Abstract
Objective:
To identify predictors of hypothalamic-pituitary-adrenal (HPA) axis recovery interval and severity of glucocorticoid withdrawal symptoms (GWS) in patients undergoing adrenalectomy for corticotropin-independent cortisol excess.
Design:
Retrospective study of patients with mild autonomous cortisol excess (MACE), moderate and severe Cushing syndrome (CS) who developed adrenal insufficiency after unilateral adrenalectomy between 1998 and 2017.
Results:
Adrenalectomy was performed in 81 patients (79% women, median age 52 years [IQR, 42–62]). HPA axis recovery occurred at a median of 4.3 months (IQR, 1.6–11.4) after adrenalectomy (severe CS vs moderate CS vs MACE: median 11.4 vs 2.8 vs 2.1 months, P<0.01). Main predictors of HPA axis recovery interval included: preoperative serum cortisol concentration after 1-mg overnight dexamethasone suppression test >10 μg/dL or >276 nmol/L (9.7 vs 1.3 months if cortisol ≤10 μg/dL or ≤276 nmol/L; P<0.01); body mass index (for every 3 kg/m2 decrease, glucocorticoid taper increased by 1 month; P<0.05); age <45 (11.4 vs 2.3 months if ≥45 years, P<0.05); duration of symptoms prior to diagnosis >1 year (11.4 vs 2.8 months if ≤1 year); moon facies (11.4 vs 2.2 months if no rounding of the face); and, myopathy (13.1 vs 2.7 months if no myopathy, P<0.05). Patients with severe CS had a higher incidence of GWS compared to patients with MACE (66.7% vs 40.0%, P<0.05) with a median of 1 and 0 events/patient, respectively.
Conclusions:
The HPA axis recovery interval was the longest for patients with severe CS. Surprisingly, patients with moderate CS recovered their HPA axis as quickly as those with MACE. GWS were observed in all groups, with more events in patients with severe CS. This study emphasizes the need to counsel patients on expectations for HPA axis recovery and address intervention for GWS based on individual preoperative parameters.
Keywords: Pituitary-Adrenal System, hypothalamic-pituitary-adrenal axis, ACTH-independent Cushing syndrome, adrenocortical hyperfunction, adrenalectomy, adrenal insufficiency, glucocorticoids, substance withdrawal syndrome
INTRODUCTION
Adrenalectomy is the mainstay of treatment for patients with corticotropin (ACTH)-independent Cushing syndrome (CS) and achieves a cure rate of virtually 100%. While overt adrenal CS is rare, biochemical abnormalities suggestive of mild autonomous cortisol excess (MACE, also known as “subclinical” CS) are much more common and are reported in at least 30% of patients with adrenal cortical adenomas1. While patients with MACE lack the classic physical features of CS, they present with significantly higher rates of metabolic abnormalities that increase cardiovascular risk, morbidity and mortality, when compared to the general population2–5.
Postoperative adrenal insufficiency is the consequence of long-standing suppression of hypothalamic corticotropin-releasing hormone secretion, pituitary ACTH secretion, and atrophy of normal adrenal cortex. Hypothalamic-pituitary-adrenal (HPA) axis suppression is present in all patients undergoing adrenalectomy for overt ACTH-independent CS, and in approximately 50% of patients with MACE6,7. Hence, following surgery most patients require glucocorticoid (GC) therapy for adrenal insufficiency replacement until the HPA axis recovers. However, despite GC therapy, patients often struggle with symptoms of fatigue, arthralgia, myalgia, decreased quality of life, depression and anxiety – symptoms attributed to a relative decrease in serum GC concentrations, so-called GC withdrawal symptoms (GWS).
GWS likely occur due to physical dependence on supraphysiologic endogenous cortisol secretion prior to curative adrenalectomy. When the source of cortisol excess is abruptly removed, behavioral and physiological changes in the body occur despite initiation of GC therapy. These changes manifest as a spectrum of signs and symptoms which collectively make up the GWS8. This syndrome is more frequently seen after discontinuation of supraphysiologic GC therapy used to treat inflammatory conditions 9.
The duration of postoperative adrenal insufficiency is reported to vary widely, from weeks to years6. Biochemical and clinical variables, such as tumor size, degree of cortisol hypersecretion and/or the presence of metabolic abnormalities, have been suggested as predictive factors for the development of postoperative adrenal insufficiency 6,7,10,11. However, it is unclear whether the degree of noted abnormalities influences the daily dose and duration of GC therapy, or the intensity of GWS after adrenalectomy. Current practice varies in regard to postoperative GC replacement—both in the GC regimens used and the different approaches to monitoring. Moreover, while clinical guidelines recommend universal peri- and postoperative GC treatment, these provide only arbitrary advice on the GC dose and taper regimen 6.
Identification of baseline variables predictive of the postoperative course in patients with CS and MACE may help provide appropriate preoperative counseling, lead to more informed use of postoperative GC therapy, and improve patients’ quality of life. In this single-center retrospective study we address the scarcely studied and controversial topic of assessment and monitoring for HPA axis recovery based on our extensive clinical experience using a much more simplified assessment approach, and in opposition to the recent guidelines. Our study objectives include: 1) to determine the HPA axis recovery interval after curative adrenalectomy for adrenal CS and MACE; and, 2) to identify which baseline demographic, clinical, and biochemical variables impact the duration and dose of the GC taper and the severity of GWS.
METHODS
This retrospective study was approved by the Mayo Clinic Institutional Review Board and included patients evaluated between January 1, 1998 and June 1, 2017. Patients were selected from an existent adrenal tumor database based on the following inclusion criteria: 1) age ≥18 years; 2) diagnosis of ACTH-independent cortisol secretory autonomy; 3) treatment with unilateral adrenalectomy; 4) requirement for postoperative GC therapy for adrenal insufficiency; and, 5) follow-up of at least 4 months.
Cortisol excess severity classification
Patients presented with varying degrees of biochemical and clinical cortisol excess. Guided by Endocrine Society guidelines on the diagnosis of CS12, ENSAT/ESE guidelines on adrenal incidentalomas1, and a systematic review on MACE2, we created a scoring system to classify biochemical and clinical abnormalities as mild, moderate, or severe.
Biochemical criteria:
We based our classification on the following: (1) increased endogenous cortisol production (24-hr urine free cortisol (UFC) ≥45 μg/24h or ≥1242 nmol/24h, 8AM serum cortisol >1.8 μg/dL or >50 nmol/L after 1-mg dexamethasone suppression test (DST), and midnight salivary cortisol ≥100 ng/dL or ≥2.8 nmol/dL; (2) loss of circadian rhythm (absence of diurnal cortisol variation based on morning and afternoon serum cortisol concentrations); and, (3) HPA-axis suppression (ACTH ≤10 pg/mL or ≤2.2 pmol/L and dehydroepiandrosterone sulfate (DHEA-S) <50 μg/dL or <1.3 μmol/L). For further details, see Supplemental Material Table S1.
Clinical criteria:
We took into account metabolic and/or physical findings related to cortisol excess (Supplemental Material Table S2). Metabolic abnormalities included: hypertension, abnormal glucose metabolism (pre-diabetes or diabetes mellitus), hyperlipidemia, cardiovascular disease, weight gain, and decreased bone density (osteopenia or osteoporosis). Physical examination findings included: central obesity, presence of supraclavicular and/or dorsocervical fat pads, rounding of the face with or without plethora, skin changes (acne, violaceous striae, thinning and/or bruising of the skin), and objective evidence of proximal muscle weakness. We classified patients as follows: MACE - metabolic abnormalities without physical findings; moderate CS - one or two physical findings with or without metabolic abnormalities; and, severe CS - three or more physical findings with or without metabolic abnormalities
Diagnosis of postoperative adrenal insufficiency
A diagnosis of postoperative adrenal insufficiency was made if patients: 1) had a 8AM serum cortisol <10 μg/dL or < 276 nmol/L on postoperative day #1; 2) developed perioperative hemodynamic instability necessitating empiric intravenous GC administration; or 3) received perioperative GC due to anticipated adrenal insufficiency after unilateral adrenalectomy. The reference range for a normal 8AM serum cortisol measurement at our laboratory is 7–25 μg/dL (193–690 nmol/L), however, at our institution we routinely use the cut-off of ≥10 μg/dL (≥276 nmol/L) for our population of patients undergoing adrenalectomy for cortisol-secreting adenomas who are investigated on postoperative day 1 while still in the hospital recovering from an invasive procedure and associated pain13,14.
Postoperative recovery of adrenal function
We collected information on the duration and intensity of postoperative GC therapy and the severity of GWS until recovery of the HPA axis. If a GC other than hydrocortisone was used, we converted the doses into a hydrocortisone equivalent dose15. In patients treated with unilateral adrenalectomy, recovery of the HPA-axis was considered to be achieved when an 8AM serum cortisol was ≥10 μg/dL or ≥276 nmol/L at 24h after the last administered dose of GC.
Classification of severity of GWS
GWS included nausea, arthralgia, myalgia, fatigue, and headache. We classified GWS into the following categories:
Mild:
Symptoms did not limit activities of daily living (ADLs) or instrumental activities of daily living (IADLs). In this situation, patients were reassured and recommended to continue their GC taper as initially planned.
Moderate:
Symptoms somewhat limited IADLs but not ADLs. In this situation, patients were advised to increase their GC dose to the previous dose at which they had no such symptoms.
Severe:
Symptoms significantly limited ADLs and IADLs. In this situation, patients were advised to double their dose of GC and then proceed with a slower taper.
Adrenal crisis:
Patients required hospital admission for intravenous GC administration due to hemodynamic instability and without evidence of another underlying etiology.
Statistical analysis
All continuous data are summarized as median and interquartile ranges (IQR). Categorical data are presented as frequencies and percentages. We used Pearson χ2 and unpaired Student t-test to make between-group comparisons for baseline nominal and ordinal variables. For baseline continuous variables, we used rank sum test. We calculated exogenous GC tapering rates with Kaplan-Meyer curves and we used the Log-Rank test for between-group comparisons. For univariate and multivariable analysis in relation to the length of taper, we used a Cox proportional hazard model. For the multivariable analysis, we included variables that are clinically relevant for the length of postoperative GC therapy. All P-values <0.05 were considered significant.
RESULTS
Baseline Characteristics
Between January 1, 1998 and June 1, 2017, a total of 116 patients underwent adrenalectomy for ACTH-independent cortisol secretory autonomy and required postoperative GC therapy for adrenal insufficiency at the Mayo Clinic in Rochester. Twenty did not follow up at our institution. Thirteen patients had bilateral adrenalectomy and were therefore excluded. Our final cohort included 81 patients (79% female, 99% Caucasian) diagnosed with ACTH-independent cortisol excess with a median age of 52 years (IQR, 42–62) (Table 1A). Patients presented with either unilateral adenoma (n=76, 93.8%) or macronodular disease (n=5, 6.2%) (Table 1B). Patients with severe CS (n=27, 33.3%) were younger at the time of diagnosis, endorsed a longer duration of symptoms prior to curative surgery and were more likely to be diagnosed non-incidentally (Table 1A).
Table 1.
Baseline characteristics of all patients (n=81) and by clinical criteria of cortisol excess severity.
| Subgroup Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|
| n=81 | MACE 30(37.0%) |
Moderate CS 24 (29.7%) |
Severe CS 27 (33.3%) |
P | MACE vs Mod CS | MACE vs Sev CS | Mod vs Sev CS | |
| A. Demographic characteristics | ||||||||
| Sex: female | 64 (79.0%) | 20 (66.7%) | 19 (79.2%) | 25 (92.6%) | 0.05 | 0.30 | 0.02 | 0.16 |
| Race: Caucasian | 80 (98.8%) | 29 (96.7%) | 24 (100%) | 27 (100%) | 0.42 | 0.36 | 0.34 | - |
| BMI, kg/m2 | 31.1 (27.3–37.7) | 28.5 (25.4–32.7) | 33.5 (29.5–39.2) | 32.3 (27.4–40.5) | 0.02 | 0.007 | 0.06 | 0.60 |
| Tobacco use: present | 31 (38.3%) | 7 (23.3%) | 13 (51.2%) | 11 (40.7%) | 0.07 | 0.02 | 0.16 | 0.34 |
| Age at the time of tumor diagnosis, years | 52 (42–62) | 60 (49–67) | 51 (44–61) | 45 (31–56) | 0.0007 | 0.06 | 0.0003 | 0.03 |
| Duration of symptoms, months | 11 (0–21) | 0 (0–8) | 6 (0–12) | 24 (12–24) | <0.0001 | 0.02 | <0.0001 | 0.002 |
| Mode of discovery: incidental | 54 (66.7%) | 25 (83.3%) | 20(83.3%) | 9 (33.3%) | <0.0001 | 1.00 | 0.0001 | 0.0003 |
| B. Tumor Characteristics and therapy | ||||||||
| Size of resected tumor, mm | 39 (30.0–48) | 40 (30–48) | 41 (33–48) | 34 (28–42) | 0.15 | 0.65 | 0.15 | 0.07 |
| Pathology: adenoma | 76 (93.8%) | 28 (93.3%) | 23 (95.8%) | 25 (92.6%) | 0.88 | 0.69 | 0.91 | 0.62 |
| C. Clinical characteristics | ||||||||
| Hypertension | 62 (76.5%) | 21 (70.0%) | 20 (83.3%) | 21 (77.8%) | 0.51 | 0.25 | 0.50 | 0.62 |
| Weight gain | 51 (63.0%) | 98 (26.7%) | 17 (70.8%) | 26 (96.3%) | <0.0001 | 0.001 | <0.0001 | 0.01 |
| Dyslipidemia | 49 (60.5%) | 17 (56.7%) | 19 (79.2%) | 13 (48.1%) | 0.07 | 0.08 | 0.52 | 0.02 |
| Abnormal Glucose metabolism a | 45 (55.6%) | 13 (43.3%) | 15 (62.5%) | 17 (63.0%) | 0.24 | 0.16 | 0.14 | 0.97 |
| Low bone density b | 30 (37.0%) | 10 (33.3%) | 9 (37.5%) | 11 (40.7%) | 0.84 | 0.75 | 0.56 | 0.81 |
| Cerebrovascular disease | 5 (6.1%) | 0 (0%) | 4 (16.7%) | 1 (3.7%) | 0.03 | 0.02 | 0.29 | 0.12 |
| D. Physical exam findings | ||||||||
| Supraclavicular or dorsocervical fat pads | 34 (42.0%) | 0 (0.0%) | 7 (29.2%) | 27 (100.0%) | <0.0001 | 0.001 | <0.0001 | <0.0001 |
| Central obesity | 32 (39.5%) | 0 (0.0%) | 7 (29.2%) | 25 (92.6%) | <0.0001 | 0.001 | <0.0001 | <0.0001 |
| Rounding of the face | 31 (38.3%) | 0 (0.0%) | 5 (20.83%) | 26 (96.3%) | <0.0001 | 0.009 | <0.0001 | <0.0001 |
| Muscle weakness | 19 (23.5%) | 0 (0.0%) | 5 (20.83%) | 14 (51.2%) | <0.0001 | 0.009 | <0.0001 | 0.02 |
| Skin Changes (thinning, easy bruising, striae) | 19 (23.5%) | 0 (0.0%) | 2 (8.3%) | 17 (63.0%) | <0.0001 | 0.11 | <0.0001 | <0.0001 |
| E. Laboratory results | ||||||||
| Number of abnormal HPA axis tests | 3 (2–4) | 2 (1–3) | 3 (2–4) | 3 (4–5) | <0.0001 | 0.07 | <0.0001 | 0.0003 |
| Urine free cortisol, μg/24h, nmol/24h, n=71 (Reference range: 3.5–45 μg/24h, 10–124 nmol/24h) |
41 (19–118) 113 (52–326) |
26.0 (17.0–44.0) 72 (47–121) |
40.0 (13.0–73.0) 110 (36–201) |
104.0 (41.0–254.0) 287 (113–701) |
<0.0001 | 0.50 | <0.0001 | 0.001 |
| Post 1-mg DST serum cortisol, μg/dL, nmol/L, n=69 (Reference range: <1.8 μg/dL, <50 nmol/L) |
5.5 (2.9–11.0) 152 (80–304) |
5.6 (3.0–9.1) 155 (81–251) |
4.6 (2.7–8.2) 127 (75–226) |
9.0 (2.8–16.8) 247 (78–462) |
0.24 | 0.59 | 0.17 | 0.14 |
| ACTH, pg/mL, pmol/L, n=71 (Reference range: 10–60 pg/dL, 2.2–13.2 pmol/L, AM draw) |
5 (5–7.5) 1.1 (1.1–1.7) |
6.6 (5–12) 1.5 (1.1–2.6) |
5 (5–7.3) 1.1 (1.1–1.6) |
5 (5–5.8) 1.1 (1.1–1.3) |
0.01 | 0.05 | 0.003 | 0.46 |
| 8AM serum cortisol, μg/dL, nmol/L, n=61 (Reference range 7–25 μg/dL, 193–690 nmol/L) |
14 (9.9–20) 386 (273–552) |
11 (9.8–18) 304 (270–497) |
13 (8.8–19.8) 359 (242–545) |
17 (14.0–21.2) 469 (386–585) |
0.05 | 0.69 | 0.02 | 0.11 |
| Afternoon serum cortisol, μg/dL, nmol/L, n=48 (Reference range 2–14 μg/dL) |
14.0 (9.4–20.0) 386 (261–552) |
9.6 (8.1–16) 265 (224–442) |
12.5 (9.3–16.5) 345 (255–457) |
17.5 (11.5–22.8) 483 (317–628) |
0.10 | 0.57 | 0.07 | 0.13 |
| Midnight salivary cortisol, ng/dL, nmol/dL, n=21 (Reference range <100 ng/dL, <2.8 nmol/dL) |
133 (56–548) 3.7 (1.5–15.1) |
56 (50–72.5) 1.5 (1.4–2.0) |
60.5 (50–117.5) 1.7 (1.4–3.2) |
382 (137.5–742) 10.5 (3.8–20.5) |
0.009 | 0.88 | 0.01 | 0.03 |
| Loss of circadian rhythm c, n=44 | 36/44 (81.8%) | 8/12 (66.7%) | 6/10 (60.0%) | 22/22 (100%) | 0.006 | 0.75 | 0.004 | 0.001 |
| DHEA-S, μg/dL, μmol/L, n=52 (Reference range: 44–332 μg/dL, 1.2–9.0 μmol/L) |
30.2 (15–55.1) 0.8 (0.4–1.5) |
29.3 (15–81.9) 0.8 (0.4–2.2) |
38.3 (27.4–56.3) 1.0 (0.7–1.5) |
22.1 (15–48.0) 0.6 (0.4–1.3) |
0.46 | 0.70 | 0.49 | 0.21 |
Continuous data are summarized as median and interquartile ranges. Categorical data are presented as frequencies and percentages. All P-values <0.05 were considered significant.
Abnormal glucose metabolism: diabetes mellitus or pre-diabetes mellitus;
Low bone density: osteopenia or osteoporosis;
Loss of circadian rhythm defined as changes in normal variability of cortisol throughout the day (i.e. elevated PM serum cortisol)
Abbreviations used: ACTH, corticotropin; BMI, body mass index; CS, Cushing syndrome; DHEA-S: Dehydroepiandrosterone sulfate; DST, dexamethasone suppression test; HPA, hypothalamic-pituitary-adrenal; and, MACE, mild autonomous cortisol excess.
The most common metabolic abnormalities present at diagnosis included hypertension (76.5%) and weight gain (63.0%), followed by dyslipidemia (60.5%), type 2 diabetes mellitus and prediabetes (55.6%), and bone disease (37.0%) (Table 1C). Patients reported a higher prevalence of weight gain in relation to the severity of CS (26.7% vs 70.8% vs 96.3% in MACE, moderate CS, and severe CS, respectively, P<0.0001). On physical examination, patients most commonly presented with supraclavicular and/or dorsocervical fat accumulation (42.0%), central obesity (39.5%), and rounding of the face with or without plethora (38.3%) (Table 1D). All standard-of-care tests investigating cortisol excess demonstrated increasing degrees of abnormality in relation to CS severity (Table 1E). Patients’ biochemical and clinical severity scores demonstrated a statistically significant positive correlation (R2=0.33, P<0.0001, supplemental material Figure S1).
Postoperative course
Serum cortisol was not measured immediately postoperatively in the majority of patients (n=48, 59.3%) because a decision was made to administer perioperative GC empirically due to a high likelihood of postoperative adrenal insufficiency. Patients who had postoperative serum cortisol measured were mainly those with a diagnosis of MACE. Forty-two patients (51.9%) received prednisone and 39 patients (48.1%) received hydrocortisone postoperatively (Table 2). The choice of GC depended on physician preference. Patients were followed for a median of 14.0 months (IQR, 6.5–36.0) after adrenalectomy (Table 2). At the time of manuscript preparation, 71 patients (87.7%) completed the GC taper.
Table 2.
Postoperative adrenal insufficiency and follow up in all patients (n=81) and by clinical severity of cortisol excess.
| n=81 | MACE 30 (37.0%) |
Moderate CS 24 (29.7%) |
Severe CS 27 (33.3%) |
P | |
|---|---|---|---|---|---|
| Median follow-up, months | 14.0 (6.5–36.0) | 18.0 (11.8–34.5) | 11.5 (3.0–40.0) | 12.0 (6.0–34.0) | 0.37 |
| Number of patients with postoperative 8AM serum cortisol 24 h off any GC | 33 (40.7%) | 16 (53.3%) | 10 (41.7%) | 7 (25.9%) | 0.11 |
| Immediate postoperative serum cortisol, μg/dL, nmol/L, n=33 (Reference range 7–25 μg/dL, 193–690 nmol/L) |
3.1 (1.3–5.6) 86 (35–155) |
3.2 (1.1–6.1) 88 (33–167) |
3.9 (1.5–6.0) 108 (40–164) |
1.6 (1.2–4.3) 44 (33–119) |
0.68 |
| Patients who received perioperative IV steroids | 63 (77.8%) | 17 (56.7%) | 22 (91.7%) | 24 (88.9%) | 0.002 |
| Hydrocortisone-equivalent dose at the start of the glucocorticoid therapy, mg/day | 40 (30–60) | 37.5 (20–40) | 40 (21.2–50) | 60 (40–80) | 0.002 |
| Type of glucocorticoid therapy: | |||||
| Hydrocortisone | 39 (48.1%) | 18 (60.0%) | 9 (37.5%) | 12 (44.4%) | 0.23 |
| Prednisone | 42 (51.9%) | 12 (40.0%) | 15 (62.5%) | 15 (55.6%) | 0.23 |
| 8AM serum cortisol at recovery, μg/dL | 11 (10.5–14.0) | 11 (10.0–15.5) | 11.0 (10.0–15.5) | 11.7 (10.0–13.3) | 0.95 |
| (Reference range 7–25 μg/dL, 193–690 nmol/L) | 306 (276–387) | 304 (290–386) | 304 (276–428) | 323 (276–368) |
Continuous data are summarized as median and interquartile ranges. Categorical data are presented as frequencies and percentages. All P-values <0.05 were considered significant.
Abbreviations used: CS, Cushing syndrome; GC, glucocorticoid; IV, intravenous; MACE, mild autonomous cortisol excess.
Postoperative GC therapy was initiated at a median daily hydrocortisone dose of 40 mg (IQR, 30–60) and lasted a median of 4.3 months (IQR, 1.6–11.4) (Figure 1A). Seventy-five percent of patients either discontinued GC or reached physiologic doses of hydrocortisone by 11.5 months. The duration of postoperative GC therapy varied based on the severity of the clinical presentation: median of 2.1 vs 2.8 vs 11.4 months in MACE, moderate CS, and severe CS, respectively (P<0.003, Figure 1B). The duration of GC therapy also correlated with the biochemical severity of CS (P<0.0001, Figure 1C). Subgroup analyses demonstrated that there was no significant difference in the median time to GC taper between patients with MACE and moderate CS, regardless of whether a clinical (P=0.58) or biochemical (P=0.16) classification of disease severity was used (Figure 1B and 1C).
Figure 1: Postoperative glucocorticoid therapy duration for all patients (n=81) and based on clinical and the biochemical severities of cortisol excess.
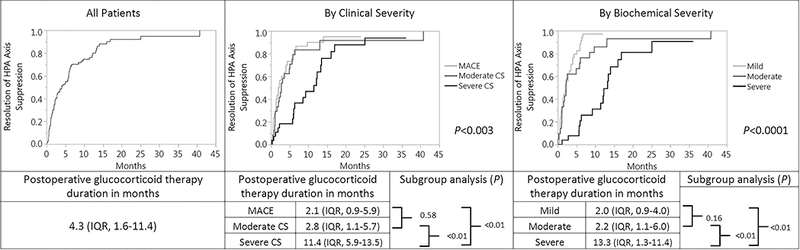
Data are summarized as median and interquartile ranges. All P-values <0.05 were considered significant.
Abbreviations used: CS, Cushing syndrome; IQR, interquartile range; MACE, mild autonomous cortisol excess.
Predictors of HPA axis recovery after adrenalectomy
Univariate analysis
Univariate analysis showed that several demographic, clinical, imaging and biochemical variables predicted the duration of postoperative GC therapy (Table 3). The duration of cortisol excess signs and symptoms >12 months, younger age at the time of diagnosis, female sex, non-incidental discovery of adrenal tumor, adrenal mass diameter <40 mm, and lower BMI– were associated with a longer HPA axis recovery interval (Tables 3A and B, and supplemental material Table S3). The type of GC used (hydrocortisone or prednisone) did not affect the duration of the taper (Table 3F and supplemental material Figure S2 and S3).
Table 3.
Univariate analysis: postoperative glucocorticoid therapy duration and demographic, clinical, biochemical and imaging characteristics.
| Characteristic | Postoperative glucocorticoid therapy duration, months | P | |
|---|---|---|---|
| A. Demographic Characteristics | |||
| Sex | Male | 1.2 (0.6–4.0) | 0.002 |
| Female | 5.7 (20.−12.3) | ||
| BMI | for each 3 kg/m2 decrease | additional 0.9 (0.8–0.9) | 0.04 |
| Age at the time of diagnosis | < 45 years old | 11.4 (5.1–14.0) | 0.001 |
| ≥ 45 years old | 2.3 (1.1–6.0) | ||
| Duration of symptoms prior to diagnosis, years | ≤ 1 year | 2.8 (1.2–6.2) | 0.0007 |
| > 1 year | 11.4 (2.2–16.9) | ||
| Mode of Discovery | Incidental | 2.4 (1.2–6.0) | 0.004 |
| Hormonal Excess | 9.2 (5.7–13.5) | ||
| B. Tumor Characteristics | |||
| Size of adrenal tumor, mm | ≥40 | 2.8 (0.9–6.0) | 0.02 |
| <40 | 6.0 (1.8–13.5) | ||
| C. Clinical Characteristics | |||
| Weight gain prior to diagnosis | No | 2.2 (1.1–5.1) | 0.01 |
| Yes | 5.9 (1.8–13.1) | ||
| D. Physical Exam Findings | |||
| Moon Facies | No | 2.2 (1.1–5.8) | <0.0001 |
| Yes | 11.4 (5.7–16.0) | ||
| Supraclavicular or dorsocervical fat pads | No | 2.3 (1.0–5.9) | 0.003 |
| Yes | 8.5 (3.1–13.5) | ||
| Skin changes | No | 2.6 (1.2–6.0) | 0.0006 |
| Yes | 12.1 (6.3–16.0) | ||
| Abdominal Obesity | No | 2.8 (1.2–6.0) | 0.007 |
| Yes | 8.5 (2.2–13.5) | ||
| Myopathy | No | 2.7 (1.2–6.2) | 0.002 |
| Yes | 13.1 (6.0–16.0) | ||
| Number of physical findings a | 0–1 | 2.2 (1.0–4.9) | <0.0001 |
| 2–3 | 5.7 (2.0–12.3) | ||
| 4–5 | 12.1 (6.3–16.9) | ||
| E. Laboratory Results | |||
| Urine free cortisol, μg/24h, nmol/24h, n=71 (Reference range: 3.5–45 μg/24h, 10–124 nmol/24h) |
≤45, ≤124 | 2.8 (1.1–6.0) | 0.0002 |
| >45, >124 | 9.2 (2.2–16.0) | ||
| Post 1-mg DST serum cortisol, μg/dL, nmol/L, n=69 (Reference range: <1.8 μg/dL, <50 nmol/L) |
≤10, ≤276 | 2.2 (1.1–5.7) | 0.0004 |
| >10, >276 | 9.7 (2.3–13.5) | ||
| ACTH, pg/mL, pmol/L, n=71 (Reference range: 10–60 pg/dL, 2.2–13.2 pmol/L, AM draw) |
>10, >2.2 | 1.3 (0.7–3.5) | 0.0006 |
| ≤10, ≤2.2 | 5.8 (2.0–13.1) | ||
| Loss of circadian rhythm b, n=44 | Yes | 9.7 (2.6–14.0) | 0.002 |
| No | 2.4 (1.8–5.5) | ||
| Number of abnormal HPA axis tests c | 1–2 | 2.0 (0.9–4.0) | <0.0001 |
| 3–4 | 5.1 (1.6–13.1) | ||
| 5–6 | 12.3 (11.4–13.5) | ||
| F. Postoperative Variables | |||
| Postoperative glucocorticoid use for taper | Prednisone | 4.0 (1.4–12.3) | 0.59 |
| Hydrocortisone | 4.0 (1.3–9.2) | ||
Data are summarized as median and interquartile ranges. All P-values <0.05 were considered significant.
Physical exam findings included: rounding and/or flushing of the face, abdominal obesity, skin changes (purple striae, thinning and/or easy bruising), subcutaneous supraclavicular and/or dorsocervical fat pads, and objective evidence of muscle weakness;
Loss of circadian rhythm defined as changes in normal variability of cortisol throughout the day, i.e. elevated PM cortisol;
Laboratory Tests included: 24hr urine free cortisol >45 μg/24h, post 1-mg DST cortisol >10 μg/dL, ACTH <10 pg/mL, midnight salivary cortisol >100 ng/dL, loss of circadian rhythm, and DHEA-S ≤50 μg/dL.
Abbreviations used: ACTH, corticotropin; BMI, body mass index; DHEA-S: Dehydroepiandrosterone sulfate; DST, dexamethasone suppression test; and, HPA, hypothalamic-pituitary-adrenal.
In addition, certain signs of CS on physical exam were associated with the duration of postoperative GC use, and included in order of strength of association: facial rounding and/or plethora, skin changes, objective myopathy, supraclavicular and/or dorsocervical fat pads, abdominal obesity and weight gain (Table 3D). The number of physical exam signs suggestive of CS at presentation correlated with the duration of GC therapy (Table 3D). However, we found no correlation between the number and degree of preoperative metabolic abnormalities and the duration of GC use (data not shown).
Similarly, the number of abnormal biochemical tests consistent with cortisol excess correlated positively with the duration of GC therapy (Table 3E). However, the only biochemical abnormalities individually associated with postoperative GC therapy duration, in order of strength of association included: 24-hr UFC >45 μg/24h or >1242 nmol/24h; serum cortisol >10 μg/dL or >276 nmol/L after the 1-mg DST; ACTH ≤10 pg/mL or ≤2.2 pmol/L; and, loss of circadian rhythm. DHEA-S suppression and elevated midnight salivary cortisol were not associated with the duration of GC use (data not shown).
Multivariable Analysis
Multivariable analysis indicated the following variables were the strongest predictors of postoperative GC therapy duration: (i) degree of endogenous cortisol excess based on biochemical classifiers (Models 2 and 4, Table 4), particularly post 1-mg DST serum cortisol (Model 4); and, (ii) age at the time of diagnosis, duration of symptoms prior to diagnosis and lower BMI (Models 1, 2 and 3). Among the physical examination findings, presence of myopathy had the strongest correlation with duration of GC therapy, followed by rounding of the face (Model 2). Female sex was another important predictor (Model 4, Table 4).
Table 4.
Multivariable analysis: postoperative glucocorticoid therapy duration adjusted for demographic, clinical, biochemical and pathology variables.
| Model 1 | OR a | 95% CI | P |
|---|---|---|---|
| Sex (Female/Male) | 0.68 | 0.37–1.30 | 0.24 |
| BMI (↓ 3 kg/m2) | 0.89 | 0.98–0.99 | 0.04 |
| Age at the time of diagnosis (↑ 10 years) | 1.3 | 1.06–1.59 | 0.01 |
| Duration of symptoms years (↑ 1 year) | 0.49 | 0.24–0.96 | 0.04 |
| Mode of discovery (Hormonal Excess/Incidental) | 0.85 | 0.45–1.54 | 0.59 |
| Size of the nodule (<40/≥40 mm) | 0.91 | 0.53–1.54 | 0.71 |
| Model 2 | OR a | 95% CI | P |
| BMI (↓ 3 kg/m2) | 0.91 | 0.83–1.04 | 0.14 |
| Age at the time of diagnosis (↑ 10 years) | 1.23 | 1.00–1.50 | 0.04 |
| Duration of symptoms years (↑ 1 year) | 0.5 | 0.23–0.94 | 0.03 |
| Cushing syndrome clinical severity (Severe CS/MACE and Moderate CS) | 1.56 | 0.67–3.49 | 0.29 |
| Cushing syndrome biochemical severity (Severe/Mild and Moderate) | 0.26 | 0.11–0.59 | 0.001 |
| Model 3 | OR | 95% CI | P |
| Sex (Female/Male) | 0.83 | 0.61–2.26 | 0.58 |
| BMI (↓ 3 kg/m2) | 0.87 | 0.77–0.96 | 0.03 |
| Age at the time of diagnosis (↑ 10 years) | 1.03 | 1.01–1.05 | 0.005 |
| Rounding of the face with or without plethora (Yes/No) | 0.40 | 015.−0.98 | 0.04 |
| Myopathy (Yes/No) | 0.41 | 0.19–0.83 | 0.01 |
| Supraclavicular or dorsocervical fat pads (Yes/No) | 1.34 | 0.56–3.16 | 0.51 |
| Skin Changes (Yes/No) | 0.74 | 0.30–1.84 | 0.52 |
| Abdominal Obesity (Yes/No) | 1.2 | 0.48–2.87 | 0.71 |
| Model 4 | OR | 95% CI | P |
| Sex (Female/Male) | 0.05 | 0.007–0.29 | 0.002 |
| BMI (↓ 3 kg/m2) | 0.90 | 0.06–2.17 | 0.27 |
| Age at the time of diagnosis (↑ 10 years) | 1.01 | 0.97–1.05 | 0.57 |
| Post 1-mg DST serum cortisol (n=82) (>10/≤10 μg/dL, >276/≤276 nmol/L) | 0.34 | 0.11–0.99 | 0.048 |
| 24-hr urine free cortisol (n=84) (>45/≤45 μg/24h, >124/≤124 nmol/24h) | 0.87 | 0.36–2.08 | 0.75 |
| ACTH (n=83) (≤10/>10 pg/mL, ≤2.2/>2.2 pmol/L) | 0.77 | 0.19–4.09 | 0.74 |
| Loss of circadian rhythm b (n=55) (Yes/No) | 0.50 | 0.14–1.85 | 0.29 |
OR>1 reflects a higher probability of a faster steroid taper, i.e. postoperative glucocorticoid therapy duration is shorter. Conversely, OR<1 reflects that the probability of a faster taper is lower, i.e. postoperative glucocorticoid therapy duration is longer;
Loss of circadian rhythm defined as changes in normal variability of cortisol throughout the day, i.e. elevated PM serum cortisol concentration.
All P-values <0.05 were considered significant.
Abbreviations used: ACTH, corticotropin; BMI, body mass index; DST, dexamethasone suppression test; MACE, mild autonomous cortisol excess; and, OR, odds ratio.
Glucocorticoid Withdrawal Events
GC withdrawal events were defined based on the communication from the patient to their provider about their symptoms until HPA axis recovery was achieved. A total of 132 GC withdrawal events were reported by patients. Sixty-seven percent of the patients with severe CS (18/27) had GWS compared to less than half of patients with moderate CS (11/24, 45.8%) and MACE (12/30, 40.0%; P=0.11; Figure 2A). Patients with severe CS had a total of 71 events (median of 1 per patient [IQR, 0–5]) vs 29 and 32 events in patients with moderate CS and MACE, respectively (median of 0 per patient [IQR 0–2] and [IQR0–1.8], respectively) (p=0.05, Figure 2B). The majority of events were classified as mild (61, 46.2%), followed by moderate (53, 40.2%), and severe (11, 8.3%) events. Adrenal crisis occurred in 7 patients (8.6%): one patient with MACE, two patients with moderate CS and four patients with severe CS (P=0.3). Although the severity of CS did not significantly correlate with the incidence of GWS (R2=0.05, P=0.11, Figure 2A), patients with severe CS had a significantly higher incidence of GWS events compared to patients with MACE (p=0.04, Figure 2A). Although we observed a trend for a higher incidence of mild, moderate and severe GWS as well as hospitalizations for adrenal crisis in patients with severe CS, this did not reach statistical significance (Figure 2B).
Figure 2: Postoperative glucocorticoid withdrawal syndrome (GWS).
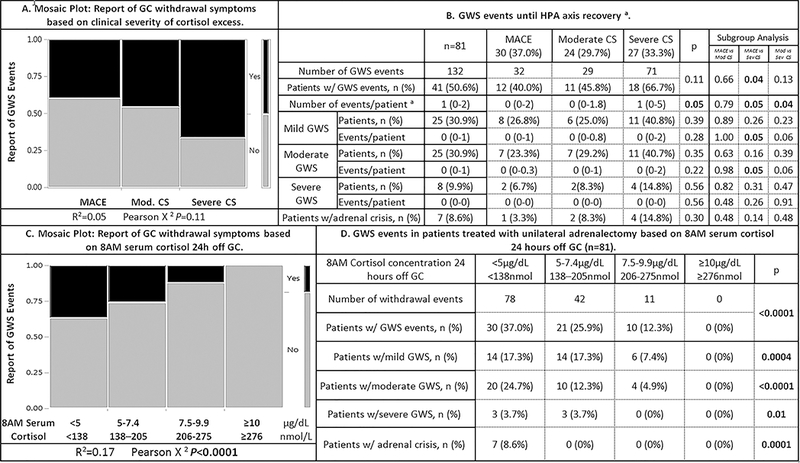
Continuous data are summarized as median and interquartile ranges. Categorical data are presented as frequencies and percentages. All P-values <0.05 were considered significant.
a Recovery of the HPA-axis was considered to be achieved when an 8AM serum cortisol was ≥10 μg/dL (≥276 nmol/L) at 24h after the last administered dose of GC.
Mild GWS: Symptoms did not limit ADLs or IADLs; Moderate GWS: Symptoms somewhat limited IADLs but not ADLs; Severe: Symptoms significantly limited ADLs and IADLs; Adrenal crisis: Patients required hospital admission for intravenous GC administration due to hemodynamic instability and without evidence of another underlying etiology.
Abbreviations used: ADL, activity of daily living; CS, Cushing syndrome; GC, Glucocorticoid; GWS, glucocorticoid withdrawal symptoms; HPA, hypothalamic-pituitary-adrenal, IADL, instrumental activity of daily living; MACE, mild autonomous cortisol excess.
GWS events were most frequent when 8AM serum cortisol 24h off GC was <5 μg/dL (<138 nmol/L; Figure 2C). However, patients still reported GC withdrawal symptoms even when 8 AM serum cortisol 24h off GC was between 5 and 9.9 μg/dL(138–275 nmol/L; Figure 2C and 2D), though the majority of events in this group were mild (31/53, 58.5%, data not shown). Patients reported no GWS events at the time when 8AM serum cortisol 24h off GC was ≥10 μg/dL (≥276 nmol/L), our biochemical definition of HPA-recovery and the time when patients would stop physiological GC replacement therapy. There was no correlation between the incidence of GWS and demographic, clinical or imaging variables (data not shown). The type of GC used (hydrocortisone or prednisone) did not affect the incidence of GWS (supplemental table S4).
DISCUSSION
In this study, we hypothesized that baseline demographic, clinical and biochemical variables predict the HPA axis recovery interval and the severity of GWS in patients treated with adrenalectomy for ACTH-independent cortisol excess.
We observed that the median time to HPA axis recovery was just over 4 months following adrenal surgery, but varied widely between 1 week and 41 months. Patients with severe CS required a longer postoperative GC taper compared to those with moderate CS or MACE. Patients with MACE had the shortest duration of GC taper with a median of 2.1 months. While a similar duration of taper in patients with MACE was reported in one small series of patients16, others have reported a much longer time to recovery of 10.317, 1918 and 29 months7. This could be explained by the differences in classification of severity of cortisol excess, evolution of the approach to patients with MACE over the years, differences in schedules to assess patients’ recovery of the HPA axis, as well as variability in the pace of GC taper towards the physiological dose. Among our patients with severe CS, the median duration of GC use was five times longer compared to patients with MACE and similar to other reports17,19. Surprisingly, we found that when compared to patients with MACE, patients with moderate CS had a similar duration of GC taper, regardless of whether clinical or biochemical classification was used. This finding suggests an overlap between clinical and biochemical features in milder forms of cortisol excess.
The main predictors of duration of postoperative GC therapy duration were: serum cortisol concentrations after 1-mg DST, younger age, and the presence of myopathy at the time of diagnosis. Less robust but also important predictors were lower BMI, the duration of symptoms prior to surgery, female sex, and the presence of rounding of face upon initial diagnosis.
We demonstrated that a serum cortisol concentration ≥10 μg/dL (≥276 nmol/L) after the 1-mg DST was the best biochemical predictor of longer postoperative HPA axis recovery. While low serum ACTH concentration and elevated 24-hr urine cortisol excretion predicted a longer HPA axis recovery time on univariate analysis, these were not significant predictors on multivariate analysis. Although elevated serum cortisol after 1-mg DST has been shown to be a good predictor of postoperative adrenal insufficiency, a recent publication with a smaller number of patients failed to show an association between this parameter and postoperative GC therapy duration 6,7. The presence of physical signs of CS, such as central fat redistribution, rounding of the face, skin changes and, especially, myopathy, strongly correlated with longer duration of HPA axis recovery interval. This is not surprising since physical stigmata of CS are more likely to be present in severe cases and in patients who have had a prolonged exposure to cortisol excess.
We found that metabolic complications recognized to be associated with subtle cortisol excess, such as low bone mineral density, hypertension, dyslipidemia and type 2 diabetes20, did not influence the duration of the postoperative GC taper. While patients who reported weight gain prior to curative surgery experienced a longer HPA axis recovery interval, these patients had overall a lower BMI. Possible explanations are that metabolic abnormalities in CS are common but not specific21,22, as well as different sensitivity of various organs to cortisol excess23. In other words, mild cortisol excess may be sufficient to affect bone and fat metabolism, but not enough to affect the severity of HPA axis suppression and then recovery.
In our study we demonstrated that GWS after adrenalectomy for ACTH-independent cortisol excess were frequent. While GWS events were most common at the time when 8 AM serum cortisol 24h off GC was <5 mcg/dL (<138 nmol/L), patients experienced GWS events even when 8 AM cortisol 24h off GC was 5–9.9 mcg/dL or 138–275 nmol/L (though at lower frequency and with milder symptomatology). However, no GWS events occurred in patients once cortisol 24h off GC was ≥10 mcg/dL (≥276 nmol/L), suggesting that this cut-off serves as a good clinical surrogate of HPA recovery. Patients with MACE or CS develop psychological and physical dependence to elevated cortisol concentrations. Therefore, GWS were encountered even when 8 AM serum cortisol was within “normal range” (>7 μg/dL or >193 nmol/L, the cut-off used at our institution). GWS occurs due to a relative decrease in cortisol concentrations after surgical cure, and consists not only of physical signs and symptoms, but also psychiatric symptoms (such as depression and anxiety), which affect recovery and overall quality of life24,25. Patients with severe CS, but not those with moderate CS, had a higher incidence and intensity of GWS when compared to patients with MACE. We found that the severity of CS is the only predictor of the number of GC withdrawal events after adrenalectomy. We found no other demographic, clinical, or biochemical independent predictors of postoperative GWS. It is important to note that while GWS were common, most were mild and required no intervention: only 40.8% of our patients with severe CS reported mild GWS not requiring intervention and 40% had symptoms which required an increase of GC dose. In another study of only 8 patients with overt CS, all complained of mild GWS and only 50% had signs or symptoms (severe weakness, dizziness, failure to thrive, and/or hypotension) requiring an increase in GC dose 26. In contradiction to a published report, the incidence and severity of GWS was not affected by the type of GC used27.
Our study has several limitations and strengths. Strengths of the study include a high level of detail in regards to preoperative symptoms, physical features of CS, and GWS. In addition, the GC taper at our institution is usually performed based on patients’ symptoms with frequent biochemical reassessments once the GC taper reaches a physiological replacement dose. This allowed a more accurate assessment of individual HPA axis recovery. We could not include all patients with ACTH-independent cortisol excess as many patients follow with a local medical provider after adrenalectomy. However, subgroup analysis of included and excluded patients demonstrated no difference in severity of cortisol excess or other baseline and immediate postoperative characteristics except for the prevalence of central obesity and serum DHEA-S concentrations (supplemental table S5). As this is a retrospective study, not all variables were available for all patients. This was especially the case for longitudinal variables in regard to resolution of metabolic and physical abnormalities. Biochemical evaluation for ACTH-independent cortisol excess varied amongst patients, a limitation that we attempted to address by creating a biochemical severity scoring system.
Notably, the approach at our institution to the diagnosis of adrenal insufficiency (and especially recovery from adrenal insufficiency) in this unique population of patients following adrenalectomy for a cortisol-secreting adenoma does not follow the Endocrine Society guidelines28,29. However, it is important to note that any test should be interpreted based on the pretest probability, and that the diagnostic accuracies of an 8AM serum cortisol concentration and the cosyntropin stimulation test for primary and secondary adrenal insufficiency are not perfect 30. The cosyntropin stimulation test simply evaluates the adrenal response to cosyntropin, the 8AM cortisol concentration assesses the entire HPA axis recovery. At the Mayo Clinic we do not use stimulation tests to assess HPA axis recovery; but rather, once the 8AM serum cortisol concentration is ≥10 xg/dL (≥276 nmol/L), daily GC are discontinued and patients are advised to use stress dose GC during sick days for a total of 12 months after GC are stopped. From the clinical practice perspective, these patients need GC replacement not only for adrenal insufficiency but also for GC withdrawal symptoms. Importantly, we now provide evidence that using an 8AM cortisol cut-off of ≥10 μg/dL (≥276 nmol/L) to define HPA axis recovery is not only safe and associated with lower healthcare burden, but that this is the cut-off at which patients no longer continue to have GC withdrawal symptoms.
In conclusion, management of postoperative adrenal insufficiency and GWS in patients undergoing adrenalectomy for ACTH-independent cortisol excess should be individualized based on preoperative clinical and biochemical factors. The HPA axis recovery interval is variable and is longest in young lean women with physical stigmata of severe CS with 1-mg DST serum cortisol concentration ≥10 μg/dL (≥276 nmol/L). On the other hand, patients with moderate CS and MACE are more likely to tolerate a more aggressive GC taper and should have an earlier evaluation for HPA axis recovery. GWS are common after adrenalectomy for ACTH-independent hypercortisolism. The frequency of reported GWS events correlate with the 8AM serum cortisol concentration 24h off GC, as no GWS events were reported at serum cortisol concentrations ≥10 mcg/dL. Few events require intervention, thus we recommend that in addition to education on adrenal insufficiency, patients are counseled on management of GWS.
Supplementary Material
Acknowledgements:
This publication was supported by Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
REFERENCES
- 1.Fassnacht M, Arlt W, Bancos I, et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol . 2016;175(2):G1–g34. [DOI] [PubMed] [Google Scholar]
- 2.Bancos I, Alahdab F, Crowley RK, et al. THERAPY OF ENDOCRINE DISEASE: Improvement of cardiovascular risk factors after adrenalectomy in patients with adrenal tumors and subclinical Cushing’s syndrome: a systematic review and meta-analysis. Eur J Endocrinol . 2016;175(6):R283–r295. [DOI] [PubMed] [Google Scholar]
- 3.Di Dalmazi G, Vicennati V, Garelli S, et al. Cardiovascular events and mortality in patients with adrenal incidentalomas that are either non-secreting or associated with intermediate phenotype or subclinical Cushing’s syndrome: a 15-year retrospective study. Lancet Diabetes Endocrinol . 2014;2(5):396–405. [DOI] [PubMed] [Google Scholar]
- 4.Clayton RN, Jones PW, Reulen RC, et al. Mortality in patients with Cushing’s disease more than 10 years after remission: a multicentre, multinational, retrospective cohort study. The lancet Diabetes & endocrinology . 2016;4(7):569–576. [DOI] [PubMed] [Google Scholar]
- 5.Morelli V, Palmieri S, Salcuni AS, et al. Bilateral and unilateral adrenal incidentalomas: biochemical and clinical characteristics. Eur J Endocrinol . 2013;168(2):235–241. [DOI] [PubMed] [Google Scholar]
- 6.Di Dalmazi G, Berr CM, Fassnacht M, Beuschlein F, Reincke M. Adrenal function after adrenalectomy for subclinical hypercortisolism and Cushing’s syndrome: a systematic review of the literature. J Clin Endocrinol Metab . 2014;99(8):2637–2645. [DOI] [PubMed] [Google Scholar]
- 7.Morelli V, Minelli L, Eller-Vainicher C, et al. Predictability of hypoadrenalism occurrence and duration after adrenalectomy for ACTH-independent hypercortisolism. In. Journal of Endocrinological Investigation 2017. [DOI] [PubMed] [Google Scholar]
- 8.Hochberg Z, Pacak K, Chrousos GP. Endocrine withdrawal syndromes. Endocrine reviews . 2003;24(4):523–538. [DOI] [PubMed] [Google Scholar]
- 9.Wolkowitz OM. Prospective controlled studies of the behavioral and biological effects of exogenous corticosteroids. Psychoneuroendocrinology . 1994;19(3):233–255. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell J, Barbosa G, Tsinberg M, Milas M, Siperstein A, Berber E. Unrecognized adrenal insufficiency in patients undergoing laparoscopic adrenalectomy. Surgical endoscopy . 2009;23(2):248–254. [DOI] [PubMed] [Google Scholar]
- 11.Eller-Vainicher C, Morelli V, Salcuni AS, et al. Post-surgical hypocortisolism after removal of an adrenal incidentaloma: is it predictable by an accurate endocrinological work-up before surgery? European Journal of Endocrinology . 2010;162(1):91–99. [DOI] [PubMed] [Google Scholar]
- 12.Nieman LK, Biller BMK, Findling JW, et al. Treatment of Cushing’s Syndrome: An Endocrine Society Clinical Practice Guideline. The Journal of Clinical Endocrinology & Metabolism . 2015;100(8):2807–2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper MS, Stewart PM. Corticosteroid Insufficiency in Acutely Ill Patients. New England Journal of Medicine . 2003;348(8):727–734. [DOI] [PubMed] [Google Scholar]
- 14.Peeters B, Langouche L, Van den Berghe G. Adrenocortical Stress Response during the Course of Critical Illness. Comprehensive Physiology . 2017;8(1):283–298. [DOI] [PubMed] [Google Scholar]
- 15.Meikle AW, Tyler FH. Potency and duration of action of glucocorticoids. Effects of hydrocortisone, prednisone and dexamethasone on human pituitary-adrenal function. Am J Med . 1977;63(2):200–207. [DOI] [PubMed] [Google Scholar]
- 16.Morioka M, Fujii T, Matsuki T, et al. Preclinical Cushing’s syndrome: Report of seven cases and a review of the literature. International Journal of Urology . 2000;7(4):126–132. [DOI] [PubMed] [Google Scholar]
- 17.Alesina PF, Hommeltenberg S, Meier B, et al. Posterior Retroperitoneoscopic Adrenalectomy for Clinical and Subclinical Cushing’s Syndrome. World Journal of Surgery . 2010;34(6):1391–1397. [DOI] [PubMed] [Google Scholar]
- 18.Reincke M, Nieke J, Krestin GP, Saeger W, Allolio B, Winkelmann W. Preclinical Cushing’s syndrome in adrenal “incidentalomas”: comparison with adrenal Cushing’s syndrome. The Journal of Clinical Endocrinology & Metabolism . 1992;75(3):826–832. [DOI] [PubMed] [Google Scholar]
- 19.Mishra AK, Agarwal A, Gupta S, Agarwal G, Verma AK, Mishra SK. Outcome of Adrenalectomy for Cushing’s Syndrome: Experience from a Tertiary Care Center. World Journal of Surgery . 2007;31(7):1425–1432. [DOI] [PubMed] [Google Scholar]
- 20.Di Dalmazi G, Vicennati V, Rinaldi E, et al. Progressively increased patterns of subclinical cortisol hypersecretion in adrenal incidentalomas differently predict major metabolic and cardiovascular outcomes: a large cross-sectional study. European Journal of Endocrinology . 2012;166(4):669–677. [DOI] [PubMed] [Google Scholar]
- 21.Terzolo M, Pia A, Ali A, et al. Adrenal incidentaloma: a new cause of the metabolic syndrome? The Journal of clinical endocrinology and metabolism . 2002;87(3):998–1003. [DOI] [PubMed] [Google Scholar]
- 22.Chiodini I, Mascia ML, Muscarella S, et al. Subclinical hypercortisolism among outpatients referred for osteoporosis. Annals of internal medicine . 2007;147(8):541–548. [DOI] [PubMed] [Google Scholar]
- 23.Gross KL, Cidlowski JA. Tissue-specific glucocorticoid action: a family affair. Trends in endocrinology and metabolism: TEM . 2008;19(9):331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dorn LD, Burgess ES, Friedman TC, Dubbert B, Gold PW, Chrousos GP. The longitudinal course of psychopathology in Cushing’s syndrome after correction of hypercortisolism. The Journal of clinical endocrinology and metabolism . 1997;82(3):912–919. [DOI] [PubMed] [Google Scholar]
- 25.Grossman A, Johannsson G, Quinkler M, Zelissen P. Therapy of endocrine disease: Perspectives on the management of adrenal insufficiency: clinical insights from across Europe. Eur J Endocrinol . 2013;169(6):R165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doherty GM, Nieman LK, Cutler GB Jr., Chrousos GP, Norton JA. Time to recovery of the hypothalamic-pituitary-adrenal axis after curative resection of adrenal tumors in patients with Cushing’s syndrome. Surgery . 1990;108(6):1085–1090. [PubMed] [Google Scholar]
- 27.Tang K, Wang L, Yang Z, et al. Comparison of hydrocortisone and prednisone in the glucocorticoid replacement therapy post-adrenalectomy of Cushing’s Syndrome. Oncotarget . 2017;8(62):106113–106120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and Treatment of Primary Adrenal Insufficiency: An Endocrine Society Clinical Practice Guideline. The Journal of Clinical Endocrinology & Metabolism . 2016;101(2):364–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fleseriu M, Hashim IA, Karavitaki N, et al. Hormonal Replacement in Hypopituitarism in Adults: An Endocrine Society Clinical Practice Guideline. The Journal of Clinical Endocrinology & Metabolism . 2016;101(11):3888–3921. [DOI] [PubMed] [Google Scholar]
- 30.Ospina NS, Al Nofal A, Bancos I, et al. ACTH Stimulation Tests for the Diagnosis of Adrenal Insufficiency: Systematic Review and Meta-Analysis. The Journal of clinical endocrinology and metabolism . 2016;101(2):427–434. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


