Abstract
Background
This phase II study was conducted to assess clinical efficacy of tasquinimod maintenance therapy in patients with metastatic castrate-resistant prostate cancer not progressing during first-line docetaxel-based therapy.
Patients and methods
Patients were randomly assigned (1 : 1) to receive tasquinimod (0.25–1.0 mg/day orally) or placebo. The primary end point was radiologic progression-free survival (rPFS); secondary efficacy end points included: overall survival (OS); PFS on next-line therapy (PFS 2) and symptomatic PFS, assessed using the Brief Pain Inventory (BPI) questionnaire and analgesic use. Quality of life was measured by the Functional Assessment of Cancer Therapy-Prostate (FACT-P) questionnaire and by the EuroQol-5 Dimension Quality of Life Instrument (EQ-5D). Adverse events were recorded.
Results
A total of 219 patients were screened and 144 patients randomized. The median duration of treatment was 18.7 weeks (range 0.6–102.7 weeks) for the tasquinimod arm and 19.2 weeks (range 0.4–80.0 weeks) for the placebo arm. Median (90% CI) rPFS was 31.7 (24.3–53.7) and 22.7 (16.1–25.9) weeks in the tasquinimod and placebo arms, respectively [HR (90% CI) 0.6 (0.4–0.9), P = 0.0162]. The median OS was not reached because only 14 deaths occurred by the cut-off date. No statistically significant differences between treatment arms were noted for symptomatic PFS, PFS 2, BPI score, FACT-P score, or EQ-5D. The incidence of any treatment emergent adverse event (TEAE) was similar in the tasquinimod and placebo arms (97.2% versus 94.3%, respectively), whereas severe TEAEs (NCI-CTC Grade 3–5) incidence was higher in the tasquinimod group (50.7% versus 27.1%).
Conclusions
Randomized trials testing new drugs as maintenance can be successfully conducted after chemotherapy in castrate-resistant prostate cancer. Maintenance tasquinimod therapy significantly reduced the risk of rPFS by 40%.
ClinicalTrials
gov identifier NCT01732549.
Keywords: tasquinimod, prostate cancer, castrate-resistant, maintenance therapy, docetaxel
Introduction
Improvement in understanding tumor–host interactions in prostate cancer has led to the development of novel immunomodulatory agents and anti-angiogenic molecules, which act on the tumor microenvironment rather than the tumor itself [1, 2]. Tasquinimod—a first-in-class, oral quinolone-3-carboxamide derivative—has immunomodulatory, anti-angiogenic, and anti-metastatic properties [3, 4]. Tasquinimod inhibits S100A9, a calcium-binding protein that promotes accumulation of myeloid-derived suppressor cells [3]. In addition to immunosuppressive effects, evidence indicates that these cells are involved in angiogenesis, invasion and metastasis [5]. In a randomized phase II study in chemotherapy-naïve patients with minimally symptomatic metastatic castrate-resistant prostate cancer (mCRPC), tasquinimod delayed disease progression by a median of 4.3 months and had an acceptable safety profile [6].
Maintenance therapy after successful first-line chemotherapy is an established concept in a number of malignancies [7, 8]. This randomized, phase II study aimed to obtain a clinical proof of concept of the clinical efficacy of tasquinimod maintenance therapy in patients with mCRPC who had not progressed following first-line docetaxel-based therapy.
Methods
Patient eligibility
Patients with mCRPC aged 18 years or older treated with first-line docetaxel in an every 3-week schedule were eligible. Docetaxel should have been administered for a minimum of six cycles with a cumulative dose of ≥360 mg/m2. Other key inclusion criteria included: Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; no progressive disease at the end of docetaxel treatment defined according to Response Evaluation Criteria In Solid Tumors (RECIST1.1) and Prostate Cancer Clinical Working Group in March 2008 (PCWG2) criteria; and no PSA increase for the three last tests. The time between each PSA test had to be preferably at least 7 days. If the value for the third PSA test was above that for the second, a fourth PSA test had to be carried out. The value of the fourth test had to be below or equal to that of the second. Patients should have an ongoing chemical or surgical castration (serum testosterone <0.5 ng/ml or <1.75 nmol/l). Key exclusion criteria included: concurrent use of other anticancer agents with the exception of ongoing treatment with luteinizing hormone-releasing hormone agonists or antagonists. Denosumab or bisphosphonate (e.g. zoledronic acid) were permitted if started ≥4 weeks before screening. Excluded were patients who had received: strontium, samarium, or radium therapy; treatment with tasquinimod; any agent with anti-angiogenic properties; ongoing corticosteroids (equivalent >10 mg/day prednisolone).
Study design and conduct
This was a multinational, multicenter, randomized, double-blind, placebo-controlled proof of concept phase II clinical trial (ClinicalTrials.gov identifier NCT01732549), conducted at 44 sites in 11 countries (Belgium, Czech Republic, Denmark, France, Germany, Hungary, Italy, Lithuania, Poland, Spain, and the UK). The study was conducted between January 2013 and May 2015. Patients were randomly assigned with a ratio of 1 : 1 to tasquinimod or placebo treatments. A list of randomization numbers stratified for presence (or absence) of visceral metastases [all prostate cancer metastatic lesions (e.g. lung, liver) except lymph nodes, local recurrence and bone] and opioid analgesic use (or not) to control for cancer-related pain, was generated with a balanced ratio (1 placebo : 1 tasquinimod). After eligibility was confirmed, patients were randomized at Baseline (Day 1), in sequential order within each centre (and within each level of strata). Placebo capsules were identical to tasquinimod capsules in appearance and excipients but excluded the active compound.
Patients received initially an oral dose of 0.25 mg/day of tasquinimod (or corresponding placebo), starting on Day 1 of the treatment period for at least 2 weeks. Once tolerability of the 0.25 mg/day dose was established, patients received a dose increase to 0.5 mg/day for at least 2 weeks, and then increased to 1 mg/day of study treatment. The treatment period continued until any criteria for treatment withdrawal was fulfilled, including disease progression or toxicity or wish to stop. Patients showing poor tolerability for the escalated doses of tasquinimod were allowed to continue study treatment at the highest individually tolerated dose.
The study was conducted under the provisions of the Declaration of Helsinki, and in accordance with the International Conference on Harmonisation (ICH) Consolidated Guideline on Good Clinical Practice. The protocol was approved by an independent ethics committee and informed consent was obtained before study entry.
Study assessments
Tumor assessment was carried out at 8-week intervals according to RECIST (v1.1, soft tissue lesions) and/or the PCWG2 (bone lesions) guidelines. Adverse events (AEs) were coded using the Medical Dictionary for Regulatory Activities (MedDRA dictionary, version 17.1) and graded using the current version of the National Cancer Institute Common Terminology Criteria (NCI-CTC) for AEs (version 4.03).
Outcomes
The primary end point was investigator-assessed radiologic progression-free survival (rPFS) including skeleton-related events, defined as the time from the date of randomization to the date of radiologic progression or death due to any cause. Radiologic progression was defined as any of the following: progression of soft tissue lesions evaluated by computed tomography (CT) scan, or magnetic resonance imaging according to the RECIST v.1.1 criteria; progression of bone lesions detected with bone scan according to PCWG2 criteria; or radiologically confirmed spinal cord compression or pathological fracture due to malignant progression. Secondary efficacy end points included: overall survival (OS), defined as the time from random assignment to death due to any cause; symptomatic PFS, defined as the time from the date of random assignment to the date of symptomatic progression or death due to prostate cancer; PFS on next-line therapy (PFS 2; defined as the time from start date of first further anticancer treatment of prostate cancer until disease progression or death [from any cause] during the study follow-up). Symptomatic PFS was assessed using the Brief Pain Inventory (BPI) questionnaire and analgesic use. Further secondary end points were time from random assignment to further treatment of prostate cancer and quality of life, measured by the Functional Assessment of Cancer Therapy - Prostate (FACT-P) questionnaire and by the EuroQol-5 Dimension Quality of Life Instrument (EQ-5D).
Safety was assessed in all patients who received at least one dose of study treatment. Safety assessments focused on AEs, vital signs, physical examination, ECOG PS, electrocardiogram, and laboratory analyses (hematology, biochemistry, and urinalysis).
Statistical analyses
The study was designed to detect a hazard ratio (HR) of 0.588, which corresponded to an increase in median PFS from 20.0 to 34.0 weeks based on published data. Assuming a one-sided significance level of 0.05, 80% power and a 1 : 1 allocation, 88 events (radiologic progression or death due to any cause) were required. Assuming a non-constant accrual period of 15 months, with all patients followed up until an event or 9 months, and a dropout rate at 3 months of 10%, 140 patients (tasquinimod, n = 70; placebo, n = 70) were required in order to observe the 88 events.
A log-rank test was used to compare the PFS and other time to event end points for tasquinimod versus placebo. The treatment effect was estimated by calculating the HR and its 90% confidence interval (CI) from a Cox proportional hazards model. Kaplan–Meier plots were also produced. Statistical evaluation was carried out using SAS software (version 9; SAS Institute, Cary, NC).
Results
Patients
A total of 219 patients were screened and 144 patients were randomized (supplementary Figure S1, available at Annals of Oncology online). At baseline, all patients were castrated with testosterone data below 1.75 nmol/l. All patients received prior docetaxel treatment. A median number of eight docetaxel cycles was received. The treatment arms were well balanced for most demographic and baseline characteristics (Table 1).
Table 1.
Baseline characteristics (intent to treat population)
| Variable | Placebo (N = 72)a | Tasquinimod (N = 71) |
|---|---|---|
| Age (years) | ||
| Median (range) | 70.0 (58–82) | 71.0 (46–85) |
| PSA (μg/l) | ||
| N | 70 | 71 |
| Median (range) | 7.40 (0.1–468.3) | 5.20 (0.1–814.4) |
| ECOG PS score, n (%) | ||
| N | 69 | 71 |
| 0 (normal activity) | 31 (42.5) | 39 (54.9) |
| 1 (restricted activity) | 38 (52.1) | 32 (45.1) |
| Gleason score at diagnosis, n (%) | ||
| N | 70 | 70 |
| 3–7 | 31 (42.5) | 38 (53.5) |
| 8–10 | 39 (53.4) | 32 (45.0) |
| Total cumulative dose of docetaxel (mg) | ||
| N | 72 | 70 |
| Median (range) | 1050.0 (450.0–1691.5) | 1050.0 (135.6–1680.0) |
| Number of cycles | ||
| N | 72 | 71 |
| Median (range) | 8.0 (6–11) | 8.0 (6–12) |
| Visceral metastases, n (%) | 16 (21.9) | 17 (23.9) |
| Opioid use at baseline, N (%) | 11 (7.6) | 10 (6.9) |
Due to data entry error, one patient was excluded from the analysis of patient characteristics in the placebo group.
ECOG PS, Eastern Cooperative Oncology Group performance status; PSA, prostate-specific antigen; SD, standard deviation.
Treatment
The median duration of treatment was 18.7 weeks (range 0.6–102.7 weeks) for the tasquinimod arm and 19.2 weeks (range 0.4–80.0 weeks) for the placebo arm. A lower proportion of patients reached the maximum dose of 1 mg in the tasquinimod compared with the placebo arm (80.3% versus 92.9%).
Outcomes
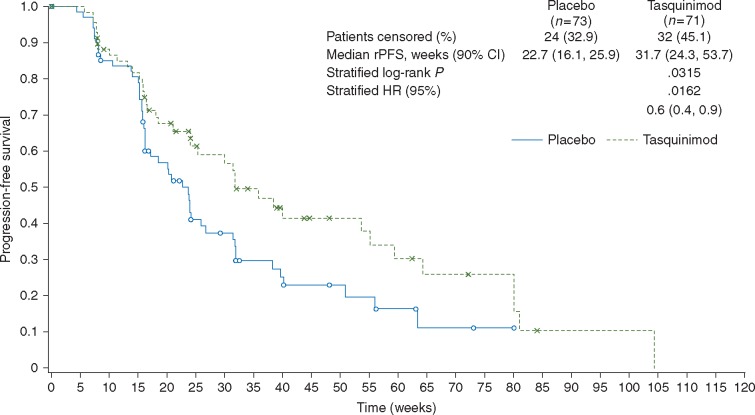
The median follow-up duration for tasquinimod- and placebo-treated patients was 59 and 53 weeks, respectively. Median rPFS data are shown in Table 1 and further illustrated in a Kaplan–Meier plot in Figure 1. The HR (90% CI) using Cox analysis was statistically significant (P = 0.0162) when adjusted for visceral metastases, opioid analgesic use and region, demonstrating a reduction in the risk of progression of 40% for tasquinimod compared with placebo.
Figure 1.
Kaplan–Meier plot for rPFS by investigator assessment (intent to treat population). CI, confidence interval; HR, hazard ratio; rPFS, radiologic progression-free survival.
The median OS was not reached as only a total of 14 deaths occurred by the cut-off date. A summary of results for the efficacy end points of PFS 2, symptomatic PFS, and time to further anticancer treatment of prostate cancer is presented in Table 2. The median time to PFS 2 was 19.3 weeks for the tasquinimod arm and 24.1 weeks for the placebo arm, and the difference was not statistically different [HR (90% CI) was 1.5 (0.5–4.8), P = 0.7375].
Table 2.
rPFS and symptomatic PFS on first-line therapy
| Placebo N = 73 | Tasquinimod N = 71 | Log-rank test P |
Cox analysisb |
||
|---|---|---|---|---|---|
| Stratifieda | HR (90% CI)c | P | |||
| Median rPFS | 22.7 (16.1–25.9) | 31.7 (24.3–53.7) | 0.0863 | 0.6 (0.4–0.9) | 0.0162 |
| Median symptomatic PFS, weeks (90% CI) | 95.3 (NE–NE) | NE (31.9–NE) | 0.5442 | 1.2 (0.7–2.1) | 0.7276 |
| Median time to further anticancer treatment, weeks (90% CI) | 29.0 (23.1–39.1) | 42.3 (32.0–58.0) | 0.1120 | 0.7 (0.5–1.0) | 0.0571 |
| Median PFS 2 (90% CI) (weeks) | 24.1 (12.6–NE) | 19.3 (7.1–30.7) | 0.5219 | 1.5 (0.5–4.8) | 0.7375 |
Time from randomization to next-line therapy for prostate cancer and PFS on the next-line therapy (PFS 2) (intent to treat population).
Log-rank test adjusting for presence (or absence) of visceral metastases, opioid analgesic use and region (Eastern Europe, Western Europe). Two-sided P value.
Cox proportional hazards model adjusting for presence (or absence) of visceral metastases, opioid analgesic use and region (Eastern Europe, Western Europe). one-sided P value.
HR tasquinimod/placebo.
CI, confidence interval; HR, hazard ratio; NE, not evaluable; PFS, progression-free survival; PFS 2, progression-free survival 2; rPFS, radiologic progression-free survival.
The median time to symptomatic PFS was not reached for tasquinimod and was 95.3 weeks for the placebo arm [HR (90% CI) was 1.2 (0.7–2.1), P = 0.7276]. The median time to further anticancer treatment of prostate cancer was in favor of tasquinimod at 42.3 weeks for the tasquinimod arm and 29.0 weeks for the placebo arm [HR (90% CI) was 0.7 (0.5–1.0)] but the result did not reach statistical significance (P = 0.0571).
Median time to deterioration (90% CI) in FACT-P was 8.1 (8.1–13.1) and 15.7 (10.6–16.3) weeks in the tasquinimod and placebo arms, respectively. HR by Cox analysis was not statistically significant [HR (90% CI) was 1.3 (0.9–1.7), P = 0.8759]. The mean FACT-P total score showed consistently larger deterioration from baseline in the tasquinimod compared with the placebo arm at all time points. Analysis of covariance of change from baseline for FACT-P scores showed mostly a larger deterioration in the tasquinimod compared with the placebo arm, but none of the treatment differences were statistically significant at the end-of-study visit.
The BPI score at baseline was similar in the two treatment arms for almost every question. At the end-of-study treatment, the mean and median scores for almost all BPI questions were higher in the tasquinimod group, indicating higher intensity of pain and degree to which pain interferes with function in the tasquinimod compared with the placebo group.
Analysis of covariance of change from baseline for EQ-5D visual analog scale (VAS) score showed a greater deterioration in the tasquinimod compared with the placebo arm at the end-of-study visit; although, the treatment difference was not statistically significant (1-sided P = 0.9824) (supplementary Table S1, available at Annals of Oncology online).
Safety
The incidence of any treatment emergent adverse event (TEAE) was similar in the tasquinimod and placebo arms (97.2% versus 94.3%, respectively). The incidence of any Grade 3–5 TEAEs was higher in the tasquinimod compared with the placebo arm (50.7% versus 27.1%) (Table 3; supplementary Table S2, available at Annals of Oncology online). Serious TEAEs occurred in 24 (33.8%) and 14 (20.0%) patients in the tasquinimod and placebo arms, respectively. Grade 3–5 serious TEAEs occurred in 18 (25.4%) and 10 (14.3%) patients in the tasquinimod and placebo arms, respectively. Only one patient receiving tasquinimod died during the study treatment period due to a non-related myocardial infarction. The most common drug-related TEAEs (all grades) were nausea (21.1% versus 8.6%), constipation (16.9% versus 7.1%), diarrhea (11.3% versus 5.7%), fatigue (15.5% versus 10%), and asthenia (15.5% versus 8.6%) and decrease appetite (28.2% versus 8.6%) in tasquinimod versus placebo arms, respectively. Of particular interest in this study were blood levels of amylase and lipase. In the tasquinimod arm, abnormal increases of grade 3 or higher were noted in two (2.8%) patients for lipase and one (1.4%) patient for amylase.
Table 3.
Summary of treatment-emergent adverse events by preferred term and system organ class with an incidence >5% of patients in any treatment group regardless of relationship (safety population); number of patients (%) reported
| System organ class/preferred term | Placebo (N = 70) |
Tasquinimod (N = 71) |
||
|---|---|---|---|---|
| All | Grades 3–5 | All | Grades 3–5 | |
| Any TEAE | 66 (94.3) | 18 (25.7) | 69 (97.2) | 36 (50.7) |
| Musculoskeletal and connective tissue disorders | 44 (62.9) | 3 (4.3) | 45 (63.4) | 13 (18.3) |
| General disorders and administration site conditions | 37 (52.9) | 3 (4.3) | 50 (70.4) | 9 (12.7) |
| Gastrointestinal disorders | 32 (45.7) | 2 (2.9) | 46 (64.8) | 7 (9.9) |
| Nervous system disorders | 19 (27.1) | 1 (1.4) | 36 (50.7) | 5 (7.0) |
| Infections and infestations | 21 (30.0) | 1 (1.4) | 22 (31.0) | 4 (5.6) |
| Metabolism and nutrition disorders | 15 (21.4) | 1 (1.4) | 28 (39.4) | 3 (4.2) |
| Vascular disorders | 12 (17.1) | 3 (4.3) | 20 (28.2) | 4 (5.6) |
| Skin and subcutaneous tissue disorders | 13 (18.6) | 0 (0.0) | 16 (22.5) | 0 |
| Investigations | 7 (10.0) | 2 (2.9) | 16 (22.5) | 5 (7.0) |
| Psychiatric disorders | 7 (10.0) | 0 (0.0) | 16 (22.5) | 3 (4.2) |
| Respiratory, thoracic and mediastinal disorders | 7 (10.0) | 0 (0.0) | 16 (22.5) | 3 (4.2) |
| Renal and urinary disorders | 10 (14.3) | 5 (7.1) | 10 (14.1) | 3 (4.2) |
| Blood and lymphatic system disorders | 2 (2.9) | 0 (0.0) | 11 (15.5) | 2 (2.8) |
| Injury, poisoning and procedural complications | 8 (11.4) | 1 (1.4) | 2 (2.8) | 1 (1.4) |
TEAE, treatment emergent adverse event.
Discussion
Analysis of the primary end point of this double-blind randomized study demonstrated that rPFS was in favor of tasquinimod [HR (90% CI): 0.6 (0.4–0.9), P = 0.0162] when adjusted for visceral metastases, opioid analgesic use, and region in patients with mCRPC who had not progressed after a first-line docetaxel-based chemotherapy. To our knowledge, this is the first completed trial of a maintenance strategy with a novel agent in mCRPC in patients not progressing after docetaxel therapy.
The median time to further anticancer treatment of prostate cancer was 42.3 weeks for the tasquinimod arm and 29.0 weeks for the placebo arm, but the overall difference between the arms was not statistically significant based on stratified log-rank test.
For the efficacy end points of PFS 2 and symptomatic PFS, HR by Cox analysis was not in favor of tasquinimod and none of the results were statistically significant. The median OS was not reached as only a total of 14 deaths occurred by the cut-off date. Quality of life analyses showed deterioration in tasquinimod-treated patients compared with placebo in FACT-P. The change in the BPI scores showed a similar trend, although not tested statistically, suggesting higher intensity of pain and degree to which pain interferes with function in the tasquinimod compared with the placebo arm. The greater deterioration in EQ-5D VAS score from baseline in the tasquinimod compared with the placebo arm was not statistically significant. Tasquinimod showed a reasonable safety profile in patients with mCRPC who had not progressed after a first-line docetaxel-based chemotherapy, which is consistent with phase II [6] and III [9] study results in pre-chemotherapy mCRPC patients.
Following positive results in a multicenter, double-blind, randomized phase II trial [6], tasquinimod was investigated in a phase III, double-blind placebo-controlled international trial in patients with asymptomatic to mildly symptomatic chemotherapy-naïve mCRPC and evidence of bone metastases. In common with the current study, results showed that tasquinimod improved rPFS compared with placebo (HR = 0.69, 95% CI 0.60–0.80) but did not extend OS (HR = 1.10, 95% CI 0.94–1.28) [10].
A number of studies have now been completed on maintenance therapy in CRPC. One of these was a randomized, double-blind, placebo-controlled trial that assessed the impact of orteronel (TAK-700), an inhibitor of extra-gonadal androgen synthesis [11], as maintenance therapy in patients with mCRPC and non-progressive disease after docetaxel [12]. Patients receiving orteronel showed a significant improvement in the primary end point of event-free survival (defined as the time from random assignment to death, or a combination of at least two outcomes that included radiographic, clinical or PSA progression): 8.5 versus 2.9 months in the orteronel and placebo arms, respectively (HR = 0.32; P = 0.001). However, the study was terminated before completing enrolment due to discontinuation of orteronel development for prostate cancer and only 47 patients were randomized. The second study was a phase II single arm trial evaluating maintenance therapy with temsirolimus, a mammalian target of rapamycin inhibitor, after docetaxel induction chemotherapy in 21 patients with CRPC [13]. Time to treatment failure (radiologic and/or symptomatic progression), the primary end point, was a median of 24.3 weeks. Both the orteronel and temsirolimus studies met their primary end point and concluded that maintenance treatment is feasible.
In conclusion, this study aimed to evaluate maintenance therapy with tasquinimod as a strategy to prolong treatment response without significantly compromising quality of life in patients with mCRPC not progressing under a first-line docetaxel-based therapy. The primary objective of delayed rPFS with tasquinimod was met, with a reduction in the risk of progression of 40% for tasquinimod compared with placebo. Although the planned number of patients was included and analysis of the outcome was possible, further development of tasquinimod in prostate cancer has been discontinued.
Supplementary Material
Acknowledgements
The authors wish to thank Jean-Christophe Pouget for the statistical support provided in the development of this work. Medical writing support was provided by Christine McKillop PhD, Medscimedia Ltd and funded by Ipsen.
Funding
Ipsen (no grant number is applicable).
Disclosure
KF: Consultant Sanofi, Janssen, Astellas. AU: None declared. LS: Consultant Non Nordisk; Research funding MSD, Ipsen, Eli Lilly, Roche, BMS, Astra Zeneca. MM: Consultant Roche, Astellas, Sanofi, Bayer, Ipsen; Research funding Sanofi, Roche, Ipsen. SL: None declared. AT-V: Honoraria Sanofi, Astellas, Pfizer, Novartis, Ipsen; Consultant Sanofi, Astellas, Pfizer, Novartis, Ipsen; Research funding Pfizer. AF: Honoraria Sanofi, Astellas, Pfizer, Novartis, Janssen Cilag, Astra Zeneca. AG: Relationship with Pfizer, Astellas, Novartis. JB: Consultant Pierre Fabre, Astellas, Pfizer, Merck, Genentech, Novartis. MAC: Honoraria Sanofi, Astellas, Janssen, Bayer. SC: Honoraria Clovis, Sanofi, Astellas, Johnson and Johnson; Consultant Clovis, Sanofi, Astellas, Johnson and Johnson. HD: Honoraria Astellas. MM: None declared. NP: Research funding Bayer Healthcare. SL: Honoraria Roche, Astra Zeneca, Pfizer, Novartis, Janssen; Consultant Roche, Pfizer, Astra Zeneca. LS: None declared. CNS: Honoraria Ipsen; Consultancy Ipsen. FB: Stock Ipsen. NG: Employment Ipsen; Stock Ipsen. GD: Consultancy Sanofi, Bayer.
References
- 1. Quinn DI, Shore ND, Egawa S. et al. Immunotherapy for castration-resistant prostate cancer: progress and new paradigms. Urol Oncol 2015; 33: 245–260. [DOI] [PubMed] [Google Scholar]
- 2. Thoreson GR, Gayed BA, Chung PH. et al. Emerging therapies in castration resistant prostate cancer. Can J Urol 2014; 21(2 Suppl 1): 98–105. [PubMed] [Google Scholar]
- 3. Raymond E, Dalgleish A, Damber JE. et al. Mechanisms of action of tasquinimod on the tumour microenvironment. Cancer Chemother Pharmacol 2014; 73: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Armstrong AJ, Häggman M, Stadler WM. et al. Long-term survival and biomarker correlates of tasquinimod efficacy in a multicenter randomized study of men with minimally symptomatic metastatic castration-resistant prostate cancer. Clin Cancer Res 2013; 19: 6891–6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ye XZ, Yu SC, Bian XW.. Contribution of myeloid-derived suppressor cells to tumor-induced immune suppression, angiogenesis, invasion and metastasis. J Genet Genomics 2010; 37: 423–430. [DOI] [PubMed] [Google Scholar]
- 6. Pili R, Häggman M, Stadler WM. et al. Phase II randomized, double-blind, placebo-controlled study of tasquinimod in men with minimally symptomatic metastatic castrate-resistant prostate cancer. J Clin Oncol 2011; 29: 4022–4028. [DOI] [PubMed] [Google Scholar]
- 7. Khalique S, Hook JM, Ledermann JA.. Maintenance therapy in ovarian cancer. Curr Opin Oncol 2014; 26: 521–528. [DOI] [PubMed] [Google Scholar]
- 8. Gerber DE, Dahlberg SE, Sandler AB. et al. Baseline tumour measurements predict survival in advanced non-small cell lung cancer. Br J Cancer 2013; 109: 1476–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carducci MA, Armstrong AJ, Pili R. et al. A phase 3, randomized, double-blind, placebo-controlled study of tasquinimod (TASQ) in men with metastatic castrate resistant prostate cancer (mCRPC). Presented at the European Cancer Congress, Vienna, Austria, 25–29 September 2015.
- 10. Sternberg C, Armstrong A, Pili R. et al. Phase III, randomized, double-blind, placebo-controlled study of tasquinimod in men with metastatic castrate-resistant prostate cancer. J Clin Oncol 2016; 34: 2636–2643. [DOI] [PubMed] [Google Scholar]
- 11. Yamaoka M, Hara T, Hitaka T. et al. Orteronel (TAK-700), a novel non-steroidal 17,20-lyase inhibitor: effects on steroid synthesis in human and monkey adrenal cells and serum steroid levels in cynomolgus monkeys. J Steroid Biochem Mol Biol 2012; 129: 115–128. [DOI] [PubMed] [Google Scholar]
- 12. Cathomas R, Crabb SJ, Mark M. et al. Orteronel switch maintenance therapy in metastatic castration resistant prostate cancer after first-line docetaxel: a multicenter, randomized, double-blind, placebo-controlled trial (SAKK 08/11). Prostate 2016; 76: 1519–1527. [DOI] [PubMed] [Google Scholar]
- 13. Emmenegger U, Booth CM, Berry S. et al. Temsirolimus maintenance therapy after docetaxel induction in castration-resistant prostate cancer. Oncologist 2015; 20: 1351–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.