Abstract
More than 80% of patients with advanced-stage Hodgkin lymphoma are now cured with contemporary treatment approaches. The ongoing challenge is how to further improve outcomes by identifying both high-risk patients who may benefit from more intensive frontline therapy to reduce the risk of relapse as well as lower-risk patients who may do just as well with less intensive therapy. Numerous trials have used an interim positron emission tomography (PET) response-adapted approach to evaluate early escalation or deescalation of therapy for patients with a positive or negative interim PET scan, respectively. Recent trials have incorporated novel agents, including brentuximab vedotin (BV) and the immune checkpoint inhibitors, in the frontline setting. Based on results of the ECHELON-1 trial, the Food and Drug Administration approved BV in combination with adriamycin, vinblastine, and dacarbazine chemotherapy for stage III to IV Hodgkin lymphoma. Improved methods to assess higher risk at diagnosis using quantitative PET metrics, such as metabolic tumor volume and total lesion glycolysis, and incorporation of emerging biomarkers may further refine patient selection for more intensive upfront therapy. The ultimate goal is to achieve the highest level of efficacy for an individual patient while minimizing the short- and long-term toxicities.
Learning Objectives
Review the randomized trials supporting an interim positron emission tomography (PET) response-adapted approach to modify therapy for patients with an early favorable or unfavorable response
Review recent trials incorporating brentuximab vedotin (BV) and the immune checkpoint inhibitors in the frontline setting for advanced-stage Hodgkin lymphoma
Introduction
Patients with advanced-stage Hodgkin lymphoma (HL) have excellent outcomes with contemporary treatment approaches. Based on the balance of efficacy and toxicity, treatment with adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD) is considered a standard of care in North America with a 5-year progression-free survival (PFS) and overall survival (OS) rates of 74% and 88%, respectively, in the era before positron emission tomography (PET).1,2 When stratified by the International Prognostic Score (IPS), the 5-year PFS and OS rates were 77% and 91% for low-risk patients (IPS of 0-2) and 67% and 84% for high-risk patients (IPS ≥ 3), respectively.3,4 Two subgroups in particular, patients older than or equal to 60 years old and the adolescent and young adult (AYA) population, have inferior outcomes with standard therapy. In the North American Intergroup E2496 trial, 6% of patients were older than or equal to 60 years old and had inferior 5-year PFS and OS rates compared with younger patients (48% vs 74%, P = .002 and 58% vs 90%, P < .0001, respectively).3 A study comparing outcomes of AYA patients (ages 17-21) treated on E2496 with those with similar characteristics treated on the Children’s Oncology Group AHOD0031 trial suggested that this population may do somewhat better when treated on pediatric protocols.4
An alternative frontline regimen developed by the German Hodgkin Study Group (GHSG) is escalated bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine, and prednisone (escBEACOPP). In the HD15 trial, the 5/10-year PFS and OS rates after 6 cycles of escBEACOPP were 90%/84% and 95%/90%, respectively, with similar outcomes across all IPS risk groups.5,6 At least 4 randomized trials have compared ABVD with some version of escBEACOPP, and all trials have shown a PFS advantage of 6% to 18% in favor of escBEACOPP.7-10 However, OS has been difficult to establish due to potentially effective salvage strategies after ABVD failure. Additionally, long-term follow-up from the Italian HD2000 trial failed to confirm a durable PFS benefit with escBEACOPP, largely due to higher mortality rates from second cancers.9 Recent long-term follow-up from the HD15 trial with a median follow-up of 8.5 years has also reported 10-year cumulative incidence rates of second cancers of 7% and 10% for patients treated with 6 and 8 cycles of escBEACOPP, respectively.6 A 3-point risk score reported treatment-related mortality of 13% in patients older than or equal to 50 years with an Eastern Cooperative Oncology Group performance status of 2, and current guidelines from the GHSG restrict using escBEACOPP to patients younger than 60 years of age.11 Frontline therapy with escBEACOPP has been used in high-risk AYA patients with 5-year PFS and OS rates of 94% and 97%, respectively, but it had a 2% risk of developing secondary leukemia at a median follow-up of 6.3 years.12 Overall, due to the increased short- and long-term toxicities of escBEACOPP, the regimen has not gained wide acceptance in North America.
Currently, PET-computed tomography is an important tool in the management of HL with an established role in staging and response assessment at the end of therapy.13 In the past decade, several studies showed that a complete response (CR) at interim PET scan after 2 to 4 cycles of ABVD is predictive of favorable outcomes independent of IPS risk group.14-16 In the latter studies, variable definitions of a positive PET scan were used, and the 3-year PFS ranged from 13% to 53% for interim PET-positive patients compared with from 90% to 96% for PET-negative patients.15-17 To reduce interreader variability, the 5-point Deauville scoring system based on comparison of 18-fluoro-deoxyglucose uptake at disease sites with that of the mediastinal blood pool and liver was developed with good concordance.17,18 This score has been adopted in most of the recent risk-adapted approaches utilizing an interim PET to optimize selection of patients with a historically worse outcome who might benefit from intensifying therapy if an interim PET was positive (Deauville 4 and 5) or deescalating therapy to reduce toxicity if interim PET was negative (Deauville 1-3).
In March 2018, the US Food and Drug Administration (FDA) approved the anti-CD30 antibody-drug conjugate brentuximab vedotin (BV) in combination with adriamycin, vinblastine, and dacarbazine (AVD) as frontline therapy for stages III and IV HL.19 The latter study did not use an interim PET-based approach, and hence, direct comparison with established paradigms is difficult. Other trials are ongoing that incorporate the recently approved immune checkpoint inhibitors nivolumab and pembrolizumab in combination with chemotherapy in the frontline setting.20 The challenge now is how to choose from the various approaches or integrate them into day-to-day clinical practice. Herein, we review the results of randomized trials that have used a PET-adapted design as well as recent studies incorporating novel agents in the frontline setting for advanced-stage HL. The ultimate goal is to achieve the highest level of efficacy for an individual patient while minimizing the short- and long-term toxicities.
Trials evaluating therapy escalation in patients with a positive interim PET scan
Historically, patients with a positive interim PET scan have had inferior outcomes compared with patients with a negative scan. Efforts to improve outcomes in interim PET-positive patients have led to strategies to escalate therapy beyond ABVD (Table 1).
Table 1.
Trials evaluating therapy escalation in interim PET-positive patients
| Trial | N | Clinical stage | Median age (range), y | Initial therapy | Escalated therapy for PET+ patients | CMR definition | % PET+ | Median follow-up, mo | PFS for PET+ patients, % (y) | OS for PET+ patients, % (y) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| US Intergroup S081621,22 | 358 | III-IV | 32 (18-60) | ABVD ×2 | EB ×6 | DS 1-3 | 22 | 45 | 64 (2) | 98 (2) | |
| United Kingdom RATHL23 | 1214 | IIB-IV or IIA unfavorable | 32 (18-79) | ABVD ×2 | EB ×3 or | DS 1-3 | 16 | 41 | 68 (3) | 86 (3) | |
| BEACOPP-14 ×4 | |||||||||||
| HD 060724 | 782 | IIB-IV | 31 (14-60) | ABVD ×2 | EB ×4 + BEACOPP ×4 ± rituximab | DS 1-3 | 19 | 43 | 60 (3) | 89 (3) | |
| GHSG HD1825 | 2101 | IIB-IV | 31 (23-44) | EB ×2 | EB ×6 + rituximab | DS 1-2 | 48 | 66 | 88.1 (5) | 93.9 (5) | |
| Control group: EB ×6 | 89.7 (5) | 96.4 (5) | |||||||||
| HD 080126 | 519 | IIB-IV | 33 (18-68) | ABVD ×2 | IGEV ×4 + ASCT | DS 1-2 | 20 | 27 | 76 (2) | 97 (2) | |
| Israeli H227 | 185 | IIB-IV, IPS 0-2 | 30 (18-60) | ABVD ×2 | EB ×4 | DS 1-3 | 12 | 55 | 68 (5) | 91 (5) |
ASCT, autologous stem cell transplantation; BEACOPP, bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine, and prednisone; BEACOPP-14, BEACOPP administered in 14-day cycles; CMR, complete metabolic response; DS, Deauville score; EB, escBEACOPP; IGEV, ifosfamide, gemcitabine, and vinorelbine; N, number of patients; RATHL, Risk-Adapted Therapy in Hodgkin Lymphoma.
The US intergroup S0816 trial conducted between 2009 and 2012 enrolled 358 patients with stages III and IV HL (median age of 32 years, range of 18-60), all of whom received 2 cycles of ABVD followed by an interim PET scan that was centrally reviewed.21,22 PET-negative patients (Deauville 1-3, 82%) received 4 additional cycles of ABVD, whereas those with PET-positive disease received further intensified therapy with 6 cycles of escBEACOPP. No radiotherapy was administered. At a median follow-up of 45 months, the 2-year PFS was 82% among PET-negative patients and 64% among PET-positive patients, with the latter far exceeding the historical 2-year PFS of 15% to 30% for interim PET-positive patients continuing on ABVD. Grades 4 and 5 toxicities were more common with escBEACOPP than ABVD (85.7% vs 36.7%, P < .001).
The United Kingdom Risk-Adapted Therapy in Hodgkin Lymphoma (RATHL) trial conducted between 2008 and 2012 included 1214 patients with stages IIB to IV or stage IIA disease with adverse features including bulk or ≥3 involved sites.23 Median age was 32 years (range of 18-79), with <10% of patients older than or equal to 60 years old. As in the US Intergroup S0816 trial, all patients received 2 cycles of ABVD followed by an interim PET. PET-positive patients (Deauville 4 and 5, 16%) received intensified therapy with either 3 cycles of escBEACOPP or 4 cycles of BEACOPP-14 (14-day cycles). At a median follow-up of 41 months, the 3-year PFS and OS rates were 68% and 86%, respectively, with no difference between patients receiving escBEACOPP and BEACOPP-14. In contrast, PET-negative patients (84%) had 3-year PFS and OS rates of 85% and 97%, respectively. Grades 3 and 4 toxicities were more common with bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP) than ABVD (81% vs 65%, respectively), and they were mainly hematologic.
The Gruppo Italiano Terapie Innovative Nei Linfomi/Fondazione Italiana Linfomi HD 0607 trial conducted between 2008 and 2014 enrolled 782 patients with stages IIB to IV HL (median age of 31 years, range of 14-60), all of whom received 2 cycles of ABVD followed by an interim PET scan.24 PET-negative patients (Deauville 1-3, 81%) continued on ABVD for a total of 6 cycles, whereas PET-positive patients (Deauville 4 and 5, 19%) were randomized to receive 4 cycles of escBEACOPP followed by 4 cycles of baseline BEACOPP with or without rituximab. With a median follow-up of 43 months, the 3-year PFS and OS rates were 60% and 89%, respectively, for PET-positive patients, with no difference in PFS between patients receiving rituximab or no rituximab (63% vs 57%, P = .53). For PET-negative patients, the 3-year PFS and OS rates were 87% and 99%, respectively.
The GHSG HD18 trial conducted between 2008 and 2014 enrolled 2101 patients with stages IIB to IV HL (median age of 31 years, range of 23-44), all of whom received 2 cycles of escBEACOPP followed by an interim PET scan.25 In contrast to the trials discussed above, a positive interim PET was defined as a Deauville score of 3 to 5. Interim PET-positive patients (48%) were randomized to receive 6 additional cycles of escBEACOPP with or without rituximab. With a median follow-up of 66 months, the 5-year PFS and OS rates were 88.1% and 93.9%, respectively, for patients receiving BEACOPP and rituximab compared with 89.7% and 96.4%, respectively, for patients receiving BEACOPP alone (P = .46 and .25, respectively). Interestingly, there was no significant difference in 5-year PFS between interim PET-positive and PET-negative patients (88.3% vs 91.4%, P = .30), although this may in part reflect the different definition of a positive interim PET used in this study.
As in the GHSG HD 18 trial, the Italian HD0801 trial conducted between 2008 and 2013 used a more conservative definition of a negative scan (Deauville 1 and 2) and evaluated a more aggressive salvage regimen for interim PET-positive patients incorporating early autologous stem cell transplantation (ASCT)26; 519 patients with stages IIB to IV HL (median age of 33 years, range of 18-68) received 2 cycles of ABVD followed by an interim PET. PET-negative patients (80%) continued on ABVD to complete 6 cycles, whereas PET-positive patients (20%) received salvage therapy with 4 cycles of ifosfamide, gemcitabine, and vinorelbine followed by ASCT with carmustine, etoposide, cytarabine, and melphalan conditioning. At a median follow-up of 27 months, PET-positive patients had a 2-year PFS of 76%, which was similar to the 2-year PFS of 81% in those with a negative PET scan. Excluding the patients who had a Deauville score of 3, the 2-year PFS for PET-positive patients with a Deauville score of 4 to 5 was still excellent at 75%. Grades 3 and 4 adverse events were primarily hematologic, and no treatment-related deaths occurred in the PET-positive patients who underwent ASCT.
An alternative strategy of patient selection based on IPS risk was used in the Israeli H2 study conducted between 2006 and 2013, which enrolled 185 patients with stages IIB to IV HL (median age of 30 years, range of 18-60).27 Low-risk patients (IPS 0-2) received initial therapy of 2 cycles of ABVD, and high-risk patients (IPS ≥ 3) received 2 cycles of escBEACOPP, both of which were followed by an interim PET scan. Of the 111 patients with IPS 0 to 2, 13 (12%) had a positive interim PET scan (Deauville 4 and 5) and subsequently received intensified therapy with 4 cycles of escBEACOPP and 30 Gy involved site radiotherapy if bulky (>10 cm) mediastinal disease. With a median follow-up of 55 months, the 5-year PFS and OS rates were 68% and 91%, respectively, for IPS 0 to 2 interim PET-positive patients.
Cumulatively, these trials suggest that, compared with historical controls, the negative prognostic impact of a positive interim PET scan after 2 cycles of ABVD may only be partially overcome by switching to intensified treatment, and currently, this strategy is recommended as one of the options in the current National Comprehensive Cancer Network (NCCN) guidelines.1 It is important to note that major limitations of these trials are the lack of a control group continuing on ABVD regardless of interim PET results as well the lack of applicability to elderly patients, because most trials either excluded or had insufficient numbers to assess outcomes in this subgroup. In general, intensification is not appropriate for elderly patients due to poor tolerance of BEACOPP.
Trials evaluating therapy deescalation in patients with a negative interim PET scan
In an effort to reduce acute and cumulative toxicity for patients with an early favorable response, several studies have evaluated therapy deescalation for patients with a negative interim PET scan (Table 2).
Table 2.
Trials evaluating therapy deescalation in interim PET-negative patients
| Trial | N | Clinical stage | Median age (range), y | Initial therapy | Subsequent therapy for PET− patients | CMR definition | % PET− | Median follow-up, mo | PFS for PET− patients, % (y) | OS for PET− patients, % (y) |
|---|---|---|---|---|---|---|---|---|---|---|
| United Kingdom RATHL23 | 1214 | IIB-IV or IIA unfavorable | 32 (18-79) | ABVD ×2 | Experimental: AVD ×4 | DS 1-3 | 84 | 41 | 84.4 (3) | 98.7 (3) |
| Control: ABVD ×4 | 85.7 (3) | 98.4 (3) | ||||||||
| Israeli H227 | 185 | IIB-IV, IPS ≥ 3 | 30 (18-60) | EB ×2 | Experimental: ABVD ×4 | DS 1-3 | 80 | 55 | 81.5 (5) | 96.8 (5) |
| Control: none | ||||||||||
| AHL 2011 LYSA28 | 823 | IIB-IV | 30 (16-60) | EB ×2 | Experimental: ABVD ×4 | DS 1-3 | 88 | 16 | 91.6 (2) | NR |
| Control: EB ×4 | 94.0 (2) | NR | ||||||||
| GHSG HD1825 | 2101 | IIB-IV | 31 (23-44) | EB ×2 | Experimental: EB ×2 | DS 1-2 | 52 | 66 | 92.2 (5) | 97.7 (5) |
| Control: EB ×4-6 | 90.8 (5) | 95.4 (5) |
CMR, complete metabolic response; DS, Deauville score; EB, escBEACOPP; N, number of patients; NR, not reported.
In the aforementioned RATHL trial, patients with a negative interim PET scan (Deauville 1-3, 84%) after 2 cycles of ABVD were randomized to either continue on ABVD for 4 additional cycles or 4 cycles of AVD with omission of bleomycin.23 At a median follow-up of 41 months, the 3-year PFS was similar between the ABVD and AVD groups (85.7% vs 84.4%, respectively), with significantly more grades 3 to 4 pulmonary events occurring in the ABVD group (15 vs 3 events, P < .05). There was also a greater reduction of the diffusing capacity of the lung for carbon monoxide among patients receiving ABVD than AVD, with an absolute difference of –7.4% (P < .001). The 3-year OS was excellent in both groups (98.4% vs 98.7%, respectively).
The AHL 2011 LYSA study conducted between 2011 and 2014 randomized 823 patients with stages IIB to IV HL (median age of 30 years, range of 16-60) to standard therapy with 6 cycles of escBEACOPP or experimental therapy utilizing an interim PET response-adapted design.28 In the latter arm (N = 401), patients received 2 cycles of escBEACOPP, and those with a positive interim PET (Deauville 4 and 5) received 4 additional cycles of BEACOPP, whereas therapy was deescalated to 4 cycles of ABVD in PET-negative patients (Deauville 1-3). At a median follow-up of 16 months, 88% of patients were PET negative, with a similar 2-year PFS in the standard and experimental arms (94% vs 91.6%, respectively) and significantly less grades 3 and 4 hematologic toxicity in patients receiving ABVD.
The aforementioned Israeli H2 trial used a similar deescalation strategy for patients with high-risk disease (IPS ≥ 3).27 After 2 cycles of escBEACOPP, interim PET-positive patients (Deauville 4 and 5) received 4 additional cycles of escBEACOPP, and therapy was deescalated to 4 cycles of ABVD in PET-negative patients (Deauville 1-3). Radiotherapy was administered to bulky disease sites at the discretion of the treating physician. Among PET-negative patients (80% of patients with IPS ≥ 3), the 5-year PFS and OS rates were 81.5% and 96.8%, respectively. The latter outcomes were similar to those of PET-negative patients with IPS 0 to 2, all of whom received 6 cycles of ABVD with 5-year PFS and OS rates of 80.4% and 96.9%, respectively. For both the AHL 2011 LYSA and Israeli H2 studies, it is likely that similar outcomes would have been achieved even with deescalation to AVD (instead of ABVD) based on the RATHL trial, which showed that AVD is equivalent to ABVD for interim PET-negative patients.23
The GHSG HD18 trial used a response-adapted design to assess whether interim PET-negative patients could receive fewer cycles of escBEACOPP without compromising efficacy25; 2101 patients with stages IIB to IV HL received 2 cycles of escBEACOPP followed by an interim PET. Patients with a negative interim PET (defined as a Deauville score of 1 or 2 in this study, 52%) were randomized to receive a total of 4, 6, or 8 cycles of escBEACOPP. The 5-year PFS was 92.2% for those receiving 4 cycles of BEACOPP vs 90.8% for those receiving 6 to 8 cycles of BEACOPP (P = .30), with a higher incidence of grades 3 and 4 hematologic toxicity and infections in the latter group.
Cumulatively, these trials suggest that therapy can be deescalated in patients with a negative interim PET without compromising efficacy and reducing toxicity. The omission of bleomycin in interim PET-negative patients after 2 cycles of ABVD has been incorporated into the NCCN guidelines as a strategy for frontline therapy.1
Trials incorporating novel agents in the frontline setting for advanced-stage HL
The anti-CD30 antibody-drug conjugate BV and immune checkpoint inhibitors, nivolumab and pembrolizumab, are FDA approved for the treatment of relapsed and refractory HL with high response rates and a favorable safety profile.29-32 Recently, studies have evaluated incorporating these agents in the frontline setting.
The ECHELON-1 trial was an international phase 3 trial conducted between 2012 and 2016; 1334 patients with stages III and IV HL were randomized to receive either standard therapy with 6 cycles of ABVD or experimental therapy with 6 cycles of BV-AVD.19 Median age was 36 years (range of 18-83), with <15% of patients older than or equal to 60 years old. In contrast to a standard definition of PFS, the primary end point of this study was a modified progression-free survival (mPFS) defined as the time to disease progression, death, or a modified progression event (lack of a CR [Deauville 3-5] after completion of frontline therapy and receipt of subsequent anticancer therapy). An interim PET scan was also performed after 2 cycles of BV-AVD; however, these results were not used to modify subsequent therapy. At a median follow-up of 24.6 months, the 2-year mPFS was modestly higher in the BV-AVD group than in the ABVD group (82.1% vs 77.2%; hazard ratio [HR], 0.77; 95% confidence interval [95% CI], 0.60-0.98; P = .04) with no significant difference in OS (96.6% vs 94.2%; HR, 0.73; 95% CI, 0.45-1.18; P = .20). In a prespecified subgroup analysis, the benefit of BV-AVD was greater in high-risk patients with IPS 4 to 7 (HR, 0.70; 95% CI, 0.46-1.07) and in North America (HR, 0.60; 95% CI, 0.40-0.90) compared with Europe (HR, 0.83; 95% CI, 0.59-1.17) or Asia (HR, 0.91; 95% CI, 0.43-1.94). Older adults older than or equal to 60 years old had similar outcomes in both arms (HR, 1.0; 95% CI, 0.58-1.72). Due to a higher incidence of febrile neutropenia in the BV-AVD arm, the trial was amended to mandate growth factor support. Patients who subsequently received primary prophylaxis with growth factors had a reduced risk of grades 3 and 4 neutropenia (29% vs 70%, respectively) and febrile neutropenia (11% vs 21%, respectively) than those who did not receive prophylaxis. There was also a higher incidence of grade 3 neuropathy in the BV-AVD arm (11% vs 2%, respectively), which resolved or improved in two-thirds of patients.
A recent post hoc analysis suggests that the mPFS benefit of BV-AVD was greatest in patients with stage IV disease (HR, 0.71; 95% CI, 0.53-0.96) and those with extranodal sites (HR, 0.70; 95% CI, 0.52-0.94).33 Interestingly, the magnitude of the mPFS benefit with BV-AVD was largely restricted to patients with a positive interim PET scan (Deauville 4 and 5) after 2 cycles of therapy (57.5% vs 42.0%; HR, 0.61; 95% CI, 0.34-1.09 compared with 85.2% vs 80.9%; HR, 0.77; 95% CI, 0.59-1.02 for those with a negative interim PET scan).34 Additionally, for patients treated in North America, similar to the mPFS, the investigator-assessed 2-year PFS (using a conventional definition) was also greater in the BV-AVD arm (88.1% vs 76.4%; HR, 0.50; 95% CI, 0.32-0.79; P = .002).35
Cumulatively, the results of the ECHELON-1 trial suggest that the addition of BV to AVD may be another approach to increase the efficacy of initial therapy, particularly among patients with the highest-risk disease, although longer follow-up will be important to define its full potential. In March 2018, BV was approved in combination with AVD by the FDA for the frontline treatment of stages III and IV HL. The NCCN guidelines have now incorporated BV-AVD as a category 2B option for stages III and IV HL and a category 2A option for select patients without baseline neuropathy and IPS 4 to 7 or inability to tolerate bleomycin.1 Although not part of the formal study design, the ECHELON-1 trial is the only study that reports outcomes of patients treated with 6 cycles of ABVD irrespective of interim PET results. The 2-year PFS of 42% in interim PET-positive patients suggests that this is a subgroup that needs alternative approaches, because results with escalation of therapy to escBEACOPP or BV-AVD seem similar, with only a modest PFS improvement of ∼57.5% to 68%.
In an attempt to reduce the toxicity of escBEACOPP, the GHSG has tested variants of the regimen in which BV has been added to frontline therapy. Bleomycin and vincristine have been omitted from these regimens to not exacerbate pulmonary toxicity or neuropathy. In one variant, dacarbazine was substituted for procarbazine in an effort to reduce the risk of secondary leukemia. In a phase 2 trial, 104 patients with stages IIB to IV HL were randomized to receive 6 cycles of brentuximab vedotin, etoposide, cyclophosphamide, adriamycin, procarbazine, and prednisone (BrECAPP) or 6 cycles of brentuximab vedotin, etoposide, cyclophosphamide, adriamycin, dacarbazine, and dexamethasone (BrECADD).36 With a median follow-up of 17 months, the 18-month PFS estimates were 95% and 89% for patients receiving BrECAPP and BrECADD, respectively, and they compared favorably with results with 6 cycles of escBEACOPP in HD15. The toxicity profile of both experimental regimens also compared favorably with escBEACOPP. For example, dose reductions were required in 40% of patients receiving BrECAPP and 31% of patients receiving BrECADD compared with 51% of patients who received escBEACOPP in the HD15 trial. Although both BrECAPP and BrECADD met the primary efficacy end points, BrECADD had a more favorable toxicity profile and is now being compared with escBEACOPP in a randomized phase 3 trial for patients with advanced-stage HL (NCT02661503).
The phase 2 CheckMate 2015 study evaluated a lead in with 4 cycles of nivolumab alone followed by 6 cycles of nivolumab with AVD for patients with stages IIB to IV HL.20 Fifty-one patients were treated with a median follow-up of 9 months. After 4 cycles of nivolumab alone, the overall response rate (ORR) as assessed by an independent review committee was 69%, with a CR rate of 18%. At the end of therapy, the ORR was 84%, with a CR rate of 67% and an mPFS of 94% at 9 months. Treatment was overall well tolerated, with grades 3 and 4 adverse events occurring in 20% of patients. Immune-related adverse events occurred in 10 patients and were typically grades 1 and 2, including 8 patients with hypothyroidism and 2 patients with elevated transaminases. Longer follow-up is required to assess the durability of the response and mPFS.
Given the single-agent activity and nonoverlapping toxicity of BV and the immune checkpoint inhibitors in relapsed HL, several ongoing phase 1 and 2 studies are evaluating frontline therapy with BV and/or checkpoint inhibitors alone or with chemotherapy in HL (Table 3). The results of these studies are eagerly awaited.
Table 3.
Ongoing trials incorporating novel agents in the frontline setting for advanced-stage HL
| Trial | Trial phase | Patient population | Treatment regimen |
|---|---|---|---|
| NCT03033914 | Phase 1 | Stage III-IV HL with IPS ≥ 3 or positive interim PET | A(B)VD followed by nivolumab |
| NCT03226249 | Phase 2 | Early- and advanced-stage HL | Pembrolizumab followed by AVD |
| NCT02758717 | Phase 2 | Age ≥60 or inability to tolerate chemotherapy due to impaired cardiac, pulmonary, or renal function | Brentuximab and nivolumab |
| NCT03331731 | Phase 2 | Age ≥65 or inability to tolerate chemotherapy due to impaired cardiac, pulmonary, or renal function | Pembrolizumab |
Future directions
Although response at interim PET is a powerful prognostic factor, it is not a perfect tool, and relapses occur in ∼20% of interim PET-negative patients, particularly those with stage IV disease or IPS 4 to 7. Better methods to define baseline risk, such as metabolic tumor volume, total lesion glycolysis (TLG), and PD-L1 amplification, are exciting areas of active investigation, and they have the potential to further refine upfront therapy.37-39 In a large international trial, baseline TLG was identified as a strong, independent predictor of outcome.40 Patients with high baseline TLG were twice as likely to have treatment failure or recurrence as those with low values, including patients who achieved a CR at interim PET scan.
Recently, in patients with a negative interim PET scan, a prognostic score based on assessment of pretherapy diagnostic tissue for expression of CD68 (≥25%), PD1 (diffuse/rosetting vs a scattered pattern) in microenvironmental cells, and STAT1 negativity in Reed–Sternberg cells identified a subset of patients who had a significantly lower 3-year PFS of 64% vs 95% for high vs low risk, respectively (P < .0001).41 Response-adapted approaches may also be improved by other techniques, such as the detection and monitoring of circulating tumor DNA.42
Conclusion
The management of advanced-stage HL continues to evolve, and choosing an optimal regimen is now more challenging than ever. An interim PET response-adapted approach as well as frontline incorporation of BV for select patients per the ECHELON-1 trial are both effective options, which are shifting treatment paradigms. Although BV-AVD was recently FDA approved for advanced-stage HL, the lack of an OS benefit, greater risk of neuropathy, neutropenia mandating granulocyte colony-stimulating factor support, and considerable cost are important limitations. Longer follow-up is also required to determine the durability of mPFS.
The optimal therapy for elderly patients and AYAs continues to be challenging, and trials specific for these subgroups are ongoing with encouraging results.43,44
At a practical level, for patients with low-risk disease, initial treatment with ABVD is a reasonable strategy with low toxicity and good predictive power from a negative interim PET scan. Most studies support that ∼80% of patients will achieve a negative interim PET scan, and based on the RATHL trial, bleomycin can be omitted from further cycles of therapy. The 3-year PFS and OS rates with the latter approach compare favorably with those achieved with BV-AVD. In contrast, for patients with higher-risk disease, initial therapy with escBEACOPP (for patients younger than 60 years old) and deescalating to AVD if interim PET is negative or if using frontline BV-AVD seems to improve disease control (Figure 1). In the absence of a randomized trial, comparison of these strategies is limited, and ultimately, the choice will require a thoughtful discussion of the pros and cons of the different strategies. Additional improvements in these already robust results will likely involve integration of other novel agents, like the immune checkpoint inhibitors, as well as refining prognostic tools to better assess baseline risk beyond the IPS.
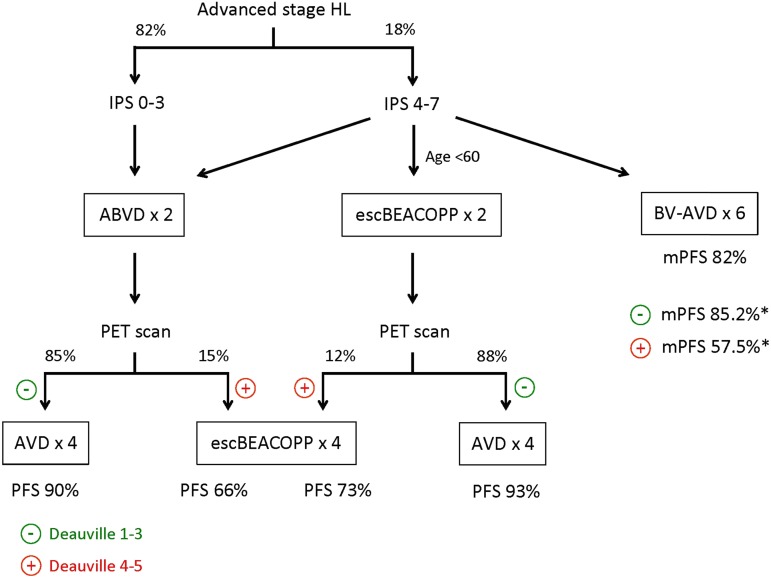
Figure 1.
Therapy options for advanced-stage HL. The flowchart shows therapy options for patients with advanced-stage HL based on IPS risk and response on interim PET scan. Percentages of patients with a positive or negative interim PET scan and PFS percentages are based on the RATHL, the AHL 2011 LYSA, and the ECHELON-1 trials, assuming that AVD is equivalent to ABVD for interim PET-negative patients. *mPFS values are for patients who had a negative or positive interim PET scan after 2 cycles of BV-AVD. Adapted with permission from Lim and Johnson.45
References
- 1.Hoppe RT, Advani RH, Ai WZ, et al. . NCCN guidelines insights: Hodgkin lymphoma, version 1.2018. J Natl Compr Canc Netw. 2018;16(3):245-254. [DOI] [PubMed] [Google Scholar]
- 2.Gordon LI, Hong F, Fisher RI, et al. . Randomized phase III trial of ABVD versus Stanford V with or without radiation therapy in locally extensive and advanced-stage Hodgkin lymphoma: an intergroup study coordinated by the Eastern Cooperative Oncology Group (E2496). J Clin Oncol. 2013;31(6):684-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evens AM, Hong F, Gordon LI, et al. . The efficacy and tolerability of adriamycin, bleomycin, vinblastine, dacarbazine and Stanford V in older Hodgkin lymphoma patients: a comprehensive analysis from the North American intergroup trial E2496. Br J Haematol. 2013;161(1):76-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henderson TO, Parsons SK, Wroblewski KE, et al. . Outcomes in adolescents and young adults with Hodgkin lymphoma treated on US cooperative group protocols: an adult intergroup (E2496) and Children’s Oncology Group (COG AHOD0031) comparative analysis. Cancer. 2018;124(1):136-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engert A, Haverkamp H, Kobe C, et al. ; Arbeitsgemeinschaft Medikamentöse Tumortherapie. Reduced-intensity chemotherapy and PET-guided radiotherapy in patients with advanced stage Hodgkin’s lymphoma (HD15 trial): a randomised, open-label, phase 3 non-inferiority trial. Lancet. 2012;379(9828):1791-1799. [DOI] [PubMed] [Google Scholar]
- 6.Engert A, Goergen H, Markova J, et al. . Reduced-intensity chemotherapy in patients with advanced-stage Hodgkin lymphoma: updated results of the open-label, international, randomised phase 3 HD15 trial by the German Hodgkin Study Group. HemaSphere. 2017;1(1):e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viviani S, Zinzani PL, Rambaldi A, et al. ; Intergruppo Italiano Linfomi. ABVD versus BEACOPP for Hodgkin’s lymphoma when high-dose salvage is planned. N Engl J Med. 2011;365(3):203-212. [DOI] [PubMed] [Google Scholar]
- 8.Mounier N, Brice P, Bologna S, et al. ; Lymphoma Study Association (LYSA). ABVD (8 cycles) versus BEACOPP (4 escalated cycles ≥ 4 baseline): final results in stage III-IV low-risk Hodgkin lymphoma (IPS 0-2) of the LYSA H34 randomized trial. Ann Oncol. 2014;25(8):1622-1628. [DOI] [PubMed] [Google Scholar]
- 9.Merli F, Luminari S, Gobbi PG, et al. . Long-term results of the HD2000 trial comparing ABVD versus BEACOPP versus COPP-EBV-CAD in untreated patients with advanced hodgkin lymphoma: a study by fondazione Italiana Linfomi. J Clin Oncol. 2016;34(11):1175-1181. [DOI] [PubMed] [Google Scholar]
- 10.Carde P, Karrasch M, Fortpied C, et al. . Eight cycles of ABVD versus four cycles of BEACOPPescalated plus four cycles of BEACOPPbaseline in stage III to IV, international prognostic score ≥ 3, high-risk Hodgkin lymphoma: first results of the phase III EORTC 20012 Intergroup Trial. J Clin Oncol. 2016;34(17):2028-2036. [DOI] [PubMed] [Google Scholar]
- 11.Wongso D, Fuchs M, Plütschow A, et al. . Treatment-related mortality in patients with advanced-stage hodgkin lymphoma: an analysis of the German Hodgkin Study Group. J Clin Oncol. 2013;31(22):2819-2824. [DOI] [PubMed] [Google Scholar]
- 12.Kelly KM, Sposto R, Hutchinson R, et al. . BEACOPP chemotherapy is a highly effective regimen in children and adolescents with high-risk Hodgkin lymphoma: a report from the Children’s Oncology Group. Blood. 2011;117(9):2596-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Juweid ME, Stroobants S, Hoekstra OS, et al. ; Imaging Subcommittee of International Harmonization Project in Lymphoma. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007;25(5):571-578. [DOI] [PubMed] [Google Scholar]
- 14.Hutchings M, Loft A, Hansen M, et al. . FDG-PET after two cycles of chemotherapy predicts treatment failure and progression-free survival in Hodgkin lymphoma. Blood. 2006;107(1):52-59. [DOI] [PubMed] [Google Scholar]
- 15.Cerci JJ, Pracchia LF, Linardi CCG, et al. . 18F-FDG PET after 2 cycles of ABVD predicts event-free survival in early and advanced Hodgkin lymphoma. J Nucl Med. 2010;51(9):1337-1343. [DOI] [PubMed] [Google Scholar]
- 16.Gallamini A, Hutchings M, Rigacci L, et al. . Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin’s lymphoma: a report from a joint Italian-Danish study. J Clin Oncol. 2007;25(24):3746-3752. [DOI] [PubMed] [Google Scholar]
- 17.Biggi A, Gallamini A, Chauvie S, et al. . International validation study for interim PET in ABVD-treated, advanced-stage hodgkin lymphoma: interpretation criteria and concordance rate among reviewers. J Nucl Med. 2013;54(5):683-690. [DOI] [PubMed] [Google Scholar]
- 18.Meignan M, Gallamini A, Meignan M, Gallamini A, Haioun C. Report on the First International Workshop on Interim-PET-Scan in Lymphoma. Leuk Lymphoma. 2009;50(8):1257-1260. [DOI] [PubMed] [Google Scholar]
- 19.Connors JM, Jurczak W, Straus DJ, et al. ; ECHELON-1 Study Group. Brentuximab vedotin with chemotherapy for stage III or IV Hodgkin’s lymphoma. N Engl J Med. 2018;378(4):331-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramchandren R, Fanale MA, Rueda A, et al. . Nivolumab for newly diagnosed advanced-stage classical Hodgkin lymphoma (cHL): results from the phase 2 Checkmate 205 Study. Blood. 2017;130:651. [Google Scholar]
- 21.Press OW, Li H, Schöder H, et al. . US intergroup trial of response-adapted therapy for stage III to IV Hodgkin lymphoma using early interim fluorodeoxyglucose-positron emission tomography imaging: Southwest Oncology Group S0816. J Clin Oncol. 2016;34(17):2020-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedberg JW, Li H, Schoder H, et al. . Long-term follow-up of SWOG S0816: response-adapted therapy of advanced stage Hodgkin lymphoma using early interim FDG-PET imaging. Haematologica. 2016;101:T003. [Google Scholar]
- 23.Johnson P, Federico M, Kirkwood A, et al. . Adapted treatment guided by interim PET-CT scan in advanced Hodgkin’s lymphoma. N Engl J Med. 2016;374(25):2419-2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gallamini A, Tarella C, Viviani S, et al. . Early chemotherapy intensification with escalated BEACOPP in patients with advanced-stage Hodgkin lymphoma with a positive interim positron emission tomography/computed tomography scan after two abvd cycles: long-term results of the GITIL/FIL HD 0607 trial. J Clin Oncol. 2018;36(5):454-462. [DOI] [PubMed] [Google Scholar]
- 25.Borchmann P, Goergen H, Kobe C, et al. . PET-guided treatment in patients with advanced-stage Hodgkin’s lymphoma (HD18): final results of an open-label, international, randomised phase 3 trial by the German Hodgkin Study Group. Lancet. 2018;390(10114):2790-2802. [DOI] [PubMed] [Google Scholar]
- 26.Zinzani PL, Broccoli A, Gioia DM, et al. . Interim positron emission tomography response-adapted therapy in advanced-stage hodgkin lymphoma: final results of the phase II part of the HD0801 study. J Clin Oncol. 2016;34(12):1376-1385. [DOI] [PubMed] [Google Scholar]
- 27.Dann EJ, Bairey O, Bar-Shalom R, et al. . Modification of initial therapy in early and advanced Hodgkin lymphoma, based on interim PET/CT is beneficial: a prospective multicentre trial of 355 patients. Br J Haematol. 2017;178(5):709-718. [DOI] [PubMed] [Google Scholar]
- 28.Casasnovas O, Brice P, Bouabdallah R, et al. . Randomized phase III study comparing an early PET driven treatment de-escalation to a not PET-monitored strategy in patients with advanced stages Hodgkin lymphoma: interim analysis of the AHL2011 Lysa Study. Blood. 2015;126(23):577. [Google Scholar]
- 29.Younes A, Gopal AK, Smith SE, et al. . Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol. 2012;30(18):2183-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ansell SM, Lesokhin AM, Borrello I, et al. . PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Younes A, Santoro A, Shipp M, et al. . Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016;17(9):1283-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen R, Zinzani PL, Fanale MA, et al. ; KEYNOTE-087. Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin Lymphoma. J Clin Oncol. 2017;35(19):2125-2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hutchings M, Radford J, Gallamini A, et al. . Brentuximab vedotin plus chemotherapy in high risk advanced-staged classical Hodgkin lymphoma (CHL) patients: results of pre-specified sub-group analyses from the ECHELON-1 study. Haematologica. 2018;2018:S112. [Google Scholar]
- 34.Chen R, Ansell S, Gallamini A, et al. . Brentuximab vedotin with chemotherapy for stage III or IV Hodgkin lymphoma (HL): impact of cycle 2 PET result on modified progression-free survival (mPFS). J Clin Oncol. 2018;36:7539. [Google Scholar]
- 35.Ramchandren R, Advani RH, Ansell SM, et al. . Brentuximab vedotin (BV) plus chemotherapy in patients with newly diagnosed advanced stage Hodgkin lymphoma (HL): North American results. J Clin Oncol. 2018;36:7541. [Google Scholar]
- 36.Eichenauer DA, Plütschow A, Kreissl S, et al. . Incorporation of brentuximab vedotin into first-line treatment of advanced classical Hodgkin’s lymphoma: final analysis of a phase 2 randomised trial by the German Hodgkin Study Group. Lancet Oncol. 2017;18(12):1680-1687. [DOI] [PubMed] [Google Scholar]
- 37.Kanoun S, Rossi C, Berriolo-Riedinger A, et al. . Baseline metabolic tumour volume is an independent prognostic factor in Hodgkin lymphoma. Eur J Nucl Med Mol Imaging. 2014;41(9):1735-1743. [DOI] [PubMed] [Google Scholar]
- 38.Akhtari M, Milgrom SA, Pinnix CC, et al. . Reclassifying patients with early-stage Hodgkin lymphoma based on functional radiographic markers at presentation. Blood. 2018;131(1):84-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roemer MGM, Advani RH, Ligon AH, et al. . PD-L1 and PD-L2 genetic alterations define classical hodgkin lymphoma and predict outcome. J Clin Oncol. 2016;34(23):2690-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pike L, Kirkwood A, Patrick P, et al. . Can baseline PET-CT features predict outcomes in advanced Hodgkin lymphoma? A prospective evaluation of UK patients in the RATHL trial (CRUK/07/033). Hematol Oncol. 2017;35(suppl 2):37-38.28591427 [Google Scholar]
- 41.Agostinelli C, Gallamini A, Stracqualursi L, et al. . The combined role of biomarkers and interim PET scan in prediction of treatment outcome in classical Hodgkin’s lymphoma: a retrospective, European, multicentre cohort study. Lancet Haematol. 2016;3(10):e467-e479. [DOI] [PubMed] [Google Scholar]
- 42.Spina V, Bruscaggin A, Cuccaro A, et al. . Circulating tumor DNA reveals genetics, clonal evolution, and residual disease in classical Hodgkin lymphoma. Blood. 2018;131(22):2413-2425. [DOI] [PubMed] [Google Scholar]
- 43.Friedberg JW, Forero-Torres A, Bordoni RE, et al. . Frontline brentuximab vedotin in combination with dacarbazine or bendamustine in patients aged ≥60 years with HL. Blood. 2017;130(26):2829-2837. [DOI] [PubMed] [Google Scholar]
- 44.Evens AM, Advani RH, Fanale MA, et al. . Sequential brentuximab vedotin (Bv) before and after adriamycin, vinblastine, and dacarbazine (Bv-AVD) for older patients with untreated classical hodgkin lymphoma (cHL): final results from a multicenter phase II study. Blood. 2017;130:733. [Google Scholar]
- 45.Lim SH, Johnson PWM. Optimizing therapy in advanced-stage Hodgkin lymphoma. Blood. 2018;131(15):1679-1688. [DOI] [PubMed] [Google Scholar]