric assessment have been developed and validated in myeloma and other cancer populations. A recently published model applied the Accumulation of Deficits approach to operationalizing frailty in older adults with myeloma and showed that frailty was prognostic, with HR for mortality of 1.63 (95% confidence interval, 1.26-2.11).14,15 The Geriatric Assessment in Hematology scale16,17 incorporates domains not assessed in the IMWG or R-MCI frailty models, including gait speed, nutrition, cognition, and depression, which as mentioned above, are prevalent in older adults with myeloma. It was developed in 164 patients with multiple myeloma, chronic lymphocytic leukemia, myelodysplastic syndrome, and acute myeloid leukemia; it has criterion validity, although data on its utility in predicting chemotherapy toxicity and survival are awaited. The Cancer and Aging Research Group Risk Prediction Tool and the Chemotherapy Risk Assessment for High Age Patients Score have been widely adopted in the geriatric oncology literature as tools to predict toxicity of chemotherapy, although their utility in hematologic malignancies is unknown.6
There is no single optimal regimen for all older patients with newly diagnosed multiple myeloma. The suggested induction regimen for this patient is influenced by both disease-related factors and her current functional status. Her high-risk cytogenetic abnormality with 17p deletion warrants consideration of a 3-drug bortezomib-based combination regimen. The IMWG consensus on the treatment of multiple myeloma with high-risk cytogenetics and the European Myeloma Network consensus on practice in older adults with myeloma both concluded that bortezomib-based regimens may partly overcome the adverse prognostic effect of 17p deletion.18,19 In the SWOG S0777 trial of lenalidomide, bortezimib, and dexamethasone (RVd) vs lenalidomide and dexamethasone (Rd), the median progression-free survival was 38 months in the high-risk patients treated with RVd compared with 16 months in the high-risk patients treated with Rd.20 In a recent review of frontline therapies for older adults with high-risk cytogenetics, Avet-Loiseau and Facon21 note that, among patients with high-risk cytogenetics, progression-free and overall survival tended to be longer with triplet regimens. Table 2 highlights regimens that have been evaluated in as frontline therapy in older adults with multiple myeloma, including response rates, progression-free survival, overall survival, and toxicities.
Table 2.
Range of reported outcomes from trials for patients with newly diagnosed multiple myeloma who are transplant ineligible
| Regimen | Overall response rate, % | Complete response rate, % | Median PFS, mo | Median OS, mo | 3-y OS in high-risk subset, % | Early deaths/death due to toxicity, % | Treatment discontinuation due to adverse events, % | Grade ≥3 fatigue, % | Grade ≥3 neuropathy, % |
|---|---|---|---|---|---|---|---|---|---|
| Proteosome inhibitor based | |||||||||
| VD/VP24,25 | 64-73 | 3-8 | 14.0-14.7 | 49.8 | NR | NR | 29 | 11 | 22 |
| VMP24,25,35,75-78 | 70-86 | 4-30 | 17.1-31 | 74%-87% 3-y OS | 56.1 | 3-6 | 2.3-34 | 2-8 | 7-20 |
| VCD-lite/VCP25,79 | 64-67 | 2-29 | 15.2-24.2 | 29.7 | NR | 7.1-7.9 | 14 | 6.1 | 6.1 |
| Immunomodulatory agent based | |||||||||
| Rd36,41,80-82 | 70-81 | 3-22 | 8.9-25.3 | 30.5-62.3 | NR | 4.6 | 7-19 | 2-11 | 0-2 |
| MPR80,83 | 68 | 3-11 | 14-24 | 62% 3-y OS | NR | 0.7-2.3 | 4-18 | 2-3 | 0-3 |
| MPR+R maintenance83-85 | 70.4-84 | 11.2-16 | 18.7-31 | 69%-70% 3-y OS | NR | 2 | 16-41 | 5 | 0-2 |
| CPR80 | 74 | 0.5 | 20 | 68% 4-y OS | NR | 3.6 | 15 | 2 | 3 |
| Proteosome inhibitor + immunomodulatory agent | |||||||||
| RVD-lite32 | 86 | 44 | 35.1 | NR | NR | NR | 4 | 16 | 2 |
| VMPT-VT78,86 | 89 | 38 | 35.3 | 61% 5-y OS | NR | 4 | 23 | 6 | 16.8 |
| VTD/VTP24,76,77 | 80-81 | 4-28 | 15.4-34 | 43-51.5 | 55 | 5 | 17-38 | 12 | 9-27 |
| Proteosome inhibitor + monoclonal antibody | |||||||||
| VMP-dara35 | 90.9 | 42.6 | NR | NR | NR | 3.2 | 4.9 | NR | 1.4 |
CPR, cyclophosphamide, prednisone, and lenalidomide; MPR, melphalan, prednisone, and lenalidomide; MPR+R, melphalan, prednisone, and lenalidomide with lenalidomide maintenance; NR, not reported; OS, overall survival; PFS, progression-free survival. VCD-lite, weekly bortezomib, cyclophosphamide, and dexamethasone; VCD, bortezomib, cyclophosphamide, and dexamethasone; VD, bortezomib and dexamethasone; VMP, bortezomib, melphalan, and prednisone; VMP-dara, bortezomib, melphalan, prednisone, and daratumumab; VMPT-VT, bortezomib, melphalan, prednisone, and thalidomide with bortezomib and thalidomide maintenance; VP, bortezomib and prednisone; VTD, bortezomib, thalidomide, and dexamethasone; VTP, bortezomib, thalidomide, and prednisone.
Although a triplet would be optimal, our patient’s current functional limitations warrant some caution with a triplet regimen at standard dosing. We know that patients enrolled in clinical trials tend to have better performance status, fewer comorbidities, and better survival than those ineligible for clinical trials, and therefore, the data from clinical trials may not directly apply to every patient in clinic.22,23 Indeed, in studies that include patients who are more vulnerable than those typically enrolled in trials of transplant-ineligible patients, survival with 3-drug regimens was similar to that of 2-drug regimens due to increased rates of toxicity.24,25
Given that the patient meets criteria for frailty based on the R-MCI, she may be at greater risk for toxicities of therapy. We know that early discontinuation of therapy due to toxicity is associated with shorter survival.26 Although the approach has not been validated in multiple myeloma, the “start low—go slow” principle used in geriatrics has been applied in treatment trials in other malignancies. In this approach, empiric initial dose modifications are followed by dose escalation if the older patient tolerates the initial dosing well.27 Empiric dose modifications based on vulnerabilities in older adults with myeloma have been proposed and are a reasonable approach (Table 3),19,28-31 although they await prospective evaluation of their impact on toxicity and efficacy. In this case, I would proceed initially with weekly subcutaneous bortezomib and dexamethasone alone, with the anticipation that there would be improvement in her renal function and possibly, her functional status as her pain improves, thereby allowing us to escalate to a triplet regimen.
Table 3.
Suggested dose modifications for older adults with myeloma
| Drug | Dose level 0 | Dose level − 1* | Dose level − 2* |
|---|---|---|---|
| Dexamethasone | 40 mg days 1, 8, 15, 22 every 4 wk | 20 mg days 1, 8, 15, 22 every 4 wk | 10 mg days 1, 8, 15, 22 every 4 wk |
| Or 20 mg on day of and day after bortezomib87 | Or 10 mg on day of and day after bortezomib | ||
| Prednisone | 2 mg/kg days 1-4 of 4- to 6-wk cycle | 1 mg/kg days 1-4 of 4- to 6-wk cycle | 0.3-0.5 mg/kg days 1-4 of 4- to 6-wk cycle |
| Or 60 mg/m2 days 1-4 of 6-wk cycle | Or 30 mg/m2 days 1-4 of 6-wk cycle | Or 10-15 mg/m2 days 1-4 of 4- to 6-wk cycle | |
| Bortezomib | 1.3 mg/m2 days 1, 4, 8, 11 every 3 wk | 1.3 mg/m2 days 1, 8, 15, 22 every 5 wk | 1.0 mg/m2 days 1, 8, 15, 22 every 5 wk |
| Ixazomib | 4 mg days 1, 8, 15 every 4 wk | 3 mg days 1, 8, 15 every 4 wk | 2.3 mg days 1, 8, 15 every 4 wk |
| Carfilzomib | 20 mg/m2 days 1, 2, 8, 9, 15, 16 in cycle 1; 27 mg/m2 in cycle 2+ every 4 wk | 20 mg/m2 days 1, 2, 8, 9, 15, 16 in cycle 1; 27 mg/m2 days 1, 8, 15 in cycle 2+ every 4 wk | 20 mg/m2 days 1, 8, 15 every 4 wk |
| Lenalidomide | 25 mg days 1-21 every 4 wk | 15 mg days 1-21 every 4 wk | 10 mg days 1-21 every 4 wk |
| Pomalidomide | 4 mg days 1-21 every 4 wk | 3 mg days 1-21 every 4 wk | 2 mg days 1-21 every 4 wk |
| Thalidomide | 100-200 mg daily | 50-100 mg daily | 50 mg every other day; 50 mg daily |
| Melphalan | 0.25 mg/kg days 1-4 every 4-6 wk | 0.18 mg/kg days 1-4 every 4-6 wk | 0.13 mg/kg days 1-4 every 4-6 wk |
| Cyclophosphamide | 300 mg/m2 days 1, 8, 15 (±22) every 4 wk | 150 mg/m2 days 1, 8, 15 every 4 wk | 75 mg/m2 days 1, 8, 15 every 4 wk |
| Daratumumab | 16 mg/kg weekly for cycles 1-2 (4-wk cycles), then every other week for cycles 3-6, then every 4 wk | No age- or frailty-related dose modifications | No age- or frailty-related dose modifications |
| Elotuzumab | 10 mg/kg weekly for cycles 1-2 (4-wk cycles), then every other week | No age- or frailty-related dose modifications | No age- or frailty-related dose modifications |
Because of her high-risk cytogenetics, a 3-drug regimen would be preferred, potentially after a 2-drug “prephase” as described above. The combination of RVd is superior to Rd alone with respect to response rate, progression-free survival, and overall survival.20 However, the rate of grade 3 neurologic toxicity using twice weekly intravenously administered bortezomib was 33%. Because of the high rate of toxicity, a modification of this regimen, termed “RVD-lite,” was designed specifically for older adults and tested in a phase 2 study. In this study, bortezomib was administered subcutaneously and weekly for 4 weeks, with 1 week off, for 9 cycles followed by 6 cycles of consolidation.32 The overall response rate was 86%, with a median progression-free survival of 35 months. Yet, importantly, this efficacy did not come at the cost of toxicity: the rate of discontinuation due to toxicity was only 4%, and the rate of grade 3 neuropathy was only 2%. As mentioned above, early discontinuation of therapy due to toxicities is associated with poorer survival, whereas a cumulative bortezomib exposure of >39 mg/m2 (which would be 7.5 cycles of RVD-lite) is associated with superior survival33; therefore, initial therapeutic selection to optimize the chances of continuing therapy is ideal. Although the combination of lenalidomide and bortezomib is not specifically approved for initial therapy, its use is supported by the National Comprehensive Cancer Network guidelines34 and the IMWG consensus on the treatment of individuals with high-risk cytogenetics.18
It is a testament to the rapid advances in our field that another new regimen (bortezomib, melphalan, prednisone [VMP], and daratumumab) was approved by the Food and Drug Administration in May 2018 for older adults with newly diagnosed multiple myeloma who are transplant ineligible.35 In our patient's case, she would not have been a candidate for the ALCYONE trial due to her renal impairment at presentation, and therefore, the role for the daratumumab-VMP regimen in this patient is unclear.
Had the patient in the case presented with different features, other considerations may have informed treatment options. For example, in a patient with standard-risk cytogenetics, the RVD-lite regimen could be considered, but there may be more room for personalization of therapy. For example, if the patient expresses a strong preference for an all orally administered regimen and her renal function allowed, Rd alone, as per the FIRST trial, would be reasonable.36 In a patient who had not experienced the functional decline seen in this patient, who met criteria as outlined above for being “fit,” induction therapy followed by consolidation with high-dose therapy and autologous stem cell transplantation could be considered. Numerous studies have shown similar outcomes between younger patients and selected fit older patients who undergo high-dose therapy and autologous stem cell transplant as well as improved survival among older patients who undergo high-dose therapy and autologous stem cell transplant compared with older adults who do not undergo this treatment.37-40
It should also be noted that steroids, particularly traditional high doses of dexamethasone (ie, cumulative doses ≥480 mg per 4-week cycle), may be particularly toxic in older adults.41,42 Dose adjustments as outlined in Table 3, with lower doses of dexamethasone or the substitution of prednisone for dexamethasone, may be appropriate.41,43
In addition to selecting the initial antimyeloma regimen, we must also attend to her supportive care. The plain radiographs, which showed compression fractures in this case, should be followed with magnetic resonance imaging of the whole spine. If there is any soft tissue component concerning for impending neurologic compromise, radiation therapy may be considered to those focal areas. Although radiation and systemic therapy are generally not coadministered, phase 1 data support the safety of concomitant bortezomib and radiation therapy.44 Concomitant treatment with radiation therapy for symptomatic focal lesions and systemic therapy for systemic disease (renal insufficiency in this case) may be required in some patients. The patient may be a candidate for vertebroplasty for her symptomatic L2 lesion and will require use of a bone-modifying agent. Given her significant renal impairment, her therapeutic options include pamidronate or denosumab. Denosumab is noninferior to zoledronic acid for the prevention of skeletal-related events; however, cost is substantially higher for denosumab. Given its ease of subcutaneous administration, denosumab is an alternative to pamidronate in myeloma patients with renal insufficiency. The IMWG recommends against the use of pamidronate or zoledronic acid in patients with a creatinine clearance <30 mL/min,45 whereas the American Society of Clinical Oncology advises slowing the rate of infusion of pamidronate to 4 to 6 hours for patients with creatinine >3 mg/dL or creatinine clearance <30 mL/min.46 Current guidelines generally recommend bone-modifying therapy for 2 years.45,46
Mrs. A. opts for RVD-lite (oral lenalidomide, subcutaneous bortezomib, and dexamethasone)
After 6 monthly cycles, reevaluation reveals M protein by serum protein electrophoresis: none; immunoglobulin A (IgA) quantification: 0.1 g/dL; β-2–microglobulin 1.4; creatinine 1.2 mg/dL; free κ 15 mg/L; free λ 25 mg/L; serum free light chain ratio 0.60 (normal); and UPEP: only trace λ.
This follow-up data show improvement in the patient’s renal function, although it is important to note that, despite her normal serum creatinine, her glomerular filtration rate using either the Chronic Kidney Disease Epidemiology Collaboration (CKD-Epi) or the Modification of Diet in Renal Disease (MDRD) equation is still <60 mL/min per 1.73 m2. In addition, her monoclonal paraprotein is negative, but given her positive urine immunofixation, she is in very good partial response. As in younger patients, depth of response in older patients correlates with progression-free and overall survival.47 Regarding duration of therapy, in the phase 2 study of RVD-lite, the initial treatment plan was for 9 35-day cycles. As long as she is tolerating the regimen without significant neuropathy, RVD-lite may be continued to increase her depth of response. However, the cumulative sensory neuropathy from bortezomib may interfere with daily functions and contribute to an increased risk of falls. Continued conversation about the patient's goals of care, preferences, and values will ensure that we do not induce toxicity that hastens functional decline in our pursuit of greater control of the myeloma.
Mrs. A. has an excellent response and opts for continued therapy for 6 more cycles
Serum M protein is not detectable, IgA is 0.5 g/dL, and serum immunofixation is negative. However, she does have grade 2 peripheral neuropathy.
The patient has attained a complete response. Achieving minimal residual disease (MRD) negativity is associated with better outcomes, overcoming the negative prognostic impact of high-risk cytogenetics.48,49 However, despite its prognostic import, it is not known how MRD assessment should influence treatment recommendations. Several lines of evidence support continuous therapy regardless of the depth of response. In the FIRST trial, continuous therapy with lenalidomide was associated with prolonged progression-free survival compared with fixed duration therapy, although it should be noted that overall survival was similar with fixed duration lenalidomide or continuous lenalidomide.36 In a pooled analysis of 3 phase 3 trials, over 1300 patients were randomized to therapy that was either of fixed duration or continuous. Continuous therapy was associated with prolongation of progression-free survival and overall survival, with an HR of 0.69 in the overall survival analysis.50 Thus, continuous treatment should be recommended as long as significant toxicity does not develop. Given that the patient now has grade 2 peripheral neuropathy, it would be appropriate to discontinue bortezomib and continue with lenalidomide alone.
Mrs. A. is treated with maintenance lenalidomide at 15 mg by mouth daily for 21 of 28 days
She remains in remission for 2.5 years but then, develops new lytic bone lesions with M protein 0.5 g/dL.
The patient has a number of therapeutic options at this point. With multiple new agents and different drug classes, there is tremendous flexibility to tailor a regimen, taking into account her prior treatment, residual toxicities of prior therapy, and her current state of health. The agents that she has not yet been exposed to include carfilzomib, ixazomib, thalidomide, pomalidomide, elotuzumab, and daratumumab as well as the conventional alkylating agents melphalan and cyclophosphamide. Given that her myeloma is symptomatic with new bony lesions and refractory to lenalidomide, ixazomib and elotuzumab are less attractive options, because they are approved in combination with lenalidomide.51,52 If her prior peripheral neuropathy has resolved, her disease may still be sensitive to bortezomib, and the combination of bortezomib, daratumumab, and dexamethasone could be considered53 as could the combination of pomalidomide, bortezomib, and dexamethasone.54 However, about 1/3 of patients with grade ≥2 neuropathy will not have improvement in neuropathy after discontinuing therapy.55 If she were exposed to further bortezomib and her neuropathy worsened, as a grade 3 neuropathy, this toxicity would mean that the neuropathy is now interfering with her ability to independently complete her activities of daily living. Patient preferences become an important consideration: 59% of older adults with cancer prioritize maintaining their independence over length of life.56 An attractive therapeutic option with less neurotoxicity would be the combination of pomalidomide, daratumumab, and dexamethasone, which was recently approved in the United States, with an overall response rate of 60% and a median progression-free survival of 8.8 months.57 Given that our patient is receiving this regimen in her second line of therapy, her likelihood of response and duration of benefit may be even greater than the typical patient in that trial given that patients who received the combination in second or third line tended to have a higher response rate than those receiving it later in their course.
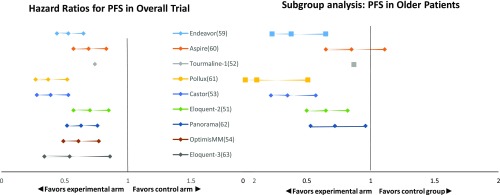
Therapeutic trials in patients with relapsed/refractory myeloma typically do not have age-based eligibility restrictions, and the outcomes of the older patients enrolled in the pivotal trials are typically similar to those of the younger patients enrolled (Figure 1).51-54,58-63 However, the proportion of older adults enrolled tends to be small; the trials tend to have exclusion criteria based on performance status, renal function, and cardiac and pulmonary comorbidities that de facto exclude older patients.58 The small proportion of older adults who are enrolled may not be representative of the older population with relapsed/refractory myeloma in general. Extrapolating from the pivotal trials and applying the results (both disease control and toxicities) to older adults who would not have been eligible for trials may overestimate their benefit and underestimate toxicity.22,23
Figure 1.
Comparison of HRs for progression-free survival in overall study population vs older subgroup in recent randomized trials in relapsed/refractory multiple myeloma. Endeavor trial: carfilzomib-dexamethasone vs bortezomib-dexamethasone (older subgroup ≥75, N = 143); Aspire trial: carfilzomib-lenalidomide-dexamethasone vs l lenalidomide-dexamethasone (older subgroup ≥65, N = 393); Tourmaline-1 trial: ixazomib-lenalidomide-dexamethasone (older subgroup >75, N = 108); Pollux trial: daratumumab-bortezomib-dexamethasone vs bortezomib-dexamethasone (older subgroup ≥75, N = 64); Castor trial: daratumumab-lenalidomide-dexamethasone vs lenalidomide-dexamethasone (older subgroup ≥65, N = 241); Eloquent-2 trial: elotuzumab-lenalidomide-dexamethasone vs lenalidomide-dexamethasone alone (older subgroup ≥65, N = 370); Panorama trial: panobinostat-bortezomib-dexamethasone vs bortezomib-dexamethasone (older subgroup ≥65, N = 323); OptimisMM trial: pomalidomide-bortezomib-dexamethasone vs bortezomib-dexamethasone (no subgroup analysis published); and Eloquent-3 trial: elotuzumab-pomalidomide-dexamethasone vs pomalidomide-dexamethasone alone (no subgroup analysis published).
She was treated with pomalidomide, dexamethasone, and daratumumab and achieved partial response
Relapse is observed with new lower back pain and L4 compression fracture.
At the time of myeloma progression, restaging the malignancy should be accompanied by “restaging the aging.” Although the frailty models described at the start of the case were developed and validated in older adults with newly diagnosed multiple myeloma, the same principles likely apply. In our case, about 5 years have intervened since the initial presentation. Our patient is now 78 years old. In addition to residual toxicities of prior therapy and the impact of the disease itself on her overall health, other aging-associated conditions may have developed. Her preexisting hypertension may have progressed to diastolic congestive heart failure, which would make carfilzomib problematic given its association with cardiac toxicities.64 Similarly, her underlying neuropathy may put her at greater risk for worsening peripheral neuropathy related to thalidomide.65 If she has developed cognitive impairment, this may be a barrier to adherence to an all orally administered regimen, such as ixazomib and dexamethasone with or without cyclophosphamide.66-68 Her social support network may now be more limited; in a survey of older caregivers of patients with cancer, over 2/3 were themselves experiencing poor health or serious health conditions, which may present a barrier to frequent visits for parenteral therapy if she is dependent on a caregiver for transportation.69 Formal assessment of the patient's comorbidities, functional status, medications, history of falls, cognition, psychosocial status, and social support will be essential in considering subsequent therapeutic options.6
Clinical trials are an important option at every therapeutic juncture. Unfortunately, older adults are systematically underenrolled in clinical trials.70 This is less commonly due to specific age restrictions, but rather, it is de facto exclusion related to comorbidities or excluded medications required for comorbid conditions. For example, our patient's creatinine clearance of <60 mL/min is a common exclusion criteria for clinical trials, even when the study drug is not renally cleared. Overly restrictive eligibility criteria limit the generalizability of results; recognition of this has led to calls to broaden inclusion criteria and provide clear rationale for exclusions.71
In summary, the approach to the care of an older adult with myeloma should include incorporation of the principles of geriatrics interwoven with disease-focused considerations. Models of frailty, incorporating just a few additional items not gathered in routine oncology assessment, can aid is stratifying the patient’s risk of toxicity and mortality and inform shared decision making.
References
- 1.Palumbo A, Avet-Loiseau H, Oliva S, et al. Revised international staging system for multiple myeloma: a report from International Myeloma Working Group. J Clin Oncol. 2015;33(26):2863-2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg PS, Barker KA, Anderson WF. Future distribution of multiple myeloma in the United States by sex, age, and race/ethnicity. Blood. 2015;125(2):410-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Extermann M, Overcash J, Lyman GH, Parr J, Balducci L. Comorbidity and functional status are independent in older cancer patients. J Clin Oncol. 1998;16(4):1582-1587. [DOI] [PubMed] [Google Scholar]
- 4.Repetto L, Fratino L, Audisio RA, et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: an Italian Group for Geriatric Oncology Study. J Clin Oncol. 2002;20(2):494-502. [DOI] [PubMed] [Google Scholar]
- 5.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59(3):255-263. [DOI] [PubMed] [Google Scholar]
- 6.Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO Guideline for Geriatric Oncology. J Clin Oncol. 2018;36(22):2326-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palumbo A, Bringhen S, Mateos M-V, et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an International Myeloma Working Group report. Blood. 2015;125(13):2068-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Handforth C, Burkinshaw R, Freeman J, et al. Comprehensive geriatric assessment and decision-making in older men with incurable but manageable (chronic) cancer. Support Care Cancer. 2018;319(1):309. [DOI] [PubMed] [Google Scholar]
- 9.Wildes TM, Fiala MA. Falls in older adults with multiple myeloma. Eur J Haematol. 2018;100(3):273-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engelhardt M, Dold SM, Ihorst G, et al. Geriatric assessment in multiple myeloma patients: validation of the International Myeloma Working Group (IMWG) score and comparison with other common comorbidity scores. Haematologica. 2016;101(9):1110-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong Y-P, Zhang Y-Z, Liao A-J, Li SX, Tian C, Lu J. Geriatric assessment to predict survival and risk of serious adverse events in elderly newly diagnosed multiple myeloma patients: a multicenter study in China. Chin Med J (Engl). 2017;130(2):130-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-M156. [DOI] [PubMed] [Google Scholar]
- 13.Engelhardt M, Domm A-S, Dold SM, et al. A concise revised Myeloma Comorbidity Index as a valid prognostic instrument in a large cohort of 801 multiple myeloma patients. Haematologica. 2017;102(5):910-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62(7):722-727. [DOI] [PubMed] [Google Scholar]
- 15.Mian HS, Wildes TM, Fiala M. Development of a Medicare health outcomes survey deficit-accumulation frailty index and its application to older patients with newly diagnosed multiple myeloma [published online ahead of print 25 July 2018]. JCO Clin Cancer Inform. doi:10.1200/CCI.18.00043. [DOI] [PMC free article] [PubMed]
- 16.Bonanad S, De la Rubia J, Gironella M, et al. ; GAH Group. Development and psychometric validation of a brief comprehensive health status assessment scale in older patients with hematological malignancies: the GAH Scale. J Geriatr Oncol. 2015;6(5):353-361. [DOI] [PubMed] [Google Scholar]
- 17.Cruz-Jentoft AJ, González B, de la Rubia J, et al. ; GAH Group. Further psychometric validation of the GAH scale: responsiveness and effect size. J Geriatr Oncol. 2017;8(3):211-215. [DOI] [PubMed] [Google Scholar]
- 18.Sonneveld P, Avet-Loiseau H, Lonial S, et al. Treatment of multiple myeloma with high-risk cytogenetics: a consensus of the International Myeloma Working Group. Blood. 2016;127(24):2955-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larocca A, Dold SM, Zweegman S, et al. Patient-centered practice in elderly myeloma patients: an overview and consensus from the European Myeloma Network (EMN). Leukemia. 2018;32:1697-1712. [DOI] [PubMed] [Google Scholar]
- 20.Durie BGM, Hoering A, Abidi MH, et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet. 2017;389(10068):519-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Avet-Loiseau H, Facon T. Front-line therapies for elderly patients with transplant-ineligible multiple myeloma and high-risk cytogenetics in the era of novel agents. Leukemia. 2018;32(6):1267-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah JJ, Abonour R, Gasparetto C, et al. Analysis of common eligibility criteria of randomized controlled trials in newly diagnosed multiple myeloma patients and extrapolating outcomes. Clin Lymphoma Myeloma Leuk. 2017;17(9):575-583.e2. [DOI] [PubMed] [Google Scholar]
- 23.Malecek M-K, Fiala M, Schroeder M, et al. Multiple myeloma patients ineligible for randomized controlled trials have poorer outcomes irrespective of treatment. Clin Lymphoma Myeloma Leuk. 2018;18(9):e363-e364. [DOI] [PubMed] [Google Scholar]
- 24.Niesvizky R, Flinn IW, Rifkin R, et al. Community-based phase IIIB trial of three UPFRONT bortezomib-based myeloma regimens. J Clin Oncol. 2015;33(33):3921-3929. [DOI] [PubMed] [Google Scholar]
- 25.Larocca A, Bringhen S, Petrucci MT, et al. A phase 2 study of three low-dose intensity subcutaneous bortezomib regimens in elderly frail patients with untreated multiple myeloma. Leukemia. 2016;30(6):1320-1326. [DOI] [PubMed] [Google Scholar]
- 26.Bringhen S, Mateos M-V, Zweegman S, et al. Age and organ damage correlate with poor survival in myeloma patients: meta-analysis of 1435 individual patient data from 4 randomized trials. Haematologica. 2013;98(6):980-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seymour MT, Thompson LC, Wasan HS, et al. ; National Cancer Research Institute Colorectal Cancer Clinical Studies Group. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): an open-label, randomised factorial trial. Lancet. 2011;377(9779):1749-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364(11):1046-1060. [DOI] [PubMed] [Google Scholar]
- 29.Wildes TM, Rosko A, Tuchman SA. Multiple myeloma in the older adult: better prospects, more challenges. J Clin Oncol. 2014;32(24):2531-2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palumbo A, Bringhen S, Ludwig H, et al. Personalized therapy in multiple myeloma according to patient age and vulnerability: a report of the European Myeloma Network (EMN). Blood. 2011;118(17):4519-4529. [DOI] [PubMed] [Google Scholar]
- 31.Zweegman S, Engelhardt M, Larocca A; EHA SWG on ‘Aging and Hematology’. Elderly patients with multiple myeloma: towards a frailty approach? Curr Opin Oncol. 2017;29(5):315-321. [DOI] [PubMed] [Google Scholar]
- 32.O’Donnell EK, Laubach JP, Yee AJ, et al. A phase 2 study of modified lenalidomide, bortezomib and dexamethasone in transplant-ineligible multiple myeloma. Br J Haematol. 2018;182(2):222-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mateos M-V, Richardson PG, Dimopoulos MA, et al. Effect of cumulative bortezomib dose on survival in multiple myeloma patients receiving bortezomib-melphalan-prednisone in the phase III VISTA study. Am J Hematol. 2015;90(4):314-319. [DOI] [PubMed] [Google Scholar]
- 34.Kumar SK, Callander NS, Alsina M, et al. NCCN guidelines insights: multiple myeloma, version 3.2018. J Natl Compr Canc Netw. 2018;16(1):11-20. [DOI] [PubMed] [Google Scholar]
- 35.Mateos M-V, Dimopoulos MA, Cavo M, et al. ; ALCYONE Trial Investigators. Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N Engl J Med. 2018;378(6):518-528. [DOI] [PubMed] [Google Scholar]
- 36.Benboubker L, Dimopoulos MA, Dispenzieri A, et al. ; FIRST Trial Team. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371(10):906-917. [DOI] [PubMed] [Google Scholar]
- 37.El Cheikh J, Kfoury E, Calmels B, et al. Age at transplantation and outcome after autologous stem cell transplantation in elderly patients with multiple myeloma. Hematol Oncol Stem Cell Ther. 2011;4(1):30-36. [DOI] [PubMed] [Google Scholar]
- 38.Muta T, Miyamoto T, Fujisaki T, et al. ; Fukuoka Blood and Marrow Transplant Group (FBMTG). Evaluation of the feasibility and efficacy of autologous stem cell transplantation in elderly patients with multiple myeloma. Intern Med. 2013;52(1):63-70. [DOI] [PubMed] [Google Scholar]
- 39.Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28(5):1122-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wildes TM, Finney JD, Fiala M, et al. High-dose therapy and autologous stem cell transplant in older adults with multiple myeloma. Bone Marrow Transplant. 2015;50(8):1075-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rajkumar SV, Jacobus S, Callander NS, et al. ; Eastern Cooperative Oncology Group. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. 2010;11(1):29-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hernández JM, García-Sanz R, Golvano E, et al. Randomized comparison of dexamethasone combined with melphalan versus melphalan with prednisone in the treatment of elderly patients with multiple myeloma. Br J Haematol. 2004;127(2):159-164. [DOI] [PubMed] [Google Scholar]
- 43.Reece DE, Trieu Y, Masih-Khan E, et al. Cyclophosphamide and bortezomib with prednisone or dexamethasone for the treatment of relapsed and refractory multiple myeloma. Clin Lymphoma Myeloma Leuk. 2016;16(7):387-394. [DOI] [PubMed] [Google Scholar]
- 44.Pugh TJ, Chen C, Rabinovitch R, et al. Phase I trial of bortezomib and concurrent external beam radiation in patients with advanced solid malignancies. Int J Radiat Oncol Biol Phys. 2010;78(2):521-526. [DOI] [PubMed] [Google Scholar]
- 45.Terpos E, Morgan G, Dimopoulos MA, et al. International Myeloma Working Group recommendations for the treatment of multiple myeloma-related bone disease. J Clin Oncol. 2013;31(18):2347-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson K, Ismaila N, Flynn PJ, et al. Role of bone-modifying agents in multiple myeloma: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2018;36(8):812-818. [DOI] [PubMed] [Google Scholar]
- 47.Lahuerta J-J, Paiva B, Vidriales M-B, et al. ; GEM (Grupo Español de Mieloma)/PETHEMA (Programa para el Estudio de la Terapéutica en Hemopatías Malignas) Cooperative Study Group. Depth of response in multiple myeloma: a pooled analysis of three PETHEMA/GEM clinical trials. J Clin Oncol. 2017;35(25):2900-2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328-e346. [DOI] [PubMed] [Google Scholar]
- 49.Paiva B, Cedena MT, Puig N, et al. ; Grupo Español de Mieloma/Programa para el Estudio de la Terapéutica en Hemopatías Malignas (GEM/PETHEMA) Cooperative Study Groups. Minimal residual disease monitoring and immune profiling in multiple myeloma in elderly patients. Blood. 2016;127(25):3165-3174. [DOI] [PubMed] [Google Scholar]
- 50.Palumbo A, Gay F, Cavallo F, et al. Continuous therapy versus fixed duration of therapy in patients with newly diagnosed multiple myeloma. J Clin Oncol. 2015;33(30):3459-3466. [DOI] [PubMed] [Google Scholar]
- 51.Lonial S, Dimopoulos M, Palumbo A, et al. ; ELOQUENT-2 Investigators. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med. 2015;373(7):621-631. [DOI] [PubMed] [Google Scholar]
- 52.Moreau P, Masszi T, Grzasko N, et al. ; TOURMALINE-MM1 Study Group. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;374(17):1621-1634. [DOI] [PubMed] [Google Scholar]
- 53.Palumbo A, Chanan-Khan A, Weisel K, et al. ; CASTOR Investigators. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(8):754-766. [DOI] [PubMed] [Google Scholar]
- 54.Richardson PG, Rocafiguera AO, Beksac M, et al. Pomalidomide (POM), bortezomib, and low-dose dexamethasone (PVd) vs bortezomib and low-dose dexamethasone (Vd) in lenalidomide (LEN)-exposed patients (pt) with relapsed or refractory mulitple myeloma (RRMM): phase 3 OPTIMISMM trial. J Clin Oncol. 2018;36(15 suppl):8001. [Google Scholar]
- 55.Richardson PG, Sonneveld P, Schuster MW, et al. Reversibility of symptomatic peripheral neuropathy with bortezomib in the phase III APEX trial in relapsed multiple myeloma: impact of a dose-modification guideline. Br J Haematol. 2009;144(6):895-903. [DOI] [PubMed] [Google Scholar]
- 56.Soto-Perez-de-Celis E, Li D, Sun C-L, et al. Patient-defined goals and preferences among older adults with cancer starting chemotherapy [abstract]. J Clin Oncol. 2018;36(15 suppl):10009. [Google Scholar]
- 57.Chari A, Suvannasankha A, Fay JW, et al. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood. 2017;130(8):974-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mian HS, Wildes TM. Novel treatments for multiple myeloma: what role do they have in older adults? Drugs Aging. 2018;35(4):289-302. [DOI] [PubMed] [Google Scholar]
- 59.Dimopoulos MA, Moreau P, Palumbo A, et al. ; ENDEAVOR Investigators. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17(1):27-38. [DOI] [PubMed] [Google Scholar]
- 60.Stewart AK, Rajkumar SV, Dimopoulos MA, et al. ; ASPIRE Investigators. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372(2):142-152. [DOI] [PubMed] [Google Scholar]
- 61.Dimopoulos MA, Oriol A, Nahi H, et al. ; POLLUX Investigators. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(14):1319-1331. [DOI] [PubMed] [Google Scholar]
- 62.San-Miguel JF, Hungria VTM, Yoon S-S, et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 2014;15(11):1195-1206. [DOI] [PubMed] [Google Scholar]
- 63.Dimopoulos M, Dytfield D, Grosicki S, et al. Elotuzumab plus pomalidomide/dexamethasone (EPD) vs PD for treatment of relapsed/refractory multiple myeloma (RRMM): results from the phase 2, randomized open-label ELOQUENT-3 study. In: Proceedings from the 23rd Congress of the European Hematology Association. 14-17 June 2018. Stockholm, Sweden. Abstract LB2606. [Google Scholar]
- 64.Shah C, Bishnoi R, Jain A, et al. Cardiotoxicity associated with carfilzomib: systematic review and meta-analysis. Leuk Lymphoma. 2018;17:1-13. [DOI] [PubMed] [Google Scholar]
- 65.Plasmati R, Pastorelli F, Cavo M, et al. Neuropathy in multiple myeloma treated with thalidomide: a prospective study. Neurology. 2007;69(6):573-581. [DOI] [PubMed] [Google Scholar]
- 66.Mislang AR, Wildes TM, Kanesvaran R, et al. Adherence to oral cancer therapy in older adults: the International Society of Geriatric Oncology (SIOG) taskforce recommendations. Cancer Treat Rev. 2017;57:58-66. [DOI] [PubMed] [Google Scholar]
- 67.Kumar SK, LaPlant B, Roy V, et al. Phase 2 trial of ixazomib in patients with relapsed multiple myeloma not refractory to bortezomib. Blood Cancer J. 2015;5(8):e338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kumar S, Grzasko N, Delimpasi S, et al. Phase 2 study of the all-oral combination of ixazomib plus cyclophosphamide and low-dose dexamethasone (ICd) in patients (Pts) with relapsed/refractory multiple myeloma (RRMM). Clin Lymphoma Myeloma Leuk. 2017;17(1 suppl):e131. [Google Scholar]
- 69.Navaie-Waliser M, Feldman PH, Gould DA, Levine C, Kuerbis AN, Donelan K. When the caregiver needs care: the plight of vulnerable caregivers. Am J Public Health. 2002;92(3):409-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hamaker ME, Stauder R, van Munster BC. Exclusion of older patients from ongoing clinical trials for hematological malignancies: an evaluation of the National Institutes of Health Clinical Trial Registry. Oncologist. 2014;19(10):1069-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim ES, Bruinooge SS, Roberts S, et al. Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and Friends of Cancer Research joint research statement. J Clin Oncol. 2017;35(33):3737-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29(25):3457-3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hurria A, Mohile S, Gajra A, et al. Validation of a prediction tool for chemotherapy toxicity in older adults with cancer. J Clin Oncol. 2016;34(20):2366-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer. 2012;118(13):3377-3386. [DOI] [PubMed] [Google Scholar]
- 75.Mateos MV, Richardson PG, Schlag R, et al. Bortezomib plus melphalan and prednisone compared with melphalan and prednisone in previously untreated multiple myeloma: updated follow-up and impact of subsequent therapy in the phase III VISTA trial. J Clin Oncol. 2010;28(13):2259-2266. [DOI] [PubMed] [Google Scholar]
- 76.Mateos M-V, Oriol A, Martínez-López J, et al. Bortezomib, melphalan, and prednisone versus bortezomib, thalidomide, and prednisone as induction therapy followed by maintenance treatment with bortezomib and thalidomide versus bortezomib and prednisone in elderly patients with untreated multiple myeloma: a randomised trial. Lancet Oncol. 2010;11(10):934-941. [DOI] [PubMed] [Google Scholar]
- 77.Mateos M-V, Oriol A, Martínez-López J, et al. GEM2005 trial update comparing VMP/VTP as induction in elderly multiple myeloma patients: do we still need alkylators? Blood. 2014;124(12):1887-1893. [DOI] [PubMed] [Google Scholar]
- 78.Palumbo A, Bringhen S, Rossi D, et al. Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: a randomized controlled trial. J Clin Oncol. 2010;28(34):5101-5109. [DOI] [PubMed] [Google Scholar]
- 79.Tuchman SA, Moore JO, DeCastro CD, et al. Phase II study of dose-attenuated bortezomib, cyclophosphamide and dexamethasone (“VCD-Lite”) in very old or otherwise toxicity-vulnerable adults with newly diagnosed multiple myeloma. J Geriatr Oncol. 2017;8(3):165-169. [DOI] [PubMed] [Google Scholar]
- 80.Magarotto V, Bringhen S, Offidani M, et al. Triplet vs doublet lenalidomide-containing regimens for the treatment of elderly patients with newly diagnosed multiple myeloma. Blood. 2016;127(9):1102-1108. [DOI] [PubMed] [Google Scholar]
- 81.Facon T, Dimopoulos MA, Dispenzieri A, et al. Final analysis of survival outcomes in the phase 3 FIRST trial of up-front treatment for multiple myeloma. Blood. 2018;131(3):301-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Quach H, Fernyhough L, Henderson R, et al. Upfront lower dose lenalidomide is less toxic and does not compromise efficacy for vulnerable patients with relapsed refractory multiple myeloma: final analysis of the phase II RevLite study. Br J Haematol. 2017;177(3):441-448. [DOI] [PubMed] [Google Scholar]
- 83.Palumbo A, Hàjek R, Delforge M, et al. ; MM-015 Investigators. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N Engl J Med. 2012;366(19):1759-1769. [DOI] [PubMed] [Google Scholar]
- 84.Zweegman S, van der Holt B, Mellqvist U-H, et al. Melphalan, prednisone, and lenalidomide versus melphalan, prednisone, and thalidomide in untreated multiple myeloma. Blood. 2016;127(9):1109-1116. [DOI] [PubMed] [Google Scholar]
- 85.Stewart AK, Jacobus S, Fonseca R, et al. Melphalan, prednisone, and thalidomide vs melphalan, prednisone, and lenalidomide (ECOG E1A06) in untreated multiple myeloma. Blood. 2015;126(11):1294-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Palumbo A, Bringhen S, Larocca A, et al. Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: updated follow-up and improved survival. J Clin Oncol. 2014;32(7):634-640. [DOI] [PubMed] [Google Scholar]
- 87.Kumar SK, Laubach JP, Giove TJ, et al. Impact of concomitant dexamethasone dosing schedule on bortezomib-induced peripheral neuropathy in multiple myeloma. Br J Haematol. 2017;178(5):756-763. [DOI] [PubMed] [Google Scholar]