Abstract
Background
Margetuximab is an anti-HER2 antibody that binds with elevated affinity to both the lower and higher affinity forms of CD16A, an Fc-receptor important for antibody dependent cell-mediated cytotoxicity (ADCC) against tumor cells. A Phase 1 study was initiated to evaluate the toxicity profile, maximum tolerated dose (MTD), pharmacokinetics, and antitumor activity of margetuximab in patients with HER2-overexpressing carcinomas.
Patients and methods
Patients with HER2-positive breast or gastric cancer, or other carcinomas that overexpress HER2, for whom no standard therapy was available, were treated with margetuximab by intravenous infusion at doses of 0.1–6.0 mg/kg for 3 of every 4 weeks (Regimen A) or once every 3 weeks (10–18 mg/kg) (Regimen B).
Results
Sixty-six patients received margetuximab (34 patients for Regimen A and 32 patients for Regimen B). The MTD was not reached for either regimen. Treatment was well-tolerated, with mostly Grade 1 and 2 toxicities consisting of constitutional symptoms such as pyrexia, nausea, anemia, diarrhea, and fatigue. Among 60 response-evaluable patients, confirmed partial responses and stable disease were observed in 7 (12%) and 30 (50%) patients, respectively; 26 (70%) of these patients had received prior HER2-targeted therapy. Tumor reductions were observed in over half (18/23, 78%) of response-evaluable patients with breast cancer including durable (>30 weeks) responders. Ex vivo analyses of patient peripheral blood mononuclear cell samples confirmed the ability of margetuximab to support enhanced ADCC compared with trastuzumab.
Conclusions
Margetuximab was well-tolerated and has promising single-agent activity. Further development efforts of margetuximab as single agent and in combination with other therapeutic agents are ongoing.
Trial Registration ID
Keywords: margetuximab, HER2, solid tumor, breast cancer, gastric cancer
Introduction
HER2, a receptor tyrosine kinase of the ErbB family, is important in tumorigenesis, tumor aggressiveness, and outcome in approximately one-fifth of breast and gastric cancers. Standard care for HER2-positive malignancies includes treatment with anti-HER2 therapies, primarily trastuzumab, which has improved outcomes for patients with metastatic [1] and early stage [2, 3] HER2-positive breast and advanced gastroesophageal cancers [4].
In addition to blocking HER2, trastuzumab mediates antibody-dependent cell-mediated cytotoxicity (ADCC), a potentially important mechanism for clinical efficacy [5]. The CD16A FcγRIIIA stimulatory receptor on natural killer (NK) cells and macrophages, important for mediating ADCC, is encoded by two alleles differing in the codon for amino acid 158: a higher-affinity V (valine) variant and a lower-affinity F (phenylalanine) variant [6]. A retrospective analysis of breast cancer patients receiving trastuzumab plus taxane therapy demonstrated superior outcomes in patients homozygous for the higher-affinity homodimer compared with patients with either lower-affinity dimer [7], suggesting that ADCC may be important in mediating clinical activity. Anti-HER2 antibodies engineered with increased affinity for both isoforms of CD16A may, therefore, enhance antitumor activity [8].
Margetuximab (MGAH22) is a monoclonal antibody (mAb) derived from 4D5, the parent antibody of trastuzumab. Margetuximab and trastuzumab bind the same epitope of HER2 with similar affinities and exhibit similar tumor-directed, effector cell-independent, anti-proliferative activity in breast cancer cells in vitro in the absence of immune effectors [9]. However, five amino acid substitutions engineered into the margetuximab IgG1 Fc domain yield increased binding to both isoforms of CD16A and reduced binding to CD32B, an inhibitory FcγR, compared with trastuzumab [9]. In ADCC assays using effector cells from donors heterozygous or homozygous for the lower-affinity 158F variant of CD16A, margetuximab had greater potency and maximum cytotoxicity than a trastuzumab surrogate with a wild-type Fc domain [9]. In similar assays using effector cells from donors homozygous for the higher-affinity 158V isoform of CD16A, margetuximab produced similar maximum cytotoxicity but lower EC50 than the trastuzumab surrogate [9]. In transgenic mice expressing the human CD16A 158F/F lower affinity FcγR, margetuximab produced superior tumor growth suppression of JIMT-1 cells, a cell line insensitive to growth inhibition by anti-HER2 antibodies, compared with the trastuzumab surrogate[10].
A first-in-human Phase 1 study was initiated to determine a recommended dose and schedule for margetuximab in patients with any relapsed HER2-overexpressing carcinoma. Pharmacokinetics, immunogenicity, and antitumor activity were also evaluated, in addition to ex vivo ADCC analyses to confirm margetuximab-enhanced effector function.
Patients and methods
Patients
Enrolled patients had histologically or cytologically confirmed carcinoma with documented HER2 overexpression by immunohistochemistry (IHC) (2+ or 3+) and disease progression during or following the last treatment regimen. Eligibility included age ≥18 years; life expectancy ≥3 months; Eastern Cooperative Oncology Group performance status ≤1; measurable disease by Response Criteria for Solid Tumors (RECIST) v1.1; adequate bone marrow, renal, and hepatic function; and left ventricular ejection fraction (LVEF) ≥50%. Key exclusion criteria included class III or IV New York Heart Association heart disease; significant pulmonary compromise; significant prior anthracycline exposure.
The study protocol was reviewed and approved by relevant institutional review boards or ethics committees and written informed consent obtained from all patients. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice.
Study design
This was a multicenter, open-label, Phase 1, dose escalation, and expansion study (NCT01148849). Two regimens of margetuximab (administered intravenously [IV] over 120 min) were evaluated: Regimen A – 0.1, 0.3, 1.0, 3.0, and 6.0 mg/kg weekly (QW) and, a less visit-intensive regimen, Regimen B – 10, 15, and 18 mg/kg once every 3 weeks (Q3W). Dose escalation and eligibility for subsequent treatment were based on safety observations and tumor assessments after the initial 7-week (43-day) treatment cycle (Cycle 1). The same margetuximab dose and regimen received during Cycle 1 was administered for subsequent cycles, however, QW for 3 weeks in Regimen A or Q3W in Regimen B.
Dose escalation followed a 3 + 3 design for Regimen A and 6 + 6 design for Regimen B. The maximum tolerated dose (MTD) was the highest dose cohort evaluated within which <33% of patients experienced dose-limiting toxicity (DLT). DLT was any margetuximab-related adverse event (AE) ≥Grade 3 in severity per Common Terminology Criteria for Adverse Events (v4.0) except treatment-emergent hematologic toxicities; and Grade 2 AEs of clinical significance (per investigator discretion) or which delayed dosing for >1 week.
Assessments
Safety assessments included AEs, clinical laboratory tests, and electrocardiograms. Cardiac function was monitored by multi-gated acquisition ventriculography or echocardiograms performed at baseline and after every other treatment cycle. Tumor response was determined using RECIST at baseline, at the end of the first cycle of treatment, and every 8- (Regimen A) or 12- (Regimen B) weeks during subsequent cycles. Patients were allowed to continue therapy until disease progression or withdrawal criteria were met.
Pharmacokinetic analyses
Pharmacokinetic blood samples were collected before, and 1, 2, 4, 6, ∼24, ∼72, and ∼96 h after, the first infusion; before, and 1h after, subsequent infusions; and post-treatment during the remainder of Cycle 1. A quantitative sandwich enzyme-linked immunosorbent assay, validated, and performed at MacroGenics, measured margetuximab concentration in serum with a lower limit of quantitation of 25.0 ng/ml.
A 2-compartment model with parallel linear and Michaelis–Menten elimination and allometric scaling of all clearance (CL) and volume parameters (with power coefficients of 0.75 for CL and inter-compartment clearance [Q], and coefficients of 1 for central volume [V1] and peripheral volumes [V2]) was used; standard errors were obtained from the NONMEM covariance step. The population pharmacokinetic analysis was conducted via non-linear mixed-effects modeling with NONMEM software, Version 7.3.0 (ICON Development Solutions, Ellicott City, MD).
ADCC analyses
Peripheral blood mononuclear cells (PBMCs) were isolated from patients in Regimen A at baseline and Day 22 (prior to the 4th infusion) of Cycle 1, incubated overnight with JIMT-1/Luc cells at an effector/target ratio of 30:1 in the presence of 0.001–1000 ng/ml of margetuximab or trastuzumab, and analyzed as described in Nordstrom et al. [9]. Specific cell lysis was calculated from luminescence counts (RLU): cytotoxicity (%) = 100 × (1−[RLU of sample/RLU of AICC control]), where AICC (antibody-independent cell-mediated cytotoxicity) control = average RLU of target cells incubated with PBMC in the absence of antibody. Data were fit to a sigmoidal dose-response function and 50% effective concentration (EC50) and percent maximum cytotoxicity values obtained.
Results
Patient characteristics
A total of 66 patients (34 Regimen A and 32 Regimen B) were enrolled and treated between August 2010 and July 2015 at 3 study sites. Baseline demographics and disease characteristics are presented in Table 1. Although patients were enrolled based on HER2-overexpression by local testing, study-specific central testing performed failed to confirm the result in 23 patients, either because the sample did not overexpress HER2 or no result was obtained. The most common tumor types enrolled were breast and gastroesophageal. Over half (45/66 [68%]) of patients received at least one prior anti-HER2 therapy in the metastatic setting. All but one breast cancer patient (26/27, 96%) received prior anti-HER2 therapy.
Table 1.
Baseline demographic and disease characteristics
| Characteristic | Regimen A, N = 34 | Regimen B, N = 32 | Total, N = 66 |
|---|---|---|---|
| Age (years) | |||
| Median (range) | 62.5 (36–83) | 57.0 (36–72) | 59 (36–83) |
| Gender, n (%) | |||
| Male | 19 | 18 | 37 |
| Female | 15 | 14 | 29 |
| Race, n (%) | |||
| Asian | 17 (50%) | 29 (91%) | 46 (69%) |
| Black | 3 (9%) | 0 | 3 (5%) |
| White | 14 (41%) | 3 (9%) | 17 (26%) |
| Tumor type, n (%) | |||
| Breast | 10 (29%) | 17 (53%) | 27 (41%) |
| Gastroesophageal | 12 (35%) | 8 (25%) | 20 (30%) |
| Colorectal | 5 (15%) | 0 | 5 (8%) |
| Gall Bladder | 0 | 2 (11%) | 2 (3%) |
| Lung | 2 (6%) | 0 | 2 (3%) |
| Othera | 5 (15%) | 5 (28%) | 10 (15%) |
| ECOG status, n (%) | |||
| 0 | 16 (47%) | 13 (72%) | 38 (58%) |
| 1 | 18 (53%) | 5 (28%) | 28 (42%) |
| HER 2 expression, n (%) | |||
| Positive (IHC 3+) | 16 (47%) | 23 (72%) | 39 (59%) |
| Positive (IHC 2+, FISH amplified) | 1 (3%) | 3 (9%) | 4 (6%) |
| Negative (IHC 0 or IHC, FISH non-amplified) | 17 (50%) | 6 (19%) | 23 (35%) |
| Number of prior chemotherapy regimens | |||
| Median (range) | 3 (1–7) | 3 (1–7) | 3.0 (1–7) |
| Prior anti-HER2 therapy, n (%) | |||
| Any | 19 (56%) | 26 (81%) | 45 (68%) |
| Trastuzumab | 15 (44%) | 25 (78%) | 40 (61%) |
| Lapatinib | 13 (38%) | 15 (47%) | 28 (42%) |
| Other | 7 (21%) | 6 (19%) | 15 (23%) |
Other tumors include rectal, sigmoid colon, ampulla of Vater, bladder, endometrial cancer, esophageal-squamous cell carcinoma, malignant tumor of lacrimal gland, salivary duct cancer, submandibular gland cancer, and transitional cell carcinoma of the renal pelvis.
Study treatment
Dose escalation and dose expansion
A MTD was not reached for either regimen. For Regimen A, 18 patients (3, 3, 3, 6, and 6 in the 0.1, 0.3, 1.0, 3.0, and 6.0 mg/kg cohorts, respectively) were evaluated during Dose Escalation. Among these patients, Cycle 1 DLT was limited to a single Grade 3 infusion-related reaction (IRR) in one of 6 patients treated at 3.0 mg/kg. The 6.0 mg/kg cohort was expanded to evaluate 19 patients overall. For Regimen B, 18 patients (6 each in the 10.0, 15.0, and 18.0 mg/kg cohorts) were evaluated during Dose Escalation without DLT reported. Based on adequate exposure during dosing, the 15.0 mg/kg cohort was expanded to evaluate 20 patients overall.
Overall, 51 (77%) patients (25 [74%] Regimen A, 26 [81%] Regimen B) completed Cycle 1 and 32 (49%) patients (11 [32%] Regimen A, 21 [66%] Regimen B) received >1 cycle (median: 5 [range: 2–43] cycles overall; 3 [2–9] Regimen A, 7 [2–43] Regimen B). Nineteen (29%) patients (14 [21%] Regimen A, 5 [8%] Regimen B) who completed Cycle 1 did not meet response criteria for continued treatment. Reasons for study discontinuation during Cycle 1 included progressive disease (PD) (10/15 [67%] patients overall; 6 [40%] Regimen A, 4 [27%] Regimen B), withdrawal by patient (3/15 patients [20%] overall), and AE (2/15 patients [13%] overall). Among patients receiving >1 cycle, reasons for study discontinuation included PD (25/32 [78%] overall; 11 [34%] Regimen A, 14 [43%] Regimen B) and AE 1/32 [3%] overall). At the time of this report, six patients continued treatment: 1, 4, and 1 at 10.0, 15.0, and 18.0 mg/kg, respectively (range: 3–43 cycles).
Margetuximab was well tolerated at all doses. Only one patient (3.0 mg/kg) discontinued due to acute toxicity manifesting as an IRR with the first margetuximab dose. A second patient (6.0 mg/kg) discontinued during Cycle 1 due to unrelated fatal acute renal failure and a third patient (6.0 mg/kg) discontinued during Cycle 3 due to margetuximab-related increased lipase. Due to Grade 2 IRR observed among the initial patients treated at 1.0 and 3.0 mg/kg QW, pre-medication guidelines were implemented for all patients receiving >3.0 mg/kg to mitigate the occurrence and severity of these events.
Sixty-six patients were evaluated for safety. Common toxicities were primarily ≤Grade 2 and included fatigue, pyrexia, anemia, nausea, vomiting, IRR, and upper respiratory tract infection (Table 2 and supplementary Table S1, available at Annals of Oncology online). IRR and fatigue were most commonly attributed to margetuximab (observed in 18%, and 14% patients overall, respectively). Grade 3/4 AEs attributed to margetuximab were infrequent and included Grade 4 increased lipase (n = 1), Grade 4 lymphocyte decreased (n = 1), Grade 3 increased blood amylase (n = 2), increased blood alkaline phosphatase (n = 1), lymphocyte decreased (n = 1), and Grade 3 IRR (n = 1) (Table 2). The elevations in lipase/amylase and amylase alone occurred in two patients receiving 3 and 5 cycles, respectively, and were not associated with signs or symptoms of pancreatitis; however, despite interruptions in drug administration, one patient was withdrawn for persistent elevations in these parameters. Overall 16 (24%) patients experienced infusion reactions (including cytokine release syndrome), all ≤Grade 2 except one. Serious AEs attributed to margetuximab included two events of IRR, both occurring during first dose administration (at 3.0 and 18.0 mg/kg).
Table 2.
Margetuximab adverse events reported in ≥9 (>15%) patients overall regardless of causality and Grade ≥3 margetuximab-related adverse events reported, by MedDRA preferred term
| Regimen A, N = 34 | Regimen B, N = 32 | Total, N = 66 | ||||
|---|---|---|---|---|---|---|
| Grade | Grade | Grade | ||||
| Any n (%) | ≥3 n (%) | Any n (%) | ≥3 n (%) | Any n (%) | ≥3 n (%) | |
| Fatigue | 8 (24) | 0 | 7 (22) | 0 | 15 (23) | 0 |
| Pyrexia | 10 (29) | 0 | 4 (13) | 0 | 14 (21) | 0 |
| Anemia | 8 (24) | 0 | 5 (16) | 0 | 13 (20) | 0 |
| Nausea | 10 (29) | 0 | 3 (9) | 0 | 13 (20) | 0 |
| Infusion-related reaction | 5 (15) | 1 (3) | 7 (22) | 0 | 12 (18) | 1 (2) |
| Upper respiratory infection | 3 (9) | 0 | 9 (28) | 0 | 12 (18) | 0 |
| Vomiting | 8 (24) | 0 | 4 (13) | 0 | 12 (18) | 0 |
| Decreased appetite | 8 (24) | 0 | 3 (9) | 0 | 11 (17) | 0 |
| Diarrhea | 4 (12) | 0 | 7 (22) | 0 | 11 (17) | 0 |
| Lymphopenia | 2 (6) | 0 | 9 (28) | 0 | 11 (17) | 0 |
| Headache | 3 (9) | 0 | 7 (22) | 0 | 10 (15) | 0 |
| Lymphocyte count decreased | 5 (15) | 2 (6) | 3 (9) | 0 | 8 (12) | 2 (3) |
| Lipase increased | 3 (9) | 0 | 5 (16) | 1 (3) | 8 (12) | 1 (2) |
| Blood amylase increased | 2 (6) | 0 | 5 (16) | 2 (6) | 7 (11) | 2 (3) |
| Blood alkaline phosphatase increased | 2 (6) | 1 (3) | 5 (16) | 0 | 7 (11) | 1 (2) |
No patients experienced an absolute decline in LVEF of >15%, a decline in LVEF <50%, or symptomatic congestive heart failure.
Pharmacokinetics
The population pharmacokinetic analysis included all 66 patients. Estimates of steady-state pharmacokinetic parameters are presented in supplementary Table S2 (available at Annals of Oncology online). Median serum concentrations of margetuximab increased as dose increased (supplementary Figure S1, available at Annals of Oncology online). At doses ≥3.0 mg/kg, simulated mean Ctrough values were approximately >20 μg/ml. Margetuximab pharmacokinetics were well described by a 2-compartment model with parallel linear and Michaelis–Menten elimination and the estimated parameters in agreement with that expected for a mAb of the human IgG1 isotype. For a typical 70-kg patient, based on all data, estimations of CL, V1, Q, and V2 were: 0.299 l/day, 3.73 l, 0.885 l/day, and 3.73 l, respectively. The distribution (T1/2dist) and terminal half-lives (T1/2term) were estimated at 1.12 and 15.5 days, respectively.
ADCC activity
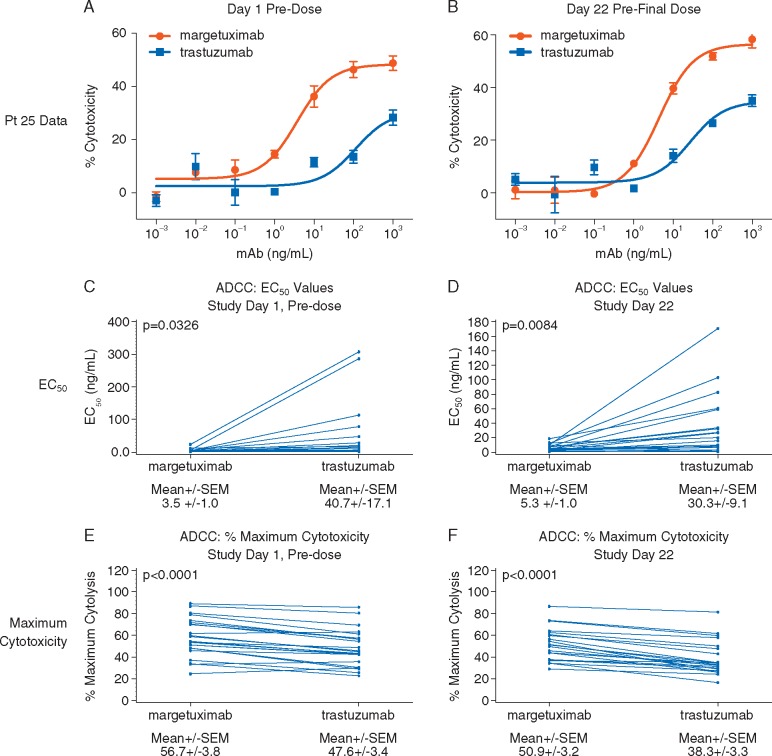
At baseline and Day 22, margetuximab mediated greater ex vivo ADCC activity compared with trastuzumab, using patient PBMCs as effectors, as indicated by lower EC50 and greater maximum cytotoxicity (Figure 1). ADCC mediated by a representative patient PBMC sample is presented in Figure 1A (baseline) and B (Day 22); pairwise comparisons of margetuximab- and trastuzumab-mediated activity across all patient samples are presented in Figure 1C and D (EC50) and 1E and F (maximum cytotoxicity).
Figure 1.
Comparison of ex vivo ADCC activity mediated by margetuximab and trastuzumab against HER2-expressing breast cancer cells using patient PBMC as effector cells. Ex vivo ADCC activity was determined against JIMT-1/Luc cells using PBMC effectors from samples collected from patients on Day 1 (baseline) and Day 22 (prior to the 4th margetuximab dose) in the presence of margetuximab or trastuzumab. Representative ADCC data from Patient 25 are shown in the top panel. Paired analyses of EC50 and maximum cytolysis values for margetuximab and trastuzumab are presented in the middle and lower panels at Day 1 (n = 24) and Day 22 (n = 22).
Antitumor activity
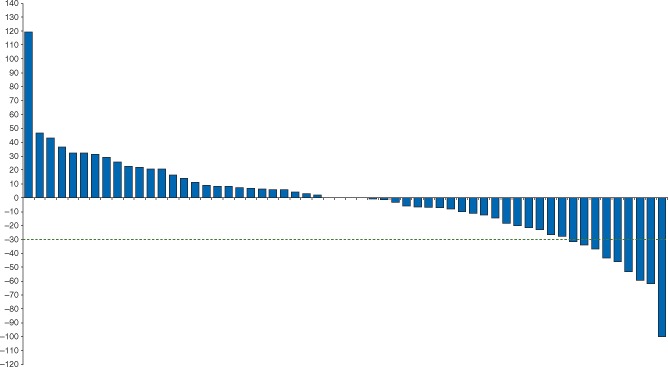
A total of 60 patients were evaluable for tumor response (i.e., received at least one dose of margetuximab and had post-baseline tumor assessment). Seven (12%) patients had confirmed partial response (PR) (4, 2, and 1 patients with breast, gastroesophageal, and lacrimal gland cancer, respectively), 31 (52%) patients had SD, and 22 (37%) patients had PD. All patients who had PRs were HER2-positive as defined as either IHC 3+ or IHC 2+ and FISH amplified. An additional 3 (5%) patients experienced a ≥30% reduction in tumor size, however, developed new lesions and, therefore, not PR per RECIST. Change in tumor size by RECIST sum at time of best response for 58 patients with both baseline and post-baseline target lesion measurements is presented in Figure 2. Among 24 breast cancer patients evaluable for response, all previously treated with at least one HER2-targeted therapy (with 4 patients receiving adjuvant trastuzumab only), 11 (48%) experienced tumor reduction (supplementary Figure S2, available at Annals of Oncology online) with confirmed responses in 4 (17%).
Figure 2.
Waterfall plot of maximum change in tumor measurements (per RECIST). 58 of 60 patients evaluable for tumor response with both pre- and post-baseline target lesion measurements.
The median progression-free survival was 14 weeks (95% CI: 6.29, 16.14) among all 66 patients and 18.1 weeks (95% CI: 12.14, 31.14) among all 27 breast cancer patients (Regimen A: 15.1 weeks [95% CI: 6.29, 40.14]; Regimen B: 21.1 weeks [95%CI: 6.14, 36.14]). Two patients previously treated with trastuzumab, pertuzumab, and ado-trastuzumab emtansine each received 1 and 20 cycles, respectively, with the latter patient currently ongoing in the study with confirmed PR. Two patients who received prior trastuzumab and ado-trastuzumab emtansine (but not pertuzumab) received 9 cycles prior to progression. A 47-year old Asian female with HER2-amplified breast cancer for whom previous trastuzumab + taxane and lapatinib + capecitabine had failed, has received 45 cycles of margetuximab (10 mg/kg Regimen B) with a continued PR.
Discussion
This is the first-in-human clinical trial of margetuximab, a next-generation Fc-modified anti-HER2 mAb. Margetuximab binds to HER2 with high affinity and produces direct growth suppression of HER2-expressing tumor cell lines. Margetuximab has enhancements that increase affinity to the activating FcγR, CD16A (both higher [158V] and lower [158F] affinity forms) and diminish affinity for the inhibitory FcγR, CD32B.
Margetuximab was well tolerated when administered IV at doses of 1.0–6.0 mg/kg weekly or 10.0–18.0 mg/kg Q3W. An MTD was not reached for either regimen. AEs were generally mild to moderate and no cardiotoxicity was observed. Both regimens were equally safe.
The post-infusion disposition of margetuximab is characterized by a rapid distribution phase with short T1/2dist of 1.12 days and slower elimination phase with longer T1/2term of 15.5 days. The T1/2term of margetuximab is shorter than the IgG1 mAbs trastuzumab (28.5 days) [11] and pertuzumab (18.9 days) [12], or endogenous IgG (20 days). In this study, the shorter estimate of the T1/2term may be underestimated as pharmacokinetic sampling was restricted to a dosing interval. A similar phenomenon was reported for trastuzumab, with a half-life of 28.5 days versus 8.3 days based on population pharmacokinetics [11] or limited pharmacokinetic sampling [14], respectively. Nevertheless, regardless of regimen, margetuximab steady state is expected in approximately 2 months (4–5 half-lives) based on T1/2term of 15.5 days.
Similar to trastuzumab and pertuzumab, margetuximab may clear faster at lower doses [11, 12]. With QW dosing, as margetuximab dose increased in a proportion of 1:3:10:30:60, first dose mean AUCinf values increased non-linearly in a proportion of 1:4:18:98:233. However, mean AUCinf between 3.0 and 6.0 mg/kg increased in an approximate proportional manner (1:2.4) for doses in 1:2 proportion. With Q3W dosing, both dose and first dose mean AUCinf values increased in proportion of 1:1.5:1.8. Steady-state values also support this contention. Since therapeutic proteins are removed from circulation by saturable and specific (target- or receptor-mediated clearance) and non-specific (proteolysis) pathways [15–17], at lower doses both elimination mechanisms are active. However, at higher doses, target-mediated clearance is saturated, resulting in slower clearance and more than proportional increases in exposure.
The Q3W regimen (10.0–18.0 mg/kg) compared with the QW regimen (6.0 mg/kg) reveals that margetuximab steady state Cmax are higher (by 0%–69%) and steady state Ctrough are lower (by 39%–61%). High Cmax is unlikely to be of clinical importance due to lack of regimen-related toxicity in the study. Furthermore, lower Ctrough is unlikely to be clinically meaningful because mean Ctrough of margetuximab exceeds 20 µg/ml, the trough concentration associated with trastuzumab clinical efficacy [11, 18, 19]. Since mean serum trough concentration for the 15 mg/kg Q3W regimen exceeded 20 µg/ml after the first dose, and similar to trastuzumab [20, 21], this regimen appears to be a feasible alternative to the weekly regimen and is currently being investigated in ongoing studies of margetuximab.
Ex vivo ADCC assays performed with PBMC samples collected from Regimen A patients demonstrated that margetuximab produced greater maximum cytoxicity and lower EC50 than a trastuzumab surrogate, which was maintained after treatment with margetuximab, confirming prior results [9].
Despite the heavily pre-treated population (median prior therapies = 3), margetuximab demonstrated evidence of clinical activity. Specifically, among response-evaluable patients with breast cancer (all with prior anti-HER2 therapy), four achieved PR. Although continued administration of trastuzumab after progression shows clinical benefit, this is only in combination with chemotherapy. The effect of single-agent margetuximab in the population studied suggests potential for antitumor activity after progression on or following other anti-HER2 regimens.
Overall, the results of this Phase 1 study indicate that single-agent margetuximab is well tolerated with promising activity in patients with HER2-expressing tumors. In patients with HER2-positive metastatic breast cancer with HER2-directed therapy failure, margetuximab shows promise for renewed responses. A randomized trial in patients with HER2-positive metastatic breast cancer with progression on prior HER2-directed therapy (SOPHIA, NCT02492711) has been initiated to evaluate the safety and efficacy of margetuximab with backbone chemotherapies. A study of margetuximab in combination with pembrolizumab (KEYTRUDA®, Merck Sharp and Dohme Corp.), an anti-PD-1 mAb is also ongoing (NCT02689284).
Supplementary Material
Acknowledgements
We wish to thank the patients who participated in this study and their families and gratefully acknowledge the participation of Dr Nancy Lewis, who serves as the independent Data Safety Monitor for this study. We would also like to acknowledge the technical support of Leilei He and Doug Smith. Editorial assistance was provided by 12 Point LLC Seattle, WA and funded by MacroGenics.
Funding
This work was supported by MacroGenics, Inc. and Green Cross Corp. No grant numbers applied.
Disclosure
YJB, GG, DYO, TMB and HAB report no disclosures. JLN, HL, GRC, PAM and JEB are employees of MacroGenics and hold stock and/or stock-options in MacroGenics. SJS and RJL are former employees of, were in leadership roles at, and hold stock in MacroGenics. SAI reports grants from AstraZeneca and advisory role without compensation for Roche and Novartis outside the submitted work.
Footnotes
Present address: Lombardi Cancer Center, Washington, USA
References
- 1. Slamon DJ, Leyland-Jones B, Shak S. et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001; 344: 783–792. [DOI] [PubMed] [Google Scholar]
- 2. Romond EH, Perez EA, Bryant J. et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005; 353: 1673–1684. [DOI] [PubMed] [Google Scholar]
- 3. Piccart-Gebhart MJ, Procter M, Leyland-Jones B. et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 2005; 353: 1659–1672. [DOI] [PubMed] [Google Scholar]
- 4. Bang YJ, Van Cutsem E, Feyereislova A. et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010; 37: 687–697. [DOI] [PubMed] [Google Scholar]
- 5. Bianchini G, Gianni L.. The immune system and response to HER2-targeted treatment in breast cancer. Lancet Oncol 2014; 15(2): e58–e68. [DOI] [PubMed] [Google Scholar]
- 6. Lehrnbecher TL, Foster CB, Zhu S. et al. Variant genotypes of the low-affinity Fcgamma receptors in two control populations and a review of low-affinity Fc gamma receptor polymorphisms in control and disease populations. Blood 1999; 94: 4220–4232. [PubMed] [Google Scholar]
- 7. Musolino A, Naldi N, Bortesi B. et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER2/neu-positive metastatic breast cancer. J Clin Oncol 2008; 26: 1789–1796. [DOI] [PubMed] [Google Scholar]
- 8. Gianni L. The “other” signaling of trastuzumab: antibodies are immunocompetent drugs. J Clin Oncol 2008; 26: 1778–1780. [DOI] [PubMed] [Google Scholar]
- 9. Nordstrom JL, Gorlatov S, Zhang W. et al. Anti-tumor activity and toxicokinetics analysis of MGAH22, an anti-HER2 monoclonal antibody with enhanced Fcγ receptor binding properties. Breast Cancer Res 2011; 13: R123.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tanner M, Kapanen AI, Junttila T. et al. Characterization of a novel cell line established from a patient with Herceptin-resistant breast cancer. Mol Cancer Ther 2004; 3: 1585–1592. [PubMed] [Google Scholar]
- 11. Bruno R, Washington CB, Lu JF. et al. Population pharmacokinetics of trastuzumab in patient with HER2+ metastatic breast cancer. Cancer Chemother Pharmacol 2005; 56: 361–369. [DOI] [PubMed] [Google Scholar]
- 12. Agus DB, Gordon MS, Taylor C. et al. Phase I clinical study of pertuzumab, a novel HER dimerization inhibitor, in patients with advanced cancer. J Clin Oncol 2005; 23: 2534–2543. [DOI] [PubMed] [Google Scholar]
- 13. Leyland-Jones B, Gelman K, Ayoub J-P. et al. Pharmacokinetics, safety, and efficacy of trastuzumab administered every three weeks in combination with paclitaxel. J Clin Oncol 2003; 21: 3965–3971. [DOI] [PubMed] [Google Scholar]
- 14. Tabrizi MA, Tseng CM, Roskos LK.. Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov Today 2006; 11: 81–88. [DOI] [PubMed] [Google Scholar]
- 15. Keizer RJ, Huitema AD, SChellens JH. et al. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet 2010; 49: 493–507. [DOI] [PubMed] [Google Scholar]
- 16. Vugmeyster Y, Xu X, Theil F-P. et al. Pharmacokinetics and toxicology of therapeutic proteins: Advances and challenges. World J Biol Chem 2012; 3: 73–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baselga J, Tripathy D, Mendelsohn J. et al. Phase II study of weekly intravenous recombinant anti-p185HER monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J Clin Oncol 1996; 14: 737–744. [DOI] [PubMed] [Google Scholar]
- 18. Pegram MD, Lipton A, Hayes DF. et al. Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J Clin Oncol 1998; 16: 2659–2671. [DOI] [PubMed] [Google Scholar]
- 19. Carbonell Castellon X, Castaneda-Soto NJ, Clemens M. et al. Efficacy and safety of 3-weekly herceptin (H) monotherapy in women with HER2-positive metastatic breast cancer (MBC): preliminary data from a phase II study. Proc Am Soc Clin Oncol 2002; 21: 19a (abstr 73). [Google Scholar]
- 20. Ghahramani P, Barton C, Leyland-Jones B.. Pharmacokinetics of Herceptin administered three-weekly compared to weekly: a simulation based on data from the clinical studies. Breast 2003; 12: S40 (suppl 1): abstr P89. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.