Abstract
Vaccination has been the most effective way to prevent or reduce infectious diseases, examples include the eradication of smallpox, and attenuation of tetanus and measles. However, there is a large segment of the population that responds poorly to vaccines, in part because they are immunocompromised due to disease, age, or pharmacologic therapy and are unable to generate long-term protection. Specialized pro-resolving mediators (SPMs) are endogenously produced lipids that have potent pro-resolving and anti-inflammatory activities. Lipoxin B4 (LXB4) is a member of the lipoxin family, with its pro-resolving effects shown in allergic airway inflammation. However, its effects on the adaptive immune system, especially on human B cells are not known. Here, we investigated the effects of LXB4 on human B cells using cells from healthy donors and donors vaccinated against influenza virus in vitro. LXB4 promoted IgG antibody production in memory B cells, and also increased the number of IgG-secreting B cells. LXB4 enhanced expression of two key transcription factors involved in plasma cell differentiation, BLIMP1 and XBP1. Interestingly, LXB4 increased expression of cyclooxygenase-2 (COX2), an enzyme that is required for efficient B cell antibody production. The effects of LXB4 are at least partially COX2-dependent as COX2 inhibitors attenuated LXB4-stimulated BLIMP1 and Xpb-1 expression as well as IgG production. Thus, our study reveals for the first time that LXB4 boosts memory B cell activation through COX2 and suggests that LXB4 can serve as a new vaccine adjuvant.
Introduction
The efficacy of vaccines relies on the ability of the adaptive immune system to generate long-term protection. Some vaccines include one or more adjuvants that help to boost protective immune responses, which have been the subject of investigation for decades. A major limitation is that some adjuvants incite unwanted immune responses such as allergic IgE-driven reactions or have overt toxicity (1). For many years, alum has been the only adjuvant routinely used in the Unites States. Only recently was MF59, an oil-in-water adjuvant, approved to be used in combination with a seasonal influenza virus vaccine (2). Given that a large segment of the population responds poorly to vaccines, because they are immunocompromised due to disease (HIV, COPD, etc), age (very young, elderly), or pharmacologic therapy (immunosuppression, chemotherapy), there is a pressing need for new adjuvants to enhance the efficacy of vaccines.
Specialized pro-resolving mediators (SPMs) are newly identified lipids that actively regulate the immune system to promote the resolution of inflammation. These endogenous lipid mediators are largely synthesized from omega-6 or omega-3 polyunsaturated fatty acids obtained from dietary sources (3–6). SPMs are classified into 4 different families termed resolvins, maresins, protectins and lipoxins. The regulatory roles of SPMs during the resolution phase on innate immune cells are now well recognized. In general, SPMs reduce neutrophil chemotaxis and transmigration, while promoting non-phlogistic monocyte recruitment (3). SPMs also increase the phagocytic activity of macrophages to uptake apoptotic neutrophils and microbial particles, accelerating the process of resolution (3). However, little is known about the effects of SPMs on adaptive immune cells such as T cells and B cells, especially during infection. So far, DHA-derived SPMs, 17-HDHA and RvD1, were discovered by us to promote human naïve B cell differentiation into antibody-secreting cells (7). Moreover, 17-HDHA and RvD1 potentiated B cell antibody production to protect against infection in a mouse model of influenza viral infection, suggesting the potential of SPMs as novel vaccine adjuvants (8). On the other hand, lipoxin A4 (LXA4) reduced human memory B cell antibody production (9), supporting the idea that the activities of SPMs are family and cell type-specific.
Lipoxins, including LXB4, are synthesized by a series of lipoxygenases from arachidonic acid, which is ultimately derived from omega-6 polyunsaturated fatty acids, unlike resolvins, protectins and maresins that are derived from omega-3 polyunsaturated fatty acids (10). LXB4 is a structurally distinct member of lipoxin family that signals in a distinct manner from LXA4. LXB4 is known to promote the resolution of allergic inflammation in the upper and lower airway of mice and also inhibited mast cell degranulation (11). However, the role of LXB4 in regulating B cell antibody production is not known.
Herein, we have investigated the effects of LXB4 on the production of IgG antibody in human B cells in vitro. This study could further lead to development of LXB4 as a novel vaccine adjuvant against respiratory viral infection. To test the hypothesis, healthy individuals or donors vaccinated against influenza virus were recruited and blood-derived B cells were treated with LXB4, followed by stimulation with a well-known memory B cell-activating stimulus. We further explored the mechanism(s) by which LXB4 regulates human memory B cell antibody production, focusing on cyclooxygenase-2 (COX2) which is important for optimal B cell differentiation, proliferation and antibody production. (12–14)
Methods and Materials
B lymphocyte isolation
Human peripheral blood B cells from healthy donors were isolated as previously described (14). Briefly, the buffy coat was separated and diluted in 1x PBS and the PBMCs were isolated using Ficoll-Paque (Amersham Biosciences, Piscataway, NJ) gradient centrifugation. B cells were then purified from the PBMCs using CD19 Dynabeads (Invitrogen, Carlsbad, CA). CD19 Dynabead-cell rosettes were disrupted using CD19 Detach beads (Invitrogen, Carlsbad, CA). Cells obtained by this method of isolation were >98% CD19+ as determined by flow cytometry. Where indicated, some experiments were performed on PBMCs. In addition, purified human B cells were stained and sorted using CD27 (clone M-T271, BD bioscience, San Jose, CA) and CD20 (clone L27, BD bioscience, San Jose, CA) using a FACSAria cell sorter (BD bioscience, San Jose, CA), purity >98%. Because a single donation generally only yielded enough B cells for a single experiment, the experiments in Figures 1–5 were performed using cells from a total of 23 unique donors. All donors gave informed written consent in accordance with the Declaration of Helsinki and the protocol was approved by the University of Rochester Research Subjects Review Board.
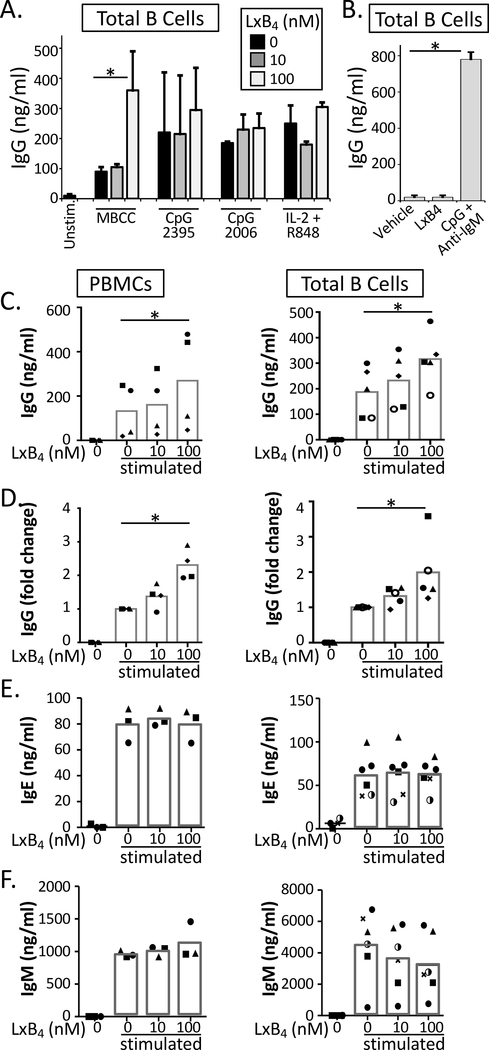
Figure 1. LXB4 enhances memory B cell IgG production.
(A) Purified CD19+ B cells were pretreated with 10 or 100 nM LXB4, followed by stimulation with different polyclonal activators as shown (MBCC, memory B cell-inducing cocktail). IgG was measured in the supernatant by ELISA after 7–8 days. (B) Purified B cells were treated with LXB4 alone (50nM) or CpG + anti-IgM as a positive control, for 7 days, and IgG was measured in the supernatant. (C–F) PBMCs or purified CD19+ B cells were stimulated with the memory B cell-inducing cocktail and treated with the indicated concentration of LXB4. IgG, IgM or IgE levels were measured in the culture supernatants after 7–8 days by ELISA. For (A) and (B), results shown are mean±S.D. for triplicate wells from one representative donor of 3 donors tested. For (C-F), each symbol represents an individual donor and represents the mean of triplicate or quadruplicate cultures. Data analyzed by repeated measures one-way ANOVA with Tukey’s posttest, *p≤0.05, **p≤0.01, ***p≤0.001.
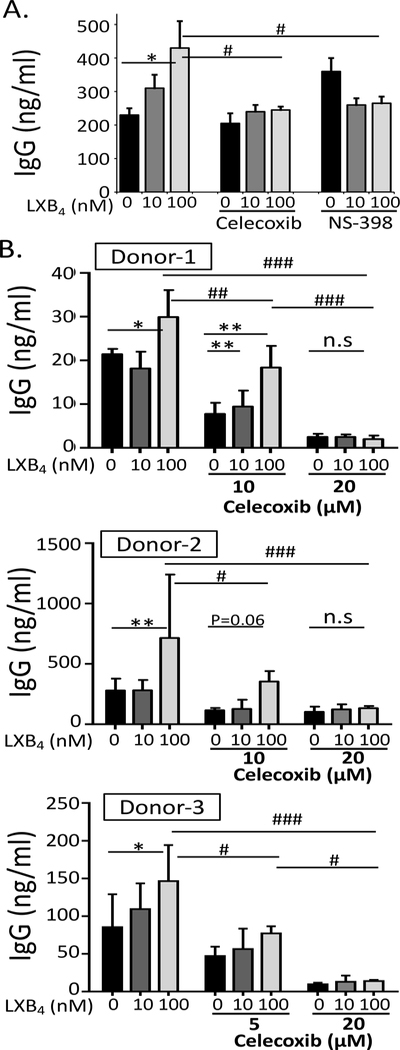
Figure 5. The stimulatory effect of LXB4 on memory B cells is mediated by COX2 activity.
(A) CD19+ B cells from healthy donors were stimulated with the memory B cell-inducing cocktail and treated with 10μM celecoxib or NS-398, prior to LXB4 treatment. Cell culture supernatants were collected at day 7–8, and IgG levels were measured by ELISA. Results shown are mean ± S.D. for triplicate wells from one representative donor of 2 donors tested. (B) B cells were stimulated with the memory B cell-inducing cocktail and treated with celecoxib at the indicated concentrations, and IgG levels were measured. Each panel is a single donor and the results shown are mean±S.D. of triplicate wells. Data analyzed by two-way ANOVA with Tukey’s posttest, *p≤0.05, **p≤0.01, ***p≤0.001 compared to vehicle alone. #p≤0.05, ##p≤0.01, ###p≤0.01 compared to vehicle plus 100 nM LXB4.
Reagents and culture conditions
Purified CD19+ B cells or PBMCs were cultured in RPMI 1640 (GIBCO/Invitrogen, Carlsbad, CA) supplemented with 5% fetal bovine serum, 2mM L-glutamine, 5×10−5M 2-mercaptoethanol, 10mM HEPES and 50μg/ml gentamicin. The memory B cell-inducing cocktail was composed of PWM (pokeweed mitogen) (1:100,000, generous gift from Dr. Shane Crotty, La Jolla Institute for Allergy & Immunology), SAC (protein A from Staphylococcus aureus) (1:10,000, Sigma Aldrich, Saint Louis, MO) and CpG ODN2395 (0.5μg/ml, Invivogen, San Diego, CA). CpG ODN2006 (0.5μg/ml) was from Invivogen, IL-2 (10ng/ml) was from R&D Systems, and Resiquimod (R848, 1μg/ml) was from Sigma. Lipoxin B4 (LXB4, 5S, 14R, 15S- trihydroxy- 6E, 8Z, 10E, 12E- eicosatetraenoic acid) (Cayman Chemical Company, Ann Harbor, MI) was suspended in ethanol and supplemented in cell culture at nanomolar concentrations. Vehicle control was defined as 1x PBS with 0.03% ethanol by volume, equivalent to the highest concentration of LXB4 used. Cells were pretreated with either vehicle control or LXB4 for 30 minutes, then were treated with the memory B cell-inducing activators or left unstimulated. Additional SPM treatments were added every 24 hours for the duration of the experiment. Celecoxib and NS-398 (R&D system, Minneapolis, MN) were dissolved in DMSO and added to cell culture at the indicated concentrations.
Enzyme-linked immunosorbent assays (ELISA) and IgG-specific ELISpot assay
Purified CD19+ B cells (5×105 cells/ml) or PBMCs (1×106 cells/ml) were cultured in triplicate in 96-well round-bottom plates for 6 days. IgG, IgM and IgE levels in the supernatant were measured by ELISA as specified by the manufacturer (Bethyl Laboratories, Montgomery, TX). For ELISpot assay, plates (Millipore, Billerica, MA) were coated with HA protein from Influenza A/California/07/2009 virus (5ug/ml, BEI Resources, Manassas, VA) or goat anti-human IgG antibodies (1:1000, Biosource, Carlsbad, CA). Cells were transferred to the ELISpot plate and incubated for a further 24 hours. IgG-secreting B cells were detected with alkaline phosphatase-conjugated mouse anti-human IgG antibody (Sigma Aldrich, Saint Louis, MO) at 1:1000 dilution. Plates were developed using Vector AP substrate kit III (Vector Laboratories, Burlingame, CA) and spots were counted using an ImmunoSpot Series 5 Analyzer (Cellular Technology, Shaker Heights, OH).
Cell proliferation and viability assays
Purified CD19+ B cells were cultured in round-bottom plates (5×105 cells/ml) in triplicate, treated with LXB4 and stimulated with the memory B cell-inducing cocktail. [3H]Thymidine (1μCi/well) was added 12 hours prior to harvest. Incorporation was measured with a Topcount Luminometer (PerkinElmer, Boston, MA). Viability was determined by staining cells at the indicated time points with 7-AAD (BD Biosciences, San Jose, CA) and determining the percent of 7-AAD-negative cells by Flow Cytometry.
Real-time PCR
Purified CD19+ B cells pretreated with LXB4 and stimulated with the memory B cell-inducing cocktail were harvested at the indicated time points. Total RNA was extracted with a Qiagen RNAeasy mini kit (Valencia, CA) using 1×106 cells/sample, and 200ng of RNA from each sample was reverse transcribed with Superscript III using the random primer method (Invitrogen, Carlsbad, CA). The cDNA levels were analyzed by quantitative real time PCR using iQ SYBR Green Supermix (Bio-Rad, Hercules, CA) and quantified with Bio-Rad iCycler software. HPRT levels were used to normalize relative gene expression. The primers used were as follows:
BLIMP1 forward, 5’-GTGTCAGAACGGGATGAAC-3’;
BLIMP1 reverse, 5′-TGTTAGAACGGTAGAGGTCC-3′,
XBP1 forward, 5′-TGGCGGTATTGACTCTTCAG-3′;
XBP1 reverse, 5′- ACGAGGTCATCTTCTACAGG-3′
HPRT forward, 5’-ATGACCAGTCAACAGGGGAC-3’;
HPRT reverse, 5’-TGCCTGACCAAGGAAAGCAA-3’.
Western blotting
Purified human B cells were lysed in RIPA buffer (150 mM NaCl, 1% NP40, 0.5% sodium deoxycholate, 50mM Tris, 0.1% SDS, pH 8.0) with protease inhibitor cocktail (Sigma Aldrich, Saint Louis, MO). Protein concentration was determined using Bio-Rad DC protein assay kit (BioRad, Hercules CA). Precast SDS-PAGE gels (Pierce/Thermo Fisher Scientific, Rockford, IL) were loaded with 10–30 μg of protein and transferred to PVDF membranes (Millipore, Billerica, MA). Western blots were probed with mouse anti-human BLIMP1, rabbit anti-human XBP1 (Novus, Littleton, CO) and mouse anti-human β-tubulin (Calbiochem Chemicals, Gibbstown, NJ). HRP conjugated goat anti-mouse or goat anti-rabbit secondary antibodies (Jackson ImmunoResearch, West Grove, PA) were used to detect specific probed antibodies. Western blots were visualized by autoradiography after incubation with ECL (Perkin Elmer Life Sciences Inc., Boston, MA).
Flow cytometry analysis
Purified CD19+ B cells were pretreated with LXB4, followed by stimulation with the memory B cell-inducing cocktail for 6 days. Cells were fixed with 4% paraformaldehyde EM grade (Electron Microscopy Sciences, Hatfield, PA) at 37°C for 10 minutes and permeabilized with BD Phosflow™ Perm Buffer III (BD Bioscience) on ice for 30 minutes. Cells were stained with anti-COX2-FITC (clone CX229, Cayman Chemical Company, Ann Harbor, MI) antibodies and incubated on ice for 30 minutes. Cell staining was analyzed using a 12-color LSRII flow cytometer (BD Biosciences) and analyzed with FlowJo software version 7.6.5 (Tree Star, Ashland, OR).
Statistical analysis
Each experiment was repeated with cells from at least three different human donors. Results are expressed as mean ± standard errors (SEM). Statistical analyses on normally distributed data were performed using repeated measure one-way analysis of variance (ANOVA) with Tukey’s posttest. Statistical analyses were performed using Prism version 6 (GraphPad, San Diego, CA).
Results
LXB4 enhances memory B cell IgG production
To investigate the effects of LXB4 on antibody production, healthy individuals were recruited and peripheral blood mononuclear cells (PBMCs) or CD19+ B cells were isolated as described (14). Cells were pretreated with 10 or 100 nanomolar LXB4, followed by stimulation to induce B cell IgG production. We tested different B cell activators including a memory B cell-inducing cocktail (MBCC) composed of Staphylococcus aureus protein A, pokeweed mitogen and CpG ODN2395, which preferentially activates memory B cells (15) (Fig. 1A). This is widely accepted (15), and we have confirmed it independently (data not shown). Note that we used a suboptimal concentration of CpG in the cocktail in order to more readily detect potential stimulatory effects of LXB4 on antibody production. After 7–8 days in culture, antibody production was measured by ELISA. LXB4 promoted antibody production from B cells stimulated with the MBCC but not other IgG inducing B cell activators tested including: CpG ODN2006 alone, CpG ODN2389 alone or IL-2/R848 cocktail (Fig. 1A). Importantly, LXB4 alone did not stimulate antibody production (Fig. 1B and Supplemental Figure 1). Although there was a variation among donors in the absolute magnitude of the antibody response, LXB4 significantly enhanced memory B cell IgG production in both PBMCs and in purified B cells upon stimulation with the MBCC in a concentration dependent manner (Fig. 1C–1D). To investigate the timing of the effect, LXB4 was added daily beginning on day 0 or on day 5 after stimulation with the MBCC. Interestingly, late addition of LXB4 did not increase antibody secretion (Supplemental Figure 2). We also found no significant effect of LXB4 on IgM or IgE antibody production (Fig. 1E–1F). Thus, LXB4 enhances IgG production by human memory B cells.
LXB4 enhances production of IgG in B cells from donors recently vaccinated against influenza virus
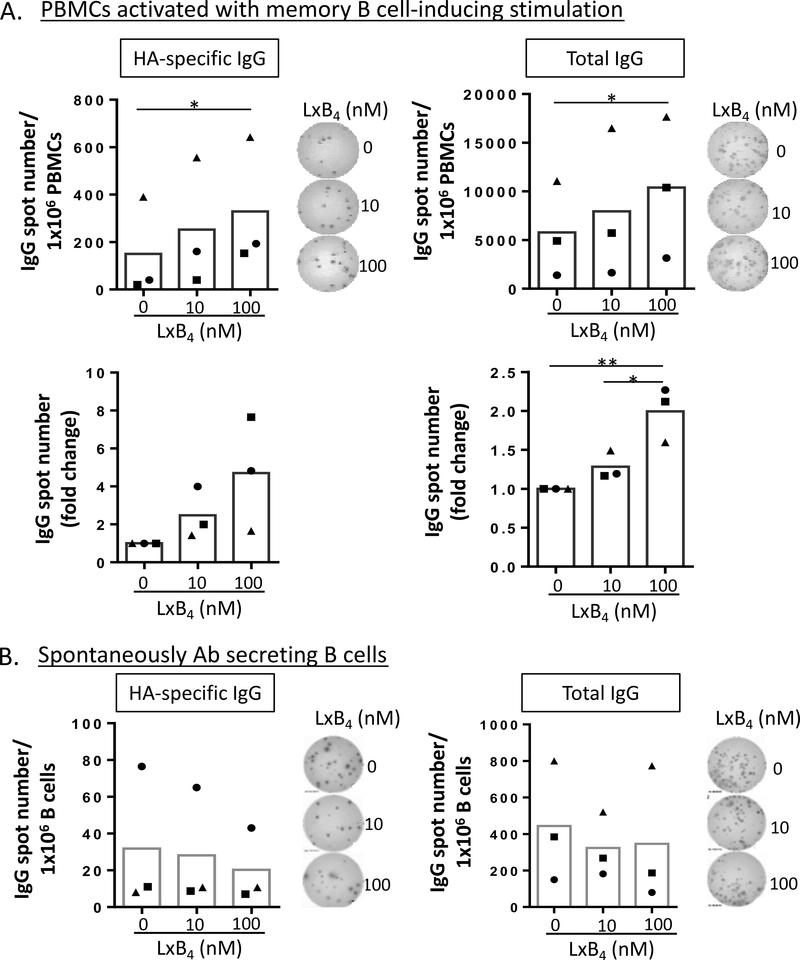
To further investigate the effects of LXB4 on antigen-specific memory B cells, we recruited donors recently vaccinated against influenza virus. Vaccination not only induces the formation of new antigen-specific memory cells and long lived plasma cells, but also re-boosts previously formed memory cells. Blood was collected 7 days after vaccination with either the 2014 trivalent vaccine or the 2015 quadrivalent vaccine, both of which include HA protein from A/California/7/2009-like virus. PBMCs from vaccinated donors were isolated and pretreated with LXB4, followed by stimulation with the MBCC and cultured for 7–8 days. The number of HA-specific IgG producing B cells or total IgG-secreting cells were counted using ELISpot assay. LXB4 increased the number of B cells secreting total or HA-specific IgG upon memory B cell-inducing stimulation (Fig. 2A). Similar to our results with total IgG levels (Figure 1), pretreatment with LXB4 resulted in a ~2-fold increase in the number of total IgG-secreting B cells (Fig. 2A). Approximately 4% of total memory B cells secreted anti-HA IgG. By taking advantage of the observation that recently vaccinated donors have high numbers of blood circulating antigen-specific antibody secreting cells in the circulation (16), we also investigated whether LXB4 could stimulate antibody production from these spontaneously IgG-secreting cells. Seven days after vaccination, blood was collected and CD19+ B cells were isolated. B cells were treated with LXB4 without additional stimulation for 24 hours, transferred to ELISpot plates and incubated a further 24 hours. There were no significant differences in the number of either HA-specific or total IgG secreting cells with LXB4 treatment (Fig. 2B). Since LXB4 does not affect antibody production by spontaneous antibody secreting B cells but does enhance antibody secretion in total PBMCs, this suggests that LXB4 may prime memory B cells to respond to activation signals, but does not further augment antibody secretion in activated B cells.
Figure 2. LXB4 promotes reactivation of memory B cells from donors recently vaccinated against influenza virus.
PBMCs (A), or CD19+ B cells (B) were isolated from donors 7 days after influenza vaccination. PBMCs were pretreated with LXB4, followed by stimulation with the memory B cell-inducing cocktail for 7–8 days (A). B cells pretreated with LXB4 without stimulation were cultured for 24 hours (B). The last day, PBMCs or B cells were transferred to anti-IgG- or HA (from A/California/7/2009)-coated ELISpot plates and further cultured overnight. The number of spots representing the number of ASC was calculated using an ImmunoSpot Series 5 Analyzer. The absolute number of HA-specific or total IgG secreting cells or the fold change induced by LXB4 are shown. Representative image from ELISpot assay is also shown. Each symbol represents an individual donor and represents means of at least triplicate cultures. Data analyzed by RM one-way ANOVA with Tukey’s posttest, *p≤0.05.
LXB4 enhances the expression of transcription factors important for plasma cell differentiation
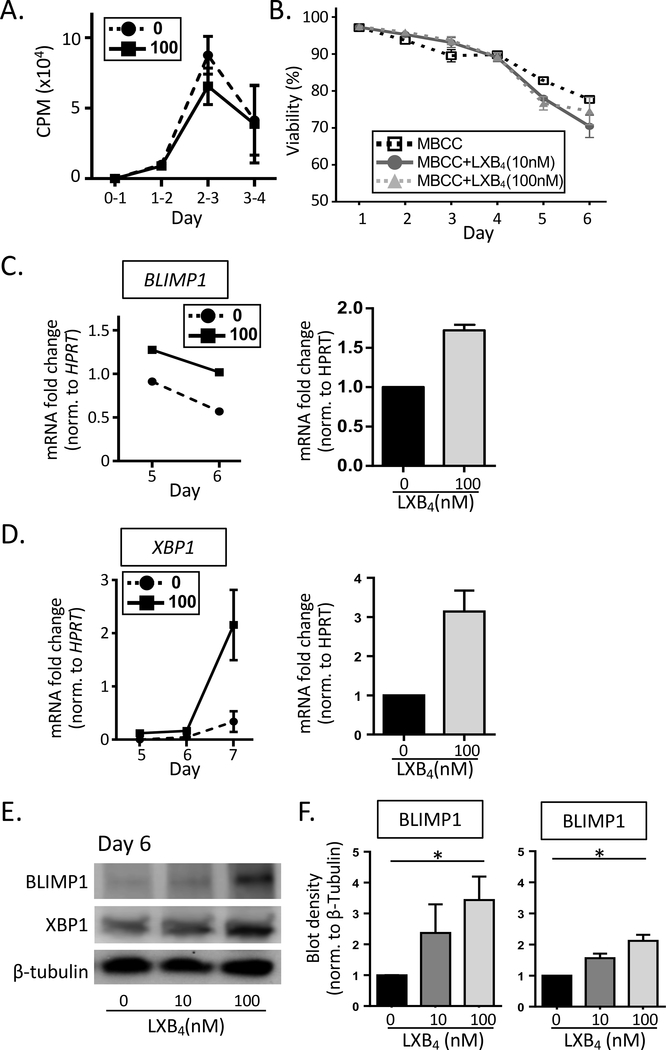
We next investigated whether or not the increase in the number of antibody-secreting cells (ASC) induced by LXB4 was a result of increased memory B cell proliferation, cell viability or differentiation. Cell proliferation was assessed by measuring 3[H]-thymidine incorporation rate in B cells pretreated with LXB4. B cell proliferation peaked on day 3 after stimulation and there were no significant difference with LXB4 treatment (Fig. 3A). LXB4 also had no significant effect on B cell survival as determined by 7-AAD staining (Fig. 3B). Next, we measured the expression of BLIMP1 and XBP1, two key transcription factors important in plasma cell differentiation. Human B cell differentiation is a well-orchestrated process with an induction of BLIMP1 that suppresses the expression of genes involved in proliferation and class switching, while inducing a different set of genes that regulate antibody secretion, one of which is XBP1 (17, 18). Here, we assessed both mRNA levels and total protein levels of BLIMP1 and XBP1 in MBCC treated B cells with or without LXB4. BLIMP1 mRNA was detected starting at day 4 (data not shown), and peaked at day 5, whereas XBP1 lags behind BLIMP1 and peaked at day 7 (Fig. 3C-D). LXB4 increased both BLIMP1 and XBP1 mRNA levels in MBCC stimulated B cells (Fig. 3C-D). To confirm that the increase in BLIMP1 and XBP1 mRNA levels with LXB4 treatment resulted in increased protein expression, we measured BLIMP1 and XBP1 protein using Western blot analysis. B cells were pretreated with LXB4, and then stimulated for 6 days, when cell lysates were collected. LXB4 strongly enhanced the total protein expression in both BLIMP1 and XBP1 (Fig. 3E-F). These results show that LXB4 promotes increased differentiation of memory B cells into antibody secreting plasma cells via upregulation of BLIMP1 and XBP1.
Figure 3. LXB4 enhances the expression of two transcription factors important for plasma cell differentiation.
CD19+ B cells from healthy individuals were pretreated with LXB4, and then stimulated with the memory B cell-inducing cocktail (MBCC )for the number of days indicated. (A) [3H] Thymidine incorporation assay in B cells pretreated with or without LXB4 (100nM), stimulated with memory B cell-inducing activators is done at different time points. Results shown are mean±S.D. for triplicate wells from one representative donor of 3 donors tested. (B) B cell viability with LXB4 treatment was measured at different time points (day 1–6) in B cells from 3 different donors using 7-AAD staining and flow cytometry, mean±S.D. is shown. The effects of LXB4 on BLIMP1 and XBP1 mRNA levels (C-D) or total protein levels (E-F) were measured using RT-qPCR or western blot, respectively. (C-D) Normalized expression of BLIMP1 and XBP1 mRNA with or without LXB4 (100 nM) were measured and shown for one representative donor. HPRT was used as a control. Each qPCR reaction was run in triplicate and the results shown are mean±SEM for 3 different donors. (E-F) Changes in total protein levels of BLIMP1 and XBP1 at day 6 post-stimulation were measured using western blot. β-tubulin was used as a control. (E) One representative western blot of 3 donors tested. (F) Densitometry for 3 donors (mean±SEM), samples normalized to vehicle control (no treatment). Data analyzed by repeated measures one-way ANOVA with Tukey’s posttest, *p≤0.05.
LXB4 enhances the expression of cyclooxygenase-2 (COX2) in human B cells
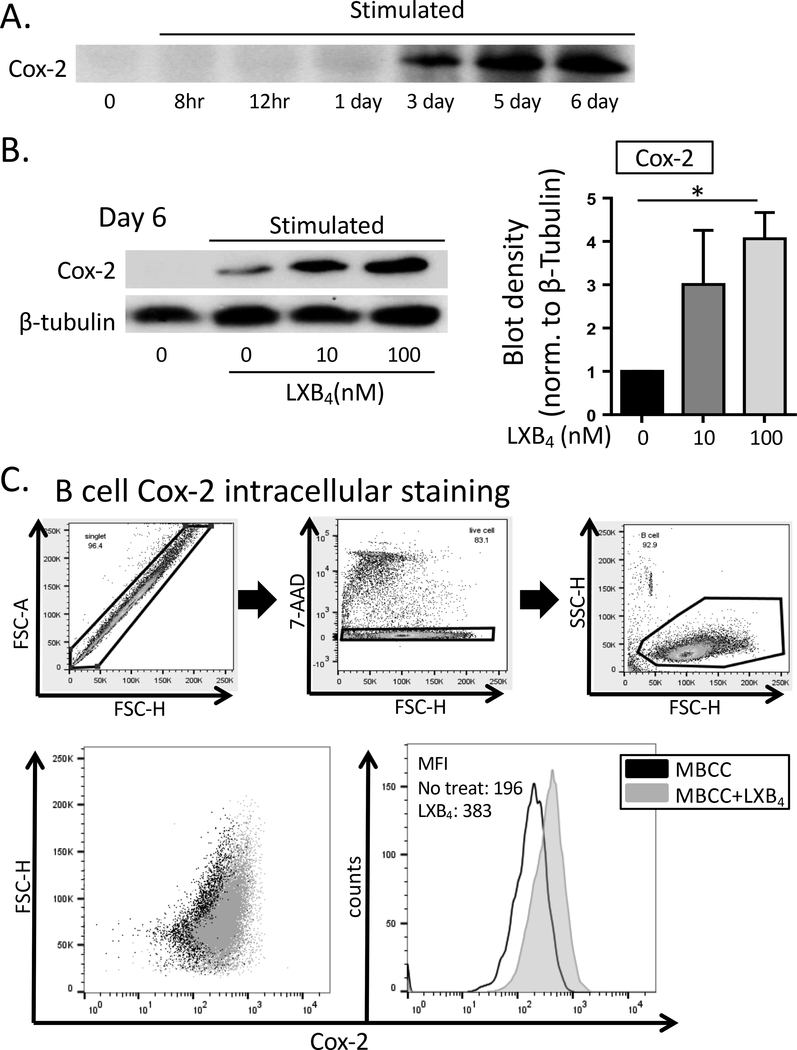
Previously, our lab has shown that human B cells express cyclooxygenase-2 (COX2) upon stimulation, and that this is very important for optimal B cell proliferation, differentiation and antibody production (12–14). COX2 increases B cell differentiation by promoting expression of BLIMP1 and XBP1, as the expression of these transcription factors was reduced in B cells treated with specific COX2 inhibitors (12). Here, we investigated whether LXB4 promoted COX2 expression, as a potential intermediate in the upregulation of plasma cell differentiation by LXB4. First, we measured COX2 expression in human B cells stimulated with the MBCC. COX2 protein levels peaked at day 5–6 after addition of MBCC (Fig. 4A), concurrent with the peak of BLIMP1 and XBP1 expression as shown in Figure 3. To measure the effects of LXB4 on COX2 expression, B cells were pretreated with LXB4, followed by stimulation with the MBCC, and COX2 protein expression was measured at different time points. Surprisingly, LXB4 significantly increased COX2 protein expression level at day 6 post-stimulation (Fig. 4B). Densitometry analysis done in 4 different donors indicated a 4-fold increase in COX2 protein levels when cells were treated with 100 nM LXB4 compared with vehicle alone (Fig. 4B). Flow cytometry using intracellular staining for COX2 confirmed the increased expression with LXB4 treatment (Fig. 4C). Finally, to determine whether COX2 is required for the antibody-stimulatory effects of LXB4, we co-treated MBCC activated B cells with LXB4 and specific inhibitors of COX2 activity. Cells were treated with either 10 μM celecoxib or 10 μM NS-398, selective COX2 inhibitors (Fig. 5A). Both COX2 inhibitors blocked the stimulatory activity of LXB4 on IgG production (Fig. 5A). Additional dose-response testing was conducted in multiple individual donors (Fig. 5B). Lower doses of celecoxib attenuate the effect of LXB4 in all 4 donors (Fig. 5A and 5B) while 20 μM celecoxib blocks all IgG production in the three donors in which this dose was used (Fig. 5B). These doses are consistent with our previously reported results (12, 13, 19, 20). Overall, these results strongly support the concepts that COX2 activity is required for optimal antibody production in B cells and that LXB4 acts upstream of COX2 to promote IgG secretion by memory B cells.
Figure 4. LXB4 enhances cyclooxygenase-2 (COX2) expression in B cells.
CD19+ B cells from healthy individuals were pretreated with LXB4, and then stimulated with the memory B cell-inducing cocktail (MBCC) for the indicated length of time. (A) The kinetics of COX2 protein expression in B cells stimulated with the memory B cell-inducing cocktail without any other treatments was assessed using western blot. One representative western blot is shown of 3 donors tested. (B) Changes in total COX2 protein levels with LXB4 were measured at day 6 post-stimulation in 4 different donors. One representative western blot is shown, the densitometry is mean ± SEM for 4 donors. Data analyzed by RM one-way ANOVA with Tukey’s posttest, *p≤0.05. (C) B cells were subjected to intracellular staining for COX2 followed by flow cytometric analysis. The gating strategy is shown. The percentage of B cells expressing COX2 and mean fluorescence intensity for COX2 staining was determined. In the diagram, black dots represent B cells stimulated without any treatment, and gray dots represent B cells pretreated with LXB4 and stimulated. Data shown from one representative donor of 4 donors tested.
Discussion
Here, we show that LXB4 enhances IgG production in human B cells stimulated with a cocktail that preferentially activates memory B cells. This was shown in primary B cells from both healthy individuals and donors vaccinated against seasonal influenza virus. The stimulatory activity of LXB4 was mediated at least in part by increased expression of COX2, which in turn enhanced the production of IgG antibody and expression of transcription factors involved in the differentiation of memory B cells to plasmablasts. It should be noted that, although we focus on memory B cells in this work, we have not investigated the effects of LXB4 on antibody production by naïve B cells, which is the subject of future studies.
In healthy humans, memory B cells comprise 30–70% of blood circulating B cells. However, this depends on many different factors, one of which is age (21). When they encounter cognate antigens, memory B cells rapidly proliferate and become early responding plasmablasts (22, 23), or participate in further germinal center reactions and are subjected to somatic hypermutation to produce higher affinity antibodies (24). Here, B cells were stimulated with pokeweed mitogen, CpG ODN (a TLR9 ligand) and Staphylococcus protein A, an IgG ligand; a cocktail previously reported to preferentially activate memory B cells (15). This MBCC stimulated IgG secretion in both PBMCs and purified B cells from healthy donors, which effect was enhanced 2–3 fold by the addition of LXB4 (Fig. 1). LXB4 also enhanced secretion of anti-influenza antibodies by B cells from recently vaccinated donors (Fig. 2). We picked a specific time point (day 7 post-vaccination) to collect enough numbers of antigen-specific memory B cells to test the effects of LXB4. LXB4 induced a ~2-fold increase in the number of IgG+ memory B cells differentiated into antibody secreting cells, and a similar ~2-fold increase in the amount of total IgG produced (Fig. 2). This suggested that LXB4 increased the frequency of memory B cell differentiation to ASCs rather than increasing the amount of IgG secreted per cell. However, it was interesting to see that the magnitude of an increase in the number of HA-specific IgG-secreting cells varied among donors, whereas increases in the number of total IgG-secreting cells were consistent. This might be due to different percentages of HA-specific memory B cells in total IgG+ memory B cells among donors, which could be affected by their individual vaccination and influenza infection histories (25). It is also possible that the effect of LXB4 is more specific to memory B cells against respiratory viral antigens, which could be further investigated in the future.
Upon re-activation, memory B cells undergo a proliferative burst, and then differentiate into ASCs. LXB4 did not affect proliferation (Fig 4A, B), but enhanced ASC differentiation via upregulation key transcription factors involved in plasma cell differentiation, namely, BLIMP1 and XBP1 (Fig. 4C-E) (26, 27). It is likely that LXB4 increases the number of B cells that express BLIMP1 and XBP1, leading to higher numbers of IgG-producing ASCs as shown in Fig. 2A-B. BLIMP1 is essential for efficient plasma cell formation and is needed for plasma cells that produce IgM, IgG and IgE class antibodies (27). BLIMP1 promotes XBP1 expression and the unfolded protein response, both necessary for antibody production and secretion, so it is not surprising that XBP1 is also increased by LXB4 (27–29).
Our studies herein show that the stimulatory effect of LXB4 on B cell antibody production was mediated by increasing the expression of COX2. Importantly, and consistent with earlier studies on COX2 and antibody production, the effect of LXB4 could be attenuated by treating B cells with the selective COX2 inhibitors, celecoxib and NS-398. Our previous work and studies by others have shown that expression of COX2 significantly increases antibody production (30–32). Cyclooxygenases (COX1 and COX2) are enzymes that regulate inflammation, at least in part, through assisting in the production of prostaglandin lipid mediators such as prostaglandin E2 (PGE2). COX1 is a constitutive enzyme with basal activity patterns whereas COX2 is a robustly inducible enzyme leading to high levels of activity. In general, COX2 is known to promote proliferation and inhibit apoptosis in various cell types (33). In B cells, COX2 inhibition using COX2 selective inhibitors such as celecoxib and NS-398 or pan-cyclooxygenase inhibitors such ibuprofen reduced B cell proliferation and markedly inhibited IgG production (12, 13, 19), while COX2 knockout mice produce significantly less IgG1, IgG2a and IgG3 antibodies in response to HPV infection (34). The fact that upregulation of COX2 expression leads to increased BLIMP1 and XBP1 suggests that one or more COX2 products plays an undiscovered role in promoting antibody production.
The timing of the effect of LXB4 is especially intriguing. LXB4 enhances antibody production when given beginning at day 0 but not day 5 of the 7–8 day in vitro assay (Supplemental Figure 2), and LXB4 has no stimulatory effect on ASCs from recently vaccinated donors that spontaneously secret anti-influenza antibodies (Fig. 2B). However, COX2, BLIMP1 and XBP1 are elevated at days 3–7 (Fig. 4). LXB4 helps differentiate memory B cells into ASC, but is not sufficient to do so in the absence of other activators. This suggests that early exposure to LXB4 primes memory B cells to more efficiently differentiate to plasmablasts. Additional studies to understand how early exposure to LXB4 leads to these delayed effects may have to await the identification and characterization of the LXB4 receptor.
Our data also shows that the MBCC cocktail stimulated production of IgM antibodies as well as IgG, but that LXB4 does not enhance production of IgM. This suggests that the mechanism of action of LXB4 may be specific for IgG, even though Blimp-1, Xbp-1 and Cox-2 are not thought to be specific for IgG; or that LXB4 may promote class-switching of IgM memory B cells to IgG ASCs. We can’t rule out that LXB4 promotes class switch to IgG, and the possibility that LXB4 might promote IgG class-switching is suggested by Figure 1F, showing a non-significant trend toward decreased IgM production with LXB4. However, prior literature suggests that the majority of memory B cells are IgG class-switched (35, 36), and that CpG preferentially activates IgG B cells over IgM B cells (37, 38), so it may be that we are just not activating IgM memory B cells in an optimal way to see an enhancing effect of LXB4. Additionally, increased expression of BLIMP1 will tend to suppress class switching. Alternatively, the priming effect of LXB4 may be specific to IgG by an unknown mechanism. We recently reported that the SPMs 17-hydroxyeicosahexaenoic acid (17-HDHA) and resolvin D1 (RvD1) selectively inhibit IgE production by limiting STAT6 occupancy on the epsilon germline transcript promoter, which is required for IgE class switch (39). Thus, there is precedent for the idea that specific SPMs can regulate specific Ig isotypes. Future studies using an IgM-specific B cell activation cocktail may be of interest.
We report here that LXB4 enhanced memory B cell IgG production upon re-activation, which is mediated by enhanced expression of COX2. LXB4 might have efficacy when added as a second adjuvant to immunizations such as seasonal influenza, where memory B cells induced by previous vaccinations or infections play important roles in responding to new infections with antigenically similar strains. Although the receptor for LXB4 is currently unknown, some studies have reported similar anti-inflammatory and pro-resolving effects for LXA4 and LXB4 (40–42). The role of LXB4 in chronic inflammatory diseases remains unclear (43, 44), and further study is needed to understand whether LXB4 could have clinical utility in enhancing vaccine efficacy or boosting memory responses in patients.
Supplementary Material
Acknowledgement
We would like to thank the University of Rochester Medical Center Blood Bank for collecting blood samples.
Grant support
This work was supported in part by NIH grants T90DE021985, R21AI103690, T32HL066988 and by the Mary Parkes Center for Asthma, Allergy and Pulmonary Care.
References
- 1.Chung EH 2014. Vaccine allergies. Clin Exp Vaccine Res 3: 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mulligan MJ, Bernstein DI, Winokur P, Rupp R, Anderson E, Rouphael N, Dickey M, Stapleton JT, Edupuganti S, Spearman P, Ince D, Noah DL, Hill H, Bellamy AR, and Group DHNVS 2014. Serological responses to an avian influenza A/H7N9 vaccine mixed at the point-of-use with MF59 adjuvant: a randomized clinical trial. JAMA 312: 1409–1419. [DOI] [PubMed] [Google Scholar]
- 3.Serhan CN 2007. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol 25: 101–137. [DOI] [PubMed] [Google Scholar]
- 4.Serhan CN, Hamberg M, and Samuelsson B 1984. Lipoxins: novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proc Natl Acad Sci U S A 81: 5335–5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, and Serhan CN 2005. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med 201: 713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, and Spite M 2009. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J Exp Med 206: 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramon S, Gao F, Serhan CN, and Phipps RP 2012. Specialized proresolving mediators enhance human B cell differentiation to antibody-secreting cells. J Immunol 189: 1036–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramon S, Baker SF, Sahler JM, Kim N, Feldsott EA, Serhan CN, Martinez-Sobrido L, Topham DJ, and Phipps RP 2014. The specialized proresolving mediator 17-HDHA enhances the antibody-mediated immune response against influenza virus: a new class of adjuvant? J Immunol 193: 6031–6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramon S, Bancos S, Serhan CN, and Phipps RP 2014. Lipoxin A(4) modulates adaptive immunity by decreasing memory B-cell responses via an ALX/FPR2-dependent mechanism. Eur J Immunol 44: 357–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fierro IM, and Serhan CN 2001. Mechanisms in anti-inflammation and resolution: the role of lipoxins and aspirin-triggered lipoxins. Braz J Med Biol Res 34: 555–566. [DOI] [PubMed] [Google Scholar]
- 11.Karra L, Haworth O, Priluck R, Levy BD, and Levi-Schaffer F 2015. Lipoxin B(4) promotes the resolution of allergic inflammation in the upper and lower airways of mice. Mucosal Immunol 8: 852–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernard MP, and Phipps RP 2010. Inhibition of cyclooxygenase-2 impairs the expression of essential plasma cell transcription factors and human B-lymphocyte differentiation. Immunology 129: 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernard MP, Bancos S, Chapman TJ, Ryan EP, Treanor JJ, Rose RC, Topham DJ, and Phipps RP 2010. Chronic inhibition of cyclooxygenase-2 attenuates antibody responses against vaccinia infection. Vaccine 28: 1363–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernard MP, and Phipps RP 2007. CpG oligodeoxynucleotides induce cyclooxygenase-2 in human B lymphocytes: implications for adjuvant activity and antibody production. Clin Immunol 125: 138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crotty S, Aubert RD, Glidewell J, and Ahmed R 2004. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J Immunol Methods 286: 111–122. [DOI] [PubMed] [Google Scholar]
- 16.Santiago FW, Fitzgerald T, Treanor JJ, and Topham DJ 2011. Vaccination with drifted variants of avian H5 hemagglutinin protein elicits a broadened antibody response that is protective against challenge with homologous or drifted live H5 influenza virus. Vaccine 29: 8888–8897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin KI, Angelin-Duclos C, Kuo TC, and Calame K 2002. Blimp-1-dependent repression of Pax-5 is required for differentiation of B cells to immunoglobulin M-secreting plasma cells. Mol Cell Biol 22: 4771–4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martins G, and Calame K 2008. Regulation and functions of Blimp-1 in T and B lymphocytes. Annu Rev Immunol 26: 133–169. [DOI] [PubMed] [Google Scholar]
- 19.Ryan EP, Pollock SJ, Murant TI, Bernstein SH, Felgar RE, and Phipps RP 2005. Activated human B lymphocytes express cyclooxygenase-2 and cyclooxygenase inhibitors attenuate antibody production. J Immunol 174: 2619–2626. [DOI] [PubMed] [Google Scholar]
- 20.Bancos S, Bernard MP, Topham DJ, and Phipps RP 2009. Ibuprofen and other widely used non-steroidal anti-inflammatory drugs inhibit antibody production in human cells. Cell Immunol 258: 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bancos S, and Phipps RP 2010. Memory B cells from older people express normal levels of cyclooxygenase-2 and produce higher levels of IL-6 and IL-10 upon in vitro activation. Cell Immunol 266: 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshida T, Mei H, Dorner T, Hiepe F, Radbruch A, Fillatreau S, and Hoyer BF 2010. Memory B and memory plasma cells. Immunol Rev 237: 117–139. [DOI] [PubMed] [Google Scholar]
- 23.Odendahl M, Mei H, Hoyer BF, Jacobi AM, Hansen A, Muehlinghaus G, Berek C, Hiepe F, Manz R, Radbruch A, and Dorner T 2005. Generation of migratory antigen-specific plasma blasts and mobilization of resident plasma cells in a secondary immune response. Blood 105: 1614–1621. [DOI] [PubMed] [Google Scholar]
- 24.Kurosaki T, Kometani K, and Ise W 2015. Memory B cells. Nat Rev Immunol 15: 149–159. [DOI] [PubMed] [Google Scholar]
- 25.Andrews SF, Huang Y, Kaur K, Popova LI, Ho IY, Pauli NT, Henry Dunand CJ, Taylor WM, Lim S, Huang M, Qu X, Lee JH, Salgado-Ferrer M, Krammer F, Palese P, Wrammert J, Ahmed R, and Wilson PC 2015. Immune history profoundly affects broadly protective B cell responses to influenza. Sci Transl Med 7: 316ra192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu CC, Dougan SK, McGehee AM, Love JC, and Ploegh HL 2009. XBP-1 regulates signal transduction, transcription factors and bone marrow colonization in B cells. EMBO J 28: 1624–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tellier J, Shi W, Minnich M, Liao Y, Crawford S, Smyth GK, Kallies A, Busslinger M, and Nutt SL 2016. Blimp-1 controls plasma cell function through the regulation of immunoglobulin secretion and the unfolded protein response. Nat Immunol 17: 323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Z, Li A, Wang Z, Pei F, Xia Q, Liu G, Ren Y, and Hu Z 2013. Blimp-1 siRNA inhibits B cell differentiation and prevents the development of lupus in mice. Hum Immunol 74: 297–301. [DOI] [PubMed] [Google Scholar]
- 29.Calame KL, Lin KI, and Tunyaplin C 2003. Regulatory mechanisms that determine the development and function of plasma cells. Annu Rev Immunol 21: 205–230. [DOI] [PubMed] [Google Scholar]
- 30.Lee H, Trott JS, Haque S, McCormick S, Chiorazzi N, and Mongini PK 2010. A cyclooxygenase-2/prostaglandin E2 pathway augments activation-induced cytosine deaminase expression within replicating human B cells. J Immunol 185: 5300–5314. [DOI] [PubMed] [Google Scholar]
- 31.Mongini PK, Inman JK, Han H, Kalled SL, Fattah RJ, and McCormick S 2005. Innate immunity and human B cell clonal expansion: effects on the recirculating B2 subpopulation. J Immunol 175: 6143–6154. [DOI] [PubMed] [Google Scholar]
- 32.Mongini PK, Inman JK, Han H, Fattah RJ, Abramson SB, and Attur M 2006. APRIL and BAFF promote increased viability of replicating human B2 cells via mechanism involving cyclooxygenase 2. J Immunol 176: 6736–6751. [DOI] [PubMed] [Google Scholar]
- 33.Sobolewski C, Cerella C, Dicato M, Ghibelli L, and Diederich M 2010. The role of cyclooxygenase-2 in cell proliferation and cell death in human malignancies. Int J Cell Biol 2010: 215158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryan EP, Malboeuf CM, Bernard M, Rose RC, and Phipps RP 2006. Cyclooxygenase-2 inhibition attenuates antibody responses against human papillomavirus-like particles. J Immunol 177: 7811–7819. [DOI] [PubMed] [Google Scholar]
- 35.Coffman RL, and Cohn M 1977. The class of surface immunoglobulin on virgin and memory B lymphocytes. J Immunol 118: 1806–1815. [PubMed] [Google Scholar]
- 36.Kurosaki T, Aiba Y, Kometani K, Moriyama S, and Takahashi Y 2010. Unique properties of memory B cells of different isotypes. Immunol Rev 237: 104–116. [DOI] [PubMed] [Google Scholar]
- 37.Hartmann G 2003. CpG: unraveling the key to B-cell function. Blood 101: 4230–4231. [Google Scholar]
- 38.Marasco E, Farroni C, Cascioli S, Marcellini V, Scarsella M, Giorda E, Piano Mortari E, Leonardi L, Scarselli A, Valentini D, Cancrini C, Duse M, Grimsholm O, and Carsetti R 2017. B-cell activation with CD40L or CpG measures the function of B-cell subsets and identifies specific defects in immunodeficient patients. Eur J Immunol 47: 131–143. [DOI] [PubMed] [Google Scholar]
- 39.Kim N, Ramon S, Thatcher TH, Woeller CF, Sime PJ, and Phipps RP 2016. Specialized proresolving mediators (SPMs) inhibit human B-cell IgE production. Eur J Immunol 46: 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Sullivan TP, Vallin KS, Shah ST, Fakhry J, Maderna P, Scannell M, Sampaio AL, Perretti M, Godson C, and Guiry PJ 2007. Aromatic lipoxin A4 and lipoxin B4 analogues display potent biological activities. J Med Chem 50: 5894–5902. [DOI] [PubMed] [Google Scholar]
- 41.Ariel A, Chiang N, Arita M, Petasis NA, and Serhan CN 2003. Aspirin-triggered lipoxin A4 and B4 analogs block extracellular signal-regulated kinase-dependent TNF-alpha secretion from human T cells. J Immunol 170: 6266–6272. [DOI] [PubMed] [Google Scholar]
- 42.Serhan CN, Takano T, Clish CB, Gronert K, and Petasis N 1999. Aspirin-triggered 15-epi-lipoxin A4 and novel lipoxin B4 stable analogs inhibit neutrophil-mediated changes in vascular permeability. Adv Exp Med Biol 469: 287–293. [DOI] [PubMed] [Google Scholar]
- 43.Croasdell A, Thatcher TH, Kottmann RM, Colas RA, Dalli J, Serhan CN, Sime PJ, and Phipps RP 2015. Resolvins attenuate inflammation and promote resolution in cigarette smoke-exposed human macrophages. Am J Physiol Lung Cell Mol Physiol 309: L888–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elajami TK, Colas RA, Dalli J, Chiang N, Serhan CN, and Welty FK 2016. Specialized proresolving lipid mediators in patients with coronary artery disease and their potential for clot remodeling. FASEB J 30: 2792–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.