Abstract
Background
The depth of invasion by basal cell carcinoma (BCC) subtypes varies.
Objective
To investigate BCC invasion depth variation by subtype and anatomic site.
Method
A prospective consecutive case series of excised BCC from 2009 to 2014 in a single Australian clinic.
Results
Descending mean depths for a total of 4,565 BCC cases by subtype were as follows: nodulocystic, 1.9 mm (n = 84, 95% CI: 1.70–2.03, P = 0.66); nodular, 1.6 mm (n = 947, 95% CI: 1.53–1.63, P < 0.0001); aggressive, 1.5 mm (n = 925, 95% CI: 1.44–1.59, P < 0.0001); superficial combined with nodular, 0.9 mm (n = 1,081, 95% CI: 0.83–0.90, P < 0.0001); and superficial, 0.3 mm (n = 1,528, 95% CI: 0.32–0.36, P < 0.0001). Deeper invasion was associated with increased chronic sunlight exposed sites. The deepest aggressive BCCs occurred on the neck with a mean depth of 1.8 mm (n = 46, 95% CI: 1.47–2.21).
Conclusion
We found significant differences in the depth of invasion for BCCs by sex, subtype, and anatomic site. For BCC with characteristics matching this study, overall adequate microscopic excision depths are proposed: superficial, 1.0 mm; superficial combined with nodular, 2.0 mm; nodular, 3.0 mm; and aggressive, 3.0 mm.
Keywords: basal cell carcinoma, superficial, nodular, infiltrating, micronodular, depth, anatomic site
Introduction
Basal cell carcinoma (BCC) presents with a range of subtypes. Presentation may be as either a single subtype BCC or combination of subtypes. These subtypes are based on architectural and cytological features [1]. Certain subtypes are more common in certain locations [2]. Along with the differing histopathological features, the rates of recurrence and positive margins are more common in certain subtypes as well as locations [3]. These differences are likely secondary to the growth pattern and depth of invasion. Little is currently known on the variation in invasion depth of BCC subtypes by anatomic location.
A recent study [4] limited to 100 cases showed that sex and site did not correlate with the depth of invasion in BCCs. Micronodular and infiltrating BCCs were found to have the deepest invasion followed by nodular and superficial BCCs. Increased tumor recurrence following excision has been recorded for BCCs that invade beyond the reticular dermis and/or display perineural invasion [5]. Furthermore, complete response rates following photodynamic therapy were found to be more strongly related to depth of invasion by facial BCC rather than subtype [6].
Recent studies have improved the understanding of the correlation between the clinical features, dermoscopy appearance, and histopathology of different BCC subtypes [7–9]. Improved identification of BCC subtypes combined with a better understanding of the depth of invasion of BCC subtype by anatomic location will lead to better clinical management. The goal of this study was to quantify BCC invasion depth by subtype and anatomic site.
Materials and Methods
This prospective study was conducted at a single mixed primary care and referral clinic in Sydney, Australia. The University of Queensland granted ethics approval for this study. All patients gave informed consent prior to each case being included in the study. Demographic data were collected from retrospective chart review. All consecutive histopathological proven cases of initial presentation BCC were collected from 2009 until the end of 2014. The intention of each surgical procedure was full excision of each BCC case. Gross excision margins were initially estimated by clinical assessment. All cases were then examined using dermoscopy to identify the visible BCC edge and mark the final surgical margin some millimeters from this visible tumor edge. A Heine Delta 20 nonpolarized dermatoscope was used to examine each BCC to identify the lesion edge. For BCCs with clinical diameters less than 5 mm, the surgical margin was typically marked 2 mm from the dermoscopy image of the tumor edge. Those BCCs with diameters greater than 5 mm had a minimum 3 mm surgical margin. Excisions were routinely performed as an ellipse down to fat. However, for the nasal ala and on selected locations on the ear (i.e., on the thin skin directly overlaying the cartilage), excisions were performed down to the perichondrium. All excised tissue was submitted in formalin for histopathological assessments in which transverse “bread-loafing” sections were examined following hematoxylin and eosin staining. Each ellipse of tissue was fully sectioned at 3-mm intervals.
Based on the histopathological assessments, cases of diagnoses other than BCC were excluded. In addition, BCC cases were also excluded if there was any historical, clinical, dermatoscopic, or histopathological evidence at the initial presentation that the case was a residual or recurrent case from a previous excision. During the study, the location of each BCC excision was recorded on an individual body map in each patient’s notes. All following excisions were checked against this body map to identify and thus exclude residual or recurrent cases. No cases were excluded based on age or anatomic site.
After histopathological assessment, each BCC case was classified into one of the following categories: superficial (only subtype present), superficial combined with nodular (no other subtypes), nodular, nodulocystic, or aggressive. The aggressive BCC category included all cases with any of the following subtypes: infiltrating, morpheaform, micronodular, and BCCs displaying squamous differentiation. These BCC subtypes were collectively allocated to the aggressive category as they commonly have histology with infiltrative architecture compared with the other BCC subtypes. The aggressive category included cases of aggressive BCC combined with superficial and/or nodular BCC subtypes.
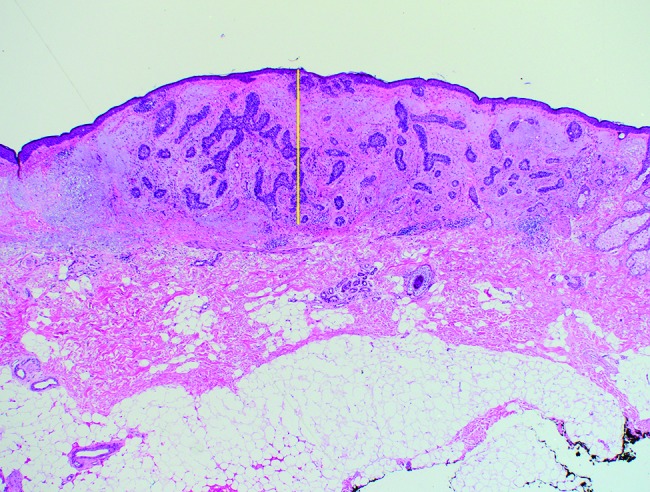
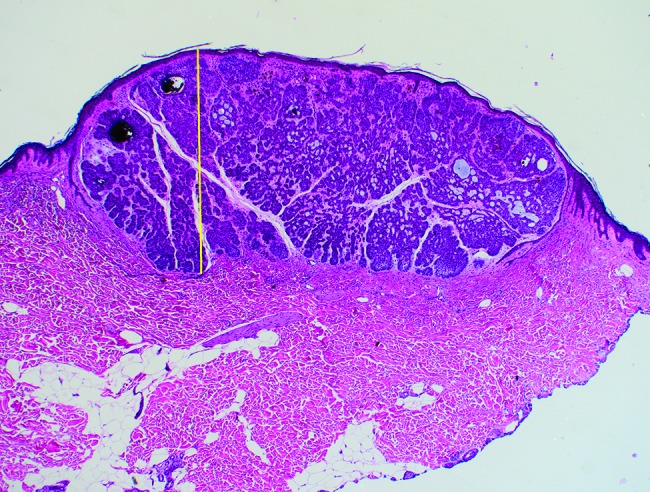
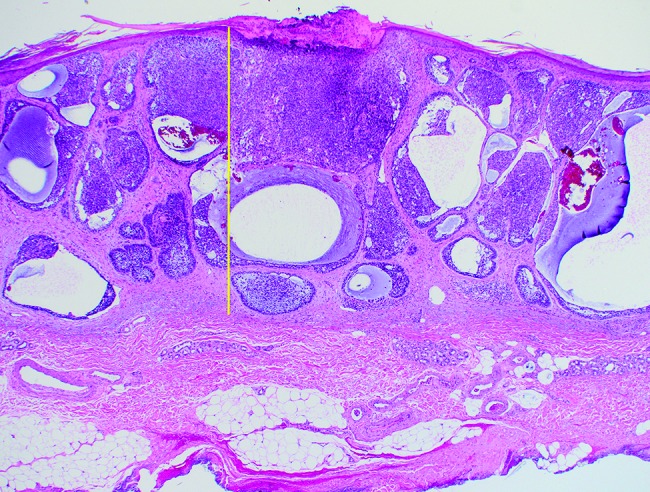
Microscopic tumor diameters were measured in the laboratory prior to further processing. Invasion depth was measured from the granular layer of the epidermis to the deepest tumor tissue in increments of 0.1 mm. Examples of depth measurements for respective BCC subtypes are shown in Figure 1 (A–D): (A) superficial BCC, (B) nodular BCC, (C) nodulocystic BCC, and (D) infiltrative BCC. Initial excisions with deep margin involvement were re-excised. If no residual BCC was reported in the second excision, the depth was recorded as the depth stated in the first excision. However, if a second excision revealed further residual BCC, the recorded residual tumor depth from the second excision was added to the depth of the first excision to provide an approximate overall depth. It is conceded that combining the 2 separate depths for a single case from 2 separate excisions provides only an approximation of the true depth. However, these combined depth data were included, as excluding the second residual depth measurements, or all 2-stage excisions, would have resulted in the collective depth data being underestimated.
Figure 1A.
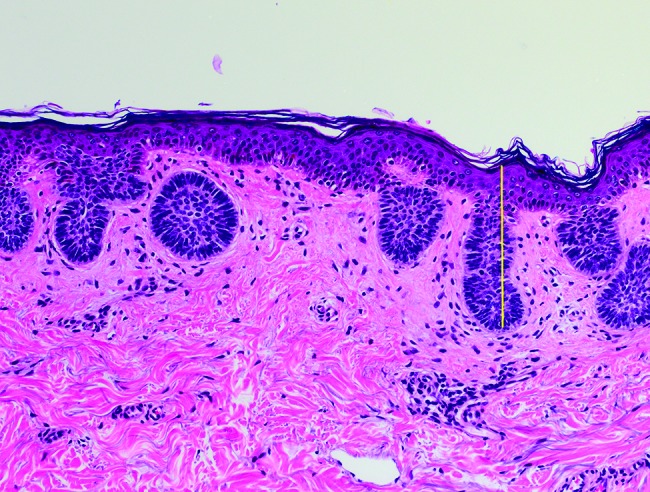
Superficial basal cell carcinoma: maximum invasion depth indicated by a vertical line from the granular layer in the epidermis down to the deepest tumor cells. [Copyright: ©2018 Pyne et al.]
Figure 1D.
Infiltrating basal cell carcinoma: maximum invasion depth indicated by the same method as in Figure 1A. [Copyright: ©2018 Pyne et al.]
Results
The patients’ tumor characteristics are presented in Table 1. Of the BCC subtypes examined, nodulocystic BCCs recorded the oldest mean ages for both men aged 76 years (95% CI: 73–79, P = 0.013) and women aged 77 years (95% CI: 73–91, P > 0.1). Conversely, superficial BCCs recorded the youngest mean ages: 67 years in men (95% CI: 66–68, P < 0.0001) and 66 years in women (95% CI: 65–68, P < 0.0004). The mean maximum microscopic tumor surface diameter for all BCC subtypes ranged from 7.6 mm for nodular BCC (95% CI: 7.3–7.8, P < 0.0001) up to 9.1 mm for aggressive subtypes (95% CI: 8.1–10.0, P < 0.0001); see Table 1. Table 2 shows the BCC subtypes as recorded in relation to separate anatomic sites.
TABLE 1.
Characteristics of basal cell carcinoma: sex, age, maximum microscopic diameter, and depth for all sites combined
| Total Cases (n = 4,565) | Superficial Only (n = 1,528) | Superficial + Nodular (n = 1,081) | Nodular (n = 947) | Nodulocystic (n = 84) | Aggressive (n = 925) |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 935 (61.2) | 712 (65.9) | 686 (72.4) | 61 (72.6) | 608 (65.7) |
| Female | 593 (38.8) | 369 (34.1) | 261 (27.6) | 23 (27.4) | 317 (34.3) |
| Age of Patients | |||||
| Male | |||||
| Mean | 67 | 71 | 70 | 76 | 73 |
| Range | 20–97 | 25–101 | 26–101 | 48–98 | 34–103 |
| 95% CI | 66–68 | 70–72 | 69–71 | 73–79 | 72–74 |
| P value | <0.0001 | <0.0005 | <0.0001 | 0.013 | 0.0002 |
| Female | |||||
| Mean | 66 | 73 | 70 | 77 | 72 |
| Range | 25–97 | 36–100 | 35–99 | 36–98 | 37–102 |
| 95% CI | 65–68 | 72–74 | 68–71 | 73–81 | 71–74 |
| P value | 0.0004 | <0.0001 | 0.0667 | >0.1 | <0.0001 |
| BCC Maximum Histological Diameter, All Sites, mm (95% CI) | 8.5 (8.3–8.7) | 8.3 (8.1–8.5) | 7.6 (7.3–7.8) | 9.0 (8.1–10.0) | 9.1 (8.8–9.4) |
| P value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| BCC Depth, All Sites, mm | 0.30 (0.32–0.36) | 0.86 (0.83–0.90) | 1.58 (1.53–1.63) | 1.86 (1.70–2.03) | 1.51 (1.44–1.59) |
| P value | <0.0001 | <0.0001 | <0.0001 | 0.66 (ns) | <0.0001 |
TABLE 2.
Basal cell carcinoma: subtypes by anatomic site (nodulocystic cases not shown)
| Total Cases (n = 4,481) | Superficial Only (n = 1,528) | Superficial + Nodular (n = 1,081) | Nodular (n = 947) | Aggressive (n = 925) |
|---|---|---|---|---|
| Scalp | 17 (16.4) | 21 (6.9) | 21 (4.5) | 21 (0.43) |
| Ear | 8 (7.7) | 34 (11.1) | 46 (9.8) | 81 (16.5) |
| Eyelid | 2 (2.0) | 18 (5.9) | 31 (6.6) | 11 (2.3) |
| Nose | 13 (12.5) | 67 (21.9) | 127 (27.0) | 120 (24.4) |
| Forehead | 14 (13.5) | 62 (20.3) | 110 (23.4) | 119 (24.2) |
| Lip | 0 | 6 (2.0) | 20 (4.3) | 12 (2.4) |
| Cheek | 13 (12.5) | 42 (13.7) | 63 (13.4) | 82 (16.7) |
| Neck | 37 (35.6) | 56 (18.3) | 53 (11.3) | 46 (9.4) |
| Shoulder | 201 (14.1) | 127 (16.4) | 96 (20.0) | 78 (18.1) |
| Chest | 109 (7.7) | 75 (9.7) | 69 (14.4) | 57 (13.2) |
| Back | 480 (33.7) | 219 (28.3) | 129 (26.8) | 134 (31.0) |
| Upper Arm | 243 (17.1) | 109 (14.1) | 43 (8.9) | 38 (8.8) |
| Forearm | 54 (3.8) | 52 (6.7) | 31 (6.4) | 24 (5.6) |
| Hand | 10 (0.7) | 8 (1.0) | 9 (1.9) | 2 (0.5) |
| Thigh | 89 (6.25) | 47 (6.1) | 38 (7.9) | 24 (5.6) |
| Leg | 227 (15.9) | 135 (17.4) | 60 (12.5) | 72 (16.7) |
| Foot | 11 (0.8) | 3 (4.0) | 6 (1.3) | 3 (0.7) |
Mean tumor depths ranged from 0.3 mm for superficial BCC (95% CI: 0.32–0.36, P < 0.0001) up to 1.86 mm for nodulocystic BCC (95% CI: 1.7–2.03, P < 0.0001); see Table 1. Across all cases, 4.1% (n = 185) were reported with deep margin involvement at the initial excision. The most common site of deep margin involvement was the nose (n = 44, 13.5% of nose cases) followed by the ear (n = 20, 11.8% of ear cases). When first excisions with an involved deep margin (n = 185) were excised a second time, 131 (71%) of these re-excision cases reported no residual tumor. From of a full total of 4,565 BCC cases, only 54 cases (1.2%) required an approximation additive depth from the 2 consecutive excisions on the same case.
For all cases combined, the following depths (to the nearest 0.1 mm) represent the 95th percentile for invasion depth within each specified subtype: superficial, 0.3 mm; superficial and nodular, 0.8 mm; nodular, 1.5 mm; nodulocystic, 1.8 mm; and aggressive, 1.2 mm. Table 3 lists mean tumor depths by anatomic site and BCC subtype. Owing to the small number of cases (n = 84), nodulocystic BCCs were not assessed by anatomic site. Table 4 provides a convenient comparison of the mean depths of BCC subtypes by sex (a comparison was made between head and neck sites combined and trunk and limb combined). Superficial BCC mean depths ranged from 0.17 mm on the cheek to 0.40 mm on the foot. Combined superficial and nodular BCC subtype depths ranged from 0.63 mm on the thigh to 1.50 mm on the lip. Nodular BCC depths ranged from 1.36 mm on the eyelid to 1.98 mm on the hand. Finally, aggressive BCC subtype depths ranged from 0.94 mm on the shoulder to 1.80 mm on the neck.
TABLE 3.
Basal cell carcinoma: tumor depth by anatomic site and subtype
| Anatomic Site | Superficial Only Mean Depth, mm (95% CI) | Superficial + Nodular, mm (95% CI) | Nodular Only Mean Depth, mm (95% CI) | Aggressive Mean Depth, mm (95% CI) |
|---|---|---|---|---|
| Scalp | 0.33 (0.14–0.53) | 0.93 (0.69–1.17) | 1.80 (1.47–2.12) | 1.48 (1.01–1.94) |
| Ear | 0.35 (N/A) | 1.47 (0.87–2.06) | 1.81(1.66–1.97) | 1.66 (1.44–1.88) |
| Eyelid | N/A | 0.87 (0.69–1.04) | 1.36 (1.17–1.54) | 1.30 (0.70–1.90) |
| Nose | 0.35 (N/A) | 1.03 (0.90–1.16) | 1.71(1.58–1.83) | 1.49 (1.29–1.69) |
| Forehead | N/A | 1.06 (0.90–1.22) | 1.51 (1.40–1.62) | 1.50 (1.40–1.66) |
| Lip | N/A | 1.50 (1.16–1.84) | 1.91 (1.10–2.25) | 1.00 (0.57–1.43) |
| Cheek | 0.17 (0.02–0.31) | 1.12(0.94–1.29) | 1.89 (1.72–2.06) | 1.44 (1.26–1.62) |
| Neck | 0.39 (0.22–0.55) | 1.14 (0.93–1.35) | 1.82 (1.58–2.05) | 1.84 (1.47–2.21) |
| Shoulder | 0.38 (0.30–0.45) | 0.74 (0.67–0.81) | 1.50 (1.31–1.70) | 0.94 (0.76–1.13) |
| Chest | 0.36 (0.31–0.41) | 0.79 (0.70–0.88) | 1.53 (1.33–1.74) | 1.05 (0.75–1.35) |
| Back | 0.36 (0.34–0.39) | 0.76 (0.70–0.82) | 1.39 (1.26–1.53) | 1.06 (0.91–1.21) |
| Upper Arm | 0.27 (0.24–0.30) | 0.74 (0.68–0.80) | 1.44 (1.24–1.63) | 1.01 (0.72–1.29) |
| Forearm | 0.24 (0.18–0.30) | 0.93 (0.80–1.06) | 1.64 (1.36–1.92) | 1.23 (1.01–1.46) |
| Hand | 0.35 (0.07–0.63) | 0.87 (0.49–1.25) | 1.98 (1.28–2.67) | N/A |
| Thigh | 0.28 (0.22–0.34) | 0.63 (0.54–0.71) | 1.53 (1.16–1.89) | 1.01 (0.57–1.46) |
| Leg | 0.33 (0.29–0.37) | 0.69 (0.63–0.76) | 1.34 (1.20–1.49) | 1.10 (0.94–1.27) |
| Foot | 0.40 (0.15–0.65) | 0.83 (0.50–1.50) | 1.48 (0.81–2.16) | N/A |
N/A (n = 3 or fewer cases).
TABLE 4.
Basal cell carcinoma: depth comparison by sex and subtype
| Male | Female | |||||
|---|---|---|---|---|---|---|
| Superficial + Nodular | Nodular | Aggressive | Superficial + Nodular | Nodular | Aggressive | |
| Head + Neck | ||||||
| Mean depth, mm | 1.1 | 1.8 | 1.7 | 1.1 | 1.6 | 1.6 |
| 95% CI | 1.0–1.2 | 1.7–1.8 | 1.6–1.8 | 0.9–1.3 | 1.5–1.7 | 1.5–1.7 |
| P value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.01 | <0.0001 |
| Case numbers (n) | 201 (26.2%) | 296 (38.6%) | 269 (35.1%) | 93 (25.6%) | 137 (37.6%) | 134 (36.8%) |
| Trunk + Limbs | ||||||
| Mean depth, mm | 0.76 | 1.5 | 1.5 | 0.76 | 1.3 | 1.0 |
| 95% CI | 0.73–0.80 | 1.4–1.6 | 1.3–1.7 | 0.71–0.81 | 1.2–1.4 | 0.92–1.2 |
| P value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Case numbers (n) | 476 (45.6%) | 336 (32.2%) | 233 (22.3%) | 266 (53.4%) | 101 (20.3%) | 131 (26.3%) |
Limitations
This study was conducted at a single community center with ambulant patients. The findings of this study should not be extrapolated to more advanced disease such as those cases typically managed in tertiary facilities. Case inclusion was based on histopathologically proven BCC. A selection bias is present as a BCC without any BCC-associated clinical or dermoscopy features may not have been excised and thus would not have been entered into the study.
Where more than one subtype of BCC presented with a tumor, the percentage of the total mass occupied by each subtype was not recorded. Many of the aggressive BCC subtype cases in this study were combined with the nodular BCC subtype cases. Thus, the single maximum depth of invasion measurement for cases of aggressive combined with nodular subtype could relate to either subtype. Superficial BCC is a straightforward histopathological diagnosis. Interobserver concordance analyses for the histological diagnoses of the different BCC subtypes were not undertaken.
Discussion
A previous French study on BCC examined 13,457 cases. Of these 13,457 cases, 15.1% were superficial BCC subtype and 6.2% were morpheaform BCC subtype [10]. Conversely, our study recorded 33.5% superficial BCC subtype cases and 20.3% aggressive BCC subtype cases. This French study also reported that 94.8% of morpheaform BCCs were located on the head while 30.6% of morpheaform BCCs were located on the nose. Similarly, our study found that the nose had the highest proportion of aggressive BCCs (24.4%, n = 120).
Our study showed that tumor invasion for BCCs was deeper among male than among female patients, as previously recorded [3]. Our study also compared the invasion depths of (1) superficial combined with nodular subtypes; (2) nodular alone; and (3) aggressive BCC subtypes across men and women. Invasion was significantly deeper in men than in women across all 3 categories of BCC subtype. This trend (i.e., that all 3 BCC subtypes were deeper in men than women) applied to the head and neck as well as the trunk and limb sites. Increased invasion depth was associated with sites exposed to higher chronic sunlight. A distal shift from the upper arm to the hand and from the thigh to the foot showed an increasing depth of invasion across all BCC subtypes except nodular BCCs on leg sites.
Data from our study indicate elliptical excisions with a microscopic depth of 1 mm appear to be quite adequate for deep margin clearance on superficial BCCs at all anatomic sites. For BCC with dimensions within the range of the study cases recorded, excisions with a microscopic 2-mm depth appear adequate for the majority of superficial combined with nodular BCCs. Nodular BCCs may require deeper excisions of up to a microscopic depth of 3 mm on the hand, neck, and scalp. Finally, aggressive BCCs may require 3 mm deep elliptical excisions on the neck. Because of cosmetic considerations, Mohs surgery may be preferred on aggressive subtype BCCs located on central facial sites and the ear rather than performing elliptical excisions.
Nodulocystic BCCs recorded the largest mean maximum microscopic tumor diameter and deepest mean depth compared with all other BCC subtypes. Another previous study reported that nodulocystic BCCs on nose and eyelid sites tend to have deeper margin involvement following excisions [11]. In our study, none of the nodulocystic cases were combined with aggressive subtype BCC.
Conclusions
Knowledge of potential tumor depth should be used to guide biopsy techniques and depth selection during excisions. Our study found that BCC subtypes vary in mean depth across both sex and anatomic site. Nodulocystic and nodular BCC subtypes presented as the deepest tumors. Head and neck BCCs were found to have an increased mean depth compared with those on trunk and limb sites. Another trend identified by this study was an increase in mean tumor depth on both the upper and lower limbs for distal sites compared with proximal limb sites.
Figure 1B.
Nodular basal cell carcinoma: maximum invasion depth indicated by the same method as in Figure 1A. [Copyright: ©2018 Pyne et al.]
Figure 1C.
Nodulocystic basal cell carcinoma: maximum invasion depth indicated by the same method as in Figure 1A. [Copyright: ©2018 Pyne et al.]
Footnotes
Funding: None.
Competing interests: The authors have no conflicts of interest to disclose.
References
- 1.Lang PG, Maize JC., Sr . Basal cell carcinoma. In: Rigel DS, Friedman RJ, Dzubow LM, Reintgen DS, Bystryn J-C, Marks R, editors. Cancer of the Skin. Philadelphia: Elsevier; 2005. pp. 101–132. [Google Scholar]
- 2.Sexton M, Jones DB, Maloney ME. Histologic pattern analysis of basal cell carcinoma: study of series of 1039 consecutive neoplasms. J Am Acad Dermatol. 1990;23:1118–1126. doi: 10.1016/0190-9622(90)70344-h. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong LTD, Magnusson MR, Guppy MPB. Risk factors for recurrence of facial basal cell carcinoma after surgical excision: a follow-up analysis. J Plast Reconstr Aesthet Surg. 2017;70:1738–1745. doi: 10.1016/j.bjps.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Welsch MJ, Troiani BM, Hale L, et al. Basal cell carcinoma characteristics as predictors of depth of invasion. J Am Acad Dermatol. 2012;67:47–53. doi: 10.1016/j.jaad.2011.02.035. [DOI] [PubMed] [Google Scholar]
- 5.Codazzi D, Van Der Velden J, Carminati M, et al. Positive compared with negative margins in a single-centre retrospective study on 3,957 consecutive excisions of basal cell carcinomas: associated risk factors and preferred surgical management. J Plast Surg Hand Surg. 2014;48:38–43. doi: 10.3109/2000656X.2013.800526. [DOI] [PubMed] [Google Scholar]
- 6.Li Q, Gao T, Jiao B, et al. Tumor thickness predicts long-term complete response of facial basal cell carcinomas in Asian skin types iv/v treated with methyl aminolaevulinate photodynamic therapy. Photomed Laser Surg. 2011;29:501–507. doi: 10.1089/pho.2010.2924. [DOI] [PubMed] [Google Scholar]
- 7.Ahnlide I, Zalaudek I, Nilsson F, Bjellerup M, Nielsen K. Preoperative prediction of histopathological outcome in basal cell carcinoma: flat surface and multiple small erosions predict superficial basal cell carcinoma in lighter skin types. Br J Dermatol. 2016;175:751–761. doi: 10.1111/bjd.14499. [DOI] [PubMed] [Google Scholar]
- 8.Lallas A, Argenziano G, Ioannides D. Dermoscopy for basal cell carcinoma subtype prediction. Br J Dermatol. 2016;175:674–675. doi: 10.1111/bjd.14657. [DOI] [PubMed] [Google Scholar]
- 9.Pyne JH, Fishburn P, Dicker A, David M. Infiltrating basal cell carcinoma: a stellate peri-tumor dermatoscopy pattern as a clue to diagnosis. Dermatol Pract Concept. 2015;5:21–26. doi: 10.5826/dpc.0502a02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scrivener Y, Grosshaans E, Cribier B. Variations of basal cell carcinomas according to gender, age, location and histopathological subtype. Br J Dermatol. 2002;147:41–47. doi: 10.1046/j.1365-2133.2002.04804.x. [DOI] [PubMed] [Google Scholar]
- 11.Gualdi G, Monari P, Crotti S, et al. Matter of margins. J Eur Acad Dermatol Venereol. 2015;29:255–261. doi: 10.1111/jdv.12504. [DOI] [PubMed] [Google Scholar]


