Abstract
There is evidence that anxiety precedes the onset of depression and that rumination contributes to this risk pathway in adolescence. This study examined inflammatory biomarkers as mediators in a risk model of depressive symptoms secondary to anxiety symptoms among adolescents who ruminate. A sample of 140 adolescents (52% female, 54% African American, 40% Caucasian, 6% biracial, mean age at T1 = 16.5 years, SD = 1.2 years) provided blood samples on two visits (T1 and T2; mean time between T1 and T2 = 13.5 months, SD = 5.9 months). Self-report anxiety, depression, and rumination measures were given at T1 and the depression measure was given again at a third visit (T3, mean months since T1 = 26.0 months, SD = 9.0 months). Higher anxiety predicted more interleukin-6, but not more C-reactive protein, for adolescents with high levels of rumination. Moderated mediation analyses (N for analysis after removing cases with missing data and outliers = 86) indicated that interleukin-6, but not C-reactive protein, at T2 mediated the relationship between anxiety symptoms at T1 and depressive symptoms at T3, conditional on rumination. Anxiety and rumination interacted such that, as rumination increased, anxiety predicted greater inflammation and depressive symptoms. These results demonstrate that established cognitive vulnerabilities for the development of depressive symptoms secondary to anxiety symptoms in adolescence might indirectly operate though biological mechanisms such as inflammation. In addition to highlighting risk factors and potential treatment targets for depression, this study suggests a potential biological mechanism underlying the effects of psychotherapies that reduce rumination on negative affect (e.g., cognitive behavioral therapy).
Introduction
Adolescence is a vulnerable period for the emergence of both anxious and depressive symptoms (Cummings, Caporino, & Kendall, 2014). For some adolescents, these symptoms progress to clinical diagnoses (van Lang et al., 2007; Letcher et al., 2017). In fact, roughly 25% and 8% of adolescents experience an anxiety or unipolar depressive disorder, respectively, in a 12-month period (Kessler et al., 2012). Symptoms of both anxiety and depression are associated with significant functional impairment and increased suicide risk in adolescents (Balázs et al., 2013). Moreover, the co-occurrence of anxiety and depression in adolescence is associated with greater symptom severity, lower global functioning, and impaired social functioning (Garber & Weersing, 2010).
Importantly, anxiety symptoms have been found to increase risk for the development of later depressive symptoms in adolescence (Cummings et al., 2014; Starr, Stroud, & Li, 2016). As 50% of individuals who experience a first depressive episode develop a second, and 80% of those who experience a second experience subsequent episodes (Burcusa & Iacono, 2007), it is imperative to identify the mechanisms underlying the pathway to depressive symptoms in adolescence to identify and intervene with youth at risk for this developmental trajectory. Indeed, identifying risk factors and mediating mechanisms for developing depressive symptoms in adolescence will allow for targeted preemptive efforts to interrupt risk pathways and decrease the disease burden of depression across the life course (Thapar et al., 2012). The current study utilizes Cohen and colleagues’ model of depression in adolescence (Cohen, Young, Gibb, Hankin, & Abela, 2014), in which anxiety interacts with cognitive vulnerabilities to predict subsequent depressive symptoms, and extends this model to include inflammatory activity as a biological mediator of the relationship between anxiety and depressive symptoms. This expanded model is described as an “immunocognitive” model of depression secondary to anxiety inasmuch as it includes both immune and cognitive processes in explaining the pathway from anxiety to depressive symptoms.
Anxiety/Depression Co-occurrence
Depression frequently develops secondary to the initial appearance of anxiety in adolescence (Bittner et al., 2004; Starr et al., 2016). Although this is the most frequent direction of transition between these two psychopathologies, it is worth noting that depressive symptoms can precede anxiety symptoms (Hamilton et al., 2016) or the two disorders may have simultaneous onset (Moffitt et al., 2007). Some researchers have postulated that anxiety is a risk factor for later depression (Cohen et al., 2014; Horn & Wuyek, 2010), whereas others view this relationship as indicative of heterotypic continuity, in which depression is a later behavioral expression of the same underlying processes manifested previously as anxiety (e.g., Costello, Mustillo, Erkanli, & Keele, 2003). Another explanation for this relationship is simply that the average age of onset for anxiety disorders is earlier than that of depression (Kessler et al., 2005). A more comprehensive understanding of the trajectories contributing to the relationship between anxiety and depression in adolescence is crucial for the refinement of preventive interventions. This is especially important during adolescence, a period of increased risk for internalizing symptoms (Cummings et al., 2014) and a crucial time for identity development (Meeus, 2011).
Rumination in Anxiety/Depression Comorbidity
Rumination, the tendency to passively focus on one’s negative mood and its causes and consequences, is a well-established risk factor for depression. In samples of undergraduates, rumination has been demonstrated to maintain or worsen depressive symptoms (Morrow & Nolen-Hoeksema, 1990; Nolen-Hoeksema, 1991) and to predict the likelihood of developing a first episode of major depression (Spasojevic & Alloy, 2001). Several previous studies have extended this research and identified rumination as an important cognitive style that contributes to the link between anxiety and later depression. In an adolescent sample, Cohen and colleagues (2014) found support for a diathesis-anxiety model, in which anxiety symptoms interact with rumination to increase risk for depressive symptoms. This model was further supported by Starr et al. (2016), who found that the tendency to ruminate specifically about anxiety symptoms moderated the sequential comorbidity between anxiety and depressive symptoms in an adolescent sample. Alternatively, Grant and colleagues (2014) found that rumination mediated the relationship between social anxiety and depression 12 weeks later in a sample of late adolescents. Similarly, McLaughlin and Nolen-Hoeksema (2011) also reported that rumination mediated the relationship between anxiety and later depression in adolescents. Thus, ruminative response styles appear to be important for understanding the relationship between anxiety and later depression during adolescence and may both moderate and mediate this sequential association. Consequently, rumination was a focus of the current study.
Depression and Inflammation
Numerous studies have demonstrated that depression is more common in individuals with chronic illnesses, especially those that involve inflammatory processes such as asthma and cardiovascular disease (Goodwin, Fergusson, & Horwood, 2004; Whooley, 2006). Similarly, studies have shown that many individuals in a depressive episode have higher levels of proinflammatory cytokines, such as interleukin-6, and other blood indicators of inflammation, such as elevated C-reactive protein, than those without depression (Dhabhar et al., 2009; Dowlati et al., 2010). Although the majority of this research has been conducted with adults, this pattern of results has been replicated with adolescent samples (Pallavi et al., 2015). Inflammation also has been associated with depressive symptoms in adolescents (see Mills, Scott, Wray, Cohen-Woods, & Baune, 2013 for a review). Importantly, whereas there is a relative dearth of literature on inflammation and depression in adolescent samples, a few longitudinal studies have demonstrated that inflammation is associated with the development of depressive symptoms in adolescence (Duivis et al., 2015; Moriarity et al., 2018) and that this relationship may be bidirectional (Miller & Cole, 2012). These results highlight the potential for inflammation to function as both a risk and a maintenance mechanism for adolescent depression. Further, the administration of certain cytokines, such as interferon-alpha and interleukin-6, as well as other immunomodulatory treatments, can induce feelings of malaise and depressive affect, suggesting that inflammation could be a significant contributor to the pathophysiology of depression (for a review, see Miller, Maletic, & Raison, 2009). Moreover, a variety of human and animal studies have documented that stressful challenges elicit an inflammatory response, which coincides with a behavioral “sickness syndrome” comprised of malaise, anorexia, somnolescence, loss of libido, decreased sociality, and impaired cognitive performance (Dantzer, O’Connor, Freund, Johnson, & Kelly, 2008; Hart, 1988). The concordance of this “sickness syndrome” with symptoms of depression has led to a new understanding of the physiological processes underlying the affective and cognitive dysfunction seen in depressive disorders. Consequently, there is strong evidence that inflammation might be an important pathophysiological mechanism for depression. However, much more research needs to be done with adolescent samples to investigate how inflammation might influence depressive symptoms during this developmental period when depression often first emerges. This is especially true given that adolescents with depression exhibit age-specific characteristics of the inflammation system (Mills et al., 2013).
Anxiety and Inflammation
Individuals with anxiety disorders often have higher concentrations of several inflammatory proteins in circulation (see Michopoulos, Powers, Gillespie, Ressler, & Jovanovic, 2016, for a review). In a cohort of 54,326 participants, Naude, Roest, Stein, de Jonge, and Doornbos (2018) found that individuals with anxiety disorders, with the exception of social anxiety disorder, exhibited significantly higher C-reactive protein than controls. Furthermore, Liukkonen and colleagues (2011) found that anxiety symptoms were predictive of elevated C-reactive protein levels in adult males. Although there is a relative dearth of literature investigating the relationship between inflammation and anxiety in adolescence, generalized anxiety disorder (GAD) was associated with higher C-reactive protein in a sample of over 1,400 adolescents (Copeland, Shanahan, Worthman, Angold, & Costello, 2012). Similarly, adolescents in the Avon Longitudinal Study of Parents and Children cohort with GAD had higher C-reactive protein than control cases, even after excluding participants with comorbid depression (Khandaker, Zammit, Lewis, & Jones, 2016). There is additional evidence for a relationship between anxiety and inflammation from studies utilizing acute lab stressors. Carroll and colleagues (2011) found that increased state anxiety in response to the Trier Social Stress Task (TSST) was associated with increased interleukin-6 levels in adults. Another study investigating responses to the TSST demonstrated that giving a speech in the context of social evaluation resulted in higher levels of tumor necrosis factor alpha (TNF-α), another inflammatory cytokine (Dickerson et al., 2009), in female undergraduates. Further, individuals who reported more stress in response to the TSST had higher proinflammatory cytokine levels than those who experienced more fear during the task (Moons, Eisenberger, & Taylor, 2010). One study also investigated the relationship between inflammation and anxiety in adolescent depression patients, finding that anxiety symptoms were positively correlated with proinflammatory interleukin-1β in female depression patients and negatively correlated with anti-inflammatory biomarkers transforming growth factor-β1 and interleukin-17A in the entire sample of depressed adolescents (Pallavi et al., 2015). Thus, there is reasonable evidence to investigate the relationship between anxiety and inflammation and its relevance to risk for depression.
Rumination and Inflammation
Some have postulated that a proinflammatory phenotype can develop over time because of repeated or chronic stress. This type of cumulative allostatic loading can then impact both neuroendocrine and immune regulation, contributing to the risk for depression (McEwen & Stellar, 1993). Certain cognitive styles also foster more frequent and enduring physiological arousal (Segerstrom & Miller, 2004), including the perseverative cognitions associated with worry and rumination, two cognitive styles associated with anxiety (Muris, Roelofs, Meesters, & Boomsma, 2004). Brosschot, Gerin, and Thayer’s (2006) perseverative cognition hypothesis posits that perseverative cognitive styles such as rumination can enhance autonomic, endocrine, and immune reactions to both physical and psychological insults, prolonging stress-related activation, and ultimately contributing to a shift in the set points for physiological regulation. Conversely, the extent of the duress induced by stressful challenges can be reduced by adaptive coping styles. For example, female young adults instructed to ruminate about a social stress task had higher cortisol levels post-stressor than participants assigned to a distraction condition (Zoccola, Figueroa, Rabideau, & Woody, 2013). The recovery time for the elevated levels of the acute phase reactant, C-reactive protein, to return to baseline also was significantly slower in the rumination condition than in the distraction condition. Although this study demonstrated an exciting connection between cognitive vulnerabilities and inflammation, additional research is needed utilizing both reports of trait rumination experienced in day-to-day life and research with adolescent samples.
The Current Study
The current study utilizes a prospective design to test whether inflammatory physiology contributes to the link between anxiety and later depressive symptoms, and whether this association is moderated by rumination in adolescents. Two primary hypotheses were tested: 1) rumination would moderate the relationship between anxiety at Time 1 (T1) and inflammation at Time 2 (T2), and 2) inflammation at T2 would mediate the relationship between anxiety symptoms at T1 and depressive symptoms at Time 3 (T3), and rumination would moderate this effect (see Fig. 1). It was expected that rumination would be associated with higher levels of inflammatory mediators and depressive symptoms. Conversely, it was predicted that lower rumination would buffer the effect of anxiety on inflammation and later depressive symptoms. Four cytokines were measured, including three with proinflammatory activity, interleukin-6, interleukin-8, tumor necrosis factor alpha, and one regulatory cytokine, interleukin-10, as well as the liver-derived acute phase reactant, C-reactive protein, which is commonly employed in clinical practice as an indicator of inflammation and infection. Because they have been found to be the most commonly associated with rumination and adolescent anxiety and depressive symptoms, only C-reactive protein and interleukin-6 were analyzed (Haapakoski, Mathieu, Ebmeier, Alenius, & Kivimäki, 2015; Khandaker et al., 2016; Zoccola et al., 2013).
Fig. 1. Immunocognitive Model of Depression Secondary to Anxiety.
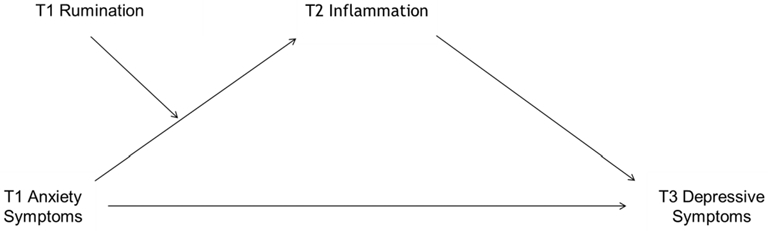
Note: Sx = symptoms.
Methods
Participants
Participants were drawn from the Adolescent Cognition and Emotion (ACE) project at Temple University, a longitudinal study of depression. Adolescents (N=640) ages 12–13 years and their mothers were recruited from the greater Philadelphia area through a combination of mailings and follow-up phone calls to families with children attending middle schools (68% of the total sample) and advertisement in local newspapers (32% of the sample). Inclusion criteria stipulated that the participant needed to be 12–13 years old, self-identify as Caucasian/White, African American/Black, or Biracial, and speak and read English. Participants were excluded if either child or mother had insufficient skill in the English language to complete the assessments or had a severe psychiatric, developmental, or learning disorder (see Alloy et al., 2012 for further details). Informed consent was obtained from all individual participants included in the study. The Temple University Institutional Review Board approved the protocol (IRB protocol #6844).
About four years after Project ACE began, voluntary blood draws were introduced into the study. The present sample included 140 adolescents (mean age at T1 (first blood draw) of this study = 16.5; SD = 1.2 years) who provided blood voluntarily on two separate visits (T1 and T2) and completed at least one follow-up session after the second blood draw (T3). This sample was 52% female, 40% Caucasian, 54% African American, and 6% biracial. Independent sample t-tests indicated that the current subsample did not differ at baseline from the total sample on depressive symptoms, anxiety symptoms, race, sex, or socioeconomic status (all ps > .05).
Procedure
At T1, adolescents provided blood and completed self-report questionnaires assessing depressive symptoms, anxiety symptoms, and cognitive response styles. At T2 (time since T1: mean = 13.5 months, SD = 5.9 months, range = 37.2 months), adolescents provided a second blood sample and completed the measures of depressive and anxiety symptoms again. Participants were invited to follow-up sessions approximately every 6 months; however, blood draws were an optional part of the study, so T2 was not necessarily the first follow-up after T1; it was whenever the participant completed a second blood draw. Depressive symptoms were re-assessed at T3, the first follow-up after T2 (time between T2 and T3: mean = 12.5 months, SD = 6.5 months, range = 33.7 months; time between T1 and T3: mean = 26.0 months, SD= 9.0 months, range = 54.2 months), to assess follow-up depressive symptoms.
Measures
Depressive symptoms.
The Children’s Depression Inventory (CDI; Kovacs, 1985) is a self-report measure of depressive symptoms designed for youths 7–17-years of age and is comprised of 27 items endorsed at one of three levels of severity (e.g., “I am sad once in a while”, “I am sad many times”, or “I am sad all the time”). It is a reliable and valid measure of depressive symptoms in youth samples (Klein, Dougherty, & Olino, 2005). The CDIs administered at baseline T1 and T3 were used in this study. Internal consistency in this sample was α = .87 at T1 and .83 at T3.
Anxiety symptoms.
The Multidimensional Anxiety Scale for Children (MASC; March, Parker, Sullivan, Stallings, & Conners, 1997) is a self-report questionnaire consisting of 39 items (e.g., I feel tense or uptight) that load onto four factors: physical symptoms of anxiety, social anxiety, separation anxiety, and harm avoidance. Items are endorsed on a 4-point Likert scale ranging from “Never” to “Often”. The MASC total from T1 was used for our main analyses inasmuch as there were no a priori hypotheses about the role of particular types of anxiety. The MASC has strong retest reliability and convergent and divergent validity (March et al., 1997; March, Sullivan, & Parker, 1999). Internal consistency in this sample was α = .88 for the MASC at T1.
Rumination.
The Children’s Response Styles Questionnaire (CRSQ; Abela, Vanderbilt, & Rochon, 2004) is a self-report instrument that measures how youth respond to sad moods. It consists of 25-items (e.g., “When I am sad, I think about how sad I feel”) probing the frequency with which dysphoria is responded to with rumination, problem solving, or distraction. Items were rated on a 4-point Likert scale (“Almost never”, “Sometimes”, “Often”, and “Almost always”). Only the rumination subscale (13 items) was used for this study. The CRSQ has demonstrated good validity and internal consistency (Abela et al., 2004). Internal consistency for the Rumination subscale in this sample was α = .89 at T1.
Immune assays.
Blood was obtained via antecubital venipuncture by a certified phlebotomist into a 10 mL vacutainer designed for freezing plasma separated from the cells within the vial (BD Hemogard with K2 EDTA). Vacutainers were stored in an ultracold freezer at −80oC, and later thawed on the day of assay. Time of day of the blood draw was recorded and participants’ body mass index (BMI) were calculated from measured height and weight.
Four cytokines were quantified by multi-cytokine array (interleukin-6, interleukin-8, interleukin-10, and tumor necrosis factor alpha), and high-sensitivity C-reactive protein was determined in singleplex assay, using an electrochemiluminescence platform and a QuickPlex SQ 120 imager for analyte detection (Meso Scale Discovery, Gaithersburg, MD). Each specimen was assayed in duplicate, with intra-assay coefficients of variation between 1.94 – 4.38%, and values referenced to a standard curve generated from 7 calibrators with known concentrations. The lower limit of detection (LLOD) for the cytokines was 0.1 pg/mL, with a large dynamic range up to 2000 pg/mL. C-reactive protein is present in blood at higher concentrations, and thus, plasma was diluted to correspond to the standard curve. Values were converted to mg/L units in keeping with the clinical literature, and were calculated down to 0.1 mg/L (Breen et al., 2011; Dabitao, Margolick, Lopez, & Bream, 2011).
Data Analysis Plan
All descriptive statistics, correlations, and analyses were conducted in SPSS (v23; IBM Corp, 2016). Moderation, mediation, and moderated mediation analyses were conducted using Models 1, 4, and 7 in the Process Macro (Hayes, 2013), respectively. Bivariate correlations between demographic and physical characteristics and primary study variables were calculated (see Tables 1 and 2). Moderation models tested whether anxiety symptoms (MASC; T1) and rumination (CRSQ; T1) interacted to predict inflammatory activity, specifically interleukin-6 or C-reactive protein (T2). Unconditional mediation models tested whether inflammation (interleukin-6 or C-reactive protein) at T2 mediated the relationship between anxiety symptoms (T1) and depressive symptoms (CDI; T3). Significant mediations were indicated by the 95% confidence intervals for the indirect effect not including 0. Moderated mediation analyses tested whether interleukin-6 and C-reactive protein (T2) mediated the relationship between anxiety symptoms (T1) and depressive symptoms (CDI; T3) at various levels of rumination (T1). Moderation, mediation, and moderated mediation analyses handled missing data with listwise deletion. Because they previously have been shown to be associated with inflammation and/or depressive symptoms, all analyses controlled for the effects of T1 age (Mills et al., 2013), T2 BMI (Howren et al., 2009), time of T2 blood draw (Dominguez-Rodriguez, Abreu-Gonzalex, & Kaski, 2009), SES (a dichotomous variable with “1” indicating eligibility for a government-funded lunch at school and “0” indicating ineligibility) (Deverts, Cohen, Kalra, & Matthews, 2012), sex (Hankin et al., 1998), and race (Alanna et al., 2011). Unconditional and moderated mediation analyses also controlled for T1 depressive symptoms. All analyses controlled for T1 levels of the biomarker used in the model and time in study. T1 depressive symptoms and inflammation were covaried to examine change in inflammation from T1 to T2 as a mediator of the interactive relationship between anxiety and rumination at T1 and change in depressive symptoms from T1 to T3. Time in study was controlled because a longer time between T1 and T3 provides more opportunity for change in depressive symptoms.
Table 1.
Bivariate Correlations and Descriptive Statistics of Primary Study Variables
| Measure | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. |
|---|---|---|---|---|---|---|---|---|
| 1. T1 Anxiety | — | |||||||
| 2. T1 Rum | .379** | — | ||||||
| 3. T1 CRP | −.229* | −.134 | — | |||||
| 4. T1 IL-6 | .052 | −.017 | .469** | — | ||||
| 5. T1 Dep | .439** | .587** | −.039 | .035 | — | |||
| 6. T2 CRP | −.053 | −.053 | .557** | .437** | −.046 | — | ||
| 7. T2 IL-6 | .044 | .075 | .447** | .477** | .078 | .521** | — | |
| 8. T3 Dep | .346** | .391** | .034 | .111 | .677** | .002 | .135 | — |
| N | 138 | 105 | 122 | 127 | 139 | 126 | 126 | 140 |
| Mean | 36.22 | 25.48 | 1.22 | 0.43 | 7.07 | 1.30 | 0.35 | 5.73 |
| SD | 15.22 | 7.82 | 1.56 | 0.30 | 6.35 | 1.69 | 0.25 | 5.16 |
Note: Rum = Rumination, CRP = C-reactive Protein, IL- = Interleukin, Dep = Depression
p < .05,
p < .01.
Table 2.
Bivariate Correlations of Biomarkers and Covariates
| Measure | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Months in Study | — | |||||||||||||||
| 2. T1 CRP | −.111 | — | ||||||||||||||
| 3. T1 IL6 | −.076 | .469** | — | |||||||||||||
| 4. T1 Dep | −.041 | −.039 | .035 | — | ||||||||||||
| 5. T1 Age | −.381** | .183* | .091 | −.132 | — | |||||||||||
| 6. Time of T2 BD | .071 | .058 | .041 | −.066 | .027 | — | ||||||||||
| 7. T2 BMI | −.032 | .472** | .442** | .098 | .137 | −.104 | — | |||||||||
| 8. T2 CRP | −.022 | .557** | .437** | −.046 | .073 | −.137 | .487** | — | ||||||||
| 9. T2 IL-6 | −.122 | .447** | .477** | .078 | −.026 | −.024 | .457** | .521** | — | |||||||
| 10. T3 Dep | .085 | .072 | .074 | .660** | .035 | −.056 | .192* | .044 | .130 | |||||||
Note: All Ns range from 114–140. CRP = C-reactive Protein, IL- = Interleukin, Dep = Depression, BD = Blood Draw, BMI = Body Mass Index
p < .05,
p < .01.
C-reactive protein values > 10 mg/L may indicate an acute infection, and thus 17 participants with C-reactive protein above 10 mg/L were excluded from subsequent analyses (Bell et al., 2017; Ferranti, Gauvreau, Ludwih, Newburger, & Rifai, 2006). Additionally, eight C-reactive protein and four interleukin-6 values that were > 3.0 standard deviations above the mean were removed as outliers. These outliers were removed, rather than winsorized, because the biomarker values were skewed even after log-transformation with the outliers in the dataset, and thus, violated the assumptions of the statistical methods used in this study. Finally, two cases were removed because their C-reactive protein values were greater than 2.5 standard deviations above the mean and the participants had indicated recent illness at the time of the blood draw. Additionally, 35 participants were missing the CRSQ at T1 due to technical difficulties, further reducing the final N for analyses. It is important to note that the sum of the participants removed for the reasons listed above is larger than the number actually removed from the analyses because of overlap between missingness on the CRSQ and removal as inflammation outliers. Even after removing outliers, raw C-reactive protein and interleukin-6 values were significantly skewed and were thus log-transformed (log(10 x value)), which normalized their distribution to an acceptable amount for the analyses.
Results
Preliminary Analyses
Descriptive statistics and bivariate correlations for the primary study variables, including the untransformed inflammation variables, are summarized in Table 1. T1 anxiety symptoms were positively correlated with T1 rumination (r =. 379, p < .01), T1 depressive symptoms (r = .439, p < .01), and T3 depressive symptoms (r = .346, p < .01) and negatively correlated with T1 C-reactive protein (r = −.229, p < .05). T1 rumination also was positively associated with T1 and T3 depressive symptoms (r = .587, p < .01; r = .391, p < .01; respectively). T1 C-reactive protein was positively correlated with T1 interleukin-6 (r = .469, p < .01), T2 C-reactive protein (r = .557, p < .01) and T2 interleukin-6 (r = .447, p < .01). T1 interleukin-6 was correlated with T2 C-reactive protein (r = .437, p < .01) and T2 interleukin-6 (r = .477, p < .01). T1 depressive symptoms was correlated with T3 depressive symptoms (r = .677, p < .01) and T2 C-reactive protein was correlated with T2 interleukin-6 (r = .521, p < .01). Independent samples t-tests were used to investigate potential differences in primary study variables as a function of gender and SES. Adolescents who qualified for a federally funded lunch had higher depressive symptoms at T3 and females had higher C-reactive protein at T1, interleukin-6 at T1 and T2, and anxiety symptoms at T1 (all p’s < .05). A one-way ANOVA found no significant differences in primary study variables between races (p > .05). Bivariate correlations between immune variables, depressive symptoms, and covariates are presented in Table 2.
Primary Analyses
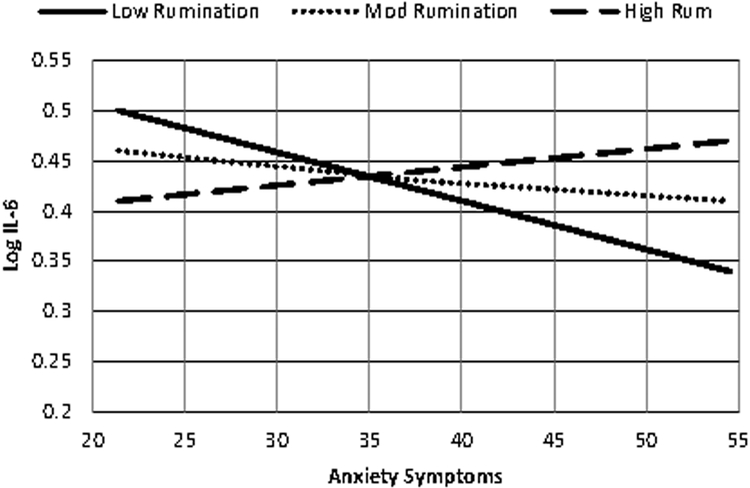
Anxiety symptoms and rumination interacted to predict interleukin-6 such that, at higher levels of rumination, anxiety predicted greater interleukin-6 (b = .0004, SE = .0002, t= 2.0985, p = .0392; see Fig. 2). This interaction did not significantly predict C-reactive protein at T2 (p = .7451).
Fig. 2. Anxiety Symptoms and Rumination Interact to Predict Log Interleukin-6.
Neither of the inflammation variables were significant mediators in the unconditional models. Additionally, the moderated mediation model including C-reactive protein as a mediator was not significant (index of moderated mediation = −.0003, SE = .0012, 95% CI: −.0029, .0021). However, consistent with our hypothesis, interleukin-6 mediated the relationship between anxiety symptoms at T1 and depressive symptoms at T3, and this relationship was conditional on rumination (index of moderated mediation = .0015, SE = .0010, 95% CI: .0001, .0042; see Table 3 and Fig. 3). Table 3 is separated into four sections, the first containing statistics for the key predictors in the a’ pathway predicting to T2 log interleukin-6, the second describing the key predictors of T3 depressive symptoms on the b’ pathway, the third showing the conditional indirect effects of T1 anxiety symptoms on T3 depressive symptoms at different levels of rumination, and the fourth reporting the index of moderated mediation. Anxiety symptoms interacted with high levels of rumination to predict higher levels of interleukin-6 at T2 and depressive symptoms at T3, and with lower levels of rumination to predict less interleukin-6 at T2 and lower depressive symptoms at T3 (b = .0004, SE = .0002, t= 2.0702, p = .0420). Follow-up analyses found that interleukin-6 mediated the conditional indirect effect of T1 anxiety symptoms on T3 depressive symptoms at low levels of rumination (−1 SD: b = −.0178, SE = .0121, 95% CI: −.0509, −.0010), but not at higher levels of rumination. Although the conditional indirect effect of T1 anxiety on T3 depressive symptoms was not significant at higher levels of rumination, it is worth noting that anxiety symptoms predicted more interleukin-6 and depressive symptoms as rumination increased (Mean: b = −.0063, SE = .0078, 95% CI: −.0327, .0026; +1 SD: b = .0053, SE = .0089, 95% CI: −.0078, .0295). In conclusion, the strength and direction of the mediation (i.e., anxiety leading to more or less interleukin-6) was conditional on trait rumination; however, interleukin-6 was only a significant mediator at low levels of rumination in this model.
Table 3.
Log IL-6 Mediates the Relationship between T1 Anxiety and T3 Depression at Different Levels of Rumination (N=86)
| Variable | Coefficient | Standard Error | t | p |
|---|---|---|---|---|
| Outcome: a’ path (predicting to T2 Log IL-6) | ||||
| T1 Anxiety | −.0126 | .0055 | −2.2813 | .0254 |
| T1 Rumination | −.0155 | .0087 | −1.7750 | .0801 |
| T1 Log IL-6 | .4128 | .1032 | 4.0013 | .0001 |
| T1 Anxiety x T1 Rumination | .0004 | .0002 | 2.0702 | .0420 |
| T1 Depression | .0014 | .0053 | .2671 | .7901 |
| R2 accounted for | 44% | |||
| Outcome: b’ path (predicting to T3 Dep) | ||||
| T1 Anxiety | .0292 | .0317 | .9227 | .3592 |
| T1 Depression | .5350 | .0831 | 6.4366 | <.0001 |
| T1 Log IL-6 | −1.9508 | 1.9558 | −.9974 | .3218 |
| T2 Log IL-6 | 3.4101 | 2.0037 | 1.7019 | .0930 |
| R2 accounted for | 55% | |||
| Conditional indirect effect of T1 Anxiety on T3 Depression at Different Levels of Rumination: | ||||
| Rumination Percentile | Effect | Boot Standard Error | Boot LLCI | Boot ULCI |
| −1 SD | −.0178 | .0121 | −.0509 | −.0010 |
| Mean | −.0063 | .0078 | −.0327 | .0026 |
| +1SD | .0053 | .0089 | −.0078 | .0295 |
| Index of Moderated Mediation | ||||
| Mediator | Index | Boot Standard Error | Boot LLCI | Boot ULCI |
| T2 Log IL-6 | .0015 | .0009 | .0001 | .0039 |
Note: All analyses controlled for the effects of T1 age, T2 BMI, T2 time of blood draw, sex, race, whether the family qualified for a free school lunch, and months in study. IL- = Interleukin.
Fig. 3. Log IL-6 Mediates the Relationship between T1 Anxiety Symptoms and T3 Depression Symptoms at Different Levels of Rumination.
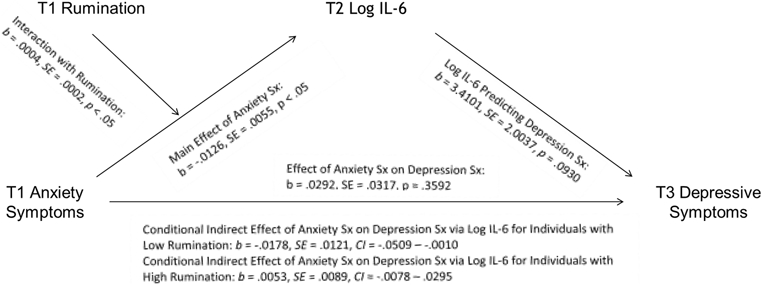
Note: Sx = symptoms. Moderated mediation analyses controlled for T1 age, depressive symptoms, and inflammation; T2 body mass index, time of T2 blood draw, as well as SES, sex, race, and time in study.
Alternate Models Tested
Inasmuch as there is evidence that rumination both moderates and mediates the temporal relationship between anxiety and depressive symptoms (Grant et al., 2014; Starr et al., 2016), sequential mediation analyses also were conducted with trait rumination mediating the relationship between anxiety symptoms at T1 and inflammation at T2 and inflammation at T2 mediating the relationship between trait rumination and depressive symptoms at T3. Covariates were identical to those described above for moderated mediation, and models were tested with both interleukin-6 and C-reactive protein as mediators. None of the mediations in either of these models was significant. Additionally, whereas the a priori hypotheses focused on anxiety symptoms generally, analyses also were run with the MASC subscales. No significant moderated or unconditional mediations were significant; however, MASC harm avoidance interacted with rumination to predict interleukin-6 (b = .0011, SE = .0004, t= 2.6075, p = .0110), such that at higher levels of rumination, greater MASC harm avoidance symptoms predicted higher interleukin-6.
Discussion
The literature on inflammation and depressive symptoms has rapidly expanded in recent years, with exciting new research and novel theoretical models (e.g., Slavich & Irwin, 2014); however, there are a number of limitations in this body of literature. First is the lack of published literature using longitudinal designs. As excessively proinflammatory phenotypes are implicated in the pathogenesis of depressive symptoms, temporal precedence of inflammation measures before depressive symptoms assessments greatly improves the ability to interpret results. This need is compounded by results suggesting that the relationship between inflammation and depressive symptoms is bidirectional in adolescents (Miller & Cole, 2012); thus, being able to account for the variance attributed to both inflammation and depressive symptoms at the same time point is crucial. Additionally, even among the few prospective studies, none has investigated inflammation as a mediator between established risk factors for depression and depressive symptoms, despite the frequent conceptualization of inflammation as a pathophysiological mechanism for depression (Slavich & Irwin, 2014). Further, whereas there are published studies investigating the link between experimentally-instructed engagement in rumination and inflammatory activity (Zoccola et al., 2013), no studies to date have investigated this relationship using trait-measures of rumination associated with depression. Crucially, there is a dearth of literature on the role of inflammation in depression using adolescent samples, a crucial period of risk for the development of depressive symptoms (Cummings et al., 2014). The current study sought to address these limitations of this area of research by testing markers of inflammation, specifically interleukin-6 and C-reactive protein, as mediators of the relationship between anxiety symptoms and depressive symptoms conditional on rumination.
Our study generated support for inflammation as a mediator of the relationship between anxiety symptoms and later depressive symptoms, and found that this relationship is conditional on rumination. Specifically, consistent with our hypothesis, results support that high levels of rumination are a risk factor, and low levels a protective factor, for the development of elevated interleukin-6, and subsequent depressive symptoms, secondary to anxiety symptoms. To our knowledge, this study is the first indicating that an inflammatory cytokine mediates the relationship between anxiety symptoms and subsequent depressive symptoms. Importantly, none of the unconditional mediations reached significance, demonstrating that accounting for the interaction between anxiety and rumination is a necessary component of this model. Considered together, this suggests that the presence of symptoms of anxiety in adolescents alone does not increase risk for proinflammatory phenotypes in a way that increases risk for depressive symptoms, but it is the combination of anxiety and how adolescents cognitively respond to negative affect that influences the trajectory toward inflammation and depressive symptoms. Further, this moderated mediation model highlights the potential for both biological and psychological treatment targets to prevent the transition to depression from anxiety in adolescence: rumination and inflammation.
C-reactive protein was not found to be a significant mediator in our models. This might be due in part to controlling for T1 inflammation in the models, as the stability in the biomarker values across the two-time points was particularly high for C-reactive protein (r = .557), one of the inflammatory markers most commonly associated with depressive symptoms. However, controlling for T1 inflammation and T1 depressive symptoms was necessary to take full advantage of our longitudinal data to best understand what processes can influence shifts toward a proinflammatory phenotype and later depressive symptoms.
The results provide further support for Cohen et al.’s (2014) diathesis-anxiety model of depression secondary to anxiety, in which anxiety symptoms interact with specific cognitive vulnerabilities to predict depressive symptoms. Similar to Cohen and colleagues’ study, the present study found that higher rumination interacted with anxiety symptoms to increase risk for proinflammatory phenotypes associated with depressive symptoms, and lower rumination interacted with anxiety symptoms to decrease risk for inflammation in adolescents. Additionally, interleukin-6 was found to mediate the relationship between anxiety and depressive symptoms at low levels of rumination, highlighting low rumination as a protective factor in this etiological pathway. Further, as both i) anxiety and depression, and ii) inflammation have been demonstrated to have a bidirectional relationship, it is likely that these variables operate in a positive feedback loop that maintains and confers risk for elevated inflammatory phenotypes and depressive symptoms (Lavigne, Hopkins, Gouze, & Bryant, 2015; Miller & Cole, 2012). These results are an important step in understanding biological and cognitive mechanisms involved in an extremely prevalent and costly psychiatric comorbidity. Notably, these results highlight two potential targets for the prevention and treatment of depression secondary to anxiety in adolescence, specifically rumination and inflammation. Additionally, these results suggest that inflammation might be a biological variable underlying some of the effects of psychotherapies that target ruminative response styles.
This study has several important strengths. It assessed a diverse community sample of adolescents. Further, adolescents are both understudied in the inflammation literature and are at elevated risk for developing depressive symptoms. Additionally, this study utilized a fully prospective design across three time points so that the temporal relationships between anxiety symptoms, inflammatory biomarkers, and depressive symptoms could be established, and baseline levels of both inflammation and depressive symptoms could be controlled. Consequently, the results demonstrate that changes in inflammation predict changes in depressive symptoms as a result of the interaction between anxiety symptoms and rumination.
These results should be considered preliminary in light of the following limitations. First, there was considerable variation in follow-up times across the three time points. Consequently, participants had different lengths of time for both their inflammatory physiology and depressive symptoms to change, which could influence the relationships between these variables. Additionally, there is evidence that the relationship between inflammation and depressive symptoms is conditional on time-to-follow-up (Moriarity et al., 2018); thus, the significance of the b’ pathway in the mediation models might have been influenced by this variability. Additionally, this study used only self-report measures of anxiety and depressive symptoms, without diagnostic clinical interviews. A test of this theoretical model with verified anxiety and depression diagnoses would ensure the clinical relevance of these findings. Furthermore, given that this sample primarily consisted of late adolescents, this model will have to be replicated in younger samples to ensure generalizability throughout adolescence.
Conclusion
Adolescence is a period of increased risk for depressive and anxious symptoms (Cummings et al., 2014). As anxiety is a risk factor for later depression (Bittner et al., 2004), the identification of cognitive and biological mechanisms that confer risk for depression secondary to anxiety in adolescence is crucial for the development of effective treatments that are both biologically and psychologically informed. As both inflammation and rumination are implicated in the pathophysiology of adolescent depression (Duivis et al., 2015; Starr et al., 2016), the current study extended the diathesis-anxiety model (Cohen et al., 2014) to include inflammation as a mediator of the relationship between the interaction of anxiety symptoms and rumination and later depressive symptoms. The results provided support for our a priori hypothesis that inflammation would mediate the relationship between anxiety symptoms and changes in depressive symptoms over time, but only when rumination is included as a moderator of the relationship between anxiety and inflammation. These results highlight the relevance of inflammatory processes and the immune system in the development of depressive symptoms secondary to anxiety symptoms during adolescence. Further, this integrated immunocognitive model highlights both psychological (rumination) and biological (inflammation) targets for depression prevention and treatment for adolescents.
Acknowledgments
Funding
This research was supported by National Institute of Mental Health Grant MH101168 to Lauren B. Alloy.
Biography
Daniel P. Moriarity, M.A. is a doctoral student in Lauren B. Alloy’s Mood and Cognition Lab at Temple University. His major research interests include how cognitive vulnerabilities and inflammation interact to confer risk for mood disorder comorbidities.
Brae Anne McArthur, Ph.D. is a Banting Postdoctoral Fellow working in the Mood and Cognition Laboratory at Temple University in Philadelphia, Pennsylvania. She received her Ph.D. in Clinical Psychology: Applied Developmental Emphasis from the University of Guelph in Guelph, Ontario. Her primary research interest is in the area of positive clinical psychology and adaptive youth functioning.
Lauren M. Ellman, Ph.D. is an Associate Professor of Psychology at Temple University. She received her doctorate in Clinical Psychology at the University of California-Los Angeles. Her major research interests include neurodevelopmental risk factors, including inflammation, for psychotic disorders (e.g. schizophrenia) and depression.
Christopher L Coe, Ph.D. is the W.B. Cannon Professor of Biopsychology at the University of Wisconsin-Madison. He received his doctorate in the behavioral neurosciences at Downstate Medical Center, SUNY. His research interests include the relationship between biomarkers and health, with a focus on resilience and vulnerability at the beginning and end of the life course, specifically the prenatal period and old age.
Lyn Y. Abramson, Ph.D. is the Sigmund Freud Professor of Psychology at the University of Wisconsin-Madison. She received her doctorate in Clinical Psychology from the University of Pennsylvania. Her major research interests include the developmental, cognitive, motivational, and cultural determinants of information processing about the self and the effects of early psychological, physical, and sexual maltreatment on the development of cognitive styles and vulnerability to depression in adulthood.
Lauren B. Alloy, Ph.D. is Laura H. Carnell Professor and Joseph Wolpe Distinguished Faculty in Psychology at Temple University. She received her doctorate in Experimental and Clinical Psychology from the University of Pennsylvania. Her major research interests include cognitive, psychosocial, developmental, and, neurobiological processes in the onset and course of depression and bipolar disorder.
Footnotes
Data Sharing Declaration
The datasets generated and/or analyzed during the current study are not publicly available but may be available from the corresponding author on reasonable request.
Conflicts of Interest
The authors report no conflict of interests.
Ethical approval
The Temple University Institutional Review Board approved the protocol (IRB protocol #6844).
Informed Consent
Written informed consent was collected from all study participants after explaining their role in the study and before starting data collection.
References
- Abela JRZ, Vanderbilt E, & Rochon A (2004). A test of the integration of the response styles and social support theories of depression in third and seventh grade children. Journal of Social and Clinical Psychology, 23(5), 653–674. https://doi.org/10.1521/jscp.23.5.653.50752 [Google Scholar]
- Alanna M, Zhao L, Ahmed Y, Stoyanova N, Hooper WC, Gibbons G, … Vaccarino V (2011). Association between depression and inflammation–differences by race and sex: the META-Health study. Psychosomatic Medicine, 73(6), 462–468. https://doi.org/10.1097/PSY.0b013e318222379c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloy LB, Black SK, Young ME, Goldstein KE, Shapero BG, Stange JP, … Abramson LY (2012). Cognitive vulnerabilities and depression versus other psychopathology symptoms and diagnoses in early adolescence. Journal of Clinical Child and Adolescent Psychology, 41(5), 539–60. https://doi.org/10.1080/15374416.2012.703123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balázs J, Miklõsi M, Keresztény Á, Hoven CW, Carli V, Wasserman C, … Wasserman D (2013). Adolescent subthreshold-depression and anxiety: Psychopathology, functional impairment and increased suicide risk. Journal of Child Psychology and Psychiatry and Allied Disciplines, 54(6), 670–677. https://doi.org/10.1111/jcpp.12016 [DOI] [PubMed] [Google Scholar]
- Bell JA, Kivimäki M, Bullmore ET, Steptoe A, Bullmore E, Vértes PE, … Carvalho LA (2017). Repeated exposure to systemic inflammation and risk of new depressive symptoms among older adults. Translational Psychiatry, 7(8), e1208 https://doi.org/10.1038/tp.2017.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner A, Goodwin RD, Wittchen HU, Beesdo K, Hofler M, & Lieb R (2004). What characteristics of primary anxiety disorders predict subsequent major depressive disorder? J Clin Psychiatry, 65(5), 618–26. [DOI] [PubMed] [Google Scholar]
- Breen EC, Reynolds SM, Cox C, Jacobson LP, Magpantay L, Mulder CB, … Norris PJ (2011). Multisite comparison of high-sensitivity multiplex cytokine assays. Clinical and Vaccine Immunology, 18(8), 1229–1242. https://doi.org/10.1128/CVI.05032-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosschot JF, Gerin W, & Thayer JF (2006). The perseverative cognition hypothesis: a review of worry, prolonged stress-related physiological activation, and health. Journal of Psychosomatic Research, 60(2), 113–24. https://doi.org/10.1016/j.jpsychores.2005.06.074 [DOI] [PubMed] [Google Scholar]
- Burcusa SL, & Iacono WG (2007). Risk for recurrence in depression. Clinical Psychology Review, 27(8), 959–985. https://doi.org/10.1016/j.cpr.2007.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JE, Low CA, Prather AA, Cohen S, Fury JM, Ross DC, & Marsland AL (2011). Negative affective responses to a speech task predict changes in interleukin (IL)-6. Brain, Behavior, and Immunity, 25(2), 232–8. https://doi.org/10.1016/j.bbi.2010.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JR, Young JF, Gibb BE, Hankin BL, & Abela JRZ (2014). Why are anxiety and depressive symptoms comorbid in youth? A multi-wave, longitudinal examination of competing etiological models. Journal of Affective Disorders, 161, 21–29. https://doi.org/10.1016/j.jad.2014.02.042.Why [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland WE, Shanahan L, Worthman C, Angold A, & Costello EJ (2012). Generalized anxiety and C-reactive protein levels: a prospective, longitudinal analysis. Psychological Medicine, 42(12), 2641–2650. https://doi.org/10.1017/S0033291712000554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ, Mustillo S, Erkanli A, & Keele G (2003). Prevalence and development of psychiatric disorders in childhood and adolescence. Archives of General Psychiatry, 60(8), 837–844. https://doi.org/10.1001/archpsyc.60.8.837 [DOI] [PubMed] [Google Scholar]
- Cummings C, Caporino N, & Kendall PC (2014). Comorbidity of anxiety and depression in children and adolescents: 20 Years After. Psychological Bulletin, 140(3), 816–845. https://doi.org/10.1037/a0034733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabitao D, Margolick JB, Lopez J, & Bream JH (2011). Multiplex measurement of proinflammatory cytokines in human serum: comparison of the Meso Scale Discovery electrochemiluminescence assay and the Cytometric Bead Array. Journal of Immunological Methods, 372(1–2), 71–77. https://doi.org/10.1038/jid.2014.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ferranti SD, Gauvreau K, Ludwih DS, Newburger JW, & Rifai N (2006). Inflammation and Changes in Metabolic Syndrome Abnormalities in US Adolescents: Findings from the 1988–1994 and 1999–2000 National Health and Nutrition Examination Surveys. Clinical Chemistry, 52(7), 1325–1330. [DOI] [PubMed] [Google Scholar]
- Deverts DJ, Cohen S, Kalra P, & Matthews KA (2012). The prospective association of socioeconomic status with C-reactive protein levels in the CARDIA study. Brain Behavior and Immunity, 26(7), 1128–1135. https://doi.org/10.1016/j.bbi.2012.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS, Burke HM, Epel ES, Mellon SH, Rosser R, Reus VI, & Wolkowitz OM (2009). Low serum IL-10 concentrations and loss of regulatory association between IL-6 and IL-10 in adults with major depression. Journal of Psychiatric Research, 43(11), 962–969. https://doi.org/10.1016/j.jpsychires.2009.05.010 [DOI] [PubMed] [Google Scholar]
- Dominguez-Rodriguez A, Abreu-Gonzalex P, & Kaski JC (2009). Inflammatory Systemic Biomarkers in Setting Acute Coronary Syndromes - Effects of the Diurnal Variation. Current Drug Targets, 10(10), 1001–1008. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, & Lanctôt KL (2010). A Meta-Analysis of Cytokines in Major Depression. Biological Psychiatry, 67(5), 446–457. https://doi.org/10.1016/j.biopsych.2009.09.033 [DOI] [PubMed] [Google Scholar]
- Duivis HE, Kupper N, Vermunt JK, Penninx BW, Bosch NM, Riese H, … de Jonge P (2015). Depression trajectories, inflammation, and lifestyle factors in adolescence: The Tracking Adolescents’ Individual Lives Survey. Health Psychology, 34(11), 1047–1057. https://doi.org/10.1037/hea0000210 [DOI] [PubMed] [Google Scholar]
- Garber J, & Weersing VR (2010). Comorbidity of Anxiety and Depression in Youth : Implications for Treatment and Prevention. Clinical Psychology: Science and Practice, 17, 263–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin RD, Fergusson DM, & Horwood LJ (2004). Asthma and depressive and anxiety disorders among young persons in the community. Psychological Medicine, 34(8), 1465–74. https://doi.org/10.1017/S0033291704002739 [DOI] [PubMed] [Google Scholar]
- Grant DM, Judah MR, Mills AC, Lechner WV, Davidson CL, & Wingate LR (2014). Rumination and excessive reassurance seeking: Mediators of the relationship between social anxiety and depression? Journal of Psychopathology and Behavioral Assessment, 36(3), 465–474. https://doi.org/10.1007/s10862-013-9399-5 [Google Scholar]
- Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, & Kivimäki M (2015). Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain, Behavior, and Immunity, 49, 206–215. https://doi.org/10.1016/j.bbi.2015.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, & Angell KE (1998). Development of depression from preadolescence to young adulthood: emerging gender differences in a 10-year longitudinal study. Journal of Abnormal Psychology, 107(1), 128–140. https://doi.org/10.1037/0021-843X.107.1.128 [DOI] [PubMed] [Google Scholar]
- Hart BL (1988). Biological basis of the behavior of sick animals. Neuroscience & Biobehavioral Reviews, 12(2), 123–137. https://doi.org/10.1016/S0149-7634(88)80004-6 [DOI] [PubMed] [Google Scholar]
- Hayes A (2013). Introduction to mediation, moderation, and conditional process analysis : a regression-based approach. New York: Guilford Press. [Google Scholar]
- Horn PJ, & Wuyek LA (2010). Anxiety disorders as a risk factor for subsequent depression. International Journal of Psychiatry in Clinical Practice, 14(4), 244–7. https://doi.org/10.3109/13651501.2010.487979 [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, & Suls J (2009). Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosomatic Medicine, 71(2), 171–186. https://doi.org/10.1097/PSY.0b013e3181907c1b [DOI] [PubMed] [Google Scholar]
- IBM Corp. (2016). IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp. [Google Scholar]
- Kessler RC, Avenevoli S, Costello EJ, Georgiades K, Green JG, Gruber MJ, … Merikangas KR (2012). Prevalence, persistence, and sociodemographic correlates of DSM-IV disorders in the National Comorbidity Survey Replication Adolescent Supplement. Archives of General Psychiatry, 69(4), 372–380. https://doi.org/10.1001/archgenpsychiatry.2011.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, & Walters EE (2005). Lifetime Prevalence and Age-of-Onset Distributions of DSM-IV Disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry, 62(6), 593–602. [DOI] [PubMed] [Google Scholar]
- Khandaker GM, Zammit S, Lewis G, & Jones PB (2016). Neurobiology of Stress Association between serum C-reactive protein and DSM-IV generalized anxiety disorder in adolescence: Findings from the ALSPAC cohort. Neurobiology of Stress, 4, 55–61. https://doi.org/10.1016/j.ynstr.2016.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein D, Dougherty LR, & Olino TM (2005). Toward Guidelines for Evidence-Based Assessment of Depression in Children and Adolescents. Journal of Clinical Child & Adolescent Psychology, 34(3), 412, 432. https://doi.org/10.1207/s15374424jccp3403 [DOI] [PubMed] [Google Scholar]
- Kovacs M (1985). The Chidlren’s Depression Inventory (CDI). Psychopharmacology Bulletin, 21(4), 995–998. [PubMed] [Google Scholar]
- Lavigne JV, Hopkins J, Gouze KR, & Bryant FB (2015). Bidirectional Influences of Anxiety and Depression in Young Children. Journal of Abnormal Child Psychology, 43(1), 163–176. https://doi.org/10.1007/s10802-014-9884-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letcher P, Sanson A, Smart D, & Toumbourou JW (2017). Precursors and Correlates of Anxiety Trajectories From Late Childhood to Late Adolescence Precursors and Correlates of Anxiety Trajectories From Late Childhood to Late Adolescence. Journal of Clinical Child & Adolescent Psychology, 41(4), 417–432. https://doi.org/10.1080/15374416.2012.680189 [DOI] [PubMed] [Google Scholar]
- Liukkonen T, Räsänen P, Jokelainen J, Leinonen M, Järvelin MR, Meyer-Rochow VB, & Timonen M (2011). The association between anxiety and C-reactive protein (CRP) levels: Results from the Northern Finland 1966 Birth Cohort Study. European Psychiatry, 26(6), 363–369. https://doi.org/10.1016/j.eurpsy.2011.02.001 [DOI] [PubMed] [Google Scholar]
- March JS, Parker JDA, Sullivan K, Stallings P, & Conners CK (1997). The Multidimensional Anxiety Scale for Children (MASC): Factor structure, reliability, and validity. Journal of the American Academy of Child and Adolescent Psychiatry, 36(4), 554–565. https://doi.org/10.1097/00004583-199704000-00019 [DOI] [PubMed] [Google Scholar]
- March JS, Sullivan K, & Parker J (1999). Test-retest reliability of the Multidimensional Anxiety Scale for Children. Journal of Anxiety Disorders, 13(4), 349–358. https://doi.org/10.1016/S0887-6185(99)00009-2 [DOI] [PubMed] [Google Scholar]
- McEwen B, & Stellar E (1993). Stress and the individual: mechanisms leading to disease. Arch Intern Med, 153, 2093–2101. [PubMed] [Google Scholar]
- McLaughlin KA, & Nolen-Hoeksema S (2011). Rumination as a transdiagnostic factor in depression and anxiety. Behaviour Research and Therapy, 49(3), 186–193. https://doi.org/10.1016/j.brat.2010.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeus W (2011). The Study of Adolescent Identity Formation 2000 – 2010: A Review of Longitudinal Research. Journal of Research on Adolescence, 21(1), 75–94. https://doi.org/10.1111/j.1532-7795.2010.00716.x [Google Scholar]
- Michopoulos V, Powers A, Gillespie CF, Ressler KJ, & Jovanovic T (2016). Inflammation in Fear- and Anxiety-Based Disorders: PTSD, GAD and Beyond. Neuropsychopharmacology, 42(April), 1–53. https://doi.org/10.1038/npp.2016.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, & Raison CL (2009). Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biological Psychiatry, 65(9), 732–41. https://doi.org/10.1016/j.biopsych.2008.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, & Cole SW (2012). Clustering of depression and inflammation in adolescents previously exposed to childhood adversity. Biological Psychiatry, 72(1), 34–40. https://doi.org/10.1016/j.biopsych.2012.02.034.CLUSTERING [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills NT, Scott JG, Wray NR, Cohen-Woods S, & Baune BT (2013). Research review: The role of cytokines in depression in adolescents: A systematic review. Journal of Child Psychology and Psychiatry and Allied Disciplines, 54(8), 816–835. https://doi.org/10.1111/jcpp.12080 [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Harrington H, Caspi A, Kim-cohen J, Goldberg D, Gregory AM, & Poulton R (2007). Depression and generalized anxiety disorder: cumulative and sequential comorbidity in a birth cohort followed prospectively to age 32 years. Archives of General Psychiatry, 64(6), 651–660. [DOI] [PubMed] [Google Scholar]
- Moons WG, Eisenberger NI, & Taylor SE (2010). Anger and fear responses to stress have different biological profiles. Brain, Behavior, and Immunity, 24(2), 215–9. https://doi.org/10.1016/j.bbi.2009.08.009 [DOI] [PubMed] [Google Scholar]
- Moriarity DP, Mac Giollabhui N, Ellman LM, Klugman J, Coe CL, Abramson LY, & Alloy LB (2018). Inflammatory proteins predict change in depressive symptoms in male and female adolescents. Manuscript under Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J, & Nolen-Hoeksema S (1990). Effects of responses to depression on the remediation of depressive affect. Journal of Personality and Social Psychology, 58(3), 519–527. https://doi.org/10.1037/0022-3514.58.3.519 [DOI] [PubMed] [Google Scholar]
- Muris P, Roelofs J, Meesters C, & Boomsma P (2004). Rumination and worry in nonclinical adolescents. Cognitive Therapy and Research, 28(4), 539–554. https://doi.org/10.1023/B:COTR.0000045563.66060.3e [Google Scholar]
- Naudé PJW, Roest AM, Stein DJ, de Jonge P, & Doornbos B (2018). Anxiety disorders and CRP in a population cohort study with 54,326 participants: The LifeLines study. World Journal of Biological Psychiatry, 1–10. https://doi.org/10.1080/15622975.2018.1433325 [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S (1991). Responses to depression and their effects on the duration of depressive episodes. Journal of Abnormal Psychology, 100(4), 569–582. https://doi.org/10.1037/0021-843X.100.4.569 [DOI] [PubMed] [Google Scholar]
- Pallavi P, Sagar R, Mehta M, Sharma S, Subramanium A, Shamshi F, … Mukhopadhyay AK (2015). Serum cytokines and anxiety in adolescent depression patients: Gender effect. Psychiatry Research, 229(1–2), 374–380. https://doi.org/10.1016/j.psychres.2015.06.036 [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, & Miller GE (2004). Psychological Stress and the Human Immune System: A Meta-Analytic Study of 30 Years of Inquiry. Psychological Bulletin, 130(4), 601–630. https://doi.org/10.1037/0033-2909.130.4.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, & Irwin MR (2014). From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychological Bulletin, 140(3), 774–815. https://doi.org/10.1037/a0035302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spasojevic J, & Alloy LB (2001). Rumination as a common mechanism relating depressive risk factors to depression. Emotion, 1(1), 25–37. https://doi.org/10.1037//1528-3542.1.1.25 [DOI] [PubMed] [Google Scholar]
- Starr LR, Stroud CB, & Li YI (2016). Predicting the transition from anxiety to depressive symptoms in early adolescence: Negative anxiety response style as a moderator of sequential comorbidity. Journal of Affective Disorders, 190, 757–763. https://doi.org/10.1016/j.jad.2015.10.065 [DOI] [PubMed] [Google Scholar]
- van Lang NDJ, Ferdinand RF, & Verhulst FC (2007). Predictors of future depression in early and late adolescence. Journal of Affective Disorders, 97, 137–144. https://doi.org/10.1016/j.jad.2006.06.007 [DOI] [PubMed] [Google Scholar]
- Whooley MA (2006). Depression and cardiovascular disease healing the broken-hearted. JAMA, 295(24), 2874–2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccola PM, Figueroa WS, Rabideau EM, & Woody A (2013). Differential effects of post-stressor rumination and distraction on C-reactive protein in healthy women. Psychosom Med, 75(3), A-41 https://doi.org/10.1037/hea0000019 [Google Scholar]


