Abstract
Background
Perioperative strategies can significantly influence long-term cancer outcomes. Dexmedetomidine, an α2-adrenoceptor agonist, is increasingly used perioperatively for its sedative, analgesic, anxiolytic, and sympatholytic effects. Such actions might attenuate the perioperative promotion of metastases, but other findings suggest opposite effects on primary tumour progression. We tested the effects of dexmedetomidine in clinically relevant models of dexmedetomidine use on cancer metastatic progression.
Methods
Dexmedetomidine was given to induce sub-hypnotic to sedative effects for 6–12 h, and its effects on metastasis formation, using various cancer types, were studied in naïve animals and in the context of stress and surgery.
Results
Dexmedetomidine increased tumour-cell retention and growth of metastases of a mammary adenocarcinoma (MADB 106) in F344 rats, Lewis lung carcinoma (3LL) in C57BL/6 mice, and colon adenocarcinoma (CT26) in BALB/c mice. The metastatic burden increased in both sexes and in all organs tested, including lung, liver, and kidney, as well as in brain employing a novel external carotid-artery inoculation approach. These effects were mediated through α2-adrenergic, but not α1-adrenergic, receptors. Low sub-hypnotic doses of dexmedetomidine were moderately beneficial in attenuating the deleterious effects of one stress paradigm, but not of the surgery or other stressors.
Conclusions
The findings call for mechanistic translational studies to understand these deleterious effects of dexmedetomidine, and warrant prospective clinical trials to assess the impact of perioperative dexmedetomidine use on outcomes in cancer patients.
Keywords: dexmedetomidine, neoplasm metastasis, perioperative care
Editor's key points.
-
•
Recent evidence points to both positive and negative effects of perioperative management on long-term outcomes in cancer surgery.
-
•
The impact of dexmedetomidine on tumour metastasis was tested in three rodent cancer models.
-
•
Dexmedetomidine had dose-dependent deleterious effects on markers of tumour-cell metastasis, which require further translational and clinical validation.
Surgery is a necessary therapeutic intervention in most patients with solid tumours, but has also been suggested to worsen the metastatic progression.1, 2 Animal studies and supporting clinical observations have indicated that specific perioperative procedures have critical impacts on long-term cancer outcomes, worsening or improving them.3, 4 The key mediating mechanisms are perioperative stress and cytokine responses, which can lead to immune suppression5, 6 and directly facilitate the progression of a residual malignant disease.7, 8 Prominent amongst these surgical stress responses are inflammatory and sympathetic responses, which were shown to, 1) suppress several aspects of anti-metastatic immunity (e.g. natural-killer cytotoxicity),9, 10 and, 2) facilitate metastatic progression through the direct effects of prostaglandins and catecholamines on a residual malignant disease or its micro-environment.8, 11
Despite being relatively short, the perioperative period involves numerous risk factors, but also presents unexploited opportunities to improve the overall patient survival.12 A prominent aspect of surgery that can be manipulated so that cancer outcomes can be potentially improved is the choice of anaesthetic/analgesic approaches, which have been suggested to impact various endocrinological, immunological, and cancer-related outcomes in animal and human studies.6, 13, 14, 15, 16, 17, 18
Dexmedetomidine is an α2-adrenoreceptor agonist that is used as a sedative and an adjuvant to anaesthetic strategies in the perioperative context. Increasingly used in the USA and recently entering the European market,19 it is unique in exerting sedative, anxiolytic, analgesic, and sympatholytic effects, and exhibiting opioid- and anaesthetic-sparing effects.20, 21, 22 Considering these effects of dexmedetomidine, specifically its sympatholytic effects, one can presume beneficial actions in the perioperative care of cancer patients. However, in vitro studies have shown increased cell survival and cell proliferation by dexmedetomidine,23, 24 and in vivo studies have reported both potentiation25, 26 and lack of effect of dexmedetomidine on tumour progression.27, 28 Notably, most in vivo studies employed chronic manipulations of α2-adrenoceptor, and assessed the growth of a primary tumour, rather than using dexmedetomidine acutely and studying the metastatic process, which are most relevant to the clinical settings in cancer patients.
We studied the influences of the short-term use of clinically relevant doses of dexmedetomidine on the progression of cancer metastases in rodent models in the context of stress and surgery. To support the generalizability of the outcomes beyond the specific characteristics of a single tumour line, we employed three types of cancer and different approaches of tumour-cell inoculation in rats and mice, studying the metastatic efficacy and progression in various organs of males and females. In two tumour models, in addition to studying the actual number of metastases developed 21 days following dexmedetomidine and tumour administration, we assessed an earlier index of metastatic efficacy—retention of tumour cells in different organs at 24 h following i.v. tumour inoculation. This index, whilst reflecting the numbers of viable tumour cells in an organ that can potentially form solid metastases,29, 30, 31 also more specifically reflects the potential initial impact of dexmedetomidine administration (12–24 h) on early processes of metastasis formation (survival of tumour cells in the circulation and extravasation into an organ).
Methods
Animals
Male and female F344 rats, and C57BL/6 and BALC/c mice (Harlan Laboratories, Jerusalem, Israel) were housed three to four per cage, under a 12:12 h light/dark cycle at 21o–23o C with ad libitum food and water. The animals were 12–20 weeks old at the beginning of the experimentation (age matched per experiment). The rats were handled daily for 4 days prior to each experiment to reduce unwanted procedural stress, and were randomly allocated to groups. Exposure to stress was counterbalanced across cages (i.e. an entire cage was subjected or not to stress) in order to prevent unwanted stress in controls. Drug administration was counterbalanced within cages. All experiments were approved by the Institutional Animal Care and Use Committee of the Tel Aviv University, Tel Aviv, Israel (protocol 10-15-002), and conformed to Animal Research: Reporting In Vivo Experiments (ARRIVE) guidelines.
Drugs and their administration
Dexmedetomidine, an α2-adrenergic agonist, and yohimbine, an α2-adrenergic antagonist (both Sigma, Rehovot, Israel), were administered (s.c. 0.5 ml; 21G needle), employing a slow release vehicle (see Supplementary material 1.1), which prevents initial high plasma levels and maintains prolonged exposure to the drugs. Phenoxybenzamine (Sigma, Rehovot, Israel), an irreversible α1-adrenoceptor antagonist, was dissolved in propylene glycol (s.c., 1 ml kg−1; 1.5 or 4 mg kg−1; see32 for dose).
Dexmedetomidine schedule
To closely simulate the dexmedetomidine clinical kinetics and impact, the doses used in most experiments—5 and 10 μg kg−1 h−1—were chosen to reach an average plasma level of 0.7 and 1.5 ng ml−1, respectively. These doses are based upon our testing of reflex loss, which were correlated to plasma concentration in a study that systematically assessed such relations.33 For further elaboration and results for these studies, see Supplementary material 3.1.
General experimental procedures
Each experimental procedure is detailed in Supplementary material 2. The general procedures for all experiments are shown in Fig. 1; changes to this procedure, if any, are detailed within specific experiments.
Fig 1.
Maintenance and radiolabelling of MADB 106, CT26, and 3LL tumour cells, and assessment of organ tumour retention.
All three tumour-cell lines were obtained from the National Institutes of Health tumour bank, tested for Mycoplasma, and maintained frozen in liquid nitrogen until use. MADB 106 is a mammary adenocarcinoma syngeneic to F344 rats, CT26 is colorectal carcinoma syngeneic to BALB/c mice, and 3LL is Lewis lung carcinoma syngeneic to C57BL/6 mice. Cells were thawed and cultured in monolayer in complete media (RPMI-1640 media supplemented with 10% heat-inactivated foetal calf serum, 50 μg mL−1 gentamicin, 2 mM L-glutamine, 0.1 mM non-essential amino acids, and 1 mM of sodium pyruvate (Biological Industries, Kibbutz Beit Haemek, Israel) in 100% humidity, 5% CO2 in air at 37°C.
DNA radiolabelling for the assessment of organ tumour retention was accomplished by adding 0.5 μCi ml−1 of 125Iododeoxyuridine (125IDUR, PerkinElmer, Hod hasharon, Israel) to the cell culture for 21 h. Cells were removed from the culture flask with trypsin solution [0.25% in phosphate-buffered saline (PBS)], and were washed with complete media.
For tumour-cell injection, rats or mice were lightly anaesthetised with isoflurane, and 4 × 105 tumour cells kg−1 in PBS (0.5 ml for rats or 100 μl for mice) containing 0.1% bovine serum albumin were injected into the tail vein or external carotid artery (ECA). For the assessment of organ tumour-cell retention, animals were sacrificed with 100% inhaled CO2 21 h after the inoculation with 125IDUR-labelled tumour cells, and specific organs were removed and placed in a γ-counter to assess the percent radioactivity retained in the organ. Tumour retention was calculated using the following formula:
| (radioactivity count of organ − background radioactivity) × 100/(radioactivity count of the total injected cell suspension − background radioactivity) |
Induction and counting of MADB 106 and CT26 experimental metastases
Rats or mice were anaesthetised with isoflurane, and unlabelled MADB 106 or CT26 tumour cells, respectively, were injected into the tail vein (rats) or spleen (mice, through a 0.5 cm abdominal incision, followed by splenectomy). Three weeks later, the animals were sacrificed, and their lungs (rats) or livers (mice) were removed, and visible surface metastases were counted by a blinded researcher. For details of the injection procedures and metastases counting, see Supplementary material 1.2 and 1.3.
External carotid artery injection procedure
This procedure enables a controlled administration of tumour cells directly to the brain without disturbing brain blood flow34 (see Supplementary material 1.4). This approach and the administration of CT26 cells through the spleen involve surgical procedures of ∼15 min.
Stress procedures
Experimental laparotomy
Briefly, rats were anaesthetised with 2.5% isoflurane, and a 4 cm midline abdominal incision was performed, opened for 20 min, and sutured with 3-0 nylon thread (see Supplementary material 1.5).
Restraint stress
Rats were placed in a perforated clear Plexiglas tube (7 cm diameter, 40 cm length) for 180 min; at 90 min, they were removed temporarily for tumour injection.
Wet-cage stress
Rats were placed for 90 min in standard transparent Plexiglas cages containing 2 cm water at room temperature (ad libitum food and water). The animals were returned to their home cages 15 min before the tumour-cell injection.
Measuring body temperature and maintaining normothermia
To prevent potential hypothermia induced by dexmedetomidine, heating pads (heating cage bedding to 39–40°C; inactive pads in controls) were activated for 8 h (duration of dexmedetomidine hypothermic effects) starting 30 min after dexmedetomidine injections. The degree of hypothermia induced by dexmedetomidine and the ability of the heating pad to induce normothermia were verified a priori (see Supplementary material 3.2).
Statistical and power analyses
One-/two-/three-way anova were used in all studies. When significant group differences were found, Fisher's protected least significant difference (PLSD) post hoc tests were used to test specific pairwise differences. For all analyses, P<0.05 was considered significant. Power analyses were conducted separately for the different outcomes, given their different variances. For the index of lung tumour retention (LTR) in MADB 106 and 3LL tumours, provided a 1.5 effect size or greater, and based on the known variance in these indices in our laboratory, a power of 80% is achieved with 7.2 animals. For the index of metastasis number in MADB 106 and CT26 tumour models, and given variance estimations from our previous studies employing these indices, 12 animals per group are needed to achieve a power of 80% provided a 2.1 effect size.
Results
MADB 106 lung tumour retention in naïve rats
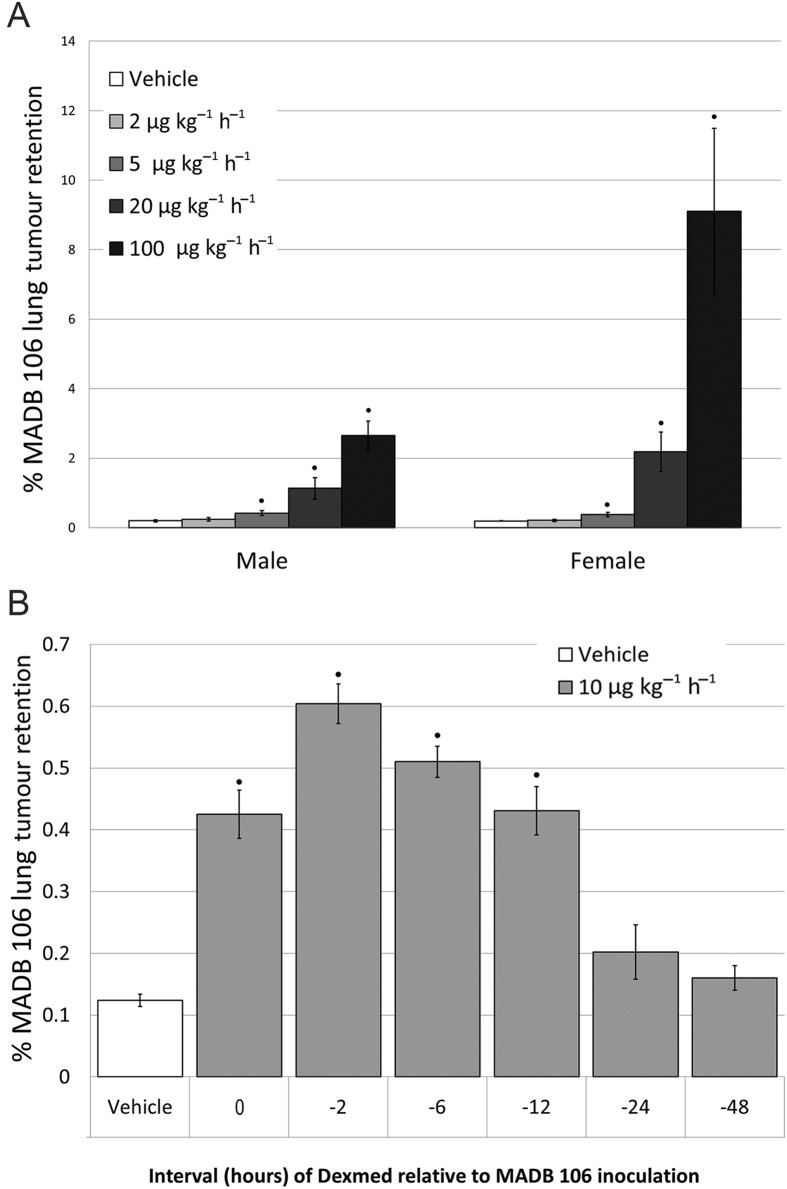
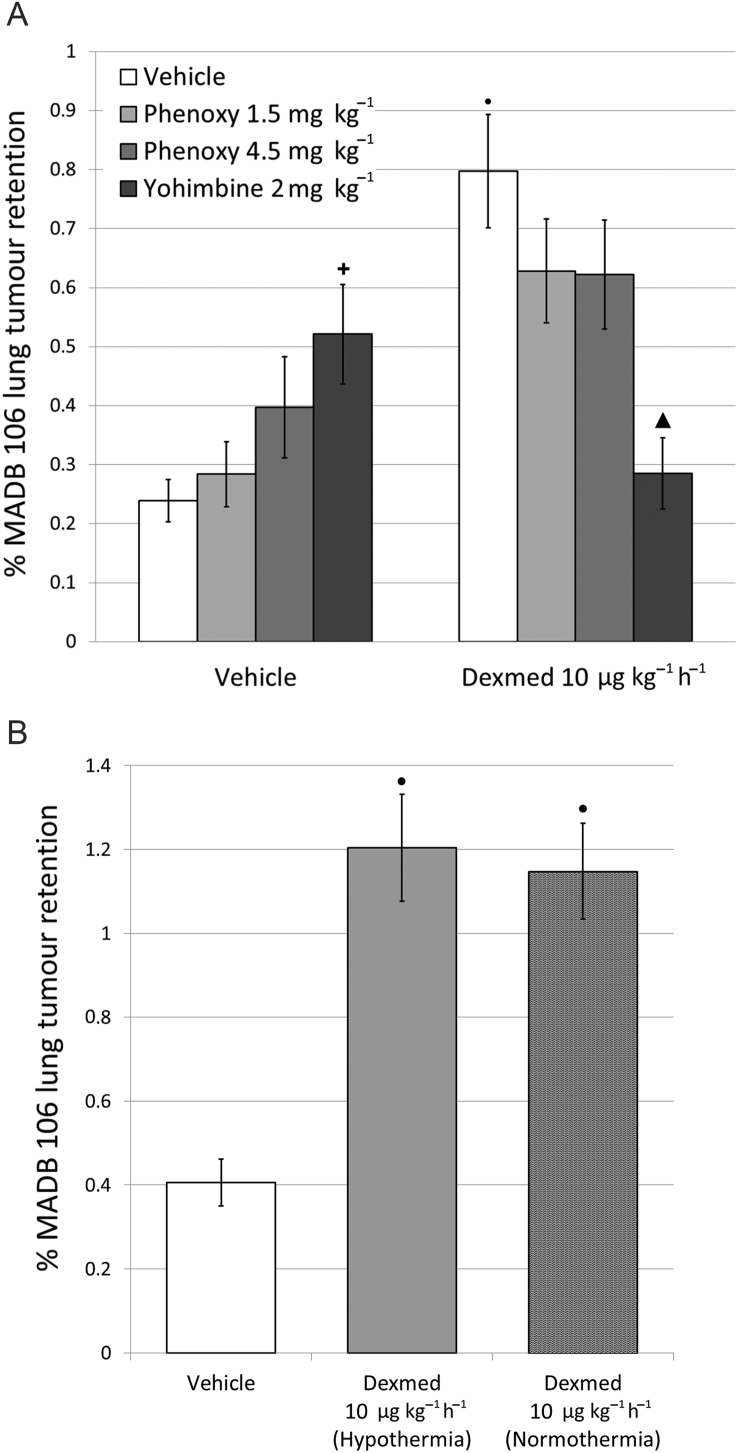
Dexmedetomidine significantly [F(4,91)=34.82, P<0.0001] increased MADB 106 lung tumour retention (LTR) dose dependently in both sexes, starting at a sub-hypnotic dose of 5 μg kg−1 h−1 (Fig. 2A). These deleterious effects occurred when dexmedetomidine was injected simultaneously with MADB 106 cells, as well as when injected 2, 6, or 12 h before (PLSD's P<0.0001) (Fig. 2B). When dexmedetomidine was given with yohimbine, a selective α2-adrenorecptor antagonist, its effects on MADB 106 LTR were prevented, but were not significantly affected when given with phenoxybenzamine, an α1-adreneceptor agonist (Fig. 3A). Hypothermia induced by dexmedetomidine did not mediate the effects of these drugs, as maintaining normothermia did not reduce their impact [F(2,40)=18.193, P<0.0001; Fig. 3B].
Fig 2.
Dexmedetomidine dose-dependently elevated MADB106 lung tumour retention in both sexes. A) Dexmededetomidine (Dexmed) elevated lung tumour retention dose-dependently, yielding significant increase in LTR compared to control levels at doses of 5 μg·kg-1·hr-1 and higher, exerting a greater impact in females. Two-way ANOVA indicated a significant main effect for dose of Dexmed (F(4,91) =34.82, p<0.0001), for sex (F(1,91) =21.96, p<0.0001), and a significant interaction (F(4,91) =11.42, p<0.0001). N=44 females and 49 males. B) Female rats (n = 50) were injected with Dexmed at different time intervals (in hr) before MADB106 tumour cells injection (-48,-24,-12,-6 or -2 and simultaneously with it (0)). One-way ANOVA indicated significant group differences (F6,49 =41.231, p<0.0001), and Fischer's PLSD pair-wise comparisons indicated that Dexmed increased LTR significantly if administered at 0, 2, 6, & 12 hr before tumour injection (p<0.0001 for all), but not at 24, or 48 hr. ● indicates significant pair-wise difference from the respective control (vehicle) group (PLSD, p < 0.05). Data presented as mean (SEM).
Fig 3.
Dexmedetomidine affects MADB106 lung tumour retention via α2 -adrenoceptors, but not via α1 -adrenoceptors, and the effects are not mediated through hypothermia. A) Female rats (n=29) received either yohimbine (α2-adrenoceptor antagonist) or phenoxybenzamine (phenoxy; α1-adreneceptor antagonist) with or without dexmedetomidine (Dexmed) (10 μg·kg-1·hr-1), one-way ANOVA indicated significant group differences (F7,28 =8.718, p<0.0001). B) Dexmededetomidine similarly increased tumour retention in male rats (n=43) both under normothermia or hypothermia conditions. One-way ANOVA indicated significant group differences (F2,40 =18.193, p<0.0001), ▴ indicates a significant increase relative to the control vehicle group (PLSD, p < 0.05) ● indicates a significant increase from vehicle group (PLSD, p < 0.05). Data presented as mean (SEM).
Impact of dexmedetomidine on MADB 106 lung tumour retention under stress and surgery conditions
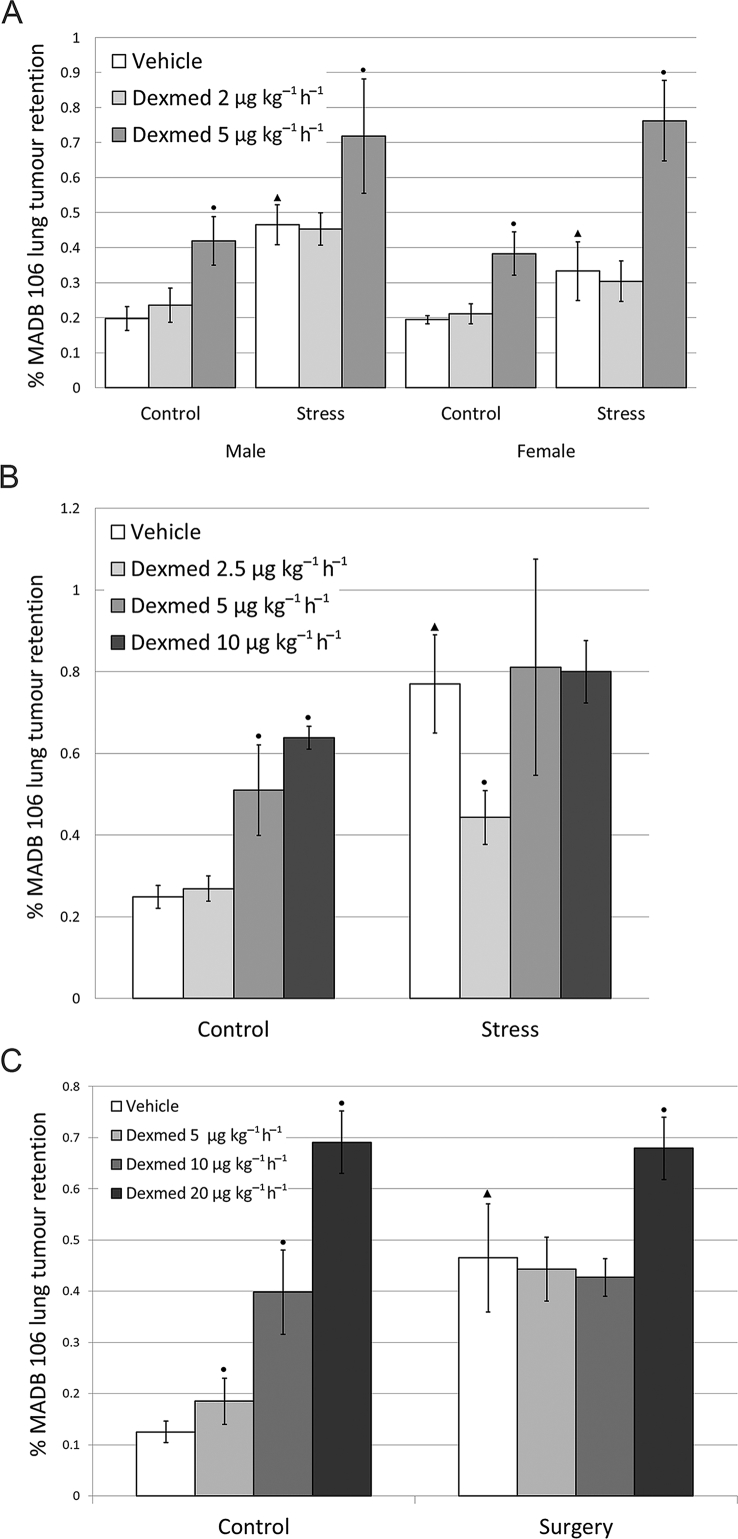
The surgery and both stress paradigms employed (wet-cage and restraint stress) significantly increased MADB 106 LTR irrespective of dexmedetomidine [surgery-F1,46 = 10.13, p = 0.0026, wet cage F(1,143)=22.154, P<0.0001, restraint-F1,56 = 12.892, p = 0.0007] (Fig. 4A–C). As expected, dexmedetomidine had deleterious effects in non-stressed and non-operated animals starting from 5 μg kg−1 h−1 (Fig. 4A–C). In animals subjected to wet-cage stress, dexmedetomidine exerted similar deleterious effects as in non-stressed animals, further increasing LTR beyond the effects of stress [F(2,143)=14.826, P<0.0001] (Fig. 4A). Interestingly, under restraint stress, dexmedetomidine interacted with the effects of stress, not exhibiting deleterious effect beyond the effects of stress, and in one low dose (2.5 μg kg−1 h−1), dexmedetomidine significantly reduced them (PLSD P=0.0077) (Fig. 4B). In animals undergoing laparotomy, dexmedetomidine did not exert deleterious effects above the effects of surgery, indicating it had no interaction with the effects of surgery. At a higher dose (20 μg kg−1 h−1), however, dexmedetomidine had deleterious effects in the operated animals (PLSD P<0.05) (Fig. 4C). We repeated this experiment in animals subjected to laparotomy four times, employing different doses of dexmedetomidine (2.5–20 μg kg−1 h−1) in both sexes, and failed to have beneficial effects in any of the conditions tested (Supplementary material 3.3).
Fig 4.
Dexmedetomidine does not attenuate the deleterious effects of wet-cage or surgery stress on MADB106 lung tumour retention, but does so at a low dose under restrainer stress. A) Wet Cage Both female and male rats (n=71, 84 respectively) were either subjected to wet-cage stress or maintained at their home cages (control). Three-way ANOVA indicated a significant main effect for the dose of dexmedetomidine (Dexmed) (F2,143 =14.826, p<0.0001) and for stress (F1,143 =22.154, p<0.0001), but no significant effect for sex and no interactions. Both wet-cage stress and Dexmed elevated tumour retention significantly. B) Restraint Male rats (n=75) were subjected to either restraint stress or maintained at their home cages. While restraint stress elevated tumour retention levels, a low dose of Dexmed (2.5 μg·kg-1·hr-1) abolished this effect (PLSD p=0.0077). Two-way ANOVA indicated a significant main effect for the dose of Dexmed (F3,56 =3.887, p=0.0136) and for stress (F1,56 =12.892, p=0.0007), with no interactions. C) Surgery Male rats (n=65) were subjected to either surgery or maintained at their home cages. Two-way ANOVA indicated a significant main effect of Dexmed (F3,46 =15.091, p<0.0001) and for surgery (F1,46 =10.13, p=0.0026) with a significant interaction (F3,46 =3.341, p=0.0272). Dexmed significantly elevated MADB106 lung tumour retention in the control groups from the dose of 5 μg·kg-1·hr-1, but in animals undergoing surgery only the highest dose of Dexmed (20 μg·kg-1·hr-1) affected tumour retention. Surgery had increased LTR.▴ indicates a significant increase in the vehicle group caused by stress. ● indicates significant pair-wise difference from the respective control (vehicle) group (PLSD, p < 0.05). Data presented as mean (SEM).
Tumour retention in other organs
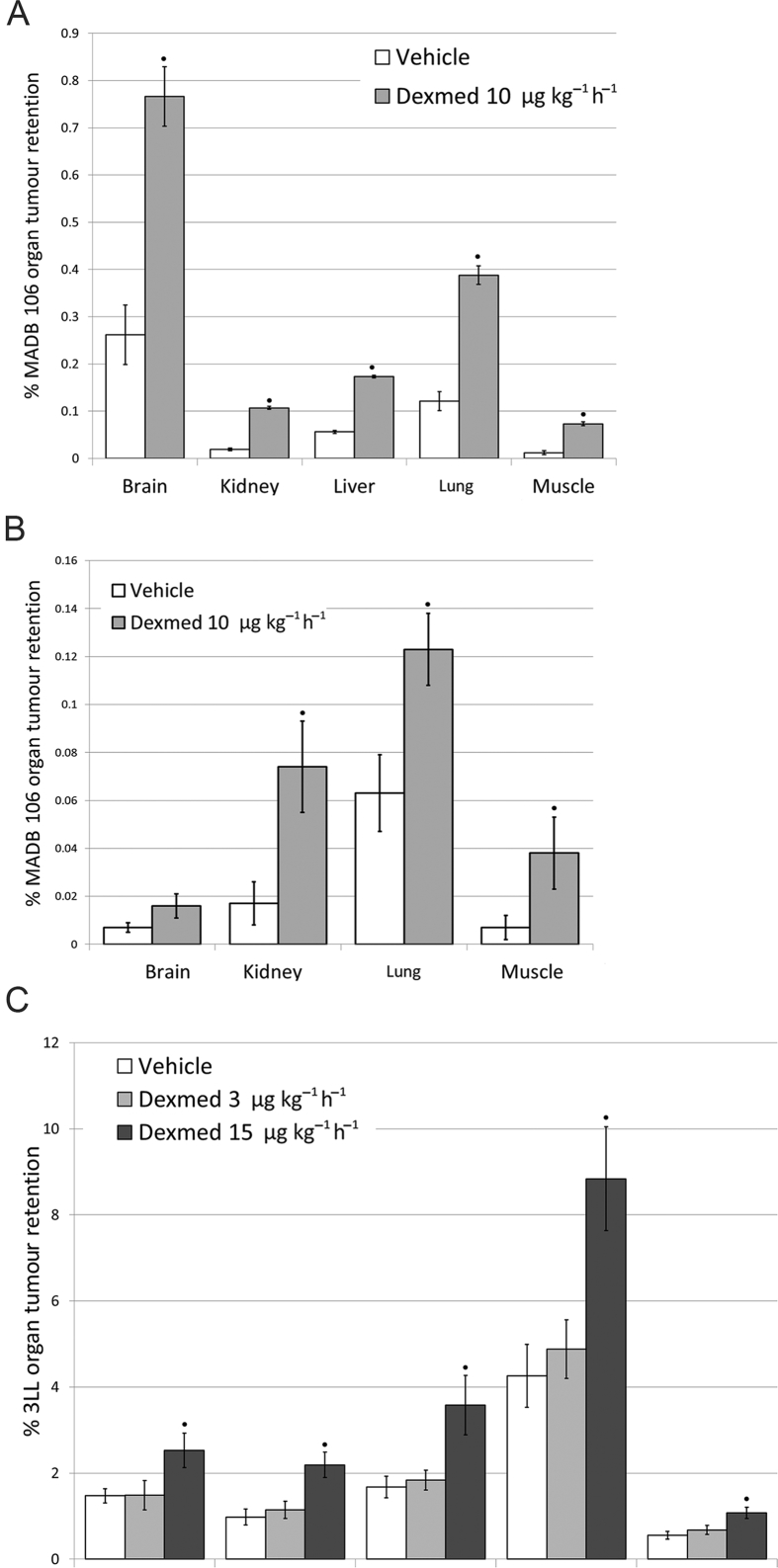
The administration of tumour cells through the external carotid artery (ECA) induced markedly greater brain tumour retention (Fig. 5A) compared to the administration through the tail vein (Fig. 5B) in rats. Importantly, dexmedetomidine increased tumour retention in rats and mice in both administration routes and in all organs tested, including brain, lung, liver, kidney, and muscle, and excluding brain in rats inoculated via the tail vein {ECA in rats (Fig. 5A) – [F(1,49)=28.861, P<0.0001] i.v. in rats (Fig. 5B) – [F(1,28)=21.905, P<0.0001] ECA in mice (Fig. 5C) – [F(2,132)=25.680, P<0.0001]} (Fig. 5A–C).
Fig 5.
Dexmedetomidine elevated tumour retention in various organs, depending on the delivery method of tumour cells in two animal models. A) ECA inoculation in rats Dexmedetomidine (Dexmed) elevated MADB106 tumour retention in the brain, liver, lung, kidney, and muscle in the context of surgery, F344 female rats (n=13). Two-way ANOVA indicated a significant main effect of Dexmed (F1,49 =28.861, p<0.0001) and a significant main effect of organ (F4,49 =35.985, p<0.0001). B) Intravenous inoculation in rats Dexmed did not elevate MADB106 tumour retention in the brain, but did so in the lungs, kidneys and muscle, F344 female rats (n=9). Two-way ANOVA Indicated significant main effect for Dexmed (F1,28 =21.905, p<0.0001) and for organ inspected (F3,28 =18.334, p<0.0001). C) ECA inoculation in mice Male C57BL/6 mice (n=15) were injected with 3LL tumour cells via the ECA. Two-way ANOVA indicated significant main effect for Dexmed dose (F2, 132 =25.680, p<0.0001) and for organ (F4, 132 =60.122, p<0.0001).● indicates significant pair-wise difference from the organ-specific control (vehicle) group (PLSD, p < 0.05). Data presented as mean (SEM).
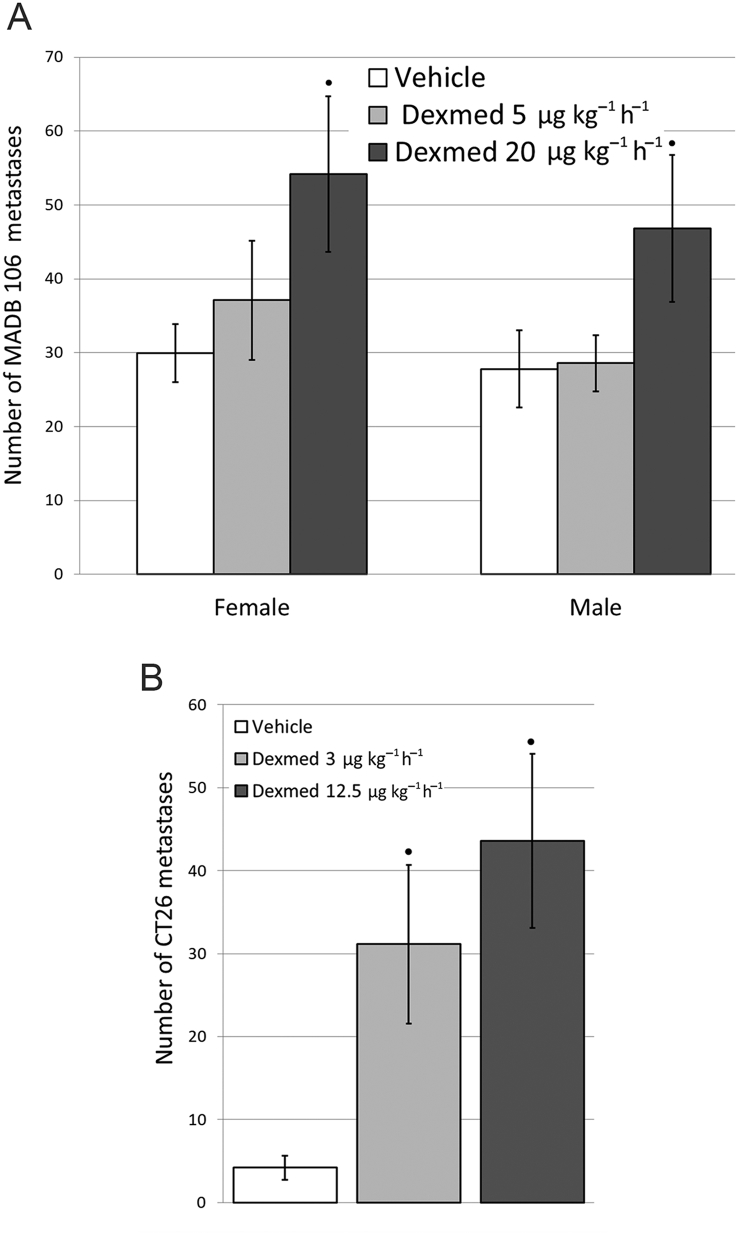
Liver metastases of CT26 colorectal cancer cells in BALB/C mice, and lung metastases of MADB 106 in F344 rats
Dexmedetomidine administered once before tumour cells caused a significant dose-dependent increase in the number of metastases counted 3 weeks later in both models—in rats only employing the higher hypnotic dose (20 μg kg−1 h−1) (F93,2=5.002, P=0.0086) (Fig. 6A), and in mice in both the low sub-hypnotic dose and the higher hypnotic dose (3 or 12.5 μg kg−1 h−1, respectively) (F2,36=4.252, P=0.022) (Fig. 6B).
Fig 6.
Dexmedetomidine elevated the number of metastases counted three weeks following tumour inoculation in two animal models. A) Male (n=45) and female F344 rats (n=53) lungs were counted for experimental metastasis retention. Two-way ANOVA indicated a significant main effect for dexmedetomidine (Dexmed) dose (F93,2 =5.002, p=0.0086), and no effects for sex nor an interaction. B) Female mice (n=39) livers were counted for experimental metastasis retention. One-way ANOVA indicated a significant effect for Dexmed (F2,36 =4.252, p=0.022). Dexmed elevated the number of liver metastasis significantly in both doses. ● indicates significant pair-wise difference from the vehicle control group (PLSD, p < 0.05). Data presented as mean (SEM).
Discussion
Relation between the experimental model and the clinical setting
The drug schedules of dexmedetomidine employed were chosen to simulate clinical plasma levels, and behavioural and physiological effects. In the clinical setting, cancer patients are exposed to dexmedetomidine during surgery for removal of the primary tumour, and/or for up to a day following it. Thus, the potential effects of dexmedetomidine on long-term cancer outcomes would most likely be mediated through its direct and indirect effects on a residual disease, especially on pre-existing micro-metastases and scattered single tumour cells in the circulation and lymphatic systems. We employed tumour models that simulate metastatic processes related to a residual disease, rather than studying the potential effects of dexmedetomidine on the development of a primary tumour. Additionally, as dexmedetomidine has known effects on stress responses and is known to reduce catecholamine release,20 we also tested its effects in two stress paradigms.
Deleterious outcomes of dexmedetomidine and their biological significance
Dexmedetomidine exerted dose-dependent deleterious effects in non-stressed animals at doses of 5 μg kg−1 h−1 and higher. Under stress conditions, the effects of dexmedetomidine on metastasis development depended on the stress/surgery paradigm and the dose of dexmedetomidine used, as can be expected given the complexity of stress and inflammatory responses. Whilst dexmedetomidine did not elevate metastatic propensity in the restraint-stress paradigm, in the wet-cage paradigm, dexmedetomidine worsened the effects of stress at all doses tested. When employing dexmedetomidine in the surgical setting, no beneficial effects were noted. In some doses and tumour models, only mild deleterious effects of dexmedetomidine were noted, whilst in other conditions, dexmedetomidine caused a consistent deleterious effect beyond the effects of surgery.
Taken together, it is likely that, when combined with stress or surgery in our studies, dexmedetomidine reduced the upsurge of catecholamines, alleviated nociception and pain, and reduced inflammation, all of which are expected to reduce metastatic progression.12 Simultaneously, however, dexmedetomidine also exerted deleterious effects through different mechanisms, and thus, the net effects at the lower to moderate doses (2.5, 5, and 10 μg kg−1 h−1, respectively) were mixed, depending on the stress paradigm and tumour model. On the other hand, at moderate and higher hypnotic doses (10–20 μg kg−1 h−1), still common in the clinical setting, the deleterious effects of dexmedetomidine dominated in all three tumour models studied, which also involved surgical procedures.
To test whether the short-term effects of dexmedetomidine on organ tumour retention translate to long-term effects, we applied two models quantifying actual metastases 3 weeks following tumour-cell administration (CT26 liver metastases in mice and MADB 106 lung metastases in rats). Dexmedetomidine increased the metastasis number in both models, indicating the biological significance of a single exposure to dexmedetomidine in the context of circulating cancer cells, and supporting the hypothesis that the effects of a relatively short perioperative exposure to dexmedetomidine could have detrimental effects on long-term clinical cancer outcomes.
To further test the generalizability of the findings, we used an additional syngeneic tumour line (3LL, Lewis lung carcinoma) and employed a different administration approach that is unique in targeting the brain, whilst also studying other organs for tumour retention. This procedure also involves surgical manipulations to expose the ECA for tumour inoculation. Dexmedetomidine had deleterious effects in all organs tested in this model, and the same outcomes were noted when F344 rats were subjected to MADB 106 ECA tumour inoculation. Natural killer (NK) cells play a role in restricting lung metastases in both models, but have no impact on brain tumour retention and brain metastases.34, 35 Thus, given the similar deleterious effects of dexmedetomidine in all organs tested, the impact of dexmedetomidine seems to be mediated, at least partly, by an organ-independent mechanism, as further discussed below.
We retrospectively analysed data from 1404 operated patients with non-small cell lung cancer (NSCLC), of which 241 were treated with dexmedetomidine perioperatively.36 The use of dexmedetomidine was associated with statistically and clinically significant lower survival rates at 5 yr post-surgery, an effect evident only in patients receiving above-median doses of dexmedetomidine (>100 μg per patient). These associative findings correspond well with the causative experimental outcomes presented here.
Potential mechanisms
Given that, in non-stressed animals, dexmedetomidine had reliable deleterious effects at mild doses and higher in all tumour models, organs, and conditions tested, we hypothesise that these effects are mediated through multiple mechanisms, including non-tumour-specific mechanisms, and generic metastatic-promoting effects. Notably, the effects of dexmedetomidine were initiated shortly following its administration, and subsided along with cessation of its behavioural effects, indicating quickly inducible and reversible mechanisms. Thus, immune mechanisms might include reduced innate anti-metastatic immunity that can quickly and transiently be modulated, specifically NK cell cytotoxicity, which controls lung and liver metastases in all tumour models used,30, 34, 35 but not brain metastasis. Non-immune mechanisms might include changes in vascular dilation and constriction, alteration in the expression of epithelial adhesion molecules, and increased capillary permeability to circulating tumour cells, all of which have been suggested to be modulated by dexmedetomidine,37, 38 as well as to impact metastatic spread.39
Study limitations
As for any tumour model used in translational studies, the generalizability of the findings to cancer progression in humans is uncertain. We employed several tumour models of metastasis, which are syngeneic to different rodent species and strains, and found converging evidences for the deleterious effects of dexmedetomidine. The absence of a definitive mediating mechanism for the effects of dexmedetomidine in any of the tumour models used is a drawback of the study, which is addressed in our ongoing studies, yielding multiple and complex potential mechanisms.40 Although the behavioural effects of dexmedetomidine in mice and rats suggested similar levels of sedation and plasma concentration as in patients, other biological effects might differ between species.
This study focused on the effects of dexmedetomidine on cancer metastasis in naïve animals, as well as in the context of stress and surgery, similar to the perioperative setting of dexmedetomidine use in cancer patients. At sub-hypnotic to hypnotic/sedative doses, dexmedetomidine significantly increased the tumour-cell retention and the actual number and growth of metastases in well-controlled experimental conditions, and in almost all of the models implemented. This suggests the generalizability of such effects of dexmedetomidine. In the context of stress or surgery, no consistent effects of dexmedetomidine were evident at low sub-hypnotic doses; however, moderate and high doses of dexmedetomidine increased metastases in all models. These findings, and the correspondence between our animal and human outcomes,36 warrant further clinical studies for different cancer types and other α2-adrenergic agonists used clinically (e.g. clonidine), and for more elaborated mechanistic research in animal models and in human cancer lines.
Authors’ contributions
Study design/planning: H.L., S.B.E., J.P.C., V.G.
Study conduct: H.L., P.M., A.B., L.S., E.R., R.H., S.B.E.
Data analysis: H.L., S.B.E.
Writing paper: H.L., S.B.E.
Revising paper: all authors.
Declaration of interest
J.P.C. received funding for consulting on a human study for Hospira Inc. (Lake Forest, IL, USA). All other authors have none to declare.
Funding
National Institutes of Health (R01 CA172138) to J.P.C.
Editorial decision: August 23, 2017
Handling editor: H. C Hemmings Jr.
Footnotes
Editorial about this article by Freeman & Buggy, BJA 2018:120:15-17
Supplementary data related to this article can be found at https://doi.org/10.1016/j.bja.2017.11.004.
Supplementary data
The following is the supplementary data related to this article:
References
- 1.Neeman E., Ben-Eliyahu S. Surgery and stress promote cancer metastasis: new outlooks on perioperative mediating mechanisms and immune involvement. Brain Behav Immun. 2013;30:S32–40. doi: 10.1016/j.bbi.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDonald P.G., O'Connell M., Lutgendorf S.K. Psychoneuroimmunology and cancer: a decade of discovery, paradigm shifts, and methodological innovations. Brain Behav Immun. 2013;30:S1–S9. doi: 10.1016/j.bbi.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buggy D.J., Borgeat A., Cata J. Consensus statement from the BJA Workshop on Cancer and Anaesthesia. Br J Anaesth. 2015;114:2–3. doi: 10.1093/bja/aeu262. [DOI] [PubMed] [Google Scholar]
- 4.Tedore T. Regional anaesthesia and analgesia: relationship to cancer recurrence and survival. Br J Anaesth. 2015;115 doi: 10.1093/bja/aev375. ii34–45. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Eliyahu S. The promotion of tumour metastasis by surgery and stress: immunological basis and implications for psychoneuroimmunology. Brain Behav Immun. 2003;17:S27–36. doi: 10.1016/s0889-1591(02)00063-6. [DOI] [PubMed] [Google Scholar]
- 6.Desmond F., McCormack J., Mulligan N., Stokes M., Buggy D.J. Effect of anaesthetic technique on immune cell infiltration in breast cancer: a follow-up pilot analysis of a prospective, randomised, investigator-masked study. Anticancer Res. 2015;35:1311–1319. [PubMed] [Google Scholar]
- 7.Belizon A., Balik E., Feingold D.L. Major abdominal surgery increases plasma levels of vascular endothelial growth factor: open more so than minimally invasive methods. Ann Surg. 2006;244:792–798. doi: 10.1097/01.sla.0000225272.52313.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armaiz-Pena G.N., Cole S.W., Lutgendorf S.K., Sood A.K. Neuroendocrine influences on cancer progression. Brain Behav Immun. 2013;30:S19–S25. doi: 10.1016/j.bbi.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melamed R., Rosenne E., Shakhar K., Schwartz Y., Abudarham N., Ben-Eliyahu S. Marginating pulmonary-NK activity and resistance to experimental tumor metastasis: suppression by surgery and the prophylactic use of a beta-adrenergic antagonist and a prostaglandin synthesis inhibitor. Brain Behav Immun. 2005;19:114–126. doi: 10.1016/j.bbi.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Yakar I., Melamed R., Shakhar G. Prostaglandin e(2) suppresses NK activity in vivo and promotes postoperative tumor metastasis in rats. Ann Surg Oncol. 2003;10:469–479. doi: 10.1245/aso.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 11.Powe D.G., Voss M.J., Zanker K.S. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget. 2010;1:628–638. doi: 10.18632/oncotarget.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horowitz M., Neeman E., Sharon E., Ben-Eliyahu S. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat Rev Clin Oncol. 2015;12:213–226. doi: 10.1038/nrclinonc.2014.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ash S.A., Valchev G.I., Looney M. Xenon decreases cell migration and secretion of a pro-angiogenesis factor in breast adenocarcinoma cells: comparison with sevoflurane. Br J Anaesth. 2014;113:i14–21. doi: 10.1093/bja/aeu191. [DOI] [PubMed] [Google Scholar]
- 14.Buckley A., McQuaid S., Johnson P., Buggy D.J. Effect of anaesthetic technique on the natural killer cell anti-tumour activity of serum from women undergoing breast cancer surgery: a pilot study. Br J Anaesth. 2014;113:i56–62. doi: 10.1093/bja/aeu200. [DOI] [PubMed] [Google Scholar]
- 15.Conrick-Martin I., Kell M.R., Buggy D.J. Meta-analysis of the effect of central neuraxial regional anesthesia compared with general anesthesia on postoperative natural killer T lymphocyte function. J Clin Anesth. 2012;24:3–7. doi: 10.1016/j.jclinane.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Tavare A.N., Perry N.J., Benzonana L.L., Takata M., Ma D. Cancer recurrence after surgery: direct and indirect effects of anesthetic agents. Int J Cancer. 2012;130:1237–1250. doi: 10.1002/ijc.26448. [DOI] [PubMed] [Google Scholar]
- 17.Meserve J.R., Kaye A.D., Prabhakar A., Urman R.D. The role of analgesics in cancer propagation. Best Pract Res Clin Anaesthesiol. 2014;28:139–151. doi: 10.1016/j.bpa.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Das J., Kumar S., Khanna S., Mehta Y. Are we causing the recurrence-impact of perioperative period on long-term cancer prognosis: review of current evidence and practice. J Anaesthesiol Clin Pharmacol. 2014;30:153–159. doi: 10.4103/0970-9185.129996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mantz J., Josserand J., Hamada S. Dexmedetomidine: new insights. Eur J Anaesthesiol. 2011;28:3–6. doi: 10.1097/EJA.0b013e32833e266d. [DOI] [PubMed] [Google Scholar]
- 20.Bhana N., Goa K.L., McClellan K.J. Dexmedetomidine. Drugs. 2000;59:263–268. doi: 10.2165/00003495-200059020-00012. discussion, 69–70. [DOI] [PubMed] [Google Scholar]
- 21.Blaudszun G., Lysakowski C., Elia N., Tramer M.R. Effect of perioperative systemic α2 agonists on postoperative morphine consumption and pain intensity: systematic review and meta-analysis of randomized controlled trials. Anesthesiology. 2012;116:1312–1322. doi: 10.1097/ALN.0b013e31825681cb. [DOI] [PubMed] [Google Scholar]
- 22.Khan Z.P., Munday I.T., Jones R.M., Thornton C., Mant T.G., Amin D. Effects of dexmedetomidine on isoflurane requirements in healthy volunteers, 1: pharmacodynamic and pharmacokinetic interactions. Br J Anaesth. 1999;83:372–380. doi: 10.1093/bja/83.3.372. [DOI] [PubMed] [Google Scholar]
- 23.Dahmani S., Rouelle D., Gressens P., Mantz J. Effects of dexmedetomidine on hippocampal focal adhesion kinase tyrosine phosphorylation in physiologic and ischemic conditions. Anesthesiology. 2005;103:969–977. doi: 10.1097/00000542-200511000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Bruzzone A., Pinero C.P., Rojas P. α(2)-adrenoceptors enhance cell proliferation and mammary tumor growth acting through both the stroma and the tumor cells. Curr Cancer Drug Targets. 2011;11:763–774. doi: 10.2174/156800911796191051. [DOI] [PubMed] [Google Scholar]
- 25.Inada T., Shirane A., Hamano N., Yamada M., Kambara T., Shingu K. Effect of subhypnotic doses of dexmedetomidine on antitumor immunity in mice. Immunopharmacol Immunotoxicol. 2005;27:357–369. doi: 10.1080/08923970500240883. [DOI] [PubMed] [Google Scholar]
- 26.Bruzzone A., Pinero C.P., Castillo L.F. Alpha2-adrenoceptor action on cell proliferation and mammary tumour growth in mice. Br J Pharmacol. 2008;155:494–504. doi: 10.1038/bjp.2008.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim Y., Kang S.-H., Hong T.-H. Effects of dexmedetomidine on the ratio of T helper 1 to T helper 2 cytokines in patients undergoing laparoscopic cholecystectomy. J Clin Anesth. 2014;26:281–285. doi: 10.1016/j.jclinane.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 28.Lamkin D.M., Sung H.Y., Yang G.S. Alpha2-Adrenergic blockade mimics the enhancing effect of chronic stress on breast cancer progression. Psychoneuroendocrinology. 2015;51:262–270. doi: 10.1016/j.psyneuen.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ben-Eliyahu S., Page G.G., Yirmiya R., Taylor A.N. Acute alcohol intoxication suppresses natural killer cell activity and promotes tumor metastasis. Nat Med. 1996;2:457–460. doi: 10.1038/nm0496-457. [DOI] [PubMed] [Google Scholar]
- 30.Shakhar G., Ben-Eliyahu S. In vivo beta-adrenergic stimulation suppresses natural killer activity and compromises resistance to tumor metastasis in rats. J Immunol. 1998;160:3251–3258. [PubMed] [Google Scholar]
- 31.Bar-Yosef S., Melamed R., Page G.G., Shakhar G., Shakhar K., Ben-Eliyahu S. Attenuation of the tumor-promoting effect of surgery by spinal blockade in rats. Anesthesiology. 2001;94:1066–1073. doi: 10.1097/00000542-200106000-00022. [DOI] [PubMed] [Google Scholar]
- 32.Smyth D.D., Umemura S., Pettinger W.A. Alpha-1 adrenoceptor selectivity of phenoxybenzamine in the rat kidney. J Pharmacol Exp Ther. 1984;230:387–392. [PubMed] [Google Scholar]
- 33.Bol C.J., Vogelaar J.P., Mandema J.W. Anesthetic profile of dexmedetomidine identified by stimulus–response and continuous measurements in rats. J Pharmacol Exp Ther. 1999;291:153–160. [PubMed] [Google Scholar]
- 34.Benbenishty A., Segev-Amzaleg N., Shaashua L., Melamed R., Ben-Eliyahu S., Blinder P. Maintaining unperturbed cerebral blood flow is key in the study of brain metastasis and its interactions with stress and inflammatory responses. Brain Behav Immun. 2017;62:265–276. doi: 10.1016/j.bbi.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sorski L., Melamed R., Lavon H., Matzner P., Rosenne E., Ben-Eliyahu S. Acute and innocuous CpG-C immune stimulation potentiates host resistance to hepatic metastases of colon cancer and protects against immunosuppressive effects of surgery. Brain Behav Immun. 2015;49:e29. [Google Scholar]
- 36.Cata J.P., Singh V., Lee B.M. Intraoperative use of dexmedetomidine is associated with decreased overall survival after lung cancer surgery. J Anaesthesiol Clin Pharmacol. 2017;33:317–323. doi: 10.4103/joacp.JOACP_299_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarmento A., Borges N., Azevedo I. Adrenergic influences on the control of blood–brain barrier permeability. Naunyn Schmiedebergs Arch Pharmacol. 1991;343:633–637. doi: 10.1007/BF00184295. [DOI] [PubMed] [Google Scholar]
- 38.Segawa T., Ito H., Inoue K., Wada H., Minatoguchi S., Fujiwara H. Dopamine releases endothelium-derived relaxing factor via alpha 2-adrenoceptors in canine vessels: comparisons between femoral arteries and veins. Clin Exp Pharmacol Physiol. 1998;25:669–675. doi: 10.1111/j.1440-1681.1998.tb02274.x. [DOI] [PubMed] [Google Scholar]
- 39.Azevedo A.S., Follain G., Patthabhiraman S., Harlepp S., Goetz J.G. Metastasis of circulating tumor cells: favorable soil or suitable biomechanics, or both? Cell Adh Migr. 2015;9:345–356. doi: 10.1080/19336918.2015.1059563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lavon H., Haldar R., Elbaz E. The perioperative use of the sedative dexmedetomidine in cancer patients may have detrimental effects. Brain Behav Immun. 2015;49:e29. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.