Abstract
Background
Incidence of locoregional neuroendocrine tumors (NETs) is rising. However, after curative resection, the patterns and risk factors associated with recurrence remain unknown. Consensus guidelines recommend surveillance every 6–12 months for up to 10 years after surgery for resected, well-differentiated NETs irrespective of patient demographics, site, grade or stage of tumor with few exceptions.
Patients and methods
From the Surveillance, Epidemiology, and End Results (SEER)-Medicare database, we identified localized and regional stage NET patients who underwent surgical resection between January 2002 and December 2011. Development of recurrence was identified by capturing at least two claims indicative of metastatic disease until 31 December 2013.
Results
Of the 2366 identified patients (median age 73 years), 369 (16%) developed metastatic disease within 5 years and only an additional 1% developed metastases between years 5 and 10 with the majority dying due to unrelated causes. The 5-year risk of developing metastases (hazard ratio, HR) varied significantly (log-rank P < 0.001) by grade: 9.9% versus 25.9% (2.2) versus 48.1% (4.4) for grades 1, 2, and ≥ 3, respectively; stage: 10.3% versus 31.1% (2.8) for localized versus regional; primary tumor size: 7.6% versus 15% (1.3) versus 26.6% (1.5) for <1, 1–2, and > 2 cm, respectively; and site: ranging from 11.3% for colon to 23.9% for pancreas.
Conclusions
Contrary to current guidelines, our study suggests that surveillance recommendations should be tailored according to patient and tumor characteristics. Surveillance past 5 years may be avoided in elderly patients with competing morbidities or low risk of recurrence. Pancreatic, lung, higher grade, and regional NETs have a higher risk of recurrence and may be considered for future adjuvant trials.
Keywords: neuroendocrine, recurrences, surgical resection, gastrointestinal, SEER-Medicare
Introduction
The incidence of neuroendocrine tumors (NETs) has been steadily rising with a growing proportion of the patients diagnosed at an earlier stage possibly due to better diagnostic techniques such as computed tomography (CT) and endoscopy in addition to improved histological classification of NETs [1, 2].
Surgical removal of the primary tumor is usually the first-line treatment for patients with locoregional stage NETs. The primary goal of surgical resection is curative, while secondary goals include symptom control and the limitation of tumor progression. The risk of recurrence after surgical resection is currently largely unknown. Due to the lack of clinical evidence, current consensus guidelines [3–5] suggest that patients be monitored with CT or magnetic resonance imaging scans for disease progression every 6–12 months after primary tumor resection without clear guidance regarding the optimal duration of surveillance. The current guidelines state that surveillance should be done for up to 10 years after surgery. Further adjuvant therapy is not recommended for the majority of patients. These guidelines are based on small, retrospective, single institution studies that have several limitations such as small size, inclusion of limited primary sites (mostly pancreatic NETs) and also referral and follow-up bias [6–10]. Therefore, a comprehensive population-based study of recurrence patterns in NETs is lacking. We chose the Surveillance, Epidemiology, and End Results (SEER)-Medicare database, even though the data are largely limited to patients > 65 years, given its unique ability to provide information regarding recurrences since most other population-based registries such as SEER and NCDB only provide overall survival data. In this paper, we aim to evaluate the recurrence patterns among elderly patients with locoregional NETs after surgical resection and determine demographic and clinical factors associated with recurrent disease.
Materials and methods
Data source
We used the SEER registry data from the National Cancer Institute (NCI) linked with Medicare claims data. The SEER registries cover approximately 28% of the US population [11]. The SEER data provide information on tumor characteristics, demographic information and follow-up for vital status for persons diagnosed with cancer in the United States. The SEER staging system was used in the current study and tumors were classified as localized (defined as an invasive neoplasm confined entirely to the organ of origin), regional (defined as a neoplasm that extended beyond the limits of the organ of origin directly into surrounding organs or tissue and/or involving regional lymph nodes) or distant NET (defined as a neoplasm that spread to parts of the body remote from the primary tumor). Of note, unlike the 2010 WHO grading nomenclature which is focused on the proportion of tumor cells that are dividing, the SEER grading system relies on histologic differentiation, classifying tumors into well differentiated (grade 1), moderately differentiated (grade 2), poorly differentiated (grade 3), and undifferentiated/anaplastic (grade 4). Undifferentiated/anaplastic cancers are commonly designated as “grade 3 neuroendocrine carcinomas,” [12] so we combined SEER grade 3 and 4 data as in prior reports [1, 13]. The Medicare claims data linked with the registry data provide additional information on the usage of health care resources, which allows researchers to identify treatments that patients received through International Classification of Diseases 9th Revision (ICD-9), Current Procedural Terminology (CPT), and Healthcare Common Procedure Coding System (HCPCS) codes in Medicare claims.
Study cohort
Our study cohort included 2366 elderly patients over age 65 who were diagnosed with localized or regional stage NETs between 1 January 2002 and 31 December 2011. The latest claims date available in the SEER-Medicare data at the time of this research was 31 December 2013. NETs were identified by International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3) codes: 8150, 8151, 8152, 8153, 8154, 8155, 8156, 8157, 8240, 8241, 8242, 8243, 8244, 8245, 8246, and 8249. As the aim of our study was to examine the development of metastases, we included only patients with localized or regional diseases at diagnosis who had only one primary cancer site and received surgical resection at the primary site. We captured the date of surgery by identifying claims indicating tumor resection based on the ICD-9 procedure codes and CPT/HCPCS codes. We required continuous Medicare Parts A and B enrollment and no enrollment in health maintenance organizations (HMO) from one month before diagnosis until three months after diagnosis to ensure the completeness of medical claims to capture surgical resection as that is the time window for the identification of the receipt of surgical resection. We also excluded cases whose claims demonstrated the development of secondary malignancy within one month of surgery as these might have been patients with metastatic spread at diagnosis who were misclassified. A detailed flow chart illustrating the inclusion/exclusion criteria of our study cohort is in supplementary Figure S1, available at Annals of Oncology online.
Identification of secondary malignancy (metastatic NET)
We identified the presence of metastatic/secondary malignancy by ICD-9 codes: 209.70, 209.71, 209.72, 209.73, 209.74, 209.79, 197.7, 198.5, 198.3, 198.81, 197.5, 198.7, 198.0, 197.0, 197.2, 197.6, 198.2, 197.4, 198.5, 196.6, 196.0, 196.1, 196.2, 196.3, 196.5, 196.8, 197.1, 197.8, 198.4, 198.6, 198.82, 198.89, 199.0, and 789.51. These codes were curated from similar manuscripts [14, 15] that validated their use to identify metastatic spread in breast, lung, colorectal and prostate cancer patients and modified to fit the typical clinical behavior of NETs. We defined patients as having metastatic disease if they had at least two claims with any of the aforementioned ICD-9 codes.
Explanatory variables
We included demographic characteristics, clinical factors, and neighborhood socioeconomic information as explanatory variables in our analyses. The demographic characteristics included in our analyses were age [65–69, 70–74, 75–79, and ≥80], gender [male versus female], race/ethnicity [non-Hispanic white, non-Hispanic black, Hispanics, or all others], region [Northeast, West, Midwest, and South], and urban/rural status [metropolitan versus non-metropolitan]. The clinical factors included tumor stage [localized, regional], primary cancer site [small intestine and cecum, appendix, colon, pancreas, larynx, bronchus, lung, trachea and other respiratory organs, rectum, and all others], histology grade [grade 1, grade 2, grade 3, grade 4, mixed histology grade, and unknown], and nodal status [negative, positive, and unknown]. We also included the following three variables in terms of quartiles as neighborhood socioeconomic status factors: median household income, percentage with at least a high school education and percentage living in poverty.
Statistical analyses
For bivariate analyses, we used Kaplan–Meier method and log-rank test to evaluate and compare the time to development of metastasis by stage, histological grade, site, and nodal status. Demographic and clinical characteristics between patients with and without development of metastasis were compared using Chi-squared tests. For multivariate analyses, we used Cox proportional hazard model to evaluate the effects of various factors on the development of metastases. Hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) were reported for time to the development of metastases. To evaluate the impact of longer survival of women on risk of recurrence, a competing risk model [16] was employed to account for the competing risk of death. The events of interest were the development of metastases or death. Patients who lost continuous enrollment in Medicare Parts A and B or started enrollment in a HMO were considered to be censored at that time point because the medical claims may have been incomplete from then on.
All statistical analyses were conducted in SAS Enterprise Guide 6.1 (SAS Institute, Cary NC). The Institutional Review Board at The University of Texas MD Anderson Cancer Center exempted this study for approval because all patients in the database had been de-identified.
Results
Table 1 provides the frequency and percentage of patients who developed metastases, were alive without observed metastases, or died without observed metastases under two timelines: 5 years and 10 years after tumor resection. The table also includes the results from chi-square test for group differences. Out of the 2366 patients, 369 (16%) developed metastatic disease within 5 years. The number rose slightly by 32 patients to 401 (17%) over the next 5 years. The number of patients who died without observed metastases rose sharply from 1053 (44%) to 1728 (73%) from 5 to 10 years.
Table 1.
Description of the study cohort by NET metastasis development within 5 and 10 years
| 5 years |
10 years |
|||||||
|---|---|---|---|---|---|---|---|---|
| Covariates | Developed metastasis | Alive and no metastasis observed | Dead and no metastasis observed | P | Developed metastasis | Alive and no metastasis observed | Dead and no metastasis observed | P |
| All | 369 (15.6%) | 944 (39.9%) | 1053 (44.1%) | 401 (16.95%) | 237 (10.02%) | 1728 (73.03%) | ||
| Age | 0.042 | 0.361 | ||||||
| <70 | 109 (15.10%) | 292 (40.44%) | 321 (44.46%) | 118 (16.34%) | 86 (11.91%) | 518 (71.75%) | ||
| 70–74 | 92 (15.44%) | 250 (41.95%) | 254 (42.62%) | 101 (16.95%) | 61 (10.23%) | 434 (72.82%) | ||
| 75–79 | 82 (16.27%) | 217 (43.06%) | 205 (40.67%) | 90 (17.86%) | 48 (9.52%) | 366 (72.62%) | ||
| ≥80 | 86 (15.81%) | 185 (34.01%) | 273 (50.18%) | 92 (16.91%) | 42 (7.72%) | 410 (75.37%) | ||
| Gender | <0.001 | 0.002 | ||||||
| Male | 168 (18.79%) | 312 (34.90%) | 414 (46.31%) | 183 (20.47%) | 87 (9.73%) | 624 (69.80%) | ||
| Female | 201 (13.65%) | 632 (42.93%) | 639 (43.41%) | 218 (14.81%) | 150 (10.19%) | 1104 (75.00%) | ||
| Race | 0.031 | 0.002 | ||||||
| Non-Hispanic White | 318 (16.41%) | 780 (40.25%) | 840 (43.34%) | 347 (17.91%) | 175 (9.03%) | 1416 (73.07%) | ||
| Non-Hispanic Black | 22 (10.43%) | 76 (36.02%) | 113 (53.55%) | 23 (10.90%) | 30 (14.22%) | 158 (74.88%) | ||
| Hispanic or Others | 29 (13.36%) | 88 (40.55%) | 100 (46.08%) | 31 (14.29%) | 32 (14.75%) | 154 (70.97%) | ||
| Stage | <0.001 | <0.001 | ||||||
| Localized | 183 (10.33%) | 782 (44.13%) | 807 (45.54%) | 209 (11.79%) | 206 (11.63%) | 1357 (76.58%) | ||
| Regional | 186 (31.31%) | 162 (27.27%) | 246 (41.41%) | 192 (32.32%) | 31 (5.22%) | 371 (62.46%) | ||
| Histologic grade | <0.001 | <0.001 | ||||||
| G1 | 174 (9.91%) | 778 (44.33%) | 803 (45.75%) | 194 (11.05%) | 206 (11.74%) | 1355 (77.21%) | ||
| G2 | 55 (25.94%) | 72 (33.96%) | 85 (40.09%) | 62 (29.25%) | 15 (7.08%) | 135 (63.68%) | ||
| G3 & G4 | 102 (48.17%) | 42 (19.81%) | 68 (32.07%) | 104 (49.05%) | Maskeda | 100 (47.16%) | ||
| Unknown | 22 (29.33%) | 18 (24.00%) | 35 (46.67%) | 23 (30.67%) | Maskeda | 48 (64.00%) | ||
| Mixed Histology | 16 (14.29%) | 34 (30.36%) | 62 (55.36%) | 18 (16.07%) | 4 (3.57%) | 90 (80.36%) | ||
| Nodal status | 0.93 | 0.285 | ||||||
| Negative | 64 (15.88%) | 159 (39.45%) | 180 (44.67%) | 67 (16.63%) | 39 (9.68%) | 297 (73.70%) | ||
| Positive | 60 (14.89%) | 169 (41.94%) | 174 (43.18%) | 62 (15.38%) | 52 (12.90%) | 289 (71.71%) | ||
| Unknown | 245 (15.71%) | 616 (39.49%) | 699 (44.81%) | 272 (17.44%) | 146 (9.36%) | 1142 (73.21%) | ||
| Region | 0.63 | 0.744 | ||||||
| Midwest | 48 (16.11%) | 114 (38.26%) | 136 (45.64%) | 52 (17.45%) | 28 (9.40%) | 218 (73.15%) | ||
| Northeast | 90 (17.31%) | 210 (40.38%) | 220 (42.31%) | 100 (19.23%) | 53 (10.19%) | 367 (70.58%) | ||
| South | 94 (14.73%) | 244 (38.24%) | 300 (47.02%) | 99 (15.52%) | 62 (9.72%) | 477 (74.76%) | ||
| West | 137 (15.05%) | 376 (41.32%) | 397 (43.63%) | 150 (16.48%) | 94 (10.33%) | 666 (73.19%) | ||
| Tumor size | <0.001 | <0.001 | ||||||
| <1 cm | 37 (7.64%) | 213 (44.01%) | 234 (48.35%) | 40 (8.26%) | 59 (12.19%) | 385 (79.55%) | ||
| 1–2 cm | 122 (15.01%) | 327 (40.22%) | 364 (44.77%) | 135 (16.61%) | 69 (8.49%) | 609 (74.91%) | ||
| >2 cm | 176 (26.59%) | 212 (32.02%) | 274 (41.39%) | 188 (28.40%) | 40 (6.04%) | 434 (65.56%) | ||
| Unknown | 34 (8.35%) | 192 (47.17%) | 181 (44.47%) | 38 (9.34%) | 69 (16.95%) | 300 (73.71%) | ||
| Primary site | <0.001 | <0.001 | ||||||
| Appendix | 12 (13.79%) | 26 (29.89%) | 49 (56.32%) | 13 (14.94%) | Maskeda | 70 (80.46%) | ||
| Colon | 13 (11.30%) | 56 (48.70%) | 46 (40.00%) | 13 (11.30%) | 21 (18.26%) | 81 (70.43%) | ||
| Larynx, bronchus, lung, trachea and other respiratory organs | 162 (17.94%) | 372 (41.20%) | 369 (40.86%) | 177 (19.60%) | 75 (8.31%) | 651 (72.09%) | ||
| Others | 54 (20.00%) | 101 (37.41%) | 115 (42.59%) | 57 (21.11%) | 25 (9.26%) | 188 (69.63%) | ||
| Pancreas | 26 (23.85%) | 30 (27.52%) | 53 (48.62%) | 33 (30.28%) | Maskeda | 72 (66.06%) | ||
| Rectum | Maskeda | 101 (54.30%) | 76 (40.86%) | Maskeda | 50 (26.88%) | 126 (67.74%) | ||
| Small intestine and cecum | 93 (13.36%) | 258 (37.07%) | 345 (49.57%) | 98 (14.08%) | 58 (8.33%) | 540 (77.59%) | ||
| Urban/Rural Status | 0.571 | 0.68 | ||||||
| Metropolitan | 307 (15.32%) | 807 (40.27%) | 890 (44.41%) | 335 (16.72%) | 204 (10.18%) | 1465 (73.10%) | ||
| Non-metropolitan | 62 (17.13%) | 137 (37.85%) | 163 (45.03%) | 66 (18.23%) | 33 (9.12%) | 263 (72.65%) | ||
| Census tract % below poverty level in quartile | 0.53 | 0.684 | ||||||
| First Quartile | 110 (17.27%) | 236 (37.05%) | 291 (45.68%) | 118 (18.52%) | 55 (8.63%) | 464 (72.84%) | ||
| Second Quartile | 83 (13.95%) | 252 (42.35%) | 260 (43.70%) | 98 (16.47%) | 65 (10.92%) | 432 (72.61%) | ||
| Third Quartile | 90 (15.41%) | 240 (41.10%) | 254 (43.49%) | 94 (16.10%) | 56 (9.59%) | 434 (74.32%) | ||
| Forth Quartile | 86 (15.64%) | 216 (39.27%) | 248 (45.09%) | 91 (16.55%) | 61 (11.09%) | 398 (72.36%) | ||
| Census tract % with at least high school diploma in quartile | 0.677 | 0.898 | ||||||
| First Quartile | 106 (17.01%) | 236 (37.88%) | 281 (45.10%) | 110 (17.66%) | 60 (9.63%) | 453 (72.71%) | ||
| Second Quartile | 87 (14.60%) | 249 (41.78%) | 260 (43.62%) | 97 (16.28%) | 64 (10.74%) | 435 (72.99%) | ||
| Third Quartile | 87 (14.22%) | 245 (40.03%) | 280 (45.75%) | 97 (15.85%) | 58 (9.48%) | 457 (74.67%) | ||
| Forth Quartile | 89 (16.64%) | 214 (40.00%) | 232 (43.36%) | 97 (18.13%) | 55 (10.28%) | 383 (71.59%) | ||
| Census tract median income in quartile | 0.737 | 0.603 | ||||||
| First Quartile | 81 (13.43%) | 244 (40.46%) | 278 (46.10%) | 87 (14.43%) | 66 (10.95%) | 450 (74.63%) | ||
| Second Quartile | 103 (16.89%) | 244 (40.00%) | 263 (43.11%) | 110 (18.03%) | 60 (9.84%) | 440 (72.13%) | ||
| Third Quartile | 94 (15.96%) | 237 (40.24%) | 258 (43.80%) | 108 (18.34%) | 58 (9.85%) | 423 (71.82%) | ||
| Forth Quartile | 91 (16.13%) | 219 (38.83%) | 254 (45.04%) | 96 (17.02%) | 53 (9.40%) | 415 (73.58%) | ||
Masked per SEER-Medicare user agreement for confidentiality.
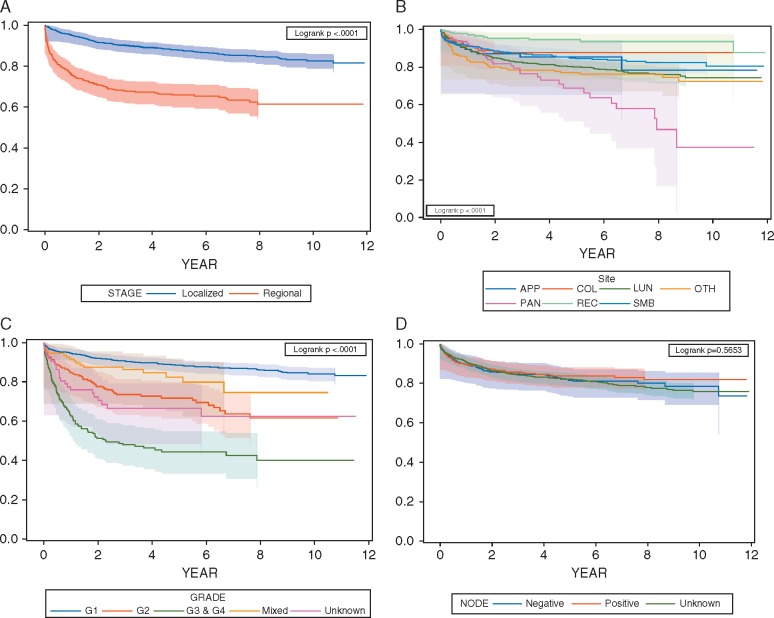
The chi-square test results suggested that there were statistically significant differences in the development of metastases by cancer stage (P < 0.001). The percentage that developed metastases within 5 years was 31% versus 10% for patients with regional versus localized disease, respectively. Higher histological grade was also significantly associated with the development of metastases (P < 0.001). Similarly, tumor size was significantly associated with the development of metastases. The larger the tumor size, the higher the percentage of patients that developed metastases. We also found significant differences by primary tumor site. Nodal status did not show a statistically significant difference possibly due to the large proportion of patients (66%) with unknown nodal status. A lower percentage of female patients developed metastases within 5 year than male patients (14% versus 19%). The significant differences in disease progression by stage, grade and site were also confirmed by log-rank test with P-values for all three < 0.001 (Figure 1A–C).
Figure 1.
Kaplan-Meier curves by (A) stage, (B) site, (C) grade, and (D) node status. Note: Censored patients were masked per SEER-Medicare user agreement for confidentiality. APP: Appendix; COL: Colon; LUN: Larynx, bronchus, lung, trachea or other respiratory organs; OTH: Other; PAN: Pancreas; REC:Rectum; SMB: Small intestine and cecum.
Table 2 provides the results from the multivariate Cox proportional hazards model. After adjusting for other factors, patients with regional stage disease were significantly more likely to develop metastases than patients with localized disease (HR = 2.81, 95% CI: 2.24–3.53). Patients with histological grade 2 or grades 3 and 4 tumors were much more likely to have metastases with HRs of 2.23 (95% CI: 1.65–3.00) and 4.34 (95% CI: 3.31–5.71), respectively, compared to patients with grade 1 tumors. Tumor size larger than 2 cm was significantly associated with higher likelihood of developing metastases (HR = 1.55, 95% CI: 1.07–2.25) compared to tumor size less than 1 cm. Compared to patients with primary tumor sites in the larynx, bronchus, lung, trachea, and other respiratory organs, patients with tumors in the small intestine and cecum (HR = 0.61, 95% CI: 0.46–0.81), colon (HR = 0.25, 95% CI: 0.14–0.46), and rectum (HR = 0.51, 95% CI: 0.27–0.99) were significantly less likely to develop metastases while pancreatic NETs had a trend towards increased risk of developing metastases (HR = 1.10, 95% CI: 0.74–1.65). Nodal status did not have a significant association with the development of metastases, which is again likely due to the large proportion of individuals with unknown nodal status. We also found that females were less likely to develop metastases (HR = 0.65, 95% CI: 0.53–0.80). The results for the competing risk model are also presented in Table 2. The significant association between tumor stage, grade, size, and site and metastatic disease were in line with the Cox proportional hazard model results; nodal status was still insignificant. The competing risk model also supported the difference in the risk of metastatic development between male and female patients (HR = 0.68, 95% CI: 0.55–0.83).
Table 2.
Cox proportional hazards model and competing risk model for disease progression among patients with local/regional stage NETs
| Cox proportional hazards model |
Competing risk model |
|||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% HR CI |
P | HR | 95% HR CI |
P | |||
| Lower limit | Upper limit | Lower limit | Upper limit | |||||
| Age | ||||||||
| 65–69 | Reference | Reference | ||||||
| 70–74 | 1.159 | 0.885 | 1.518 | 0.2831 | 1.114 | 0.848 | 1.464 | 0.4366 |
| 75–79 | 1.139 | 0.86 | 1.509 | 0.3649 | 1.087 | 0.811 | 1.458 | 0.5758 |
| ≥80 | 1.263 | 0.948 | 1.684 | 0.1106 | 1.112 | 0.828 | 1.492 | 0.4809 |
| Gender | ||||||||
| Male | Reference | Reference | ||||||
| Female | 0.648 | 0.528 | 0.795 | <0.0001 | 0.676 | 0.548 | 0.834 | 0.0003 |
| Race | ||||||||
| Non-Hispanic White | Reference | Reference | ||||||
| Non-Hispanic Black | 0.849 | 0.553 | 1.302 | 0.4529 | 0.812 | 0.517 | 1.275 | 0.3654 |
| Hispanic or Others | 0.861 | 0.585 | 1.267 | 0.4473 | 0.848 | 0.586 | 1.229 | 0.3838 |
| Stage | ||||||||
| Localized | Reference | Reference | ||||||
| Regional | 2.81 | 2.241 | 3.525 | <0.0001 | 2.662 | 2.112 | 3.356 | <0.0001 |
| Histologic grade | ||||||||
| G1 | Reference | Reference | ||||||
| G2 | 2.225 | 1.65 | 2.999 | <0.0001 | 2.275 | 1.686 | 3.071 | <0.0001 |
| G3 & G4 | 4.343 | 3.305 | 5.707 | <0.0001 | 3.857 | 2.887 | 5.154 | <0.0001 |
| Mixed | 1.41 | 0.719 | 2.765 | 0.3176 | 1.223 | 0.631 | 2.368 | 0.5509 |
| Unknown | 2.344 | 1.486 | 3.697 | 0.0002 | 2.217 | 1.389 | 3.539 | 0.0008 |
| Tumor size | ||||||||
| < 1 cm | Reference | Reference | ||||||
| 1–2 cm | 1.312 | 0.909 | 1.893 | 0.1472 | 1.311 | 0.897 | 1.916 | 0.1613 |
| >2 cm | 1.549 | 1.067 | 2.25 | 0.0215 | 1.553 | 1.052 | 2.291 | 0.0266 |
| Unknown | 1.005 | 0.638 | 1.584 | 0.9813 | 0.96 | 0.604 | 1.525 | 0.8618 |
| Nodal status | ||||||||
| Negative | Reference | Reference | ||||||
| Positive | 0.828 | 0.583 | 1.177 | 0.2923 | 0.833 | 0.581 | 1.195 | 0.3219 |
| Unknown | 0.992 | 0.754 | 1.303 | 0.9514 | 0.973 | 0.725 | 1.305 | 0.8539 |
| Region | ||||||||
| Midwest | Reference | Reference | ||||||
| Northeast | 0.93 | 0.638 | 1.356 | 0.7069 | 0.989 | 0.662 | 1.476 | 0.9551 |
| South | 0.837 | 0.583 | 1.203 | 0.3367 | 0.835 | 0.57 | 1.222 | 0.3539 |
| West | 0.741 | 0.513 | 1.07 | 0.1103 | 0.748 | 0.508 | 1.101 | 0.1409 |
| Primary Site | ||||||||
| Small intestine and cecum | 0.614 | 0.463 | 0.813 | 0.0007 | 0.634 | 0.476 | 0.844 | 0.0018 |
| Appendix | 0.803 | 0.364 | 1.768 | 0.585 | 0.886 | 0.403 | 1.949 | 0.7643 |
| Colon | 0.252 | 0.139 | 0.455 | <0.0001 | 0.25 | 0.133 | 0.469 | <0.0001 |
| Larynx, bronchus, lung, trachea, and other respiratory organs | Reference | Reference | ||||||
| Other | 0.986 | 0.708 | 1.372 | 0.9324 | 0.863 | 0.591 | 1.261 | 0.4467 |
| Pancreas | 1.103 | 0.735 | 1.654 | 0.6368 | 1.139 | 0.763 | 1.702 | 0.5243 |
| Rectum | 0.514 | 0.269 | 0.985 | 0.045 | 0.544 | 0.279 | 1.057 | 0.0726 |
| Urban/Rural Status | ||||||||
| Metropolitan | Reference | Reference | ||||||
| Non-Metropolitan | 1.185 | 0.85 | 1.651 | 0.3161 | 1.203 | 0.86 | 1.682 | 0.2798 |
| Census tract % below poverty level in quartile | ||||||||
| First quartile | Reference | Reference | ||||||
| Second quartile | 0.719 | 0.506 | 1.022 | 0.066 | 0.752 | 0.524 | 1.079 | 0.122 |
| Third quartile | 0.714 | 0.451 | 1.133 | 0.1527 | 0.784 | 0.491 | 1.25 | 0.3062 |
| Forth quartile | 0.914 | 0.52 | 1.605 | 0.7536 | 1.018 | 0.57 | 1.819 | 0.9512 |
| Census tract median income in quartile | ||||||||
| First quartile | Reference | Reference | ||||||
| Second quartile | 1.349 | 0.93 | 1.955 | 0.1147 | 1.396 | 0.957 | 2.037 | 0.0831 |
| Third quartile | 1.403 | 0.857 | 2.294 | 0.1779 | 1.459 | 0.89 | 2.391 | 0.1345 |
| Forth quartile | 1.099 | 0.612 | 1.973 | 0.7528 | 1.156 | 0.644 | 2.075 | 0.6265 |
| Census tract % with high school diploma in quartile | ||||||||
| First quartile | Reference | Reference | ||||||
| Second quartile | 0.952 | 0.69 | 1.314 | 0.7661 | 0.963 | 0.695 | 1.335 | 0.8219 |
| Third quartile | 0.727 | 0.504 | 1.047 | 0.0864 | 0.734 | 0.506 | 1.064 | 0.1022 |
| Forth quartile | 0.778 | 0.516 | 1.172 | 0.2303 | 0.806 | 0.527 | 1.234 | 0.321 |
Discussion
We found that the risk of developing metastases differed significantly by stage, grade, and tumor size as noted in prior studies. We also established the prognostic importance of primary site in the development of recurrent disease. While some previous small-scale studies have shown some of these differences [6–10], the magnitude of such differences was not well-known or validated in a larger population before this study.
The steadily rising incidence of early, resectable NETs [1, 2] underscores the urgent need to have concrete, evidence-based guidelines for post-resection NET surveillance. Reflecting the lack of quality data, current National Comprehensive Cancer Network (NCCN) guidelines suggest surveillance for up to 10 years with evaluation every 6–12 months irrespective of site or grade for well-differentiated NETs with very few exceptions such as in the case of early and small rectal, gastric or appendiceal NETs that do not require long-term follow-up [3]. In sharp contrast, the current study reveals significant differences in risk of recurrence depending on site, grade and stage. Therefore, future surveillance guidelines for resected NETs could take these factors into consideration to customize the guidelines depending on the magnitude of risk to reduce unnecessary testing. We found that in our cohort of elderly patients above 65 years old, few patients were observed to develop metastatic disease after 5 years post-resection. As patients may have been lost to follow-up due to either the end of the study period (31 December 2013) or ending continuous enrollment in Medicare Part A and B (or beginning HMO enrollment), we further examined subgroups of patients with complete 5 year and 10 year follow-up. We found that out of the 2366 patients, 1643 had complete 5-year follow-up and 1102 had complete 10-year follow-up. Among the 1643 patients who had complete 5-year follow-up, 369 developed metastatic disease. In comparison, among the 1102 patients who had complete 10-year follow-up, 401 developed metastatic disease. Therefore, this analysis confirms our finding that the development of metastases did not increase significantly between years 5 and 10 after surgical resection among elderly patients. Future studies could evaluate whether NET surveillance could be stopped after 5 years in elderly patients with competing comorbidities. One previous study showed that comorbidities were associated with higher risk of mortality among elderly local/regional stage NET patients with carcinoid syndrome [17].
Another important unanswered question in the management of well-differentiated resected NETs is the role of adjuvant therapy. The design and initiation of adjuvant trials have been hampered by a lack of data regarding the optimal subgroups to be targeted, and the time to recurrence in these patients that would be necessary for power and sample size calculations is currently unclear. The current study addresses this issue to a certain extent by identifying subgroups at higher risk of recurrence: elderly patients with regional stage, higher grade tumors, and those arising in certain primary sites including the pancreas. These findings on association between stage, site, grade and risk of recurrence are in line with prior smaller-scale studies on some NETs [6–10]. Future studies should validate the findings from our study across age groups and primary sites to pave the way for adjuvant trials in these subgroups. Our study showed that women had a lower risk of recurrence even when competing risk of death was considered. This is probably due to the lower grade and smaller tumor size noted in women. In our cohort, 85% of the women had grade 1 or 2 disease, while 80% of the men had grade 1 or 2 disease; 26% of the women had tumor sizes greater than 2 cm, while 31% of the men had tumor sizes greater than 2 cm. Such differences are consistent with the findings from another study by our group based on SEER data, where we also found lower grade and better survival among women with NETs [18, 19].
This population-based study inherits some of the common limitations of observational studies. Because our data are limited to the SEER-Medicare population, we focused on elderly NET patients, which explain the high percentage of patients who died without observed metastases in 10 years. This high mortality rate limits the applicability of our results to the younger population for long-term follow up; thus, our conclusions are limited to the elderly population. Our current analyses did not show a significant association between nodal status and the development of metastases probably due to the large proportion (66%) of patients with unknown nodal status in our analyzed data. The staging is based on local, regional, and metastatic classification rather than the more formal TNM staging systems. The grading of tumors in the SEER registry is by tumor differentiation rather than on numerical cutoffs of proliferation and may not match grading based on Ki-67 levels. Although the SEER registry provided information on the initial treatment, it did not provide the exact date of surgery. Hence we had to capture the surgery date based on the Medicare claims and excluded some patients for whom we were not able to identify the exact date of surgery. Quality of surgery was not accounted for as the data do not include such information. The identification of recurrence is based on ICD-9 codes in medical claims; therefore, it does not capture recurrences that were untreated or uncoded in the claims data. An interesting future direction will be to validate the algorithm based on claims data to identify recurrence by comparing chart review and the algorithm. It is confirming that the overall recurrence rate we observed in our cohort is reasonably consistent with previous studies in the literature. For example, we found 17% of the patients had recurrence within 10 years, which is close to the 18% recurrence rate found in another study based on chart review of NET patients [8]. We found that 10% of localized-stage patients and 31% of regional-stage patients had recurrence within 5 years, which is in line with relapse-free 5-year survival rates of 90%, 70%, and 53% for stage I, II, and III patients, respectively, excluding patients referred after their metastatic recurrence at a single institution [9]. Finally, information regarding surveillance patterns and methods are unavailable in the SEER-Medicare database. Nevertheless, this is the largest population-based study, to the best of our knowledge, on the development of metastases among NET patients that quantified the significant differences by various clinical factors.
Supplementary Material
Acknowledgements
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Funding
This work was supported in part by Ipsen Pharma. The funder sponsored the purchase of SEER-Medicare data and provided funding for analytical support. All authors had unrestricted access to the final study data on request, were responsible for data interpretation, manuscript preparation, and the decision to submit for publication, and attest to the completeness and accuracy of the data and statistical analysis. No grant number is applicable.
Key Message
This study found that the risk of recurrence after surgical resection differed greatly by clinical, pathological, and demographic factors in elderly patients with neuroendocrine tumors. Contrary to current guidelines, the results suggest that surveillance recommendations should be tailored according to patient and tumor characteristics.
Disclosure
The authors have declared no conflicts of interest.
References
- 1. Yao JC, Hassan M, Phan A. et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008; 26: 3063–3072. [DOI] [PubMed] [Google Scholar]
- 2. Modlin IM, Latich I, Zikusoka M. et al. Gastrointestinal carcinoids: the evolution of diagnostic strategies. J Clin Gastroenterol 2006; 40: 572–582. [DOI] [PubMed] [Google Scholar]
- 3. Kulke MH, Shah MH, Benson AB III. et al. Neuroendocrine tumors, version 1.2015. J Natl Compr Canc Netw 2015; 13: 78–108. [DOI] [PubMed] [Google Scholar]
- 4. Kunz PL, Reidy-Lagunes D, Anthony LB. et al. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas 2013; 42: 557–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kulke MH, Siu LL, Tepper JE. et al. Future directions in the treatment of neuroendocrine tumors: consensus report of the National Cancer Institute Neuroendocrine Tumor clinical trials planning meeting. J Clin Oncol 2011; 29: 934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boninsegna L, Panzuto F, Partelli S. et al. Malignant pancreatic neuroendocrine tumour: lymph node ratio and Ki67 are predictors of recurrence after curative resections. Eur J Cancer 2012; 48: 1608–1615. [DOI] [PubMed] [Google Scholar]
- 7. Casadei R, Ricci C, Pezzilli R. et al. Are there prognostic factors related to recurrence in pancreatic endocrine tumors? Pancreatology 2010; 10: 33–38. [DOI] [PubMed] [Google Scholar]
- 8. Kim SJ, Kim JW, Oh DY. et al. Clinical course of neuroendocrine tumors with different origins (the pancreas, gastrointestinal tract, and lung). Am J Clin Oncol 2012; 35: 549–556. [DOI] [PubMed] [Google Scholar]
- 9. Strosberg JR, Cheema A, Weber JM. et al. Relapse-free survival in patients with nonmetastatic, surgically resected pancreatic neuroendocrine tumors: an analysis of the AJCC and ENETS staging classifications. Ann Surg 2012; 256: 321–325. [DOI] [PubMed] [Google Scholar]
- 10. Ter-Minassian M, Chan JA, Hooshmand SM. et al. Clinical presentation, recurrence, and survival in patients with neuroendocrine tumors: results from a prospective institutional database. Endocr Relat Cancer 2013; 20: 187–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. National Cancer Institute: Surveillance, Epidemiology and End Results (SEER), 2015; http://seer.cancer.gov/about/overview.html (27 April 2017, date last accessed).
- 12. Pasaoglu E, Dursun N, Ozyalvacli G. et al. Comparison of World Health Organization 2000/2004 and World Health Organization 2010 classifications for gastrointestinal and pancreatic neuroendocrine tumors. Ann Diagn Pathol 2015; 19: 81–87. [DOI] [PubMed] [Google Scholar]
- 13. Halperin DM, Shen C, Dasari A. et al. Frequency of carcinoid syndrome at neuroendocrine tumour diagnosis: a population-based study. Lancet Oncol 2017; 18: 525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hassett MJ, Ritzwoller DP, Taback N. et al. Validating billing/encounter codes as indicators of lung, colorectal, breast, and prostate cancer recurrence using 2 large contemporary cohorts. Med Care 2014; 52: e65–e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lamont EB, Herndon JE 2nd, Weeks JC. et al. Measuring disease-free survival and cancer relapse using Medicare claims from CALGB breast cancer trial participants (companion to 9344). J Natl Cancer Inst 2006; 98: 1335–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fine JP, Gray RJ.. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509. [Google Scholar]
- 17. Shen C, Shih YC, Xu Y. et al. Octreotide long-acting repeatable use among elderly patients with carcinoid syndrome and survival outcomes: a population-based analysis. Cancer 2014; 120: 2039–2049. [DOI] [PubMed] [Google Scholar]
- 18. Shen C, Dasari A, Zhao B. et al. Incidence and prevalence of neuroendocrine tumors in the United States 1973–2012. In NANETS Symposium 2016, Jackson, Wyoming.
- 19. Dasari A, Shen C, Halperin D. et al. Survival trends of neuroendocrine tumors and associated prognostic factors. In NANETS Symposium, 2016, Jackson, Wyoming.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.