Abstract
After an initial 3 to 6 months of anticoagulation for venous thromboembolism (VTE), clinicians and patients face an important question: “Do we stop anticoagulants or continue them indefinitely?” The decision is easy in some scenarios (eg, stop in VTE provoked by major surgery). In most scenarios, which are faced on a day-to-day basis in routine practice, it is a challenging decision because of uncertainty in estimates in the long-term risks (principally major bleeding) and benefits (reducing recurrent VTE) and the tight trade-offs between them. Once the decision is made to continue, the next question to tackle is “Which anticoagulant?” Here again, it is a difficult decision because of the uncertainty with regard to estimates of efficacy and the safety of anticoagulant options and the tight trade-offs between choices. We conclude with the approach that we take in our clinical practice.
Learning Objectives
Recognize absolute long-term risks for recurrent VTE in subcategories of VTE patients
Recognize absolute long-term risks for major bleeding with anticoagulants
Develop an approach to determine who should stay on anticoagulants indefinitely (and which anticoagulants to choose)
Introduction
The benefits of initial short-term anticoagulation for venous thromboembolism (VTE) are clear.1 Whether to continue anticoagulants after 3 to 6 months of anticoagulation is a common, and, at times, vexing, clinical question. Ultimately, patients, clinicians, and policymakers must balance the benefits and burdens of ongoing anticoagulation.
VTE subgroups
Germane to this discussion is that the risk for recurrent VTE varies considerably between subgroups of patients with VTE. First, we must consider what risk factors were present at the time of initial VTE and classify the VTE; VTE may be unprovoked or be provoked by a major transient risk factor, a minor transient risk factor, or a persistent risk factor.2 Patients with VTE provoked by major transient risk factors (eg, major surgery [eg, general anesthesia for > 30 minutes] or major immobilization [eg, ≥3 days bedridden]) have a 1-year risk for recurrent VTE after discontinuing anticoagulants as low as 1%. As discussed in the section entitled “VTE provoked by major transient risk factor,” they can safely discontinue anticoagulants after short-term therapy.3 At the other extreme, patients with recurrent unprovoked VTE, VTE associated with persistent malignancy or potent thrombophilia (antithrombin deficiency,4 anti-phospholipid antibody syndrome,5 or “double-hit” thrombophilias), likely have a risk for recurrent VTE > 10% per year. In between, unselected patients with a first unprovoked VTE have a 1-year risk for recurrent VTE of ∼8%, a 5-year risk of ∼30%, and a 20-year risk of ∼40%.6,7 Patients with VTE provoked by minor transient risk factors (eg, minor surgery, hospitalization < 3 days, leg injury associated with reduced mobility < 3 days) have a risk for recurrent VTE of ∼5% in the first year and ∼15% at 5 years.2,8 Over 50% of patients have unprovoked VTE or weakly provoked VTE; hence, this therapeutic dilemma is common.9
Intermediate-duration anticoagulation only delays recurrent VTE
Ideally, we would have randomized controlled trials (RCTs) that compare short-term to indefinite anticoagulation with long-term time horizons to determine whether anticoagulation should be continued indefinitely in each of these important VTE subgroups. Unfortunately, these RCTs have not been undertaken nor are they likely to be conducted given the long-time horizons required to complete such RCTs. Nonetheless, RCTs have been conducted comparing short-term and intermediate-term anticoagulation and have provided us with valuable information. Extending anticoagulation (eg, 2 years then stop) simply delays the risk for recurrence until after anticoagulation is stopped compared with short-term anticoagulation during longer-term follow-up10 (ie, in the long run, intermediate-term anticoagulant groups catch up to short-term anticoagulant groups after discontinuing anticoagulants). The key lesson that we learned from these trials is that we need to identify which patients benefit from indefinite suppression of recurrent VTE risk with anticoagulants because of a high risk for recurrent VTE. Because we do not have evidence from indefinite vs short-term anticoagulant RCTs, we must arrive at recommendations through projected long-term benefit/risk analysis for each of these VTE patient subgroups to answer this common clinical question.
Which recurrent VTE thresholds should we use to decide who can stop?
The International Society of Thrombosis and Hemostasis Scientific Subcommittee suggests that patients with a risk for recurrent VTE < 5% at 1 year or 15% at 5 years are unlikely to benefit from indefinite anticoagulant therapy.11 We can use decision analysis to simulate the trade-offs between continuing anticoagulants indefinitely vs stopping anticoagulants after short-term therapy. The simplest decision analysis would focus on mortality differences. The major competing mortality risks would be death from recurrent VTE while off anticoagulants compared with death from major bleed from discontinuing anticoagulants. As simple as this may sound, it is complicated by the indefinite time horizon of the question and the high early risk for recurrent VTE with a decreased risk for recurrent VTE in later years that competes with the relatively steady risk for major bleed in “anticoagulant-experienced” patients (ie, the benefits of continued anticoagulation accrue early, whereas the risks of anticoagulants continue to accrue over time). Further complicating matters, a recurrent VTE and a major bleed do not have the same mortality risk. A case fatality rate is defined as the number of deaths from an event divided by the number of events and is a measure of the lethality of events. The case fatality rates for major bleed are invariably twofold to threefold higher than the case fatality rates for recurrent VTE12 (ie, if you have a major bleed you are 2 to 3 times more likely to die from that major bleed than you would be from dying from VTE if you had a recurrent VTE). To estimate the risk for fatal VTE and fatal major bleeding, we can multiply the risk for these events by their case fatality rates to estimate fatal event rates. The burdens and benefits are not simply death from these 2 competing events; however, this is the key trade-off that drives decision making for most patients, clinicians, and policymakers. Other burdens that we do not consider include the fact that recurrent VTE increases the risk for postthrombotic syndrome and chronic thromboembolic pulmonary hypertension, as well as the fact that anticoagulants cause minor bleeds, which are associated with the taking of medications, cost, laboratory monitoring, and concern about elective and urgent reversal. For simplicity, we will also ignore the fact that anticoagulants are not perfect at preventing recurrent VTE (but with 80%-90% relative risk reductions they are close to perfect).13 We will take into account that there is an ∼0.5% risk for major bleed per year in patients with unprovoked VTE off anticoagulants and assume an incremental major bleeding risk ∼ 0.8% on anticoagulants, as has been observed in many vitamin K antagonist studies (as discussed in the section entitled “Which anticoagulants,” this incremental risk may be lower with direct oral anticoagulants [DOACs]).13
Let’s explore examples of these major trade-offs in VTE subgroups.
VTE provoked by major transient risk factor
In patients with VTE provoked by a major transient risk factor, we would expect a risk for recurrent VTE at 1 year to be ∼1% and then return to near the usual population risk of 0.2% per year. Over a 20-year horizon, we would expect the risk of recurrent VTE to be ∼5%. In the patient with average bleeding risk who is “anticoagulant experienced” (ie, has been on anticoagulants for 3-6 months), the incremental risk of major bleed is ∼0.8% per year.14 Over a 20-year horizon, we would expect ∼16% to experience major bleed. A no-brainer! There is no long-term benefit from continuing anticoagulants, especially given that major bleeds are twofold to threefold more likely to be fatal than recurrent VTE events.
Recurrent unprovoked VTE
On the other extreme, in patients with recurrent unprovoked VTE with an estimated risk of recurrent VTE of 10% per year, we might expect a 20-year risk of recurrent VTE > 80%. Again, in the patient with average bleeding risk, we would expect a 20-year risk for major bleed of 16%. Even using a threefold higher case fatality rate for major bleed, we would expect a net mortality benefit from continuing anticoagulants.
First unprovoked VTE
Where things get tricky is with the most common VTE subgroup: first unprovoked VTE. As stated above, patients with a first unprovoked VTE have a 20-year risk for recurrent VTE ∼40%. In the patient with average bleeding risk, we would expect a 20-year risk for major bleed ∼16%. When we consider that major bleeds are twofold to threefold more likely to cause death than recurrent VTE, we see that there is a tight balance between these competing risks.
VTE provoked by minor transient risk factor
The minor transient risk factor group also requires careful consideration. The quality of the absolute recurrent VTE risk data are poor, limited, and heterogenous (eg, definitions of “minor” and ”transient”).8 Nonetheless, it suggests that these patients have absolute risks for recurrent VTE of ∼15% at 5 years. Again, with a major bleed risk of ∼0.8% per year, you would expect a 5-year risk for major bleed of ∼4%. When we consider that major bleeds are twofold to threefold more likely to cause death than recurrent VTE, we see that there is a tight balance between these competing risks at 5 years.
Bleeding risk stratification
Current guidelines suggest that anticoagulants be continued indefinitely in unprovoked VTE patients with nonhigh bleeding risk.15,16 If a patient has a yearly bleeding risk on anticoagulants > 3% (ie, high bleeding risk), we would expect a 20-year cumulative risk for major bleed of ∼60%. Given the twofold to threefold higher case fatality rate of major bleed, no matter the risk of recurrent VTE, it is very unlikely that these patients with high bleeding risk will derive a mortality benefit from indefinite anticoagulation. However, a major limitation is that we do not have adequately validated bleeding risk scores that allow us to identify anticoagulant-experienced VTE patients at high bleeding risk (ie, >3% per year).
Bleeding risk scores have been developed for atrial fibrillation patients on anticoagulants and for VTE patients during the initial period of anticoagulation.17-21 However, these patient populations have higher bleeding risk than anticoagulant-experienced VTE patients and likely have different bleeding risk factors/predictors. Furthermore, several VTE bleeding risk scores include “cancer” as a risk factor that immediately limits applicability to unprovoked VTE patient populations or VTE provoked by minor transient risk factor patient populations.17,18,20 Indeed, when several of the atrial fibrillation and initial VTE bleeding risk scores were examined in anticoagulant-experienced VTE patients, they performed poorly.22 There is an unmet need for validated tools to predict bleeding risk in anticoagulant-experienced VTE patients that focus on identifying the patient with high bleeding risk who will not benefit from extended anticoagulant therapy (>3% per year). Until such tools are developed and validated, clinicians must rely on their individual clinical judgment.
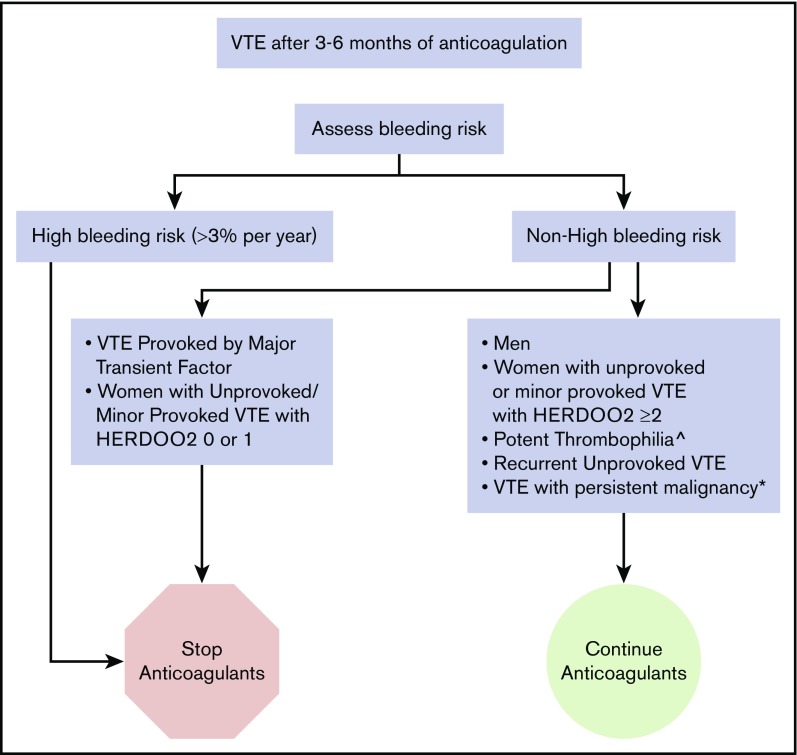
In our practice, we use the variables that are consistently predictors of bleeding across scores/studies (anemia, antiplatelet drug use, older age, chronic renal dysfunction, history of major bleed) to form a “clinical impression” (ie, educated guess) of who may be at >3% per year risk (eg, patients with ≥2 of these factors). We discontinue anticoagulants in almost all of these patients after short-term anticoagulant therapy for any VTE (Figure 1). The exception to the rule may be in limited very thrombogenic circumstances (such as high-titer triple-positive antiphospholipid antibody [APLA] [eg, catastrophic APLA]) in which patients may benefit from ongoing anticoagulation despite high bleeding risk.
Figure 1.
How we treat VTE patients who have completed 3 to 6 months of anticoagulation. ^Antiphospholipid antibody syndrome, “double-hit” and antithrombin deficiency. *On treatment, palliative or <6 months postcurative intervention.
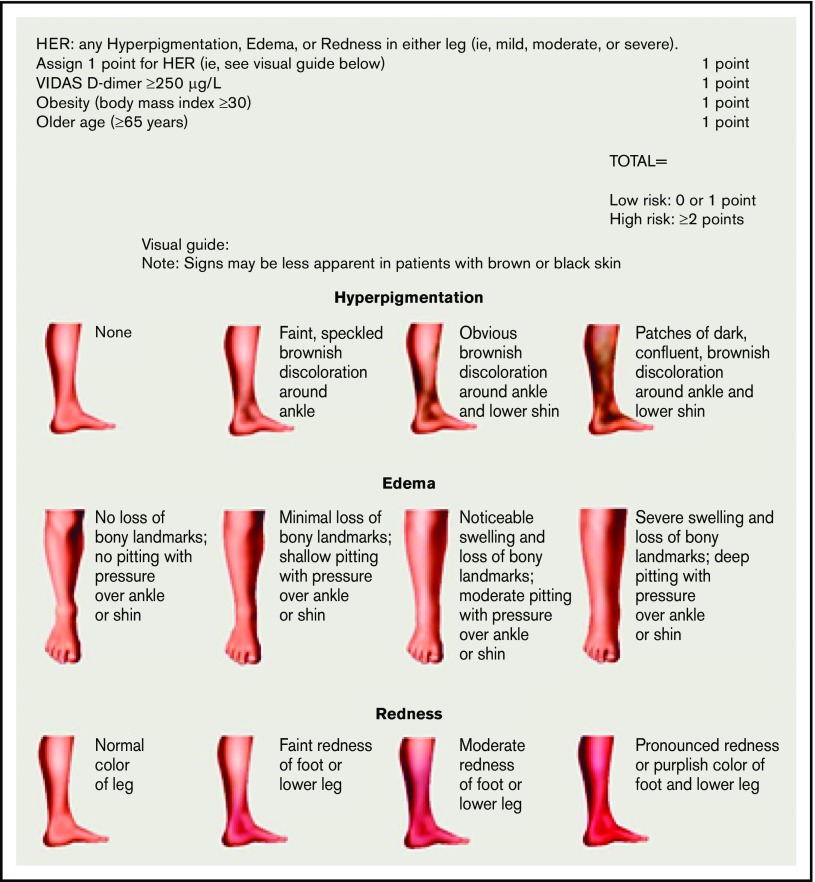
Figure 2.
HERDOO2 score to identify low-risk women with unprovoked or weakly provoked VTE who can discontinue anticoagulants after short-term therapy (reproduced from Rodger et al30 with permission).
Recurrent VTE risk stratification
Another approach is to identify subgroups of unprovoked VTE patients or VTE provoked by minor transient risk factors that are of sufficiently low risk (eg, <5% at 1 year or <15% at 5 years) that they would be unlikely to benefit from indefinite anticoagulation. Investigators have explored single risk factors alone to see if any individual risk factor was powerful enough to identify a low-risk group. Unfortunately, the absence of residual venous thrombosis in compression leg vein imaging after deep vein thrombosis,23 D-dimer on or off anticoagulants,24 and female gender25 were not sufficient by themselves to identify a subgroup who could stop anticoagulants and meet these low thresholds. Similarly, repeated D-dimer testing or combinations of predictors of recurrent VTE have been explored with mostly disappointing results (point estimates close to 5% at 1 year and/or sample sizes too small to ensure risk < 5% at 1 year). The DULCIS investigators explored an approach using residual venous obstruction and serial age- and gender-specific D-dimer thresholds (tested at days 0, 15, 30, 60, and 90 after stopping anticoagulants) to decide who could safely stop anticoagulants. Patients with persistently normal D-dimer after discontinuing anticoagulants had a low risk for recurrent VTE (3%; 95% confidence interval [CI], 2.0-4.4). Importantly, the proportion of patients with persistently normal D-dimers was large (∼50%).26 Of note, this approach did not work as well in the unprovoked VTE subgroup. Overall, although this approach appears to be safe and applicable to many, the need for frequent D-dimer testing, the use of age-/gender-/D-dimer reagent–specific cutoffs, and the variable initial anticoagulant duration based on residual venous obstruction make this a cumbersome approach for patients and clinicians.
The DASH Score and Vienna Prediction model have also been developed to risk stratify patients with unprovoked VTE, but they have not been prospectively validated in management studies and, as such, are not ready for clinical use.27,28
The HERDOO2 score was derived and validated to identify a low-risk group of women who could discontinue anticoagulants.29,30 Women with 0 or 1 HERDOO2 predictors (Figure 2) who discontinued anticoagulants had a risk for recurrent VTE that was low enough to discontinue anticoagulants. About 50% of women with unprovoked VTE or VTE provoked by minor transient risk will be categorized as low risk using the HERDOO2 rule. Disappointingly, we were unable to identify a low-risk group of men.
On the research front, it is clear that we still have a long way to go. The majority of “high-risk” patients do not develop recurrent VTE, even in the long run.7 Hence, we must continue to search for better predictors of recurrent VTE risk so that the burdens of indefinite anticoagulation are borne by those who will benefit.
How we put it all together
In patients at high risk for bleeding, determined using “clinical judgment” based on risk factors for bleeding on anticoagulants to identify patients likely to have a major bleed risk > 3% per year, we suggest that you stop anticoagulants after short-term therapy (3-6 months) (Figure 1). In patients with nonhigh bleeding risk and recurrent unprovoked VTE or VTE associated with potent thrombophilia (eg, “double-hit” thrombophilia, APLA, and antithrombin deficiency), we continue anticoagulants indefinitely. In patients with nonhigh bleeding risk and a first unprovoked VTE or a first VTE provoked by minor transient risk factors, we suggest applying the HERDOO2 score in women and discontinuing anticoagulants in low-risk women with 0 or 1 HERDOO2 points. In men and women with ≥2 HERDOO2 points with unprovoked VTE or VTE provoked by minor transient risk factors, we suggest a discussion based on patient preferences and values; however, in our experience, most patients agree to continue anticoagulants given a projected, albeit small, mortality benefit (eg, 1%-2% more likely to be alive at 20 years).
Which anticoagulants?
The elusive “ideal anticoagulant” would be effective, with no burdens of administration, inexpensive, reversible, and cause no bleeding. If such a breakthrough were to occur, all patients with a greater-than-minimal risk for recurrent VTE would likely benefit from ongoing anticoagulants (eg, we would only stop anticoagulants after VTE provoked by major surgery). Some investigators have suggested that DOACs are well on the way to this ideal, but we must be cautious that, in real-world studies, the risk for major bleeding has been reported to be as high as 3.8% with DOACs, perhaps due to DOAC use in patients excluded from the DOAC registration trials.31
Aspirin, which is not an anticoagulant per se, has a modest effect on reducing the risk of recurrent VTE. In 2 RCTs, aspirin reduced the risk of recurrent VTE in unprovoked VTE patients by 32% compared with no aspirin.32 Aspirin use was associated with a major bleeding risk of 0.5% per year (as above, similar to the risk of major bleeding in unprovoked VTE patients off anticoagulants). In the EINSTEIN Choice RCT comparing rivaroxaban with aspirin for 12 months in VTE patients already treated with anticoagulants for 6 to 12 months, rivaroxaban 10 mg/d or 20 mg/d was more effective than aspirin, with comparable risks of major bleeding.33 The risk of major bleed was 0.5%, 0.4%, and 0.3% in the 20-mg rivaroxaban, 10-mg rivaroxaban, and aspirin groups, respectively (again, note that these risks are similar to the absolute risks of major bleeding in unprovoked VTE patients off anticoagulants). Notably, unprovoked VTE patients in the aspirin arms of these 3 RCTs had a substantial residual risk for recurrent VTE > 5% per year. Overall, aspirin is not good enough; anticoagulants are better at suppression of recurrent VTE and should be used if ongoing suppression of recurrent VTE risk is warranted. However, in resource-challenged environments, aspirin is a better option than no anticoagulants if ongoing suppression of recurrent VTE risk is warranted and anticoagulants are not available.
RCTs comparing an extended duration of anticoagulant therapy with placebo (or no intervention) after completing an initial period of at least 3 months of anticoagulant therapy for VTE are not directly helpful in allowing us to pick one anticoagulant over another for patients in whom indefinite suppression of recurrent VTE is warranted.34-36 Indeed, anticoagulants have not been extensively tested head to head in the setting of long-term secondary prevention of VTE. One trial compared dabigatran (150 mg, twice a day) with warfarin, with no differences in recurrent VTE or major bleeding risk during treatment of secondary prevention; however, it showed an increase in acute coronary syndrome in the dabigatran arm, discouraging its use in this setting.36 A network meta-analysis of >12 000 VTE patients explored anticoagulant options for secondary VTE prevention and suggested that warfarin is slightly better than DOACs, but at a cost of higher bleeding risk.13 However, network meta-analysis only provides indirect comparisons of treatment options. The COVET trial (NCT03196349), exploring warfarin vs apixaban vs rivaroxaban in >3000 VTE patients treated with anticoagulants for >3 months, is underway and should provide valuable insights and perhaps guide us on anticoagulant choice after an initial 3 months of anticoagulation.
Several trials have explored the hypothesis that perhaps “less is more” and have tested the hypothesis that lower doses of anticoagulants may be as effective and safe after an initial 3 months of anticoagulants. Disappointingly, vitamin K antagonists with a lower target international normalized ratio (1.5-2.0) compared with the usual target international normalized ratio2,3 for secondary prevention in VTE patients did not demonstrate a lower risk for major bleeding.37 The AMPLIFY Extension study compared apixaban (2.5 mg, twice a day or 5 mg, twice a day) with placebo for 12 months in VTE patents who had completed 3 to 12 months of initial anticoagulant therapy. In a secondary analysis comparing apixaban, 2.5 mg, twice a day with 5 mg, twice a day, the risk of recurrent VTE or VTE-related death was similar (hazard ratio, 0.97; 95% CI, 0.46-2.02). However, as can be gleaned from the 95% CIs, the risk of recurrent VTE might be double in the lower-dose group. The major bleeding risks were low in both apixaban arms (2.5 mg, twice a day: 0.2%; 95% CI, 0.1-0.9 and 5 mg, twice a day: 0.1%; 95% CI, 0-0.7) and were comparable to placebo (0.5%; 95% CI, 0-1.2). In a secondary analysis of the EINSTEIN Choice study (see above), rivaroxaban (10 mg, once a day vs 20 mg, once a day) was administered to patients with VTE; similar risks for recurrent VTE (hazard ratio, 1.34; 95% CI, 0.65-2.75) were seen. However, as can be gleaned from the 95% CIs, the risk of recurrent VTE might be 50% higher in the lower-dose group. The major bleeding risks were low in both rivaroxaban groups (20 mg, once a day: 0.5%; 95% CI, 0.2-1.2 and 10 mg, once a day: 0.4%; 95% CI, 0.2-1.0) (hazard ratio, 1.23; 95% CI, 0.37-4.03). The RENOVE trial (NCT03285438) is designed to directly compare low-dose DOACs with higher-dose DOACs and hopefully determine whether lower-dose DOACs have a better risk/benefit balance in the long-term secondary suppression of recurrent VTE risk.
In our practice, in patients who require ongoing suppression of recurrent VTE risk, we discuss 3 options: vitamin K antagonists, apixaban, and rivaroxaban. We do not offer aspirin unless patients are adamant that they will not take anticoagulants. We do not offer dabigatran because many other options are available, and these other options do not appear to have an increased risk for acute coronary syndromes. Until the results of head-to-head trials in a secondary VTE-prevention setting are completed, we tell patients that there is no clear choice among vitamin K antagonists, apixaban, and rivaroxaban. Vitamin K antagonists might be slightly more effective, are inexpensive, and are easily reversible, but they require routine monitoring and likely have a higher bleeding risk. Apixaban requires twice a day administration (which may affect compliance/persistence), is more expensive than vitamin K antagonists, and is not reversible (no specific reversal agents are currently on the market), but it does not require monitoring and might have the lowest bleeding risk. Rivaroxaban is given once a day, is more expensive than vitamin K antagonists, and is not reversible (no specific reversal agents are currently on the market), but it does not require monitoring and likely has a lower bleeding risk than vitamin K antagonists.
In conclusion, the days of managing all VTE patients in a uniform manner (eg, 3 months of anticoagulants then stop) have come and gone. Clinical research has established that the risk of recurrent VTE is substantially different in VTE subgroups. Patients with unprovoked VTE or VTE provoked by minor transient risk factors, a large subgroup, have substantial long-term risk for recurrent VTE. On the one hand, recurrent VTE and major bleeding risk-stratification tools will add further complexity to the clinical care of VTE patients, on the other hand, they ensure that the right patients shoulder the burdens of long-term anticoagulant suppression of recurrent VTE risk.
References
- 1.Barritt DW, Jordan SC. Anticoagulant drugs in the treatment of pulmonary embolism. A controlled trial. Lancet. 1960;1(7138):1309-1312. [DOI] [PubMed] [Google Scholar]
- 2.Kearon C, Ageno W, Cannegieter SC, Cosmi B, Geersing GJ, Kyrle PA; Subcommittees on Control of Anticoagulation, and Predictive and Diagnostic Variables in Thrombotic Disease. Categorization of patients as having provoked or unprovoked venous thromboembolism: guidance from the SSC of ISTH. J Thromb Haemost. 2016;14(7):1480-1483. [DOI] [PubMed] [Google Scholar]
- 3.Baglin T, Luddington R, Brown K, Baglin C. Incidence of recurrent venous thromboembolism in relation to clinical and thrombophilic risk factors: prospective cohort study. Lancet. 2003;362(9383):523-526. [DOI] [PubMed] [Google Scholar]
- 4.Sokol J, Timp JF, le Cessie S, et al. . Mild antithrombin deficiency and risk of recurrent venous thromboembolism: results from the MEGA follow-up study. J Thromb Haemost. 2018;16(4):680-688. [DOI] [PubMed] [Google Scholar]
- 5.Kearon C, Parpia S, Spencer FA, et al. . Antiphospholipid antibodies and recurrent thrombosis after a first unprovoked venous thromboembolism. Blood. 2018;131(19):2151-2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kyrle PA, Kammer M, Eischer L, et al. . The long-term recurrence risk of patients with unprovoked venous thromboembolism: an observational cohort study. J Thromb Haemost. 2016;14(12):2402-2409. [DOI] [PubMed] [Google Scholar]
- 7.Rodger MA, Scarvelis D, Kahn SR, et al. . Long-term risk of venous thrombosis after stopping anticoagulants for a first unprovoked event: a multi-national cohort. Thromb Res. 2016;143:152-158. [DOI] [PubMed] [Google Scholar]
- 8.Iorio A, Kearon C, Filippucci E, et al. . Risk of recurrence after a first episode of symptomatic venous thromboembolism provoked by a transient risk factor: a systematic review. Arch Intern Med. 2010;170(19):1710-1716. [DOI] [PubMed] [Google Scholar]
- 9.White RH, Murin S, Wun T, Danielsen B. Recurrent venous thromboembolism after surgery-provoked versus unprovoked thromboembolism. J Thromb Haemost. 2010;8(5):987-997. [DOI] [PubMed] [Google Scholar]
- 10.Couturaud F, Sanchez O, Pernod G, et al. ; PADIS-PE Investigators. Six months vs extended oral anticoagulation after a first episode of pulmonary embolism: The PADIS-PE Randomized Clinical Trial. JAMA. 2015;314(1):31-40. [DOI] [PubMed] [Google Scholar]
- 11.Kearon C, Iorio A, Palareti G; Subcommittee on Control of Anticoagulation of the SSC of the ISTH. Risk of recurrent venous thromboembolism after stopping treatment in cohort studies: recommendation for acceptable rates and standardized reporting. J Thromb Haemost. 2010;8(10):2313-2315. [DOI] [PubMed] [Google Scholar]
- 12.Carrier M, Le Gal G, Wells PS, Rodger MA. Systematic review: case-fatality rates of recurrent venous thromboembolism and major bleeding events among patients treated for venous thromboembolism. Ann Intern Med. 2010;152(9):578-589. [DOI] [PubMed] [Google Scholar]
- 13.Castellucci LA, Cameron C, Le Gal G, et al. . Efficacy and safety outcomes of oral anticoagulants and antiplatelet drugs in the secondary prevention of venous thromboembolism: systematic review and network meta-analysis. BMJ. 2013;347(7924):f5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castellucci LA, Le Gal G, Rodger MA, Carrier M. Major bleeding during secondary prevention of venous thromboembolism in patients who have completed anticoagulation: a systematic review and meta-analysis. J Thromb Haemost. 2014;12(3):344-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kearon C, Akl EA, Ornelas J, et al. . Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report [published correction appears in Chest 2016;150(4):988]. Chest. 2016;149(2):315-352. [DOI] [PubMed] [Google Scholar]
- 16.Konstantinides G. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(45):3145-3146. [DOI] [PubMed] [Google Scholar]
- 17.Kuijer PM, Hutten BA, Prins MH, Büller HR. Prediction of the risk of bleeding during anticoagulant treatment for venous thromboembolism. Arch Intern Med. 1999;159(5):457-460. [DOI] [PubMed] [Google Scholar]
- 18.Ruíz-Giménez N, Suárez C, González R, et al. ; RIETE Investigators. Predictive variables for major bleeding events in patients presenting with documented acute venous thromboembolism. Findings from the RIETE Registry. Thromb Haemost. 2008;100(1):26-31. [DOI] [PubMed] [Google Scholar]
- 19.Di Nisio M, Raskob G, Büller HR, et al. . Prediction of major and clinically relevant bleeding in patients with VTE treated with edoxaban or vitamin K antagonists. Thromb Haemost. 2017;117(4):784-793. [DOI] [PubMed] [Google Scholar]
- 20.Klok FA, Hösel V, Clemens A, et al. . Prediction of bleeding events in patients with venous thromboembolism on stable anticoagulation treatment. Eur Respir J. 2016;48(5):1369-1376. [DOI] [PubMed] [Google Scholar]
- 21.Di Nisio M, Ageno W, Rutjes AWS, Pap AF, Büller HR. Risk of major bleeding in patients with venous thromboembolism treated with rivaroxaban or with heparin and vitamin K antagonists. Thromb Haemost. 2016;115(2):424-432. [DOI] [PubMed] [Google Scholar]
- 22.Riva N, Bellesini M, Di Minno MN, et al. . Poor predictive value of contemporary bleeding risk scores during long-term treatment of venous thromboembolism: A multicentre retrospective cohort study. Thromb Haemost. 2014;112(3):511-521. [DOI] [PubMed] [Google Scholar]
- 23.Donadini MP, Ageno W, Antonucci E, et al. . Prognostic significance of residual venous obstruction in patients with treated unprovoked deep vein thrombosis: a patient-level meta-analysis. Thromb Haemost. 2014;111(1):172-179. [DOI] [PubMed] [Google Scholar]
- 24.Kearon C, Spencer FA, O’Keeffe D, et al. ; D-dimer Optimal Duration Study Investigators. D-dimer testing to select patients with a first unprovoked venous thromboembolism who can stop anticoagulant therapy: a cohort study. Ann Intern Med. 2015;162(1):27-34. [DOI] [PubMed] [Google Scholar]
- 25.Douketis J, Tosetto A, Marcucci M, et al. . Risk of recurrence after venous thromboembolism in men and women: patient level meta-analysis. BMJ. 2011;342:d813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palareti G, Cosmi B, Legnani C, et al. ; DULCIS (D-dimer and ULtrasonography in Combination Italian Study) Investigators. D-dimer to guide the duration of anticoagulation in patients with venous thromboembolism: a management study. Blood. 2014;124(2):196-203. [DOI] [PubMed] [Google Scholar]
- 27.Tosetto A, Iorio A, Marcucci M, et al. . Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH). J Thromb Haemost. 2012;10(6):1019-1025. [DOI] [PubMed] [Google Scholar]
- 28.Eichinger S, Heinze G, Jandeck LM, Kyrle PA. Risk assessment of recurrence in patients with unprovoked deep vein thrombosis or pulmonary embolism: the Vienna prediction model. Circulation. 2010;121(14):1630-1636. [DOI] [PubMed] [Google Scholar]
- 29.Rodger MA, Kahn SR, Wells PS, et al. . Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ. 2008;179(5):417-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodger MA, Le Gal G, Anderson DR, et al. ; REVERSE II Study Investigators. Validating the HERDOO2 rule to guide treatment duration for women with unprovoked venous thrombosis: multinational prospective cohort management study. BMJ. 2017;356:j1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beyer-Westendorf J. What have we learned from real-world NOAC studies in venous thromboembolism treatment? Thromb Res. 2018;163:83-91. [DOI] [PubMed] [Google Scholar]
- 32.Simes J, Becattini C, Agnelli G, et al. ; INSPIRE Study Investigators (International Collaboration of Aspirin Trials for Recurrent Venous Thromboembolism). Aspirin for the prevention of recurrent venous thromboembolism: the INSPIRE collaboration. Circulation. 2014;130(13):1062-1071. [DOI] [PubMed] [Google Scholar]
- 33.Weitz JI, Lensing AWA, Prins MH, et al. ; EINSTEIN CHOICE Investigators. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med. 2017;376(13):1211-1222. [DOI] [PubMed] [Google Scholar]
- 34.Agnelli G, Buller HR, Cohen A, et al. ; AMPLIFY-EXT Investigators. Apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2013;368(8):699-708. [DOI] [PubMed] [Google Scholar]
- 35.EINSTEIN Investigators; Bauersachs R, Berkowitz SD, Brenner B, et al. . Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363(26):2499-2510. [DOI] [PubMed] [Google Scholar]
- 36.Schulman S, Kearon C, Kakkar AK, et al. ; RE-SONATE Trial Investigators. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013;368(8):709-718. [DOI] [PubMed] [Google Scholar]
- 37.Kearon C, Ginsberg JS, Kovacs MJ, et al. ; Extended Low-Intensity Anticoagulation for Thrombo-Embolism Investigators. Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med. 2003;349(7):631-639. [DOI] [PubMed] [Google Scholar]