Abstract
Background
Occupational exposure to acrylamide was associated with excess mortality from pancreatic cancer, though in the absence of dose-risk relationship. Few epidemiological studies have examined the association between acrylamide from diet and pancreatic cancer risk.
Patients and methods
We considered this issue in a combined set of 1975 cases of pancreatic cancer and 4239 controls enrolled in six studies of the Pancreatic Cancer Case–Control Consortium (PanC4). We calculated pooled odds ratios (ORs) and their 95% confidence intervals (CI) by estimating study-specific ORs through multivariate unconditional logistic regression models and pooling the obtained estimates using random-effects models.
Results
Compared with the lowest level of estimated dietary acrylamide intake, the pooled ORs were 0.97 (95% CI, 0.79–1.19) for the second, 0.91 (95% CI, 0.71–1.16) for the third, and 0.92 (95% CI, 0.66–1.28) for the fourth (highest) quartile of intake. For an increase of 10 µg/day of acrylamide intake, the pooled OR was 0.96 (95% CI, 0.87–1.06), with heterogeneity between estimates (I2 = 67%). Results were similar across various subgroups, and were confirmed when using a one-stage modelling approach.
Conclusions
This PanC4 pooled-analysis found no association between dietary acrylamide and pancreatic cancer.
Keywords: acrylamide, case–control studies, pancreatic neoplasms, pooled-analysis, risk factors
Introduction
The association between dietary acrylamide intake and risk of several common cancers has been widely debated [1–5], following the findings of a Swedish study reporting the formation of acrylamide in foods, particularly starchy foods such as potatoes and breads, cooked at high temperatures [6].
Epidemiological data on the association between dietary acrylamide and pancreatic cancer are scant. It is of particular interest to investigate this association because two studies of acrylamide exposure in the occupational setting [7,8] have shown moderately increased mortality from pancreatic cancer, in the absence of a dose–risk relationship. When the results of those studies were pooled, the summary standardized mortality ratios (SMR) of pancreatic cancer were 1.54 (95% confidence interval, CI, 0.95–2.36) for any occupational exposure to acrylamide and 1.67 (95% CI, 0.83–2.99) for high exposure [4]. In contrast, a recent systematic review and meta-analysis of epidemiological data considering exposure to dietary acrylamide reported relative risks (RR) of pancreatic cancer of 0.93 (95% CI, 0.76–1.12) for high versus low acrylamide intake and of 0.99 (95% CI, 0.95–1.03) for an increase in intake equal to 10 µg/day. The estimates were based on four studies (three cohort and one case–control study), with a total of 1732 cases [5].
With the aim to provide additional evidence on this topic, given the scant amount of available epidemiological data, we examined the role of acrylamide intake on a combined set of 1975 cases of pancreatic cancer (i.e. more than those included in the meta-analysis) and over 4000 controls enrolled in six studies from the Pancreatic Cancer Case–Control Consortium (PanC4) [9].
Materials and methods
PanC4 is an International consortium of scientists set up to investigate the aetiology of pancreatic cancer, through pooled analyses of shared data (http://www.panc4.org ; date last accessed: 12 December 2016). This investigation is based on six case–control studies [10–15] with comprehensive food frequency questionnaires, including information on the major food items contributing acrylamide intake (e.g. potato products cooked at high temperatures, breads, coffee) as well as calculation of total energy intake. They included a total of 1975 cases and 4239 controls. The main characteristics of the studies are described in supplementary Table S1, available at Annals of Oncology online. A number of participants in three case–control studies included [11, 12, 14] lacked >10% of the required information to estimate acrylamide intake (i.e. they had not completed the food frequency questionnaires), and were therefore excluded from the analyses. Another PanC4 participating study from Shanghai, China [16], could not be included in the investigation since data on content of dietary acrylamide in Chinese foods was not available at the time of data analysis. Cases and controls were interviewed in-person in all studies except the Queensland (Australia) study, where interviews were conducted either over the telephone or face-to-face, with a self-administered food frequency questionnaire.
For the present analyses, the original datasets were restructured either by the original study investigators or by our central coordinators using a uniform format for data harmonization. From each study, individual data on socio-demographic characteristics, anthropometric measures, tobacco smoking and history of diabetes were collected, whenever available.
The list of foods containing dietary acrylamide was derived from international databases [17–19], and included coffee, breads, potato products, various breakfast cereals, biscuits and cookies, gingerbread and spiced cakes, chocolate products (such as brownies and candy bars), several types of snacks and pastries, fried fish, fried chicken, pizza, tacos, fried rice and beer. Each participating study was asked to provide information on individual consumption of all these food items of cases and controls, whenever available in food frequency questionnaires, together with their cooking method whenever relevant (particularly for potato products). The total number of food items containing acrylamide in each study ranged from 14 (in the Louisiana State University study) to 33 (in the University of Minnesota study). Subjects who had a maximum of 10% of missing information among the food items contributing to total acrylamide were maintained in the analyses, by assigning to the missing food item the study-specific median frequency of consumption of that item. Information on total energy intake was also collected, for adjustment purposes. Data on the average acrylamide content of foods were derived from area-specific resources. Thus, for studies from USA, we applied measures of acrylamide levels made available from the United States Food and Drug Administration (Total Diet Study 2003–2006 [18]); for the Italian study, we used data from the European Food Safety Agency [17] and the Agence Française de Sécurité Sanitaire des Aliments (AFSSA) [20], integrated with estimates on specific Italian dietary items [21, 22]; and for the Australian study, we used data from two complementary reports from the Government of South Australia (on non-carbohydrate based foods [23]) and from Croft et al. (on carbohydrate based foods [24]).
Statistical analysis
A two-stage modelling approach was used to estimate the association between dietary acrylamide intake and risk of pancreatic cancer. In the first stage, we considered the association between acrylamide intake and risk of pancreatic cancer separately for each study, by estimating the odds ratios (ORs) and their 95% CI through multivariate unconditional logistic regression models [25], including a priori defined terms, when available, for age, sex, race/ethnicity, education, smoking habits, diabetes, body mass index, and total energy intake. The Italian study was further adjusted for two study-specific covariates, i.e. period of interview and study centre. We modelled exposure using both study-specific quartiles and a continuous measure (i.e. 10 µg/day) of acrylamide intake. In the second stage, summary (pooled) effect estimates were computed using a random-effects model [26].
Heterogeneity between studies was examined using the χ2 statistic [27] and quantified through the I2 [28].
Subgroup analyses were conducted to examine whether the effect of high versus low acrylamide intake was heterogeneous across strata (through the χ2 statistic [27]) of sex, age, smoking habit, obesity, diabetes and geographic area of the study.
In addition to the two-stage analysis, we performed an aggregate analysis by pooling data from all six studies into a single large dataset (one-stage analysis). The association between acrylamide intake and the risk of pancreatic cancer was then assessed through multivariate unconditional logistic regression models [25], adjusted for study and the same covariates reported above for the two-stage modelling approach. This analysis was conducted by including in the model the study-specific quartiles of acrylamide intake or, in an additional sensitivity analysis, quartiles of acrylamide intake based on the distribution of all 4239 controls. We also conducted a sensitivity analysis in which each study was excluded one at a time to ensure that the magnitude of the overall estimates was not dependent on any specific study, and another one by excluding all subjects that had any missing information in food items contributing to total acrylamide intake (i.e. about 10% of cases and 8% of controls).
Results
Supplementary Table S2, available at Annals of Oncology online, presents the distribution of pancreatic cancer cases and controls according to sex, age and other selected covariates.
Table 1 gives information on estimates of dietary acrylamide intake in the six studies. The mean daily intake of acrylamide in cases ranged between 20.3 µg in the Louisiana State University study and 33.6 µg in the Italian study, and in controls between 16.8 and 31.2 µg (in the same studies). The median acrylamide intake of controls varied from a lowest value of 14.7 µg/day in the Louisiana State University study to a highest of 28.2 µg/day in the Italian study. The main food categories contributing to total acrylamide intake in each study, and the corresponding contribution proportions, are reported in supplementary Table S3, available at Annals of Oncology online.
Table 1.
Information on daily acrylamide intake in the studies considered in the analyses. International Pancreatic Cancer Case–Control Consortium (PanC4)
| Study centre | Mean intake±SD, cases (μg/day) | Mean intake±SD, controls (μg/day) | 25th percentile (μg/day)a | Median intake (μg/day)a | 75th percentile (μg/day)a | Mean intake±SD per kg of BW, cases (μg/day/kg BW) | Mean intake±SD per kg of BW, controls (μg/day/kg BW) |
|---|---|---|---|---|---|---|---|
| MD Anderson, USA | 20.5±12.5 | 22.2±13.7 | 12.5 | 19.0 | 28.0 | 0.25±0.16 | 0.27±0.17 |
| Italy | 33.6±15.9 | 31.2±17.2 | 19.1 | 28.2 | 39.6 | 0.47±0.24 | 0.43±0.24 |
| UCSF, USA | 22.7±13.7 | 20.7±14.5 | 11.9 | 17.6 | 26.0 | 0.31±0.18 | 0.29±0.20 |
| LSU, USA | 20.3±14.8 | 16.8±11.2 | 8.3 | 14.7 | 22.2 | 0.25±0.18 | 0.22±0.16 |
| UMN, USA | 25.3±14.4 | 26.0±14.6 | 16.5 | 23.1 | 31.9 | NAb | NAb |
| QIMR, Australia | 22.8±16.6 | 20.0±11.1 | 12.5 | 17.8 | 25.5 | 0.29±0.22 | 0.26±0.16 |
Among controls.
Information on body weight was not available in the University of Minnesota study.
BW, body weight; LSU, Louisiana State University; NA, not available; QIMR, QIMR Berghofer Medical Research Institute; SD, standard deviation; UCSF, University of California, San Francisco; UMN, University of Minnesota.
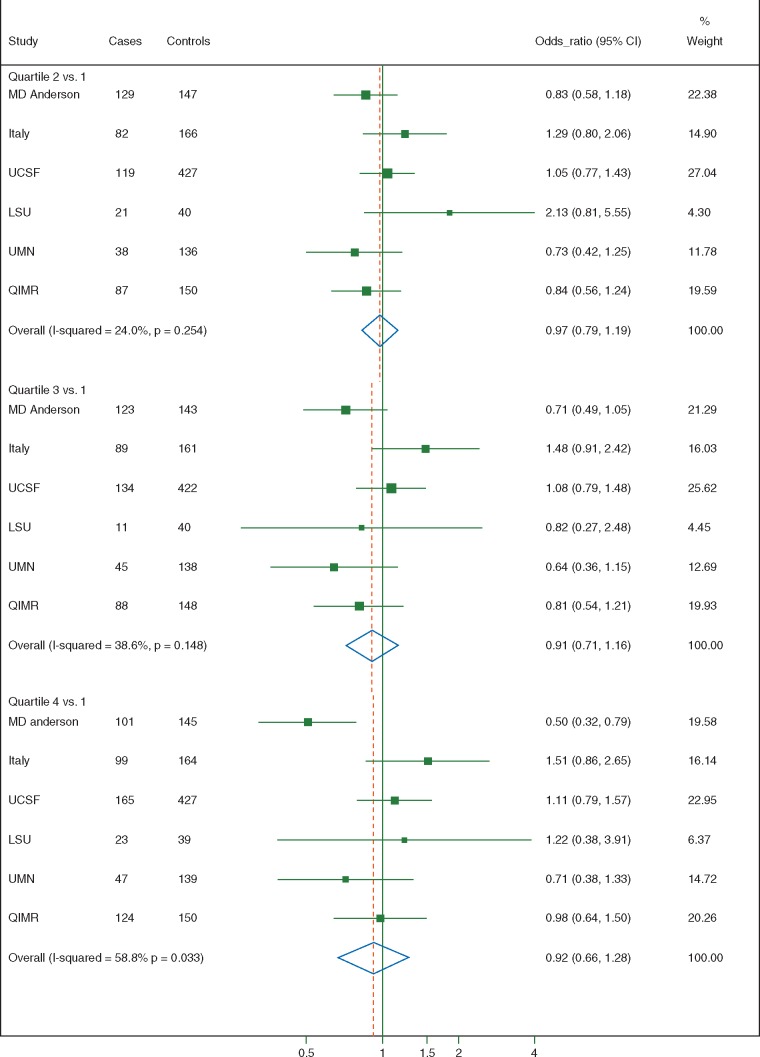
Figure 1 shows a forest plot, displaying the ORs and 95% CIs for each study and the pooled estimate, according to study-specific quartiles of acrylamide intake. Compared to the lowest study-specific quartile of dietary acrylamide intake, the pooled ORs were 0.97 (95% CI, 0.79–1.19) for the second, 0.91 (95% CI, 0.71–1.16) for the third and 0.92 (95% CI, 0.66–1.28) for the fourth (highest) quartile of intake. Heterogeneity of the risk estimates between participating studies was low for the second (I2 = 24%), low to moderate for the third (I2 = 39%) and moderate to high for the fourth quartile of intake (I2 = 59%) [28]. Four sensitivity analyses were conducted. In the first one, we pooled all studies into a single dataset and conducted an aggregate (one-stage) analysis. This gave an OR of 0.96 (95% CI, 0.80–1.16) for the highest versus lowest study-specific quartile of intake. The second one used the pooled dataset and calculated quartiles of intake based on the distribution in all controls, rather than being study-specific. This analysis found an OR of 0.98 (95% CI, 0.81–1.19) for the highest versus the lowest quartile. In the third one, we excluded one study at a time, and found ORs ranging from 0.84 (95% CI, 0.60–1.17, when excluding the Italian study) to 1.07 (95% CI, 0.86–1.33, when excluding the MD Anderson study) for the highest versus lowest quartile of intake. In the fourth one, we excluded all subjects that had any missing information in food items contributing to total acrylamide: the pooled OR for high versus low acrylamide intake, based on 1766 cases and 3885 controls, was 0.96 (95% CI, 0.74–1.26).
Figure 1.
Study-specific and pooled odds ratios (OR), and corresponding 95% confidence intervals (CI), of pancreatic cancer according to quartiles of acrylamide intake, with the lowest intake being the reference. International Pancreatic Cancer Case–Control Consortium (PanC4).
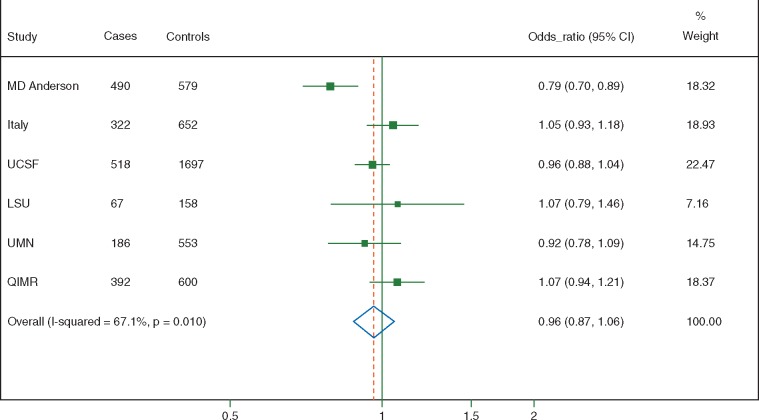
Figure 2 shows the ORs and the corresponding 95% CIs for each study and the pooled estimate for an increase of 10 µg/day of dietary acrylamide intake. The latter was 0.96 (95% CI, 0.87–1.06), with high heterogeneity between estimates (I2 = 67%). When studies were pooled into a single dataset (one-stage analysis), the OR for an increase of 10 µg/day of dietary acrylamide intake was 0.98 (95% CI, 0.93–1.02).
Figure 2.
Study-specific and pooled odds ratios (OR), and corresponding 95% confidence intervals (CI), of pancreatic cancer according to an increase of 10 µg/day in acrylamide intake. International Pancreatic Cancer Case–Control Consortium (PanC4).
Table 2 presents the results of analyses of high compared to low dietary acrylamide intake, stratified by sex, age, smoking habit, BMI, diabetes and study area. None of the summary estimates in any subgroup examined was significantly different from unity. No significant differences were observed between subgroups (all P values were >0.05).
Table 2.
Pooled odds ratios (OR), and corresponding 95% confidence intervals (CI), of pancreatic cancer for high versus low acrylamide intake, overall and in strata of selected covariates. International Pancreatic Cancer Case–Control Consortium (PanC4)
| Covariate | Low intake (1st quartile) |
High intake (4th quartile) |
OR (95% CI)a | P valueb | ||
|---|---|---|---|---|---|---|
| Cases, n (%) | Controls, n (%) | Cases, n (%) | Controls, n (%) | |||
| Overall | 450 (22.8) | 1057 (24.9) | 559 (28.3) | 1064 (25.1) | 0.92 (0.66–1.28) | – |
| Sex | ||||||
| Male | 207 (18.0) | 471 (20.2) | 375 (32.6) | 695 (29.8) | 0.78 (0.44–1.36) | |
| Female | 243 (29.4) | 586 (30.7) | 184 (22.3) | 369 (19.3) | 1.16 (0.67–2.01) | 0.32 |
| Age | ||||||
| <65 | 181 (19.0) | 469 (23.1) | 299 (31.3) | 549 (27.1) | 0.88 (0.51–1.51) | |
| ≥65 | 269 (26.3) | 588 (26.6) | 260 (25.5) | 515 (23.3) | 0.94 (0.72–1.23) | 0.83 |
| Smoking habit | ||||||
| Never smokers | 204 (27.7) | 528 (27.8) | 179 (24.3) | 416 (21.9) | 1.08 (0.79–1.47) | |
| Former cigarette smokers | 167 (22.4) | 414 (24.0) | 220 (29.4) | 459 (26.6) | 0.79 (0.50–1.23) | |
| Current cigarette smokers | 72 (16.2) | 95 (18.8) | 144 (32.5) | 164 (32.5) | 0.56 (0.22–1.46) | 0.29 |
| Body mass index (BMI)c | ||||||
| <30 kg/m2 | 315 (22.3) | 768 (24.8) | 402 (28.4) | 779 (25.2) | 0.93 (0.60–1.43) | |
| ≥30 kg/m2 | 75 (20.9) | 142 (24.7) | 107 (29.8) | 143 (24.9) | 0.90 (0.46–1.78) | 0.94 |
| Diabetes | ||||||
| No | 347 (22.2) | 957 (25.0) | 442 (28.3) | 953 (24.9) | 0.92 (0.65–1.30) | |
| Yesd | 96 (24.5) | 100 (24.4) | 111 (28.3) | 111 (27.1) | 0.79 (0.41–1.51) | 0.69 |
| Study area | ||||||
| USA (4 studies) | 305 (24.2) | 744 (24.9) | 336 (26.6) | 750 (25.1) | 0.80 (0.50–1.26) | |
| Other (2 studies) | 145 (20.3) | 313 (25.0) | 223 (31.2) | 314 (25.1) | 1.17 (0.77–1.77) | 0.23 |
ORs from multivariate logistic regression models adjusted for age, sex, race/ethnicity, education, smoking habits, diabetes, body mass index, and total energy intake.
P value for heterogeneity between strata.
Information was not available in the University of Minnesota study, thus, the OR estimates on acrylamide intake in strata of BMI are based on five studies.
OR estimate is based on five studies, since for the LSU study, there were too few diabetic patients for the model to converge.
Discussion
This large individual-level pooled-analysis from the PanC4 study reported no evidence of increased pancreatic cancer risk associated with estimated intake of acrylamide. Pooled risk estimates for increasing acrylamide intake were below unity, and none of the studies included showed significant positive associations between dietary acrylamide and pancreatic cancer. These findings were not substantively changed by a number of sensitivity analyses, and were consistent in strata of smoking habit and other individual-level and study-level covariates.
Only three cohort studies [29–31] and one case–control study (included in this investigation, too [32]) have previously reported results on acrylamide from diet in relation to the risk of pancreatic cancer. None showed increases in risk in participants with high compared to low acrylamide intake. The largest analysis, from the European Prospective Investigation into Cancer and Nutrition (EPIC) study, was based on 865 cases and reported ORs of 0.77 (95% CI, 0.58–1.04) for high versus low acrylamide and 0.95 (95% CI, 0.89–1.01) for each 10 µg/day increase in intake [30]. Overall, our results are consistent with those from a meta-analysis of the four previous studies [5], thus confirming in a larger dataset—and with the advantages of an individual data approach—the lack of an association between acrylamide intake and pancreatic cancer risk. With further reference to potential associations in specific population subgroups, an inverse relation emerged among obese subjects in the EPIC analysis [30]. This was not, however, confirmed in our dataset.
We computed the estimates of acrylamide intake using food frequency questionnaires combined with databases of mean content of acrylamide in foods. Still, the amount of acrylamide varies widely within different samples of the same food item, due to variations in cooking procedures (e.g. duration of cooking and temperature), food characteristics (e.g. potato variety) and storage, or product brand (e.g. for breakfast cereals and chips). Further, we estimated intakes in populations from different continents, enrolled during different time periods, and acrylamide food contents vary across geographic areas (e.g. higher values for white bread in Europe than in USA) and over the years. We tried to overcome these problems by using area-specific databases of acrylamide content in foods. As a consequence, a Chinese study of the PanC4 [16] was not included in this investigation, since area-specific information on acrylamide content in food was not available at the time of analysis. With reference to variations in acrylamide food contents over the years, manufacturers’ measurements reported a substantial downward trend since 2002, more for potato crisps/chips than for other foods [33]. None of the acrylamide-rich foods, and notably coffee, has in any case been associated with pancreatic cancer risk [34]. Early symptoms of disease might also have led to diet modifications in pancreatic cancer cases. Epidemiological studies using biomarkers of acrylamide exposure would be needed to overcome most of these limitations. Strengths of this study are its large size and the consortium, individual-level, data approach, with consequent availability of detailed and harmonized information for relevant covariates. Also, we performed various sensitivity analyses to assess the robustness of results, and no meaningful differences emerged.
Acrylamide may play a role in the aetiology of cancer through its oxidization to glycidamide, a chemically reactive genotoxic metabolite [35], and—for selected body sites, such as endometrial and ovarian cancer—by affecting hormonal balances in humans [36]. To date, however, epidemiological studies found no evidence of any relationship for cancers of the digestive organs [5]. Findings from this PanC4 pooled-analysis further support a lack of association between dietary acrylamide and pancreatic cancer.
Funding
The project was conducted thanks to funding from the Italian Ministry of Health, General Directorate of European and International Relations, and the Italian Foundation for Research on Cancer (FIRC). V.R. was supported by a fellowship from the Italian Foundation for Research on Cancer (FIRC #18107). R.E.N. is funded by a fellowship from the National Health and Medical Research Council (NHMRC, Australia). The Queensland Pancreatic Cancer Study was funded by a project grant from the NHMRC.
Disclosure
The authors have declared no conflicts of interest.
Supplementary Material
References
- 1. Hogervorst J, Duell E, Schouten L. et al. Reaction on the acrylamide and cancer review by Lipworth and colleagues. Eur J Cancer Prev 2013; 22: 194–198. [DOI] [PubMed] [Google Scholar]
- 2. Lipworth L, Sonderman JS, Tarone RE, McLaughlin JK.. Review of epidemiologic studies of dietary acrylamide intake and the risk of cancer. Eur J Cancer Prev 2012; 21: 375–386. [DOI] [PubMed] [Google Scholar]
- 3. Lipworth L, Sonderman JS, Tarone RE, McLaughlin JK.. Acrylamide: a human cancer risk?. Eur J Cancer Prev 2013; 22: 193–194. [DOI] [PubMed] [Google Scholar]
- 4. Pelucchi C, La Vecchia C, Bosetti C. et al. Exposure to acrylamide and human cancer–a review and meta-analysis of epidemiologic studies. Ann Oncol 2011; 22: 1487–1499. [DOI] [PubMed] [Google Scholar]
- 5. Pelucchi C, Bosetti C, Galeone C, La Vecchia C.. Dietary acrylamide and cancer risk: an updated meta-analysis. Int J Cancer 2015; 136: 2912–2922. [DOI] [PubMed] [Google Scholar]
- 6. Tareke E, Rydberg P, Karlsson P. et al. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J Agric Food Chem 2002; 50: 4998–5006. [DOI] [PubMed] [Google Scholar]
- 7. Marsh GM, Youk AO, Buchanich JM. et al. Mortality patterns among workers exposed to acrylamide: updated follow up. J Occup Environ Med 2007; 49: 82–95. [DOI] [PubMed] [Google Scholar]
- 8. Swaen GM, Haidar S, Burns CJ. et al. Mortality study update of acrylamide workers. Occup Environ Med 2007; 64: 396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bertuccio P, La Vecchia C, Silverman DT. et al. Cigar and pipe smoking, smokeless tobacco use and pancreatic cancer: an analysis from the International Pancreatic Cancer Case-Control Consortium (PanC4). Ann Oncol 2011; 22: 1420–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Talamini R, Polesel J, Gallus S. et al. Tobacco smoking, alcohol consumption and pancreatic cancer risk: a case-control study in Italy. Eur J Cancer 2010; 46: 370–376. [DOI] [PubMed] [Google Scholar]
- 11. Luckett BG, Su LJ, Rood JC, Fontham ET.. Cadmium exposure and pancreatic cancer in South Louisiana. J Environ Public Health 2012; 2012: 180186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hassan MM, Bondy ML, Wolff RA. et al. Risk factors for pancreatic cancer: case-control study. Am J Gastroenterol 2007; 102: 2696–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chan JM, Wang F, Holly EA.. Sweets, sweetened beverages, and risk of pancreatic cancer in a large population-based case-control study. Cancer Causes Control 2009; 20: 835–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tran B, Whiteman DC, Webb PM. et al. Association between ultraviolet radiation, skin sun sensitivity and risk of pancreatic cancer. Cancer Epidemiol 2013; 37: 886–892. [DOI] [PubMed] [Google Scholar]
- 15. Zhang J, Dhakal IB, Gross MD. et al. Physical activity, diet, and pancreatic cancer: a population-based, case-control study in Minnesota. Nutr Cancer 2009; 61: 457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ji BT, Chow WH, Dai Q. et al. Cigarette smoking and alcohol consumption and the risk of pancreatic cancer: a case-control study in Shanghai, China. Cancer Causes Control 1995; 6: 369–376. [DOI] [PubMed] [Google Scholar]
- 17. European Food Safety Agency (EFSA). Results on acrylamide levels in food from monitoring years 2007-2009 and exposure assessment. EFSA J 2011; 9: 2133. [Google Scholar]
- 18. Food and Drug Administration (FDA). Survey data on acrylamide in food: total diet study results. 2006. http://www.fda.gov/Food/FoodborneIllnessContaminants/ChemicalContaminants/ucm053566.htm (12 December 2016, date last accessed).
- 19. FAO/WHO. Summary and conclusions of the sixty-fourth meeting of the joint FAO/WHO Expert Committee on Food Additives (JECFA). Geneva: FAO/WHO; 2005. [Google Scholar]
- 20. Agence Française de Sécurité Sanitaire des Aliments (AFSSA). Acrylamide: point d’information n° 3. Maisons-Alfort: AFSSA 2005. http://www.anses.fr/sites/default/files/documents/RCCP2002sa0300b.pdf (12 December 2016, date last accessed).
- 21. Tateo F, Bononi M, Andreoli G.. Acrylamide levels in cooked rice, tomato sauces and some fast food on the Italian market. J Food Compos Anal 2007; 20: 232–235. [Google Scholar]
- 22. Sagratini G. HPLC–MS validation of QualisaFoo® biosensor kit for cost-effective control of acrylamide levels in Italian coffee. Food Control 2007; 18: 1267–1271. [Google Scholar]
- 23. Government of South Australia (GSA). A survey of acrylamide in non-carbohydrate based foods. Food Policy and Programs Branch, Public Health; Department of Health; Government of South Australia 2006.
- 24. Croft M, Tong P, Fuentes D, Hambridge T.. Australian survey of acrylamide in carbohydrate-based foods. Food Addit Contam 2004; 21: 721–736. [DOI] [PubMed] [Google Scholar]
- 25. Breslow NE, Day NE.. Statistical methods in cancer research. Vol 1: The analysis of case-control studies. Geneva: IARC Science Publication; 1980. [PubMed] [Google Scholar]
- 26. DerSimonian R, Laird N.. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 27. Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 1987; 9: 1–30. [DOI] [PubMed] [Google Scholar]
- 28. Higgins JP, Thompson SG, Deeks JJ, Altman DG.. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hogervorst JG, Schouten LJ, Konings EJ. et al. Dietary acrylamide intake is not associated with gastrointestinal cancer risk. J Nutr 2008; 138: 2229–2236. [DOI] [PubMed] [Google Scholar]
- 30. Obon-Santacana M, Slimani N, Lujan-Barroso L. et al. Dietary intake of acrylamide and pancreatic cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Ann Oncol 2013; 24: 2645–2651. [DOI] [PubMed] [Google Scholar]
- 31. Hirvonen T, Kontto J, Jestoi M. et al. Dietary acrylamide intake and the risk of cancer among Finnish male smokers. Cancer Causes Control 2010; 21: 2223–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pelucchi C, Galeone C, Talamini R. et al. Dietary acrylamide and pancreatic cancer risk in an Italian case–control study. Ann Oncol 2011; 22: 1910–1915. [DOI] [PubMed] [Google Scholar]
- 33. EFSA. Scientific Opinion on acrylamide in food. EFSA Panel on Contaminants in the Food Chain (CONTAM). EFSA J 2015; 13: 4104. [Google Scholar]
- 34.World Cancer Research Fund (WCRF) and American Institute for Cancer Research (AICR). Continuous Update Project Report. Food, Nutrition, Physical Activity, and the Prevention of Pancreatic Cancer, 2012. http://wcrf.org/int/research-we-fund/continuous-update-project-findings-reports/pancreatic-cancer (12 December 2016, date last accessed).
- 35. International Agency for Research on Cancer (IARC). Some industrial chemicals. IARC Monographs on the evaluation of carcinogenic risks to humans, Vol. 60. Lyon: IARC; 1994. [Google Scholar]
- 36. Hogervorst JG, Baars BJ, Schouten LJ. et al. The carcinogenicity of dietary acrylamide intake: a comparative discussion of epidemiological and experimental animal research. Crit Rev Toxicol 2010; 40: 485–512. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.