Abstract
Serum PCB congener concentrations were measured in 602 adults living near a PCB pollution source in eastern Slovakia. We created iso-concentration maps for 21 PCB congeners by geocoding each participant’s place of residence and kriging. Concentrations of PCB congeners were inversely associated with the distance of the participants’ residence from the source of pollution. Congener-specific risk factors were derived, particularly for PCBs 52 and 153. We observed that the spatial distribution of serum concentrations was influenced by micro-climatic parameters and physicochemical properties of the congeners. PCB congener profiles strongly correlated with that of the PCB commercial product Delor 106, which was manufactured in the region. The iso-concentration maps indicate that the zones with the highest predicted congener concentration have a mean area of approximately 235.75 ± 188.56 km2 and the mean enrichment of concentration of congeners in serum in these zones is about 5.12 ± 1.36. We estimate that depending on congener approximately 23 457±18 762 individuals with PCB concentrations exceeding health-based guidance values live in these zones.
Keywords: Polychlorinated biphenyls, Contaminated site, POPs, Human biomonitoring, Kriging, Geographic information system
Graphical Abstract
1. Introduction
Polychlorinated biphenyls (PCBs) are synthetic chemicals used for a variety of commercial and industrial applications. Exposure generally occurs as a mixture, rather than as a single PCB. The different types of PCBs, called congeners, are distinguished by the number and location of chlorines. The same properties that made PCBs desirable (e.g., water insolubility and chemical stability) contribute to their persistence in the environment, as they do not easily degrade. The food chain is currently considered the primary source of human exposure to PCBs (ATSDR 2014; Sonneborn et al., 2008). Transfer from mothers to infants is also an important exposure route, as PCBs readily cross the placenta (Lancz et al., 2015a), and transmission also occurs via breastfeeding (Lancz et al., 2015b). PCBs continue to be measurable in a high proportion of samples from the general population, including pregnant women (CDC, 2018). PCBs are persistent, with an estimated in vivo half-life in children of 3–9 years (Grandjean et al., 2008) and 5–10 years in adolescents (Wimmerová et al., 2011). Depending on the congener, PCBs may be carcinogenic to humans (Group 1) (IARC, 2016). The U.S. government has also expressed concern over PCB exposure: According to the U.S. Department of Health and Human Services Agency for Toxic Substances and Disease Registry, in 2017, PCBs ranked 5th out of more than 250 chemicals, given their frequency, toxicity, and potential for human exposure (ATSDR, 2017).
We recently described the spatial distribution of human exposure to PCBs in selected regions around a former production site, the Chemko Strážske plant, in the Michalovce district of Slovakia. Blood serum concentration from participants living in these regions served as an exposure biomarker (Wimmerová et al., 2015). Maps were produced using kriging, an interpolation technique in which the surrounding measured values are weighted to derive a predicted value for an unmeasured location. Weights were based on the distance between the measured points, the prediction locations, and the overall spatial arrangement among the measured points (GIS dictionary, 2018). One of the main conclusions was that in eastern Slovakia, humans can be affected at distances of up to approximately 70 km from the original point source of PCB contamination. For residents in this area, ΣPCB serum concentrations in adults approached limits established by the French Agency for Food, Environmental and Occupational Health & Safety (ANSES, 2010)1, the limits suggested by the European Food Safety Authority (EFSA, 2005)2 and health-based guidance values of the German Human Biomonitoring Commission (HBM-I and -II values) (Apel et al., 2017)3. The extent of exposure was markedly dependent on distance and direction from the source and on microclimatic characteristics of the region (Wimmerová et al., 2015). The present study continues this line of inquiry with the addition of measured congener-specific PCB concentrations, whose addition permits inferences on a congener by congener basis, which may provide additional details about how PCBs are distributed in this region.
The aim of the present work was to study the congener-specific spatial distribution of PCB serum concentrations using geostatistic prediction and to quantify the exposure risk from enrichment of a particular PCB congener in blood serum of subjects living in exposure zones.
One of the objectives of the study was compare the PCB congener signature in blood serum of participants with those of 4 commercial PCB products manufactured in the local, historical PCB manufacturer, Chemko Strážske.
2. Material and methods
2.1. Study population
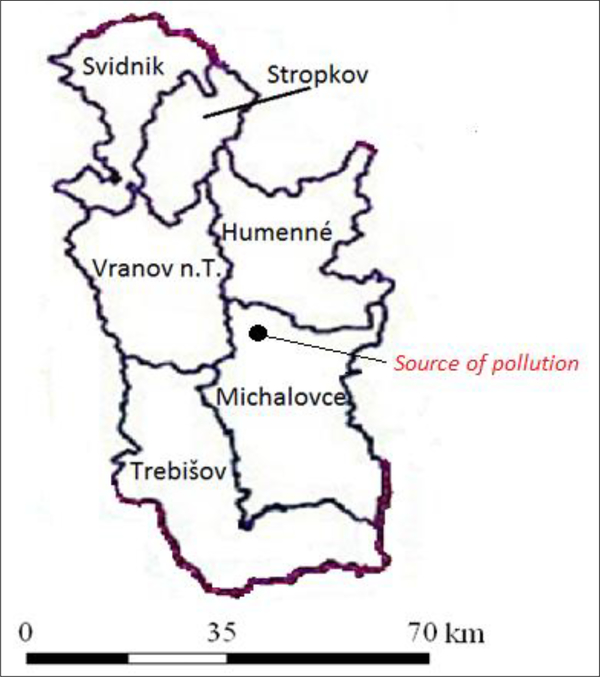
We recruited 602 volunteers with permanent residence for more than 20 years in equal numbers of 150 in each of the 4 districts, Michalovce, Vranov nad Topľou, Humenné and Trebišov (Figure 1). Participants were equally distributed among sexes and 3 age groups (30–40, 41–50 and 51–60 years). Exclusion criteria were pregnancy, acute injuries, oncological diseases, diseases affecting blood pressure, and diagnosed hypertension of 2nd or 3rd stage (systolic blood pressure ≥159 mm Hg and diastolic blood pressure ≥99 mm Hg). We planned to study association between PCB exposure and incidence of hypertension (subclinical, small increases of blood pressure). Overt hypertension has been therefore excluded. Among the participants no one was a former employee of the Chemko plant.
Figure 1.
The map showing the districts (Vranov n.T., Humenné, Trebišov and Michalovce) from which the study volunteers were recruited and the position of the source of pollution, the Chemko Strážske factory.
2.2. Blood collection, clinical examination, health questionnaires
Each participant underwent a clinical examination (general physical, anthropometric data, blood pressure measurement) and blood sampling. Details on the handling of specimens and isolation of serum have been presented elsewhere (Jusko et al. 2010). The study protocol was approved by the Institutional Review Board at the Slovak Medical University. All participants gave informed consent for participation in the study. After enrollment, participants were administered a questionnaire by trained staff which elicited information about sociodemographic status, including education, occupation, marital status, tobacco and alcohol use, diet, family living environment, and health status. Geographic position of the place of residence of each subject was determined by a commercial GPS device or from Google Maps.
2.3. PCB and lipid measurement
Concentrations of 21 PCB congeners (IUPAC no. 28, 52, 74, 99, 101, 105, 114, 118, 123, 138, 153, 156, 157, 167, 170, 180,187+182, 189, 194, 196+203, and 199) were determined as described elsewhere (Drobná et al. 2011; Čonka et al. 2005; US EPA 2008). The sum of the 21 individual PCB congeners analyzed represents ΣPCBs. Serum samples were treated by modified solid-phase extraction (SPE). Each of the serum samples was thawed, spiked with a known amount of 13C-labelled compounds. Serum mixed with an equivalent amount of water: 1-propanol (85 : 15, v/v) mixture was applied to a conditioned SPE column (2 g C18, endcapped; Alltech, Deerfield, Illinois, USA). The analytes were eluted with an n-hexane: dichloromethane (1: 1, v/v) mixture, and the eluate was concentrated. The extract was cleaned-up on a multi-layer florisil–silica/H2SO4 column and eluated with n-hexane: dichloromethane (9: 1, v/v). The eluate was concentrated under a gentle nitrogen stream just to dryness. 13C -labelled recovery standard solution was added immediately prior to GC injection.
The measurements were performed using isotope-dilution method by a high-resolution mass spectrometer (HRMS, MAT 95 XP; Thermo Finnigan, Bremen, Germany) coupled to an HP 6890 gas chromatograph (Hewlett-Packard, Palo Alto, California, USA) with a DB-5ms column (60 m × 0.25 mm × 0.25 μm) using splitless mode injection. Helium was used as a carrier gas at a constant flow of 0.8 ml·min−1. HRMS was operated at a resolution of 10 000 in the positive ionization mode at 53 eV. The proportion of the two most abundant ions of natural (12C) compounds and 13C-labelled ones monitored in the selected ion monitoring mode together with retention time matching provided sufficient identification criteria. Calibration was completed through the analysis of five calibration standard solutions, each containing the measured 12C and above-mentioned 13C-labelled compounds.
2.4. Quality assurance and quality control
The serum samples were treated and analyzed in sets of 10 together with one blank sample. A certified reference material of human serum (1589a, PCBs, Pesticides and Dioxins/Furans in Human Serum, NIST, Gaithersburg, MD 20899) was used for checking the analytical process accuracy. All analytical measurements were carried out at the National Reference Centre for Dioxins and Related Compounds (Department of Toxic Organic Pollutants, Slovak Medical University), which has been certified by the Slovak National Accreditation Service (ISO/IEC 17 025:2005, certification no. S-111) and regularly participates in inter-laboratory studies and proficiency tests on dioxins and PCBs in food and feed (EU-RL for halogenated persistent organic pollutants in Feed and Food, Freiburg, Germany) and interlaboratory comparison program on PCBs and OCPs in blood serum (G-EQUAS) organized by Institute and Out-Patient Clinic for Occupational, Social and Environmental Medicine of the Friedrich-Alexander University in Erlangen, Germany.
For the values below limits of detection (LOD), we took the LOD value divided by the square root of two if the PCB congener had fewer than 20 % of values below the LOD; otherwise, we used LOD values divided by two (Persky et al., 2001; Weisskopf et al., 2005). We estimated total serum lipids using the enzymatic summation method (Akins et al., 1989). PCB concentrations reported in this study are serum lipid adjusted.
2.5. Statistical analysis and kriging
The univariate distributions of serum PCB congener concentrations were positively skewed. We applied Box-Cox transformation to approach normal distributions using Sigma XL version 8.07 (SigmaXL Inc.).
The exposure predictors have been chosen using literature sources and results of bivariate analysis between PCB congener concentrations and age, sex, education, smoking (number of cigarettes /day, passive smoking, rate of smoking at home), consumption of foodstuffs known to contain PCBs (fish, pork, beef, poultry, eggs, pork lard, butter, milk and dairy products), and origin of food (homemade, retail chain, both). Predictors of the PCB serum concentration were examined by Spearman correlation, Mann-Whitney, and Kruskal-Wallis tests. We adjusted the PCB serum concentrations for age, sex, education and smoking by Mixed model from SPSS 19.0 (IBM Support). A p-value <0.05 was considered as statistically significant. We assume that after concentration data adjustment for age, gender, education and smoking the sole significant exposure predictor (determinant) is the distance from the source of pollution.
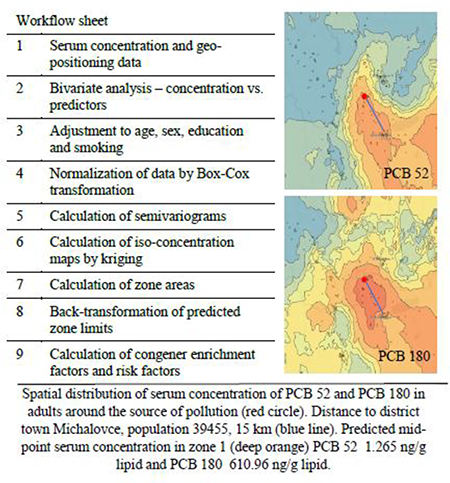
ArcGIS (Esri, Redlands, CA USA) was used to produce iso-concentration maps. The residential address of each participant, along with latitude and longitude coordinates, were entered to create maps providing information on the spatial pattern of each PCB congener quantified in serum of volunteers. The respective zones between the neighboring iso-concentration lines were colored by spectrum colors with the red end denoting the highest serum PCB concentrations and the blue end the lowest serum PCB concentrations. The optional output variance of prediction raster created contains the kriging variance at each output raster cell. Assuming the kriging errors are normally distributed, there is a 95.5 % probability that the actual z value at the cell is the predicted raster value, plus or minus two times the square root of the value in the prediction raster (ArcGIS software). The areas of the respective zones were calculated from the kriging maps after adjustment for Slovakia state borders by combining the Python programming language and the Calculate Geometry tool arcGIS. Finally, we manually cross-checked the selected zones.
Each zone resulting from kriging was defined with an upper and lower serum concentration limit. The areas with the highest concentration were denoted as zone 1, with less concentration as zone 2, etc. We calculated the midpoint PCB congener serum concentration for each zone using the formula for a midpoint of a class interval (Sciencing, 2017) as
Midpoint serum concentration = (Upper limit concentration + Lower limit concentration)/2.
We limited our analysis to the highest 3 zones, as the midpoint serum concentration in zone 3 was close to the median PCB serum concentrations of all participants. For summed zones 1+2 the midpoints were calculated as
Midpoint serum congener concentration for summed zones 1+2 = (Upper limit concentration of zone 1 + Lower limit concentration of zone 2)/2.
Similarly, we proceeded when summing zones 1+2+3.
To assess potential risk resulting from living in the polluted area, we used the outcomes of kriging PCB biomonitoring data. Congener-specific risk was assumed to increase with the size of the zone and with enrichment of the serum concentration. Increasing area of the zone increases the number of exposed individuals. For each PCB congener, we evaluated the risk resulting from the combination of the zone size and the predicted concentration enrichment in serum. We defined the PCB congener enrichment factor (CEF) as
CEF = (Midpoint serum concentration of PCB congener in zone n)/(Median PCB congener serum concentration in subjects of the cohort)
When assessing the risk, we calculated the risk factor (RF) using two methods: 1) expressing the size of the zone either as an absolute value in km2 or 2) as a fraction of areas of zones 1+2+3, as follows:
RF (1) = (Area of zone n km2).{ (Midpoint serum concentration of PCB congener in zone n)/(Median PCB congener serum concentration of subjects of the cohort)}
or
RF (2) = {(Area of zone n km2)/(Sum of areas 1+2+3 km2)}.{(Midpoint serum concentration of PCB congener in zone n)/(Median PCB congener serum concentration of subjects of the cohort)}.
3. Results
3.1. Study subjects and exposures
The number of participants in the study was 602, 307 were female and 295 male. Of these, 80% were married, 36% had a basic education (8–9 years), 45% completed high school, and 19% attained a university degree. Forty-six percent of participants reported previously smoking, and 27% were current smokers. The unadjusted serum concentrations of ΣPCBs in volunteers from the four districts of eastern Slovakia are shown in Table 1. The highest serum levels were observed in the Michalovce district, home of the manufacturing facility that once produced PCBs. The congener profile of PCBs in serum of volunteers from the 4 districts is shown in Table 2. PCB 153 was the most abundant congener (by median), followed by (in order) PCBs 180, 170, and 138. The results of bivariate analyses in Table 3 show that statistically significant predictors are older age, male gender, less education, and smoking more than 3 months in life. No association was observed between total PCB serum levels and alcohol intake and some dietary patterns, including food rich in animal fat (eggs, meat, butter, milk and milk products). The results of bivariate analyses between serum concentration of sum PCBs and source of food are in Table 4
Table 1.
Unadjusted concentration of ΣPCBs (ng/g lipids) in blood serum of subjects from the four districts of eastern Slovakia.
| District | Population | N | Mean | Standard deviation | Geometric mean | Percentiles |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 25 | 50 | 75 | 95 | ||||||
| Humenné | 62 561 | 150 | 776.5 | 717.06 | 599.1 | 207.5 | 371.3 | 557.2 | 877.5 | 1956.8 |
| Michalovce | 110 713 | 150 | 2871.3 | 7084.7 | 1404.5 | 278.1 | 720.9 | 1333.0 | 2603.4 | 9154.5 |
| Trebišov | 105 605 | 151 | 728.3 | 587.6 | 597.6 | 246.6 | 373.9 | 590.8 | 910.8 | 1683.6 |
| Vranov n.T. | 80 607 | 151 | 727.8 | 757.9 | 527.5 | 139.7 | 331.6 | 509.8 | 849.6 | 1758.0 |
SD - Standard deviation
GM - Geometric mean
Table 2.
Descriptive statistics of PCB congener concentration (ng/g lipids) in blood serum of participants (n=602).
| PCB congener | Count | % over LOD | Arithmetic mean serum concentration | SD | GM serum concentration | GM Standard deviation | Percentiles | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 25 | 50 | 75 | 95 | |||||||
| 28 | 591 | 100 | 2.27 | 4.036 | 1.341 | 2.8 | 0.26 | 0.756 | 1.378 | 2.506 | 6.369 |
| 52 | 525 | 99.8 | 0.579 | 1.989 | 0.235 | 3.994 | 0.018 | 0.101 | 0.253 | 0.646 | 1.853 |
| 74 | 592 | 100 | 19.319 | 42.612 | 9.421 | 2.886 | 2.052 | 4.52 | 8.582 | 15.798 | 71.037 |
| 99 | 592 | 99.5 | 6.275 | 9.337 | 3.497 | 2.807 | 0.777 | 1.786 | 3.111 | 6.422 | 23.765 |
| 101 | 489 | 94.5 | 0.562 | 2.112 | 0.175 | 4.249 | 0.015 | 0.077 | 0.185 | 0.439 | 1.738 |
| 105 | 592 | 97.6 | 2.084 | 3.477 | 1.104 | 3.012 | 0.248 | 0.581 | 1.018 | 2.008 | 6.809 |
| 114 | 592 | 86.6 | 0.635 | 0.984 | 0.281 | 4.207 | 0.017 | 0.156 | 0.325 | 0.655 | 2.571 |
| 118 | 591 | 100 | 13.502 | 23.14 | 8.175 | 2.5 | 2.193 | 4.222 | 7.475 | 14.311 | 41.4 |
| 123 | 591 | 41.4 | 0.708 | 4.68 | 0.108 | 4.051 | 0.015 | 0.046 | 0.096 | 0.203 | 2.011 |
| 138 | 592 | 100 | 115.53 | 266.97 | 70.209 | 2.461 | 18.541 | 39.462 | 66.428 | 117.18 | 331.71 |
| 153 | 592 | 100 | 326.24 | 692.35 | 204.368 | 2.349 | 59.086 | 114.46 | 191.96 | 337.77 | 962.19 |
| 156 | 592 | 100 | 22.492 | 51.959 | 13.77 | 2.334 | 3.787 | 7.936 | 12.966 | 22.261 | 53.751 |
| 157 | 592 | 99.7 | 2.017 | 4.239 | 1.209 | 2.562 | 0.32 | 0.707 | 1.131 | 2.023 | 5.658 |
| 167 | 592 | 100 | 6.153 | 11.179 | 3.831 | 2.432 | 1.029 | 2.092 | 3.689 | 6.671 | 17.412 |
| 170 | 592 | 100 | 171.97 | 610.26 | 92.752 | 2.439 | 25.514 | 52.914 | 86.462 | 151.15 | 446.93 |
| 180 | 592 | 100 | 335.75 | 1142.2 | 180.238 | 2.437 | 49.946 | 101.91 | 167.09 | 287.34 | 882.42 |
| 187+182 | 592 | 100 | 75.939 | 169.87 | 38.496 | 2.828 | 8.719 | 18.792 | 33.19 | 70.776 | 269.8 |
| 189 | 592 | 99.8 | 5.864 | 36.664 | 2.679 | 2.579 | 0.728 | 1.482 | 2.473 | 4.29 | 14.387 |
| 194 | 592 | 100 | 62.231 | 569.82 | 21.273 | 2.672 | 5.443 | 11.297 | 19.211 | 33.903 | 153.92 |
| 196+203 | 592 | 100 | 21.265 | 52.518 | 10.838 | 2.708 | 2.656 | 5.513 | 9.654 | 18.516 | 74.92 |
| 199 | 592 | 100 | 88.291 | 587.16 | 32.073 | 2.925 | 7.179 | 15.474 | 27.876 | 57.691 | 267.24 |
| ΣPCB | 592 | 1279.5 | 3711.9 | 719.182 | 2.421 | 199.59 | 396.06 | 658.1 | 1171.7 | 3541.2 | |
GM - Geometric mean
SD - Standard deviation
Table 3.
Examination of statistically significant predictors of serum concentration of sum PCBs. Results of bivariate analyses.
| Sum PCBs | N | Mean Rank | p | |
|---|---|---|---|---|
| Age | 592 | - | <0.001 | |
| Gender | Male | 290 | 318.06 | 0.003 |
| Female | 302 | 275.79 | ||
| Education | Primary school and high school without GCSE | 212 | 311.00 | 0.022 |
| High school with GCSE | 263 | 299.60 | ||
| University | 115 | 257.57 | ||
| Smoking more than 3 months in life | no | 317 | 277.03 | 0.003 |
| yes | 275 | 318.95 |
Table 4.
Relationship (tested by Kruskal-Wallis test) between origin of consumed food and serum concentration of sum PCBs.
| Food | Source | N | % | Mean Rank | p |
|---|---|---|---|---|---|
| Fish | homemade | 19 | 3.16 | 334.21 | 0.316 |
| retail | 499 | 82.89 | 295.16 | ||
| both | 78 | 12.96 | 272.62 | ||
| missing | 6 | 1.00 | |||
| Pork | homemade | 73 | 12.13 | 281.30 | 0.805 |
| retail | 420 | 69.77 | 295.35 | ||
| both | 103 | 17.11 | 294.76 | ||
| missing | 6 | 1.00 | |||
| Beef | homemade | 30 | 4.98 | 269.70 | 0.923 |
| retail | 495 | 82.23 | 275.75 | ||
| both | 34 | 5.65 | 285.12 | ||
| missing | 43 | 7.14 | |||
| Poultry | homemade | 74 | 12.29 | 274.40 | 0.492 |
| retail | 378 | 62.79 | 299.33 | ||
| both | 145 | 24.09 | 290.11 | ||
| missing | 5 | 0.83 | |||
| Eggs | homemade | 184 | 30.56 | 283.26 | 0.593 |
| retail | 221 | 36.71 | 295.67 | ||
| both | 191 | 31.73 | 300.82 | ||
| missing | 6 | 1.00 | |||
| Pork lard | homemade | 133 | 22.09 | 281.79 | 0.225 |
| retail | 320 | 53.16 | 292.99 | ||
| both | 124 | 20.60 | 262.89 | ||
| missing | 25 | 4.15 | |||
| Butter | homemade | 3 | 0.50 | 258.67 | 0.402 |
| retail | 578 | 96.01 | 295.16 | ||
| both | 15 | 2.49 | 237.47 | ||
| missing | 6 | 1.00 | |||
| Milk | homemade | 7 | 1.16 | 271.71 | 0.202 |
| retail | 548 | 91.03 | 292.72 | ||
| both | 33 | 5.48 | 239.09 | ||
| missing | 14 | 2.33 | |||
| Dairy products | homemade | 7 | 1.16 | 287.43 | 0.660 |
| retail | 549 | 91.20 | 291.83 | ||
| both | 40 | 6.64 | 317.00 | ||
| missing | 6 | 1.00 | |||
| Fruits and vegetables | homemade | 134 | 22.26 | 262.53 | 0.013 |
| retail | 132 | 21.93 | 279.24 | ||
| both | 329 | 44.19 | 310.79 | ||
| missing | 7 | 1.16 |
3.2. Spatial distribution of PCB congener serum concentrations and exposure risk
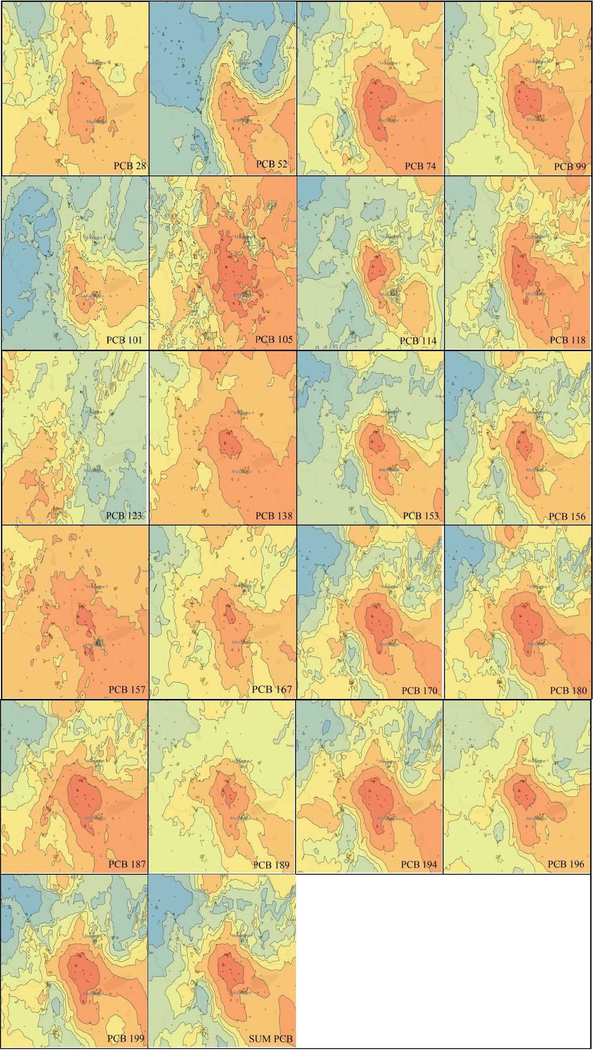
Kriging was used to generate iso-concentration maps of blood serum concentrations of the PCB congeners listed in section 2.3. (Figure 2). PCB 123 was included, however owing to only 40 % of samples >LOD was not further evaluated. High resolution maps with Chemko plant and the district town Michalovce marked can be found in supplemental material. Specific information on the spatial distribution of each congener serum concentration and associated exposure risk were ascertained by visual inspection of each map, utilizing the RF and CEF calculations outlined in section 2.4.
Figure 2.
Iso-concentration maps for individual PCB congeners. The zones with highest enrichment are marked with the deepest orange color. The midpoint concentrations of a particular PCB congener in the zones and the size of the zones are shown in Table 3.
3.2.1. Visual inspection of iso-concentration maps
The spatial distribution of PCBs is congener, residence, and local climate dependent. The exposure decreases with increasing distance from the exposure source (the former PCB manufacturing facility), and this distance is the dominant predictor of exposure. The prevailing winds blow predominantly from the north-west to south-east and this pattern can be observed in the distribution pattern of serum concentrations. While the exposure gradient (width of zones) with most congeners steeply decreases in north-west direction, in the opposite direction, the zones are elongated and frequently fall beyond the examined area. Such configuration prevents determination of areas of several zones 2 (except PCBs 114, 153, 156, 167, 189 and 196) and most zones 3. In spite of the complexity of the entire system, the external environment, and human subjects living in it (Beyer and Biziuk, 2009), marked similarities in the pattern of spatial distribution of the serum concentration of PCB congeners 153, 156, 170, 180, 189, 194 and 196 are visible. Relationships between physicochemical properties of the congeners and serum concentration distribution pattern can also be inferred. The areas of the zones 1 of the low chlorinated congeners (PCBs 28, 52, 74, and 99) are larger compared with the high chlorinated ones, most probably owing to higher volatility of low chlorinated PCBs.
3.2.2. Assessment of risk parameters
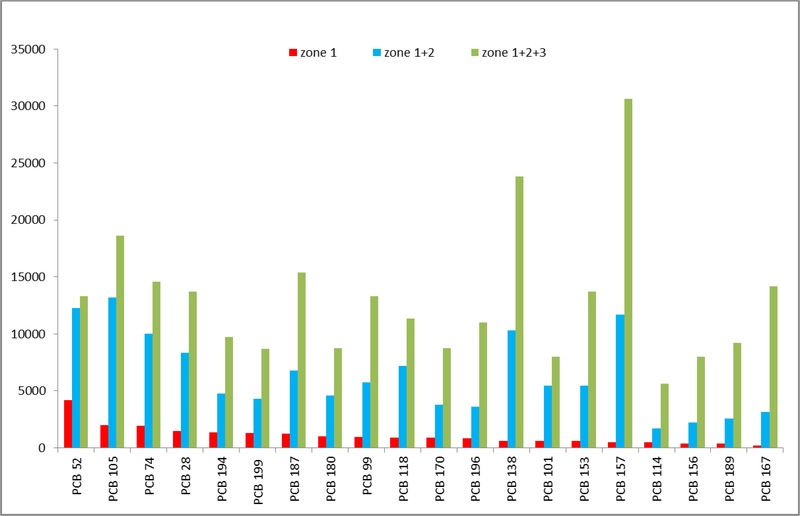
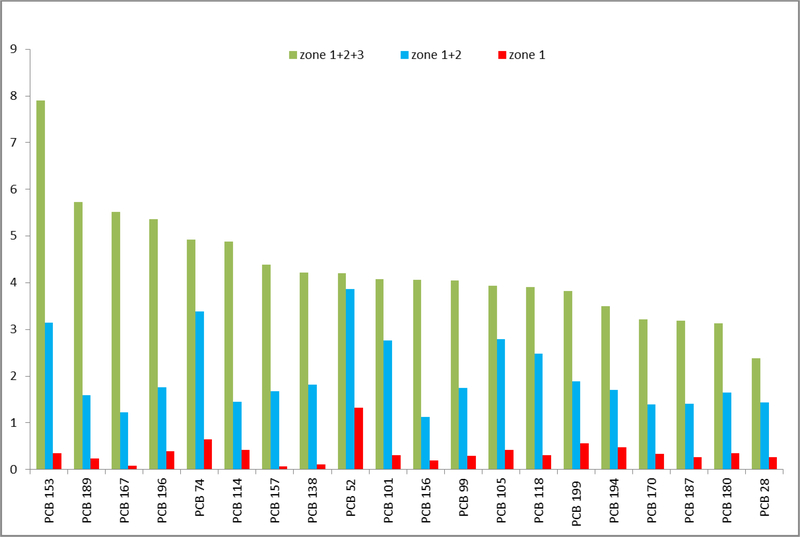
The number of inhabitants to be exposed increases with the area of the central zones. The areas of the zones, the midpoint congener serum concentrations in the given zone/s and CEFs are shown in Table 5. We were not able to determine the zone areas of PCB 105. PCB 52 had the largest area of zone 1. For summed areas of zones 1+2 it was replaced by another low chlorinated congener PCB 28. The RFs were calculated two ways. RFs (1) expressing absolute value of the area are shown in Figure 3. The greatest value of RF (1) for zone 1 was attained by PCB 52, whereas for zones 1+2 by PCB 105 and 1+2+3 by PCB 157. RFs (2), expressing zone areas as a fraction of areas of zones 1+2+3, are shown in Figure 4. PCB153 presents the greatest exposure risk for residents living in combined zones 1+2+3.
Table 5.
The congener enrichment factors (CEFs), area in km2 of zone 1 and combined areas of zones 1+2 and 1+2+3 for individual PCB congeners and the midpoint congener serum concentration in ng/g lipid. The congeners were ranked based on the size of zone/s. The mean±SD area for all congeners of zone 1 is 235.75 ± 188.56 km2, zones 1+2 1523.7 ± 902.44 km2 and zones 1+2+3 3196.8 ± 1535.92 km2.
| PCB Congener | Zone 1 | PCB Congener | Zones 1+2 | PCB Congener | Zones 1+2+3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Area km2 | Midpoint concentration | CEF | Area km2 | Midpoint concentration | CEF | Area km2 | Midpoint concentration | CEF | |||
| 52 | 839 | 1.265 | 4.99 | 28 | 3269 | 3.504 | 2.54 | 157 | 6980 | 4.962 | 4.39 |
| 28 | 507 | 4.042 | 2.93 | 105 | 3198 | 4.208 | 4.13 | 28 | 5777 | 3.275 | 2.38 |
| 105 | 421 | 4.775 | 4.69 | 52 | 2775 | 1.12 | 4.43 | 138 | 5650 | 279.74 | 4.21 |
| 187+182 | 337 | 123.25 | 3.71 | 157 | 2548 | 5.183 | 4.58 | 187+182 | 4836 | 105.535 | 3.18 |
| 194 | 326 | 78.678 | 4.09 | 138 | 2344 | 291.59 | 4.39 | 105 | 4737 | 4.008 | 3.94 |
| 74 | 324 | 50.637 | 5.9 | 187+182 | 2041 | 110.11 | 3.32 | 52 | 3182 | 1.065 | 4.21 |
| 199+201 | 282 | 125.31 | 4.5 | 74 | 1936 | 44.52 | 5.19 | 99 | 3285 | 12.59 | 4.05 |
| 180 | 267 | 610.96 | 3.66 | 118 | 1762 | 30.59 | 4.09 | 74 | 2964 | 42.268 | 4.92 |
| 170 | 238 | 325.04 | 3.76 | 180 | 1403 | 545.96 | 3.27 | 118 | 2903 | 29.218 | 3.91 |
| 118 | 195 | 34.302 | 4.59 | 99 | 1320 | 13.484 | 4.33 | 180 | 2795 | 552.68 | 3.13 |
| 99 | 189 | 15.545 | 4.99 | 194 | 1301 | 70.166 | 3.65 | 194 | 2787 | 67.125 | 3.49 |
| 196+203 | 127 | 61.24 | 6.34 | 101 | 1256 | 0.8 | 4.32 | 170 | 2716 | 278.35 | 3.22 |
| 138 | 126 | 328.4 | 4.94 | 170 | 1125 | 290.58 | 3.36 | 167 | 2574 | 20.335 | 5.51 |
| 101 | 125 | 0.906 | 4.89 | 199+201 | 1069 | 111.569 | 4.0 | 199+201 | 2268 | 106.68 | 3.83 |
| 157 | 96 | 5.882 | 5.2 | 153 | 662 | 1578.9 | 8.22 | 196+203 | 2053 | 51.703 | 5.36 |
| 156 | 82 | 62.094 | 4.79 | 196+203 | 646 | 54.175 | 5.61 | 156 | 1971 | 52.645 | 4.06 |
| 114 | 81 | 1.908 | 5.88 | 167 | 543 | 21.496 | 5.83 | 101 | 1965 | 0.756 | 4.08 |
| 153 | 65 | 1765.5 | 9.2 | 156 | 523 | 55.156 | 4.25 | 153 | 1735 | 1515.7 | 7.9 |
| 189 | 57 | 16.616 | 6.72 | 189 | 428 | 14.774 | 5.97 | 189 | 1603 | 14.174 | 5.73 |
| 167 | 31 | 24.599 | 6.67 | 114 | 325 | 1.675 | 5.16 | 114 | 1155 | 1.584 | 4.88 |
Figure 3.
Risk factors, RFs (1), for population of the polluted area computed from the absolute size of the zones predicted by kriging and the enrichment of the PCB congeners (CEFs) in the blood serum. The congeners were ranked with respect to the RFs in Zone 1.
Figure 4.
Risk factors, RFs (2), for population of the polluted area computed from the relative size of the zones predicted by kriging and the enrichment of the PCB congeners (CEFs) in the blood serum. The congeners were ranked with respect to the RFs in Zones 1+2+3.
3.2.3. Behavior of congeners from the pollution source to human exposure
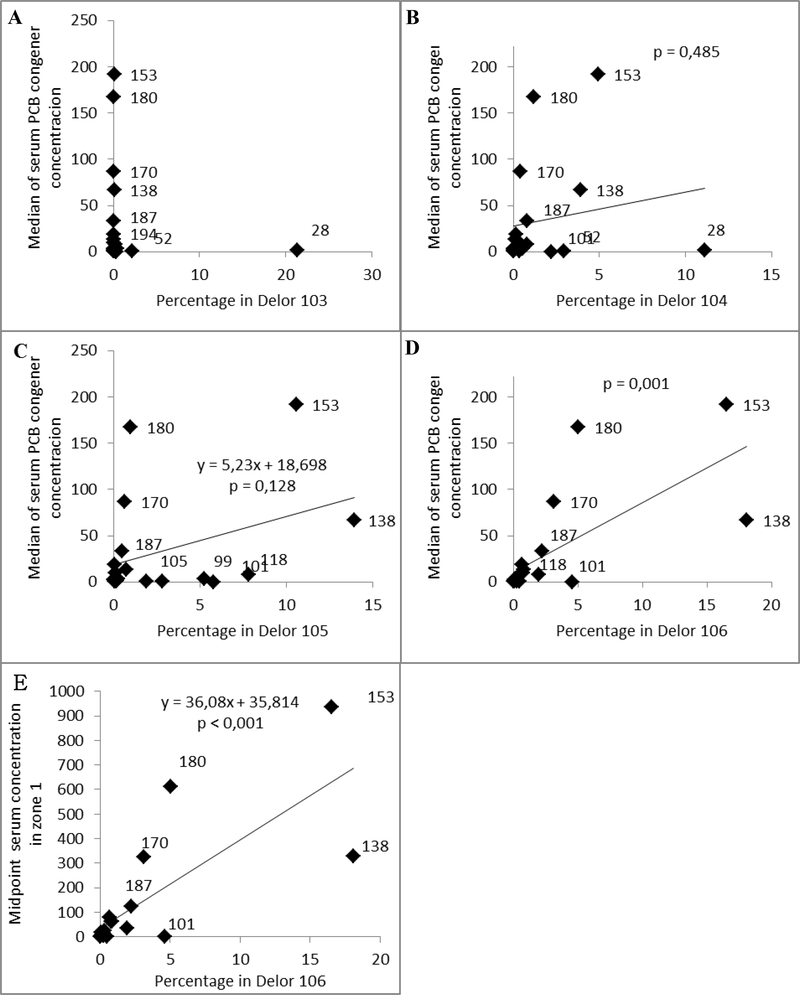
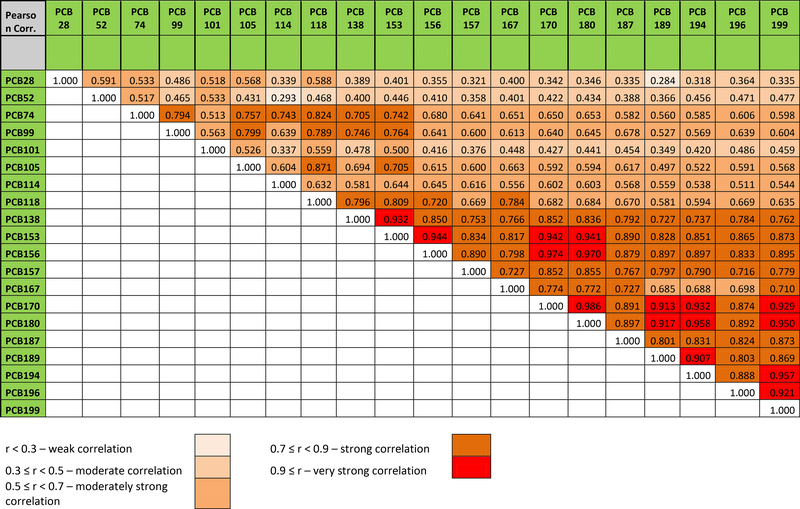
To understand the relationship between historical PCB production in Michalovce and human exposure, we compared the four commercial PCB products (Delors 103,104, 105, and 106) manufactured by Chemko Strážske to the congener-specific concentrations among the participants in our study. Based on the homologue composition, the approximate chlorine contents by weight of Delors 103,104, 105, and 106 were estimated to be 44, 50, 57 and 60%, respectively (Taniyasu et al., 2003). We then correlated the % (w/w) of PCB congeners of each of the Delor mixtures against the median PCB congener concentration (ng/g lipid) in blood serum of participants in our cohort (Figure 5, A-D). The serum PCB congener pattern in study participants correlated strongly with that of Delor 106. In addition, we observed that the Delor 106 signature correlated with the midpoint concentrations of PCB congeners in zone 1. The resistance to metabolism and the long survival of the high chlorinated PCBs in the human body is also reflected in correlation pattern of PCB congeners in serum (Figure 6). The matrix shows that the correlation between concentrations of congeners in serum increases with increasing chlorination. The correlation level between the concentration of the hexachlorobiphenyls, heptachlorobiphenyls and octachlorobiphenyls was r ≥0.7 with the exception of PCB 167 and PCB congeners 189, 194 and 196 with r slightly less than 0.7. The serum concentration of congeners with lower chlorination was less interrelated with highly chlorinated congeners.
Figure 5.
Correlation between percentage of PCB congeners (w/w) in various types of Delors and median PCB congener concentrations in blood serum of participants of the cohort (Figures A-D). Figure E shows correlation between percentage of PCB congeners (w/w) in Delors 106 and midpoint serum concentration of PCB congeners in zone 1.
Figure 6.
Pearson’s correlation coefficients between the concentration of PCB congeners in blood serum of particpants (n=602) of the cohort. For all colored fields p≤0.001.
3.2.4. Estimate of exposed population residing in zone 1
To estimate the mean number of persons residing in the highest exposure zones 1 has been attempted based on general demographic data on Slovakia. The population density for the entire examined area (the 4 districts shown in Figure 1) of 3616.45 km2 is 99.5 (population per km2). Applying this value to the mean areas of 235.75±188.56 km2 of zone 1, assuming an even population distribution across the whole area, we obtain a population estimate of 23 457±18 762 persons.
4. Discussion
We have determined a series of PCB congeners in blood serum of 602 adults residing in an area of 3616.45 km2 (7.4% of the territory of Slovakia) polluted by organochlorines (Wimmerová et al., 2015). For each PCB congener, an iso-concentration map defining zones with predicted congener serum concentration was developed. We purposely restricted quantification of exposure risk to most central zones due to methodical difficulties with determining the areas of zones >2. The mean± SD area of zones 1 was 235.75 ± 188.56 km2 which represents 6.52 ± 5.21 % of the total examined area of 3616.45 km2. The mean ± SD CEFs in zones 1 was 5.12 ± 1.36 (Table 5). A person permanently living in zone 1, with a probability defined in section 2.4., attains an enrichment of a particular serum congener concentration by its CEF value. The greatest enrichment was observed with PCBs 153, 189, 167, and 196+208, 9.2, 6.72, 6.67, and 6.34, respectively.
We understand that the current PCB body burden in our cohort is a result of historical and recent intakes. A previous study demonstrates that PCB congeners 153, 138, and 180 contain a phenyl group with 2,4,5-substitution, and are resistant to biotransformation and elimination (Megson et al., 2013). Correspondingly in the present study, serum concentrations of these congeners were the highest among all congeners: PCBs 180, 153, and 138 (335.75, 326.24, and 115.53 ng/g lipid, respectively). Such behavior corresponds to our data on interrelations between PCB congeners in serum shown in the correlation matrix (Figure 6). These correlations indicate exposure to highly chlorinated biphenyls and their long survival in environment and consequently in human body.
Before analyzing the concentration data by kriging, we adjusted the serum concentrations for potential confounders. We confirmed the following exposure predictors: age, sex, education, and smoking more than 3 months in life. No association was observed between total PCB serum levels and alcohol intake and some dietary patterns, including food rich in animal fat (eggs, meat, butter, milk and milk products).
It is interesting that in surveys made on a site polluted by PCBs in Brescia, Italy in 2003 and 2013, in agreement with our data, no association was found between total PCB serum levels and tobacco smoking, alcohol intake and some dietary patterns (eggs, meat, butter, milk and cheese) (Magoni et al., 2016; Apostoli et al., 2005; Donato et al., 2006). Questionnaire data from an independent study has shown that most participants even from rural region had urban way of life and purchased in supermarkets most of the food items known as potential vectors of PCBs, however with low PCB level (Salgovicová and Pavlovicová, 2007).
The currently predicted exposure to PCBs in zones 1 is several orders of magnitude higher than health-based guidance values for sum of marker PCBs in blood (ANSES, 2010; EFSA, 2005; Apel et al., 2017). In particular, from the data for the Michalovce district (population 110 713) the 50th percentile is 1333 ng/g lipids. Conversion of wet weight HBM-II: 7 μg/L to lipid-based limit gives 1000 ng/g serum lipid (sum of the three most abundant congeners 138, 153 and 180 times two) (Apel et al., 2017). The value of 1333 ng/g lipid indicates that every second inhabitant exceeds the HBM-II limit in the Michalovce district. Note that a similar comparison with the French (ANSES, 2010) and EFSA limits (EFSA, 2005) for two other exposed population groups has been done previously (Wimmerová et al., 2015).
Moreover, the “background” PCB level in eastern Slovakia, the starting level for CEF calculations, is high compared with other world regions. So, while NHANES data (CDC, 2011) for PCB 153 are approximately 20 ng/g lipids depending on age, gender, and race, the currently measured median PCB 153 concentration (Table 2) in our cohort was approximately 190 ng/g lipids.
The current study was targeting spatial distribution of serum concentration of PCB congeners in environmentally exposed adult population living around a source of pollution. The PCB body burden is an end stage of the largely unknown process following the entrance of the chemical stressor into the environment. Our prior contribution to this issue has shown from data gathered during years 2002–2004 on delivering women that the PCB level in serum was associated with the consumption of fat from locally sourced food products, however distance from pollution source was not taken into consideration (Sonneborn et al., 2008). At that time on average, more than a third of the fat consumed by these women from pork, lard and eggs came from locally produced foods. However, since that time the consumption pattern of the Slovak population has dramatically changed, especially after joining the EU in 2004, which has been reflected in questionnaire data of the current project summarized in (Table 4). A trend towards consumption of food purchased in retail chains has been observed. Bivariate analysis between food source and concentration of PCBs in serum (Table 4) did not show an association between consumption of any food products and PCB exposure. According to our data from the area of the 4 studied districts, the suspected PCB vectors, fruits and vegetables (Donato et al., 2006; Magoni et al., 2016), do not play role in PCB concentration in blood (Table 4).
In spite of this change in nutritional habits the PCB serum level in population of the most polluted district Michalovce during a period of more than a decade did not decrease markedly. Comparison of the current mean concentration of ΣPCBs of 2871 ng/g lipids for adults (n= 149, age (mean ±SD) 45.3±8.4) of Michalovce district (Table 1) with the value of 3105 ng/g lipids for a corresponding population sample (n= 1008, age (mean ±SD) 44.6±12.5) of Michalovce district in year 2001 (Petrik et al., 2006) shows that the exposure of adults in Michalovce district decreased negligibly over 14 years. This is in contrast to kinetics of the PCB exposure of residents around a former PCB production facility in Brescia, northern Italy, where a ban on locally produced food of plant origin was associated with a decrease of PCB serum levels within 10 years to about one third, the level observed in industrialized countries in last decades (Raffetti et al., 2017; Magoni et al., 2016). The differences between the exposure scenarios in Brescia and Strážske have to be stressed. While PCB polluted water was the source of exposure in Brescia, which was discharged in irrigation channels and hence accumulated in the soil of a nearby agricultural area, (Donato et al., 2006) in Strážske, such practice was never used.
Considering the significantly reduced oral intake of PCB vectors, the question arises which sources and mechanisms contribute to preserve the present (2015) almost constant PCB serum level of the adult population of the Michalovce district, comparable to the level observed in an age- and region-matched adult population 13 years ago (Petrik et al., 2006)? It may be inferred that in this population, redistribution of historic pollution is expected to be the major source of PCB exposure. This redistribution involves volatilization from soil and water into the atmosphere with subsequent transport in air and removal from the atmosphere via wet/dry deposition of PCBs bound to particulates (CIRCABC EU, 2011). In support of identification exposure routes other as oral intake may serve several observations. Tree bark samples were collected to identify the relative amounts and congener profiles of atmospheric PCBs dissolved into bark lipids from the gas phase in Anniston, Alabama, USA, where PCBs were manufactured from the 1920s until 1971. Results from Anniston show that organisms living near the PCB plant and landfills were exposed to very high concentrations of atmospheric PCB and a mixture of PCB congeners that may have included high molecular mass compounds near the plant and landfills (Hermanson, et al., 1989; Hermanson and Hites, 1990; Hermanson et al., 2003). Important from our perspective is that exposures since the end of production have remained high near that area, PCB congener profiles show persistent congeners 31 + 28, 52, 66, 153, 138, and 180 and bark PCB concentrations were dropping exponentially at a distance of about 7 km (Hermanson and Johnson, 2007). These results are applicable to other sites where PCBs were produced in a high temperature process. The Strážske exposure scenario bears many similarities to that of Anniston site. The same technology has been used, there are big stores of PCB distillation residues at the neighborhood of the producing facility and there is an adjacent creek which has been heavily contaminated with PCBs from surface drainage at the PCB plant. PCB off-gassing from these sources may contribute to uptakes alternate to the oral route.
The importance of the Brescia Caffaro contaminated site and its surrounding areas have been confirmed as primary source in driving PCB concentrations in air (DiGuardo et al., 2017). Importance of atmospheric pollution by PCBs demonstrates a study on outdoor air at 34 homes surrounding New Bedford Harbor during dredging of highly contaminated harbor sediments. Air concentrations were higher in neighborhoods closest to the harbor and contained slightly greater proportions of volatile PCB congeners (Vorhees et al., 1997). Increased PCB soil concentrations were described in Michalovce area (Kocan et al., 2001; Dömötörová et al., 2012) however, data suggests that soil contaminated with PCBs contribute little to human body burden as measured by serum concentrations (Kimbrough et al., 2010). PCBs may be taken up by some plants, but that is not typical (ATSDR, 2000). If dermal absorption can be excluded as an important contributor to PCB body burden in our cohort we consider inhalation an alternate exposure route. Air as a source of PCB exposure was nearly completely ignored until a decade ago (Robertson and Ludewig, 2011). Indeed, recently much attention is paid to inhalation exposures to low chlorinated PCBs (Basra et al., 2018; Carpenter 2015; Lehmann et al., 2015; Marek et al., 2017). These exposures are clearly dependent on congener volatility related to boiling point. However, there are many observations that the hexa, hepta, octa, and nona chlorinated biphenyls contribute to environmental exposures by inhalation. The atmospheric PCBs are predominantly in the gaseous phases showing prevailing occurrence of slightly chlorinated congeners, whereas the highly chlorinated ones are mainly under particulate form (Blanchard et al., 2006). Several authors reported significant concentrations of the highly chlorinated congeners in air samples (Vilavert et al., 2014; Norström et al., 2010; Cetin et al., 2017; Hao et al., 2017; Ampleman et al., 2015). Airborne emissions from sources of legacy pollutants may lead to inhalation exposure at levels comparable to, and sometimes higher than, dietary exposure (Currado et al., 1998; Harrad et al., 2006; Ampleman et al., 2015).
In our previous paper on spatial distribution of PCB serum concentrations (Wimmerová et al., 2015) we stated that no reports were published on distance from the point source as the main exposure determining factor. Interestingly, in a recent study an inverse correlation between total WHO-TEQ and distance to source of pollution was confirmed by multiple linear regression models (Chen et al., 2015). An argument for air transport of PCB may be the correspondence of orientation of the elongated exposure zones for all congeners with prevailing winds. It is not plausible that distance from the pollution source may drive the outcomes of PCB oral intake.
Conclusions
We assume that after adjustment to age, sex, smoking and education the serum concentration of 20 PCB congeners in serum of environmentally exposed adults is dependent on the distance of the subjects’ residencies from the source of pollution. Iso-concentration maps demonstrate the influence of micro-climatic parameters and physicochemical properties of the congeners on spatial distribution of their serum concentration. Highest congener-specific risk factors considering size of the zone with highest exposure and the congener serum enrichment were observed for PCBs 52 and PCB 153. The PCB serum signature correlated best with manufactured PCB product containing 60% of chlorine. We suggest examine the hypothesis that airborne emissions from local sources may lead to inhalation exposure at levels comparable to dietary exposure.
Supplementary Material
Highlights.
We determined PCB congeners in serum from 602 adults living in PCB polluted area.
We created PCB congener-specific iso-concentration maps using kriging.
The mean area of zones with highest predicted concentration was 235.75 ± 188.56 km2.
The mean serum congener concentration enrichments in these zones was 5.12 ± 1.36
Depending on the congener 23 457±18 762 inhabitants live in these particular zones.
Acknowledgments
This project has been funded by Ministry of Health, Slovak Republic, through projects 2007/07-SZU-03, 2012/41-SZU-5 and 2012/47-SZU-11; Slovak Research and Development Agency, through projects APVV-0571–12 and APVV-0444–11; the project “Center of Excellence of Environmental Health”, ITMS No. 26240120033, based on the supporting Operational Research and Development Program financed from the European Regional Development Fund. This work also received support from U.S. National Institutes of Health grant NIH R01-CA096525 and P30-ES001247. The authors would like to thank Drs. M. Veliký, Humenné, J. Stašková, Michalovce, Ľ Rosiarová, Vranov n.T. and T. Konevičová, Trebišov for their assistance with recruiting human subjects and all the volunteers of this study who gave blood.
Footnotes
ANSES proposed a critical concentration of 700 ng total PCB/g of plasma lipids as a threshold for pregnant women; women of childbearing age; lactating women and children under 3 years of age with a maximum limit of 1800 ng total PCB/g of plasma lipids for the rest of the population
EFSA suggests a value of approximately1000 ng total PCB/g of plasma lipids for the entire population
German Human Biomonitoring Commission suggests HBM-II-Value for ∑ of PCB (138 + 153 + 180) in serum × 2 for infants, small children and women of child-bearing age 1000 ng/g lipids.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akins JR, Waldrep KT, Bernert JR (1989). The estimation of total serum lipids by a completely enzymatic ‘summation’ method. Clin Chim Acta. 184: 219–226. [DOI] [PubMed] [Google Scholar]
- Ampleman MD, Martinez A, DeWall J, Rawn DF, Hornbuckle KC, Thorne PS (2015). Inhalation and dietary exposure to PCBs in urban and rural cohorts via congener-specific measurements. Environ Sci Technol. 49: 1156–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANSES (2010) OPINION of the French Food Safety Agency on interpreting the health impact of PCB concentration levels in the French population https://www.anses.fr/sites/default/files/documents/RCCP2008sa0053EN.pdf
- Apel P, Angerer J, Wilhelm M, Kolossa-Gehring M (2017). New HBM values for emerging substances, inventory of reference and HBM values in force, and working principles of the German Human Biomonitoring Commission. Int J Hyg Environ Health. 220:152–166. [DOI] [PubMed] [Google Scholar]
- Apostoli P, Magoni M, Bergonzi R, Carasi S, Indelicato A, Scarcella C, Donato F (2005). Assessment of reference values for polychlorinated biphenyl concentration in human blood. Chemosphere. 61:413–421. [DOI] [PubMed] [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry). (2000). Toxicological profile for polychlorinated biphenyls. USDHHS, PHS, US Department of Health and Human Services, Atlanta, Georgia. [PubMed] [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry). (2014). Case studies in environmental medicine, Polychlorinated Biphenyls (PCBs) Toxicity Course: WB 2460, 2014. https://www.atsdr.cdc.gov/csem/csem.asp?csem=30&po=4
- ATSDR (Agency for Toxic Substances and Disease Registry). (2017). Substance Priority List, 2017. https://www.atsdr.cdc.gov/spl/index.html
- Basra K, Scammell MK, Benson EB, Heiger-Bernays W (2018). Ambient Air Exposure to PCBs: Regulation and Monitoring at Five Contaminated Sites in EPA Regions 1, 2, 4, and 5. New Solut. Jan 1:1048291118763620. [DOI] [PubMed]
- Beyer A, Biziuk M (2009). Environmental fate and global distribution of polychlorinated biphenyls. Rev Environ Contam Toxicol. 201:137–158. doi: 10.1007/978-1-4419-0032-6_5. [DOI] [PubMed] [Google Scholar]
- Blanchard M, Teil MJ Chevreuil M (2006). The Seasonal Fate of PCBs in Ambient Air and Atmospheric Deposition in Northern France. J of Atmospheric Chemistry. 53: 123–144.| [Google Scholar]
- Carpenter DO (2015). Exposure to and health effects of volatile PCBs. Rev Environ Health. 30: 81–92. [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention). (2011). Fourth National Report on Human Exposure to Environmental Chemicals 2009 and the Updated Tables. http://www.cdc.gov/exposurereport/.
- CDC (Centers for Disease Control and Prevention). (2018). Fourth National Report on Human Exposure to Environmental Chemicals Updated Tables. March 2018. https://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Volume1_Mar2018.pdf
- Cetin B, Yurdakul S, Keles M, Celik I, Ozturk F, Dogan C (2017). Atmospheric concentrations, distributions and air-soil exchange tendencies of PAHs and PCBs in a heavily industrialized area in Kocaeli, Turkey. Chemosphere. 183: 69–79. [DOI] [PubMed] [Google Scholar]
- Chen X, Chen JS, Zhang L, Li JG, Yao L, Self SG, Sun X, Tang NJ (2015). Levels of PCDDs, PCDFs and dl-PCBs in the blood of childbearing-aged women living in the vicinity of a chemical plant in Tianjin: a primary study. Chemosphere. 118:1–4. [DOI] [PubMed] [Google Scholar]
- CIRCABC EU (2011). PCB NDL EQS draft dossier 2011. https://www.google.com/search?rlz=1C2QJDB_enSK604SK604&biw=1680&bih=895&ei=RxjXWrzQGonwUP28iagG&q=NDL-PCBs_EQS_20110119&oq=NDLPCBs_EQS_20110119&gs_l=psyab.12...22172.50972.0.58774.13.13.0.0.0.0.117.1222.11j2.13.0....0...1.1.64.psyab..0.0.0....0.TYF42bnMvRc
- Conka K, Drobna B, Kocan A, Petrik J (2005). Simple solid-phase extraction method for determination of polychlorinated biphenyls and selected organochlorinepesticides in human serum. J. Chromatogr A. 1084: 33–38. [DOI] [PubMed] [Google Scholar]
- Currado GM, Harrad S (1998). Comparison of polychlorinated biphenyl concentrations in indoor and outdoor air and the potential significance of inhalation as a human exposure pathway. Environ. Sci.Technol 32: 3043–3047. [Google Scholar]
- Di Guardo A, Terzaghi E, Raspa G, Borin S, Mapelli F, Chouaia B, Zanardini E, Morosini C, Colombo A, Fattore E, Davoli E, Armiraglio S, Sale VM, Anelli S, Nastasio P (2017). Differentiating current and past PCB and PCDD/F sources: The role of a large contaminated soil site in an industrialized city area. Environ Pollut. 223: 367–375. [DOI] [PubMed] [Google Scholar]
- Dömötörová M, Sejáková ZS, Kočan A, Čonka K, Chovancová J, Fabišiková A (2012). PCDDs, PCDFs, dioxin-like PCBs and indicator PCBs in soil from five selected areas in Slovakia. Chemosphere. 89: 480–485. [DOI] [PubMed] [Google Scholar]
- Donato F, Magoni M, Bergonzi R, Scarcella C, Indelicato A, Carasi S, Apostoli P (2006). Exposure to polychlorinated biphenyls in residents near a chemical factory in Italy: the food chain as main source of contamination. Chemosphere. 64: 1562–1572. [DOI] [PubMed] [Google Scholar]
- Drobna B, Fabišikova A, Conka K, Chovancova J, Dömötörova M, Wimmerova S, Sovcikova E, Kocan A (2011). Differences between dioxin-like PCB, non-dioxin-like PCB, polychlorinated dibenzo-p-dioxin and dibenzofuran intake from human milk and infant milk formula by infants in the Michalovce district (Slovakia). Journal of Food and Nutrition Research. 50: 106–117. [Google Scholar]
- EFSA (European Food Safety Authority). (2005). Opinion of the scientific panel on contaminants in the food chain on a request from the commission related to the presence of non-dioxin-like polychlorinated biphenyls (PCB) in feed and food. EFSA J. 284:1–137. [Google Scholar]
- GIS Dictionary. (2018). ESRI. http://support.esri.com/en/other-resources/gisdictionary/term/kriging
- Grandjean P, Budtz-Jørgensen E, Barr DB, Needham LL, Weihe P, Heinzow B (2008). Elimination half-lives of polychlorinated biphenyl congeners in children. Environ Sci Technol. 42: 6991–6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Li Y, Wang T, Hu Y, Sun H, Matsiko J, Zheng S, Wang P, Zhang Q (2017). Distribution, seasonal variation and inhalation risks of polychlorinated dibenzo-p-dioxins and dibenzofurans, polychlorinated biphenyls and polybrominated diphenyl ethers in the atmosphere of Beijing, China. Environ Geochem Health. 26: 1–12. doi: 10.1007/s10653-017-9961-2. [DOI] [PubMed] [Google Scholar]
- Harrad S, Hazrati S, Ibarra C (2006). Concentrations of polychlorinated biphenyls in indoor air and polybrominated diphenyl ethers in indoor air and dust in Birmingham, United Kingdom: Implications for human exposure. Environ. Sci. Technol 40: 4633–4638. [DOI] [PubMed] [Google Scholar]
- Hermanson MH, Hites RA (1989). Long-term measurements of atmospheric polychlorinated biphenyls in the vicinity of Superfund dumps. Environ. Sci. Technol 23: 1253–1258. [Google Scholar]
- Hermanson MH, Hites RA (1990). Polychlorinated biphenyls in tree bark. Environ. Sci. Technol 24: 666–671. [Google Scholar]
- Hermanson MH, Johnson GW (2007). Polychlorinated biphenyls in tree bark near a former manufacturing plant in Anniston, Alabama. Chemosphere. 68: 191–198. [DOI] [PubMed] [Google Scholar]
- Hermanson MH, Scholten CA, Compher K (2003). Variable air temperature response of gas-phase atmospheric polychlorinated biphenyl concentrations near a former production facility. Environ. Sci. Technol 37: 4038–4042. [DOI] [PubMed] [Google Scholar]
- IARC Monographs (2016). Polychlorinated biphenyls and polybrominated biphenyls volume 107 IARC monographs on the evaluation of carcinogenic risks to humans. Lyon, France. [PMC free article] [PubMed] [Google Scholar]
- Jusko TA, De Roos AJ, Schwartz SM, Lawrence BP, Palkovičova L, Nemessanyi T, Drobná B, Fabisikova A, Kočan A, Sonneborn D, Jahnova E, Kavanagh TJ, Trnovec T, Hertz-Picciotto I (2010). A cohort study of developmental polychlorinated biphenyl (PCB) exposure in relation to post-vaccination antibody response at 6 months of age. Environ Res. 110: 388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrough RD, Krouskas CA, Carson ML, Long TF, Bevan C, Tardiff RG (2009). Human uptake of persistent chemicals from contaminated soil: PCDD/Fs and PCBs. Regul Toxicol Pharmacol. 57: 43–54. [DOI] [PubMed] [Google Scholar]
- Kocan A, Petrik J, Jursa S, Chovancova J, Drobna B (2001). Environmental contamination with polychlorinated biphenyls in the area of their former manufacture in Slovakia. Chemosphere. 43: 595–600. [DOI] [PubMed] [Google Scholar]
- Lancz K, Murínová L, Patayová H, Drobná B, Wimmerová S, Sovčíková E, Kováč J, Farkašová D, Hertz-Picciotto I, Jusko TA, Trnovec T (2015a). Ratio of cord to maternal serum PCB concentrations in relation to their congener-specific physicochemical properties. Int J Hyg Environ Health. 218: 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancz K, Hertz-Picciotto I, Jusko TA, Murínová L, Wimmerová S, Sovčíková E, Dedík L, Strémy M, Drobná B, Farkašová D, Trnovec T (2015b). Duration of breastfeeding and serum PCB 153 concentrations in children. Environ Res. 36: 35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann GM, Christensen K, Maddaloni M, Phillips LJ (2015). Evaluating health risks from inhaled polychlorinated biphenyls: research needs for addressing uncertainty. Environ Health Perspect. 123:109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magoni M, Donato F, Speziani F, Leonardi L, Orizio G, Scarcella C, Gaia A, Apostoli P (2016). Substantial decline of polychlorinated biphenyls serum levels 10years after public health interventions in a population living near a contaminated site in Northern Italy. Environ Int. 95: 69–78. [DOI] [PubMed] [Google Scholar]
- Marek RF, Thorne PS, Herkert NJ, Awad AM, Hornbuckle KC (2017). Airborne PCBs and OH-PCBs Inside and Outside Urban and Rural U.S. Schools. Environ Sci Technol. 51: 7853–7860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megson D, O’Sullivan G, Comber S, Worsfold PJ, Lohan MC, Edwards MR, Shields WJ, Sandau CD, Patterson DG Jr. (2013). Elucidating the structural properties that influence the persistence of PCBs in humans using the National Health and Nutrition Examination Survey (NHANES) dataset. Sci Total Environ. 461/462: 99–107. [DOI] [PubMed] [Google Scholar]
- Norström K, Czub G, McLachlan MS, Hu D, Thorne PS, Hornbuckle KC (2010). External exposure and bioaccumulation of PCBs in humans living in a contaminated urban environment. Environ Int. 36: 855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persky V, Turyk M, Anderson HA, Anderson HA, Hanrahan LP, Falk C, Steenport DN, Chatterton R Jr., Freels S (2001).Great Lakes Consortium. The effects of PCB exposure and fish consumption on endogenous hormones. Environ Health Perspect. 109: 1275–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrik J, Drobna B, Pavuk M, Jursa S, Wimmerova S, Chovancova J (2006). Serum PCBs and organochlorine pesticides in Slovakia: age, gender, and residence as determinants of organochlorine concentrations. Chemosphere. 65: 410–418. [DOI] [PubMed] [Google Scholar]
- Raffetti E, Speziani F, Donato F, Leonardi L, Orizio G, Scarcella C, Apostoli P, Magoni M (2017). Temporal trends of polychlorinated biphenyls serum levels in subjects living in a highly polluted area from 2003 to 2015: a follow-up study. Int J Hyg Environ Health. 220: 461–467. [DOI] [PubMed] [Google Scholar]
- Robertson LW, Ludewig G (2011). Polychlorinated Biphenyl (PCB) carcinogenicity with special emphasis on airborne PCBs. Gefahrst Reinhalt Luft. 71: 25–32. [PMC free article] [PubMed] [Google Scholar]
- Salgovicová D, Pavlovicová D (2007). Exposure of the population of the Slovak Republic to dietary polychlorinated biphenyls. Food Chem Toxicol. 45:1641–1649. [DOI] [PubMed] [Google Scholar]
- Sciencing (2017). https://sciencing.com/midpoint-interval-8628422.html
- Sonneborn D, Park HY, Babinska K, Palkovicova L, Trnovec T, Kocan A, Nguyen DV, Hertz-Picciotto I (2008). Serum PCB concentrations in relation to locally produced food items in eastern Slovakia. J Expo Sci Environ Epidemiol. 18: 581–587. [DOI] [PubMed] [Google Scholar]
- Taniyasu S, Kannan K, Holoubek I, Ansorgova A, Horii Y, Hanari N, Yamashita N, Aldous KM (2003). Isomer-specific analysis of chlorinated biphenyls, naphthalenes and dibenzofurans in Delor: polychlorinated biphenyl preparations from the former Czechoslovakia. Environ Pollut. 126: 169–178. [DOI] [PubMed] [Google Scholar]
- US EPA (United States Environmental Protection Agency). (2008). Method 1668, Revision B Chlorinated Biphenyl Congeners in Water, Soil, Sediment, Biosolids, and Tissue by HRGC/HRMS, 128 pp.
- Vilavert L, Nadal M, Schuhmacher M, Domingo JL (2014). Seasonal surveillance of airborne PCDD/Fs, PCBs and PCNs using passive samplers to assess human health risks. Sci Total Environ. 1: 733–740. [DOI] [PubMed] [Google Scholar]
- Vorhees DJ, Cullen AC, Altshul LM (1997). Exposure to Polychlorinated Biphenyls in Residential Indoor Air and Outdoor Air near a Superfund Site. Environ. Sci. Technol 31: 3612–3618. [Google Scholar]
- Weisskopf MG, Anderson HA, Hanrahan LP, Kanarek MS, Falk CM, Steenport DM, Draheim LA (2005). Great Lakes Consortium. Maternal exposure to Great Lakes sport-caught fish and dichlorodiphenyl dichloroethylene, but not polychlorinated biphenyls, is associated with reduced birth weight. Environ Res. 97: 149–162. [DOI] [PubMed] [Google Scholar]
- Wimmerová S, Lancz K, Tihányi J, Sovčíková E, Kočan A, Drobná B, Palkovičová L, Jurečková D, Fabišiková A, Conka K, Trnovec T (2011). Half-lives of serum PCB congener concentrations in environmentally exposed early adolescents. Chemosphere. 82: 687–691. [DOI] [PubMed] [Google Scholar]
- Wimmerová S, Watson A, Drobná B, Šovčíková E, Weber R, Lancz K, Patayová H, Richterová D, Koštiaková V, Jurečková D, Závacký P, Strémy M, Jusko TA, Palkovičová Murínová Ľ, Hertz-Picciotto I, Trnovec T (2015). The spatial distribution of human exposure to PCBs around a former production site in Slovakia. Environ Sci Pollut Res Int. 22:14405–14415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.