Introduction
The mitochondrion is a double-membrane organelle consisting of an outer membrane, intermembrane space, inner membrane, cristae, and matrix. It is the “energy plant” that provides most of the energy for cells. Mitochondria also participate in various processes such as calcium homeostasis, cell death, and cell growth during development and aging [1]. Mitochondrial abnormalities are a common phenomenon in aging and age-related neurodegenerative diseases such as Alzheimer’s disease (AD). Mitochondrial dysfunction is even taken to be a marker for aging, an indispensable risk factor for AD [1]. Anti-oxidants and factors that maintain mitochondrial homeostasis have beneficial effects in AD therapy. Thus, the mitochondrion is emerging as a novel target for AD therapy.
Mitochondrial Dysfunction is an Early and Common Feature of AD
AD is a neurodegenerative disease characterized by deficits in learning, memory, and other cognitive functions. Its major pathological characteristics are the presence of amyloid-β (Aβ) plaques and neurofibrillary tangles (NFTs) [2]. The plaques are mainly composed of Aβ that is produced by sequential proteolytic cleavage of amyloid precursor protein (APP) by β- and γ-secretases [3, 4]. NFTs consist of hyperphosphorylated tau, a multifunctional protein involved in microtubule assembly and stabilization [5, 6]. In addition to these two pathological markers, mitochondrial dysfunction has been taken to be an early feature of both familial and sporadic AD [7]. Glucose uptake is reduced in the neurons of AD patients. Altered morphology of mitochondria such as changed size, disruption of cristae, and intra-mitochondrial accumulation of osmiophilic materials, concurrent with enhanced oxidative damage and changes in mitochondrial mass, enzymes, and DNA, have also been found in the brains of AD patients [7, 8]. Some of these mitochondrial dysfunctions occur prior to the appearance of Aβ plaques and NFTs. The mitochondrial cascade hypothesis even proposes that mitochondrial dysfunction is a trigger for AD pathology including Aβ accumulation, formation of NFTs, and neurodegeneration [7].
Mitochondrial Dysfunction Triggers and Accelerates the Pathogenesis of AD
Consistent with the cascade hypothesis, mitochondrial dysfunction suppresses α-secretase activity, while enhancing the levels of β-secretase and PS1, a core protein in γ-secretase complex, thus enhancing Aβ production [9]. Mitochondrial dysfunction also enhances tau phosphorylation, making cells more susceptible to either Aβ- or tau-induced toxicity [9]. One of the mechanisms underlying the above effects of mitochondrial dysfunction is oxidative stress, which is induced by damaged mitochondria. Extensive oxidative stress is an early manifestation of AD [9]. The levels of oxidative markers are directly correlated with the severity of cognitive impairment as well as the symptomatic progression from mild cognitive impairment to AD [10]. Extensive oxidative stress causes the oxidative modification of proteins and lipids, eventually leading to neuronal dysfunction. In addition, oxidative stress can upregulate the levels of BACE1 and PS1, the key enzyme/protein for Aβ generation. Oxidative stress is also involved in Aβ- or tau-induced toxicity [9]. Consistently, antioxidants such as curcumin and the mitochondria-targeted antioxidant mitoQ attenuate AD pathology in mouse models of AD [9, 11]. Some antioxidants are even under clinical trials in therapy for mild cognitive impairment and AD [11].
Aβ and Tau Accumulation Cause Mitochondrial Dysfunction
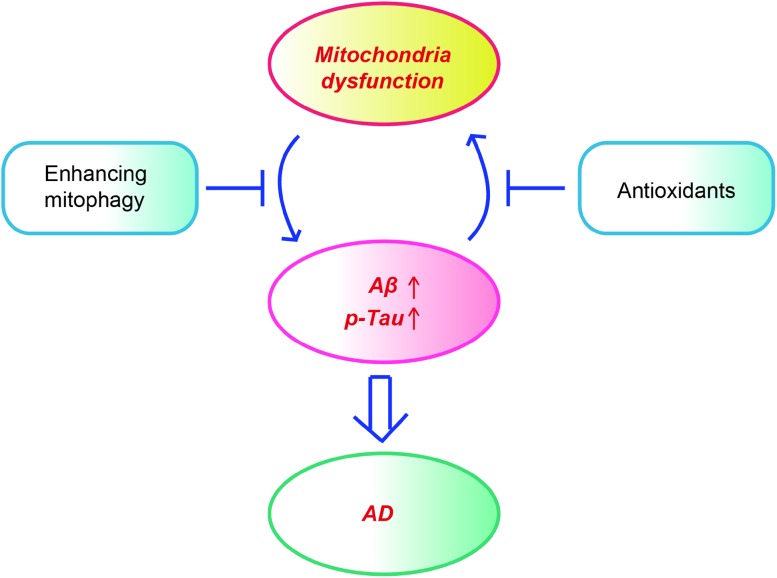
It is worth noting that the two pathological species, oligomeric Aβ and hyperphosphorylated tau, also cause mitochondrial damage. Aβ is translocated to mitochondria, where it interacts with various proteins to modulate mitochondrial functions. For example, Aβ regulates the mitochondrial permeability transition by interacting with adenine nucleotide translocase, the voltage-dependent anion channel, and cyclophilin D. Aβ modulates ATP generation via binding to ATP synthase subunit α [12]. Intracellular Aβ, rather than extracellular Aβ, enhances nitric oxide production while reducing mitochondrial activity [13]. The Aβ-enhanced nitric oxide production promotes the S-nitrosylation of Drp1, a dynamin-like protein that plays a role in mitochondrial fission. Blocking the nitrosylation of Drp1 by mutating cysteine abrogates the Aβ-induced mitochondrial dysfunction and suppresses synaptic plasticity [14]. Aβ also interacts with the mitochondrial protein Aβ-binding ethanol dehydrogenase (ABAD), preventing the binding of NAD+ to ABAD, thereby changing the mitochondrial permeability transition and decreasing the activity of respiratory chain enzymes [15]. Consistently, supplementation with NAD+ precursors like nicotinamide riboside and nicotinamide mononucleotide ameliorates the cognitive deficits, synaptic plasticity, and tau phosphorylation in mouse models of AD [16, 17]. Hyperphosphorylated tau also causes mitochondrial damage by disturbing mitochondrial dynamics [6], increasing the mitochondrial membrane potential [5] and reducing the expression of components of the mitochondrial respiratory chain complex and antioxidant enzymes [18]. Therefore, a vicious cycle also exists between mitochondrial dysfunction and Aβ or tau pathology. Accumulation of Aβ or hyperphosphorylated tau causes mitochondrial dysfunction, which in turn exacerbates Aβ accumulation and tau hyperphosphorylation and the latter further damages mitochondria (Fig. 1).
Fig. 1.
Mitochondrial dysfunction and pathological hallmarks form a vicious cycle in AD pathology. Mitochondrial dysfunction triggers and accelerates the accumulation of Aβ and tau, which in turn exaggerate mitochondrial damage. Anti-oxidant agents and enhancing mitophagy have beneficial effects in mouse models of AD.
Compromised Mitophagy Contributes to the Accumulation of Damaged Mitochondria in AD
Damaged mitochondria are mainly degraded by autophagy, a process known as mitophagy. Autophagy is a highly-conserved system for the degradation of abnormally aggregated proteins and organelles. Cargos are encapsulated in autophagosomes and then transferred to lysosomes for degradation. Mitophagy is an important mitochondrial quality-control system in both aging and AD. Once mitochondria are damaged, PTEN-induced putative kinase protein 1 is recruited and accumulates on the surface of damaged mitochondria, and it further recruits parkin from the cytosol to the mitochondria. Parkin, an E3 ubiquitin ligase, can ubiquitinate outer mitochondrial membrane (OMM) proteins, thus activating the ubiquitin-proteasome system and resulting in the degradation of OMM proteins [19]. Mitophagy/autophagy is impaired in AD [19]. One of the mechanisms underlying this impairment is lysosomal dysfunction. In this context, it is worth noting that mutations in PS1, reduce lysosomal hydrolase activity [20]. The intracellular accumulation of wild-type tau also induces a mitophagy deficit by the dose-dependent allocation of tau protein to the mitochondria. In addition, the downregulation of autophagy factors such as Beclin1 and SIRT1/3 also contributes to impaired mitophagy/autophagy in AD brains [21–25]. Consistently, enhancing autophagy/mitophagy by suppressing mTOR or by mTOR-independent means ameliorates AD-like pathology such as those of Aβ and tau and the cognitive deficits in mouse models of AD [26–29].
Mitophagy receptors play essential roles in selecting damaged mitochondria for degradation by autophagy. One of the characteristic features of these receptors is that they contain an LC3-interacting region, through which they bind to LC3. Once the damaged mitochondria are recognized by mitophagy receptors, these receptors recruit assembling autophagosomes (phagophores) to the damaged mitochondria and trigger the extension and closure of phagophores to form autophagosomes. In this way, damaged mitochondria are recruited to autophagosomes and degraded when the autophagosomes fuse with lysosomes [19]. Limited numbers of mitophagy receptors have been identified so far: P62, NBR1, Nix, TAxBP1, NDP51, optineurin, FUNDC1, and Ambra1 [19]. Our group recently reported that disrupted in schizophrenia 1 (DISC1), which is a risk factor for psychiatric disorders such as schizophrenia, bipolar disorder, and autism spectrum disorder, acting as a novel mitophagy receptor, promotes the clearance of mitochondria damaged by oligomeric Aβ [30]. DISC1 levels are downregulated in the brains of AD patients and APP/PS1 transgenic mice [30, 31], consistent with the previous report that DISC1 is genetically associated with AD [32]. We further found downregulation of DISC1 by treatment with oligomeric Aβ. DISC1 harbors a classical LC3-interacting motif, through which DISC1 directly binds to LC3. Overexpression of wild-type DISC1, rather than mutant DISC1 lacking LC3 binding, prevents oligomeric Aβ-induced synaptic loss and cognitive deficits, concomitant with rescue of mitochondrial dysfunction [30].
In summary, mitochondrial dysfunction is one of the early features of AD pathology. It triggers and accelerates Aβ accumulation and tau hyperphosphorylation, which in turn further damage mitochondria. Compromised mitophagy partly accounts for the accumulation of damaged mitochondria in the brain. Enhancing mitophagy or ameliorating mitochondrial dysfunction has beneficial effects in mouse models of AD, highlighting that the mitochondrion is a potential therapeutic target for AD (Fig. 1). Research on the molecular mechanisms underlying mitochondrial dysfunction at different stages of AD pathology, especially in the early stage, may suggest novel strategies/targets for AD therapy. Considering that mitochondrial dysfunction is an early feature of AD, further investigation on how altered mitochondrial metabolites are linked to AD may reveal novel biomarkers for the diagnosis of AD.
Acknowledgements
This perspective article was supported by the National Natural Science Foundation of China (81870897, 81671111, and 81601111), the Natural Science Foundation of Jiangsu Province, China (BK20181436), the Priority Academic Program Development of Jiangsu Higher Education Institutions, the Suzhou Clinical Research Center of Neurological Disease (Szzx201503), a Jiangsu Provincial Medical Key Discipline Project (ZDXKB2016022), the Jiangsu Provincial Special Program of Medical Science (BL2014042), and the Jiangsu Key Laboratory of Translational Research and Therapy for Neuro-Psycho-Diseases (BM2013003).
Conflict of interest
The authors have no conflicting financial interests.
References
- 1.Srivastava S. The mitochondrial basis of aging and age-related disorders. Genes (Basel) 2017, 8. [DOI] [PMC free article] [PubMed]
- 2.Zhu Y, Zhang H. A mouse model of Alzheimer’s disease with transplanted stem-cell-derived human neurons. Neurosci Bull. 2017;33:766–768. doi: 10.1007/s12264-017-0180-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang A, Wang C, Song B, Zhang W, Guo Y, Yang R, et al. Attenuation of beta-amyloid toxicity in vitro and in vivo by accelerated aggregation. Neurosci Bull. 2017;33:405–412. doi: 10.1007/s12264-017-0144-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu Y, Li Y, Zhang Y. Yeast two-hybrid screening for proteins that interact with the extracellular domain of amyloid precursor protein. Neurosci Bull. 2016;32:171–176. doi: 10.1007/s12264-016-0021-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu Y, Li XC, Wang ZH, Luo Y, Zhang X, Liu XP, et al. Tau accumulation impairs mitophagy via increasing mitochondrial membrane potential and reducing mitochondrial Parkin. Oncotarget. 2016;7:17356–17368. doi: 10.18632/oncotarget.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li XC, Hu Y, Wang ZH, Luo Y, Zhang Y, Liu XP, et al. Human wild-type full-length tau accumulation disrupts mitochondrial dynamics and the functions via increasing mitofusins. Sci Rep. 2016;6:24756. doi: 10.1038/srep24756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swerdlow RH, Burns JM, Khan SM. The Alzheimer’s disease mitochondrial cascade hypothesis: progress and perspectives. Biochim Biophys Acta. 2014;1842:1219–1231. doi: 10.1016/j.bbadis.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pickett EK, Rose J, McCrory C, McKenzie CA, King D, Smith C, et al. Region-specific depletion of synaptic mitochondria in the brains of patients with Alzheimer’s disease. Acta Neuropathol. 2018;136:747–757. doi: 10.1007/s00401-018-1903-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao Y, Zhao B. Oxidative stress and the pathogenesis of Alzheimer’s disease. Oxid Med Cell Longev. 2013;2013:316523. doi: 10.1155/2013/316523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ansari MA, Scheff SW. Oxidative stress in the progression of Alzheimer disease in the frontal cortex. J Neuropathol Exp Neurol. 2010;69:155–167. doi: 10.1097/NEN.0b013e3181cb5af4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mecocci P, Polidori MC. Antioxidant clinical trials in mild cognitive impairment and Alzheimer’s disease. Biochim Biophys Acta. 2012;1822:631–638. doi: 10.1016/j.bbadis.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt C, Lepsverdize E, Chi SL, Das AM, Pizzo SV, Dityatev A, et al. Amyloid precursor protein and amyloid beta-peptide bind to ATP synthase and regulate its activity at the surface of neural cells. Mol Psychiatry. 2008;13:953–969. doi: 10.1038/sj.mp.4002077. [DOI] [PubMed] [Google Scholar]
- 13.Keil U, Bonert A, Marques CA, Scherping I, Weyermann J, Strosznajder JB, et al. Amyloid beta-induced changes in nitric oxide production and mitochondrial activity lead to apoptosis. J Biol Chem. 2004;279:50310–50320. doi: 10.1074/jbc.M405600200. [DOI] [PubMed] [Google Scholar]
- 14.Cho DH, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z, et al. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324:102–105. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lustbader JW, Cirilli M, Lin C, Xu HW, Takuma K, Wang N, et al. ABAD directly links Abeta to mitochondrial toxicity in Alzheimer’s disease. Science. 2004;304:448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Hu X, Yang Y, Takata T, Sakurai T. Nicotinamide mononucleotide protects against beta-amyloid oligomer-induced cognitive impairment and neuronal death. Brain Res. 2016;1643:1–9. doi: 10.1016/j.brainres.2016.04.060. [DOI] [PubMed] [Google Scholar]
- 17.Hou Y, Lautrup S, Cordonnier S, Wang Y, Croteau DL, Zavala E, et al. NAD(+) supplementation normalizes key Alzheimer’s features and DNA damage responses in a new AD mouse model with introduced DNA repair deficiency. Proc Natl Acad Sci U S A. 2018;115:E1876–E1885. doi: 10.1073/pnas.1718819115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.David DC, Hauptmann S, Scherping I, Schuessel K, Keil U, Rizzu P, et al. Proteomic and functional analyses reveal a mitochondrial dysfunction in P301L tau transgenic mice. J Biol Chem. 2005;280:23802–23814. doi: 10.1074/jbc.M500356200. [DOI] [PubMed] [Google Scholar]
- 19.Kerr JS, Adriaanse BA, Greig NH, Mattson MP, Cader MZ, Bohr VA, et al. Mitophagy and Alzheimer’s disease: cellular and molecular mechanisms. Trends Neurosci. 2017;40:151–166. doi: 10.1016/j.tins.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JH, Yu WH, Kumar A, Lee S, Mohan PS, Peterhoff CM, et al. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell. 2010;141:1146–1158. doi: 10.1016/j.cell.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang EF, Scheibye-Knudsen M, Chua KF, Mattson MP, Croteau DL, Bohr VA. Nuclear DNA damage signalling to mitochondria in ageing. Nat Rev Mol Cell Biol. 2016;17:308–321. doi: 10.1038/nrm.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Julien C, Tremblay C, Emond V, Lebbadi M, Salem N, Jr, Bennett DA, et al. Sirtuin 1 reduction parallels the accumulation of tau in Alzheimer disease. J Neuropathol Exp Neurol. 2009;68:48–58. doi: 10.1097/NEN.0b013e3181922348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tseng AH, Shieh SS, Wang DL. SIRT3 deacetylates FOXO3 to protect mitochondria against oxidative damage. Free Radic Biol Med. 2013;63:222–234. doi: 10.1016/j.freeradbiomed.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Yang W, Zou Y, Zhang M, Zhao N, Tian Q, Gu M, et al. Mitochondrial Sirt3 expression is decreased in APP/PS1 double transgenic mouse model of Alzheimer’s disease. Neurochem Res. 2015;40:1576–1582. doi: 10.1007/s11064-015-1630-1. [DOI] [PubMed] [Google Scholar]
- 25.Salminen A, Kaarniranta K, Kauppinen A, Ojala J, Haapasalo A, Soininen H, et al. Impaired autophagy and APP processing in Alzheimer’s disease: The potential role of Beclin 1 interactome. Prog Neurobiol. 2013;106–107:33–54. doi: 10.1016/j.pneurobio.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Caccamo A, Magri A, Medina DX, Wisely EV, Lopez-Aranda MF, Silva AJ, et al. mTOR regulates tau phosphorylation and degradation: implications for Alzheimer’s disease and other tauopathies. Aging Cell. 2013;12:370–380. doi: 10.1111/acel.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, et al. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS One. 2010;5:e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du J, Liang Y, Xu F, Sun B, Wang Z. Trehalose rescues Alzheimer’s disease phenotypes in APP/PS1 transgenic mice. J Pharm Pharmacol. 2013;65:1753–1756. doi: 10.1111/jphp.12108. [DOI] [PubMed] [Google Scholar]
- 29.Portbury SD, Hare DJ, Sgambelloni C, Perronnes K, Portbury AJ, Finkelstein DI, et al. Trehalose improves cognition in the transgenic Tg2576 mouse model of Alzheimer’s disease. J Alzheimers Dis. 2017;60:549–560. doi: 10.3233/JAD-170322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z, Lu M, Zhang Y, Ji W, Lei L, Wang W, et al. DISC1 protects synaptic plasticity in a transgenic mouse model of Alzheimer’s disease as a mitophagy receptor. Aging Cell 2018. [DOI] [PMC free article] [PubMed]
- 31.Deng QS, Dong XY, Wu H, Wang W, Wang ZT, Zhu JW, et al. Disrupted-in-schizophrenia-1 attenuates amyloid-beta generation and cognitive deficits in APP/PS1 transgenic mice by reduction of beta-site APP-cleaving enzyme 1 Levels. Neuropsychopharmacology. 2016;41:440–453. doi: 10.1038/npp.2015.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen L, Jia J. An overview of genome-wide association studies in Alzheimer’s disease. Neurosci Bull. 2016;32:183–190. doi: 10.1007/s12264-016-0011-3. [DOI] [PMC free article] [PubMed] [Google Scholar]