Abstract
Osteoarthritis (OA) in the temporomandibular joint (TMJ) is a degenerative disease in the adult, which is characterized by the pathological degeneration of condylar cartilage. Axin1 plays a critical role in the regulation of cartilage development and homeostasis. To determine the role of Axin1 in TMJ tissue at the adult stage, we generated Axin1Agc1ER mice, in which Axin1 was deleted in aggrecan‐expressing chondrocytes at 2 months of age. Histology, histomorphometry, and immunostaining analyses were performed using TMJ tissues harvested from 4- and 6-month-old mice after tamoxifen administration. Total RNA isolated from TMJ cartilage of 6-month- old mice was used for gene expression analysis. Progressive OA-like degeneration was observed in condylar cartilage in Axin1 knockout (KO) mice with loss of surface continuity and the formation of vertical fissures. In addition, reduced alcian blue staining in condylar cartilage was also found in Axin1 KO mice. Immunostaining and reverse transcription quantitative polymerase chain reaction (qRT-PCR) assays revealed disturbed homeostasis in condylar cartilage with increased expressions of MMP13 and Adamts5 and decreased lubricin expression in Axin1-deficient chondrocytes. Less proliferative cells with increased hypertrophic and apoptotic activities were presented in the condylar cartilage of Axin1Agc1ER KO mice. As a scaffolding protein, the deletion of Axin1 stimulated not only the β-catenin but also the fibroblast growth factor (FGF) signaling via ERK1/2 activation. The qRT-PCR results showed an increased expression of Fgfr1 in Axin1 KO cartilage. Overall, the deletion of Axin1 in condylar chondrocytes altered the β-catenin and FGF/ERK1/2 signaling pathways, thus cooperatively contribute to the cartilage degeneration.
Keywords: axis inhibition protein (axin1), fibroblast growth factor (FGF)/ERK, osteoarthritis (OA), temporomandibular joint (TMJ), β-catenin
Graphical Abstract
The contents of this page will be used as part of the graphical abstract of html only. It will not be published as part of main.
We have generated Axin1 conditional knockout (KO) mice by targeting aggrecan-expressing condylar cartilage cells in the temporomandibular joint (TMJ). The Axin1 conditional KO mice showed TMJ osteoarthritis (OA)-like phenotype, probably through activation of β-catenin and fibroblast growth factor (FGF) signaling.
1 |. INTRODUCTION
As one of the most frequently used joints in humans, temporomandibularjoint (TMJ) acts like a sliding hinge, connecting the jawbone to the skull. TMJ is a bilateral synovial joint composed of the articulareminence fossa of the temporal bone, the condylar process of the mandibular bone, and a fibrocartilaginous disc between them. The mandibular condylar cartilage is characterized as the articularfibrocartilage tissue. Structurally, the superficial layer of condylar cartilage is comprised of oriented collagen to resist loading and movement. The proliferative layer contains chondroprogenitor cells that contribute to the development and maintenance of TMJ cartilage. The deeper layer contains flattened mature chondrocytes that produce aggerecan- and collagen-enriched extracellular matrix, and then terminally differentiate into hypertrophic chondrocytes.
The disorders in TMJ (TMD) have a high incidence in adults resulting in severe craniofacial pain and reduced life quality. Among TMDs, the osteoarthritis (OA)-like degenerative changes in TMJ is a highly prevalent disease. The genetic predisposition contributes to the development of TMJ OA, although the molecular mechanisms remain largely unknown. TMJ OA is a type of OA disease sharing similar degenerative changes with OA found in other joints. The pathological changes of TMJ OA include the condylar chondrocyteapoptosis, cartilage loss, and subchondral bone sclerosis. According to the OARSI or modified Mankin scoring assessment, the phenotype of degenerative condylar cartilage may show the formation of chondrocyte clusters with hypocellularity, surface discontinuity with matrix loss, and formation of vertical fissures and cartilage erosion in more severe cases (Pauli et al., 2012; Zhou et al., 2016). At the molecular level, the imbalance of matrix catabolic and anabolic activities contributes to the pathological progression of condylar cartilage. The impaired secretion of matrix components, such as collagens and glycosaminoglycans (GAGs), and the increased synthesis of degradative enzymes, such as matrix metalloproteinases(MMPs) and a disintegrin and metalloproteinase with thrombospondinmotifs (Adamts), disturb the cartilage homeostasis in TMJ during OA development (Chen et al., 2016).
Axis inhibition protein (Axin1) is expressed in the cartilaginousarea of the axial and appendicular skeleton during embryonic axisdevelopment (Zeng et al., 1997). The deletion of Axin1 leads to earlyembryonic mortality with the axis determination defects (Chia &Costantini, 2005; Chia, Kim, Itoh, Sokol, & Costantini, 2009).Significant deformities in craniofacial and axial skeleton were also shown in Axin2-deficient mice during endochondral bone development(Dao et al., 2010). To investigate the role of Axin1 at lateembryonic and postnatal stages, we generated mouse models withconditional knockout (KO) alleles for Axin1 by creating loxP sites flanking the exon2 of the Axin1 gene (Axin1flox/flox; Xie, Jiang, & Chen,2011). These mouse models provide a valuable tool to investigate therole of Axin1 in a tissue-specific and inducible manner.
In the absence of Wnt ligands, Axin1 and its homology Axin2 facilitate the enzyme GSK-3β in a destruction complex to degradeexcess β-catenin proteins in the cytoplasm. In the presence of Wnt signals, Axin1 and Axin2 are recruited to the Wnt receptors, leadingto the release of β-catenin and its translocation into the nucleus to activate the Wnt signaling pathway. In previous studies, wedemonstrated that the conditional activation of β-catenin signaling in chondrocytes induces the OA-like cartilage degeneration in joints including TMJ (Wang et al., 2012; Wang et al., 2015; Zhu et al., 2009),suggesting the critical role of Wnt/β-catenin signaling in OA pathogenesis.
Fibroblast growth factor (FGF) family members and their tyrosinekinase receptors (FGFR1-4) play an important role in maintaining chondrocyte homeostasis (Ellman, An, Muddasani, & Im, 2008; Ellman et al., 2013). FGF exerts biological function via binding to specific FGFRs to activate intracellular signaling molecules, including ERK1/2, PI3K/AKT, PlCγ, PKC, and Stats (Brewer, Mazot, & Sorian, 2016). Individual FGF and FGFR have different functions in chondrogenesis, and the effects of different FGF molecules on OA progression are still controversial. Specifically, FGFR1 triggers the intracellular Raf1- MEK-ERK1/2 cascade to activate target gene Mmp13, leading to the cartilage degeneration during OA development (Im et al., 2007; Li et al., 2012; Li et al., 2015; Muddasani, Zhao, Rangan, Mikecz, & Im, 2005; Weng et al., 2012; Yan et al., 2011). Conversely, FGFR3 exerts a chondroprotective effect to attenuate cartilage degeneration in induced-OA mouse models (Davidson et al., 2005; Yasuda et al., 2012; Zhou, Wang et al., 2016; Zhou, Xie et al., 2016).
It has been reported that the deletion of Axin2 could target FGF ligands and stimulate FGFR1 expression during calvarial develop-ment and in the development of craniosynostosis (Yu et al., 2005). The balance of Wnt and FGF signaling was further demonstrated by effects of these signaling pathways on endochondral bone formation and craniofacial development (Maruyama, Mirando, Deng, & Hsu, 2010). A recent study reported that Axin1 could act as an inhibitor of Raf1-MEK-ERK1/2 cascade in fibroblasts in a β-catenin-dependent manner (Jeon et al., 2007), suggesting that the Wnt/β-catenin and FGF signaling cross-talk may occur during chondrogenesis. In chondrocytes, Wnt enhance FGF-mediated suppression of chondro-cyte differentiation (Buchtova et al., 2015), whereas FGFs activate Wnt/β-catenin signaling in an ERK-dependent manner (Krejci et al., 2012; Tamai et al., 2004). Simultaneous activation of the FGF and Wnt/β-catenin pathways leads to the increased expression of matrix degradation enzymes and the loss of cartilage extracellular matrix (Buchtova et al., 2015). These findings imply that the interaction of Wnt/β-catenin and FGF signaling pathways during chondrogenesis may play a role in OA progression.
In the present study, we generated Axin1Agc1ER mice by crossing Axin1flox/flox mice with Agc1-CreERT2 mice and observed the OA-like degenerative changes in TMJ cartilage. Because the phenotype observed in Axin1 KO mice is quite different from that found in β- catenin activation mice (Hui et al., 2018), we hypothesize that Axin1 may interact with other signaling pathways in condylar cartilage. Our studies demonstrate that deletion of Axin1 in condylar chondrocytes may activate both β-catenin and FGF signaling to affect cartilage growth and development during postnatal and adult life.
2 |. MATERIALS AND METHODS
2.1 |. Animals
The animal protocol of this study has been approved by the Institutional Animal Care and Use Committee of the Rush University Medical Center, and all experimental procedures were carried out in accordance with the approved guidelines. Agc1-CreERT2 transgenic mice were obtained from Jackson Laboratories (Bar Harbor, ME) and Axin1flox/flox mice were generated in our lab (Xie et al., 2011). Axin1Agc1ER mice were generated by crossing Axin1flox/flox mice with Agc1-CreERT2 mice and the Cre-negative littermates were used ascontrols. Tamoxifen (Sigma, St. Louis, MO) was administered into 2-month-old mice via intraperitoneal injection (1mg per 10g body weight for 5 consecutive days), n=7 in each group.
2.2 |. Histology and histomorphometry
TMJ specimens were harvested from Axin1Agc1ER mice and Crenegative control mice. Samples were fixed in 10% neutral buffered formalin (VWR, Radnor, PA) for 3 days, decalcified with formic acid (Decal Chemical Corp., Suffern, NY) for 21 days, and embedded in paraffin. Four-micrometer-thick sagittal sections at three different levels (50μm apart) were cut from the medial compartment of the TMJ. The alcian blue/hematoxylin (AB/H&E) staining was performed, and these sections were used to analyze the TMJ cartilage by modified Mankin scoring system shown in Supporting Information Table S1 (Liao et al., 2016; Pauli et al., 2012; Zhou et al., 2016). AB-positive cartilage area was measured using the OsteoMeasure software (OsteoMetrics, Inc., Atlanta, GA).
2.3 |. Micro-CT analysis
Both sides of the undecalcified TMJ specimen of 6-month-old Crenegative and Axin1 cKO mice were scanned by a vivaCT 40 micro-CT system (Scanco Medical, Bruttisellen, Switzerland). Serial 11-µm 2D images were achieved at 70kV and 113mA.
2.4 |. Immunohistochemistry and immunofluorescence
The decalcified sections were deparaffinized, rehydrated, and followed by the antigen retrieval using Antigen Unmasking solution (H-3300; Vector Laboratories) at 95°C for 10min. Endogenous peroxidase activity was quenched by 3% H2O2 for 15min. Slides were then blocked with normal goat serum (S-1000; Vector Laboratories) in 1% BSA for 1hr at room temperature. The specimens were incubated with primary antibodies at 4°C overnight. For immunohistological staining, biotinylated secondary antibody (BA-9200; Vector Laboratories) was added for 1hr followed by horseradish peroxidase-conjugated streptavidin–biotin staining (VECTASTAIN Elite ABC HRP kit; Vector Laboratories, Burlingame, CA; PK-6100; ImmPACT DAB Peroxidase Substrate; Vector Laboratories, PK-6100). Slides were then counterstained with CAT H&E (CATHE-GL; Biocare Medical),Q12 dehydrated and cleared by xylene for coversliping. For immunofluorescence staining, appropriate secondary antibody conjugated to a fluorescence probe was added to slides for 1hr and mounted by anti-fading mounting media (Vector Laboratories). Primary antibodies: Axin1 (1:100, 2087; Cell Signaling Technology, Danvers, MA); MMP13 (1:400, ab39012; Abcam, Cambridge, UK); Adamts5 (1:500, ab41037; Abcam), lubricin (1:500, ab28484; Abcam); PCNA (1:5000 ab18197; Abcam); β-catenin (1:100, 610154; BD Biosciences, San Jose, CA); pERK1/2 (1:400, 4370; Cell Signaling Technology; Wang et al., 2018).
2.5 |. Apoptosis assay
The terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNNEL) assay was performed using the assay kit (G3250; Promega, Madison, WI) according to the manufacturer’s instructions.
2.6 |. qRT-PCR assay
Total RNA was extracted from primary TMJ cartilage tissues of Axin1Agc1CreER mice and Cre-negative littermates at the ages of 6 months with TRIzol reagent (Invitrogen, Carlsbad, CA). One-microgram RNA was used to reversely transcribe into complementary DNA (cDNA) using an iScripts cDNA Synthesis kit (Quanta Biosciences, MD, USA). Real-time polymerase chain reaction (PCR) amplification was carried out with specific primers by SYBR Green real-time PCR kit (Quanta Biosciences). The primer sequences are listed in Table 1.
TABLE 1.
Primer names and sequences
| Primer names | Primer sequences |
|---|---|
| Fgfr1, forward | 5′-GATGCGGGGGAGTATACGTG-3′ |
| Fgfr1, reverse | 5′-AGGTAGAGCGGTGAGGTCAT-3′ |
| Fgfr2, forward | 5′-CACAACCAATGAGGAATACTTGGA-3′ |
| Fgfr2, reverse | 5′-GTAGGTTCCTGGTGCTGTCC-3′ |
| Fgfr3, forward | 5′-GATCATGCGGGAATGTTGGC-3′ |
| Fgfr3, reverse | 5′-CATGGGTGAACACCGAGTCA-3′ |
| Mmp13, forward | 5′TTCTTCTTGTTGAGCTGGACTC-3′ |
| Mmp13, reverse | 5′-CTGTGGAGGTCACTGTAGACT-3′ |
| Adamts5, forward | 5′-GGAGCGAGGCCATTTACAAC-3′ |
| Adamtd5, reverse | 5′-CGTAGACAAGGTAGCCCACTTT-3′ |
| Col10a1, forward | 5′-TTCTGCTGCTAATGTTCTTGACC-3 |
| Col10a1, reverse | 5′-GGGATGAAGTATTGTGTCTTGGG-3′ |
| Ihh, forward | 5′-CAGCTGCTTTGGACTGCTTG-3′ |
| Ihh, reverse | 5′-CATGCCTTGTTCTGCATGGG-3′ |
2.7 |. Statistical analysis
Data were presented as the mean±standard error. Statistical difference between two groups was evaluated using unpaired Student t test. For data involved in multiple groups, two-way analysis of variance was performed followed by Turkey’s post-hoc test. *p<0.05 and **p<0.01 are considered as the significant difference between groups.
3 |. RESULTS
3.1 |. Axin1 deletion in TMJ cartilage at adult stage
In previous studies, we demonstrated that Agc1-CreERT2 mice could efficiently target TMJ cartilage with high efficiency at postnatal stage (Hui et al., 2018). To investigate the role of Axin1 in adult TMJ cartilage homeostasis, we generated Axin1Agc1ER KO mice by crossing Agc1-CreERT2 mice with Axin1flox/flox mice. Tamoxifen was administered to 2-month-old mice for 5 days, and the condylar cartilage was harvested at 4 and 6 months of age. The Axin1 protein levels were significantly decreased in Axin1 KO mice, illustrated by immunohistochemistry (IHC) staining (Figure 1). In contrast, Axin2 mRNA expression was slightly increased in condylar cartilage of Axin1 KO mice but did not reach the significant difference (data not shown).
FIGURE 1.
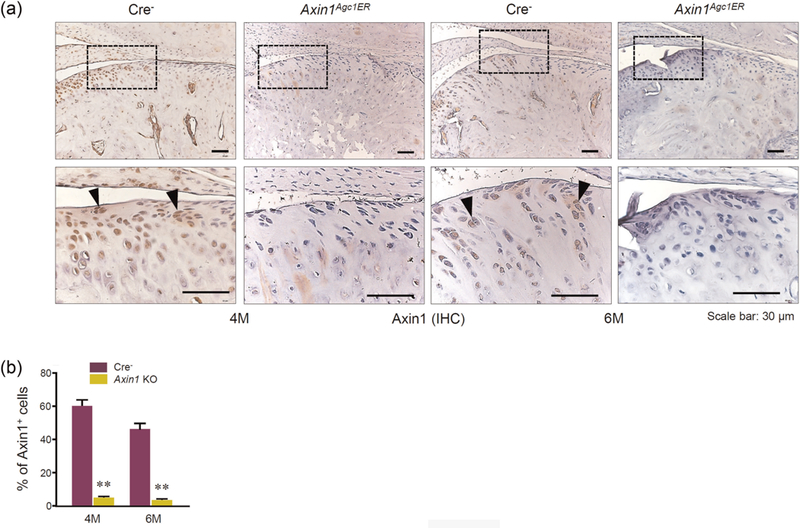
Axin1 deletion in TMJ cartilage at adult stage. Tamoxifen was administrated into 2-month-old Axin1Agc1ER mice and Cre-negative control mice (1mg/10g body weight, i.p. injecton, daily for 5 days). TMJ tissues were harvested from mice at the age of 4 and 6 months of age. (a) Results of Immunohistochemistry (IHC) illustrated that Axin1 protein levels were significantly decreased in condylar cartilage of Axin1Agc1ER conditional KO mice compared with Cre-negative mice (black arrowheads indicate Axin1 positive cells). (b) The ratios of Axin1 positive cells were counted and calculated. Values represent mean±standard error, **p < 0.01, two-way analysis of variance followed by the Tukey’s post-hoc test. i.p.: intraperitoneal; KO: knockout; TMJ: temporomandibular joint
3.2 |. Deletion of Axin1 in TMJ cartilage induces OA-like defects
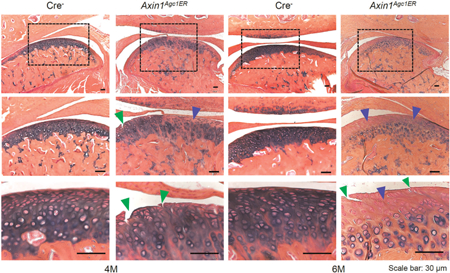
TMJ tissues were harvested at the age of 4 and 6 months after tamoxifen injection in Axin1Agc1ER KO and Cre-negative control mice. Histologic analysis illustrated that the Cre-negative mice exhibited intact cartilage surface with well-organized chondrocytes and evenly stained AB. In contrast, 4-month-old Axin1Agc1ER KO mice showed early signs of TMJ OA with less AB staining and clusters of chondrocytes. The formation of surface fissures was observed at the cartilage layer in half of the Axin1 KO mice (4/7). At 6 months of age, the surface fissures occurred in every Axin1 KO mouse (all seven mice). The cartilage fissures were further extended into the hypertrophic chondrocyte layer in some cases (Figure 2a, green arrowheads). In addition, progressive loss of AB staining, the formation of chondrocyte clusters with hypocellularity was presentat the condylar cartilage (Figure 2a). We then quantified changes in condylar cartilage area and thickness by tracing AB staining positive areas using the OsteoMeasure system, as previously described (Hui et al., 2018; Wang et al., 2015). Results of histomorphometric analysis showed the statistically significant reduction in areas and thickness of TMJ cartilage in 6-month-old Axin1Agc1ER KO mice compared with the Cre-negative control mice (Figures 2b,c). Analysis with modified Mankin scoring system further revealed the significant cartilage degradation of TMJ cartilage in 4- and 6-month-old Axin1 KO mice compared with the age-matched control mice (Figure 2d). During OA development, the degenerative changes in condylar cartilage are often associated with subchondral bone alterations. Micro-CT analysis of the mandibular condyles showed significant subchondral sclerosis in 6-month-old Axin1 conditional KO mice. The increases in bone volume fraction (%) and trabecular thickness were observed at the TMJ subchondral bone of Axin1 KO mice compared with the Cre-negative mice (Supporting Information Figure S1).
FIGURE 2.
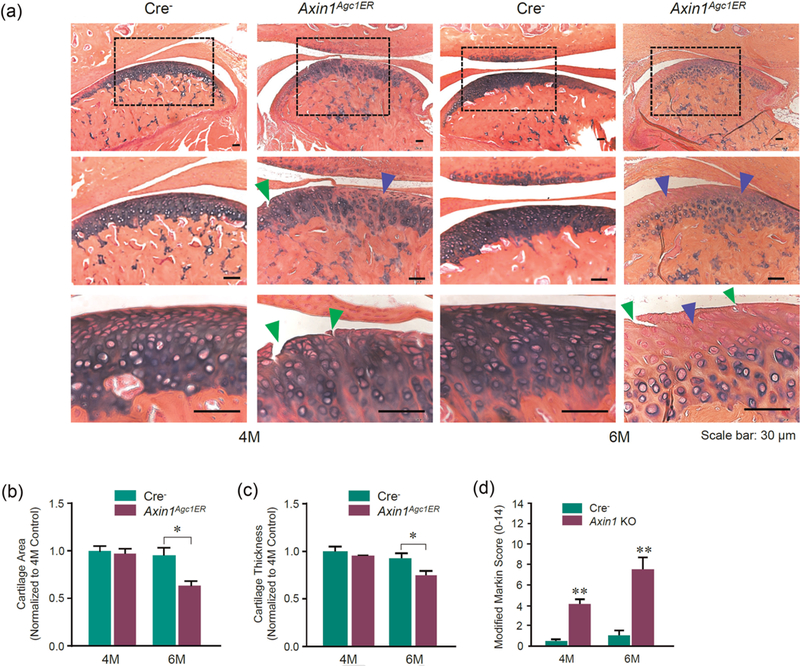
Axin1 conditional deletion in TMJ cartilage induces progressive OA-like defects. Alcian blue/hematoxylin orange G staining was performed using TMJ samples from Axin1 conditional KO mice and Cre-negative mice. (a) The 4-month-old Axin1Agc1ER KO mice presented early signs of OA-like defects with less alcian blue staining (blue arrowheads) and clusters of chondrocytes. The formation of surface fissures (green arrowheads) was observed at the cartilage layer in half of the mice (4/7). At 6 months of age, the surface fissures were found in all seven Axin1 conditional KO mouse. The progressive loss of Alcian blue staining with hypocellularity was present at the condylar cartilage layers. (b,c) The condylar cartilage areas and cartilage thickness were quantified by tracing Alcian blue positive stained areas using the OsteoMeasure system. (d) The severity of OA-like histologic defects was graded by modified Mankin scoring system. Values represent mean±standard error, *P < 0.05 and **P < 0.01, two-way analysis of variance followed by the Tukey’s post-hoc test; n = 7 mice per group. KO: knockout; OA: Osteoarthritis; TMJ: temporomandibular joint
3.3 |. Deletion of Axin1 in condylar cartilage accelerates cartilage degradation in TMJ tissue
To further investigate alterations in TMJ cartilage, IHC staining was performed to detect changes in the expression of matrix degradation enzymes. The results revealed the significantly upregulated expression ofMMP13 and Adamts5 proteins in condylar cartilage, especially at the superficial layer of 6-month-old Axin1Agc1ER KO mice (Figures 3a–d). Given that the disrupted continuity of TMJ cartilage surface was observed in Axin1 KO mice, we examined changes in expression of lubricin, which is essential for lubrication in the joint fluid (Hill, Duran, & Purcell, 2014). We found that the expression of lubricin was downregulated at the superficial and proliferative layers of condylar cartilage (Figures 3e,f). Immunofluorescent assays showed an enhanced expression of Col-X at the hypertrophic layer in 4-month-old Axin1 KO mice and expanded to the superficial layer of TMJ cartilage in 6-month-old Axin1 KO mice (Figures 3g,h). The upregulation of Col-X expression indicates that chondrocyte hypertrophy was induced by the deletion of Axin1.
FIGURE 3.
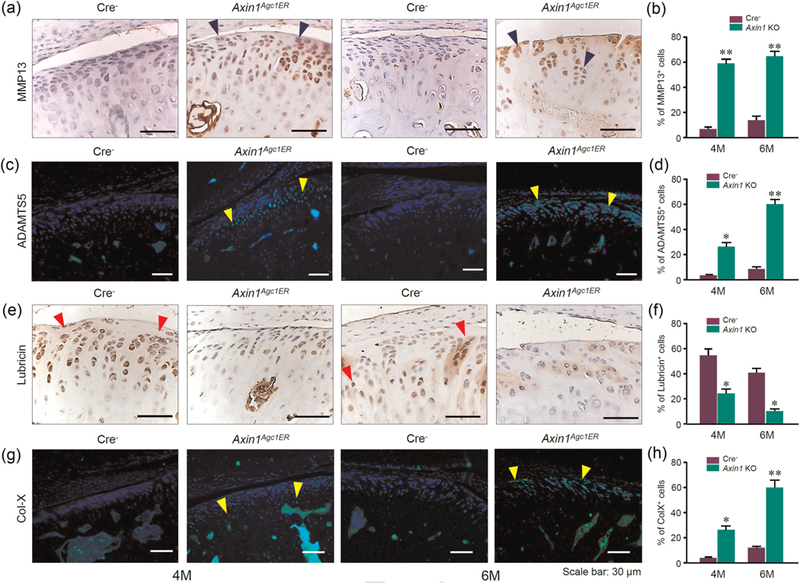
Pathological changes of TMJ cartilage in Axin1Agc1ER conditional KO mice. Disturbed homeostasis of TMJ cartilage in 4- and 6-month-old mice were analyzed by immunostaining. (a,c) The expression levels of MMP13 (blue arrowheads) and ADAMTS5 (yellow arrowheads) were significantly increased, especially at the superficial layer of cartilage in Axin1 conditional KO mice compared with control mice. (e) IHC staining of lubricin showed the numbers of lubricin-positive cells (red arrowheads) at the superficial, and proliferative zones were reduced in Axin1 KO mice. (g) Collagen X-positive cells were significantly increased in Axin1 KO mice and the Col X-positive cells were extended to the superficial layer of cartilage in 6-month-old Axin1 conditional KO mice. (b,d,f,h) The ratios of immunoreactive positive cells were quantified. Values represent mean ± standard error, *p < 0.05 and **p < 0.01, two-way analysis of variance followed by the Tukey’s post-hoc test. IHC: immunohistochemistry; KO: knockout; TMJ: temporomandibular joint
Furthermore, results of PCNAQ13 staining showed that the numbers of PCNA-positive cells were significantly reduced at the superficial layer of 4- and 6-month-old Axin1 KO mice (Figures 4a,b) suggesting reduced chondrocyte proliferation in Axin1 KO mice. We then performed TUNNEL assay to determine changes in chondrocyte apoptosis in Axin1 KO mice. Results showed that the increased TUNNEL-positive cells were distributed at the hypertrophic layer in 4-month-old Axin1 KO mice, and then expended to the superficial layer in 6-month-old Axin1 KO mice (Figures 4c,d). Taken together, the deletion of Axin1 in chondrocytes at adult stage disturbed TMJ tissue homeostasis resulting in OA-like degenerative defects.
FIGURE 4.
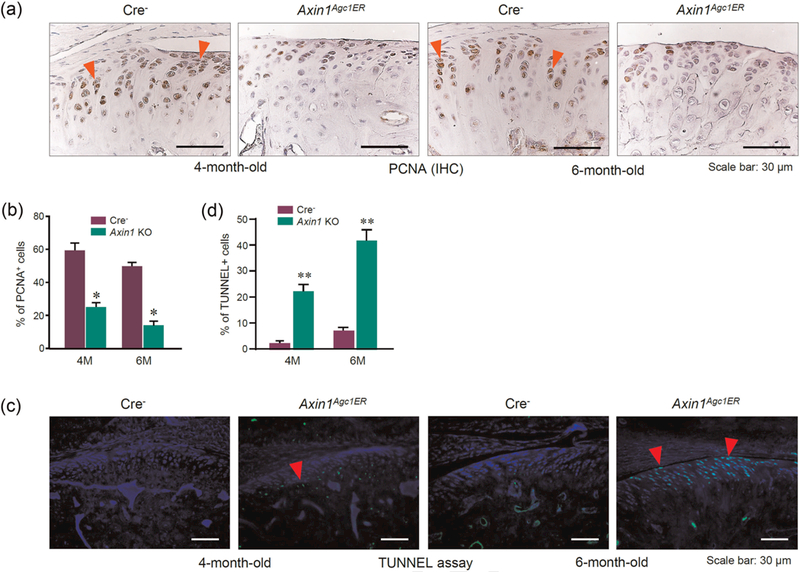
Changes in the proliferation and apoptosis of TMJ cartilage in Axin1Agc1ER conditional KO mice. TMJ cartilage in 4- and 6-monthold mice was analyzed by immunostaining. (a) Results of PCNA IHC staining revealed that the numbers of PCNA-staining-positive proliferative chondrocytes (red arrowheads) were reduced in Axin1 KO mice compared with Cre-negative controls. (c) TUNNEL assay was performed and demonstrated that the apoptotic cells were increased at the hypertrophic layer in 4-month-old Axin1 KO mice and the superficial layer in 6-month-old Axin1 KO mice. (b,d) The ratios of immunoreactive positive cells were quantified. Values represent mean ± standard error, *p < 0.05 and **p < 0.01, two-way analysis of variance followed by the Tukey’s post-hoc test. IHC: immunohistochemistry; KO: knockout; PCNA: XXX; TMJ: temporomandibular joint
3.4 |. Cross-talking of Wnt/β-catenin and FGF/ERK signaling contributes to the TMJ OA phenotype in Axin1 KO mice
As Axin1 is a scaffolding protein acting as a negative regulator of Wnt/β-catenin signaling, we then examined changes in β-catenin protein levels by IHC assay in Axin1 KO mice. Results showed that the deletion of Axin1 significantly increased β-catenin protein levels in condylar cartilage (Figure 5).
FIGURE 5.
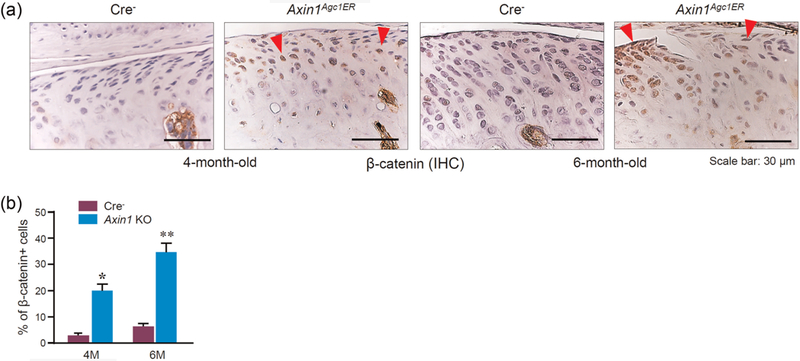
Enhanced β-catenin expression in Axin1 conditional KO mice. (a) Results of IHC staining revealed the upregulated β-catenin expression in TMJ cartilage of Axin1 KO mice. The data from 4- and 6-month-old mice were shown. Red arrowheads: β-catenin staining positive cells. (b) The ratio of β-catenin positive cells was quantified. Values represent mean ± standard error, *P < 0.05 and **P < 0.01, two-way analysis of variance followed by the Tukey’s post-hoc test. IHC: Immunohistochemistry; KO: knockout; TMJ: temporomandibular joint
Furthermore, total RNA was extracted from condylar cartilage isolated from 6-month-old Axin1 KO mice or Cre-negative control mice. Expression of Col10a1, Mmp13, and Adamts5 was examined. The results showed that expression of Col10a1, Mmp13, and Adamts5 was significantly increased in Axin1 KO mice (Figure 6a-c). These findings suggest that cartilage degradation observed in Axin1 KO mice may be partially due to the upregulation of matrix degradation enzymes.
FIGURE 6.
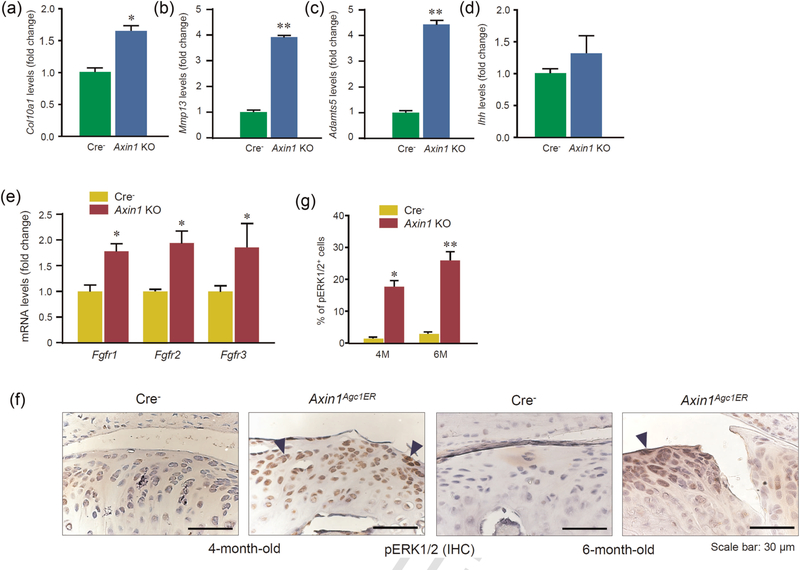
Enhanced FGF/ERK signaling in Axin1 conditional KO mice. (a–e) Total RNAs were extracted from condylar cartilage of 6-monthold Axin1 KO and Cre-negative mice followed by the real-time PCR assay. Expression of Col10a1, Mmp13, Adamts5, Ihh, Fgfr1, Fgfr2, and Fgfr3 was analyzed. Significant upregulation of Col10a1, Mmp13, Adamts5, Fgfr1, Fgfr2, and Fgfr3 expression was observed. Unpaired Student’s t test, n=5 mice per group. (f) Results of IHC staining revealed the upregulation of pERK1/2 expression in TMJ cartilage of Axin1 KO mice. (g) The ratio of pERK1/2 positive cells was quantified. Values represent mean ± standard error, *P < 0.05 and **P < 0.01, two-way analysis of variance followed by the Tukey’s post-hoc test. ERK: XXX; FGF: fibroblast growth factor; IHC: immunohistochemistry; KO: knockout; PCR: polymerase chain reaction
Wnt signaling has been demonstrated to interact with other signaling pathways, such as IhhQ14 and FGF signaling. It has been shown that Wnt and FGF signaling affects chondrogenesis during craniofacial development (Maruyama et al., 2010; Yu et al., 2005). In this study, we also examined changes in the expression of Ihh and Fgfr1-3. We found that there was no significant change in Ihh expression in Axin1 KO mice (Figure 6d). In contrast, expression of Fgfr1, Fgfr2, and Fgfr3 was significantly increased in condylar cartilage of Axin1 KO mice (Figure 6e). We then examined changes in the expression of FGF downstream molecule pERK1/2 and found that the expression of pERK1/2 was also significantly increased in TMJ cartilage in Axin1 KO mice (Figures 6f,g). These findings suggest that upregulation of FGFR1/ERK signaling may also contribute to the TMJ OA phenotype observed in Axin1 KO mice. This is consistent with the previous report and suggests that Axin1 may serve as a linker for Wnt/FGF signaling cross-talk (Castellone et al., 2009; Jeon et al., 2007).
4 |. DISCUSSION
Axin1 plays a critical role in the regulation of cartilage development and maintenance. Both Axin1 and its homology Axin2 null mutant mice exhibited severe developmental deformities (Yan et al., 2009). As a multidomain scaffolding protein, Axin1 interacts with multiple proteins to regulate canonical Wnt/β-catenin signaling and other signaling pathways. To investigate the role of Axin1 in TMJ tissue at adult stage, we generated Axin1 conditional KO mice by specific deletion of Axin1 in aggrecan-expressing chondrocytes at the adult stage. OA-like degenerative defects of TMJ cartilage were observed, including loss of AB-positive cartilage area, accelerated chondrocyte hypertrophy, and impaired integrity of cartilage surface. Both β-catenin and FGFR1/ERK signaling pathways were activated by Axin1 deletion in chondrocytes. Axin1 may serve as a linker between Wnt and FGF signaling pathways in a pERK1/2 dependent manner, thus cooperatively regulating the initiation and progression of TMJ OA.
Vertical fissure formation at the cartilage surface was observed in Axin1 KO mice at 4-months age and higher incidence rate occurred at 6-months age. The biosynthesis of GAG and proteoglycan was significantly decreased in the Axin1 KO mice, demonstrated by the reduced AB staining, while increased catabolism activities were demonstrated by higher expression of matrix degradation enzymes, such as MMP13 and Adamts5. Lubricin is also named as proteoglycan 4 and is responsible for boundary lubrication during joint movement(Hill et al., 2014). The decreased lubricin expression in Axin1 KO mice may also contribute to TMJ OA development associated with the loss of cartilage integrity. This is consistent with other previous studies (Elsaid et al., 2008; Hill et al., 2014; Koyama et al., 2014).
The consequence of disturbed cartilage homeostasis may be associated with enhanced Wnt/β-catenin and FGF signaling pathways caused by the deletion of Axin1. Our previous studies demonstrated that the activation of β-catenin signaling in chondrocytes leads to degeneration in TMJ, knee joints, and intervertebral disc, and Mmp13 and Adamts5 may act as important downstream target genes (Wang et al., 2012; Wang et al., 2015; Zhu et al., 2009). The role of individual FGFs in cartilage is complicated (Ellman et al., 2008; Ellman et al., 2013; Li et al., 2012; Zhong, Huang, Karperien, & Post, 2015). FGFR1 is activated by the FGF2, which is released from cartilage ECM upon joint injury. Activation of FGFR1 phosphorylates intracellular ERK1/2, and the activated pERK1/2 is then translocated into the nucleus, combine with Elk-1 to act on its responsive element in target genes, such as Mmp13 in human articular chondrocytes (Ellman et al., 2008; Im et al., 2007; Muddasani et al., 2005; Yan et al., 2011). Deletion of Fgfr1 in chondrocytes attenuated cartilage degeneration in vivo and antagonized the IL-1β-induced MMP13 upregulation in vitro (Weng et al., 2012). Simultaneous activation of FGFR1 and Wnt/β-catenin signaling in chondrocytes cooperatively suppressed the chondrocyte differentiation and ECMQ15 synthesis in vitro (Buchtova et al., 2015). The stimulation of FGFR1 found in the present study may promote the cartilage degeneration via ERK1/2 pathway. Conversely, FGFR3 is required to maintain cartilage homeostasis during adult stage (Davidson et al., 2005; Zhou et al., 2016). Deletion of Fgfr3 in cartilage induced OA-like degenerative changes in TMJ, along with the upregulation of Runx2 via Ihh pathway (Yasuda et al., 2012; Zhou et al., 2016). In our study, Fgfr3 is also upregulated in condylar cartilage of Axin1 KO mice with no significant changes in Ihh expression (Figure 6d), suggesting that FGFR3 may not play an important role in TMJ OA development. The role of FGFR3 in TMJ cartilage homeostasis needs to be further investigated. Few studies about the functions of FGFR2 and FGFR4 in OA development have been reported. A recent study showed that high levels of FGFR2 with increased FGF1 expression were found in the synovial fluid and articular cartilage in patients with OA (Li et al., 2015), suggesting that increased expression of Fgfr2 in Axin1 KO mice may also contribute to the progression of TMJ OA.
In previous studies, we found that the activation of β-catenin in aggrecan-expressing chondrocytes promoted the formation of hypertrophic round cells at the superficial and deep layers of condylar cartilage. In this study, the deletion of Axin1 increased the expression of β-catenin in condylar cartilage, with no hypertrophic chondrocyte accumulation at the superficial layer, suggesting that Axin1 may regulate TMJ cartilage function through other mechanisms in addition to its role in β-catenin activation. These findings suggest that Axin1 may act as a scaffolding protein to integrateβ-catenin and FGF signaling by activating downstream effector pERK1/2. Besides, Axin2, the homology of Axin1, has been reported to function during endochondral bone formation showing restricted expression in hypertrophic chondrocytes of the ribs, vertebra, and long bone (Dao et al., 2010). Loss of Axin2 accelerated hypertrophic differentiation and results in disrupted endochondral bone growth in mutant mice. Interestingly, Axin1 does not apparently preserve endochondral bone formation in Axin2-/- mice, in turn, Axin2 cannot compensate for the deletion of Axin1 as Axin1-/- mice are embryonically lethal (Chia & Costantini, 2005; Chia et al., 2009; Dao et al., 2010).
Collectively, deletion of Axin1 in TMJ chondrocytes activates both β-catenin and FGF/ERK signaling pathways, leading to the OA-like degenerative changes in TMJ tissue. The detail molecular mechanism related to the integration of Axin1 and FGFR1/ERK signals during OA development needs to be further investigated.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to express our gratitude to Ms. Lily Yu for her help on processing and staining histological samples. This study was supported by National Institutes of Health Grants R01AR054465 and R01AR070222 to D. Chen. This study was also partiallysupported by the grant from Shenzhen Science and Technology Innovation Committee, China (grant # JCYJ20160331114205502) to D. Chen and National Natural Science Foundation of China (grant # 81630066) to G. Xiao.
Funding information
National Natural Science Foundation of China, Grant/Award Number: 81630066; National Institute of Arthritis and Musculoskeletal and Skin Diseases, Grant/Award Numbers: R01AR070222, R01AR054465; Shenzhen Science and Technology Innovation Committee, China, Grant/Award Number: JCYJ20160331114205502
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- Brewer JR, Mazot P, & Soriano P (2016). Genetic insights into the mechanisms of Fgf signaling. Genes & Development, 30, 751–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchtova M, Oralova V, Aklian A, Masek J, Vesela I, Ouyang Z, … Krejci P (2015). Fibroblast growth factor and canonical WNT/betacatenin signaling cooperate in suppression of chondrocyte differentiation in experimental models of FGFR signaling in cartilage. Biochimica et Biophysica Acta, 1852, 839–850. [DOI] [PubMed] [Google Scholar]
- Castellone MD, De Falco V, Rao DM, Bellelli R, Muthu M, Basolo F, … Santoro M (2009). The beta-catenin axis integrates multiple signals downstream from RET/papillary thyroid carcinoma leading to cell proliferation. Cancer Research, 69, 1867–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Shen J, Zhao W, Wang T, Han L, Hamilton JL, & Im H-J (2016). Osteoarthritis: Toward a comprehensive understanding of pathological mechanism. Bone Research, 5, 16044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia IV, & Costantini F (2005). Mouse Axin and Axin2/conducting proteins are functionally equivalent in vivo. Molecular and Cellular Biology, 25, 4371–4376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia IV, Kim MJ, Itoh K, Sokol SY, & Costantini F (2009). Both the RGS domain and the six C-terminal amino acids of mouse Axin are required for normal embryogenesis. Genetics, 181, 1359–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao DY, Yang X, Flick LM, Chen D, Hilton MJ, & O’Keefe RJ (2010). Axin2 regulates chondrocyte maturation and axial skeletal development. Journal of Orthopedic Research, 28, 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson D, Blanc A, Filion D, Wang H, Plut P, Pfeffer G, … Henderson JE (2005). Fibroblast growth factor (FGF) 18 signals through FGF receptor 3 to promote chondrogenesis. Journal of Biological Chemistry, 280, 20509–20515. [DOI] [PubMed] [Google Scholar]
- Ellman MB, An HS, Muddasani P, & Im HJ (2008). Biological impact of the fibroblast growth factor family on articular cartilage and intervertebral disc homeostasis. Gene, 420, 82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman MB, Yan D, Ahmadinia K, Chen D, An HS, & Im HJ (2013). Fibroblast growth factor control of cartilage homeostasis. Journal of Cellular Biochemistry, 114, 735–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsaid KA, Fleming BC, Oksendahl HL, Machan JT, Fadale PD, Hulstyn MJ, … Jay GD (2008). Decreased lubricin concentrations and markersof joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthritis & Rheumatism, 58, 1707–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A, Duran J, & Purcell P (2014). Lubricin protects the temporomandibular joint surfaces from degeneration. PLoS One, 9, e106497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui T, Zhou Y, Wang T, Li J, Zhang S, Liao L, … Chen D (2018). Activation of β-catenin signaling in aggrecan-expressing cells in temporomandibular joint causes osteoarthritis-like defects. International Journal of Oral Science, 10, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im HJ, Muddasani P, Natarajan V, Schmid TM, Block JA, Davis F, … Loeser RF (2007). Basic fibroblast growth factor stimulates matrix metalloproteinase-13 via the molecular cross-talk between the mitogen-activated protein kinases and protein kinase Cdelta pathways in human adult articular chondrocytes. Journal of Biological Chemistry, 282, 11110–11121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon SH, Yoon JY, Park YN, Jeong WJ, Kim S, Jho EH, … Choi KY (2007). Axin inhibits extracellular signal-regulated kinase pathway by Ras degradation via beta-catenin. Journal of Biological Chemistry, 282, 14482–14492. [DOI] [PubMed] [Google Scholar]
- Koyama E, Saunders C, Salhab I, Decker RS, Chen I, Um H, … Nah HD (2014). Lubricin is Required for the Structural Integrity and Post-natal Maintenance of TMJ. Journal of Dental Research, 93, 663–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krejci P, Aklian A, Kaucka M, Sevcikova E, Prochazkova J, Masek JK, … Balek L (2012). Receptor tyrosine kinases activate canonical WNT/beta-catenin signaling via MAP kinase/LRP6 pathway and direct beta-catenin phosphorylation. PLoS One, 7, e35826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Wang B, He CQ, Yang YQ, Guo H, Chen Y, & Du TH (2015). Upregulation of fibroblast growth factor 1 in the synovial membranes of patients with late stage osteoarthritis. Genetics and Molecular Research, 14, 11191–11199. [DOI] [PubMed] [Google Scholar]
- Li X, Ellman MB, Kroin JS, Chen D, Yan D, Mikecz K, … Im HJ (2012). Species-specific biological effects of FGF-2 in articular cartilage: Implication for distinct roles within the FGF receptor family. Journal of Cellular Biochemistry, 113, 2532–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao L, Zhang S, Gu J, Takarada T, Yoneda Y, Huang J, … Chen D (2016). Deletion of Runx2 in articular chondrocytes decelerates the progression of DMM-induced osteoarthritis in adult mice. Scientific Reports, 7, 2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama T, Mirando AJ, Deng CX, & Hsu W (2010). The balance of WNT and FGF signaling influences mesenchymal stem cell fate during skeletal development. Science Signal, 3, ra40–ra40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muddasani P, Zhao LJ, Rangan J, Mikecz K, & Im HJ (2005). Basic fibroblast growth factor stimulates MMP-13 expression via FGFR1-dependent activation of MAPK and NFκB that converge to activate Elk-1 in human adult articular chondrocytes. Orthopedic Trauma, 30, 137. [Google Scholar]
- Pauli C, Whiteside R, Heras FL, Nesic D, Koziol J, Grogan SP, … Lotz MK (2012). Comparison of cartilage histopathology assessment systems on human knee joints at all stages of osteoarthritis development. Osteoarthritis and Cartilage, 20, 476–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamai K, Zeng X, Liu C, Zhang X, Harada Y, Chang Z, & He X (2004). A mechanism for Wnt coreceptor activation. Molecular Cell, 13, 149–156. [DOI] [PubMed] [Google Scholar]
- Wang T, Li S, Yi D, Zhou GQ, Chang Z, Ma PX, … Chen D (2018). CHIP regulates bone mass by targeting multiple TRAF family members in bone marrow stromal cells. Bone Research, 6, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Li S, Xie W, Shen J, Im H-J, Holz JD, & Chen D (2015). Activation of β-catenin signaling leads to temporomandibular joint defects. European Cell & Materials, 28, 223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Tang D, Shu B, Wang B, Jin H, Hao S, … Chen D (2012). Conditional activation of β-catenin signaling in mice leads to severedefects in intervertebral disc tissue. Arthritis & Rheumatism, 64, 2611–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng T, Yi L, Huang J, Luo F, Wen X, Du X, … Chen L (2012). Genetic inhibition of fibroblast growth factor receptor 1 in knee cartilage attenuates the degeneration of articular cartilage in adult mice. Arthritis & Rheumatism, 64, 3982–3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie R, Jiang R, & Chen D (2011). Generation of Axin1 conditional mutant mice. Genesis, 49, 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D, Chen D, Cool SM, van Wijnen AJ, Mikecz K, Murphy G, & Im HJ (2011). Fibroblast growth factor receptor 1 is principally responsible for fibroblast growth factor 2-induced catabolic activities in human articular chondrocytes. Arthritis Research & Therapy, 13, R130. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yan Y, Tang D, Chen M, Huang J, Xie R, Jonason JH, … Chen D (2009). Axin2 controls bone remodeling through the β-catenin-BMP signaling pathway in adult mice. Journal of Cell Science, 122, 3566–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda T, Nah HD, Laurita J, Kinumatsu T, Shibukawa Y, Shibutani T, … Koyama E (2012). Muenke syndrome mutation, FgfR3P(2)(4)(4) R, causes TMJ defects. Journal of Dental Research, 91, 683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HM, Jerchow B, Sheu TJ, Liu B, Costantini F, Puzas JE, & Hsu W (2005). The role of Axin2 in calvarial morphogenesis and craniosynostosis. Development, 132, 1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek TJ, Perry WL III, … Costantini F (1997). The mouse fused locus encodes axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell, 90, 181–192. [DOI] [PubMed] [Google Scholar]
- Zhong L, Huang X, Karperien M, & Post JN (2015). The regulatory role of signaling crosstalk in hypertrophy of MSCs and human articular chondrocytes. International Journal of Molecular Science, 16, 19225–19247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Wang Z, Tang J, Li W, Huang J, Xu W, … Chen L (2016). Exogenous fibroblast growth factor 9 attenuates cartilage degradation and aggravates osteophyte formation in post-traumatic osteoarthritis. Osteoarthritis and Cartilage, 24, 2181–2192. [DOI] [PubMed] [Google Scholar]
- Zhou S, Xie Y, Li W, Huang J, Wang Z, Tang J, … Chen L (2016). Conditional deletion of Fgfr3 in chondrocytes leads to osteoarthritis-like defects in temporomandibular joint of adult mice. Scientific Reports, 6, 24039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Tang D, Wu Q, Hao S, Chen M, Xie C, … Chen D (2009). Activation of β-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult β-catenin conditional activation mice. Journal of Bone and Mineral Research, 24, 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


