Abstract
The ventral pallidum (VP) is a crucial component of the limbic loop of the basal ganglia and participates in the regulation of reward, motivation, and emotion. Although the VP receives afferent inputs from the central histaminergic system, little is known about the effect of histamine on the VP and the underlying receptor mechanism. Here, we showed that histamine, a hypothalamic-derived neuromodulator, directly depolarized and excited the GABAergic VP neurons which comprise a major cell type in the VP and are responsible for encoding cues of incentive salience and reward hedonics. Both postsynaptic histamine H1 and H2 receptors were found to be expressed in the GABAergic VP neurons and co-mediate the excitatory effect of histamine. These results suggested that the central histaminergic system may actively participate in VP-mediated motivational and emotional behaviors via direct modulation of the GABAergic VP neurons. Our findings also have implications for the role of histamine and the central histaminergic system in psychiatric disorders.
Keywords: Ventral pallidum, Histamine, H1 receptor, H2 receptor, Motivation, Emotion
Introduction
The ventral pallidum (VP), a major structure in the limbic subdivision of the basal ganglia circuitry, has attracted increasing attention in recent years. Through major reciprocal connections with the accumbens, extended amygdala, limbic thalamus, substantia nigra, lateral habenula, and ventral tegmental area (VTA) [1–4], the VP plays a critical role in brain functions related to reward, motivation, and emotion. Both incentive salience and reward hedonic information are encoded by VP neurons [5, 6]. In humans, bilateral lesions of the VP lead to a lack of motivation and pleasure [7]. In rodents, optogenetic or chemogenetic inactivation of VP neurons suppresses reward-seeking behaviors [6, 8], while manipulation of VP parvalbumin-positive neurons induces depressive-like phenotypes [4]. Therefore, the VP is of great importance for regulation of motivational and emotional states.
The central histaminergic system, originating exclusively in the tuberomammillary nucleus of the hypothalamus, is likely to be a general modulator of the activity of the whole brain [9–11]. It extensively innervates various brain regions and holds a key position in the regulation of many basic physiological functions, including the sleep-wake cycle, energy and endocrine homeostasis, and motor control, as well as reward and motivation [9, 11, 12]. Interestingly, it has been revealed that moderate densities of histaminergic fibers [13, 14] and high densities of histamine receptors [15, 16] are distributed throughout the basal ganglia. However, the effect of histamine on neuronal activity of the VP is still far from clear. Thus, in the present study, by whole-cell patch clamp recording from brain slices and immunostaining, the effect of histamine on GABAergic VP neurons, a major cell type in the VP, and the underlying receptor mechanisms were investigated. Our results showed that histamine directly depolarizes the GABAergic VP neurons via co-activation of postsynaptic H1 and H2 receptors.
Materials and Methods
Animals and Brain Slice Preparations
Based on a rat brain atlas [17], coronal slices (300 μm thick) containing the VP were cut with a Vibroslicer (VT 1200 S, Leica, Wetzlar, Germany) from Sprague-Dawley rats of either sex aged 14–18 days. The slices were incubated in oxygenated artificial cerebrospinal fluid (ACSF, composition in mmol/L: 124 NaCl, 2.5 KCl, 1.25 NaH2PO4, 1.3 MgSO4, 26 NaHCO3, 2 CaCl2, and 10 D-glucose) at 35 ± 0.5°C for at least 1 h. Then the slices were maintained at room temperature for ~ 20 min before recordings. For recording sessions, the slices were transferred to a submerged recording chamber and continuously perfused with oxygenated ACSF at 2 mL/min maintained at room temperature. All experimental procedures were performed in accordance with the US National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication 85-23, revised 2011) and were approved by the Experimental Animal Care and Use Committee of Nanjing University.
Whole-Cell Patch Clamp Recordings
Whole-cell patch clamp recordings were made as previously described [18–22] with borosilicate glass pipettes (3–5 MΩ). The pipettes were filled with (in mmol/L): 140 K-methylsulfate, 7 KCl, 2 MgCl2, 10 HEPES, 0.1 EGTA, 4 Na2-ATP, 0.4 GTP-Tris, and 4% biocytin (Sigma-Aldrich, St. Louis, MO), pH 7.25. During recording sessions, VP neurons were visualized under a light microscope (BX51WI, Olympus, Tokyo, Japan). Patch-clamp recordings were acquired with an Axopatch-200B amplifier (Axon Instruments, Foster City, CA) and the signals were fed into a computer through a Digidata-1550 interface (Axon Instruments) for data capture and analysis (pClamp 10.4, Axon Instruments). Recordings of whole-cell currents were low-pass filtered at 2 kHz and digitized at 10 kHz and recordings of membrane potentials were low-pass filtered at 5 kHz and digitized at 20 kHz. Neurons were held at a membrane potential of – 60 mV and characterized by injection of rectangular voltage pulses (5 mV, 50 ms) to monitor the whole-cell membrane capacitance, series resistance, and membrane resistance. Neurons were excluded from the study if the series resistance was not stable or exceeded 20 MΩ. To identify GABAergic VP neurons, which characteristically have a prominent hyperpolarization-activated cyclic nucleotide-gated (HCN) channel current (Ih) [22], a series of 1-s negative hyperpolarizing voltage steps (ranging from – 60 to – 110 mV in 10-mV steps) was used in voltage-clamp recordings.
Drug Application
We briefly (1 min) bathed the slices with histamine (3, 10, and 30 μmol/L; Tocris, Bristol, UK) to stimulate the recorded neurons. Tetrodotoxin (TTX, 0.3 μmol/L; Alomone Labs, Jerusalem, Israel) was used to assess the postsynaptic effect of histamine. Selective antagonists for histamine H1 and H2 receptors, mepyramine (3 μmol/L; Tocris) and ranitidine (3 μmol/L; Tocris) as well as selective agonists for H1 and H2 receptors, 2-pyridylethylamine (2-PyEA; 30, 100, and 300 μmol/L; Sigma) and dimaprit (30, 100, and 300 μmol/L; Tocris) were applied to assess the underlying postsynaptic receptor mechanisms. All drugs were stored frozen and dissolved in distilled water, and dilutions were freshly prepared in ACSF and equilibrated with 95% O2 and 5% CO2 before perfusing the slices. In all experiments, the drugs were delivered by bath application.
Immunostaining
Immunofluorescence staining was performed as previously described [18–23]. Rats (230–250 g) were given an overdose of sodium pentobarbital and perfused transcardially with 100 mL saline, followed by 450–500 mL 4% sodium phosphate-buffered paraformaldehyde. The brains were post-fixed in the same fixative for 12 h at 4°C, then cryoprotected with 30% sucrose for 48 h. Frozen coronal sections (15 μm thick) were cut on a freezing microtome (CM3050S, Leica) and mounted on gelatin-coated slides. The slices were rinsed with PBS containing 0.1% Triton X-100 and then incubated for 30 min in 10% normal bovine serum in PBS containing 0.1% Triton X-100. Sections were incubated overnight at 4°C with the primary antibodies mouse anti-GABA (1:1000; Sigma), goat anti-H1 receptor (1:200; Everest Biotech, Bicester, UK), rabbit anti-H1 receptor (1:200; Alamone Labs, Jerusalem, Israel), and/or goat anti-H2 receptor (1:200; Everest Biotech). After a complete wash in PBS, the sections were incubated in the related secondary antibodies (1:2,000; Life Technologies, Carlsbad, CA) conjugated to Alexa 488- and Alexa 594- for 2 h at room temperature in the dark for double immunostaining. The slides were washed and mounted in Fluoromount-G mounting medium (Southern Biotech, Birmingham, AL). Incubation replacing the primary antiserum with control immunoglobulins and/or omitting the primary antiserum served as negative controls.
For immunohistochemical identification of the recorded GABAergic VP neurons, brain slices containing biocytin-filled neurons in the whole-cell patch clamp recordings were fixed, dehydrated, re-sectioned, and then incubated with primary antibodies against GABA (1:1000; Sigma). Micrographs were captured with an inverted laser scanning confocal microscope (TCS SP8, Leica). Digital images from the microscope were recorded with LAS X Viewer software (Leica). Image processing and cell counts were performed with Image Pro Plus 6.0 software (Media Cybernetics, Rockville, MD).
Statistical Analysis
All data were analyzed with SPSS 17.0 (SPSS, Chicago, IL) and are presented as box and whisker plots, with the boxes and whisker lengths denoting the 25th and 75th percentiles and the 10th and 90th percentiles, respectively. The paired-t test was used for analysis of the data. P-values < 0.05 were considered to be statistically significant.
Results
Histamine Excites GABAergic VP Neurons
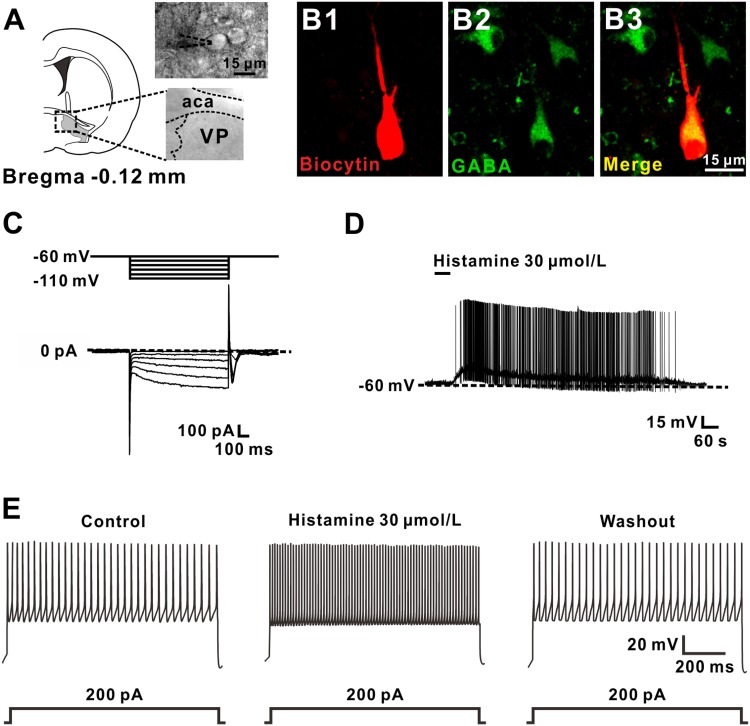
A total of 51 GABAergic VP neurons with input resistances > 300 MΩ and capacitance of 135.8 ± 19.7 pF were identified and recorded. To identify GABAergic neurons in the VP, we filled all recorded neurons with biocytin and then immunostained them with GABA after recording. The somal diameters of the recorded GABAergic VP neurons co-labelled by biocytin and GABA were ~15 μm (Fig. 1A, B). Moreover, these GABAergic VP neurons exhibited a slow hyperpolarization-activated inwardly-rectifying current (Ih) in response to hyperpolarizing voltage steps from a holding potential of –60 mV (Fig. 1C). These morphological and electrophysiological properties are consistent with previous reports [24]. Of the 51 GABAergic VP neurons recorded, 45 (88.24%) were excited by histamine. In current-clamp recordings, brief bath application of histamine (30 μmol/L) evoked a strong depolarization of GABAergic neurons in the VP, which was sufficient to induce neuronal firing (Fig. 1D, n = 6). Moreover, a 1-s depolarizing current of 200 pA was injected to evoke firing of GABAergic VP neurons. The depolarizing current-elicited firing was significantly enhanced by histamine (Fig. 1E, n = 5).
Fig. 1.
Histamine depolarizes GABAergic VP neurons. A Diagram and a coronal brain section showing the location of the VP and GABAergic neurons in the VP ~ 15 μm in diameter (aca, anterior commissure, anterior part; VP, ventral pallidum). B Identification of a GABAergic VP neuron by immunostaining the recorded biocytin-filled neuron with GABA. C A series of 1-s hyperpolarizing voltage steps (from −60 to −110 mV in 10-mV steps) was used to measure inwardly-rectifying currents in the recorded neurons. Note that GABAergic VP neurons exhibited Ih currents. D Histamine depolarized and evoked firing of a GABAergic VP neuron. E Histamine increased the firing response to a 1-s suprathreshold current of 200 pA.
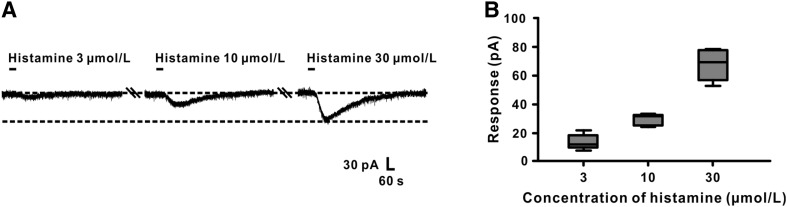
In addition, in voltage clamp recordings, histamine induced a significant inward current in GABAergic VP neurons in a concentration-dependent manner (Fig. 2A). The group data in Fig. 2B showed that application of 3, 10, and 30 μmol/L histamine elicited inward currents of 11.8 ± 2.4, 32.5 ± 1.8, and 68.4 ± 6.0 pA, respectively. These results indicated that histamine exerts an excitatory effect on GABAergic neurons in the VP.
Fig. 2.
Histamine excites GABAergic VP neurons in a dose-dependent manner. A Histamine elicited an inward current in a GABAergic VP neuron in a concentration-dependent manner. B Group data of the tested neurons (n = 5).
Histamine-Induced Excitation of GABAergic VP Neurons is a Direct Postsynaptic Effect
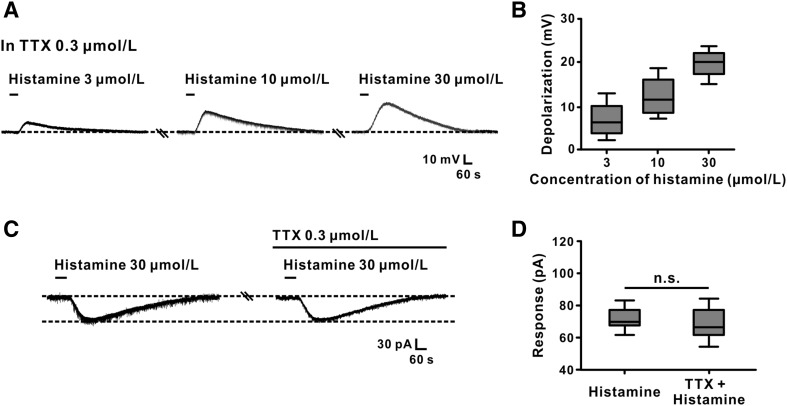
TTX was used to investigate whether the histamine-induced excitation of GABAergic VP neurons is a direct postsynaptic effect. In the presence of 0.3 μmol/L TTX, 3, 10, and 30 μmol/L histamine still concentration-dependently evoked depolarization in VP neurons (n = 5) of 6.7 ± 1.8 mV, 12.7 ± 2.1 mV, and 19.8 ± 1.4 mV, respectively (Fig. 3A, B). Paired t-test analysis showed that TTX did not block the inward current induced by histamine (73.3 ± 7.7 pA vs 71.0 ± 8.1 pA; n = 5, t4 = 2.123, P = 0.1010; Fig. 3C, D). These results clearly confirmed that the excitation of GABAergic VP neurons by histamine is a direct postsynaptic effect.
Fig. 3.
Histamine-induced excitation of GABAergic VP neurons is a direct postsynaptic effect. A Histamine depolarized a GABAergic VP neuron in a concentration-dependent manner in the presence of TTX. B Group data of the tested neurons. C TTX did not block the inward current induced by histamine. D Group data of the tested neurons. n.s. indicates no significant difference.
Histamine H1 and H2 Receptors Co-mediate the Excitatory Effect of Histamine on GABAergic VP Neurons
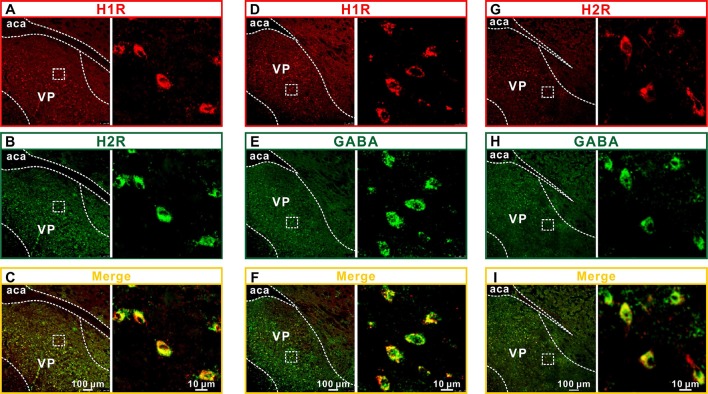
Four distinct histamine receptor subtypes (H1, H2, H3, and H4) have been identified [25, 26]. Among them, H1, H2 and H4 are postsynaptic but H4 is predominantly expressed in the periphery [11, 27]. Therefore, we assessed the expression of H1 and H2 receptors in the VP. Double immunostaining for H1 and H2 showed that they were not only present in the rat VP but also co-localized in the same neurons (Fig. 4A–C). Moreover, double immunostaining for H1/H2 receptors and GABA showed that H1 and H2 localized on the GABAergic VP neurons (Fig. 4D–I).
Fig. 4.
Histamine H1 and H2 receptors are expressed on GABAergic VP neurons. A–C Double immunofluorescence staining for H1 (A) and H2 (B) in rat VP neurons. D–F Double immunofluorescence staining for GABA (D) and H1 (E) in rat VP neurons. G–I Double immunofluorescence staining for GABA (G) and H2 (H) in rat VP neurons. aca, anterior commissure, anterior part; VP, ventral pallidum.
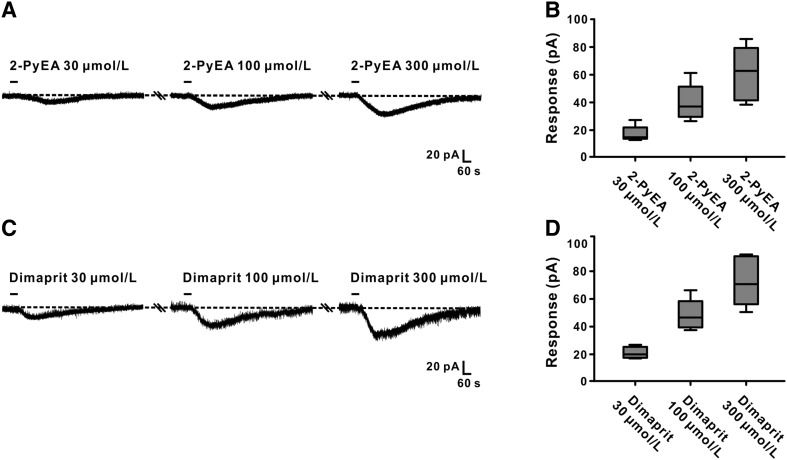
We used selective agonists and antagonists for H1 and H2 receptors in patch clamp recordings to further determine whether both receptors mediate the excitatory effect of histamine on GABAergic VP neurons. Both 2-PyEA (30, 100 and 300 μmol/L, a selective agonist for H1) and dimaprit (30, 100 and 300 μmol/L, a selective agonist for H2) mimicked the histamine-induced inward current in GABAergic VP neurons in a concentration-dependent manner: 16.0 ± 2.6, 38.8 ± 6.0, and 60.1 ± 8.9 pA (n = 5; Fig. 5A, B) and 20.2 ± 1.9, 47.9 ± 5.0, and 72.8 ± 8.1 pA (n = 5; Fig. 5C, D), respectively.
Fig. 5.
Agonists for H1 and H2 receptors mimic the excitatory effect of histamine on GABAergic VP neurons. A 2-PyEA (selective agonist for H1) mimicked the histamine-induced inward currents in a concentration-dependent manner. B Group data for the neurons tested. C Dimaprit (selective agonist for H2) concentration-dependently mimicked the histamine-induced inward currents. D Group data of the neurons tested.
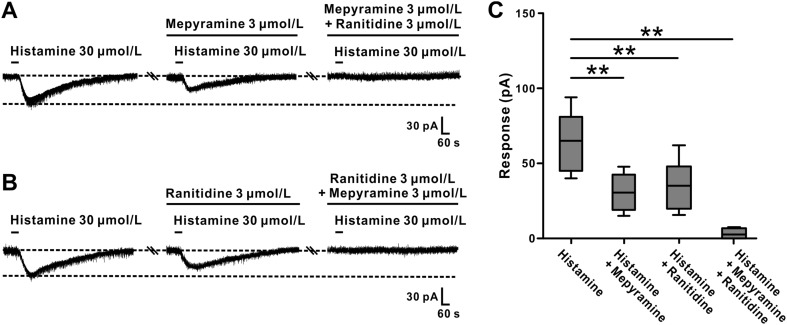
In addition, the inward currents induced by histamine were partially blocked by 3 μmol/L mepyramine (a selective H1 receptor antagonist) or 3 μmol/L ranitidine (a selective H2 receptor antagonist) from 65.4 ± 8.4 pA to 30.9 ± 4.9 pA (n = 6, t5 = 9.273, P < 0.01; Fig. 6A, C) and from 68.3 ± 12.0 pA to 36.3 ± 6.6 pA (n = 6, t5 = 5.498, P < 0.01), respectively. These results indicated that both histamine H1 and H2 receptors are involved in the excitatory effect of histamine on GABAergic VP neurons. Furthermore, combined application of mepyramine and ranitidine totally blocked the histamine-induced excitation (n = 6, t5 = 7.432, P < 0.01; Fig. 6A–C), strongly suggesting that histamine excites GABAergic VP neurons by co-activation of histamine H1 and H2 receptors.
Fig. 6.
Antagonists for H1 and H2 receptors block the excitatory effect of histamine on GABAergic VP neurons. A The inward current induced by histamine was partly blocked by mepyramine (selective antagonist for H1) and totally blocked by combined application of mepyramine and ranitidine (selective antagonist for H2). B The inward current induced by histamine was partly blocked by ranitidine and totally blocked by combined application of ranitidine and mepyramine. C Group data of the neurons tested. **P < 0.01.
Discussion
The basal ganglia contain high densities of histamine receptors [15, 16] and are one of the targets of the central histaminergic system. Although the effects of histamine on several structures scattered in the motor loop of the basal ganglia, including the substantia nigra [28] and globus pallidus [29], have been reported, the role of histamine and the central histaminergic system in the VP, a key structure in the limbic loop of the basal ganglia, remains enigmatic. In the present study, by means of whole-cell patch clamp recording and immunofluorescent staining techniques, we reveal a direct postsynaptic activation of histamine on GABAergic VP neurons, the main population of neurons in the VP.
Neuroanatomical studies have demonstrated that a majority of GABAergic VP neurons project to structures in the mesocorticolimbic system, including the nucleus accumbens, VTA, and mediodorsal thalamus [3]. Therefore, GABAergic neurons are implicated as the most important neuronal population in the VP for encoding cues for incentive salience and reward hedonics. Silencing of GABAergic VP neurons projecting to the VTA blocks the primed reinstatement of cocaine-seeking [8]. Moreover, the parvalbumin-positive VP neurons that project to the VTA also subserve the symptoms of social anhedonia [4]. In the present study, we found a strong postsynaptic depolarization/excitation effect of histamine on the GABAergic neurons in the VP. We therefore suggest that histamine and the central histaminergic system may influence mesocorticolimbic activity via direct modulation of the VP GABAergic projection neurons, and ultimately regulate motivational and emotional behaviors.
Histamine is well known to hold a key position in a variety of complex behaviors, ranging from feeding to arousal [9]. However, the role of brain histamine in motivation is still unclear. Notably, potential links between histamine and reward behaviors have recently been explored. Methamphetamine-induced behavioral sensitization is significantly exaggerated in l-histidine decarboxylase knockout mice [30], which are genetically unable to synthetize histamine. A similar facilitation of methamphetamine addiction has also been reported in mice with double-knockout of H1/H2 receptor genes [31]. These results suggest that the central histaminergic system may participate in methamphetamine-induced behavioral sensitization. On the other hand, H3-receptor-knockout mice, which show enhanced histamine neurotransmission, exhibit a greater extent of wakefulness driven by palatable food motivation [32]. In addition, a lack of motivation induced by a novel object or environment has been reported in H1-receptor-knockout mice [33]. This evidence strongly suggests that histamine and the central histaminergic system may be actively involved in motivational processing driven by different reinforcers (addictive drugs, palatable food, and novel objects), although they may play different roles in distinct motivations by the mediation of different receptor subtypes. Although histamine receptors are widely expressed in various neural cells including astrocytes [34], our results show that histamine H1 and H2 receptors are expressed in GABAergic VP neurons and consequently co-mediate the excitatory effect of histamine on these neurons. This suggests that the central histaminergic system may regulate motivational behaviors by directly modulating the projection neurons in the VP, a critical structure for motivation and emotion, via both H1 and H2 receptors.
In conclusion, the present study reveals a direct excitatory action of histamine on GABAergic neurons in the VP via co-activation of postsynaptic H1 and H2 receptors. Through biasing neuronal activity in the VP, the central histaminergic system and histamine may hold a key position in maintaining excitability and setting an appropriate level of sensitivity to external reward and emotional signals. In this way, the central histaminergic system and histamine may actively participate in the regulation of motivational and emotional behaviors.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81671107, 31330033, 91332124, 31471112, 31600834, and the NSFC and the Research Grants Council Joint Research Scheme 31461163001); the Ministry of Education of China (Fundamental Research Funds for the Central Universities 020814380071, 020814380048 and 020814380091) and the China Postdoctoral Science Foundation (2017T100351).
Conflict of interest
All authors claim that there are no conflicts of interest.
Contributor Information
Lei Yu, Email: yulei_nju@163.com.
Jing-Ning Zhu, Email: jnzhu@nju.edu.cn.
References
- 1.Zahm DS, Zaborszky L, Alheid GF, Heimer L. The ventral striatopallidothalamic projection: II. The ventral pallidothalamic link. J Comp Neurol. 1987;255:592–605. doi: 10.1002/cne.902550410. [DOI] [PubMed] [Google Scholar]
- 2.Maurice N, Deniau JM, Menetrey A, Glowinski J, Thierry AM. Position of the ventral pallidum in the rat prefrontal cortex-basal ganglia circuit. Neuroscience. 1997;80:23–534. doi: 10.1016/S0306-4522(97)00002-X. [DOI] [PubMed] [Google Scholar]
- 3.Tripathi A, Prensa L, Mengual E. Axonal branching patterns of ventral pallidal neurons in the rat. Brain Struct Funct. 2013;8:1133–1157. doi: 10.1007/s00429-012-0451-0. [DOI] [PubMed] [Google Scholar]
- 4.Knowland D, Lilascharoen V, Pacia CP, Shin S, Wang EH, Lim BK. Distinct ventral pallidal neural populations mediate separate symptoms of depression. Cell. 2017;170:284–297. doi: 10.1016/j.cell.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith KS, Berridge KC. The ventral pallidum and hedonic reward: neurochemical maps of sucrose ‘‘liking’’ and food intake. J Neurosci. 2005;25:8637–8649. doi: 10.1523/JNEUROSCI.1902-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richard JM, Ambroggi F, Janak PH, Fields HL. Ventral pallidum neurons encode incentive value and promote cue-elicited instrumental actions. Neuron. 2016;90:1165–1173. doi: 10.1016/j.neuron.2016.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller JM. Anhedonia after a selective bilateral lesion of the globus pallidus. Am J Psychiatry. 2006;163:786–788. doi: 10.1176/ajp.2006.163.5.786. [DOI] [PubMed] [Google Scholar]
- 8.Mahler SV, Vazey EM, Beckley JT, Keistler CR, McGlinchey EM, Kaufling J, et al. Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nat Neurosci. 2014;7:577–585. doi: 10.1038/nn.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haas HL, Panula P. The role of histamine and the tuberomamillary nucleus in the nervous system. Nat Rev Neurosci. 2003;4:121–130. doi: 10.1038/nrn1034. [DOI] [PubMed] [Google Scholar]
- 10.Zhu JN, Yung WH, Chow BKC, Chan YS, Wang JJ. The cerebellar-hypothalamic circuits: potential pathways underlying cerebellar involvement in somatic-visceral integration. Brain Res Rev. 2006;52:93–106. doi: 10.1016/j.brainresrev.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Haas HL, Sergeeva OA, Selbach O. Histamine in the nervous system. Physiol Rev. 2008;88:1183–1241. doi: 10.1152/physrev.00043.2007. [DOI] [PubMed] [Google Scholar]
- 12.Li B, Zhu JN, Wang JJ. Histaminergic afferent system in the cerebellum: structure and function. Cerebellum Ataxias. 2014;1:5. doi: 10.1186/2053-8871-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panula P, Pirvola U, Auvinen S, Airaksinen MS. Histamine-immunoreactive nerve fibres in the rat brain. Neuroscience. 1989;28:585–610. doi: 10.1016/0306-4522(89)90007-9. [DOI] [PubMed] [Google Scholar]
- 14.Cumming P, Damsma G, Fibiger HC, Vincent SR. Characterization of extracellular histamine in the striatum and bed nucleus of the stria terminalis of the rat: an in vivo microdialysis study. J Neurochem. 1991;56:1797–1803. doi: 10.1111/j.1471-4159.1991.tb02083.x. [DOI] [PubMed] [Google Scholar]
- 15.Cumming P, Laliberte C, Gjedde A. Distribution of histamine H3 binding in forebrain of mouse and guinea pig. Brain Res. 1994;664:276–279. doi: 10.1016/0006-8993(94)91985-2. [DOI] [PubMed] [Google Scholar]
- 16.Vizuete ML, Traiffort E, Bouthenet ML, Ruat M, Souil E, Tardivel-Lacombe J, et al. Detailed mapping of the histamine H2 receptor and its gene transcripts in guinea-pig brain. Neuroscience. 1997;80:321–343. doi: 10.1016/S0306-4522(97)00010-9. [DOI] [PubMed] [Google Scholar]
- 17.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 7. San Diego: Academic Press; 2014. [Google Scholar]
- 18.Yu L, Zhang XY, Cao SL, Peng SY, Ji DY, Zhu JN, et al. Na+ -Ca2+ exchanger, leak K+ channel and hyperpolarization-activated cyclic nucleotide-gated channel comediate the histamine-induced excitation on rat inferior vestibular nucleus neurons. CNS Neurosci Ther. 2016;22:184–193. doi: 10.1111/cns.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, Zhuang QX, Li B, Wu GY, Yung WH, Zhu JN, et al. Selective modulation of histaminergic inputs on projection neurons of cerebellum rapidly promotes motor coordination via HCN channels. Mol Neurobiol. 2016;53:1386–1401. doi: 10.1007/s12035-015-9096-3. [DOI] [PubMed] [Google Scholar]
- 20.Li B, Zhang XY, Yang AH, Peng XC, Chen ZP, Zhou JY, et al. Histamine increases neuronal excitability and sensitivity of the lateral vestibular nucleus and promotes motor behaviors via HCN channel coupled to h2 receptor. Front Cell Neurosci. 2017;10:300. doi: 10.3389/fnagi.2017.00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao HR, Zhuang QX, Zhang YX, Chen ZP, Li B, Zhang XY, et al. Orexin directly enhances the excitability of globus pallidus internus neurons in rat by co-activating OX1 and OX2 receptors. Neurosci Bull. 2017;33:365–372. doi: 10.1007/s12264-017-0127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Chen ZP, Zhuang QX, Zhang XY, Li HZ, Wang JJ, et al. Role of corticotropin-releasing factor in cerebellar motor control and ataxia. Curr Biol. 2017;27:2661–2669. doi: 10.1016/j.cub.2017.07.035. [DOI] [PubMed] [Google Scholar]
- 23.Wang ZC, Li LH, Bian C, Yang L, Lv N, Zhang YQ. Involvement of NF-κB and the CX3CR1 signaling network in mechanical allodynia induced by tetanic sciatic stimulation. Neurosci Bull. 2017;34:64–73. doi: 10.1007/s12264-017-0149-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bengtson CP, Osborne PB. Electrophysiological properties of anatomically identified ventral pallidal neurons in rat brain slices. Ann N Y Acad Sci. 1999;877:691–694. doi: 10.1111/j.1749-6632.1999.tb09303.x. [DOI] [PubMed] [Google Scholar]
- 25.Bongers G, de Esch I, Leurs R. Molecular pharmacology of the four histamine receptors. Adv Exp Med Biol. 2010;709:11–19. doi: 10.1007/978-1-4419-8056-4_2. [DOI] [PubMed] [Google Scholar]
- 26.Seifert R, Strasser A, Schneider EH, Neumann D, Dove S, Buschauer A. Molecular and cellular analysis of human histamine receptor subtypes. Trends Pharmacol Sci. 2013;34:33–58. doi: 10.1016/j.tips.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jutel M, Akdis M, Akdis CA. Histamine, histamine receptors and their role in immune pathology. Clin Exp Allergy. 2009;39:1786–1800. doi: 10.1111/j.1365-2222.2009.03374.x. [DOI] [PubMed] [Google Scholar]
- 28.Anichtchik OV, Rinne JO, Kalimo H, Panula P. An altered histaminergic innervation of the substantia nigra in Parkinson’s disease. Exp Neurol. 2000;163:20–30. doi: 10.1006/exnr.2000.7362. [DOI] [PubMed] [Google Scholar]
- 29.Chen K, Wang JJ, Yung WH, Chan YS, Chow BK. Excitatory effect of histamine on neuronal activity of rat globus pallidus by activation of H2 receptors in vitro. Neurosci Res. 2005;53:288–297. doi: 10.1016/j.neures.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 30.Brabant C, Quertemont E, Anaclet C, Lin JS, Ohtsu H, Tirelli E. The psychostimulant and rewarding effects of cocaine in histidine decarboxylase knockout mice do not support the hypothesis of an inhibitory function of histamine on reward. Psychopharmacology (Berl) 2007;190:251–263. doi: 10.1007/s00213-006-0603-0. [DOI] [PubMed] [Google Scholar]
- 31.Iwabuchi K, Kubota Y, Ito C, Watanabe T, Yanai K. Methamphetamine and brain histamine: a study using histamine-related gene knockout mice. Ann N Y Acad Sci. 2004;1025:129–134. doi: 10.1196/annals.1316.016. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi K, Suwa H, Ishikawa T, Kotani H. Targeted disruption of H3 receptors results in changes in brain histamine tone leading to an obese phenotype. J Clin Invest. 2002;110:1791–1799. doi: 10.1172/JCI15784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zlomuzica A, Viggiano D, De Souza Silva MA, Ishizuka T, Gironi Carnevale UA, Ruocco LA, et al. The histamine H1-receptor mediates the motivational effects of novelty. Eur J Neurosci. 2008;27:1461–1474. doi: 10.1111/j.1460-9568.2008.06115.x. [DOI] [PubMed] [Google Scholar]
- 34.Fang Q, Hu WW, Wang XF, Yang Y, Lou GD, Jin MM, et al. Histamine up-regulates astrocytic glutamate transporter 1 and protects neurons against ischemic injury. Neuropharmacology. 2014;77:156–166. doi: 10.1016/j.neuropharm.2013.06.012. [DOI] [PubMed] [Google Scholar]