Abstract
Background
There is increasing evidence that metastatic colorectal cancer (mCRC) is a genetically heterogeneous disease and that tumours arising from different sides of the colon (left versus right) have different clinical outcomes. Furthermore, previous analyses comparing the activity of different classes of targeted agents in patients with KRAS wild-type (wt) or RAS wt mCRC suggest that primary tumour location (side), might be both prognostic and predictive for clinical outcome.
Methods
This retrospective analysis investigated the prognostic and predictive influence of the localization of the primary tumour in patients with unresectable RAS wt mCRC included in six randomized trials (CRYSTAL, FIRE-3, CALGB 80405, PRIME, PEAK and 20050181), comparing chemotherapy plus EGFR antibody therapy (experimental arm) with chemotherapy or chemotherapy and bevacizumab (control arms). Hazard ratios (HRs) and 95% confidence intervals (CIs) for overall survival (OS) and progression-free survival (PFS) for patients with left-sided versus right-sided tumours, and odds ratios (ORs) for objective response rate (ORR) were estimated by pooling individual study HRs/ORs. The predictive value was evaluated by pooling study interaction between treatment effect and tumour side.
Results
Primary tumour location and RAS mutation status were available for 2159 of the 5760 patients (37.5%) randomized across the 6 trials, 515 right-sided and 1644 left-sided. A significantly worse prognosis was observed for patients with right-sided tumours compared with those with left-sided tumours in both the pooled control and experimental arms for OS [HRs = 2.03 (95% CI: 1.69–2.42) and 1.38 (1.17–1.63), respectively], PFS [HRs = 1.59 (1.34–1.88) and 1.25 (1.06–1.47)], and ORR [ORs = 0.38 (0.28–0.50) and 0.56 (0.43–0.73)]. In terms of a predictive effect, a significant benefit for chemotherapy plus EGFR antibody therapy was observed in patients with left-sided tumours [HRs = 0.75 (0.67–0.84) and 0.78 (0.70–0.87) for OS and PFS, respectively] compared with no significant benefit for those with right-sided tumours [HRs = 1.12 (0.87–1.45) and 1.12 (0.87–1.44) for OS and PFS, respectively; P value for interaction <0.001 and 0.002, respectively]. For ORR, there was a trend (P value for interaction = 0.07) towards a greater benefit for chemotherapy plus EGFR antibody therapy in the patients with left-sided tumours [OR = 2.12 (1.77–2.55)] compared with those with right-sided tumours [OR = 1.47 (0.94–2.29)]. Exclusion of the unique phase II trial or the unique second-line trial had no impact on the results. The predictive effect on PFS may depend of the type of EGFR antibody therapy and on the presence or absence of bevacizumab in the control arm.
Conclusion
This pooled analysis showed a worse prognosis for OS, PFS and ORR for patients with right-sided tumours compared with those with left-sided tumours in patients with RAS wt mCRC and a predictive effect of tumour side, with a greater effect of chemotherapy plus EGFR antibody therapy compared with chemotherapy or chemotherapy and bevacizumab, the effect being greatest in patients with left-sided tumours. These predictive results should be interpreted with caution due to the retrospective nature of the analysis, which was carried out on subpopulations of patients included in these trials, and because none of these studies contemplated a full treatment sequence strategy.
Keywords: colorectal cancer, prognostic, predictive value, tumour side, anti-EGFR treatment, randomized trial
Introduction
Primary tumours arising from the left and right sides of the colon have distinct clinical and molecular characteristics [1–9], which may be a reflection of the differences in embryological origin of the normal tissue of the left and right sides of the colon. In particular, the proximal colon from the caecum to a point approximately half to two-thirds of the way along the transverse colon (right side) is derived from the embryonic midgut, whilst the distal third of the transverse colon to the rectum (left side) is derived from the embryonic hindgut. Consistent with this difference in embryological origin, gene expression microarray analysis has suggested that biopsies of adult colonic epithelium can be reliably classified on the basis of their gene expression profiles as being derived from either the right or left sides of the colon [10]. Certainly, the physiological functions of the right and left colon differ and exposure to nutrients and carcinogens varies. Also, the vascular support systems for the two sides of the colon are unique with the left and right sides of the colon being supported by the inferior and superior mesenteric arteries, respectively. However, the unique biological functions specific to the left and right sides of the colon are not fully understood and the pathways that initiate, control and maintain ‘sidedness’ remain to be fully defined [11].
The incidence rates of left- and right-sided colorectal cancers (CRC) also differ markedly, with approximately two-thirds of CRCs derived from the left side, and the remaining one-third from the right side [3]. Thus, left- and right-sided CRCs are increasingly being viewed as separate tumour types. Clinically, this is of paramount importance, as therapeutic regimens and treatment approaches may not be similarly effective across these two tumour types. This is a relatively new concept, and to date, primary tumour localization has not been a factor in guiding the selection of the most appropriate therapy for patients with metastatic CRC (mCRC). Also, predictive molecular signatures for CRC treatment efficacy are largely unknown, with RAS and maybe BRAF tumour mutation status currently the only guides for systemic therapy decision-making [12].
Clinically, right-sided tumours are more common in women, and are likely to be diploid, more commonly associated with poor prognostic indicators such as RAS and BRAF tumour mutations [13], microsatellite instability, CpG island methylator phenotype (CIMP)-high, mutagenic metabolites of cytochrome p450, MAPK signalling and mucinous histology [14, 15]. Left-sided tumours on the other hand are more common in men, more commonly associated with chromosomal instability, KRAS, DCC and P53 mutations, HER1 and HER2 gene amplification, aneuploidy and gene expression profiles consistent with sensitivity to EGFR-targeted antibody therapy [16, 17]. Also, hereditary non-polyposis CRC is more likely to develop on the right side of the colon, whilst familial adenomatous polyposis is associated with the development of tumours on the left side of the colon [14, 18].
Thus, patients with right-sided tumours are generally associated with a worse prognosis than those with left-sided tumours [16, 19–22] and endoscopically the appearance of right- and left-sided tumours is different [14]. Mucosal microbiota organization is also different with invasive bacterial aggregates (biofilms) identified almost universally (89%) on right-sided tumours [23].
A recent retrospective analysis of the impact of tumour location on clinical outcome in patients with chemotherapy-refractory KRAS wild-type (wt) mCRC from the NCICCTG CO.17 trial [24] showed the addition of cetuximab to best supportive care to significantly benefit patients with left-sided tumours in terms of progression-free survival (PFS) but not those patients with right-sided tumours, with a significant interaction between tumour location and treatment effect (P = 0.002) [24]. Other trials have now confirmed this observation for the predictive value of tumour location for patients with KRAS exon 2 wt, RAS wt and RAS wt/BRAF wt disease receiving EGFR antibody therapy as part of a systemic therapy approach [25–30]. Also, although an initial trial [31] suggested that the addition of the VEGF-targeted agent bevacizumab to chemotherapy may also primarily benefit patients with left-sided tumours, this was not supported by the data of Loupakis et al. [16]. Furthermore, the results of a recent prospective trial suggested that the efficacy outcomes for bevacizumab were greatest in patients with right-sided tumours [32].
Thus, the current pooled analysis was principally designed to study the prognostic and predictive effects of tumour side on overall survival (OS), PFS and objective response rate (ORR) in patients with RAS wt mCRC who had received first-line or second-line chemotherapy with or without EGFR-targeted monoclonal antibodies in six randomized trials. The influence on the results of line of therapy and inclusion or not of bevacizumab in the control arm was also investigated.
Methods
The present pooled analyses are based on data from the randomized CRYSTAL (NCT00154102) [33], PRIME (NCT00364013) [34], PEAK (NCT00819780) [35], FIRE-3 (NCT00433927) [36, 37], CALGB 80405 (NCT00265850) [38] and 20050181 (NCT00339183) [39] trials in patients with mCRC, based on the details of publications and slide presentations presented at an ESMO Special Symposium held as part of the ESMO 2016 Annual Conference on 10 October 2016 in Copenhagen and at the ESMO Asia 2016 meeting in Singapore on 18 December 2016. As this is a symposium report, the trials included were based on the presented trial data and the associated available data for the presented trials. The COIN [40], OPUS [41] and Chinese phase III [42] trials were excluded because no data were available with regard to primary tumour localization.
Trials
The methodological and study details for all six trials have already been published and/or presented extensively. Briefly, in the CRYSTAL and PRIME trials patients with a first occurrence of ‘unresectable’ mCRC were randomly assigned to receive first-line chemotherapy with FOLFIRI ± cetuximab or FOLFOX4 ± panitumumab, respectively [33, 34], whilst the PEAK and FIRE-3 trials investigated FOLFOX6 plus either panitumumab or bevacizumab and FOLFIRI plus either cetuximab or bevacizumab, respectively, in patients with previously untreated, unresectable mCRC [35, 36]. The CALGB 80405 trial investigated chemotherapy (FOLFIRI or FOLFOX6, investigator’s choice) in combination with either cetuximab or bevacizumab in patients with previously untreated mCRC [38], whilst the 20050181 trial, the only one of the six trials to investigate treatment in the second-line setting, investigated FOLFIRI plus or minus panitumumab [39]. The latter second-line trial was included to look at the effect of primary tumour localization independently of treatment line.
In all six trials patients were deemed to be treated until disease progression or unacceptable toxicity. The trial designs, treatment regimens, eligibility criteria and RAS (and BRAF) mutational analyses for all six trials, have previously been reported in detail [33–36, 38, 39, 43–50]. All six trials had received approval from the relevant ethics committees and were conducted in accordance with the Declaration of Helsinki. All patients provided informed consent before their participation.
Patients
Patients with RAS wt (KRAS exon 2–4 wt; NRAS exon 2–4 wt) disease, from the randomized CRYSTAL, PRIME, PEAK, FIRE-3, CALGB 80405 and 20050181 trials, were selected for analysis based on their tumour location as recorded /or reported in the individual patient case report forms of their respective trials. Primary tumours originating in the appendix, caecum, ascending colon, hepatic flexure and transverse colon were classified as right-sided, except in the case of the CALGB 80405 trial where tumours in the transverse colon were omitted from the analysis. Primary tumours originating in the splenic flexure, descending colon, sigmoid colon and rectum were classified as left-sided in all six trials. If tumours within an individual patient were sited in both left-sided and right-sided locations and the origin could not be ascribed to either side, the patient was excluded from the analysis.
Endpoints
The endpoints investigated were OS, PFS and ORR. The primary endpoint for the CRYSTAL, PRIME and PEAK trials was PFS, for the FIRE-3 trial was ORR (complete and partial responses), for the 20050181 trial was PFS and OS and for the CALGB 80405 OS. OS and PFS were defined as the time from randomization to death and to disease progression or death from any cause, respectively. Objective response was assessed according to RECIST version 1.0 and centrally reviewed except for patients in the CALGB 80405 trial.
Data collection
Analyses were based on aggregated retrospective data [hazard ratio (HR) or odds ratio (OR)] comparing outcome by tumour side in each arm or treatment arms by tumour side in patients with confirmed RAS wt primary tumours, extracted from publications or meeting slides, or provided by the investigators. These data were validated by the investigators before the analyses.
Statistical analysis
All the individual trial analyses were retrospective. For both prognostic and predictive analyses, OS, PFS and ORR according to treatment arm were assessed in the patient subgroups defined by their RAS wt tumour status, according to whether the primary tumours were right-sided or left-sided.
All variables associated with tumour localization (tumour sidedness) were investigated. The endpoint definitions used in the current analysis were identical to those used in the original clinical studies. The prognostic value of tumour side was studied by comparing patient outcome in patients with right-sided or left-sided RAS wt tumours using the HRs and ORs in the experimental arm and control arms separately. The predictive value of tumour side was studied by comparing the HRs or ORs of the chemotherapy plus EGFR antibody therapy (experimental) arm versus the control arm, which was either chemotherapy alone or chemotherapy plus bevacizumab. HRs were adjusted according to covariates, but were variable taking into account the differences between studies. The ORs were not adjusted. A HR for treatment (predictive) effect of <1 and an OR of >1 favour the EGFR antibody-containing experimental arm. A HR for prognostic effect >1 and an OR <1 indicates a worse prognosis for right-sided tumours. A HR/OR of interaction, which is used to summarise the predictive effect, is the ratio of the HR or OR for the treatment effect, i.e. right-sided divided by left-sided.
The pooling of the HRs/ORs was based on a two-step analysis corresponding to stratified Cox proportional hazards and logistic regression models, respectively, with fixed effect models. Heterogeneity was evaluated using the Cochrane test (P < 0.10) and I2 [51]. In the case of heterogeneity a random effect model was used.
For the predictive analysis, treatment interaction tests (likelihood ratio test within Cox proportional hazards and logistic regression models, respectively) were used to assess the difference in HRs and ORs for patients with left-sided and right-sided tumours. The HRs/ORs of interaction were pooled as proposed by Fisher et al. [52].
To investigate the impact of the main differences in study characteristics (Table 1) on the results, the following strategies were adopted. To investigate the impact of study phase and treatment line, sensitivity analyses excluding the only phase II study, and the only study of second-line treatment, respectively, were carried out. To compare the studies according to the choice of, EGFR antibody therapy (cetuximab versus panitumumab) and treatment modalities in the control arm (chemotherapy alone or chemotherapy plus bevacizumab), the results of the two groups of studies (subsets) defined by each of these characteristics were analysed separately and compared by interaction tests. All tests were both sided and all statistical analyses were carried out using SAS version 9.3 software (SAS Institute, Cary, NC).
Table 1.
Source of patients for the analyses
| Trial name | Trial characteristics |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phase of trial | Chemo-therapy backbone | Bevacizumab in control arm? | Anti-EGFR therapy | Treatment line | Randomized | With KRAS evaluable | With KRAS Wta | With all RAS wt | With all RAS wt and tumour side confirmed | |
| CRYSTAL [28, 45, 46] | III | FOLFIRI | No | Cetuximab | 1st | 1217 | 1063 | 666 | 367b | 364 |
| FIRE-3 [28, 36] | III | FOLFIRI | Yes | Cetuximab | 1st | 752 | NA | 609£ | 400c | 394 |
| PRIME [43, 44] | III | FOLFOX4 | No | Panitumumab | 1st | 1183 | 1096 | 656 | 512 | 416 |
| PEAK [35] | II | FOLFOX6 | Yes | Panitumumab | 1st | 285 | 285 | 285 | 170d | 143 |
| CALGB 80405 [27, 38] | III | FOLFIRI/ FOLFOX6 | Yes | Cetuximab | 1st | 1137 | 1137 | 1137 | 526e | 474 |
| 20050181 [48] | III | FOLFIRI | No | Panitumumab | 2nd | 1186 | 1083 | 597 | 421 | 368 |
Exon 2 (CRYSTAL, FIRE-3) sometimes from available tissue to test (CALGB 80405).
Not always easy to determine, taken from publication or slide presentation. Sometimes refers to all ITT population (PRIME, PEAK, AMGEN181) sometimes from the KRAS wt.
Only 430 patients were assessable for other RAS mutations.
A total of 475 patients were tested successfully for the other KRAS mutations.
Extended RAS analysis was carried out in 250 patients with 233 patients with KRAS or RAS results. Out of the 221 patients with KRAS exon 2 wt at this stage, 170 were RAS wt.
Out of 670 patients tested for all RAS; £592 patients if only those receiving study treatment are considered and 493 patients if only those receiving study treatment and had assessable CT-scan are considered.
EGFR, epidermal growth factor receptor; NA, not available; wt, wild-type.
Results
Of the 5760 patients originally randomized across the 6 trials, a total of 2159 patients (37.6%) with RAS wt mCRC and defined primary tumour location were included in the overall analysis (Figure 1). These comprised: 364 from the CRYSTAL trial, 416 from the PRIME trial, 143 from the PEAK trial, 394 from FIRE-3, 474 from the CALGB 80405 trial and 368 from the second-line 20050181 trial (Table 1). Of these, 515 (23.9%) were right-sided. Patient characteristics according to tumour location for the six trials are presented in supplementary Tables S1 and S2, available at Annals of Oncology online. The efficacy outcome data for the individual trials will be presented below followed by the results of the pooled analyses.
Figure 1.
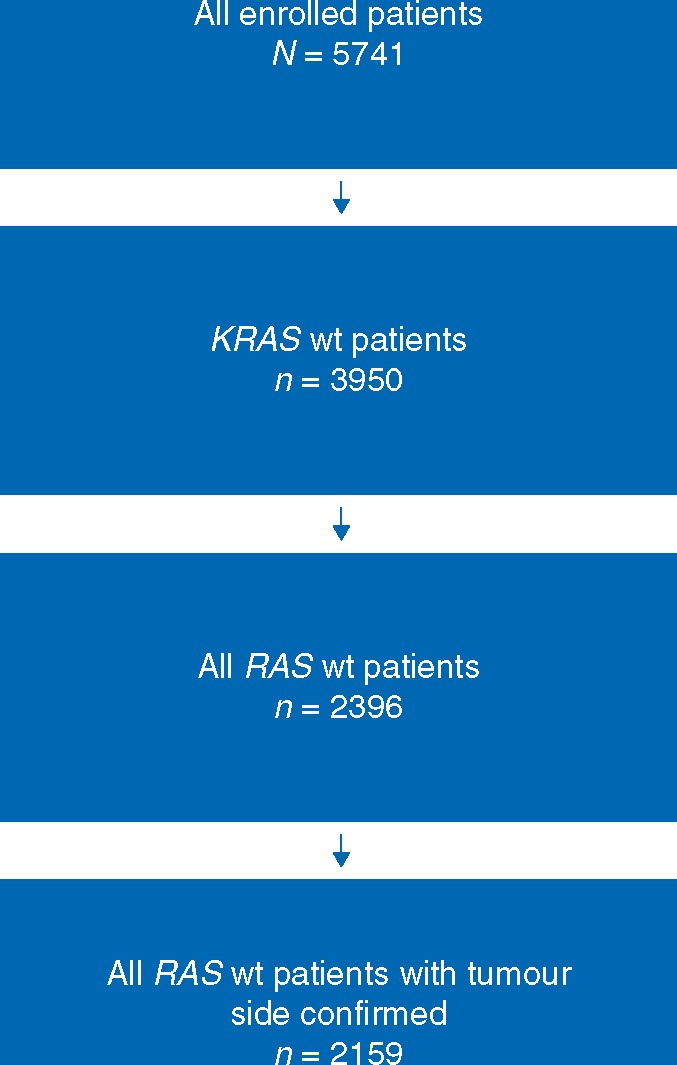
FLOW chart showing the origin of the 2159 patients with RAS wt mCRC from six randomized trials used to investigate the prognostic and predictive significance of right-sided versus left-sided tumour location on treatment outcomes in patients treated with chemotherapy plus EGFR-targeted antibody therapies or chemotherapy ± bevacizumab; wt, wild-type.
Efficacy outcomes according to treatment arm and primary tumour location
Panitumumab trials
There were differences in baseline characteristics between patients with right-sided tumours in the two treatment arms of the PRIME trial in terms of liver involvement, and in the PEAK trial in terms of Eastern Cooperative Oncology Group performance status (ECOG PS) and liver-limited disease (LLD) (supplementary Table S1, available at Annals of Oncology online). Additionally, in the PEAK trial the incidence of BRAF mutant tumours was 40.9% and 7.1% for right-sided patients in the FOLFOX6 plus panitumumab and FOLFOX6 plus bevacizumab arms, respectively. In the PRIME trial more patients with left-sided tumours had LLD in both treatment arms and in the PEAK trial more patients with left-sided tumours had an ECOG PS of 0 in the panitumumab arm (supplementary Table S1, available at Annals of Oncology online). There were no marked differences in the baseline characteristics between those patients with right-sided and those with left-sided disease in the 20050181 second-line trial (supplementary Table S2, available at Annals of Oncology online).
The prognostic HRs for OS in the chemotherapy plus panitumumab arm according to primary tumour location, right- versus left-sided, were 1.58 (1.02–2.45), 2.68 (1.31–5.46) and 2.01 (1.29–3.13) for the PRIME, PEAK and 20050181 trials, respectively. In both the first- and second-line settings patients with right-sided tumours had a worse prognosis irrespective of the treatment received (Table 2). For all three trials the treatment outcomes were better (numerically higher) in patients with left-sided tumours compared with those with right-sided tumours for OS and ORR (Table 3). This was true also for PFS in the PRIME and 20050181 trials. In patients with left-sided tumours panitumumab in combination with chemotherapy appears to be significantly superior to chemotherapy alone in the PRIME trial for OS, PFS and ORR and in the 20050181 trial for ORR, and numerically superior to bevacizumab in combination with chemotherapy in terms of clinical outcomes in the PEAK trial, but this was not true in the case of patients with right-sided tumours (Table 3).
Table 2.
Prognostic results for RAS wild-type PRIME, PEAK and 20050181 panitumumab trial patients, according to treatment
| PRIME |
PEAK |
20050181 (second-line setting) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FOLFOX4 |
FOLFOX4+panitumumab |
FOLFOX6+bevacizumab |
FOLFOX6+panitumumab |
FOLFIRI |
FOLFIRI+panitumumab |
|||||||
|
N=208 |
N=208 |
N=68 |
N=75 |
N=187 |
N=181 |
|||||||
| Parameter | Right- sided tumours | Left- sided tumours | Right- sided tumours | Left- sided tumours | Right- sided tumours | Left- sided tumours | Right- sided tumours | Left- sided tumours | Right- sided tumours | Left- sided tumours | Right- sided tumours | Left- sided tumours |
| n=49 | n=159 | n=39 | n=169 | n=14 | n=54 | n=22 | n=53 | n=39 | n=148 | n=31 | n=150 | |
| OS | ||||||||||||
| Median, months | 15.4 | 23.6 | 11.1 | 30.3 | 21.0 | 32.0 | 17.5 | 43.4 | 8.1 | 16.6 | 10.3 | 20.1 |
| HR (95% CI)a | 1.27 (0.88–1.83) | 1.58 (1.02–2.45) | 2.86 (1.40–5.84) | 2.68 (1.31–5.46) | 1.51 (0.96–2.37) | 2.01 (1.29–2.13) | ||||||
| P value | 0.20 | 0.04 | 0.004 | 0.007 | 0.07 | 0.002 | ||||||
| PFS | ||||||||||||
| Median, months | 7.0 | 9.2 | 7.5 | 12.9 | 12.6 | 11.5 | 8.7 | 14.6 | 2.4 | 5.8 | 4.8 | 8.0 |
| HR (95% CI)a | 1.19 (0.82–1.74) | 1.20 (0.79–1.81) | 1.20 (0.63–2.30) | 1.61 (0.83–3.12) | 1.36 (0.89–2.09) | 1.40 (0.92–2.13) | ||||||
| P value | 0.36 | 0.40 | 0.58 | 0.16 | 0.16 | 0.12 | ||||||
| ORR | ||||||||||||
| Rate, % | 34.8 | 52.6 | 42.1 | 67.9 | 50.0 | 57.4 | 63.6 | 64.2 | 2.6 | 13.2 | 13.3 | 49.7 |
| Odds ratio (95% CI) | 0.48 (0.26–0.90) | 0.34 (0.18–0.65) | 0.74 (0.27–2.04) | 0.98 (0.44–2.17) | 0.18 (0.02–1.37) | 0.16 (0.05–0.46) | ||||||
| P value | 0.02 | <0.001 | 0.56 | 0.96 | 0.10 | <0.001 | ||||||
HRs were adjusted for BRAF status, adjuvant chemotherapy and ECOG PS.
CI, confidence interval; FOLFIRI, fluorouracil, leucovorin and irinotecan; FOLFOX, fluorouracil, leucovorin and oxaliplatin; HR, hazard ratio; NA, not available; ORR, objective response rate; OS, overall survival; PFS, progression-free survival.
Table 3.
Efficacy results for RAS wild-type PRIME, PEAK and 20050181 panitumumab trial patients according to primary tumour location
| PRIME (RAS wt) |
PEAK (RAS wt/BRAF wt) |
20050181 (second-line setting) (RAS wt) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Right-sided N=88 |
Left-sided N=328 |
Right-sided N=36 |
Left-sided N=107 |
Right-sided N=70 |
Left-sided N=298 |
|||||||
| Parameter | FOLFOX4 | FOLFOX4 +pani | FOLFOX4 | FOLFOX4 +pani | FOLFOX6 +bev | FOLFOX6 +pani | FOLFOX6 +bev | FOLFOX6 +pani | FOLFIRI | FOLFIRI +pani | FOLFIRI | FOLFIRI +pani |
| n = 49 | n = 39 | n = 159 | n = 169 | n = 14 | n = 22 | n = 54 | n = 53 | n = 39 | n = 31 | n = 148 | n = 150 | |
| OS | ||||||||||||
| Median, months | 15.4 | 11.1 | 23.6 | 30.3 | 21.04 | 17.4 | 32.0 | 43.4 | 8.1 | 10.3 | 16.6 | 20.1 |
| HR (95.5% CI)a | 0.87 (0.55–1.37) | 0.73 (0.57–0.93) | 0.67 (0.30–1.50) | 0.77 (0.46–1.28) | 1.14 (0.68–1.89) | 0.96 (0.75–1.23) | ||||||
| P value | 0.55 | 0.012 | 0.32 | 0.31 | 0.62 | 0.75 | ||||||
| P value for interaction | 0.51 | 0.77 | 0.55 | |||||||||
| PFS | ||||||||||||
| Median, months | 7.0 | 7.5 | 9.2 | 12.9 | 12.6 | 8.7 | 11.5 | 14.6 | 2.4 | 4.8 | 5.8 | 8.0 |
| HR (95.5% CI)a | 0.80 (0.51–1.26) | 0.72 (0.57–0.90) | 1.04 (0.50–2.18) | 0.68 (0.45–1.04) | 0.75 (0.45–1.27) | 0.88 (0.69–1.12) | ||||||
| P value | 0.33 | 0.005 | 0.91 | 0.07 | 0.28 | 0.30 | ||||||
| P value for interaction | 0.68 | 0.32 | 0.58 | |||||||||
| ORR | ||||||||||||
| Rate, % | 34.8 | 42.1 | 52.6 | 67.9 | 50.0 | 63.6 | 57.4 | 64.1 | 2.6 | 13.3 | 13.2 | 49.7 |
| OR (95.5% CI) | 1.36 (0.60–3.08) | 1.91 (1.33–2.72) | 1.75 (0.57–5.41) | 1.33 (0.72–2.46) | 5.69 (0.60–53.63) | 6.49 (3.73–11.30) | ||||||
| P value | 0.46 | <0.001 | 0.33 | 0.37 | 0.13 | <0.001 | ||||||
| P value for interaction | 0.46 | 0.67 | 0.91 | |||||||||
HRs were adjusted for BRAF status, adjuvant chemotherapy and ECOG PS.
Bev, bevacizumab; CI, confidence interval; FOLFIRI, fluorouracil, leucovorin and irinotecan; FOLFOX, fluorouracil, leucovorin and oxaliplatin; HR, hazard ratio; OR, odds ratio: ORR, objective response rate; OS, overall survival; pani, panitumumab; PFS, progression-free survival; wt, wild-type.
Cetuximab trials
Numerical differences in patient baseline characteristics were also observed in the CRYSTAL, FIRE-3 and CALGB 80405 trials. In the CRYSTAL trial, fewer patients with right-sided tumours in the FOLFIRI plus cetuximab arm had an ECOG PS of 0 and more had received prior adjuvant therapy. Also, patients with right-sided tumours treated with FOLFIRI plus cetuximab less frequently received second-line therapy than patients with right-sided tumours receiving FOLFIRI alone (data not shown). More patients with right-sided tumours in the FIRE-3 trial had an ECOG PS of 0 in the FOLFIRI plus bevacizumab arm than in the cetuximab arm (supplementary Table S1, available at Annals of Oncology online) [28]. In the CALGB 80405 trial more patients with left-sided tumours had the primary in place, more had liver-only disease and more had liver metastases but less extrahepatic disease than those patients with right-sided tumours (supplementary Table S1, available at Annals of Oncology online).
In terms of prognosis, patients with left-sided primary tumours were superior to those with right-sided tumours in the CRYSTAL and FIRE-3 trials for the different treatment combinations although, it was less pronounced for the FOLFIRI plus bevacizumab arm than for the FOLFIRI plus cetuximab arm in the FIRE-3 trial [28]. In the case of patients in the CRYSTAL trial receiving FOLFIRI plus cetuximab this difference between patients with right- and left-sided tumours was statistically significant (Table 4). In the CALGB 80405 trial clinical outcomes were consistently superior for PFS and OS in patients with left-sided tumours compared with those with right-sided tumours (Table 4). The difference in median OS between patients with right-and left-sided tumours treated with chemotherapy plus cetuximab was 25.7 months in favour of those with left-sided primaries with a statically significant HR of 1.82 (adjusted P value of 0.001).
Table 4.
Prognostic results for RAS wild-type CRYSTAL, FIRE-3 and CALGB 80405 cetuximab trial patients, according to treatment
| CRYSTAL |
FIRE-3 |
CALGB 80405 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FOLFIRI |
FOLFIRI+cetuximab |
FOLFIRI+bevacizumab |
FOLFIRI+cetuximab |
FOLFIRI/FOLFOXa+bevacizumab |
FOLFIRI/FOLFOXa+cetuximab |
|||||||
|
N=189 |
N=175 |
N=199 |
N=195 |
N=230 |
N=244 |
|||||||
| Parameter | Right- sided tumours | Left- sided tumours | Right- sided tumours | Left- sided tumours | Right- sided tumours | Left- sided tumours | Right- sided tumours | Left- sided tumours | Right- sided tumours | Left- sided tumours | Right- sided tumours | Left- sided tumours |
| n = 51 | n = 138 | n = 33 | n = 142 | n = 50 | n = 149 | n = 38 | n = 157 | n = 78 | n = 152 | n = 71 | n = 173 | |
| OS | ||||||||||||
| Median, months | 15.0 | 21.7 | 18.5 | 28.7 | 23.0 | 28.0 | 18.3 | 38.3 | 29.2 | 32.6 | 13.6 | 39.3 |
| HR (95% CI) | 1.35 (0.93–1.97) | 1.93 (1.24–2.99) | 1.48 (1.02–2.16) | 2.84 (1.86–4.33) | 1.14 (0.80–1.61) | 1.82 (1.27–2.56) | ||||||
| P value | 0.12 | 0.003 | 0.04 | <0.001 | 0.47b | <0.001b | ||||||
| PFS | ||||||||||||
| Median, months | 7.1 | 8.9 | 8.1 | 12.0 | 9.0 | 10.7 | 7.6 | 10.7 | 10.2 | 11.2 | 7.5 | 12.7 |
| HR (95% CI) | 1.54 (0.96–2.46) | 1.77 (1.08–2.91) | 1.38 (0.99–1.94) | 2.00 (1.36–2.93) | 1.01 (0.73–1.41) | 1.64 (1.19–2.22) | ||||||
| P value | 0.07 | 0.02 | 0.06 | <0.001 | 0.95b | 0.002b | ||||||
| ORR | ||||||||||||
| Rate, % | 33.3 | 40.6 | 42.4 | 72.5 | 50.0 | 61.7 | 52.6 | 68.8 | 39.7 | 57.9 | 42.3 | 69.4 |
| Odds ratio (95% CI) | 0.73 (0.39–1.38) | 0.28 (0.13–0.61) | 0.62 (0.36–1.07) | 0.51 (0.25–1.03) | 0.48 (0.29–0.78) | 0.32 (0.20–0.53) | ||||||
| P value | 0.33 | 0.001 | 0.09 | 0.06 | 0.003 | <0.001 | ||||||
Investigator choice.
Adjusted for treatment arm, protocol chemotherapy, prior adjuvant therapy, prior radiotherapy, age, sex, synchronous disease, in place primary, liver metastases.
CI, confidence interval; FOLFIRI, fluorouracil, leucovorin and irinotecan; FOLFOX, fluorouracil, leucovorin and oxaliplatin; HR, hazard ratio; ORR, objective response rate; OS, overall survival; PFS, progression-free survival.
As for the panitumumab trials, the location of a patient’s primary tumour was also associated with a difference in treatment effect. In the CRYSTAL trial the addition of cetuximab to FOLFIRI in the treatment of patients with RAS wt left-sided tumours was associated with a significant improvement in OS, PFS and ORR (Table 5) [28]. In contrast, no benefit from the addition of cetuximab to FOLFIRI was observed in terms of treatment outcome in patients with right-sided tumours (Table 5) [28]. In the FIRE-3 trial patients with RAS wt left-sided tumours treated with FOLFIRI plus cetuximab benefited significantly in terms of OS and PFS compared with patients with right-sided tumours (Table 4). Indeed in the case of patients in the FIRE-3 trial receiving FOLFIRI plus cetuximab there was a dramatic 20-month difference in median OS favouring patients with left-sided tumours over those with right-sided tumours (Tables 4 and 5). Furthermore, patients with left-sided tumours treated with FOLFIRI plus cetuximab had a significantly longer median OS than patients with left-sided tumours treated with FOLFIRI plus bevacizumab (HR = 0.63; P = 0.002), whilst a non-significant numerical advantage in ORR was observed (Table 5). For patients with RAS wt right-sided tumours no significant differences in ORR, PFS or OS were observed, for those patients treated with FOLFIRI plus cetuximab versus those treated with FOLFIRI plus bevacizumab (Table 5). Post hoc statistical modelling confirmed a significant interaction between primary tumour location and treatment of OS and PFS but not ORR for the CRYSTAL trial and OS, but not PFS or ORR for the FIRE-3 trial [28]. In the CALGB 80405 trial cetuximab and bevacizumab were also shown to have different treatment effects according to primary tumour localization with primary tumour localization predictive for treatment outcome (Table 5).
Table 5.
Efficacy results for RAS wild-type CRYSTAL, FIRE-3 and CALGB 80405 cetuximab trial patients, according to tumour location
|
CRYSTAL |
FIRE-3 |
CALGB 80405 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Right-sided tumours N=84 |
Left-sided tumours N=280 |
Right-sided tumours N=88 |
Left-sided tumours N=306 |
Right-sided tumours N=149 |
Left-sided tumours N=325 |
|||||||||
| Parameter | FOLFIRI | FOLFIRI +cetux | FOLFIRI | FOLFIRI +cetux | FOLFIRI +bev | FOLFIRI +cetux | FOLFIRI +bev | FOLFIRI +cetux | CTa+bev | CTa+cetux | CTa+bev | CTa+cetux | ||
| n = 51 | n = 33 | n = 138 | n = 142 | n = 50 | n = 38 | n = 149 | n = 157 | n = 78 | n = 71 | n = 152 | n = 173 | |||
| OS | ||||||||||||||
| Median, months | 15.0 | 18.5 | 21.7 | 28.7 | 23.0 | 18.3 | 28.0 | 38.3 | 29.2 | 13.7 | 32.6 | 39.3 | ||
| HR (95% CI) | 1.08 (0.65–1.81) | 0.65 (0.50–0.86) | 1.31 (0.81–2.11) | 0.63 (0.48–0.85) | 1.36 (0.93–1.99) | 0.77 (0.59–0.99) | ||||||||
| P value | 0.77 | 0.002 | 0.27 | 0.002 | 0.11b | 0.05b | ||||||||
| P value for interaction | 0.09 | 0.01 | 0.02 | |||||||||||
| PFS | ||||||||||||||
| Median, months | 7.1 | 8.1 | 8.9 | 12.0 | 9.0 | 7.6 | 10.7 | 10.7 | 10.2 | 7.5 | 11.2 | 12.7 | ||
| HR (95% CI) | 0.87 (0.47–1.62) | 0.50 (0.34–0.72) | 1.44 (0.92–2.26) | 0.90 (0.71–1.14) | 1.64 (1.15–2.36) | 0.84 (0.66–1.06) | ||||||||
| P value | 0.66 | <0.001 | 0.11 | 0.38 | 0.007b | 0.15b | ||||||||
| P value for interaction | 0.13 | 0.07 | 0.002 | |||||||||||
| ORR | ||||||||||||||
| Rate, % | 33.3 | 42.4 | 40.6 | 72.5 | 50.0 | 52.6 | 61.7 | 68.8 | 39.7 | 42.3 | 57.9 | 69.4 | ||
| Odds ratio (95% CI) | 1.45 (0.58–3.64) | 3.99 (2.40–6.62) | 1.11 (0.48–2.59) | 1.37 (0.85–2.19) | 1.11 (0.61–2.01)b | 1.65 (1.16–2.34)b | ||||||||
| P value | 0.43 | <0.001 | 0.81 | 0.19 | 0.73 | 0.005 | ||||||||
| P value for interaction | 0.06 | 0.67 | 0.26 | |||||||||||
Investigator choice of FOLFIRI/FOLFOX.
Adjusted for treatment arm, protocol chemotherapy, prior adjuvant therapy, prior radiotherapy, age, sex, synchronous disease, in place primary, liver metastases.
Bev, bevacizumab; cetux, cetuximab; CI, confidence interval; CT, chemotherapy; FOLFIRI, fluorouracil, leucovorin and irinotecan; FOLFOX, fluorouracil, leucovorin and oxaliplatin; HR, hazard ratio; ORR, objective response rate; OS, overall survival; PFS, progression-free survival.
Prognostic role of tumour location for patients receiving either chemotherapy alone or chemotherapy plus bevacizumab (control arm): pooled analysis
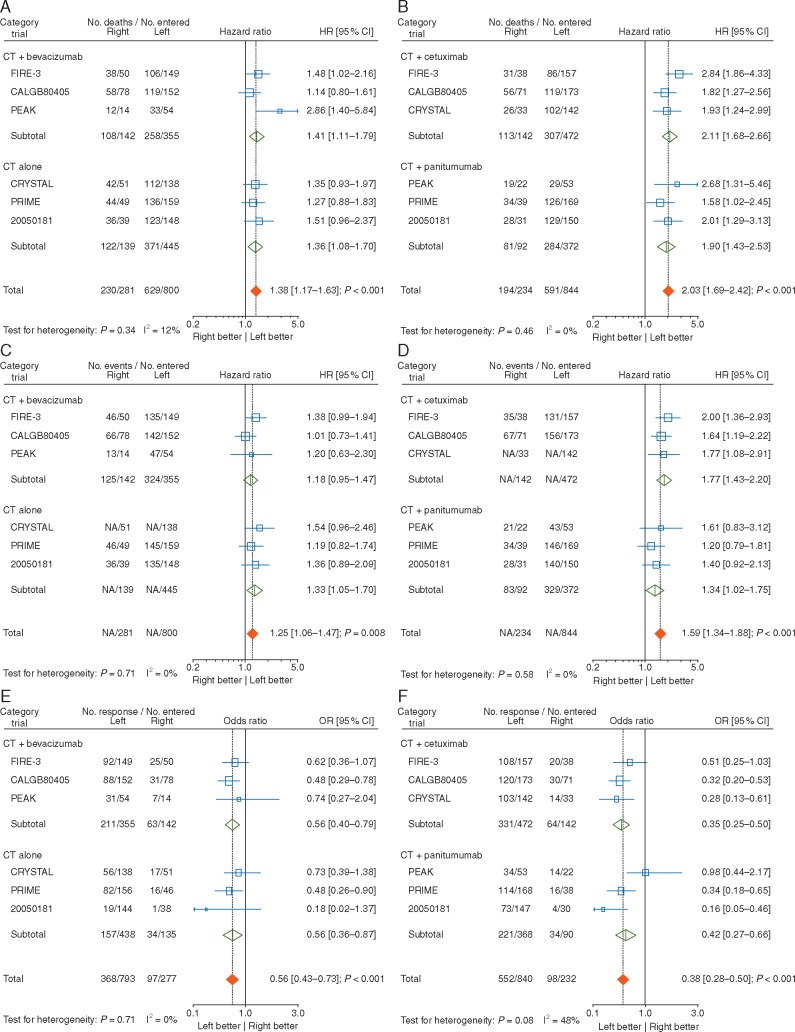
The pooled analyses (Figures 2A, C and E; Table 6), showed the overall HR for OS to be 1.38 [1.17–1.63] (P < 0.001) in the absence of any significant heterogeneity between the trials (P = 0.34; I2 = 12%) showing a negative prognostic effect of right-sided tumour location, but less pronounced than in the experimental arm. The results for PFS were similar with an overall HR of 1.25 [1.06–1.47] (P = 0.008; P value for heterogeneity = 0.71; I2 = 0%). Results for ORR also favoured left-sided tumour location with an overall OR of 0.56 [0.43–0.73] (P < 0.001), in the absence of inter study heterogeneity (P = 0.71; I2 = 0%).
Figure 2.
Forest plots for the prognostic analyses of tumour location (right versus left side) in the control and experimental arms (chemotherapy plus EGFR antibody therapy) for—overall survival, (A) and (B), respectively, progression-free survival, (C) and (D), respectively, and objective response rate, (E) and (F), respectively. CI, confidence interval; EGFR, epidermal growth factor receptor; HR, hazard ratio; NA, not available; OR, odds ratio.
Table 6.
Trials’ subset and sensitivity analyses for prognostic analysis in control arm for OS, PFS and ORR
| Main analysis | Trials’ subset analysis |
Sensitivity analyses |
|||
|---|---|---|---|---|---|
| Bevacizumab in control arm |
Only phase III (without PEAK) | Only first line (without 20050181) | |||
| Parameter | Yes | No | |||
| OS | |||||
| HR (95% CI) | 1.38 (1.17–1.63) | 1.41 (1.11–1.79) | 1.36 (1.08–1.70) | 1.32 (1.12–1.57) | 1.36 (1.14–1.63) |
| P value | <0.001 | 0.005 | 0.008 | 0.001 | <0.001 |
| P value for interactiona | 0.82 | ||||
| P value for heterogeneity | 0.34 | 0.07 | 0.84 | 0.92 | 0.36 |
| PFS | |||||
| HR (95% CI) | 1.25 (1.06–1.47) | 1.18 (0.95–1.47) | 1.33 (1.05–1.70) | 1.25 (1.06–1.48) | 1.23 (1.03–1.47) |
| P value | 0.008 | 0.14 | 0.02 | 0.01 | 0.02 |
| P value for interactiona | 0.47 | ||||
| P value for heterogeneity | 0.71 | 0.43 | 0.70 | 0.71 | 0.74 |
| ORR | |||||
| OR (95% CI) | 0.56 (0.43–0.73) | 0.56 (0.40–0.79) | 0.56 (0.36–0.87) | 0.55 (0.41–0.72) | 0.57 (0.44–0.75) |
| P value | <0.001 | <0.001 | 0.009 | <0.001 | <0.001 |
| P value for interactiona | 0.98 | ||||
| P value for heterogeneity | 0.71 | 0.67 | 0.34 | 0.76 | 0.89 |
Test comparing the HRs between the two trial subsets (with/without bevacizumab).
CI, confidence interval; HR, hazard ratio; OR, odds ratio; ORR, objective response rate; OS, overall survival; PFS, progression-free survival.
There was no significant difference (P value for interaction >0.05) in the prognostic effect according to presence or absence of bevacizumab in the control arm for the three endpoints. Exclusion of the phase II study (PEAK) or the second-line 20050181 study data led to similar results (Table 6) for all three endpoints.
Prognostic role of tumour location for patients receiving chemotherapy plus EGFR antibody therapy (experimental arm): pooled analysis
The pooled analyses (Figures 2B, D and F; Table 7), showed the overall HR for OS to be 2.03 [1.69–2.42] P < 0.001 in the absence of any heterogeneity between the trials (P = 0.46; I2 = 0%) confirming a clear negative prognostic effect of right-sided tumour location. The results for PFS were similar with an overall HR of 1.59 [1.34–1.88] (P < 0.001; P value for heterogeneity = 0.58: I2 = 0%). Results for ORR were also in favour of the left-sided tumour location with an overall OR of 0.38 [0.28–0.50] (P < 0.001), but with some inter study heterogeneity (P = 0.08; I2 = 48%). Use of a random effect model confirmed this result with an OR of 0.38 [0.25–0.57].
Table 7.
Trials’ subset and sensitivity analyses for prognostic analysis in the experimental arm for OS, PFS and ORR
| Main analysis | Trials’ subset analysis |
Sensitivity analyses |
|||
|---|---|---|---|---|---|
| Type of anti-EGFR |
Only phase III (without PEAK) | Only first line (without 20050181) | |||
| Parameter | Cetuximab | Panitumumab | |||
| OS | |||||
| HR (95% CI) | 2.03 (1.69–2.42) | 2.11 (1.68–2.66) | 1.90 (1.43–2.53) | 1.99 (1.65–2.39) | 2.03 (1.67–2.47) |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| P value for interactiona | 0.57 | ||||
| P value for heterogeneity | 0.46 | 0.25 | 0.44 | 0.54 | 0.46 |
| PFS | |||||
| HR (95% CI) | 1.59 (1.34–1.88) | 1.77 (1.43–2.20) | 1.34 (1.02–1.75) | 1.59 (1.33–1.89) | 1.63 (1.35–1.96) |
| P value | <0.001 | <0.001 | 0.03 | <0.001 | <0.001 |
| P value for interactiona | 0.11 | ||||
| P value for heterogeneity | 0.58 | 0.73 | 0.73 | 0.58 | 0.65 |
| ORR | |||||
| OR (95% CI) | 0.38 (0.28–0.50) | 0.35 (0.25–0.50) | 0.42 (0.27–0.66) | 0.33 (0.24–0.44) | 0.40 (0.30–0.53) |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| P value for interactiona | 0.55 | ||||
| P value for heterogeneity | 0.08 | 0.50 | 0.02 | 0.64 | 0.22 |
Test comparing the HRs between the two trial subsets (cetuximab, panitumumab).
HR, hazard ratio; OR, odds ratio; CI, confidence interval; ORR, objective response rate; OS, overall survival; PFS, progression-free survival.
There was no significant difference (P value for interaction >0.05) in the prognostic effect according to which EGFR antibody therapy was used for the three endpoints. Also, exclusion of either the phase II study (PEAK) or the second-line 20050181 study data led to similar results (Table 7) for all three endpoints. However, after exclusion of the PEAK study, there was no longer significant heterogeneity for ORR.
Predictive role of tumour location: pooled analysis
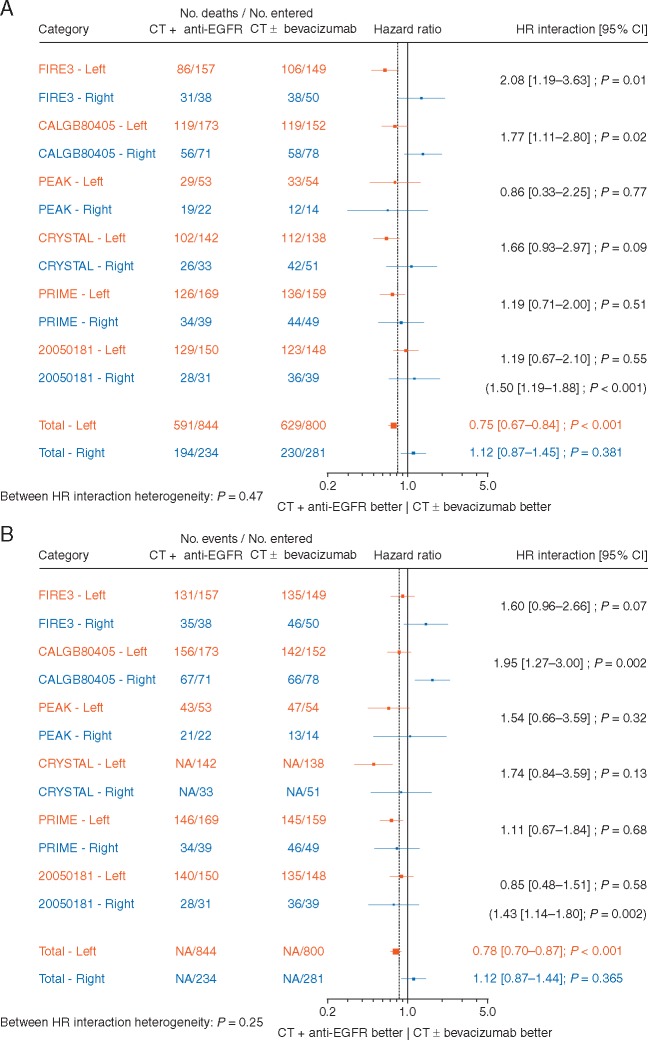
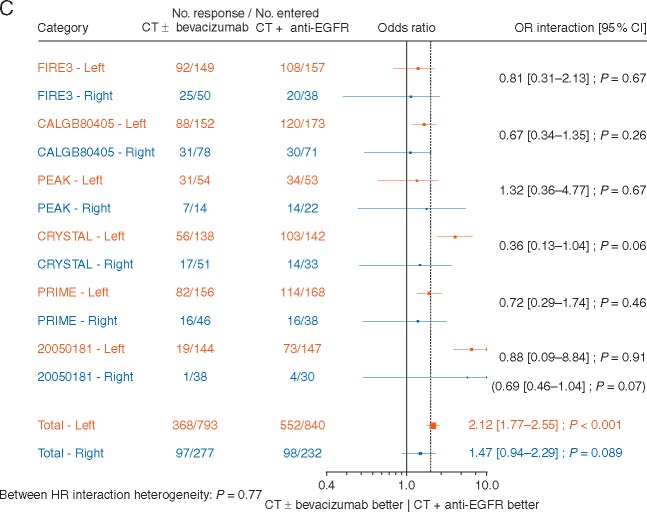
The predictive effect of chemotherapy plus EGFR antibody therapy compared with chemotherapy alone, or chemotherapy plus bevacizumab was significantly different for patients with left- and right-sided tumours (Figure 3A and B) both for OS (HR 1.50, P value for interaction <0.001) and PFS (HR 1.43, P = 0.002). A significant benefit of chemotherapy plus EGFR antibody therapy was observed in patients with the left-sided tumours [HRs 0.75 (0.67–0.84); P < 0.001 and 0.78 (0.70–0.87); P < 0.001 for OS and PFS, respectively] compared with no benefit in those with right-sided tumours [HRs 1.12 (0.87–1.45); P = 0.381 and 1.12 (0.87–1.44); P = 0.365 for OS and PFS, respectively]. For ORR (Figure 3C), there was a trend (P value for interaction = 0.07) towards a greater benefit for chemotherapy plus EGFR antibody therapy in the patients with left-sided tumours [OR = 2.12 (1.77–2.55); P < 0.001] compared with those with right-sided tumours [OR = 1.47 (0.94–2.29); P = 0.089]. There was no significant inter study heterogeneity for the three endpoints.
Figure 3.
Forest plots for predictive analyses of tumour location (right versus left side) in trials comparing chemotherapy plus EGFR antibody therapy (experimental arm) with chemotherapy alone or chemotherapy plus bevacizumab (control arm)—(A) overall survival, (B) progression-free survival and (C) objective response rate. CI, confidence interval; CT, chemotherapy; EGFR, epidermal growth factor receptor; HR, hazard ratio; NA, not available; OR, odds ratio.
Predictive role of tumour location according to study characteristics
The predictive role of tumour side was not significantly different between studies with or without bevacizumab in the control arm in terms of OS (P for interaction = 0.26, Table 8). However, the predictive role was significant for studies with bevacizumab (P = 0.001) but was not significant for studies without bevacizumab (P = 0.09). In the studies with cetuximab a significant predictive role of tumour side was observed (P < 0.001), but not in those with panitumumab (P = 0.47) with a significant difference in the HR of interaction between the two study groups (P = 0.048, Table 8). With regard to PFS, the use of bevacizumab in the control arm and the use of cetuximab instead of panitumumab in the experimental arm are associated with different treatment effects in patients with left-sided tumours compared with those with right-sided tumours, with the experimental treatment superior to the control treatment in the patients with left-sided tumours and the opposite being the case for those with right-sided tumours. Use of bevacizumab in the control arm and the type of EGFR antibody therapy used, had no impact on the borderline predictive effect of tumour side on ORR.
Table 8.
Trials’ subset and sensitivity analyses for predictive analysis on OS, PFS and ORR
| Main analysis | Trials subset analyses |
Sensitivity analyses |
|||||
|---|---|---|---|---|---|---|---|
| Bevacizumab in control arm |
Type of anti-EGFR |
Only phase III (without PEAK) | Only first line (without 20050181) | ||||
| Parameter | Yes | No | Cetuximab | Panitumumab | |||
| OS | |||||||
| HRLeft (95% CI) | 0.75 (0.67–0.84) | 0.71 (0.59–0.85) | 0.78 (0.67–0.90) | 0.69 (0.59–0.80) | 0.83 (0.70–0.98) | 0.75 (0.67–0.84) | 0.70 (0.62–0.80) |
| HRRight (95% CI) | 1.12 (0.87–1.45) | 1.22 (0.84–1.78) | 1.02 (0.72–1.45) | 1.25 (0.89–1.76) | 0.94 (0.64–1.40) | 1.16 (0.89–1.51) | 1.10 (0.83–1.46) |
| HRInteraction (95% CI) | 1.50 (1.19–1.88) | 1.72 (1.23–2.39) | 1.32 (0.96–1.81) | 1.82 (1.35–2.47) | 1.14 (0.80–1.62) | 1.55 (1.22–1.96) | 1.57 (1.22–2.01) |
| P value HRInteractiona | <0.001 | 0.001 | 0.09 | <0.001 | 0.47 | <0.001 | <0.001 |
| P value for interactionb | 0.26 | 0.048 | |||||
| PFS | |||||||
| HRLeft (95% CI) | 0.78 (0.70–0.87) | 0.84 (0.72–0.98) | 0.73 (0.63–0.85) | 0.79 (0.68–0.92) | 0.78 (0.66–0.91) | 0.79 (0.71–0.89) | 0.76 (0.67–0.86) |
| HRRight (95% CI) | 1.12 (0.87–1.46) | 1.48 (1.05–2.09) | 0.82 (0.57–1.19) | 1.42 (1.01–1.98) | 0.83 (0.56–1.21) | 1.13 (0.87–1.46) | 1.20 (0.91–1.58) |
| HRInteraction (95% CI) | 1.43 (1.14–1.80) | 1.76 (1.30–2.39) | 1.12 (0.80–1.56) | 1.79 (1.32–2.41) | 1.06 (0.75–1.50) | 1.42 (1.13–1.80) | 1.58 (1.23–2.02) |
| P value HRInteractiona | 0.002 | <0.001 | 0.52 | <0.001 | 0.72 | 0.003 | <0.001 |
| P value for interactionb | 0.05 | 0.03 | |||||
| ORR | |||||||
| ORLeft (95% CI) | 2.12 (1.77–2.55) | 1.50 (1.16–1.94) | 3.01 (2.33–3.90) | 1.93 (1.51–2.47) | 2.38 (1.81–3.12) | 2.22 (1.83–2.69) | 1.85 (1.53–2.25) |
| ORRight (95% CI) | 1.47 (0.94–2.29) | 1.19 (0.67–2.11) | 1.69 (0.84–3.40) | 1.19 (0.68–2.07) | 2.08 (0.98–4.39) | 1.43 (0.90–2.28) | 1.27 (0.81–2.01) |
| ORInteraction (95% CI)a | 0.69 (0.46–1.04) | 0.79 (0.47–1.32) | 0.56 (0.29–1.07) | 0.62 (0.38–1.01) | 0.87 (0.43–1.75) | 0.62 (0.38–1.01) | 0.87 (0.43–1.75) |
| P value ORInteractiona | 0.07 | 0.37 | 0.08 | 0.06 | 0.70 | 0.06 | 0.70 |
| P value for interactionb | 0.42 | 0.43 | |||||
Test comparing HRLeft and HRRight.
Test comparing the HRs between trial subsets (with/without bevacizumab; cetuximab; panitumumab).
CI, confidence interval; HR, hazard ratio; OR, odds ratio; ORR, objective response rate; OS, overall survival; PFS, progression-free survival.
Exclusion of the phase II study (PEAK) or the single second-line study (20050181) data led to similar results (Table 8) for the three endpoints, except for the exclusion of the 20050181 study data for ORR, when the difference between tumour sides became non-significant.
Discussion/summary of the evidence
In relation to the prognostic value, the individual trial data for all six trials showed treatment outcomes to be better in patients with left-sided tumours than in those with right-sided tumours, confirming the evidence from previous studies [16, 19–21] and a recent meta-analysis of 66 studies [22].
In relation to the predictive value of primary tumour location, the individual trial data showed the treatment benefit from the addition of EGFR antibody therapy to chemotherapy to be greatest in those patients who had left-sided primary tumours as seen in patients receiving chemotherapy plus EGFR antibody therapy versus chemotherapy alone in the first-line PRIME and CRYSTAL trials and in the second-line 20050181 trial. The individual trial data for all six trials showed patients with left-sided tumours receiving chemotherapy plus EGFR antibody therapy to have superior treatment outcomes in terms of OS, when compared with patients with right-sided tumours receiving the same therapy. Furthermore, patients with left-sided RAS wt tumours treated with chemotherapy plus EGFR antibody therapy also achieved better outcomes in terms of response, and PFS compared with the comparator arms in most of the six trials. Also, in the FIRE-3 and CALGB 80405 trials patients with left-sided tumours receiving chemotherapy plus EGFR antibody therapy (cetuximab) did significantly better than those receiving chemotherapy plus bevacizumab. Limited, if any, benefit appeared to be conferred by the addition of EGFR antibody therapy (cetuximab) to chemotherapy in the treatment of patients with right-sided tumours, except for the CRYSTAL trial in terms of ORR but not PFS or OS and a trend in the second-line 20050181 trial for ORR (Tables 3 and 5). However, analysis of treatment outcome for patients with right-sided tumours in the individual FIRE-3 trial (Table 5) suggested that patients with right-sided RAS wt tumours might benefit from chemotherapy plus bevacizumab compared with cetuximab in terms of OS (HR 1.31, P = 0.27) and PFS (HR 1.44, P = 0.11), but not for ORR. Thus, these data suggest that there may be a subset of patients with RAS wt tumours that might benefit from treatment with chemotherapy plus bevacizumab in terms of PFS and OS, namely those with right-sided tumours.
Certainly, the present pooled analysis confirmed the observation that left-sided tumour localization was associated with an anti-EGFR (cetuximab) disease control expression signature [17, 53]. Furthermore, there was no significant difference in prognosis depending on which EGFR antibody therapy was used or if bevacizumab was or was not used in the control arm. Conversely, the predictive effect depended significantly (test of interaction <0.05 for OS and PFS) on which EGFR antibody therapy was used. A significant predictive effect was observed in the subset of trials involving cetuximab where comparison of the treatment arms favoured the experimental arm in patients with left-sided tumours and the control arm in those with right-sided tumours (Table 8), but not in the case of patients receiving panitumumab. Also, a significant effect of the presence or absence of bevacizumab in the control arm was observed at least for PFS (Table 8), but as the variations in the treatment used in the control and experimental arms were not independent (Table 1), it is difficult to draw conclusions.
Thus, the pooled analysis data strongly suggest that patients with left-sided RAS wt tumours achieve a benefit from being treated with chemotherapy plus EGFR antibody therapy that is not seen in those with right-sided tumours. The question is, given the fact that not all the trials involving chemotherapy plus EGFR antibody therapy were included in the analysis and the potential selection biases associated with a retrospective analysis of aggregated data from mCRC patients initially accrued independently of RAS analysis and that only involved 37.5% of the patients randomized in the six trials selected (based on the available data for tumour RAS mutation status and tumour sidedness), can these data be used to support a change in clinical practice? Certainly, the absence of stratification according to tumour side and RAS mutation in the different trials, the heterogeneity of the treatments compared in the six trials (which impact on some of the results), as well as the presence of some imbalances in the covariates between the two arms when considering separately the patients with right-sided and left-sided tumours (supplementary Tables S1 and S2, available at Annals of Oncology online) and the variation in the adjustment of these covariables from one trial to another, lend a note of caution to the interpretation of these results. Also, the increased HRs in the studies with bevacizumab included in the control arm mentioned above, suggesting that bevacizumab might be more beneficial in patients with right-sided tumours, could be a consequence of either a poorer outcome in patients receiving EGFR antibody therapy or a better outcome in those receiving bevacizumab. Unfortunately, as it would be inappropriate to make cross trial comparisons between the FOLFIRI control arm of the CRYSTAL trial and the FOLFIRI plus bevacizumab arm of the FIRE-3 or the FOLFOX arm of the PRIME trial and the FOLFOX plus bevacizumab arm of the PEAK trial, no firm conclusions can be drawn. Also, it should be noted that the influence of tumour BRAF mutation status was not investigated as these data were not available for the patients in three of the trials and the pooling of the data for the remaining three trials would involve small patient numbers, particularly for patients with right-sided tumours, rendering the results of any analysis unreliable.
The predictive effect of primary tumour location for EGFR-antibody therapy in patients from the PRIME and CRYSTAL trials with chemotherapy in the control arm, conducted as part of a recently published meta-analysis on the prognosis and efficacy of targeted agents according to tumour sidedness also reached the conclusion that left-sided primary tumour localization was predictive of a benefit from the addition of EGFR-antibody therapy to standard chemotherapy in terms of OS, PFS and ORR in patients with RAS wt tumours [54]. The test for interaction between treatment efficacy and sidedness however did not reach statistical significance. A separate meta-analysis of data from the FIRE-3, CALGB 80405 and PEAK trials with bevacizumab-containing control arms was also carried out, and the results and conclusions were generally in line with our own results.
What implications will these data have for our clinical practice?
As mentioned previously the data presented in this manuscript are derived from unplanned retrospective analyses. However, not all decisions relating to the development of treatment algorithms have the benefit of being supported by top level evidence, and previous examples of pragmatic decision-making in the treatment of patients with mCRC have included for example the choice of first-line treatment for the ‘conversion’ of colorectal liver metastases to resectability based on patient series and cross-trial comparisons [55], the use of new non-validated treatment indicators such as depth of response and early tumour shrinkage [56], the best treatment of patients with BRAF mutant disease based on an unplanned subgroup analysis of a subgroup of <30 patients in a randomized trial and observational data [57], and others.
The most recent ESMO consensus guidelines [12] define tumour characteristics, patient characteristics and treatment characteristics as the drivers of decision-making in the first-line treatment setting, as well as therapeutic goal differentiating between ‘disease stabilization’ and the necessity for tumour volume reduction. Along with RAS and BRAF tumour mutation status, tumour biology is also listed as a differentiation factor, and this analysis strongly contributes to the evidence suggesting that there is a clear difference in tumour biology between tumours of the right and left sides of the colon. Currently the preferred treatment option for patients with RAS wt/BRAF wt (all wt) tumours is doublet chemotherapy plus EGFR antibody therapy and possibly, in very selected patients, FOLFOXIRI plus bevacizumab.
However, it was argued based on the evidence from both, the individual trial findings, and the present prognostic and predictive pooled analysis data, that a distinction needs to be made between the treatment approaches for patients with right- versus left-sided tumours.
For the treatment of patients with left-sided RAS wt (BRAF wt) tumours going forward the preferred therapy option for patients would be a chemotherapy doublet plus EGFR antibody therapy, independent of treatment goal, for the majority of patients.
In the case of patients with right-sided RAS wt tumours the preferred therapy option for patients where cytoreduction is the goal would be a chemotherapy triplet (e.g. FOLFOXIRI) with bevacizumab. However, given the findings from the analysis of ORR here, a doublet plus EGFR antibody therapy remains an option. For patients where disease stabilization is the goal a chemotherapy doublet with or without bevacizumab would be the treatment of choice, and due to the poorer outcomes associated with patients with right-sided tumours, intensification to a chemotherapy triplet could be considered.
Independent from tumour localization, RAS mutant tumour status is known to be a very strong negative predictor for EGFR antibody therapy whilst, RAS wt tumour status is a relatively strong predictive marker for the efficacy of EGFR antibody therapy [43, 45, 46, 58]. BRAF tumour mutations are strong negative prognostic markers but might be (non-significant) predictive markers for intensive therapy [57].
Therefore, with all of the caveats resulting from this analysis, which was carried out retrospectively, and involved a limited number of patients from the individual trials, a relatively small number of right-sided patients, and—most importantly—did not consider a preference for distinct treatment sequences, as only the randomization to the respective treatment line was analysed, it provides evidence in the first-line treatment setting to:
Reinforce the use of EGFR antibody therapy in patients with mCRC and left-sided RAS wt tumours.
Promote the idea that patients with right-sided RAS wt tumours might be better treated with chemotherapy alone or chemotherapy plus bevacizumab—except maybe if the goal is tumour size reduction as the ORRs were higher (but not PFS and OS).
Emphasise that in the absence of data on specific treatment sequences, there is no reason that EGFR-antibody therapy should be avoided in cases of disease progression or treatment intolerance independent of primary tumour location.
Promote the concept of a ‘continuum of care’ and the sequential use of all therapies, including bevacizumab where appropriate, in the treatment of patients with mCRC.
However, the developmental, genetic, physiological and biological differences associated with the different locations in the colon and rectum are clearly more complex than simple right- and left-sidedness and one might predict that a comprehensive evaluation of molecular features in left- and right-sided CRCs will contribute to improvements in treatment outcomes in the future. Going forward, new randomized controlled trials should have to stratify patients according to primary tumour location and if the sequence in which the currently available therapies are delivered matters we need more trials based on tumour location and molecular characteristics.
Supplementary Material
Acknowledgements
Anne Kinsella (Cancer Communications and Consultancy Ltd, Knutsford, Cheshire, UK) is acknowledged for her assistance in the preparation of the manuscript, funded by ESMO.
Funding
All expenses relating to the special symposia were covered by ESMO.
Disclosure
DA has reported honoraria/consultancy for Roche, Merck-Serono, Bayer, Amgen; research funding from Roche. J-YD has reported advisory boards/symposia/lectures for Amgen, Roche, Merck-Serono, Sanofi and Bayer. MP has reported honoraria/consultancy/advisory boards for Amgen, Bayer, Sanofi and Servier; research funding from Amgen, Bayer and Roche. H-JL has reported advisory board/lecture and clinical trial support from Merck KGaA and Roche/Genetech. AV has reported consultancy/advisory boards for Genentech, Merck-Serono and Roche; research funding from BMS, Genentech, Merck-Serono, and Roche. VH has reported speaker’s honoraria/advisory boards for Merck-Serono, Roche, Sanofi, Amgen and Lilly; research funding from Merck-Serono, Roche, Sanofi, Amgen and Lilly. EVC has reported research grants Bayer, Boehringer, Amgen, Celgene, Ipsen, Lilly, Merck, Novartis, Roche, and Sanofi. JT has reported consultancy/advisory role for Amgen, Bayer, Boehringer Ingelheim, Celgene, Chugai, Lilly, MSD, Merck-Serono, Novartis, Roche, Sanofi, Symphogen, Taiho and Takeda; institutional funding from Novartis, Pharma Mar, Roche, Janssen-Cilag, Symphogen, Agendia, Debiopharm, Servier and Amgen. AC has reported member of speaker’s bureau for Roche and Merck-Serono; advisory boards for Merck-Serono, Roche, Amgen, Bayer and Lilly. FC has reported: advisory boards for Merck-Serono, Roche, Amgen, Bayer, Lilly, AstraZeneca; research funding for Bayer and Roche. All remaining authors have declared no conflicts of interest.
References
- 1. Richman S, Adlard J.. Left and right sided large bowel cancer. Br Med J 2002; 324: 931–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hansen IO, Jess P.. Possible better long-term survival in left versus right-sided colon cancer - a systematic review. Dan Med J 2012; 59: A4444.. [PubMed] [Google Scholar]
- 3. Meza R, Jeon J, Renehan AG, Luebeck EG.. Colorectal cancer incidence trends in the United States and United Kingdom: evidence of right- to left-sided biological gradients with implications for screening. Cancer Res 2010; 70: 5419–5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aarts F, de Hingh I, de Wilt JHW. et al. Differences in outcome between right- and left-sided colon cancer: a population based study. J Clin Oncol 2013; 31(suppl 4): Abstract 493. [Google Scholar]
- 5. Verhulst J, Ferdinande L, Demetter P, Ceelen W.. Mucinous subtype as prognostic factor in colorectal cancer: a systematic review and meta-analysis. J Clin Pathol 2012; 65: 381–388. [DOI] [PubMed] [Google Scholar]
- 6. Domingo E, Ramamoorthy R, Oukrif D. et al. Use of multivariate analysis to suggest a new molecular classification of colorectal cancer. J Pathol 2013; 229: 441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Slattery ML, Curtin K, Wolff RK. et al. A comparison of colon and rectal somatic DNA alterations. Dis Colon Rectum 2009; 52: 1304–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maus MKH, Hanna DL, Stephens C. et al. Gene expression profiles and tumor locations in colorectal cancer (left vs. right vs. rectum). J Clin Oncol 2013; 31(suppl 15): Abstract 3527. [Google Scholar]
- 9. Missiaglia E, Jacobs B, D'Ario G. et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol 2014; 25: 1995–2001. [DOI] [PubMed] [Google Scholar]
- 10. LaPointe LC, Dunne R, Brown GS. et al. Map of differential transcript expression in the normal human large intestine. Physiol Genomics 2008; 33: 50–64. [DOI] [PubMed] [Google Scholar]
- 11. Hamada H, Meno C, Watanabe D, Saijoh Y.. Establishment of vertebrate left-right asymmetry. Nat Rev Genet 2002; 3: 103–113. [DOI] [PubMed] [Google Scholar]
- 12. Van Cutsem E, Cervantes A, Adam R. et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016; 27: 1386–1422. [DOI] [PubMed] [Google Scholar]
- 13. Guinney J, Dienstmann R, Wang X. et al. The consensus molecular subtypes of colorectal cancer. Nat Med 2015; 21: 1350–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim SE, Paik HY, Yoon H. et al. Sex- and gender-specific disparities in colorectal cancer risk. World J Gastroenterol 2015; 21: 5167–5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yamauchi M, Morikawa T, Kuchiba A. et al. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut 2012; 61: 847–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Loupakis F, Yang D, Yau L. et al. Primary tumor location as a prognostic factor in metastatic colorectal cancer. J Natl Cancer Inst 2015; 107: dju427.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Laurent-Puig P, Grisoni M-L, Heinemann V. et al. MiR-31-3p is a predictive biomarker of cetuximab response in the FIRE-3 trial. Ann Oncol 2016; 27(suppl 6): Abstract 457o. [Google Scholar]
- 18. Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer 2002; 101: 403–408. [DOI] [PubMed] [Google Scholar]
- 19. Modest DP, Schulz C, von Weikersthal LF. et al. Outcome of patients with metastatic colorectal cancer depends on the primary tumor site (midgut vs. hindgut): analysis of the FIRE1-trial (FuFIRI or mIROX as first-line treatment). Anticancer Drugs 2014; 25: 212–218. [DOI] [PubMed] [Google Scholar]
- 20. Seligmann JF., Elliott F, Richman SD. et al. Primary tumour location (PTL) as a prognostic and predictive factor in advanced colorectal cancer: data from 20175 patients in randomised trials. Ann Oncol 2014; 25 (suppl 4): iv172. [Google Scholar]
- 21. Zhang Y, Ma J, Zhang S. et al. A prognostic analysis of 895 cases of stage III colon cancer in different colon subsites. Int J Colorectal Dis 2015; 30: 1173–1183. [DOI] [PubMed] [Google Scholar]
- 22. Petrelli F, Tomasello G, Borgonovo K. et al. Prognostic survival associated with left-sided vs right-sided colon cancer: a systematic review and meta-analysis. JAMA Oncol 2016. DOI: 10.1001/jamaoncol.2016.4227. [DOI] [PubMed] [Google Scholar]
- 23. Dejea CM, Wick EC, Hechenbleikner EM. et al. Microbiota organization is a distinct feature of proximal colorectal cancers. Proc Natl Acad Sci USA 2014; 111: 18321–18326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brule SY, Jonker DJ, Karapetis CS. et al. Location of colon cancer (right-sided versus left-sided) as a prognostic factor and a predictor of benefit from cetuximab in NCIC CO.17. Eur J Cancer 2015; 51: 1405–1414. [DOI] [PubMed] [Google Scholar]
- 25. Moretto R, Cremolini C, Rossini D. et al. Location of primary tumor and benefit from anti-epidermal growth factor receptor monoclonal antibodies in patients with RAS and BRAF wild-type metastatic colorectal cancer. Oncologist 2016; 21: 988–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen K-H, Shao Y-Y, Chen H-M. et al. Primary tumor site is a useful predictor of cetuximab efficacy in the third-line or salvage treatment of KRAS wild-type (exon 2 non-mutant) metastatic colorectal cancer: a mationwide cohort study. BMC Cancer 2016; 16: 327.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Venook A, Niedzwiecki D, Innocenti F. et al. Impact of primary tumor location on overall suvival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): analysis of CALGB/SWOG 80405 (Alliance). J Clin Oncol 2016; 34 (suppl): Abstract 3504. [Google Scholar]
- 28. Tejpar S, Stintzing S, Ciardiello F. et al. Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: Retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA Oncol 2016. DOI: 10.1001/jamaoncol.2016.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Benedix F, Kube R, Meyer F. et al. Comparison of 17,641 patients with right- and left-sided colon cancer: differences in epidemiology, perioperative course, histology, and survival. Dis Colon Rectum 2010; 53: 57–64. [DOI] [PubMed] [Google Scholar]
- 30. Schrag D, Weng S, Brooks G. et al. The relationship between primary tumor sidedness and prognosis in colorectal cancer. J Clin Oncol 2016; 34(suppl): Abstract 3505. [Google Scholar]
- 31. Boisen MK, Johansen JS, Dehlendorff C. et al. Primary tumor location and bevacizumab effectiveness in patients with metastatic colorectal cancer. Ann Oncol 2013; 24: 2554–2559. [DOI] [PubMed] [Google Scholar]
- 32. Wong HL, Lee B, Field K. et al. Impact of primary tumor site on bevacizumab efficacy in metastatic colorectal cancer. Clin Colorectal Cancer 2016; 15: e9–e15. [DOI] [PubMed] [Google Scholar]
- 33. Van Cutsem E, Kohne CH, Hitre E. et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009; 360: 1408–1417. [DOI] [PubMed] [Google Scholar]
- 34. Douillard JY, Siena S, Cassidy J. et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 2010; 28: 4697–4705. [DOI] [PubMed] [Google Scholar]
- 35. Schwartzberg LS, Rivera F, Karthaus M. et al. PEAK: a randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol 2014; 32: 2240–2247. [DOI] [PubMed] [Google Scholar]
- 36. Heinemann V, von Weikersthal LF, Decker T. et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol 2014; 15: 1065–1075. [DOI] [PubMed] [Google Scholar]
- 37. Stintzing S, Modest DP, Rossius L. et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): a post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol 2016; 17: 1426–1434. [DOI] [PubMed] [Google Scholar]
- 38. Venook A, Niedzwiecki D, Lenz HJ. et al. CALGB/SWOG 80405: Phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC). J Clin Oncol 2014; 32(suppl 15): abstract LBA 3. [Google Scholar]
- 39. Peeters M, Price TJ, Cervantes A. et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol 2010; 28: 4706–4713. [DOI] [PubMed] [Google Scholar]
- 40. Maughan TS, Adams RA, Smith CG. et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet 2011; 377: 2103–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bokemeyer C, Kohne CH, Ciardiello F. et al. FOLFOX4 plus cetuximab treatment and RAS mutations in colorectal cancer. Eur J Cancer 2015; 51: 1243–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ye LC, Liu TS, Ren L. et al. Randomized controlled trial of cetuximab plus chemotherapy for patients with KRAS wild-type unresectable colorectal liver-limited metastases. J Clin Oncol 2013; 31: 1931–1938. [DOI] [PubMed] [Google Scholar]
- 43. Douillard JY, Oliner KS, Siena S. et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 2013; 369: 1023–1034. [DOI] [PubMed] [Google Scholar]
- 44. Douillard JY, Siena S, Cassidy J. et al. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol 2014; 25: 1346–1355. [DOI] [PubMed] [Google Scholar]
- 45. Van Cutsem E, Kohne CH, Lang I. et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 2011; 29: 2011–2019. [DOI] [PubMed] [Google Scholar]
- 46. Van Cutsem E, Lenz HJ, Kohne CH. et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol 2015; 33: 692–700. [DOI] [PubMed] [Google Scholar]
- 47. Peeters M, Douillard JY, Van Cutsem E. et al. Mutant KRAS codon 12 and 13 alleles in patients with metastatic colorectal cancer: assessment as prognostic and predictive biomarkers of response to panitumumab. J Clin Oncol 2013; 31: 759–765. [DOI] [PubMed] [Google Scholar]
- 48. Peeters M, Oliner KS, Price TJ. et al. Analysis of KRAS/NRAS mutations in a phase III study of panitumumab with FOLFIRI compared with FOLFIRI alone as second-line treatment for metastatic colorectal cancer. Clin Cancer Res 2015; 21: 5469–5479. [DOI] [PubMed] [Google Scholar]
- 49. Lenz HJ, Niedzwiecki D, Innocenti F. et al. CALGB/SWOG 80405: phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with expanded RAS analyses untreated metastatic adenocarcinoma of the colon or rectum (mCRC). Ann Oncol 2014; 25(suppl 4): Abstract 501o. [Google Scholar]
- 50. Modest DP, Ricard I, Heinemann V. et al. Outcome according to KRAS-, NRAS- and BRAF-mutation as well as KRAS mutation variants: pooled analysis of five randomized trials in metastatic colorectal cancer by the AIO colorectal cancer study group. Ann Oncol 2016; 27: 1746–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Higgins JP, Thompson SG.. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 52. Fisher DJ, Copas AJ, Tierney JF, Parmar MK.. A critical review of methods for the assessment of patient-level interactions in individual participant data meta-analysis of randomized trials, and guidance for practitioners. J Clin Epidemiol 2011; 64: 949–967. [DOI] [PubMed] [Google Scholar]
- 53. Khambata-Ford S, Garrett CR, Meropol NJ. et al. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol 2007; 25: 3230–3237. [DOI] [PubMed] [Google Scholar]
- 54. Holch JW, Ricard I, Stintzing S. et al. The relevance of primary tumour location in patients with metastatic colorectal cancer: a meta-analysis of first-line clinical trials. Eur J Cancer 2017; 70: 87–98. [DOI] [PubMed] [Google Scholar]
- 55. Adam R, De Gramont A, Figueras J. et al. The oncosurgery approach to managing liver metastases from colorectal cancer: a multidisciplinary international consensus. Oncologist 2012; 17: 1225–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Heinemann V, Stintzing S, Modest DP. et al. Early tumour shrinkage (ETS) and depth of response (DpR) in the treatment of patients with metastatic colorectal cancer (mCRC). Eur J Cancer 2015; 51: 1927–1936. [DOI] [PubMed] [Google Scholar]
- 57. Cremolini C, Loupakis F, Antoniotti C. et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol 2015; 16: 1306–1315. [DOI] [PubMed] [Google Scholar]
- 58. Bokemeyer C, Bondarenko I, Hartmann JT. et al. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol 2011; 22: 1535–1546. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.