Abstract
Objective
Cognitive reserve has been proposed to explain the discrepancy between clinical symptoms and the effects of aging or Alzheimer’s pathology. Functional magnetic resonance imaging (fMRI) may help elucidate how neural reserve and compensation delay cognitive decline and identify brain regions associated with cognitive reserve. This systematic review evaluated neural correlates of cognitive reserve via fMRI (resting-state and task-related) studies across the cognitive aging spectrum (i.e., normal cognition, mild cognitive impairment, and Alzheimer’s disease).
Method
This review examined published articles up to March 2017. There were 13 cross-sectional observational studies that met the inclusion criteria, including relevance to cognitive reserve, subjects 60 years or older with normal cognition, mild cognitive impairment, and/or Alzheimer’s disease, at least one quantitative measure of cognitive reserve, and fMRI as the imaging modality. Quality assessment of included studies was conducted using the Newcastle–Ottawa Scale adapted for cross-sectional studies.
Results
Across the cognitive aging spectrum, medial temporal regions and an anterior or posterior cingulate cortex-seeded default mode network were associated with neural reserve. Frontal regions and the dorsal attentional network were related to neural compensation. Compared to neural reserve, neural compensation was more common in mild cognitive impairment and Alzheimer’s disease.
Conclusions
Neural reserve and compensation both support cognitive reserve, with compensation more common in later stages of the cognitive aging spectrum. Longitudinal and intervention studies are needed to investigate changes between neural reserve and compensation during the transition between clinical stages, and to explore the causal relationship between cognitive reserve and potential neural substrates.
Keywords: Cognitive reserve, Functional magnetic resonance imaging, Neural reserve, Neural compensation, Aging, Alzheimer’s disease
Introduction
Current epidemiological literature has documented a nonlinear relationship between clinical symptoms and pathological severity of Alzheimer’s disease (AD) or aging (Bennett et al., 2006; Bennett, Wilson, Boyle, Buchman, & Schneider, 2012; Buchman & Bennett, 2012), suggesting that other factors may explain the discrepancy (Stern, 2002). Cognitive reserve (CR) attempts to bridge such discrepancy and refers to inter-individual differences in preserved factors, such as education (Amieva et al., 2014; Bennett et al., 2003; Scarmeas, Albert, Manly, & Stern, 2006), premorbid IQ (Rentz et al., 2004; Starr & Lonie, 2008), leisure activities (Akbaraly et al., 2009; Sattler, Toro, Schonknecht, & Schroder, 2012), with some controversy surrounding bilingualism (Abutalebi et al., 2015; Chertkow et al., 2010; Gollan, Salmon, Montoya, & Galasko, 2011) and occupation (Anttila et al., 2002; Pool et al., 2016; Singh-Manoux et al., 2011). Differences in CR underlie individual variability in tolerating or compensating for the effects of aging or AD pathology and in performing cognitive tasks (Stern, 2002, 2009). Since most of the CR proxies appear to be modifiable, understanding the mechanisms supporting the impact of CR on aging and AD pathology, as well as the clinical manifestations, will provide insight for identifying new therapeutic targets or strategies for delaying cognitive decline seen across the cognitive aging spectrum.
A variety of imaging techniques have investigated the potential neural substrates of CR. One topic review of structural and functional imaging found that higher CR was related to greater brain structural damage and higher compensatory functional activity in older adults (Bartres-Faz & Arenaza-Urquijo, 2011). Another recent review of positron emission tomography (PET) suggested that AD patients with high CR tend to show hypermetabolism in dorsolateral prefrontal cortex but hypometabolism in AD-associated posterior regions (Morbelli & Nobili, 2014). Current literature suggests that differences in neural recruitment and efficiency may be one of the mechanisms by which CR provides protection against aging or AD pathology (Barulli & Stern, 2013; Sole-Padulles et al., 2009). Two neural implementations have been proposed to support CR: (1) neural reserve, in which certain brain regions or networks are resistant to the effect of neurodegeneration or AD pathology; and (2) neural compensation, in which alternative brain regions or networks not typically used in healthier or younger adults are recruited in order to compensate for regions that have degenerated or been affected by AD pathology (Stern, 2002). Findings from previous studies suggest that CR in young adults may be more relevant to neural reserve, while CR in older adults, including those with AD pathology, may be more relevant to neural compensation (Habeck et al., 2003; Stern et al., 2005, 2012); however, these hypotheses have only been tested discretely. Nonetheless, functional magnetic resonance imaging (fMRI) may be particularly useful for investigating such neural hypotheses. In the context of CR, task-related fMRI has been used to identify brain regions or networks that link certain responses to cognitive tasks with CR, while resting-state fMRI (rsfMRI) has been employed to evaluate intrinsic neural activity across or within temporally coherent brain networks linked to CR (Dennis & Thompson, 2014).
Several systematic reviews on CR have been published: two examined the relationship between education and dementia in the context of CR (Meng & D’Arcy, 2012; Sharp & Gatz, 2011) while another reviewed different proxies of CR and dementia (Harrison et al., 2015); however, none of these reviews systematically compared the two potential neural mechanisms underlying CR. Using fMRI to investigate the neural correlates of CR along the aging to AD-associated neurodegenerative continuum may help elucidate exactly how neural reserve and compensation delay cognitive decline, as well as determine a generalized functional network that underlies CR, which may ultimately assist in identifying foci for intervention. Hence, the present review systematically evaluated fMRI studies (resting-state and task-related fMRI) related to CR across the cognitive aging spectrum [i.e., normative cognition, mild cognitive impairment (MCI) and AD], focusing on two aims: (1) to examine the potential neural correlates of CR; and (2) to compare two competitive neural mechanisms—neural reserve versus neural compensation—underlying CR. According to Stern (2002), neural correlates in healthy young adults would be expected related to neural reserve, as this population exhibits intact neural capacity in supporting cognitive capacity. Individuals experiencing age- and/or AD pathophysiology-related cognitive decline would be expected to rely more on neural compensation, which involves brain regions not commonly used in younger or healthier counterparts.
Methods
Search Strategy
Studies were searched via PubMed, PsycINFO, and Web of Science from January 2000 to March 2017. Potential candidate studies were identified first using the following search word combinations: CR-relevant (cognitive reserve, neural reserve, neural compensation, education, intelligence, IQ, or occupation), cognitive aging-relevant (aging, old, elderly, mild cognitive impairment, dementia, or Alzheimer’s disease), and fMRI-relevant [fMRI, blood-oxygen-level-dependent (BOLD), functional imaging, functional connectivity, amplitude of low frequency fluctuation/ALFF, regional homogeneity/ReHo, centrality, rest, or resting]. Reference lists of retrieved studies were also searched manually to find additional potential studies.
Inclusion and Exclusion Criteria
Studies were required to be relevant to CR and assess populations of healthy older adults and/or patients with MCI or AD who averaged 60 years or older. Additionally, studies were required to include at least one quantitative measure of CR with fMRI (task-related and/or resting-state) as an imaging modality. Inclusion of a CR proxy was required to differentiate between studies that investigated how brain activity varies as a function of CR and studies that focused on how activity varies as a function of AD pathology or cognitive performance. Exclusion criteria included reserve related to brain structure or cellular/molecular factors, dementia other than Alzheimer’s, exclusive use of non-fMRI imaging techniques, non-English languages, case studies, reviews, and meta-analyses.
Data Extraction and Quality Assessment
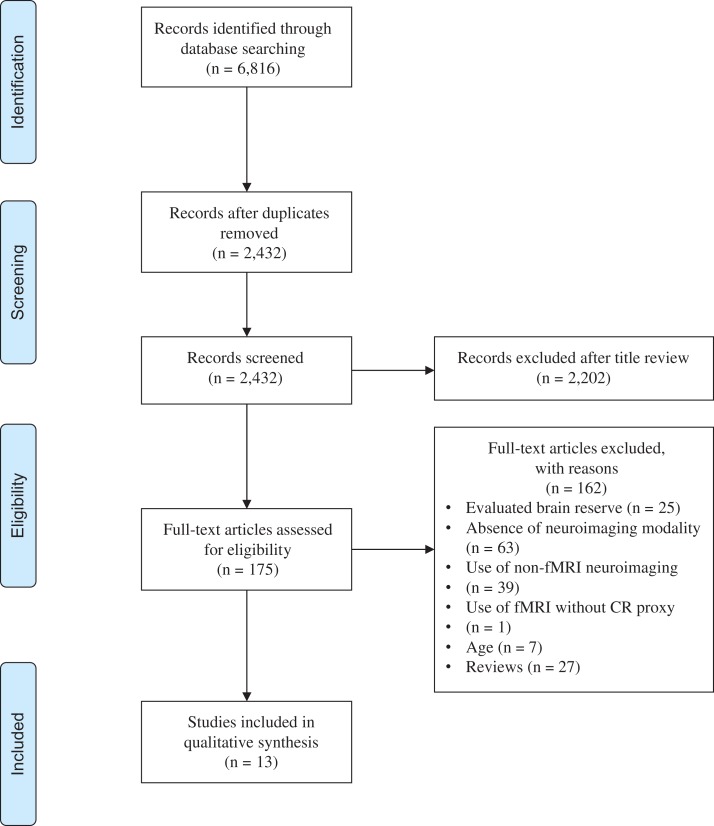
Abstracts were screened for relevance and candidate studies were reviewed thoroughly for criteria fulfillment. Two authors (MA and FL) independently reviewed studies which met inclusion criteria to confirm eligibility. Study characteristics (i.e., population, sample size, mean age), fMRI paradigm, CR proxy, and main findings were extracted. Data extraction was completed independently by the two authors, and disagreements regarding extracted data or study inclusion were resolved by discussion (see Fig. 1 flow diagram). Quality assessment of the included studies was evaluated based on the Newcastle–Ottawa Scale adapted for cross-sectional studies (Herzog et al., 2013). Study quality was rated as good, fair, or poor based on thresholds described previously (McPheeters et al., 2012). A summary of the quality assessment is shown in Table 1, and study characteristics of the included studies are shown in Table 2.
Fig. 1.
Flow chart of the method used to identify eligible studies for qualitative synthesis. (Adapted from the PRISMA Group) (Moher, Liberati, Tetzlaff, Altman, & Group, 2009).
Table 1.
Modified Newcastle–Ottawa Quality Assessment Scale for included studies
| Reference | Design | Selection | Comparability based on design and analysis | Outcome | ||||
|---|---|---|---|---|---|---|---|---|
| Representativeness of the sample | Sample size | Non-respondents | Ascertainment of exposure | Assessment of the outcome | Statistical test | |||
| Bartres-Faz et al. (2009) | Cross-sectional correlational | ++ | + | ++ | ||||
| Bastin et al. (2012) | Cross-sectional correlational | + | ++ | + | ++ | + | ||
| Arenaza-Urquijo et al. (2013) | Cross-sectional correlational | + | ++ | + | ++ | |||
| Marques, Soares, Magalhaes, Santos, and Sousa (2015) | Cross-sectional correlational | + | ++ | + | ++ | + | ||
| Marques et al. (2016) | Cross-sectional correlational | + | ++ | + | ++ | + | ||
| Springer, McIntosh, Winocur, and Grady (2005) | Cross-sectional case–control | + | ++ | ++ | ++ | + | ||
| Stern et al. (2008) | Cross-sectional case–control | + | ++ | ++ | ++ | + | ||
| Waiter et al. (2008) | Cross-sectional case–control | + | ++ | ++ | ++ | + | ||
| Solé-Padullés et al. (2009) | Cross-sectional case–control | + | ++ | ++ | ++ | + | ||
| Bosch et al. (2010) | Cross-sectional case–control | + | ++ | ++ | ++ | + | ||
| Steffener, Reuben, Rakitin, and Stern (2011) | Cross-sectional case–control | + | ++ | ++ | ++ | + | ||
| Bozzali et al. (2015) | Cross-sectional case–control | + | ++ | ++ | ++ | + | ||
| Franzmeier et al. (2017) | Cross-sectional case–control | + | ++ | ++ | ++ | + | ||
Note: Each + represents 1 point.
Good quality: 4–5 points in the selection domain, 1–2 points in the comparability domain, and 2–3 points in the outcome domain. Fair quality: 3 points in the selection domain, 1–2 points in the comparability domain, and 2–3 points in the outcome domain. Poor quality: 1–2 points in the selection domain, or 0 points in the comparability domain, or 0–1 point(s) in the outcome domain.
Table 2.
Study characteristics and main findings
| Reference | Study design | Main sample | CR proxy | Imaging modality/paradigm | Main findings |
|---|---|---|---|---|---|
| Springer et al. (2005) | Cross-sectional case–control | 19 healthy older adults, mean age 73.9; 14 young adults, mean age 23.4 | Education | Classification task (nonliving vs. living) and perceptual judgements (large vs. small; capital vs. lowercase) | High CR in older adults correlated with higher frontal and DMN activity with lower medial temporal activity. High CR in young adults was related to lower frontal activity and greater medial temporal activity |
| Bartres-Faz et al. (2009) | Cross-sectional correlational | 15 healthy older adults, mean age 68.3 | Composite score of education-occupation attainment, premorbid IQ, and social, physical, and leisure/cognitively stimulating activities | n-back task | Higher CR was associated with greater GMV in frontoparietal regions |
| Stern et al. (2008) | Cross-sectional case–control | 18 healthy older adults, mean age 74.4; 40 young adults, mean age 25.1 (letter task). 21 older adults, mean age 75.8; 24 young adults, mean age 24 (shape task) | Premorbid IQ | Two delayed-item-recall tasks (letter and shape) | In older adults, higher CR was related to less expression of a frontotemporal network. In young adults, higher CR correlated with greater expression of the frontotemporal network and less expression of second network involving bilateral MFG/SFG |
| Waiter et al. (2008) | Cross-sectional case–control | 25 “cognitive sustainers”a; 15 “cognitive decliners,”a mean age 68 | Premorbid IQ | Inspection time task | Older adults with high CR exhibited patterns of FC and brain activity similar to those seen in young adults |
| Solé-Padullés et al. (2009) | Cross-sectional case–control | 16 mild AD, mean age 76.5; 12 aMCI, mean age 74.25; 16 older adults, mean age 73.31 | Composite score of education-occupation attainment; premorbid IQ; and social, physical, and leisure/cognitively stimulating activities | Visual encoding task | High CR in AD patients was associated with greater activity in left ACC, left lingual gyrus, and right cerebellum. Positive correlations between CR and activity in right STG and left SPL were stronger in AD compared to controls. High CR in controls was associated with less activity in frontal regions and bilateral cerebellum. No significant correlations between CR and brain activity for MCI were observed |
| Bosch et al. (2010) | Cross-sectional case–control | 15 mild AD, mean age 75.27; 15 aMCI, mean age 74.63; 15 healthy older adults, mean age 72.2 | Composite score of education-occupation attainment; premorbid IQ; and social, physical, and leisure/cognitively stimulating activities | Speech comprehension task | In MCI/AD, higher CR was related to greater activity in areas processing speech and less activity in DMN regions. These relationships were inverted in healthy controls |
| Steffener et al. (2011) | Cross-sectional case–control | 15 healthy older adults, mean age 74.5; 37 young adults, mean age 25 | Premorbid IQ | Delayed-item-recall task | Higher CR was related to lower expression of a frontoparietal network in both age groups, which reduced expression of a second network involving right PHG. Less PHG activity was related to higher task performance in older adults only |
| Bastin et al. (2012) | Cross-sectional correlational | 74 healthy older adults, mean age 70.6 | Education and vocabulary | rsfMRI | Higher CR was related to lower metabolism in DMN and DAN regions |
| Arenaza-Urquijo et al. (2013) | Cross-sectional correlational | 39 healthy older adults, mean age 67.14 | Education | rsfMRI | Higher CR was related to stronger FC of ACC with DMN regions |
| Bozzali et al. (2015) | Cross-sectional case–control | 11 mild AD, mean age 74.6; 18 aMCI, mean age 68.9; 16 older adults, mean age 65 | Education | rsfMRI | Higher CR was related to higher FC of PCC; this association was strongest in AD and weakest in controls |
| Marques et al. (2015) | Cross-sectional correlational | 97 healthy older adults, mean age 64.94 | Education | rsfMRI | Higher CR was related to stronger FC in a large and distributed network, as well as weaker FC in a focal network |
| Marques et al. (2016) | Cross-sectional correlational | 100 healthy older adults, mean age 64.8 | Residual variance in episodic memory | rsfMRI | Higher CR correlated with greater local efficiency and clustering in cuneus and occipital regions, as well as the strength and betweeness centrality of right ITG |
| Franzmeier et al. (2017) | Cross-sectional case–control | 76 aMCI (ADNI), mean age; 93 aMCI (ISD); 36 healthy controls, mean age 75 | Education and premorbid IQ | rsfMRI | Amnestic MCI patients with high CR exhibited higher memory performance despite lower anti-correlation between the anterior DMN and DAN |
Note: ADNI = Alzheimer’s Disease Neuroimaging Initative; ISD = Institute for Stroke and Dementia; DMN = default mode network; DAN = dorsal attention network; FC = functional connectivity; GMV = gray matter volume; ACC = anterior cingulate cortex; PCC = posterior cingulate cortex; ITG = inferior temporal gyrus; MFG = medial frontal gyrus; SFG = superior frontal gyrus; STG = superior temporal gyrus; SPL = superior parietal lobule; PHG = parahippocampal gyrus.
aCognitive sustainers and decliners based on premorbid IQ measured at age 11 and 70.
Results
Number of Studies
An initial literature search yielded 6,816 records. A total of 2,432 records were screened, from which 175 records were retrieved for assessment. Many evaluated studies utilized a quantitative measure of CR; however, these were excluded due to an absence of neuroimaging (n = 63) or the use of a non-fMRI imaging modality (n = 39). Further reasons for exclusion are shown in Fig. 1. In total, 13 cross-sectional observational studies successfully met inclusion criteria.
Study Characteristics
Regarding the sample characteristics, three studies compared healthy older adults and young adults, six included healthy older adults alone, and four included a comparison of MCI and/or early AD with healthy older adults. Regarding fMRI, seven studies used task-related fMRI and six studies used rsfMRI. Task-related fMRI paradigms probed working memory (n = 3), semantic (n = 2), and visual/visuospatial memory (n = 2). In terms of CR proxies, the studies in this review predominantly used education and/or premorbid IQ. Education was measured in years, and IQ was measured with the North American Reading Test (NART) or the vocabulary subtest of the Wechsler Adult Intelligence Scale-Revised (WAIS-R). One recent study (Marques et al., 2016) employed residual variance in episodic memory, a latent model of CR that was recently developed to provide a direct quantitative measure of CR as opposed to measuring a proxy for CR (e.g., education) (Reed et al., 2010). The included studies predominantly measured one proxy (n = 8) (Arenaza-Urquijo et al., 2013; Bozzali et al., 2015; Marques et al., 2015, 2016; Springer et al., 2005; Steffener et al., 2011; Stern et al., 2008; Waiter et al., 2008), while two studies measured two proxies (Bastin et al., 2012; Franzmeier et al., 2017) and three studies obtained a composite CR score comprising of education-occupation attainment, premorbid IQ, and a questionnaire of leisure activities using factor analysis (Bartres-Faz et al., 2009; Bosch et al., 2010; Sole-Padulles et al., 2009).
Quality Assessment
The 13 included studies received fair or poor ratings (Table 2). In the selection domain, representativeness of the sample and ascertainment of exposure were well demonstrated across all studies, except for one study, which did not provide sufficient information to determine the source(s) of its subjects (Bartres-Faz et al., 2009). None of the included studies disclosed response rate or conducted power analysis for sample size estimation. Regarding comparability, controlling for CR was considered the most important factor, followed by cognition. All of the studies controlled for CR, while eight out of the 13 studies controlled for both CR and cognition. In the outcome domain, all thirteen studies performed well in assessment of the neural outcomes. The majority of the studies also showed performed well in statistical tests, except for two studies, which did not provide sufficient detail (Arenaza-Urquijo et al., 2013; Bartres-Faz et al., 2009). Overall, 11 out of 13 studies received a “fair” quality rating, while two studies received a “poor” quality rating.
Aim 1: Neural correlates of CR
In this section we examined the potential neural correlates of CR. Findings are grouped by brain region or neural networks.
Frontal Regions
Six task-related fMRI studies reported CR in relation to frontal regions (Bartres-Faz et al., 2009; Bosch et al., 2010; Sole-Padulles et al., 2009; Springer et al., 2005; Steffener et al., 2011; Waiter et al., 2008). Springer and colleagues (2005) found that higher CR was related to increased bilateral frontal activity in older adults compared to decreased frontal activity in young adults during a semantic memory task. However, a later study reported that, while healthy older adults exhibited increased activity of frontal regions [left inferior frontal gyrus (IFG)/insula, left medial frontal gyrus (MFG) into precentral gyrus, and bilateral MFG/superior frontal gyrus (SFG)] compared to young adults, higher CR was related to lower activity in these regions in both groups during a working memory task (Steffener et al., 2011). Another study examining healthy older adults found that high-CR individuals exhibited greater activity in right anterior cingulate cortex (ACC) than low-CR individuals during an inspection time task (Waiter et al., 2008). Bartrés-Faz and colleagues (2009) found that higher CR correlated with less activity of right IFG/precentral gyrus in healthy older adults during a working memory task, albeit this relationship became nonsignificant after controlling for gray matter volume (GMV).
Regarding MCI and AD, a study found that higher CR correlated with higher activity in MFG/SFG and a region surrounding left IFG/precentral gyrus in amnestic MCI (aMCI) patients compared to healthy controls during a speech comprehension task; AD patients exhibited an even stronger positive correlation between CR and left IFG/precentral gyrus activity. Additionally, greater activity of left IFG/precentral gyrus in aMCI and less activity of right ACC in aMCI/AD correlated with higher comprehension scores (Bosch et al., 2010). Another study found that aMCI/AD patients with high CR exhibited greater frontal activity (left precentral gyrus, right MFG, and left ACC) compared to healthy controls during a visual encoding task; this effect was predominantly seen in the AD group (Sole-Padulles et al., 2009).
Temporal or Other Regions
Three task-related fMRI studies (Sole-Padulles et al., 2009; Springer et al., 2005; Steffener et al., 2011) and one rsfMRI study (Marques et al., 2016) reported CR in relation to temporal or other regions. A study found that high CR was related to lower activity in left inferior/medial temporal regions [inferior temporal gyrus (ITG), parahippocampal gyrus (PHG), and fusiform gyrus (FG)] in healthy older adults, but higher activity in medial temporal lobe (MTL) regions [PHG, right FG, right medial temporal gyrus (MTG), and left superior temporal gyrus (STG)] in young adults during a semantic memory task (Springer et al., 2005). Solé-Padullés and colleagues (2009) reported that high CR in healthy older adults was associated with less activity in right MTG, right claustrum, left thalamus, and cerebellum during a visual encoding task. Additionally, an rsfMRI study found that high CR was related to the strength and betweeness centrality of ITG in healthy older adults, as well as greater local efficiency and clustering in bilateral cuneus and occipital regions (Marques et al., 2016).
Regarding MCI/AD, Solé-Padullés and colleagues (2009) found that higher CR was associated with greater activity of right lingual gyrus in the AD group during a visual encoding task. Positive correlations between a composite CR score and activity in right STG and left SPL were stronger in AD compared to controls.
Neural Networks
Three task-related fMRI studies (Bosch et al., 2010; Steffener et al., 2011; Stern et al., 2008) and five rsfMRI studies (Arenaza-Urquijo et al., 2013; Bastin et al., 2012; Bozzali et al., 2015; Franzmeier et al., 2017; Marques et al., 2015) examined CR in relation to neural networks. Stern and colleagues (2008) identified two CR-related functional networks in young and older adults during verbal and object working memory tasks. For the first network (left MFG, bilateral IFG/precentral gyrus, and right frontal subgyral), young adults showed greater deactivation with increasing CR during the object task, whereas older adults showed less deactivation. For the second network (bilateral MFG/SFG), young adults exhibited stronger deactivation with higher CR in both tasks; however, older adults’ recruitment of the network did not reach significance. In another fMRI study, high CR in both young and older adults was associated with less activity of a neural network involving temporal and limbic regions (left HPC, left insula, right MTG/STG, right cingulate, and cerebellum) during a working memory task. Reduced activity of this network was related to lower recruitment of a second network (right PHG), which in turn, improved task performance only in older adults (Steffener et al., 2011). One rsfMRI study found that higher CR was correlated with stronger functional connectivity of the default mode network (DMN) [ACC with right HPC, right posterior cingulate cortex (PCC), left inferior frontal lobe (IFL), and left angular gyrus] in healthy older adults (Arenaza-Urquijo et al., 2013). Another rsfMRI study found that higher CR was related to stronger functional connectivity in a large and distributed network [key nodes in left lingual gyrus; SFG; cuneus; right precentral gyrus; and middle right temporal pole] in healthy older adults, while related to weaker functional connectivity in a focal network (key nodes in vermis 1 and 12 of the cerebellum) (Marques et al., 2015). Additionally, an rsfMRI/PET study found that high CR in healthy older adults was significantly related to lower resting-state metabolism in regions of the DMN (right posterior temporoparietal cortex) and dorsal attention network (DAN; left anterior intraparietal sulcus) (Bastin et al., 2012).
For MCI/AD, Bosch and colleagues (2010) found that higher CR correlated with less activity in DMN regions during a speech comprehension task, specifically right ACC and left PCC/PCu in MCI compared to controls; high CR was related to even less PCC/PCu activity in AD compared to controls. Both patient groups also showed a negative correlation between right ACC activity and language comprehension scores. An rsfMRI study investigated within-network functional connectivity of the DMN and found that PCC connectivity showed a positive correlation with CR, which was most pronounced in AD patients, less so in MCI, and weakest in controls (Bozzali et al., 2015). Another recent rsfMRI study reported that the anterior DMN–DAN anti-correlation was not significantly different between aMCI patients and controls; however, a reduced anti-correlation was associated with lower memory performance for low-CR but not high-CR patients (Franzmeier et al., 2017).
Aim 2: Comparison of neural reserve versus neural compensation underlying CR
In this section we further examined whether the neural correlates revealed from Aim 1 were attributed to neural reserve or neural compensation in supporting CR.
Out of the six studies that reported a relationship between CR and frontal activity, four task-related studies supported a positive correlation between CR and higher frontal activity in healthy older adults compared to young adults (Bartres-Faz et al., 2009; Springer et al., 2005; Steffener et al., 2011; Waiter et al., 2008); however, the results from one study were nonsignificant after controlling for GMV (Bartres-Faz et al., 2009). Two task-related studies supported a positive correlation between CR and frontal activity in MCI/AD compared to controls (Bosch et al., 2010; Sole-Padulles et al., 2009). Taking into account the “fair” quality rating of five of the studies (Bosch et al., 2010; Sole-Padulles et al., 2009; Springer et al., 2005; Steffener et al., 2011; Waiter et al., 2008) and the “poor” quality rating of one study (Bartres-Faz et al., 2009), these findings may suggest that expression of more active frontal regions is a potential component of functional networks underlying neural compensation in cognitive aging.
Out of the five studies that reported a relationship between CR and areas other than the frontal lobe, four task-related studies supported a negative correlation between CR and temporal regions, including MTG/STG, HPC, and PHG, in healthy older adults, which was inverted in young adults (Sole-Padulles et al., 2009; Springer et al., 2005; Steffener et al., 2011; Stern et al., 2008), and one rsfMRI study supported a positive relationship between CR and network properties of ITG, cuneus, and occipital regions in healthy older adults (Marques et al., 2016). All five studies received a “fair” quality rating. Thus, MTL regions may reflect the capacity of neural reserve, as these areas are some of the first to degenerate in aging; nonetheless, it should be noted that contributions of the MTL to CR may still be inconclusive. Additionally, one of the task-related studies examining MCI/AD supported a positive correlation between CR and superior temporoparietal regions in AD compared to controls, suggesting that these regions underwent functional reorganization and may represent loci of neural compensation (Sole-Padulles et al., 2009).
Out of the two task-related studies that found a relationship between CR and neural networks, one study supported a negative relationship between CR and DMN activity in healthy older adults (Steffener et al., 2011), while the other study supported the same correlation in MCI/AD (Bosch et al., 2010). Out of the five rsfMRI studies that found a relationship between CR and default neural networks, two studies reported that higher CR correlated with stronger functional connectivity of distributed networks, including the DMN, in healthy older adults (Arenaza-Urquijo et al., 2013; Marques et al., 2015), while one study reported stronger within-network connectivity in PCC for MCI/AD but not controls (Bozzali et al., 2015). Findings from this latter study indicated that AD patients exhibit the strongest functional connectivity within DMN regions (Bozzali et al., 2015). An rsfMRI/FDG-PET study found a negative correlation between CR and resting-state metabolism in DMN and DAN regions (Bastin et al., 2012), while a recent study suggested that a reduced anterior DMN–DAN anti-correlation in aMCI has less of a negative affect on memory performance in high-CR compared to low-CR patients (Franzmeier et al., 2017). Given that six studies received a “fair” quality rating (Bastin et al., 2012; Bosch et al., 2010; Bozzali et al., 2015; Franzmeier et al., 2017; Marques et al., 2015; Steffener et al., 2011), and one study received a “poor” quality rating (Arenaza-Urquijo et al., 2013), the findings implicate the DMN in neural reserve; however, specific regions within the DMN, such as PCC, may functionally reorganize in patients and contribute to neural compensation.
Discussion
Regions related to neural reserve were areas actively linked to high CR in young adults, such as MTL regions (e.g., PCC, PHG, FG, MTG, STG, and HPC). Regions related to neural compensation were areas associated with high CR in the cognitive aging spectrum when compared to younger or healthier counterparts, such as frontal regions (e.g., MFG, SFG, IFG, precentral gyrus) or frontal lobe-associated networks (e.g., DAN). Consequently, among cognitively normal older adults with high CR, we often observe lower MTL activity (due to lower neural reserve) with higher frontal activity (due to higher neural compensation) (Springer et al., 2005; Steffener, Brickman, Rakitin, Gazes, & Stern, 2009; Steffener et al., 2011). Notably, significant MTL expression was not identified in studies examining MCI/AD; however, higher CR was related to even higher frontal activity in MCI/AD patients compared to their cognitively normal counterparts (Bartres-Faz et al., 2009; Bosch et al., 2010; Sole-Padulles et al., 2009). Summarizing these findings, a decline in the ratio between MTL versus frontal region activity in supporting CR seems to reflect the progress of cognitive decline and neurodegeneration.
Findings from this review indicate that the DMN underlies neural reserve in the context of CR. However, due to its involvement via multiple anterior and posterior regions during passive, self-referential states or while monitoring the external environment (Buckner, Andrews-Hanna, & Schacter, 2008; Simic, Babic, Borovecki, & Hof, 2014), the relationship of the DMN with CR warrants more investigation. The PCC and ACC are considered integral hubs of the DMN and have extensive connections within themselves, as well as with other DMN regions. Previous studies have reported disconnections from PCC to cortical and subcortical regions, particularly the HPC (Dunn et al., 2014; Teipel & Grothe, 2016) and PHG (Cha et al., 2013; Liu et al., 2016), which may precede gray matter atrophy within the PCC (Gili et al., 2011). Furthermore, findings from this review suggest that higher CR correlates with stronger functional connectivity of the ACC with other DMN regions, including the PCC, in healthy older adults (Arenaza-Urquijo et al., 2013). Converging these separate lines of evidence, a link between CR and an ACC- or PCC-centered DMN across cognitive aging spectrum may reflect neural reserve in this network.
Several factors may contribute to differences in neural reserve and compensation across the aging spectrum. First, clinical status likely plays a large role in these differences; older adults show evidence of compensation when compared to young adults but not when compared to MCI/AD. MCI tends to exhibit a level of compensation between AD and controls; however, the extent to which MCI exhibits intermediate differences may partially stem from the pathophysiologic heterogeneity of the group (Edmonds et al., 2016; Nettiksimmons, DeCarli, Landau, & Beckett, 2014). Second, while findings in this review were predominantly independent of GM atrophy, results from one study became nonsignificant after controlling for GMV, suggesting that brain indices affected by both age-related cognitive decline and AD pathology may account for some differences in CR. Third, the relationship of CR with frontal and MTL activity was mixed across studies across diverse cognitive tasks (e.g., working memory, episodic or semantic memory, and visuospatial memory) (Bosch et al., 2010; Sole-Padulles et al., 2009), suggesting that different brain regions may show a relationship with CR, depending on the task paradigm. Lastly, the size of networks involved in supporting CR appears to play a role in neural reserve versus compensation. Large-scale networks, such as the DMN that includes anterior and posterior regions, may contribute to neural reserve (Arenaza-Urquijo et al., 2013); however, individual regions within the network may reorganize to compensate for AD-related neurodegeneration (Bozzali et al., 2015). Noticeably, studies outside the CR context have also reported distributed networks in healthy older adults (Marques et al., 2016; Springer et al., 2005) while high local/regional intensity in MCI/AD (Daianu et al., 2016; Zhao et al., 2012). Future studies will need to clarify the relationship between aging/neurodegeneration, size of networks, and CR.
Findings from this review should be interpreted cautiously given the following limitations. First, due to the limited available literature related to both fMRI and CR, we were only able to identify cross-sectional studies that met the inclusion criteria of the present review. The included studies received a fair or poor quality rating, which underlines how the inherent design of cross-sectional studies restricts findings from supporting causal conclusions. While we attempted to initiate a mechanistic understanding of the neural correlates of CR, the causal relationship between neural function and CR has to be identified from stronger sources of evidence, such as randomized controlled trials. Second, we excluded studies published in languages other than English and studies published before 2000, which would affect the comprehensiveness of the findings. Third, our review did not include a meta-analysis given the heterogeneity of population and CR proxies; with the accumulation of studies relevant to neuroimaging and CR, conducting a meta-analysis to quantitatively validate our findings is needed.
Although cross-sectional findings have broadened the current understanding of how CR is operationalized, they merely provide a snapshot of CR in different population groups, thereby potentially biasing how results are interpreted. Previous longitudinal studies have observed an increase in the engagement of mental and physical leisure activities in older adults (Barnes et al., 2013; Cheng et al., 2014), while our recent randomized controlled trial involving computerized vision-based speed of processing training improved the efficiency of the DMN among older adults with MCI (Lin et al., 2016). Therefore, as the next step, longitudinal studies can provide insight into changes between neural reserve and compensation as older adults transition between clinical stages. Ideally, these studies would clarify whether a shift from neural reserve to compensation occurs or whether there is parallel processing of both. Intervention studies that modify neural correlates of CR or modify CR later in life will help determine the exact causal relationship between CR and relevant neural correlates.
Conclusions
Cross-sectional studies have revealed potential neural correlates of CR across the aging spectrum. Medial temporal regions and an anterior or posterior cingulate-seeded default mode network were associated with neural reserve, while frontal regions and the dorsal attentional network were associated with neural compensation. However, these findings are derived solely from cross-sectional studies, underling the need for longitudinal neuroimaging studies of CR or intervention studies to clarify the causal relationships between CR and its neural correlates, as well as how neural reserve and compensation evolve as older adults transition between clinical stages.
Acknowledgments
The authors are grateful to their colleague Ping Ren, who provided editing suggestions.
Funding
This work was supported by the National Institutes of Health (grant number NR015452, AG053193) to F.L.
Conflict of Interest
The authors disclose no conflicts of interest.
References
- Abutalebi J., Guidi L., Borsa V., Canini M., Della Rosa P. A., Parris B. A., et al. (2015). Bilingualism provides a neural reserve for aging populations. Neuropsychologia, 69, 201–210. doi:10.1016/j.neuropsychologia.2015.01.040. [DOI] [PubMed] [Google Scholar]
- Akbaraly T. N., Portet F., Fustinoni S., Dartigues J. F., Artero S., Rouaud O., et al. (2009). Leisure activities and the risk of dementia in the elderly: Results from the Three-City Study. Neurology, 73, 854–861. doi:10.1212/WNL.0b013e3181b7849b. [DOI] [PubMed] [Google Scholar]
- Amieva H., Mokri H., Le Goff M., Meillon C., Jacqmin-Gadda H., Foubert-Samier A., et al. (2014). Compensatory mechanisms in higher-educated subjects with Alzheimer’s disease: A study of 20 years of cognitive decline. Brain, 137, 1167–1175. doi:10.1093/brain/awu035. [DOI] [PubMed] [Google Scholar]
- Anttila T., Helkala E. L., Kivipelto M., Hallikainen M., Alhainen K., Heinonen H., et al. (2002). Midlife income, occupation, APOE status, and dementia: A population-based study. Neurology, 59, 887–893. [DOI] [PubMed] [Google Scholar]
- Arenaza-Urquijo E. M., Landeau B., La Joie R., Mevel K., Mezenge F., Perrotin A., et al. (2013). Relationships between years of education and gray matter volume, metabolism and functional connectivity in healthy elders. Neuroimage, 83, 450–457. doi:10.1016/j.neuroimage.2013.06.053. [DOI] [PubMed] [Google Scholar]
- Bartres-Faz D., & Arenaza-Urquijo E. M. (2011). Structural and functional imaging correlates of cognitive and brain reserve hypotheses in healthy and pathological aging. Brain Topography, 24, 340–357. doi:10.1007/s10548-011-0195-9. [DOI] [PubMed] [Google Scholar]
- Barnes D. E., Santos-Modesitt W., Poelke G., Kramer A. F., Castro C., Middleton L. E., & Yaffe K. (2013). The Mental Activity and eXercise (MAX) trial: a randomized controlled trial to enhance cognitive function in older adults. JAMA Internal Medicine (Tokyo, Japan), 173 (9), 797–804. doi:10.1001/jamainternmed.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartres-Faz D., Sole-Padulles C., Junque C., Rami L., Bosch B., Bargallo N., et al. (2009). Interactions of cognitive reserve with regional brain anatomy and brain function during a working memory task in healthy elders. Biological Psychology, 80, 256–259. doi:10.1016/j.biopsycho.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Barulli D., & Stern Y. (2013). Efficiency, capacity, compensation, maintenance, plasticity: Emerging concepts in cognitive reserve. Trends in Cognitive Sciences, 17, 502–509. doi:10.1016/j.tics.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastin C., Yakushev I., Bahri M. A., Fellgiebel A., Eustache F., Landeau B., et al. (2012). Cognitive reserve impacts on inter-individual variability in resting-state cerebral metabolism in normal aging. Neuroimage, 63, 713–722. doi:10.1016/j.neuroimage.2012.06.074. [DOI] [PubMed] [Google Scholar]
- Bennett D. A., Schneider J. A., Arvanitakis Z., Kelly J. F., Aggarwal N. T., Shah R. C., et al. (2006). Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology, 66, 1837–1844. doi:10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- Bennett D. A., Wilson R. S., Boyle P. A., Buchman A. S., & Schneider J. A. (2012). Relation of neuropathology to cognition in persons without cognitive impairment. Annals of Neurology, 72, 599–609. doi:10.1002/ana.23654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett D. A., Wilson R. S., Schneider J. A., Evans D. A., de Leon C. F. M., Arnold S. E., et al. (2003). Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology, 60, 1909–1915. [DOI] [PubMed] [Google Scholar]
- Bosch B., Bartres-Faz D., Rami L., Arenaza-Urquijo E. M., Fernandez-Espejo D., Junque C., et al. (2010). Cognitive reserve modulates task-induced activations and deactivations in healthy elders, amnestic mild cognitive impairment and mild Alzheimer’s disease. Cortex; A Journal Devoted to the Study of the Nervous System and Behavior, 46, 451–461. doi:10.1016/j.cortex.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Bozzali M., Dowling C., Serra L., Spano B., Torso M., Marra C., et al. (2015). The impact of cognitive reserve on brain functional connectivity in Alzheimer’s disease. Journal of Alzheimer’s Disease: JAD, 44, 243–250. doi:10.3233/JAD-141824. [DOI] [PubMed] [Google Scholar]
- Buchman A. S., & Bennett D. A. (2012). Amyloid pathology in persons with “normal” cognition. Neurology, 78, 228–229. doi:10.1212/WNL.0b013e31824367c2. [DOI] [PubMed] [Google Scholar]
- Buckner R. L., Andrews-Hanna J. R., & Schacter D. L. (2008). The brain’s default network: Anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences, 1124(1), 1–38. doi:10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Cha J., Jo H. J., Kim H. J., Seo S. W., Kim H. S., Yoon U., et al. (2013). Functional alteration patterns of default mode networks: Comparisons of normal aging, amnestic mild cognitive impairment and Alzheimer’s disease. European Journal of Neuroscience, 37, 1916–1924. doi:10.1111/ejn.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S. T., Chow P. K., Song Y. Q., Yu E. C., Chan A. C., Lee T. M., & Lam J. H. (2014). Mental and physical activities delay cognitive decline in older persons with dementia. The American Journal of Geriatric Psychiatry : Official Journal of the American Association for Geriatric Psychiatry, 22 (1), 63–74. doi:10.1016/j.jagp.2013.01.060. [DOI] [PubMed] [Google Scholar]
- Chertkow H., Whitehead V., Phillips N., Wolfson C., Atherton J., & Bergman H. (2010). Multilingualism (but not always bilingualism) delays the onset of Alzheimer disease: Evidence from a bilingual community. Alzheimer Disease and Associated Disorders, 24, 118–125. doi:10.1097/WAD.0b013e3181ca1221. [DOI] [PubMed] [Google Scholar]
- Daianu M., Mezher A., Mendez M. F., Jahanshad N., Jimenez E. E., & Thompson P. M. (2016). Disrupted rich club network in behavioral variant frontotemporal dementia and early-onset Alzheimer’s disease. Human Brain Mapping, 37, 868–883. doi:10.1002/hbm.23069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis E. L., & Thompson P. M. (2014). Functional brain connectivity using fMRI in aging and Alzheimer’s disease. Neuropsychology Review, 24, 49–62. doi:10.1007/s11065-014-9249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn C. J., Duffy S. L., Hickie I. B., Lagopoulos J., Lewis S. J., Naismith S. L., et al. (2014). Deficits in episodic memory retrieval reveal impaired default mode network connectivity in amnestic mild cognitive impairment. Neuroimage Clinical, 4, 473–480. doi:10.1016/j.nicl.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds E. C., Eppig J., Bondi M. W., Leyden K. M., Goodwin B., Delano-Wood L., et al. (2016). Heterogeneous cortical atrophy patterns in MCI not captured by conventional diagnostic criteria. Neurology, 87, 2108–2116. doi:10.1212/WNL.0000000000003326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzmeier N., Buerger K., Teipel S., Stern Y., Dichgans M., & Ewers M., Alzheimer’s Disease Neuroimaging Initiative (ADNI). (2017). Cognitive reserve moderates the association between functional network anti-correlations and memory in MCI. Neurobiology of Aging, 50, 152–162. doi:10.1016/j.neurobiolaging.2016.11.013. [DOI] [PubMed] [Google Scholar]
- Gili T., Cercignani M., Serra L., Perri R., Giove F., Maraviglia B., et al. (2011). Regional brain atrophy and functional disconnection across Alzheimer’s disease evolution. Journal of Neurology, Neurosurgery, and Psychiatry, 82, 58–66. doi:10.1136/jnnp.2009.199935. [DOI] [PubMed] [Google Scholar]
- Gollan T. H., Salmon D. P., Montoya R. I., & Galasko D. R. (2011). Degree of bilingualism predicts age of diagnosis of Alzheimer’s disease in low-education but not in highly educated Hispanics. Neuropsychologia, 49, 3826–3830. doi:10.1016/j.neuropsychologia.2011.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeck C., Hilton H. J., Zarahn E., Flynn J., Moeller J., & Stern Y. (2003). Relation of cognitive reserve and task performance to expression of regional covariance networks in an event-related fMRI study of nonverbal memory. Neuroimage, 20, 1723–1733. [DOI] [PubMed] [Google Scholar]
- Harrison S. L., Sajjad A., Bramer W. M., Ikram M. A., Tiemeier H., & Stephan B. C. M. (2015). Exploring strategies to operationalize cognitive reserve: A systematic review of reviews. Journal of Clinical and Experimental Neuropsychology, 37, 253–264. doi:10.1080/13803395.2014.1002759. [DOI] [PubMed] [Google Scholar]
- Herzog R., Alvarez-Pasquin M. J., Diaz C., Del Barrio J. L., Estrada J. M., & Gil A. (2013). Are healthcare workers' intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health, 13, 154 doi:10.1186/1471-2458-13-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F., Heffner K. L., Ren P., Tivarus M. E., Brasch J., Chen D. G., & Tadin D. (2016). Cognitive and Neural Effects of Vision-Based Speed-of-Processing Training in Older Adults with Amnestic Mild Cognitive Impairment: A Pilot Study. Journal of the American Geriatrics Society, 64 (6), 1293–1298. doi:10.1111/jgs.14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. Q., Zhang X. Q., Yu C. S., Duan Y. Y., Zhuo J. J., Cui Y., et al. (2016). Impaired parahippocampus connectivity in mild cognitive impairment and Alzheimer’s disease. Journal of Alzheimer’s disease : JAD, 49, 1051–1064. doi:10.3233/Jad-150727. [DOI] [PubMed] [Google Scholar]
- Marques P., Moreira P., Magalhaes R., Costa P., Santos N., Zihl J., et al. (2016). The functional connectome of cognitive reserve. Human Brain Mapping, 37, 3310–3322. doi:10.1002/hbm.23242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques P., Soares J. M., Magalhaes R., Santos N. C., & Sousa N. (2015). The bounds of education in the human brain connectome. Scientific Reports, 5, 12812 doi:10.1038/srep12812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPheeters M. L., Kripalani S., Peterson N. B., Idowu R. T., Jerome R. N., Potter S. A., et al. (2012). Closing the quality gap: Revisiting the state of the science (vol. 3: Quality improvement interventions to address health disparities). Evidence Report/Technology Assessment, 208 (3), 1–475. [PMC free article] [PubMed] [Google Scholar]
- Meng X. F., & D’Arcy C. (2012). Education and dementia in the context of the cognitive reserve hypothesis: A systematic review with meta-analyses and qualitative analyses. PLoS One, 7, e38268 doi:10.1371/journal.pone.0038268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D. G., & Group P. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Journal of Clinical Epidemiology, 62, 1006–1012. doi:10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Morbelli S., & Nobili F. (2014). Cognitive reserve and clinical expression of Alzheimer’s disease: Evidence and implications for brain PET imaging. American Journal of Nuclear Medicine and Molecular Imaging, 4, 239–247. [PMC free article] [PubMed] [Google Scholar]
- Nettiksimmons J., DeCarli C., Landau S., & Beckett L. (2014). Biological heterogeneity in ADNI amnestic mild cognitive impairment. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 10, 511–521.e1. doi:10.1016/j.jalz.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pool L. R., Weuve J., Wilson R. S., Bultmann U., Evans D. A., & de Leon C. F. M. (2016). Occupational cognitive requirements and late-life cognitive aging. Neurology, 86, 1386–1392. doi:10.1212/Wnl.0000000000002569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed B. R., Mungas D., Farias S. T., Harvey D., Beckett L., Widaman K., et al. (2010). Measuring cognitive reserve based on the decomposition of episodic memory variance. Brain, 133, 2196–2209. doi:10.1093/brain/awq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentz D. M., Huh T. J., Faust R. R., Budson A. E., Scinto L. F., Sperling R. A., et al. (2004). Use of IQ-adjusted norms to predict progressive cognitive decline in highly intelligent older individuals. Neuropsychology, 18, 38–49. doi:10.1037/0894-4105.18.1.38. [DOI] [PubMed] [Google Scholar]
- Sattler C., Toro P., Schonknecht P., & Schroder J. (2012). Cognitive activity, education and socioeconomic status as preventive factors for mild cognitive impairment and Alzheimer’s disease. Psychiatry Research, 196, 90–95. doi:10.1016/j.psychres.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Scarmeas N., Albert S. M., Manly J. J., & Stern Y. (2006). Education and rates of cognitive decline in incident Alzheimer’s disease. Journal of Neurology, Neurosurgery, and Psychiatry, 77, 308–316. doi:10.1136/jnnp.2005.072306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp E. S., & Gatz M. (2011). Relationship between education and dementia an updated systematic review. Alzheimer Disease & Associated Disorders, 25, 289–304. doi:10.1097/WAD.0b013e318211c83c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simic G., Babic M., Borovecki F., & Hof P. R. (2014). Early failure of the default-mode network and the pathogenesis of Alzheimer’s disease. CNS Neuroscience & Therapeutics, 20, 692–698. doi:10.1111/cns.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Manoux A., Marmot M. G., Glymour M., Sabia S., Kivimaki M., & Dugravot A. (2011). Does cognitive reserve shape cognitive decline? Annals of Neurology, 70, 296–304. doi:10.1002/ana.22391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solé-Padullés C., Bartrés-Faz D., Junqué C., Vendrell P., Rami L., Clemente I. C., et al. (2009). Brain structure and function related to cognitive reserve variables in normal aging, mild cognitive impairment and Alzheimer’s disease. Neurobiology of Aging, 30, 1114–1124. doi:10.1016/j.neurobiolaging.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Springer M. V., McIntosh A. R., Winocur G., & Grady C. L. (2005). The relation between brain activity during memory tasks and years of education in young and older adults. Neuropsychology, 19, 181–192. doi:10.1037/0894-4105.19.2.181. [DOI] [PubMed] [Google Scholar]
- Starr J. M., & Lonie J. (2008). Estimated pre-morbid IQ effects on cognitive and functional outcomes in Alzheimer disease: A longitudinal study in a treated cohort. BMC Psychiatry, 8, 27 doi:10.1186/1471-244X-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffener J., Brickman A. M., Rakitin B. C., Gazes Y., & Stern Y. (2009). The impact of age-related changes on working memory functional activity. Brain Imaging and Behavior, 3, 142–153. doi:10.1007/s11682-008-9056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffener J., Reuben A., Rakitin B. C., & Stern Y. (2011). Supporting performance in the face of age-related neural changes: Testing mechanistic roles of cognitive reserve. Brain Imaging and Behavior, 5, 212–221. doi:10.1007/s11682-011-9125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. (2002). What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society: JINS, 8, 448–460. [PubMed] [Google Scholar]
- Stern Y. (2009). Cognitive reserve. Neuropsychologia, 47, 2015–2028. doi:10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y., Habeck C., Moeller J., Scarmeas N., Anderson K. E., Hilton H. J., et al. (2005). Brain networks associated with cognitive reserve in healthy young and old adults. Cerebral Cortex, 15, 394–402. doi:10.1093/cercor/bhh142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y., Rakitin B. C., Habeck C., Gazes Y., Steffener J., Kumar A., et al. (2012). Task difficulty modulates young-old differences in network expression. Brain Research, 1435, 130–145. doi:10.1016/j.brainres.2011.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y., Zarahn E., Habeck C., Holtzer R., Rakitin B. C., Kumar A., et al. (2008). A common neural network for cognitive reserve in verbal and object working memory in young but not old. Cerebral Cortex, 18, 959–967. doi:10.1093/cercor/bhm134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teipel S., & Grothe M. J. (2016). Does posterior cingulate hypometabolism result from disconnection or local pathology across preclinical and clinical stages of Alzheimer’s disease? European Journal of Nuclear Medicine and Molecular Imaging, 43, 526–536. doi:10.1007/s00259-015-3222-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waiter G. D., Fox H. C., Murray A. D., Starr J. M., Staff R. T., Bourne V. J., et al. (2008). Is retaining the youthful functional anatomy underlying speed of information processing a signature of successful cognitive ageing? An event-related fMRI study of inspection time performance. Neuroimage, 41, 581–595. doi:10.1016/j.neuroimage.2008.02.045. [DOI] [PubMed] [Google Scholar]
- Zhao X. H., Liu Y., Wang X. B., Liu B., Xi Q., Guo Q. H., et al. (2012). Disrupted small-world brain networks in moderate Alzheimer’s disease: A resting-state fMRI study. PLoS One, 7, e33540 doi:10.1371/journal.pone.0033540. [DOI] [PMC free article] [PubMed] [Google Scholar]