Abstract
Drosophila dEAAT2, a member of the excitatory amino-acid transporter (EAAT) family, has been described as mediating the high-affinity transport of taurine, which is a free amino-acid abundant in both insects and mammals. However, the role of taurine and its transporter in hearing is not clear. Here, we report that dEAAT2 is required for the larval startle response to sound stimuli. dEAAT2 was found to be enriched in the distal region of chordotonal neurons where sound transduction occurs. The Ca2+ imaging and electrophysiological results showed that disrupted dEAAT2 expression significantly reduced the response of chordotonal neurons to sound. More importantly, expressing dEAAT2 in the chordotonal neurons rescued these mutant phenotypes. Taken together, these findings indicate a critical role for Drosophila dEAAT2 in sound transduction by chordotonal neurons.
Electronic supplementary material
The online version of this article (10.1007/s12264-018-0255-1) contains supplementary material, which is available to authorized users.
Keywords: Drosophila dEAAT2, Taurine, Chordotonal neurons, Sound transduction
Introduction
Taurine (2-aminoethane-sulfonic acid) is one of the most abundant free amino-acids in mammals [1]. Although it is not incorporated into proteins, taurine has various physiological functions, such as osmoregulation, neuroprotection, Ca2+ modulation, antioxidant defense, and membrane stabilization [1–3]. It also plays a role in neural development [4, 5]. Several studies have shown that taurine plays an important role in the functional development of the auditory system [6, 7]. Taurine supplementation in the diet has an effect on hearing development in preterm infants [8–11]. In addition, taurine acts as a neuromodulator. In the central auditory pathway, it reduces neuronal excitability and depresses synaptic transmission in the inferior colliculus by activating glycine receptors [12, 13]. Moreover, it acts as a neuromodulator to strengthen glycinergic and GABAergic neurotransmission in rat anteroventral cochlear nucleus neurons [14]. In the cochlea, taurine is abundant in supporting cells [15–17], but its role in the peripheral auditory pathway remains unclear.
In mammals, a high intracellular taurine level is maintained by a Na+- and Cl–-dependent taurine transporter (TauT/SLC6A). Taurine transporter knockout (taut−/−) mice show a strongly reduced taurine level [18] and this occurs in various diseases, including retinal degeneration, reduced olfactory function, unspecific hepatitis, and liver fibrosis [19]. However, the function of the TauT in hearing remains poorly understood.
Excitatory amino-acid transporters (EAATs) are membrane proteins that mediate the clearance of glutamate, aspartate, and other molecules released during synaptic transmission in the nervous system [20, 21]. Previous studies have shown that the glutamate/aspartate transporter (GLAST; EAAT1), which occurs in the mammalian organ of Corti [22, 23], is the main glutamate transporter involved in the modulation of auditory transmission between inner hair cells and spiral ganglion neurons [24]. To date, two EAAT members have been identified in Drosophila: dEAAT1 and dEAAT2 [25]. dEAAT1 is a glutamate transporter and plays an essential role in regulating locomotion in Drosophila [26, 27]. dEAAT2 is the only taurine transporter identified in Drosophila [28]. A previous study has shown that disrupted dEAAT2 expression significantly reduces the taurine level [29]. However, the role of dEAAT2 in hearing remains elusive. The fruit fly D. melanogaster is a prominent model system in neuroscience [30–32]. The chordotonal organs are specialized for hearing in most insects including Drosophila [33]. Studies have shown that the chordotonal organs share transduction mechanisms similar to vertebrate hair cells [34, 35]. In adult Drosophila, the chordotonal neurons of Johnston’s organ in the second antennal segment mediate hearing as well as sensing gravity and wind [36, 37]. We previously reported that larval chordotonal neurons, like their counterparts in adult Drosophila, are required for sound transduction [38]. Thus, Drosophila is a suitable model in which to study the function of dEAAT2 in hearing.
Therefore, in the present study we explored the role of Drosophila dEAAT2 in larval chordotonal neurons in sound transduction.
Materials and Methods
Drosophila Stocks
All flies were raised on a standard cornmeal medium at 25 °C with 60% relative humidity under a 12 h light/dark cycle. The following strains were used: w1118, IAV-Gal4, NOMPC-Gal4, UAS-GFP, UAS-CD8-GFP, UAS-GCaMP6, UAS-CD4-tdTomato, dEaat2MI (stock #36215), dEAAT2-Gal4, and UAS-dEAAT2-EGFP. All the fly lines, except for dEAAT2-Gal4, UAS-dEAAT2-EGFP, UAS-GCaMP6, and UAS-CD4-tdTomato, were from the Bloomington Drosophila Stock Center at Indiana University (Bloomington, IN). The UAS-GCaMP6 and UAS-CD4-tdTomato strains were gifts from Yuh-Nung Jan (University of California, San Francisco, CA) [39]. The dEAAT2-Gal4 and UAS-dEAAT2-EGFP transgenic lines were constructed in our laboratory.
Generation of Transgenic Flies and Confirmation of the dEaat2MI Mutation
The dEAAT2-Gal4 was generated by amplifying the Drosophila dEAAT2 promoter region from the genomic DNA of w1118 via the polymerase chain reaction (PCR). The following primers with specific restriction sites were used: forward 5′-GAATTCGGCAACCTTTGGCGGACTCCCCATCAA-3′; reverse 5′-GGATCCTGTCCAAATGTCCAAGCTTCCGGGTG-3′.
And then the PCR fragment was inserted into the EcoR III/BamH I sites located before the GAL4 sequence in the pCaSpeR-GAL4 vector.
To generate the UAS-dEAAT2-EGFP construct, dEAAT2 cDNA was prepared by RT-PCR. Then the dEAAT2-coding sequence was amplified by PCR using the following primers with specific restriction sites: forward 5′-GAATTCATGGGTCCCCCCACCTCAACTG-3′; reverse 5′-GGTACCGACCCTGCGGTTACAATGCTCCT-3′.
The reverse primer was designed to remove the stop codon from the dEAAT2 sequence. The purified PCR products were cloned into a pUAST-EGFP vector at the EcoR I and Kpn I sites. The UAS-dEAAT2-EGFP and dEAAT2-Gal4 recombinant plasmids were then injected into w1118 embryos. Transgenic flies were obtained by P-element-mediated germ-line transformations.
We confirmed the presence of the Minos element insertion in dEaat2MI strain DNA by PCR using the following primers: Primer 1: 5′-GTTCGTCAGCATCAGCTCTATAGTCCC-3′; Primer 2: 5′-TAGGATCCGTTGACCTGCAGGTCGA-3′; Primer 3: 5′-GTATTCAGCGTATGCGTCCGCGTAC-3′.
We also examined the transcript levels of dEAAT2 in the dEaat2MI strain using quantitative reverse-transcription PCR. Total RNA was extracted from 10 larvae using TRIzol reagent (Thermo Fisher Scientific, Shanghai, China). The cDNA was synthesized using HiScript® Reverse Transcriptase (Vazyme, Nanjing, China) following the manufacturer’s instructions. After an 8-fold dilution of cDNA, we performed real-time quantitative PCR using ChamQ™ SYBR® qPCR Master Mix (Vazyme, Nanjing, China) and the CFX96 Real-time System (Bio-Rad, Hercules, CA). Actin 5C (Primers: 5′-TGTGACGAAGAAGTTGCTGC and 5′-CTCATCACCCACGTACGAGT) was used as an internal control. Relative quantification was calculated using the 2−∆Ct formula. The primers for dEaat2 were as follows: Primer 4: 5′-AAGCTCGATTGTGATGGGGT-3′; Primer 5: 5′-CTCATCACCCACGTACGAGT-3′.
Behavioral Tests
Startle Response Assay
Each third instar larva was carefully picked up and washed twice with phosphate-buffered saline (PBS). Then groups of 20 larvae were gently transferred to a 2% agar plate, which was placed on top of a loudspeaker. For the startle assay, custom software was written to use MATLAB to generate a sine tone of 500 Hz. The sound signal was amplified by the loudspeaker. The sound intensity was measured with a CEL-63x Series Sound Level Meter (Casella CEL, Kempston, UK) following the manufacturer’s instructions. At the same sound intensity, the freely-moving larvae were stimulated with a 2-s sound pulse repeated 3 times. When exposed to a sound, they exhibited startle behaviors: pausing, turning, mouthhook retraction, and backward locomotion. To evaluate the sound response, a larva was counted as responsive when it exhibited any of the above behaviors more than once in response to sound stimuli. The ratio of larvae that had startle responses was calculated for different sound intensities.
Touch Sensitivity Assay
All flies were raised at 25 °C in an incubator. Groups of 20 third-instar larvae were carefully picked up and washed with PBS. A single larva was then gently transferred to a 2% agar plate. During linear locomotion on the plate, the larva was gently touched with an eyelash on the anterior thoracic segment, and this was repeated 4 times. When touched, the larva stopped crawling, contracted the head, and then turned to either side or retracted. The behavioral response was scored as 0 for those with no response, 1 for stopping and hesitation, 2 for retracting the head or turning, and 3 for a wave of retraction. The sum of responses in 4 trials served as the touch response score, and wild-type (WT) larvae had a score of ~ 6.
In Vivo Ca2+ Imaging
Functional Ca2+ imaging can be used to study neurons in the peripheral nervous system [40, 41]. In vivo Ca2+ imaging of larval chordotonal neurons was performed as described previously [38]. In brief, a freely-moving third-instar larva was picked up and rinsed with PBS at room temperature (25 °C). The larva was then pressed between two coverslips with the body lateral side up. The imaging data were acquired on a Zeiss LSM510 confocal microscope (Zeiss, Jena, Germany) with a 20× objective lens. The genetically-coded Ca2+ indicator GCaMP6 was used to measure the Ca2+ signal. GCaMP6 and red fluorescent proteins were excited respectively by a 488-nm and a 543-nm laser, and the fluorescence signals were collected. The soma was selected to measure GCaMP6 fluorescence intensity. All recordings were from a lateral cluster of five (lch5) chordotonal neurons which are in the fifth abdominal segments (A5) of larvae. The average GCaMP6 signal from the first 5 s before the sound stimulus was taken as F0, and ∆F/F0 was calculated for each data point. GCaMP6 signals from the soma were analyzed.
Electrophysiological Recording
Extracellular recordings were made as previously described [38]. The third-instar larvae were dissected in a modified hemolymph-like saline (in mmol/L: NaCl 103, KCl 3, HEPES 5, trehalose 10, glucose 10, sucrose 7, NaHCO3 26, NaH2PO4 1, MgCl2 4, pH 7.25). Before use, 2 mmol/L Ca2+ (in the form of CaCl2) was added to the saline. Major muscles covering the chordotonal neurons were carefully removed under microscopy. Glass electrodes with a tip diameter of 10 μm for electrophysiological recordings were pulled with a P-97 puller (Sutter Instruments, Novato, CA) and filled with external solution. Recordings were made from vchA chordotonal neurons stimulated with a 1-s sound stimulus. Signals were amplified using an Axopatch-200B amplifier (Molecular Devices, Sunnyvale, CA) recorded at a sample rate of 10 kHz and low-pass filtered at 1 kHz. Data were acquired with a Digidata 1550B (Molecular Devices) acquisition system, and analyzed off-line using pClamp 10.4 software (Molecular Devices).
The extracellularly-recorded action potentials (APs) of vchA chordotonal neurons were detected by a threshold-based search of the single-unit recordings. A time-window of fixed length (1 s) was used before and during sound stimulation. The numbers of APs in the two time windows were calculated. To obtain the adaptation rate of chordotonal neurons, the post-stimulus time histograms for 6 neurons were averaged and fitted to a curve.
Statistical Analyses
All data were analyzed using Prism6 (GraphPad) and are presented as mean ± SEM. For behavioral data, the χ2 and two-tailed Mann–Whitney tests were used for significance testing. The two-tailed unpaired Student’s t test was used for significance testing in the Ca2+-imaging experiments. Two-tailed Mann–Whitney tests were used for the electrophysiological recordings. Statistical significance was defined as P < 0.05. All experiments were repeated three times.
Results
Drosophila dEAAT2 is Expressed in Larval Chordotonal Neurons
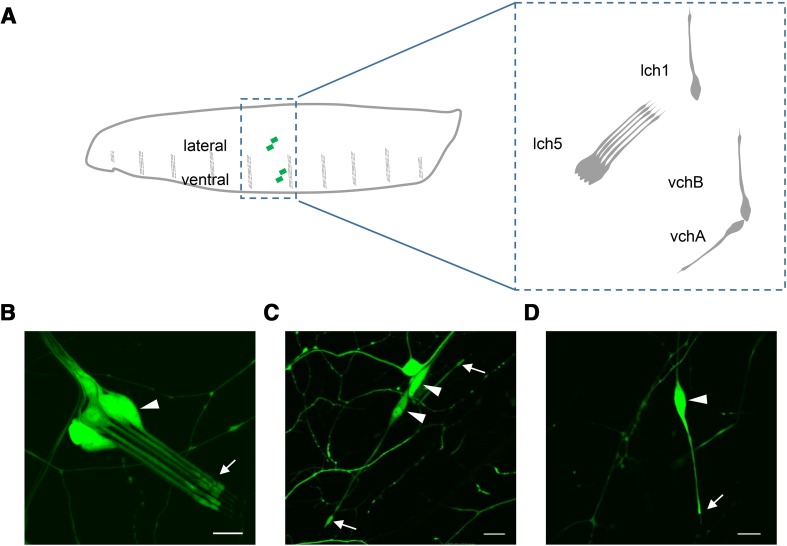
A previous study had shown that Drosophila dEAAT2 is expressed in larval peripheral sensory neurons, including a lateral cluster of five chordotonal neurons (lch5) and multidendritic neurons [29]. We previously reported that chordotonal neurons are required for the startle response of larvae to sound [38]. Larval chordotonal neurons contain three singlet chordotonal neurons (vchA, vchB, and lch1) and lch5 (Fig. 1A), and these neurons respond to sound with increased AP firing [38]. To explore the potential role of dEAAT2 in hearing, we first investigated whether it is expressed in larval auditory sensory organs by using the Gal4/UAS system. We generated a dEAAT2-Gal4 transgene containing a 4.4-kb genomic fragment upstream of the dEAAT2 translation initiation codon. The dEAAT2-Gal4 transgenic line was then used to drive the expression of green fluorescent protein (GFP). Similar to the previous study [29], GFP signals were detected in third-instar larval chordotonal and multidendritic neurons, including lch5 (Fig. 1B), the three singlet chordotonal neurons (vchA, vchB, and lch1) (Fig. 1C, D) and class I neurons (Fig. S1B).
Fig. 1.
dEAAT2 is expressed in chordotonal neurons. A Schematic of chordotonal neurons in an abdominal hemisegment of the larval body wall. Green diamonds mark the chordotonal neurons (left). The chordotonal neurons include three singlet neurons (vchA, vchB, and lch1) and a lateral cluster of five neurons (lch5) (right). B–D All four chordotonal neurons were labeled by dEAAT2-Gal4: lch5 (B), vchA and vchB (C), and lch1 (D). Arrowheads, cell bodies; arrows, terminal dendritic structures. Green indicates GFP signals. Scale bars, 10 μm.
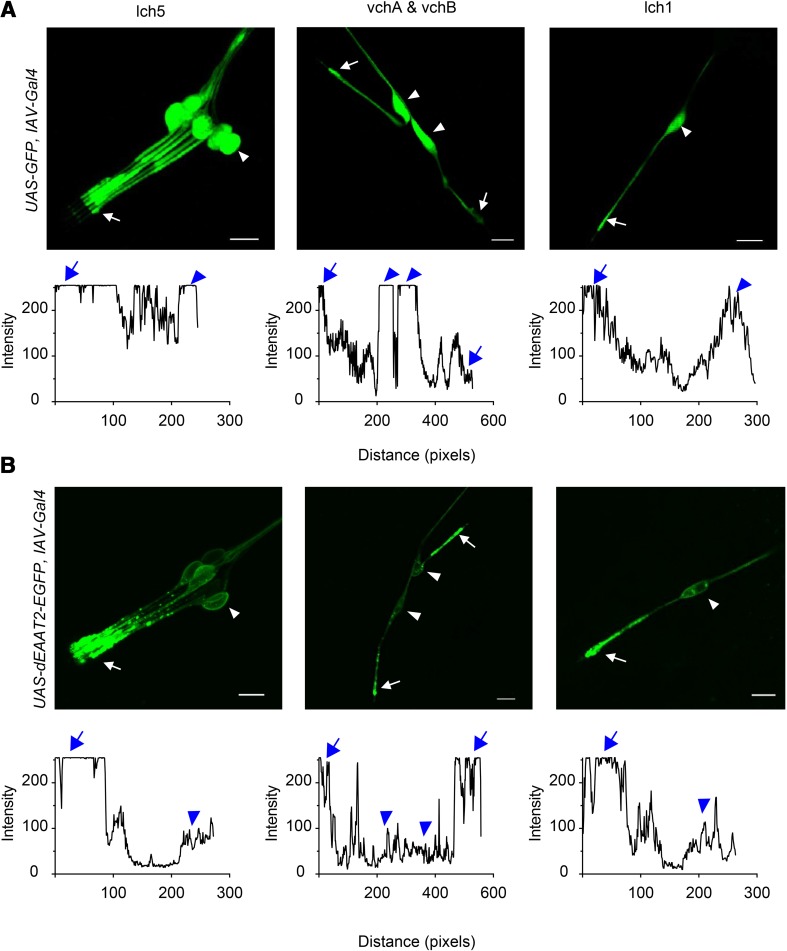
To further identify the subcellular localization of dEAAT2 in larval chordotonal neurons, we constructed a UAS-dEAAT2-EGFP transgenic line in which the enhanced GFP (EGFP) coding sequence was inserted into the C-terminal region of dEAAT2. IAV-Gal4 was then used to drive the expression of GFP or the dEAAT2::EGFP fusion protein in the larval chordotonal neurons. When expressing GFP alone, we observed a uniform distribution of GFP fluorescence in the chordotonal neurons (Fig. 2A). In contrast, when expressing the dEAAT2::EGFP fusion protein, we found that EGFP labeling was present in all regions of the chordotonal neurons, including somata, dendrites, and axon. The dEAAT2::EGFP fusion protein was enriched in the distal dendritic region of chordotonal neurons (Fig. 2B). Thus, it seems likely that dEAAT2 is localized to dendritic tips. In Drosophila larvae, the ciliated dendrite is bathed in K+-enriched receptor lymph secreted by the supporting cell [42, 43] and plays an important role in mechanotransduction [44]. These results indicate that dEAAT2 is involved in the sound sensation of larvae.
Fig. 2.
Subcellular localization of dEAAT2 protein in chordotonal neurons. A Upper panels: GFP fluorescence signals are uniformly distributed in chordotonal neurons when expressing GFP alone. Lower panels: pixel intensity of GFP along chordotonal neurons plotted against distance. B Upper panels: confocal images showing that dEAAT2::EGFP protein is enriched in the distal dendritic region of chordotonal neurons. Lower panels: pixel intensity of dEAAT2::EGFP along chordotonal neurons plotted against distance. Green, dEAAT2::EGFP protein signals; arrowheads, cell bodies; arrows, terminal dendritic structures. Scale bars, 10 μm.
dEAAT2 is Required for Sensing Sound in Larvae
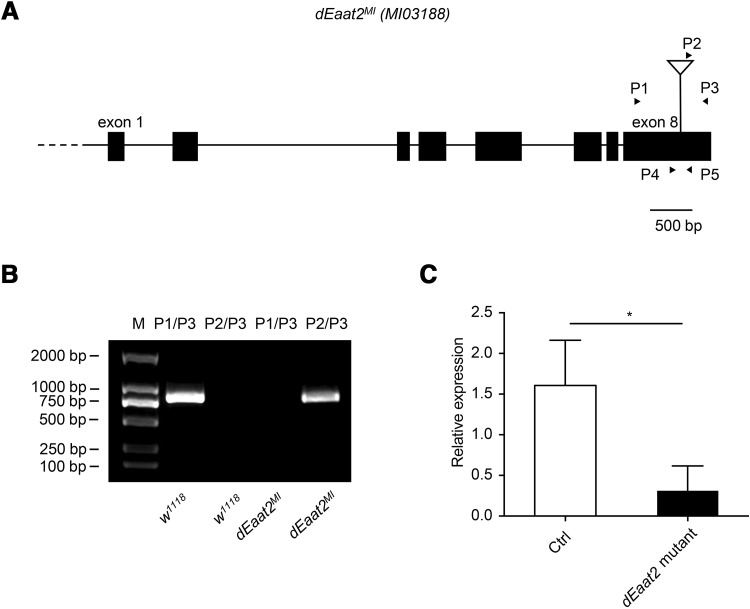
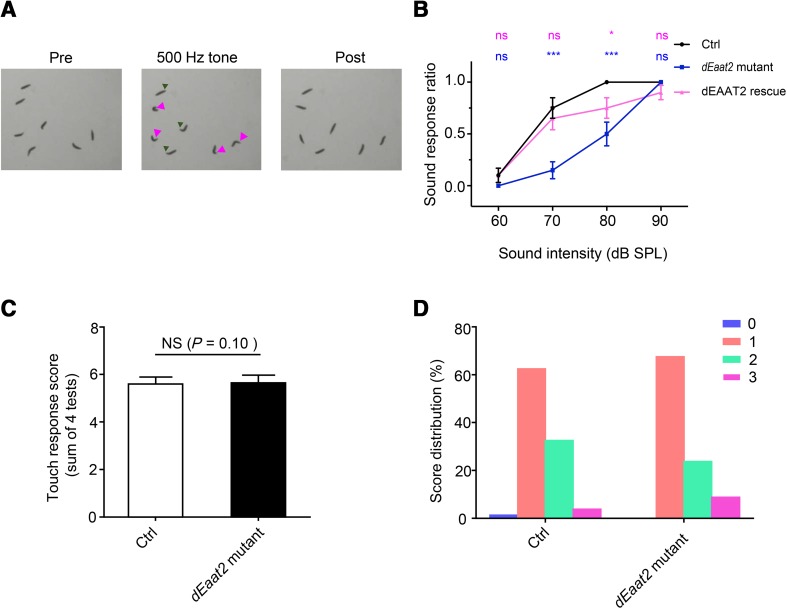
To investigate the function of Drosophila dEAAT2 in hearing transduction, we obtained a Minos element insertion in dEaat2 exon 8 (dEaat2MI) (Fig. 3A), the presence of which was confirmed by PCR (Fig. 3B). The transcript levels of dEAAT2 were reduced in the dEaat2MI line (Fig. 3C). We then examined the startle behavior of dEAAT2 mutant larvae in response to sound. When exposed to a 500-Hz sound (2 s duration at 8-s intervals), the WT larvae exhibited a series of startle behaviors, including pausing, turning, mouthhook retraction, and/or backward locomotion (Fig. 4A, Movie S1). To quantify this behavior, the number of larvae that showed responses was calculated. Neither the WT nor the dEAAT2 mutant larvae had startle-freeze reactions at a low sound intensity (60 dB) (Fig. 4B). Compared with the WT larvae, the responses of the dEAAT2 mutant were significantly reduced at 70 dB and 80 dB (Fig. 4B). At 90 dB, both the WT and dEAAT2 mutant larvae showed strong startle responses (Fig. 4B). NOMPC-Gal4 was used to drive the expression of the UAS-dEAAT2-EGFP rescuing transgene in the dEaat2MI background. We found that this mutant phenotype was rescued by expressing dEAAT2 in chordotonal neurons (Fig. 4B). These results revealed an important role of dEAAT2 in the sound response of chordotonal neurons.
Fig. 3.
Confirmation of the dEaat2MI mutation. A Boxes represent exons; straight lines are introns. The location of the Minos insertion (dEaat2MI) is indicated by a triangle. Arrows indicate the positions of the primers used for mutant verification. P1-5 means primer 1-5. B Genomic PCR confirming the presence of the Minos insertion in the dEaat2 gene, using primers as indicated in A.A 750 bp genomic fragment of w1118 was amplified using P1 and P3; a 800 bp genomic fragment containing Minos element sequence of dEaat2MI was amplified using P2 and P3. C dEAAT2 mRNA levels assessed by quantitative reverse-transcription (q-RT) PCR (*P < 0.05, unpaired t-test).
Fig. 4.
dEAAT2 is required for the larval startle response to sound stimuli. A Startle response behaviors to sound stimuli in control larvae. Left, freely-moving larvae; center, larvae exhibiting a series of startle responses to sound (pause and contraction indicated by green arrowheads; turning, by magenta arrowheads); right, larvae recovered 5 s after the removal of the sound stimulus. B Larval startle responses to a 500-Hz sound of increasing intensity. The dEaat2 mutant showed a reduced response, which was rescued by expressing dEAAT2 in chordotonal neurons. n = 20/group. Two-tailed Mann–Whitney tests of data sets against the WT background control were performed at each sound intensity. P values are indicated above each data point colored according to the genotype. C The dEaat2 mutant showed touch sensitivity similar to control larvae. The histogram shows the responses in 4 trials of individual larvae of different genotypes. n = 20/group. The χ2 test was used to test for the statistical significance of differences between control and dEaat2 mutant. D Score distribution of control and dEaat2 mutant larvae. 0, no response; 1, pause; 2, recoil; 3, reverse contraction. Ctrl: w1118. dEaat2 mutant: dEaat2MI/dEaat2MI; +/+. dEAAT2 rescue: dEaat2MI/dEaat2MI; NOMPC-Gal4/USA-dEAAT2-EGFP. NS, not significant; *P < 0.05. Error bars represent ± SEM.
In addition to chordotonal neurons, class III dendritic arborization (da) neurons were also labeled by dEAAT2-Gal4 (Fig. S1A). As class III neurons contribute to gentle touch sensation [45], we next tested the touch sensitivity of dEAAT2 mutant larvae using a previously-established behavioral assay [45]. Both the WT and dEAAT2 mutant larvae showed various behavioral responses to gentle touch, including pause, recoil, and reverse contraction (Fig. 4D), with no difference in the scores between the WT and the dEAAT2 mutant (Fig. 4C). Taken together, the results suggested that dEAAT2 is required for sound perception, but not for tactile sensation.
Reduced Calcium Response of lch5 Chordotonal Neurons in the dEaat2 Mutant
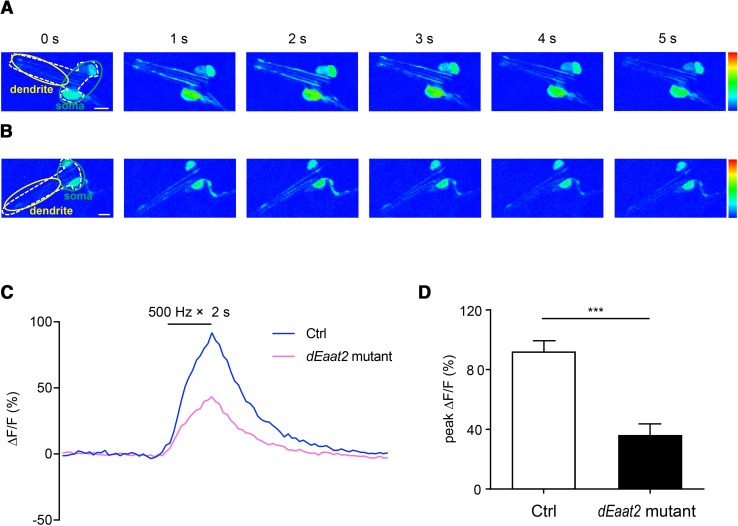
As there was no evident morphological defect in the lch5 chordotonal neurons of the dEaat2 mutant (data not shown), dEAAT2 might be involved in sound transduction rather than neural development. To determine the role of dEAAT2 in sound transduction, we first performed in vivo Ca2+ imaging of the lch5 chordotonal neurons of larvae with or without the dEAAT2 mutation. In a previous study, we showed that the lch5 neurons are a cluster of five chordotonal neurons, and less affected by movements. Moreover, the Ca2+ response of lch5 neurons is sensitive and stable [38]. NOMPC-Gal4 was used to drive the expression of the Ca2+ indicator GCamP6 in chordotonal neurons. When exposed to a 500-Hz sound at 90 dB, the Ca2+ level in the cell bodies of lch5 chordotonal neurons increased by 91.7% in WT larvae, and only increased by 35.7% in dEaat2 mutant larvae (Fig. 5D). In addition, the Ca2+ signal in dendrites was also elicited by sound stimuli (Fig. 5A). However, we did not find any difference in behavioral responses at the same intensity (90 dB) compared to WT larvae (Fig. 4B). This discrepancy can be explained by the possibility that the defective activity of chordotonal neurons at 90 dB might be compensated for in the central nervous system, or the behavioral assay using the startle response was not sensitive enough to detect a difference.
Fig. 5.
The Ca2+ response of lch5 chordotonal neurons to sound. A and B Sound-evoked Ca2+ signals in control (A) and the dEaat2 mutant (B). White dashed lines, border of lch5 chordotonal neurons; green outline, somata of lch5 chordotonal neurons; yellow outline, dendrites (rainbow color range: 0–255; scale bars, 10 μm). C Average Ca2+ responses to a 500-Hz tone of 90 dB in the cell bodies of lch5 chordotonal neurons. Black bar, sound stimulation. n = 5 in each case. D Statistical analysis of the Ca2+ responses in control and dEaat2 mutant larvae. The dEaat2 mutant showed a reduced Ca2+ response to a 500-Hz sound. Ctrl: +/+; NOMPC-Gal4/UAS-GCamP6, UAS-CD4-tdTomato. dEaat2 mutant: dEaat2MI/dEaat2MI; NOMPC-Gal4/UAS-GCamP6, UAS-CD4-tdTomato (n = 10/group; two-tailed unpaired Student’s t-test; ***P < 0.001; error bars represent ± SEM).
Decreased Action Potential Firing Responses of vchA Chordotonal Neurons in the dEaat2 Mutant
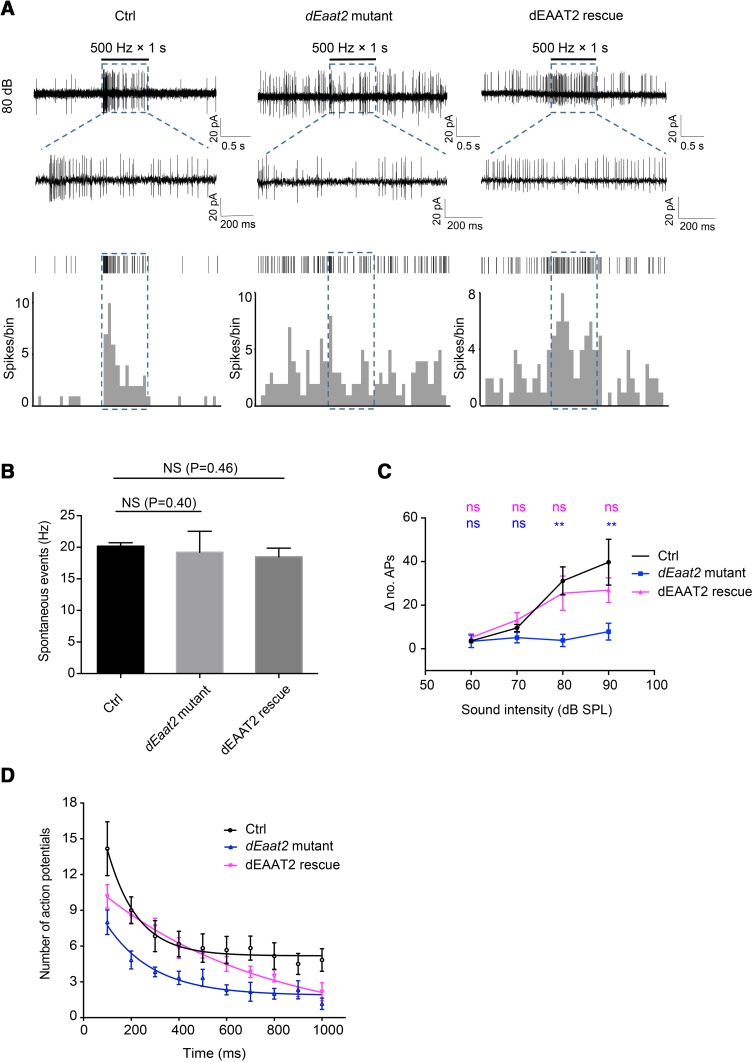
To further examine the response of chordotonal neurons in dEaat2 mutant larvae at 60 dB–90 dB, the AP firing rates of chordotonal neurons were recorded. We recorded extracellularly from vchA chordotonal neurons. In the absence of sound stimulation, these neurons fired spontaneously in both the WT and dEaat2 mutant larvae (Fig. 6A, Fig. S2–3), and there was no difference in this spontaneous firing rate (Fig. 6B). When exposed to a 500-Hz sound at 70 dB, the WT responded with increased AP firing (Fig. S2B). At 80 (Fig. 6A) and 90 dB (Fig. S3), greater numbers of APs were elicited from the vchA chordotonal neuron in WT larvae. In contrast, the dEaat2 mutant had significantly reduced responses at 80 dB and 90 dB (Fig. 6C).
Fig. 6.
dEaat2 mutant has a decreased AP firing response of vchA chordotonal neurons to sound. A AP firing responses of vchA chordotonal neurons to a 500-Hz sound at 80 dB in control (left), dEaat2 mutant (center), and dEAAT2 rescue (right) larvae. Upper panels: original traces; middle panels: raster plots of spikes; lower panels: post-stimulus time histograms of spikes (bin width, 100 ms). Black bars indicate sound stimulation; blue boxes and dashed lines indicate expanded views of spiking activity during delivery of a prolonged 500 Hz sound. B Statistical analysis of the rate of spontaneous firing in control (n = 6), dEaat2 mutant (n = 5), and dEAAT2 rescue larvae (n = 6; two-tailed Mann–Whitney tests). C Summary of AP firing response of vchA chordotonal neurons in the control, dEaat2 mutant, and dEAAT2 rescue larvae. ∆no. APs: increase of the number of APs in 1 s after sound onset compared to 1 s before sound stimulus onset. The dEaat2 mutant exhibited dramatically reduced firing responses at 80 dB and 90 dB, and this was rescued by expressing dEAAT2 in the chordotonal neurons (n = 6/group; two-tailed Mann–Whitney tests; P values are indicated above each data point colored according to the genotype). D Exponential decay curve of response at 90 dB in a 100-ms bin. A 1-s sound stimulus was used (n = 6/group; τ, time constant of adaptation rate). vchA chordotonal neurons of control larvae adapted quickly to sound with a τ of 125 ms; the dEaat2 mutant showed an adaptation rate similar to control (τ = 198 ms). dEAAT2 rescue exhibited an adaptation rate different from control larvae (τ = 698 ms). Ctrl: +/+; NOMPC-Gal4/UAS-CD8-GFP. dEaat2 mutant: dEaat2MI/dEaat2MI; NOMPC-Gal4/UAS-CD8-GFP. dEAAT2 rescue: dEaat2MI/dEaat2MI; NOMPC-Gal4/UAS-dEAAT2-EGFP. NS, not significant; **P < 0.01; error bars represent ± SEM.
We found a decreased startle response in dEaat2 mutant larvae at 70 dB (Fig. 4B), but did not find any difference in the AP firing response at the same intensity compared to WT larvae (Fig. 6C). It is possible that our electrophysiological recording at low sound intensity was not sensitive enough to detect the difference. Consistent with the results of Ca2+ imaging, chordotonal neurons of the dEaat2 mutant showed a decreased response to sound at 90 dB (Fig. 6C).
The vchA chordotonal neurons of WT larvae adapted quickly to a prolonged sound stimulus of 90 dB with a τ of 125 ms (Fig. 6D). The dEaat2 mutant had an adaptation rate similar to WT larvae (Fig. 6D). We found that the defect in firing response was rescued by dEAAT2 expression (Fig. 6C). Interestingly, dEAAT2 rescue larvae showed an adaptation rate different from the WT (τ = 698 ms) (Fig. 6D). Together, the findings indicate that dEAAT2 is required for sound transduction in larval chordotonal neurons.
Discussion
In the current study, multiple lines of evidence were provided to support the hypothesis that dEAAT2, a taurine/aspartate transporter, is critical for sound transduction in Drosophila larvae. First, we found that dEAAT2 was required for the larval startle response to sound stimuli. Second, dEAAT2 was enriched in the distal regions of chordotonal neurons. Third, the sound-induced Ca2+ response and the AP firing response of chordotonal neurons in the dEAAT2 mutant were dramatically reduced. Moreover, the mutant phenotype was rescued by expressing dEAAT2 in chordotonal neurons, indicating that dEAAT2 functions in Drosophila chordotonal neurons for sound transduction.
In D. melanogaster, the chordotonal neurons of Johnston’s organ in the second antennal segment mediate hearing as well as sensing gravity and wind [36, 37]. The ciliated dendrites of chordotonal neurons play an important role in sound transduction [44]. Genes required for Johnston’s organ function in Drosophila have been identified, such as ato (atonal), cut, ck (crinkled), nompA (no mechanoreceptor potential A), nompB (no mechanoreceptor potential B), btv (beethoven), rempA (reduced mechanoreceptor potential A), tilB (touch insensitive larva B), nompC (no mechanoreceptor potential C), iav (Inactive), nan (Nanchung), and unc (uncoordinated) [35, 46, 47]. Three of these genes, ato, ck, and cut, are required for Johnston’s organ development [48, 49]. Ion channels of the transient receptor potential family encoded by Nompc, nan, and iav, are important for the mechanotransduction of hearing in Drosophila [50, 51].
Unlike other EAAT orthologues, dEAAT2 has been reported to mediate the high-affinity transport of aspartate, an excitatory amino-acid, and taurine. Taurine is found at high concentrations in mammals and has been associated with a variety of physiological functions in the central nervous system, from development to cytoprotection [4, 5]. Particularly, it has long been known that taurine is highly concentrated in the mammalian retina [52, 53]. In the rat, taurine is critical for the development and maintenance of retinal function and promotes the development of rod photoreceptors [54]. Taurine is critical for photoreceptor function both as an antioxidant and as an osmolyte [55, 56]. Moreover, a previous study showed that taurine is involved in the activation of cyclic GMP-gated channels, an important step in the phototransduction process [57]. It also acts in various ways to regulate synaptic signal transmission in retinal ganglion cells [57, 58]. In addition, the olfactory bulb contains a high level of taurine. In slices of rat olfactory bulb, the selective inhibitory effects of taurine by activating GABAA receptors suggest that taurine plays a role in olfaction [59]. Considering its broad distribution and its functional significance, taurine deserves detailed study. Many of its functions rely on its intracellular concentration, which is maintained by a high-affinity TauT transporter that takes up taurine from the extracellular compartment [60]. A decrease of taurine levels in a variety of tissues has been reported in taut−/− mice, and these taurine-deficient mice show severe retinal degeneration [61] and reduced olfactory function [19].
In insects, taurine is the second most abundant amino-acid after glutamate, but little is known about its function(s) in the nervous system. Taurine has been detected in Drosophila mushroom bodies, an integrative structure implicated in learning and memory [62] and dEAAT2 is a taurine transporter in Drosophila. Here, we found that dEAAT2 is involved in sound transduction in Drosophila.
In conclusion, we have investigated the expression and functional role of Drosophila dEAAT2 in the peripheral auditory system. These findings show that dEAAT2 is required for auditory transduction in Drosophila. Analyses of dEaat2 gene functions in Drosophila hearing add to an appreciation of the importance of taurine in the auditory pathway.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Blooming Drosophila Stock Center and Yuh-Nung Jan for fly lines and the Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, for the microinjection of plasmids into Drosophila embryos. The research was supported by funds from The Ministry of Science and Technology of China (2017YFA0103900 and 2016YFA0502800), The National Natural Science Foundation of China (31571083), The Program for Professor of Special Appointment (Eastern Scholar of Shanghai; TP2014008), The Shanghai Rising-Star Program (14QA1400800) and a grant from the Young 1000 Talent Program of China to ZY. The research was also supported by The National Natural Science Foundation of China (81470701), The National Natural Science Foundation of China (81771882) and The Fundamental Research (Discipline Layout) Foundation from Shenzhen Committee of Science, Technology and Innovation (JCYJ20170817111912585) to FC.
Compliance with ethical standards
Conflict of interest
All authors claim that there are no conflicts of interest.
Contributor Information
Fangyi Chen, Email: chenfy@sustc.edu.cn.
Zhiqiang Yan, Email: zqyan@fudan.edu.cn.
References
- 1.Huxtable R. Physiological actions of taurine. Physiol Rev. 1992;72:101–163. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- 2.Green TR, Fellman JH, Eicher AL, Pratt KL. Antioxidant role and subcellular location of hypotaurine and taurine in human neutrophils. Biochim Biophys Acta. 1991;1073:91–97. doi: 10.1016/0304-4165(91)90187-L. [DOI] [PubMed] [Google Scholar]
- 3.Ripps H, Shen W. Taurine: a “very essential” amino acid. Mol Vis. 2012;18:2673. [PMC free article] [PubMed] [Google Scholar]
- 4.Sturman J, Gaull G. Taurine in the brain and liver of the developing human and monkey. J Neurochem. 1975;25:831–835. doi: 10.1111/j.1471-4159.1975.tb04414.x. [DOI] [PubMed] [Google Scholar]
- 5.Sturman JA, Hayes KC. The biology of taurine in nutrition and development. Adv Nutr Res 1980: 231–299.
- 6.Davies W, Harding N, Kay I, Hopkins P. The role of taurine in mammalian hearing. In: Huxtable RJ, Michalk D (eds). Taurine in Health and Disease. Advances in Experimental Medicine and Biology. Boston, MA. Springer 1994, 359: 393–398. [DOI] [PubMed]
- 7.Periman M. Taurine and auditory system maturation. Pediatrics. 1989;83:796–798. [PubMed] [Google Scholar]
- 8.Davies WE, Hopkins PC, Rose SJ, Dhillon SK. The influence of different taurine diets on hearing development in normal babies. Adv Exp Med Biol. 1996;403:631–637. doi: 10.1007/978-1-4899-0182-8_70. [DOI] [PubMed] [Google Scholar]
- 9.Dhillon SK, Davies WE, Hopkins PC, Rose SJ. Effects of dietary taurine on auditory function in full term infants. Adv Exp Med Biol. 1998;442:507–514. doi: 10.1007/978-1-4899-0117-0_61. [DOI] [PubMed] [Google Scholar]
- 10.Tyson JE, Lasky R, Flood D, Mize C, Picone T, Paule CL. Randomized trial of taurine supplementation for infants ≤ 1,300-gram birth weight: effect on auditory brainstem-evoked responses. Pediatrics. 1989;83:406–415. [PubMed] [Google Scholar]
- 11.Verner AM, McGuire W, Craig JS. Effect of taurine supplementation on growth and development in preterm or low birth weight infants. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD006072.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu H, Wang W, Tang ZQ, Xu TL, Chen L. Taurine acts as a glycine receptor agonist in slices of rat inferior colliculus. Hear Res. 2006;220:95–105. doi: 10.1016/j.heares.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Xu H, Zhou KQ, Huang YN, Chen L, Xu TL. Taurine activates strychnine-sensitive glycine receptors in neurons of the rat inferior colliculus. Brain Res. 2004;1021:232–240. doi: 10.1016/j.brainres.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Song NY, Shi HB, Li CY, Yin SK. Interaction between taurine and GABA(A)/glycine receptors in neurons of the rat anteroventral cochlear nucleus. Brain Res. 2012;1472:1–10. doi: 10.1016/j.brainres.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Harding N, Davies W. Cellular localisation of taurine in the organ of Corti. Hear Res. 1993;65:211–215. doi: 10.1016/0378-5955(93)90214-L. [DOI] [PubMed] [Google Scholar]
- 16.Horner KC, Aurousseau C. Immunoreactivity for taurine in the cochlea: its abundance in supporting cells. Hear Res. 1997;109:135–142. doi: 10.1016/S0378-5955(97)00057-9. [DOI] [PubMed] [Google Scholar]
- 17.Usami S, Ottersen OP. The localization of taurine-like immunoreactivity in the organ of Corti: a semiquantitative, post-embedding immuno-electron microscopic analysis in the rat with some observations in the guinea pig. Brain Res. 1995;676:277–284. doi: 10.1016/0006-8993(95)00098-B. [DOI] [PubMed] [Google Scholar]
- 18.Warskulat U, Flögel U, Jacoby C, Hartwig HG, Thewissen M, Merx MW, et al. Taurine transporter knockout depletes muscle taurine levels and results in severe skeletal muscle impairment but leaves cardiac function uncompromised. FASEB J. 2004;18:577–579. doi: 10.1096/fj.03-0496fje. [DOI] [PubMed] [Google Scholar]
- 19.Warskulat U, Borsch E, Reinehr R, Heller-Stilb B, Roth C, Witt M, et al. Taurine deficiency and apoptosis: findings from the taurine transporter knockout mouse. Arch Biochem Biophys. 2007;462:202–209. doi: 10.1016/j.abb.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 20.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/S0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 21.Torres GE, Amara SG. Glutamate and monoamine transporters: new visions of form and function. Curr Opin Neurobiol. 2007;17:304–312. doi: 10.1016/j.conb.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, et al. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 23.Rebillard G, Ruel J, Nouvian R, Saleh H, Pujol R, Dehnes Y, et al. Glutamate transporters in the guinea-pig cochlea: partial mRNA sequences, cellular expression and functional implications. Eur J Neurosci. 2003;17:83–92. doi: 10.1046/j.1460-9568.2003.02429.x. [DOI] [PubMed] [Google Scholar]
- 24.Glowatzki E, Cheng N, Hiel H, Yi E, Tanaka K, Ellis-Davies GC, et al. The glutamate-aspartate transporter GLAST mediates glutamate uptake at inner hair cell afferent synapses in the mammalian cochlea. J Neurosci. 2006;26:7659–7664. doi: 10.1523/JNEUROSCI.1545-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Besson MT, Soustelle L, Birman S. Identification and structural characterization of two genes encoding glutamate transporter homologues differently expressed in the nervous system of Drosophila melanogaster. FEBS Lett. 1999;443:97–104. doi: 10.1016/S0014-5793(98)01695-0. [DOI] [PubMed] [Google Scholar]
- 26.Besson MT, Soustelle L, Birman S. Selective high-affinity transport of aspartate by a Drosophila homologue of the excitatory amino-acid transporters. Curr Biol. 2000;10:207–210. doi: 10.1016/S0960-9822(00)00339-0. [DOI] [PubMed] [Google Scholar]
- 27.Stacey SM, Muraro NI, Peco E, Labbé A, Thomas GB, Baines RA, et al. Drosophila glial glutamate transporter Eaat1 is regulated by fringe-mediated notch signaling and is essential for larval locomotion. J Neurosci. 2010;30:14446–14457. doi: 10.1523/JNEUROSCI.1021-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Besson MT, Ré DB, Moulin M, Birman S. High affinity transport of taurine by the Drosophila aspartate transporter dEAAT2. J Biol Chem. 2005;280:6621–6626. doi: 10.1074/jbc.M412440200. [DOI] [PubMed] [Google Scholar]
- 29.Besson M, Sinakevitch I, Melon C, Iché-Torres M, Birman S. Involvement of the Drosophila taurine/aspartate transporter dEAAT2 in selective olfactory and gustatory perceptions. J Comp Neurol. 2011;519:2734–2757. doi: 10.1002/cne.22649. [DOI] [PubMed] [Google Scholar]
- 30.Tian Y, Zhang ZC, Han J. Drosophila studies on aurism spectrum disorders. Neurosci Bull. 2017;33:737–746. doi: 10.1007/s12264-017-0166-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan Y. Sandman is a Sleep Switch in Drosophila. Neurosci Bull. 2016;32:503–504. doi: 10.1007/s12264-016-0062-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ou J, Gao Z, Song L, Ho MS. Analysis of glial distribution in Drosophila adult brains. Neurosci Bull. 2016;32:162–170. doi: 10.1007/s12264-016-0014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Field LH, Matheson T. Chordotonal organs of insects. Adv Insect Physiol. 1998;27:1–228. doi: 10.1016/S0065-2806(08)60013-2. [DOI] [Google Scholar]
- 34.Boekhoff-Falk G. Hearing in Drosophila: development of Johnston’s organ and emerging parallels to vertebrate ear development. Dev Dyn. 2005;232:550–558. doi: 10.1002/dvdy.20207. [DOI] [PubMed] [Google Scholar]
- 35.Senthilan PR, Piepenbrock D, Ovezmyradov G, Nadrowski B, Bechstedt S, Pauls S, et al. Drosophila auditory organ genes and genetic hearing defects. Cell. 2012;150:1042–1054. doi: 10.1016/j.cell.2012.06.043. [DOI] [PubMed] [Google Scholar]
- 36.Kamikouchi A, Inagaki HK, Effertz T, Hendrich O, Fiala A, Göpfert MC, et al. The neural basis of Drosophila gravity-sensing and hearing. Nature. 2009;458:165–171. doi: 10.1038/nature07810. [DOI] [PubMed] [Google Scholar]
- 37.Yorozu S, Wong A, Fischer BJ, Dankert H, Kernan MJ, Kamikouchi A, et al. Distinct sensory representations of wind and near-field sound in the Drosophila brain. Nature. 2009;458:201–205. doi: 10.1038/nature07843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang W, Yan Z, Jan LY, Jan YN. Sound response mediated by the TRP channels NOMPC, NANCHUNG, and INACTIVE in chordotonal organs of Drosophila larvae. Proc Natl Acad Sci U S A. 2013;110:13612–13617. doi: 10.1073/pnas.1312477110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han C, Jan LY, Jan YN. Enhancer-driven membrane markers for analysis of nonautonomous mechanisms reveal neuron–glia interactions in Drosophila. Proc Natl Acad Sci U S A. 2011;108:9673–9678. doi: 10.1073/pnas.1106386108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson M, Zheng Q, Dong X. Investigation of pain mechanisms by calcium imaging approaches. Neurosci Bull. 2018;34:194–199. doi: 10.1007/s12264-017-0139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li K, Gong Z. Feeling hot and cold: thermal sensation in Drosophila. Neurosci Bull. 2017;33:317–322. doi: 10.1007/s12264-016-0087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eberl DF. Feeling the vibes: chordotonal mechanisms in insect hearing. Curr Opin Neurobiol. 1999;9:389–393. doi: 10.1016/S0959-4388(99)80058-0. [DOI] [PubMed] [Google Scholar]
- 43.Roy M, Sivan-Loukianova E, Eberl DF. Cell-type-specific roles of Na+/K+ ATPase subunits in Drosophila auditory mechanosensation. Proc Natl Acad Sci U S A. 2013;110:181–186. doi: 10.1073/pnas.1208866110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Todi SV, Sharma Y, Eberl DF. Anatomical and molecular design of the Drosophila antenna as a flagellar auditory organ. Microsc Res Tech. 2004;63:388–399. doi: 10.1002/jemt.20053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan Z, Zhang W, He Y, Gorczyca D, Xiang Y, Cheng LE, et al. Drosophila NOMPC is a mechanotransduction channel subunit for gentle-touch sensation. Nature. 2013;493:221. doi: 10.1038/nature11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kernan M, Cowan D, Zuker C. Genetic dissection of mechanotransduction: Drosophila mutations defective in mechanoreception. Neuron. 1994;12:1195–1206. doi: 10.1016/0896-6273(94)90437-5. [DOI] [PubMed] [Google Scholar]
- 47.Eberl DF, Duyk GM, Perrimon N. A genetic screen for mutations that disrupt an auditory response in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1997;94:14837–14842. doi: 10.1073/pnas.94.26.14837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jarman AP, Grau Y, Jan LY, Jan YN. atonal is a proneural gene that directs chordotonal organ formation in the Drosophila peripheral nervous system. Cell. 1993;73:1307–1321. doi: 10.1016/0092-8674(93)90358-W. [DOI] [PubMed] [Google Scholar]
- 49.Ebacher DJ, Todi SV, Eberl DF, Boekhoff-Falk GE. cut mutant Drosophila auditory organs differentiate abnormally and degenerate. Fly. 2007;1:86–94. doi: 10.4161/fly.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gong Z. TRPV channel subunits, inactive and nanchung, mediate hearing in Drosophila. J Neurosci. 2004;24:9059–9066. doi: 10.1523/JNEUROSCI.1645-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Effertz T, Wiek R, Göpfert MC. NompC TRP channel is essential for Drosophila sound receptor function. Curr Biol. 2011;21:592–597. doi: 10.1016/j.cub.2011.02.048. [DOI] [PubMed] [Google Scholar]
- 52.Reddy D. Distribution of free ammo acids and related compounds in ocular fluids, lens, and plasma of various mammalian species. Invest Ophthalmol. 1967;6:478–483. [PubMed] [Google Scholar]
- 53.Heinämäki A, Muhonen A, Piha R. Taurine and other free amino acids in the retina, vitreous, lens, irisciliary body, and cornea of the rat eye. Neurochem Res. 1986;11:535–542. doi: 10.1007/BF00965323. [DOI] [PubMed] [Google Scholar]
- 54.Altshuler D, Turco JL, Rush J, Cepko C. Taurine promotes the differentiation of a vertebrate retinal cell type in vitro. Development. 1993;119:1317–1328. doi: 10.1242/dev.119.4.1317. [DOI] [PubMed] [Google Scholar]
- 55.Rego AC, Santos MS, Oliveira CR. Oxidative stress, hypoxia, and ischemia-like conditions increase the release of endogenous amino acids by distinct mechanisms in cultured retinal cells. J Neurochem. 1996;66:2506–2516. doi: 10.1046/j.1471-4159.1996.66062506.x. [DOI] [PubMed] [Google Scholar]
- 56.Petrosian AM, Haroutounian JE. The role of taurine in osmotic, mechanical, and chemical protection of the retinal rod outer segments. Adv Exp Med Biol 1998: 407–413. [DOI] [PubMed]
- 57.Militante J, Lombardini J. Pharmacological characterization of the effects of taurine on calcium uptake in the rat retina. Amino Acids. 1998;15:99–108. doi: 10.1007/BF01345283. [DOI] [PubMed] [Google Scholar]
- 58.Jiang Z, Bulley S, Guzzone J, Ripps H, Shen W. The modulatory role of taurine in retinal ganglion cells. Adv Exp Med Biol 2013: 53–68. [DOI] [PMC free article] [PubMed]
- 59.Belluzzi O, Puopolo M, Benedusi M, Kratskin I. Selective neuroinhibitory effects of taurine in slices of rat main olfactory bulb. Neuroscience. 2004;124:929–944. doi: 10.1016/j.neuroscience.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 60.Pramod AB, Foster J, Carvelli L, Henry LK. SLC6 transporters: structure, function, regulation, disease association and therapeutics. Mol Aspects Med. 2013;34:197–219. doi: 10.1016/j.mam.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heller-Stilb B, van Roeyen C, Rascher K, Hartwig HG, Huth A, Seeliger MW, et al. Disruption of the taurine transporter gene (taut) leads to retinal degeneration in mice. FASEB J. 2002;16:231–233. doi: 10.1096/fj.01-0691fje. [DOI] [PubMed] [Google Scholar]
- 62.Strausfeld NJ, Sinakevitch I, Vilinsky I. The mushroom bodies of Drosophila melanogaster: an immunocytological and golgi study of Kenyon cell organization in the calyces and lobes. Microsc Res Tech. 2003;62:151–169. doi: 10.1002/jemt.10368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.