Abstract
Background
Metastatic melanoma patients can have durable responses to systemic therapy and even long-term survival. However, a large subgroup of patients does not benefit. Tumour metabolic alterations may well be involved in the efficacy of both targeted and immunotherapy. Knowledge on in vivo tumour glucose uptake and its heterogeneity in metastatic melanoma may aid in upfront patient selection for novel (concomitant) metabolically targeted therapies. The aim of this retrospective study was to provide insight into quantitative 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) parameters and corresponding intra- and inter-patient heterogeneity in tumour 18F-FDG uptake among metastatic melanoma patients. Consecutive, newly diagnosed stage IV melanoma patients with a baseline 18F-FDG PET/CT scan performed between May 2014 and December 2015 and scheduled to start first-line systemic treatment were included. Volume of interests (VOIs) of all visible tumour lesions were delineated using a gradient-based contour method, and standardized uptake values (SUVs), metabolically active tumour volume (MATV) and total lesion glycolysis (TLG) were determined on a per-lesion and per-patient basis. Differences in quantitative PET parameters were explored between patient categories stratified by BRAFV600 and RAS mutational status, baseline serum lactate dehydrogenase (LDH) levels and tumour programmed death-ligand 1 (PD-L1) expression.
Results
In 64 patients, 1143 lesions ≥ 1 ml were delineated. Median number of lesions ≥ 1 ml was 6 (range 0–168), median maximum SUVpeak 9.5 (range 0–58), median total MATV 29 ml (range 0–2212) and median total TLG 209 (range 0–16,740). Per-patient analysis revealed considerable intra- and inter-patient heterogeneity. Maximum SUVs, MATV, number of lesions and TLG per patient did not differ when stratifying between BRAFV600 or RAS mutational status or PD-L1 expression status, but were higher in the patient group with elevated LDH levels (> 250 U/l) compared to the group with normal LDH levels (P < 0.001). A subset of patients with normal LDH levels also showed above median tumour 18F-FDG uptake.
Conclusions
Baseline tumour 18F-FDG uptake in stage IV melanoma is heterogeneous, independent of mutational status and cannot be fully explained by LDH levels. Further investigation of the prognostic and predictive value of quantitative 18F-FDG PET parameters is of interest.
Electronic supplementary material
The online version of this article (10.1186/s13550-018-0453-x) contains supplementary material, which is available to authorized users.
Keywords: Stage IV melanoma, 18F-FDG PET/CT, Metabolism, SUV, LDH
Background
Novel therapies, especially immunotherapy, have revolutionized the treatment of stage IV metastatic melanoma over the past decade. One-year overall survival (OS) rates have improved to 50–75% and a subset of patients shows durable responses [1]. Still, a considerable number of patients do not respond, especially those with elevated serum lactate dehydrogenase (LDH) levels [1].
The metabolic reprogramming that characterizes cancer cells may well be involved in the efficacy of antitumour immune responses [2, 3]. Cancer cells metabolize a substantial amount of the consumed glucose through glycolysis only—even under aerobic conditions—in order to generate sufficient biomass for rapid cellular proliferation [4, 5]. Novel therapeutic agents interfering with this altered glucose metabolism have shown hints of anticancer activity in (pre)clinical studies, for example in breast cancer, non-small cell lung cancer and glioblastoma [6, 7]. Additionally, preclinical data suggest metabolically targeted therapies can improve antitumour immune response and susceptibility to adjuvant chemo- and radiotherapy [8–10]. In patients, however, such treatments can result in toxicity in highly glucose-dependent healthy tissues, such as the kidney [7, 11]. Furthermore, recent in vitro studies demonstrate that not all melanomas rely on altered glucose metabolic pathways to the same extent [12, 13]. This underlines the need for upfront selection of patients with highly glucose-dependent tumours in order to maximize the benefit of (concomitant) metabolic therapies and ensure a sufficiently broad therapeutic window.
Metastatic melanoma is clinically renowned for its high uptake of the glucose analogue 18F-fluorodeoxyglucose (18F-FDG) on positron emission tomography/computed tomography (PET/CT) scans. Whole-body 18F-FDG PET/CT is therefore part of standard care staging procedures at baseline in stage IV disease, where it is used in a qualitative fashion to provide information on the presence and location of metastases. However, quantitative 18F-FDG PET/CT scan analysis has been completely unvisited in stage IV melanoma so far and could provide a wealth of knowledge on quantitative tumour glucose uptake in vivo, potentially useful for upfront patient selection for metabolically targeted therapies. The aim of this retrospective study was to provide an overview of tumour 18F-FDG uptake and corresponding intra- and inter-patient heterogeneity in metastatic melanoma patients using quantitative 18F-FDG PET/CT scan analysis.
Patients and methods
Patients
Patients for this retrospective study were selected from a prospectively maintained database containing all melanoma patients registered at the Department of Medical Oncology of the University Medical Center Groningen (UMCG), the Netherlands, from 2012 onwards. All patients ≥ 18 years of age with histologically proven cutaneous or mucosal metastatic melanoma (American Joint Committee on Cancer [AJCC] 7th edition stage IV melanoma [14]) without prior systemic treatment and with a baseline 18F-FDG PET/CT scan performed between May 2014 and December 2015 were eligible for inclusion (n = 108). Exclusion criteria were unknown or inadequate adherence to European Association of Nuclear Medicine (EANM) PET/CT scan acquisition guidelines [15] (e.g. PET/CT scan not performed at our hospital) (n = 26), no indication for start of first-line systemic treatment within 2 months of baseline PET/CT scan (n = 10), concurrent malignancy or other malignancy within the previous 10 years (n = 5) and/or no PET-positive lesions (n = 3). Ultimately, 64 patients were included (Additional file 1: Figure S1). The Medical Ethics Committee approved the study. Consultation of the local objection registry verified that none of the selected patients had objected to use of their personal data for research purposes. Patients were pseudonymized, and data were stored on a secured server following local data management regulations.
18F-FDG PET/CT imaging
18F-FDG PET/CT scans were acquired using a Siemens Biograph mCT PET/CT system (Siemens/CTI, Knoxville, TN) accredited by the European Association of Nuclear Medicine (EANM) Research Limited (EARL). Scan acquisition and reconstructions were performed following the recommendations of the EANM guideline for oncology 18F-FDG imaging [15]. Patients were instructed to fast and avoid exercise at least 4–6 h prior to intravenous 18F-FDG injection at an activity of 3 MBq/kg. Serum glucose levels before tracer injection were < 8.3 mmol/l. Whole-body PET/CT scanning (from the top of the skull to the bottom of the feet) was performed 60 min after 18F-FDG injection with 1–3 min per bed position. Prior to the PET acquisition, patients underwent a low-dose CT (LD-CT) scan during tidal breathing for attenuation correction (80–140 kVp, quel. ref. 30 mAs and pitch of 1).
18F-FDG PET/CT scan analysis and volume of interest delineation
All PET/CT scans were initially reported by a nuclear medicine physician as part of routine patient care. Quantitative scan analysis and identification and delineation of all tumour lesions for this study were performed by one investigator (EH) and verified by a board-certified nuclear medicine physician with expertise in melanoma (AB).
PET(/CT) and gradient PET images were displayed side-to-side, and volume of interests (VOIs) were delineated on the gradient PET images using a gradient-based manual contouring method (in-house developed software program). Gradient PET images are derived directly from reconstructed PET images and depict the relative change in counts between neighbouring voxels (Δ standardized uptake value [SUV]), which is typically the highest around tumour borders. Gradient PET images consequently provide an image where the borders of the lesion are most intense. Use of gradient PET data enables a (manual) VOI delineation method where lesion border location is independent of colour scale, in contrast to manual contouring on regular PET images. Additional motives for choosing gradient-based delineation were a lack of systematic delineation studies in metastatic melanoma and inaccuracy of EARL-recommended semi-automatic delineation methods for delineation of large heterogeneous tumour lesions or small yet highly 18F-FDG-avid lesions [15].
A region of interest (ROI) was manually drawn around each tumour lesion on consecutive transaxial slices. Subsequently, the observer adjusted a %-threshold based on maximum SUV (SUVmax) until the VOI borders optimally corresponded with the location of the steepest gradient on the gradient PET images as judged visually. SUVmax, mean SUV (SUVmean), peak SUV (SUVpeak, i.e. a 1.2-cm3 spheric region positioned to yield the highest average value), metabolically active tumour volume (MATV) and total lesion glycolysis (TLG, the product of SUVmean and MATV) were determined for each VOI. SUVs were corrected for serum glucose level and lean body mass according to the Janmahasatian formula [15].
Lesions with an MATV < 1 ml were excluded from the final quantitative analysis to prevent partial volume effects. PET parameters were analysed on a per-patient, per-location and per-lesion basis. Patient’s maximum SUV and median SUV reflect respectively the highest and median value derived from all lesions ≥ 1 ml within that patient. Interquartile range (IQR) SUVpeak was derived from the SUVpeaks of all individual lesions delineated in one patient as a measure for intra-patient 18F-FDG uptake heterogeneity. Total MATV or total TLG equals the sum of respectively MATV or TLG of all lesions ≥ 1 ml within that patient.
CT and brain MRI scan analysis
Previously, PET-negative (i.e. with SUVmax < 1.5) melanoma metastases have been described, and we excluded three eligible patients upfront due to the presence of only PET-negative lesions [16]. Therefore, we aimed to evaluate the first 20 included patients for the presence of PET-negative lesions with a diameter ≥ 1 cm on baseline contrast-enhanced CT (ce-CT) scan performed within 1 month of the baseline PET/CT. ce-CT scan was available in 12 of the 20 patients and revealed only 2 additional 18F-FDG PET-negative lesions ≥ 1 cm on top of the total of 491 PET-positive lesions > 1 ml in these patients (0.4%). Due to this limited additional value, ce-CT analysis was omitted for the remaining patients.
High physiological background 18F-FDG uptake prevents accurate detection and quantification of brain metastases. Therefore, the presence of brain lesions was additionally evaluated on baseline cerebral MRI scans or cerebral ce-CT. Quantitative data from brain lesions were not incorporated in per-patient PET parameters. When brain lesions were measurable (longest axis on MRI > 1 cm according to Response Assessment in Neuro-Oncology Brain Metastases [RANO-BM] criteria [17]) and 18F-FDG-avid, SUVpeak and SUVmax were measured.
Data acquisition
Patient and tumour characteristics, baseline serum LDH levels and respectively tumour BRAF and RAS mutation status were retrospectively determined from the electronic patient file. Pre-treatment serum LDH levels were derived from the date closest to the baseline PET/CT scan. When pre-treatment archival tumour biopsies for a distant metastasis were available, PD-L1 immunohistochemistry (IHC) was performed as previously described elsewhere using the 22C3 anti-PD-L1 antibody (DAKO, Merck) on Ventana BenchMark ULTRA platform [18]. Tissue derived from primary melanomas, local recurrences, in-transit cutaneous metastases or lymph node metastases was excluded. Scoring was performed by two board-certified pathologists (GFHD, NAH) and performed according to the manufacturer’s instructions.
Statistical analysis
Variables were assessed for normal distribution by Q-Q plots. Independent Mann-Whitney U tests were used to assess differences in PET parameters between LDH, BRAF and RAS groups, respectively, and Kruskal-Wallis tests for differences between metastatic locations and PD-L1 expression groups. Spearman’s rank correlation was used for the correlation between lesion MATV and SUVpeak. A P value < 0.05 (two-sided) was considered statistically significant. Statistical analysis was performed using SPSS Statistics, version 23.0 (IBM Corp., Armonk, NY).
Results
PET parameters on a per-patient basis
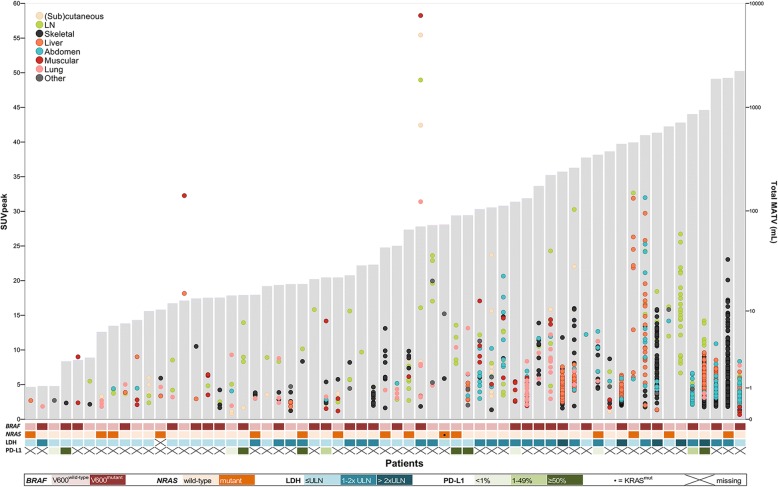
Patient characteristics are presented in Table 1. BRAFV600 mutational status did not differ between patients with normal or elevated serum LDH (42.9% vs. 57.1%; P = 0.260). Patient’s maximum SUVpeak showed a broad range (0–58; median 9.5) between patients (Fig. 1 and Table 2). Furthermore, intra-patient 18F-FDG uptake heterogeneity was observed, with SUVpeak IQR ranging from 0 to 42.4 (median 2.1). The number of lesions, SUVs, total MATV and total TLG (per-patient basis) did not differ between BRAFV600 mutant vs. wild-type patients (Table 3), RAS mutated vs. wild-type patients and BRAFV600/RAS mutant vs. BRAFV600+RAS wild-type patients (data not shown). Patients with an elevated LDH level (> 250 U/l) had more lesions ≥ 1 ml (median 17 vs. 4, P < 0.001), a higher total MATV (127 vs. 14 ml, P < 0.001), higher maximum SUVpeak (13.3 vs. 8.7, P = 0.011), SUVmax (15.8 vs. 11.3, P = 0.026) and SUVmean (9.0 vs. 6.0, P = 0.009) and higher total TLG (1180 vs. 67, P < 0.001) (Table 3). Of the 13 tumour specimens that were available for PD-L1 IHC, 4 showed < 1% PD-L1 expression, 3 1–49% and 6 ≥ 50%. PD-L1 expression status did not correlate with any of the PET parameters (data not shown).
Table 1.
Patient characteristics
| Characteristic | All patients (n = 64) |
|---|---|
| Gender | |
| Male | 40 (62.5%) |
| Female | 24 (37.5%) |
| Age (years) at baseline PET/CT | 59 (45–69) (range 25–80) |
| World Health Organization performance | |
| 0 | 45 (70.3%) |
| 1 | 7 (10.9%) |
| ≥ 2 | 7 (11.0%) |
| Missing | 5 (7.8%) |
| Histology primary melanoma | |
| Cutaneous | 47 (73.4%) |
| Mucosal | 4 (6.3%) |
| Primary melanoma unknown/missing | 13 (20.3%) |
| M-stage at baseline PET/CT | |
| M1a | 1 (1.6%) |
| M1b | 2 (3.1%) |
| M1c | 61 (95.3%) |
| No. of different metastatic locationsa | |
| 1 | 3 (4.7%) |
| 2 | 6 (9.4%) |
| > 2 | 55 (85.9%) |
| Organ involvement | |
| (Sub)cutaneous | 39 (60.9%) |
| Lymph nodes | 54 (84.4%) |
| Lungs | 40 (62.5%) |
| Muscular | 25 (39.1%) |
| Skeletal | 39 (60.9%) |
| Liver | 24 (37.5%) |
| Abdomenb | 30 (46.9%) |
| Otherc | 14 (21.9%) |
| Brain metastasesd | |
| Yes | 22 (34.4%) |
| 18F-FDG-avide | 11 (17.2%) |
| Not 18F-FDG-avid | 11 (17.2%) |
| No | 35 (54.7%) |
| Missing | 7 (10.9%) |
| BRAF mutation status | |
| BRAFV600 mutation | 31 (48.4%) |
| No BRAFV600 mutation | 33 (51.6%) |
| RAS mutation status | |
| RAS mutationf | 15 (23.4%) |
| No RAS mutation | 49 (76.6%) |
| Baseline serum LDH (U/l) | 246 (192–327) (range 92–11,371) |
| Normal | 35 (54.7%) |
| Elevatedg | 28 (43.7%) |
| > 1–2× ULN | 23 (35.9%) |
| > 2× ULN | 5 (7.8%) |
| Missing | 1 (1.6%) |
| Interval between baseline PET/CT and LDH measurement (days) | 0 (− 7 to + 3) (range − 39 to + 11) |
Data are displayed as n (%) or median (interquartile range)
LDH lactate dehydrogenase, ULN upper limit of normal
aIncluding brain metastases
bNumber of patients with lesions in the abdominal cavity/peritoneum (n = 27; 42.2% of all patients), adrenal gland (n = 12; 18.8%), bowel (n = 6; 9.4%), spleen (n = 3; 4.7%), kidney (n = 2; 3.1%), gallbladder (n = 1; 1.6%), stomach (n = 1; 1.6%), rectum (n = 1; 1.6%) and/or pancreas (n = 1; 1.6%)
cNumber of patients with lesions in the vaginal or nasal mucosa (n = 4; 6.3%), myelum (n = 1; 1.6%), shoulder joint (n = 2; 3.1%), breast (n = 2; 3.1%), pericardium (n = 3; 4.7%), heart (n = 2; 3.1%) and/or abdominal or thoracic wall of undetermined tissue of origin (n = 2; 3.1%)
dBased on MRI brain (n = 53) or, when missing, contrast enhanced CT (n = 4)
eI.e. distinguishable from normal brain tissue
fNRAS (n = 14) and KRAS (n = 1)
gI.e. > 250 U/l
Fig. 1.
Individual tumour lesions (≥ 1 ml) and their SUVpeak displayed per patient. For each patient (x-axis; n = 64), individual tumour lesions are plotted against their SUVpeak (left y-axis). Grey shaded bars represent the patient’s total MATV (right y-axis). The heatmap displays respectively the patient’s LDH level and tumour BRAF and NRAS status and PD-L1 expression. Three patients are not displayed since they only had lesions < 1 ml, which resulted in SUVs, a MATV and TLG of 0. LDH lactate dehydrogenase, LN lymph node, PD-L1 programmed death-ligand 1, ULN upper limit of normal
Table 2.
18F-FDG PET tumour lesion parameters on a per-patient basis
| All patients (n = 64) | Range | |
|---|---|---|
| No. of lesions | ||
| All | 18 (11–51) | 1–417 |
| ≥ 1 mla,b | 6 (2–16) | 0–168 |
| SUVpeak | ||
| Maximum | 9.5 (5.5–15.5) | 0–58.3 |
| Median | 4.3 (3.2–8.6) | 0–25.2 |
| SUVpeak interquartile rangec | 2.1 (0–5.1) | 0–42.4 |
| SUVmax | ||
| Maximum | 11.8 (7.3–18.0) | 0–67.2 |
| Median | 6.1 (4.1–11.5) | 0–37.6 |
| SUVmean | ||
| Maximum | 7.2 (4.7–10.7) | 0–30.2 |
| Median | 4.3 (3.0–7.3) | 0–18.5 |
| Total MATV (ml) | 29.2 (12.2–234) | 0–2212 |
| Total TLG | 209 (46.2–1510) | 0–16,740 |
Data are displayed as median (interquartile range)
aThree patients had only lesions < 1 ml
bI.e. all lesions included in quantitative analyses
cI.e. interquartile range of the different SUVpeaks measured within one patient, measure of intra-patient heterogeneity
Table 3.
18F-FDG PET lesion parameters on a per-patient basis, stratified by LDH or BRAFV600 mutation status
| LDH groupsa | P | BRAFV600 groups | P | |||
|---|---|---|---|---|---|---|
| Normalb (n = 35) | Elevated (n = 28) | Wild-type (n = 33) | Mutant (n = 31) | |||
| No. of lesions | ||||||
| All | 13 (7–17) | 46 (20–140) | < 0.001 | 17 (10–34) | 18 (11–64) | 0.510 |
| ≥ 1 mlc | 4 (2–6) | 17 (7–48) | < 0.001 | 6 (3–14) | 6 (2–26) | 0.984 |
| SUVpeak | ||||||
| Maximum | 8.7 (4.4–13.1) | 13.3 (7.1–23.5) | 0.011 | 10.1 (5.6–18.9) | 8.8 (5.2–13.9) | 0.310 |
| Median | 3.9 (2.7–7.4) | 5.5 (3.5–8.9) | 0.203 | 5.3 (3.3–9.0) | 4.0 (3.2–8.3) | 0.317 |
| SUVpeak interquartile ranged | 1.2 (0–3.3) | 3.3 (1.5–6.7) | 0.002 | 2.7 (0–5.1) | 2.0 (0–4.7) | 0.380 |
| SUVmax | ||||||
| Maximum | 11.3 (6.1–16.6) | 15.8 (9.0–27.7) | 0.026 | 13.0 (7.3–22.2) | 11.6 (7.2–17.4) | 0.344 |
| Median | 5.3 (3.7–9.4) | 8.0 (4.9–12.0) | 0.171 | 7.4 (4.5–11.7) | 5.3 (4.0–11.7) | 0.394 |
| SUVmean | ||||||
| Maximum | 6.0 (4.1–8.7) | 9.0 (6.1–15.4) | 0.009 | 8.4 (4.7–12.3) | 6.9 (4.7–8.8) | 0.274 |
| Median | 3.9 (2.8–6.2) | 5.3 (3.4–7.6) | 0.128 | 4.8 (3.2–7.9) | 3.9 (2.9–7.1) | 0.256 |
| Total MATV (ml) | 14 (6–65) | 127 (29–512) | < 0.001 | 44 (10–185) | 29 (13–238) | 0.861 |
| Total TLG | 67 (18–448) | 1180 (200–2998) | < 0.001 | 281 (43–1541) | 199 (64–1568) | 0.984 |
Data are displayed as median (interquartile range)
LDH lactate dehydrogenase
aOne patient had a missing LDH value
bThree patients with normal LDH had only lesions < 1 ml
cAll lesions included in subsequent quantitative analyses
dInterquartile range of the different SUVpeaks measured within one patient, measure of intra-patient heterogeneity
PET parameters on a per-lesion and per-location basis
In total, 3408 tumour lesions were delineated, of which 1143 had an MATV ≥ 1 ml. Median lesion SUVpeak was 5.0 (range 0–58), median MATV was 2.4 ml (range 1.0–1921) and median TLG 11 (range 1.1–11,206) (Additional file 2: Table S1). Lesion SUVpeak and MATV were moderately correlated (correlation coefficient 0.521, P < 0.001). The highest numbers of separate lesions were observed in bone (n = 504, 44% of all lesions ≥ 1 ml), liver (n = 241, 21%) and lymph nodes (n = 125, 11%) (Additional file 3: Figure S2a), and total measured MATV was highest in the abdomen (5683 ml, 39%) followed by bone (3864 ml, 27%) and lymph nodes (2321 ml, 16%) (Additional file 3: Figure S2b). No major differences between metastatic locations concerning individual lesion’s MATV and SUVpeak were observed (Additional file 4: Figure S3).
Brain metastases were present in 22 patients, and 16 had measurable disease according to RANO-BM criteria [17]. In 11 of these patients, brain metastases were visible as hypermetabolic lesions on 18F-FDG PET/CT. Median SUVpeak and SUVmax of these lesions were 7.2 (range 4.8–36.8) and 9.0 (6.5–45.4), respectively.
Overall survival
Following the baseline PET/CT scan, patients commenced standard systemic treatment consisting of either immune checkpoint inhibition, BRAF(/MEK) inhibition and/or dacarbazine chemotherapy. Given the various systemic treatments used, overall survival analysis was performed for exploratory purposes only (Kaplan-Meier overall survival curves in Additional file 5: Figure S4).
Discussion
We show major intra- and inter-patient heterogeneity in tumour lesion 18F-FDG uptake among metastatic melanoma patients. Presence of tumours with above median 18F-FDG uptake was independent of tumour mutational status and did not fully coincide with high serum LDH level. This suggests that tumour 18F-FDG uptake is an independent feature and that 18F-FDG PET parameters might be suitable as a selection tool for novel metabolic therapies.
This is the first large study providing an overview of intra- and inter-patient differences in tumour glucose consumption in metastatic melanoma patients using quantitative whole-body imaging of 18F-FDG uptake. Previous melanoma studies on 18F-FDG PET/CT imaging focused on its diagnostic accuracy for qualitative lesion detection and/or used quantitative parameters derived from the primary melanoma or only a limited number of (the most intense) lesions for response evaluation or prognostic models. By performing quantitative evaluation of all tumour lesions, we highlight the utility of 18F-FDG PET/CT in demonstrating heterogeneity of glucose uptake among metastatic melanoma patients. Preliminary estimates of the influence of tumour 18F-FDG uptake on survival support further prospective investigation as a prognostic biomarker.
Compared to previous studies, we found a higher proportion of bone metastases and a lower incidence of lung and soft tissue metastases. Two previous studies in metastatic melanoma using 18F-FDG PET/CT ± other imaging methods qualitatively report metastases predominantly to the lung, liver, lymph nodes and skin/soft tissue [19, 20]. This difference might be explained by differing patient populations, especially since our study also included patients with an unknown primary melanoma and subsequent widespread (skeletal) metastases (n = 8), as opposed to the study performed by Schoenewolf et al. [19]. Furthermore, we excluded lesions with an MATV < 1 ml to minimize partial volume effects. Three patients had only lesions with an MATV < 1 ml, which all concerned metastases in the lymph nodes, lung, subcutis and/or muscles. The small MATV at these locations, resulting in the exclusion of these lesions for the analysis, further explains the smaller fraction of soft tissue, lymph node and subcutaneous lesions in our PET-based study.
BRAFV600 mutant melanoma cells rely heavily on glycolysis with high glycolytic rates induced by activation of the mitogen-activated protein kinase (MAPK) pathway [21, 22]. BRAFV600 wild-type melanomas (approximately 50% of melanomas) often have alternative mutations in the MAPK-pathway including RAS or MEK1/2 that are also associated with glycolytic dependency and increased glucose uptake [23–25]. In thyroid carcinoma, BRAFV600E tumours show increased expression of glucose transporter (GLUT) and higher SUVs compared to BRAFV600 wild-type tumours [26, 27]. We found no difference in tumour glucose uptake and MATV between patients with and without a BRAFV600 or RAS mutation. Overexpression of other proteins stimulating glucose consumption in the BRAF/RAS wild-type population may explain this observation. Potentially relevant proteins include MEK1/2 (8% of melanomas), involved in the MAPK-pathway [25], and mTOR (10.4% of primary melanomas) or PDK1, involved in the PI3K-Akt-mTOR pathway [28, 29]. Furthermore, patient’s BRAF status is determined based on one tumour tissue sample, not uncommonly the (excised) primary melanoma, and consequently does not necessarily represent the mutational status of all metastases within a patient [30].
Patients with an elevated serum LDH level—a well-established prognostic biomarker for both worse survival and poor treatment response—had higher tumour 18F-FDG uptake as well as higher metabolic tumour volume compared to those with normal LDH levels. However, we also observed tumours with high 18F-FDG in patients with (still) relatively low MATV and normal LDH levels. Moreover, several patients with an elevated LDH level had only tumour lesions with relatively low 18F-FDG uptake. LDH is a cytoplasmic enzyme that catalyses the interconversion of pyruvate and lactate downstream of glycolysis. A high LDH serum level is generally regarded as a marker of cell damage or necrosis, but the exact source of serum LDH levels is unknown. The biological role of LDH in glucose metabolism has also been suggested as an underlying mechanism and in vitro data suggest differential reliance on aerobic glycolysis and oxidative phosphorylation between patients with normal and elevated serum LDH [3, 13]. Unfortunately, meaningful multivariate approaches to unravel the interrelations between tumour volume, tumour glucose consumption and a proposed metabolic factor underlying serum LDH levels were prohibited by collinearity in our data.
Metabolic targeting may constitute a promising novel approach for patients with tumours with high glucose uptake identified by 18F-FDG PET. Furthermore, glycolysis results in extracellular accumulation of lactate and low pH, which are known to impair immune cell function and contribute to an immunosuppressive tumour microenvironment [3, 5]. Metabolic interference combined with immunotherapy might thus be attractive for improving immunotherapy response, for instance in the poorly responding group of metastatic melanoma patients with elevated LDH levels. Metabolic cancer therapies have numerous specific metabolic targets and so far, studies into the correlation between melanoma expression of specific glycolytic transporters and enzymes, such as GLUT1 and hexokinase (HK), and 18F-FDG uptake are limited and contradictive [31, 32]. New studies are needed to integrate tumour 18F-FDG uptake and other clinical biomarkers with tumour dependence upon specific metabolic pathways and targetable metabolic transporters and enzymes.
Limitations of our study include its retrospective nature and patient heterogeneity in treatment, which allowed only preliminary estimates of the influence of tumour PET parameters on survival. The lack of ce-CT in several patients and its more detailed anatomical lesion information could have resulted in erroneous inclusion of physiological PET-positive lesions or exclusion of malignant PET-positive lesions, respectively. Since tumour measurements were performed on PET images only, necrotic areas (observed in three patients) and brain metastases (n = 22) are not incorporated in the MATV.
Conclusions
Tumour 18F-FDG uptake is heterogeneous within and among metastatic melanoma patients. High 18F-FDG uptake is independent of BRAF/RAS mutation status and does not fully correlate with serum LDH levels. This suggests 18F-FDG PET metabolic parameters could serve as an (additional) selection tool for melanoma patients potentially benefiting from metabolic therapies. Further investigation of the prognostic and predictive value of quantitative 18F-FDG PET parameters is warranted.
Additional files
Figure S1. Patient selection. Flow diagram showing the selection of eligible patients. (DOCX 547 kb)
Table S1. 18F-FDG PET lesion parameters on a per-lesion basis. (DOCX 14 kb)
Figure S2. Number of tumour lesions per metastatic location (A) and total MATV (B) per location. In total, 1143 tumour lesions ≥ 1 ml were identified in 64 patients. The outer ring in (A) displays the distribution when lesions < 1 ml are incorporated as well (total lesion n = 3408), showing only minor differences. Total MATV of all 1143 lesions was 14,560 ml (B). LN = lymph node. (DOCX 796 kb)
Figure S3. Individual tumour lesion SUVpeak (A) and MATV (B) per metastatic location. SUVpeak (A) and MATV (B) of individual lesions ≥ 1 ml (total n = 1143), displayed per metastatic location. Boxes represent interquartile range and whiskers respectively 25th and 75th percentile + 1.5 interquartile range. (DOCX 805 kb)
Figure S4. Kaplan-Meier overall survival estimates stratified by LDH levels and PET parameters. Following baseline 18F-FDG PET/CT scan, 30 patients (46.9%) started with immunotherapy, 20 patients (31.3%) started with BRAF(/MEK) inhibition, and 5 patients (7.8%) commenced dacarbazine chemotherapy. Nine patients (14.1%) did not receive any systemic treatment. Twenty-one of the included 64 patients (32.8%) were still alive at the time of analysis (17.9 months after the last included baseline PET/CT scan). Curves display overall survival of all patients (n = 64) stratified by normal vs. elevated (i.e. > 250 U/l) LDH levels and patient population median of respectively maximum SUVpeak (A), total MATV (B) and total TLG (C). LDH = lactate dehydrogenase. (DOCX 271 kb)
Acknowledgements
The authors acknowledge JH van Snick and NA ‘t Hart for their technical assistance.
Funding
EH received a Van Walree Grant of the Royal Netherlands Academy of Arts and Sciences to present these findings at the AACR Metabolism and Cancer 2018 Conference.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 18F-FDG
18F-fluorodeoxyglucose
- AJCC
American Joint Committee on Cancer
- Akt
Protein kinase B
- ce
Contrast-enhanced
- CT
Computed tomography
- EANM
European Association of Nuclear Medicine
- EARL
EANM Research Limited
- HK
Hexokinase
- IHC
Immunohistochemistry
- LD
Low-dose
- LDH
Lactate dehydrogenase
- MAPK
Mitogen-activated protein kinase
- MATV
Metabolically active tumour volume
- MEK
Mitogen-activated protein kinase kinase
- MRI
Magnetic resonance imaging
- mTOR
Mammalian target of rapamycin
- PD-L1
Programmed death-ligand 1
- PET
Positron emission tomography
- PI3K
Phosphoinositide 3-kinase
- RANO-BM
Response Assessment in Neuro-Oncology Brain Metastases
- ROI
Region of interest
- SUV
Standardized uptake value
- TLG
Total lesion glycolysis
- UMCG
University Medical Center Groningen
- VOI
Volume of interest
Authors’ contributions
EH, AB, RB, WS, GH, EV and MJ contributed to the study concepts and design. EH, AB, RB, GD and MJ contributed to the data acquisition and quality control of the data. EH, AB, RB, WS, GD, GH, EV and MJ contributed to the data analyses and interpretation. EH, AB, RB, WS and MJ contributed to the statistical analysis. EH, AB, RB and MJ contributed to the manuscript preparation. EH, AB, RB, WS, GD, EV, GH and MJ contributed to the manuscript review and editing. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This retrospective study was approved by the Institutional Medical Ethics Committee (case number 2016/474), and the need for informed consent was waived. Consultation of the local objection registry verified that none of the selected patients had objected to use of their personal data for research purposes.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ellen C. de Heer, Email: e.c.de.heer@umcg.nl
Adrienne H. Brouwers, Email: a.h.brouwers@umcg.nl
Ronald Boellaard, Email: r.boellaard@umcg.nl.
Wim J. Sluiter, Email: w.j.sluiter@umcg.nl
Gilles F. H. Diercks, Email: g.f.h.diercks@umcg.nl
Geke A. P. Hospers, Email: g.a.p.hospers@umcg.nl
Elisabeth G. E. de Vries, Email: e.g.e.de.vries@umcg.nl
Mathilde Jalving, Email: m.jalving@umcg.nl.
References
- 1.Ugurel S, Röhmel J, Ascierto PA, Flaherty KT, Grob JJ, Hauschild A, et al. Survival of patients with advanced metastatic melanoma: the impact of novel therapies. Eur J Cancer. 2016;53:125–134. doi: 10.1016/j.ejca.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Brand A, Singer K, Koehl GE, Kolitzus M, Schoenhammer G, Thiel A, et al. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab. 2016;24:657–671. doi: 10.1016/j.cmet.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Blank CU, Haanen JB, Ribas A, Schumacher TN. The “cancer immunogram”. Science. 2016;352:658–660. doi: 10.1126/science.aaf2834. [DOI] [PubMed] [Google Scholar]
- 4.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michelakis ED, Sutendra G, Dromparis P, Webster L, Haromy A, Niven E, et al. Metabolic modulation of glioblastoma with dichloroacetate. Sci Transl Med. 2010;2:31ra34. doi: 10.1126/scitranslmed.3000677. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Outschoorn UE, Peiris-Pagés M, Pestell RG, Sotgia F, Lisanti MP. Cancer metabolism: a therapeutic perspective. Nat Rev Clin Oncol. 2017;14:11–31. doi: 10.1038/nrclinonc.2016.60. [DOI] [PubMed] [Google Scholar]
- 8.Vartanian A, Agnihotri S, Wilson MR, Burrell KE, Tonge PD, Alamsahebpour A, et al. Targeting hexokinase 2 enhances response to radio-chemotherapy in glioblastoma. Oncotarget. 2016;7:69518–69535. doi: 10.18632/oncotarget.11680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bénéteau M, Zunino B, Jacquin MA, Meynet O, Chiche J, Pradelli LA, et al. Combination of glycolysis inhibition with chemotherapy results in an antitumor immune response. Proc Natl Acad Sci U S A. 2012;109:20071–20076. doi: 10.1073/pnas.1206360109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganapathy-Kanniappan S, Geschwind JF. Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol Cancer. 2013;12:152. doi: 10.1186/1476-4598-12-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garon EB, Christofk HR, Hosmer W, Britten CD, Bahng A, Crabtree MJ, et al. Dichloroacetate should be considered with platinum-based chemotherapy in hypoxic tumors rather than as a single agent in advanced non-small cell lung cancer. J Cancer Res Clin Oncol. 2014;140:443–452. doi: 10.1007/s00432-014-1583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shestov AA, Mancuso A, Lee SC, Guo L, Nelson DS, Roman JC, et al. Bonded cumomer analysis of human melanoma metabolism monitored by 13C NMR spectroscopy of perfused tumor cells. J Biol Chem. 2016;291:5157–5171. doi: 10.1074/jbc.M115.701862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho J, de Moura MB, Lin Y, Vincent G, Thorne S, Duncan LM, et al. Importance of glycolysis and oxidative phosphorylation in advanced melanoma. Mol Cancer. 2012;11:76. doi: 10.1186/1476-4598-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–354. doi: 10.1007/s00259-014-2961-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strobel K, Dummer R, Husarik DB, Pérez Lago M, Hany TF, Steinert HC. High-risk melanoma: accuracy of FDG PET/CT with added CT morphologic information for detection of metastases. Radiology. 2007;244:566–574. doi: 10.1148/radiol.2442061099. [DOI] [PubMed] [Google Scholar]
- 17.Lin NU, Lee EQ, Aoyama H, Barani IJ, Barboriak DP, Baumert BG, et al. Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol. 2015;16:270–278. doi: 10.1016/S1470-2045(15)70057-4. [DOI] [PubMed] [Google Scholar]
- 18.Ilie M, Khambata-Ford S, Copie-Bergman C, Huang L, Juco J, Hofman V, et al. Use of the 22C3 anti–PD-L1 antibody to determine PD-L1 expression in multiple automated immunohistochemistry platforms. PLoS One. 2017;12:e0183023. doi: 10.1371/journal.pone.0183023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schoenewolf NL, Belloni B, Simcock M, Tonolla S, Vogt P, Scherrer E, et al. Clinical implications of distinct metastasizing preferences of different melanoma subtypes. Eur J Dermatology. 2014;24:236–241. doi: 10.1684/ejd.2014.2292. [DOI] [PubMed] [Google Scholar]
- 20.Frauchiger AL, Mangana J, Rechsteiner M, Moch H, Seifert B, Braun RP, et al. Prognostic relevance of lactate dehydrogenase and serum S100 levels in stage IV melanoma with known BRAF mutation status. Br J Dermatol. 2016;174:823–830. doi: 10.1111/bjd.14347. [DOI] [PubMed] [Google Scholar]
- 21.Hall A, Meyle KD, Lange MK, Klima M, Sanderhoff M, Dahl C, et al. Dysfunctional oxidative phosphorylation makes malignant melanoma cells addicted to glycolysis driven by the (V600E)BRAF oncogene. Oncotarget. 2013;4:584–599. doi: 10.18632/oncotarget.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hardeman KN, Peng C, Paudel BB, Meyer CT, Luong T, Tyson DR, et al. Dependence on glycolysis sensitizes BRAF-mutated melanomas for increased response to targeted BRAF inhibition. Sci Rep. 2017;7:42604. doi: 10.1038/srep42604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973–977. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerr EM, Gaude E, Turrell FK, Frezza C, Martins CP. Mutant Kras copy number defines metabolic reprogramming and therapeutic susceptibilities. Nature. 2016;531:110–113. doi: 10.1038/nature16967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richtig G, Hoeller C, Kashofer K, Aigelsreiter A, Heinemann A, Kwong LN, et al. Beyond the BRAFV600E hotspot: biology and clinical implications of rare BRAF gene mutations in melanoma patients. Br J Dermatol. 2017;177:936–944. doi: 10.1111/bjd.15436. [DOI] [PubMed] [Google Scholar]
- 26.Choi EK, Chong A, Ha JM, Jung CK, O JH, Kim SH. Clinicopathological characteristics including BRAF V600E mutation status and PET/CT findings in papillary thyroid carcinoma. Clin Endocrinol. 2017;87:73–79. doi: 10.1111/cen.13335. [DOI] [PubMed] [Google Scholar]
- 27.Yoon M, Jung SJ, Kim TH, Ha TK, Urm SH, Park JS, et al. Relationships between transporter expression and the status of BRAF V600E mutation and F-18 FDG uptake in papillary thyroid carcinomas. Endocr Res. 2016;41:64–69. doi: 10.3109/07435800.2015.1066803. [DOI] [PubMed] [Google Scholar]
- 28.Yan K, Si L, Li Y, Wu X, Xu X, Dai J, et al. Analysis of mTOR gene aberrations in melanoma patients and evaluation of their sensitivity to PI3K-AKT-mTOR pathway inhibitors. Clin Cancer Res. 2016;22:1018–1027. doi: 10.1158/1078-0432.CCR-15-0939. [DOI] [PubMed] [Google Scholar]
- 29.Pópulo H, Caldas R, Lopes JM, Pardal J, Máximo V, Soares P. Overexpression of pyruvate dehydrogenase kinase supports dichloroacetate as a candidate for cutaneous melanoma therapy. Expert Opin Ther Targets. 2015;19:733–745. doi: 10.1517/14728222.2015.1045416. [DOI] [PubMed] [Google Scholar]
- 30.Riveiro-Falkenbach E, Santos-Briz A, Ríos-Martín JJ, Rodríguez-Peralto JL. Controversies in intrapatient melanoma BRAFV600E mutation status. Am J Dermatopathol. 2017;39:291–295. doi: 10.1097/DAD.0000000000000710. [DOI] [PubMed] [Google Scholar]
- 31.Park SG, Lee JH, Lee WA, Han KM. Biologic correlation between glucose transporters, hexokinase-II, Ki-67 and FDG uptake in malignant melanoma. Nucl Med Biol. 2012;39:1167–1172. doi: 10.1016/j.nucmedbio.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Yamada K, Brink I, Bissé E, Epting T, Engelhardt R. Factors influencing [F-18] 2-fluoro-2-deoxy-D-glucose (F-18 FDG) uptake in melanoma cells: the role of proliferation rate, viability, glucose transporter expression and hexokinase activity. J Dermatol. 2005;32:316–334. doi: 10.1111/j.1346-8138.2005.tb00903.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Patient selection. Flow diagram showing the selection of eligible patients. (DOCX 547 kb)
Table S1. 18F-FDG PET lesion parameters on a per-lesion basis. (DOCX 14 kb)
Figure S2. Number of tumour lesions per metastatic location (A) and total MATV (B) per location. In total, 1143 tumour lesions ≥ 1 ml were identified in 64 patients. The outer ring in (A) displays the distribution when lesions < 1 ml are incorporated as well (total lesion n = 3408), showing only minor differences. Total MATV of all 1143 lesions was 14,560 ml (B). LN = lymph node. (DOCX 796 kb)
Figure S3. Individual tumour lesion SUVpeak (A) and MATV (B) per metastatic location. SUVpeak (A) and MATV (B) of individual lesions ≥ 1 ml (total n = 1143), displayed per metastatic location. Boxes represent interquartile range and whiskers respectively 25th and 75th percentile + 1.5 interquartile range. (DOCX 805 kb)
Figure S4. Kaplan-Meier overall survival estimates stratified by LDH levels and PET parameters. Following baseline 18F-FDG PET/CT scan, 30 patients (46.9%) started with immunotherapy, 20 patients (31.3%) started with BRAF(/MEK) inhibition, and 5 patients (7.8%) commenced dacarbazine chemotherapy. Nine patients (14.1%) did not receive any systemic treatment. Twenty-one of the included 64 patients (32.8%) were still alive at the time of analysis (17.9 months after the last included baseline PET/CT scan). Curves display overall survival of all patients (n = 64) stratified by normal vs. elevated (i.e. > 250 U/l) LDH levels and patient population median of respectively maximum SUVpeak (A), total MATV (B) and total TLG (C). LDH = lactate dehydrogenase. (DOCX 271 kb)
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.