Abstract
Background
The phosphatidylinositol-3-kinase delta (PI3Kδ) inhibitor idelalisib has been shown to block downstream intracellular signaling, reduce the production of prosurvival chemokines and induce apoptosis in classical Hodgkin lymphoma (HL) cell lines. It has also been shown to inhibit regulatory T cells and myeloid-derived suppressor cells in other tumor models. We hypothesized that inhibiting PI3Kδ would have both direct and indirect antitumor effects by directly targeting the malignant cells as well as modulating the inflammatory microenvironment. We tested this hypothesis in a phase II study.
Patients and methods
We enrolled 25 patients with relapsed/refractory HL with a median age of 42 years and who had previously received a median of five therapies including 18 (72%) with failed autologous stem cell transplant, 23 (92%) with failed brentuximab vedotin, and 11 (44%) with prior radiation therapy. Idelalisib was administered at 150 mg two times daily; an increase to 300 mg two times daily was permitted at the time of disease progression.
Results
The overall response rate to idelalisib therapy was 20% (95% confidence interval: 6.8%, 40.7%) with a median time to response of 2.0 months. Seventeen patients (68%) experienced reduction in target lesions with one complete remission and four partial remissions. The median duration of response was 8.4 months and median progression-free survival was 2.3 months. The most common grade ≥3 adverse event was elevation of alanine aminotransferase (two patients, 8%). Diarrhea/colitis was seen in three patients and was grade 1–2. There was one adverse event leading to death (hypoxia).
Conclusions
Idelalisib was tolerable and had modest single-agent activity in heavily pretreated patients with HL. Rational combinations with other novel agents may improve response rate and duration of response.
Clinical trial registration
ClinicalTrials.gov # NCT01393106.
Keywords: PI3Kδ, idelalisib therapy, Hodgkin lymphoma
Introduction
The phosphatidylinositide 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) pathway is essential for cell cycle regulation and constitutively activated in Hodgkin lymphoma (HL). Although treatment of advanced-stage HL has improved, ∼10%–30% of patients will not achieve complete response (CR) [1], and 20%–30% of responding patients subsequently relapse after treatment [2, 3].
Relapsed or refractory HL remains a therapeutic challenge. The standard treatment of relapsed HL is salvage therapy and autologous stem cell transplant (ASCT) for eligible patients [4] followed by brentuximab vedotin (BV) for maintenance or subsequent relapse [5, 6]. Other chemotherapy regimens such as gemcitabine, vinorelbine, and doxorubicin (GVD) are often effective for patients in whom transplant failed [7].
Patients with HL who fail ASCT and BV may respond to programmed cell death 1 (PD1) blockade [8, 9]. Drugs that block the cytotoxic T lymphocyte-associated antigen 4, PD1, and its ligand (PDL1) inhibit immune checkpoints and drive tumor-specific T cells away from a regulatory T cell (Treg) phenotype toward an effector phenotype [8–13]. In general, these approaches are palliative and novel targets and therapies are needed.
Idelalisib is a selective inhibitor of phosphatidylinositol-3-kinase delta (PI3Kδ) that inhibits HL growth and survival [14, 15]. Idelalisib may also bias T cell differentiation away from a Treg and toward a T effector phenotype via blockade of PI3K signaling [16]. Based on the above preclinical data, the current study evaluated the efficacy and safety of idelalisib in relapsed and refractory HL.
Patients and methods
Study design and treatments
This was a phase II, open-label, single-arm, two-stage trial at three US centers evaluating idelalisib monotherapy in patients with relapsed or refractory HL (ClinicalTrials.gov # NCT01393106). The study tested the alternative hypothesis that the overall response rate (ORR) was ≥45% against the null hypothesis that the ORR was ≤20% using Simon’s minimax two-stage design with >80% power and a significance level of 0.05 [17] with a planned sample size of 21 patients. Stage 1 required a response in 3 of the first 13 patients. The hypothesis could be rejected if responses occurred in ≥8 of 21 patients. The final analysis was carried out when all patients had been on study for at least 24 weeks.
Idelalisib was administered orally at a starting dose of 150 mg two times daily until tumor progression or unacceptable toxicity. The dose was increased to 300 mg two times daily for patients with disease progression after 8 weeks of therapy.
All study protocols were approved by the institutional review board and conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization guidelines for Good Clinical Practice. All patients provided written consent before participation in the study.
Patient eligibility
Eligible patients met all of the following inclusion criteria to participate in the study: relapsed or refractory HL after previous myeloablative therapy with ASCT or after ≥2 prior chemotherapy-containing regimens; ≥12 years of age; fluorodeoxyglucose (FDG)-avid adenopathy; and a Karnofsky performance score of ≥60 (Eastern Cooperative Oncology Group performance score of 0–2).
Study objectives and assessments
The primary objective was to estimate the ORR in this HL patient population using standard criteria [18]. The FDG-positron-emission tomography occurred at eight weeks to confirm CR; otherwise, computed tomography was used.
Secondary endpoints were time to response (TTR), duration of response (DOR), progression-free survival (PFS), percent change from baseline in sum of the product of the greatest perpendicular diameters (SPD) of target lymph nodes and overall survival (OS). Adverse events (AEs) were graded using the Common Terminology Criteria for Adverse Events (CTCAE), version 4.03. The study also characterized changes in patient-reported health-related quality of life (HRQL) using the Functional Assessment of Cancer Therapy‐Lymphoma (FACT-Lym).
Statistical analysis
The intent-to-treat (ITT) analysis set included all 25 patients who received at least one dose of idelalisib and was used in the primary analysis of ORR, PFS, SPD, OS, safety, drug administration, and compliance. The per-protocol (PP) analysis set included 24 patients and was used for secondary analysis of ORR, PFS, and SPD. The responding analysis set included those patients who achieved a best response of CR or partial response (PR) and was used in the analysis of TTR and DOR. The ORR was calculated as proportion of patients with a CR or PR, and a corresponding two-sided 95% exact binomial confidence interval (CI) was constructed.
The exact binomial test was used in the final analysis because the accrual could not be limited to exactly 21 patients and nonresponding patients could be included in the final analysis as responding if they experienced a late response. Kaplan–Meier (KM) methods were calculated for PFS and OS following idelalisib monotherapy and 95% CIs were calculated using the Greenwood’s formula with (complementary) log–log transformation.
Results
Patients
From September 2011 to August 2014, 25 patients with relapsed or refractory classical HL were enrolled, received study drug and were included in the ITT population. The majority of patients were female (56%) and white (76%); median age was 42 years (Table 1). Patients had received a median of five prior therapies (range 2–9); 23 patients (92%) had received BV, 24 (96%) had received adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD), 18 (72%) had undergone ASCT, and 11 (44%) had received prior radiation therapy. Exposure to idelalisib ranged from 0.5 to 25.2 months, with a median of 3.6 months. Five patients had at least one dose reduction; two patients received a dose reduction to 75 mg two times daily. Five patients received idelalisib at 300 mg two times daily at the time of disease progression. Reasons for permanent discontinuation of study drug included progressive disease in 20 patients (80%), AEs in three patients (12%), lack of response in one patient (4%) and voluntary withdrawal by one patient (4%) (supplementary Figure S1, available at Annals of Oncology online). The majority of patients (64%) discontinued study drug before week 16.
Table 1.
Demographics and baseline characteristicsa
| Idelalisib | |
|---|---|
| N = 25b | |
| Male, n (%) | 11 (44) |
| Race, n (%) | |
| White | 19 (76) |
| Black or African American | 2 (8) |
| Asian | 1 (4) |
| Other | 3 (12) |
| Ethnicity, n (%) | |
| Hispanic or Latino | 3 (12) |
| Not Hispanic or Latino | 22 (88) |
| Agec, years, median (range) | 42 (21–80) |
| BMId, kg/m2, mean (SD) | 25 (5.94) |
| ECOG performance status, n (%) | |
| 0 | 10 (40) |
| 1 | 10 (40) |
| 2 | 1 (4) |
| Missing | 4 (16) |
| Time since diagnosise, years, mean (SD) | 3.9 (4.26) |
| Prior therapies, median (range) | 5 (2–9) |
| Type of previous treatment, n (%) | |
| BV | 23 (92) |
| ASCT | 18 (72) |
| Radiation therapy | 11 (44) |
| Classical HL pathologic subtype, n (%) | |
| Nodular sclerosis | 23 (92) |
| Lymphocyte rich | 2 (8) |
| Disease stage at screening, n (%) | |
| I–II | 9 (36) |
| III–IV | 16 (64) |
The population used for this analysis includes all patients in the intent-to-treat set who received ≥1 dose of idelalisib.
Includes all patients treated during this study.
Age (years) = (date of first dose of study medication – date of birth + 1)/365.25.
BMI (kg/m2) = weight/height2.
Time since diagnosis is calculated in months as (date of randomization to the primary study – date of diagnosis)/30.4375.
ASCT, autologous stem cell transplant; BMI, body mass index; BV, brentuximab vedotin; ECOG, Eastern Cooperative Oncology Group; HL, Hodgkin lymphoma; SD, standard deviation.
Efficacy evaluation
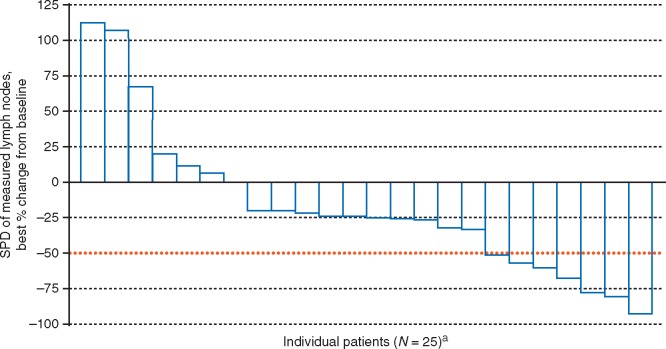
The ORR for all 25 patients was 20% (95% CI: 6.8%, 40.7%). One patient (4%) had CR and four (16%) experienced PR. Seven patients (28%) experienced stable disease, 12 (48%) experienced progressive disease and 1 patient (4%) was classified as not evaluable (Table 2). For the 24-patient PP analysis set, the ORR was similar (20.8%; 95% CI: 7.1%, 42.2%). Four PRs were seen in the 23 patients who received prior BV. Of the response-evaluable patients, 17 (68%) experienced reduction in target lesions (Figure 1). Escalation to 300 mg two times daily following disease progression did not result in any responses. The responses were not significantly different depending on the classical HL pathologic subtype (Table 2). However, we have observed a significant difference in response depending on the ECOG performance status (Table 2).
Table 2.
Overall response rate (ORR)a
| Response in patients who failed prior therapy | ||||||
|---|---|---|---|---|---|---|
| All patients N = 25b | Failure of brentuximab and no ASCT (n = 7) | Failure of brentuximab and ASCT (n = 16) | Failure of ASCT (n = 18) | Failure of radiation therapy (n = 11) | ||
| Overall response ratec | 5 (20) | 0 | 4 (25) | 5 (27.8) | 1 (9.1) | |
| 95% CId | 6.8, 40.7 | 7.3, 52.4 | 10.0, 35.5 | 0.2, 41.3 | ||
| CR | 1 (4) | 0 | 0 | 1 (5.6) | 0 | |
| PR | 4 (16) | 0 | 4 (25) | 4 (22.2) | 1 (9.1) | |
| SD | 7 (28) | 0 | NR | NR | NR | |
| PD | 12 (48) | 0 | NR | NR | NR | |
| NE | 1 (4) | 0 | NR | NR | NR | |
| Disease characteristics at baseline by response | ||||||
| Nodular sclerosis | Lymphocyte-rich | P-valuee | Stage I–II | Stage III–IV | P-valuee | |
| CR + PR, n =5 | 4 (80) | 1 (20) | 0.2200 | 1 (20) | 4 (80) | 0.1127 |
| SD, n = 7 | 6 (85.7) | 1 (14.3) | 5 (71.4) | 2 (28.6) | ||
| PD + NE, n =13 | 13 (100) | 0 | 3 (23.1) | 10 (76.9) | ||
| ECOG performance status at baseline by response | ||||||
| ECOG 0 | ECOG 1 | ECOG 2 | Missing | P-valuee | ||
| CR + PR, n =5 | 2 (40) | 0 | 0 | 3 (60) | 0.0443 | |
| SD, n =7 | 2 (28.6) | 4 (57.1) | 0 | 1 (14.3) | ||
| PD + NE, n =13 | 6 (46.2) | 6 (46.2) | 1 (7.7) | 0 | ||
Data presented as n (%).
All percentages are based on the number of patients in the intent-to-treat analysis set. Patients who did not have any postbaseline tumor assessments were considered nonresponders. Complete response was confirmed by positron-emission tomography.
Includes all patients treated during this study.
Overall response rate was the percentage of patients who had best overall response of complete response and partial response. Patients who did not have sufficient baseline or on-study tumor assessment to characterize response were included in the denominator.
95% CI for response rate was based on the exact binomial method.
P-values were obtained using Fisher’s exact test.
ASCT, autologous stem cell transplant; CI, confidence interval; CR, complete response; ECOG, Eastern Cooperative Oncology Group; NE, not evaluable; NR, not reported; PD, progressive disease; PR, partial response, SD, stable disease.
Figure 1.
Waterfall plot of percent change in SPD in nodal lesions. SPD, sum of the product of the greatest perpendicular diameters of target lymph nodes as documented radiographically. The red line represents a % change of −50% and is a criterion for lymphadenopathy response [20]. aOne patient was non-evaluable.
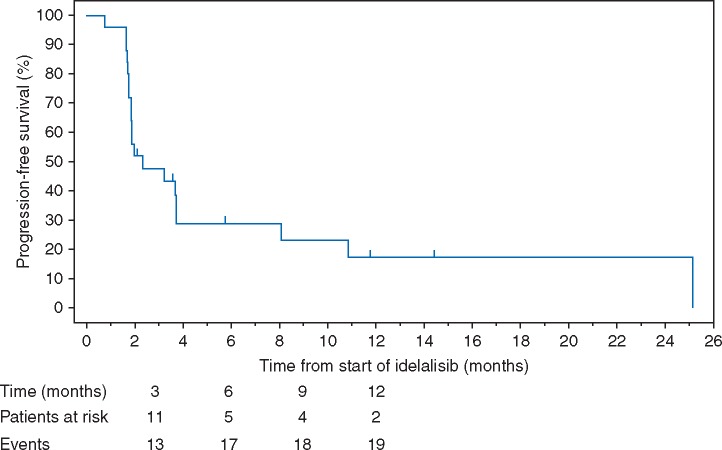
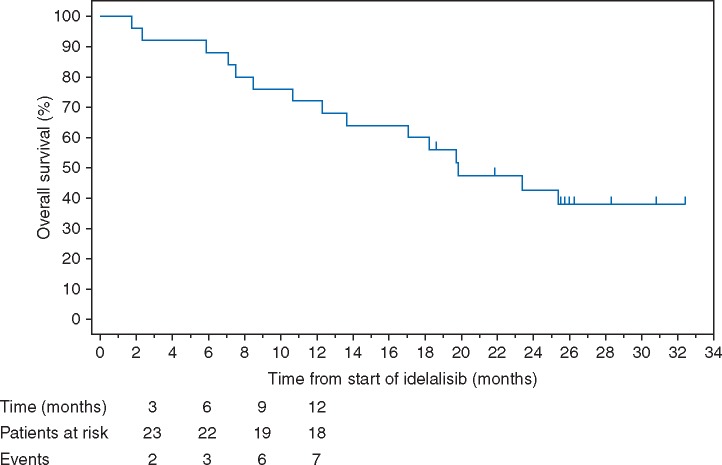
Median TTR was 2.0 months (range from 1.9 to 16.8 months). Median DOR was 8.4 months (95% CI: 1.8, not estimable). Median PFS was 2.3 months (95% CI: 1.8, 3.7; Figure 2). The median OS was 19.8 months (95% CI: 12.3, not estimable; Figure 3).
Figure 2.
Kaplan–Meier curve for progression-free survival (PFS). Progression-free survival was defined as the time from the start of idelalisib treatment to the date of documented disease progression or death due to any cause, whichever came first. The KM estimate of median PFS was 2.3 months. The KM estimate of the proportion of patients with PFS at six months was 28.9%.
Figure 3.
Kaplan–Meier curve for overall survival (OS). Overall survival was defined as the time from study day one until the date of death due to any cause, including data from long-term follow-up. The KM estimate of median OS was 19.8 months. The KM estimate of the proportion of surviving patients at six months was 88%. This figure depicts the KM curve for OS for the intent-to-treat analysis set, including long-term follow-up data.
Safety evaluation
Twenty-four patients (96%) reported at least one AE (Table 3). Eight patients (32%) had fatigue; seven (28%) had pyrexia; and six (24%) each had increased levels of aspartate aminotransferase (AST), constipation, and vomiting. AEs of interest were rash (grades 1–3), observed in three patients (12%), diarrhea/colitis (grade 1 or 2) in three patients (12%), and pneumonitis (grade 2) reported in one patient (4%). Serious AEs (SAEs) were reported for nine patients (36%) and six (24%) experienced SAEs related to idelalisib (Table 3).
Table 3.
Overall summary of AEsa
| AEs | Idelalisib |
|---|---|
| N = 25b | |
| Any AE | 24 (96) |
| AEs related to study drug | 19 (76) |
| Grade ≥3 AEs | 12 (48) |
| AEs leading to dose change or temporary interruption of study drug | 9 (36) |
| Grade ≥3 AEs related to study drug | 6 (24) |
| AEs leading to permanent discontinuation of study drug | 2 (8) |
| AEs leading to death during study | 1 (4) |
| Any SAE | 9 (36) |
| SAEs related to study drug | 6 (24) |
| Maculopapular rash | 2 (8) |
| Colitis | 1 (4) |
| Febrile neutropenia | 1 (4) |
| Herpes zoster | 1 (4) |
| Hypocalcemia | 1 (4) |
| Hypokalemia | 1 (4) |
| Hypomagnesemia | 1 (4) |
| Hypoxia | 1 (4) |
| Hospitalization | 1 (4) |
| Lung infection | 1 (4) |
| Platelet count decreased | 1 (4) |
| Pneumonia | 1 (4) |
| Pneumonitis | 1 (4) |
| Productive cough | 1 (4) |
| Pyrexia | 1 (4) |
| Skin infection | 1 (4) |
| Vomiting | 1 (4) |
| Deaths during study drug or long-term follow-up | 15 (60) |
| Deaths during long-term follow-up | 14 (56) |
| Deaths during study drug up to 30 days post-last study treatment | 1 (4) |
Data presented as n (%). The severity of AEs was graded by the investigator according to the Common Terminology Criteria for Adverse Events, version 4.03.
All percentages are based on the number of patients in the intent-to-treat analysis set.
Includes all patients treated during this study.
AE, adverse event; SAE, serious adverse event.
Fifteen deaths (60%) occurred during the study or long-term follow-up. One death occurred on study; this patient was receiving palliative chemotherapy at the time of death, 24 days after discontinuing idelalisib. The other 14 deaths occurred during long-term follow-up (>30 days following last dose of study drug) including disease progression (7), unknown causes (6), and respiratory insufficiency with renal failure after stem cell transplant (1).
Alanine aminotransferase (ALT; 5 patients) or AST (4 patients) ≥grade 3 elevations were observed in five patients but were generally transient and asymptomatic. Four of these patients (80%) resolved to grade 1 or less; and one patient had increased ALT/AST levels at the last study visit. Three of these patients temporarily interrupted idelalisib therapy, one patient received a dose reduction and one received his last dose of idelalisib the day before the grade 3 AST/ALT elevation.
The most commonly reported ≥grade 3 hematologic laboratory abnormalities were lymphopenia (12), neutropenia (2), anemia (1), thrombocytopenia (1), and leukopenia (1). Immunophenotyping showed that five patients (20%) had ≥ grade 3 decrease in CD4+ lymphocytes. The range of FACT-Lym scores did not change significantly over the course of the study.
Discussion
In this trial, idelalisib treatment had a modest response in patients with multiply-relapsed HL, demonstrating the first evidence of clinical activity for this class of drugs in this malignancy. Overall response rates were comparable to other single-agent studies with gemcitabine, lenalidomide, rituximab, or panobinostat (ORRs 19%–27%), although in those studies, most patients had not previously received BV [19]. At the time of enrollment in this study, most patients had recurrent disease following BV and high-dose therapy with ASCT, and despite these high-risk features, the ORR with idelalisib monotherapy was 20%, with most responses occurring early. Furthermore, nearly 70% achieved at least some disease control. Rates of AEs observed were comparable to those seen using idelalisib in heavily pretreated indolent non-HL patients in which 7% developed pneumonia, 4% developed colitis, and 8.8% (11 patients) died within 30 days of receiving idelalisib [20].
Though options for patients with relapsed HL have increased since the design of this trial, only a minority will be cured with BV [21]. More recently, PDL1 and PDL2 alterations have been identified as hallmarks of classical HL, and the use of anti-PD1 therapy is currently approved for direct modulation of the immune evasion that also plays a major role in most of HL cases [22]. However, even with the use of nivolumab or pembrolizumab, most responses have been partial [8, 23]. Preclinical solid tumor models showed inactivation of PI3Kδ-promoted antitumor immunity by inhibiting regulatory T cells and myeloid-derived suppressor cells [24]. Other studies suggest that PI3Kδ inhibition can reduce anti-inflammatory cytokines, such as IL-10, and PDL1 expression on tumor cells [25, 26]. Based on these data, one could hypothesize that the addition of idelalisib to anti-PD1 therapy has the potential to increase clinical efficacy of immune checkpoint inhibition in HL. However, with a risk for autoimmune and inflammatory AEs with each of these single agents, this approach needs to be evaluated in careful prospective trials.
In summary, this is the first study to demonstrate the efficacy of PI3Kδ inhibition by idelalisib in patients with multiple-relapsed HL. The therapy was well tolerated and the ORR was comparable to other single agents used for patients with multiple lines of prior treatment. Deriving meaningful benefit from PI3Kδ inhibitors such as idelalisib in HL may be aided by combination therapy, possibly with immunotherapeutic agents such as checkpoint inhibitors in carefully designed clinical trials.
Supplementary Material
Acknowledgements
The authors wish to thank all the patients and their families who contributed to this study, all the participating research nurses and data coordinators. The authors acknowledge medical writing assistance provided by Paraskevi Viviana Vogiatzi, MD, PhD, of AlphaBioCom, LLC, and funded by Gilead Sciences, Inc.
Funding
This work was supported by Gilead Sciences, Inc., Foster City, CA, USA. No grant number is applicable.
Disclosure
AKG: Research funding: Gilead, Spectrum, Pfizer, BioMarin, Cephalon/Teva, Emergent/Abbott, Janssen, Merck, Millennium, Piramal, Seattle Genetics, Biogen Idec and BMS. Consultancy: Gilead, Spectrum, Pfizer, Janssen, Seattle Genetics. AKG also received philanthropic support from Frank and Betty Vandermeer. Honoraria: Millennium, Seattle Genetics, Sanofi-Aventis. MAF: Research funding: Gilead. CHM: Research funding: Seattle Genetics, Pharmacyclics, Merck. Consultancy: Celgene Corporation, Genentech BioOncology, Merck, Seattle Genetics. ARS: Research funding: Spectrum, Seattle Genetics, Novartis. Consultancy: Celgene, BMS, Spectrum. Honoraria: Celgene, BMS. SM: Employment: Gilead. WY: Employment: Gilead. Ownership interests: Gilead stocks. AY: Research funding: Seattle Genetics. Honoraria: Seattle Genetics, Takeda Pharmaceuticals International Co. Advisory/scientific boards: Seattle Genetics. AJM: Research funding: Seattle Genetics. Advisory board: Seattle Genetics. Honorarium: Seattle Genetics.
References
- 1. Kuruvilla J. Standard therapy of advanced Hodgkin lymphoma. Hematology Am Soc Hematol Educ Program 2009; 497–506. doi:10.1182/asheducation-2009.1.497. [DOI] [PubMed] [Google Scholar]
- 2. Kuruvilla J, Keating A, Crump M.. How I treat relapsed and refractory Hodgkin lymphoma. Blood 2011; 117: 4208–4217. [DOI] [PubMed] [Google Scholar]
- 3. Follows GA, Ardeshna KM, Barrington SF. et al. Guidelines for the first line management of classical Hodgkin lymphoma. Br J Haematol 2014; 166: 34–49. [DOI] [PubMed] [Google Scholar]
- 4. Keller SF, Kelly JL, Sensenig E. et al. Late relapses following high-dose autologous stem cell transplantation (HD-ASCT) for Hodgkin's lymphoma (HL) in the ABVD therapeutic era. Biol Blood Marrow Transplant 2012; 18: 640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eichenauer DA, Engert A, Andre M. et al. Hodgkin's lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014; 25(Suppl 3): iii70–iii75. [DOI] [PubMed] [Google Scholar]
- 6. Onishi M, Graf SA, Holmberg L. et al. Brentuximab vedotin administered to platinum-refractory, transplant-naive Hodgkin lymphoma patients can increase the proportion achieving FDG PET negative status. Hematol Oncol 2015; 33: 187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bartlett NL, Niedzwiecki D, Johnson JL. et al. Gemcitabine, vinorelbine, and pegylated liposomal doxorubicin (GVD), a salvage regimen in relapsed Hodgkin's lymphoma: CALGB 59804. Ann Oncol 2007; 18: 1071–1079. [DOI] [PubMed] [Google Scholar]
- 8. Ansell SM, Lesokhin AM, Borrello I. et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 2015; 372: 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moskowitz CH, Ribrag V, Michot J-M. et al. PD-1 Blockade with the Monoclonal Antibody Pembrolizumab (MK-3475) in Patients with Classical Hodgkin Lymphoma after Brentuximab Vedotin Failure: Preliminary Results from a Phase 1b Study (KEYNOTE-013). Blood 2014; 124: 290–290. [Google Scholar]
- 10. Raimondi G, Shufesky WJ, Tokita D. et al. Regulated compartmentalization of programmed cell death-1 discriminates CD4+CD25+ resting regulatory T cells from activated T cells. J Immunol 2006; 176: 2808–2816. [DOI] [PubMed] [Google Scholar]
- 11. Tai X, Van Laethem F, Pobezinsky L. et al. Basis of CTLA-4 function in regulatory and conventional CD4(+) T cells. Blood 2012; 119: 5155–5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Francisco LM, Salinas VH, Brown KE. et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med 2009; 206: 3015–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yamamoto R, Nishikori M, Kitawaki T. et al. PD-1-PD-1 ligand interaction contributes to immunosuppressive microenvironment of Hodgkin lymphoma. Blood 2008; 111: 3220–3224. [DOI] [PubMed] [Google Scholar]
- 14. Lannutti BJ, Meadows SA, Herman SE. et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood 2011; 117: 591–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meadows SA, Vega F, Kashishian A. et al. PI3Kdelta inhibitor, GS-1101 (CAL-101), attenuates pathway signaling, induces apoptosis, and overcomes signals from the microenvironment in cellular models of Hodgkin lymphoma. Blood 2012; 119: 1897–1900. [DOI] [PubMed] [Google Scholar]
- 16. Patton DT, Garden OA, Pearce WP. et al. Cutting edge: the phosphoinositide 3-kinase p110 delta is critical for the function of CD4+CD25+Foxp3+ regulatory T cells. J Immunol 2006; 177: 6598–6602. [DOI] [PubMed] [Google Scholar]
- 17. Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials 1989; 10: 1–10. [DOI] [PubMed] [Google Scholar]
- 18. Cheson BD, Pfistner B, Juweid ME. et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007; 25: 579–586. [DOI] [PubMed] [Google Scholar]
- 19. Alinari L, Blum KA.. How I treat relapsed classical Hodgkin lymphoma after autologous stem cell transplant. Blood 2016; 127: 287–295. [DOI] [PubMed] [Google Scholar]
- 20. Gopal AK, Kahl BS, de Vos S. et al. PI3Kdelta inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med 2014; 370: 1008–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gopal AK, Chen R, Smith SE. et al. Durable remissions in a pivotal phase 2 study of brentuximab vedotin in relapsed or refractory Hodgkin lymphoma. Blood 2015; 125: 1236–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roemer MG, Advani RH, Ligon AH. et al. PD-L1 and PD-L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. J Clin Oncol 2016; 34: 2690–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Armand P, Shipp MA, Ribrag V. et al. PD-1 blockade with pembrolizumab in patients with classical Hodgkin lymphoma after brentuximab vedotin failure: safety, efficacy, and biomarker assessment. Blood 2015; 126: 584–584. Abstract 584. [Google Scholar]
- 24. Ali K, Soond DR, Pineiro R. et al. Inactivation of PI(3)K p110delta breaks regulatory T-cell-mediated immune tolerance to cancer. Nature 2014; 510: 407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marshall NA, Galvin KC, Corcoran AM. et al. Immunotherapy with PI3K inhibitor and Toll-like receptor agonist induces IFN-gamma+IL-17+ polyfunctional T cells that mediate rejection of murine tumors. Cancer Res 2012; 72: 581–591. [DOI] [PubMed] [Google Scholar]
- 26. Oh T, Ivan ME, Sun MZ. et al. PI3K pathway inhibitors: potential prospects as adjuncts to vaccine immunotherapy for glioblastoma. Immunotherapy 2014; 6: 737–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.