Abstract
While inflammatory bowel disease (IBD) might be a risk factor in the development of brain dysfunctions, the underlying mechanisms are largely unknown. Here, mice were treated with 5% dextran sodium sulfate (DSS) in drinking water and sacrificed on day 7. The serum level of IL-6 increased, accompanied by elevation of the IL-6 and TNF-α levels in cortical tissue. However, the endotoxin concentration in plasma and brain of mice with DSS-induced colitis showed a rising trend, but with no significant difference. We also found significant activation of microglial cells and reduction in occludin and claudin-5 expression in the brain tissue after DSS-induced colitis. These results suggested that DSS-induced colitis increases systemic inflammation which then results in cortical inflammation via up-regulation of serum cytokines. Here, we provide new information on the impact of colitis on the outcomes of cortical inflammation.
Electronic supplementary material
The online version of this article (10.1007/s12264-018-0288-5) contains supplementary material, which is available to authorized users.
Keywords: Cortical inflammation, Dextran sodium sulfate, Colitis, Endotoxin, Blood-brain barrier
Introduction
Crohn disease and ulcerative colitis are the major forms of inflammatory bowel disease (IBD) [1], characterized by chronic diarrhea and abdominal pain [2]. However, many patients develop severe complications over time, such as cognitive dysfunction and depression [3, 4]. Intestinal inflammation might be a risk factor for the development of Parkinson’s disease [5], Alzheimer’s disease [6], and autism [7, 8], and rats with colitis show increased hippocampal slice excitability [9]. However, the mechanisms underlying the links between IBD and changes in the central nervous system (CNS) are largely unknown.
Peripheral inflammation can induce CNS inflammation [10]. One way for peripheral inflammation to alter function in the CNS is through disruption of the blood-brain barrier (BBB) [11]. The BBB is comprised of endothelial cells that line the capillaries of the brain. Its unique characteristics include tight junctions (TJs), which may change in the pathological process [12]. The movements underlying leukocyte–endothelial cell adhesive interactions are finely regulated by several cell adhesion molecules (CAMs). Brain endothelial VCAM-1 is markedly up-regulated in animals with colitis [13], indicating migration of leukocytes across the BBB. A slight loss in endothelial barrier antigen immunoreactivity has been reported in dextran sodium sulfate (DSS)-induced colitis, showing a marker of BBB disruption [5]. Disruption of the BBB could allow inflammatory mediators or other circulating products to enter the brain parenchyma [14].
Several reports have provided evidence that colitis disturbs the composition of gut microbiota and increases the levels of gut microbiota endotoxin [15, 16]. Therefore, it has been hypothesized that bacterial translocation occurs due to impaired colonic barrier function, thus leading to a deleterious pro-inflammatory systemic immune response [17]. Endotoxin can lead to nuclear factor-kB activation when gut-derived endotoxin enters the bloodstream [18]. Furthermore, systemic administration of endotoxin can also induce the expression of pro-inflammatory products, and it is a general stimulator of microglia in the brain [19, 20]. More importantly, when accompanied by disruption of the BBB, gut microbiota endotoxin may enter the brain parenchyma and result in marked neuroinflammation. Here, we explored whether endotoxin produced by the gut plays a role in the development of neuroinflammation under conditions of an impaired BBB in a DSS-induced colitis model.
Materials and Methods
Mice
Seven- to eight-week-old male C57BL/6 mice weighing 18 ± 2 g were housed at a constant temperature under a 12-h light-dark cycle with unlimited access to a standard diet and water. The animal studies were approved by the Animal Care and Use Committee of the Institute of Basic Medical Sciences.
Induction of Colitis
We used a well-characterized chemical model to initiate experimental IBD by giving mice access to 5% DSS (MP Biomedicals, Santa Ana, CA) in drinking water for 7 days. Mice were sacrificed on days 3 and 7 after the beginning of DSS administration in some experiments. Four independent experiments were carried out (4–6 mice/group in each experiment). Both the cortex and the hippocampus from each group were isolated for protein and RNA analysis All samples were run individually. Trunk blood was collected and circulating cytokines were measured in the serum using ELISA (Neobioscience Technology Co, Ltd, Shenzhen, China). Exogenous endotoxin was measured in the plasma using Limulus amebocyte lysate (LAL; Lot No: 170908; Chinese Horseshoe Crab Reagent Manufactory Co., Ltd, Xiamen, China). Additional groups of DSS-treated and control mice (5 mice/group) were sacrificed on day 7 and perfused with paraformaldehyde (4% in 0.1 mol/L phosphate buffer, pH 7.4) for immunofluorescence and confocal laser-scanning studies.
Lipopolysaccharide (LPS) Administration
In a separate experiment, the mice were given an intraperitoneal (i.p.) injection of a certain concentration of 0.5 mg/kg LPS (Sigma, St. Louis, MO) diluted in normal saline. Mice were sacrificed at 6 h and 24 h, and brain and trunk blood from each group were collected for LAL tests. This experiment was carried out twice.
Quantitative Real-Time PCR
Total RNA was extracted from cortical and intestinal tissue homogenates using TRIzol reagent (Invitrogen, Carlsbad, CA). cDNA was synthesized using an MLV (Murine Leukemia Virus) reverse transcription kit (TaKaRa, Kusatsu, Shiga, Japan) according to the manufacturer’s instructions. Real-time PCR was performed with SYBR Green master mix (Applied Biosystems, Waltham, MA) using a real-time PCR detection system as recommended by the manufacturer. The specific primers used were as follows: β-actin: forward: 5′-ACTGTCGAGTCGCGTCCA-3′, reverse: 5′-GTCATCCATGGCGAACTGGT-3′; IL-1β: forward: 5′-TTCAGGCAGGCAGTATCACTC-3′, reverse: 5′-GAAGGTCCACGGGAAAGACAC-3′; IL-6: forward: 5′-AGTCCTTCCTACCCCAATTTCC-3′, reverse: 5′-TTGGTTAGCCACTCCTTC-3′; tumor necrosis factor (TNF)-α: forward: 5′-CCCTCACACTCAGATCATCTTCT-3′, reverse: 5′-GCTACGACGTGGGCTACAG-3′. Gene-specific expression was normalized to β-actin expression.
Measurements of Serum IL-1β, IL-6, and TNF-α
ELISA analyses were performed to quantify the levels of inflammatory cytokines and interleukins in mouse serum. Blood samples were clotted for 2 h at room temperature before centrifugation (4°C, 2000 g, 30 min). The concentrations of IL-1β, IL-6, and TNF-α in the resulting supernatants were measured using a Quantikine ELISA kit (Neobioscience Technology Co, Ltd, Shenzhen, China) following the manufacturer’s protocol. The results are shown as the concentration of cytokine per milliliter of serum. The samples were aliquoted and stored at − 80°C.
LAL Assay for Endotoxin
Brain and plasma endotoxin concentrations were measured using the LAL kit (Lot No: 170908; Chinese Horseshoe Crab Reagent Manufactory). Total protein was extracted from half of the mouse brain samples which were homogenized in 1 mL endotoxin-free water. The clear supernatants were collected for LAL assays. For plasma analysis, the collected blood was added to an equal volume of heparin anticoagulant before centrifugation for 2 min at 3000 rpm. Samples of the resulting plasma were diluted in pyrogen-free water. The concentration of endotoxin that remained was quantified by coagulation of the LAL reagent. The samples were incubated at 70°C for 10 min to inactivate endogenous inhibitors of the LAL test, prior to the LAL assay. The concentrations of bacterial endotoxin were determined using the LAL kit according to the manufacturer’s protocol.
Immunofluorescence Staining
The mice were anesthetized with 1% sodium pentobarbital through intraperitoneal injection and perfused with chilled 0.9% saline to wash out circulating blood, followed by 4% paraformaldehyde. After each brain was dehydrated and frozen-sectioned at 40 μm, the sections were blocked with 5% donkey serum (0.2% PBST containing 5% donkey serum and 5% BSA) for 30 min at 37°C and then incubated with specific primary antibody (Iba-1, 1:400, WAKO, Tokyo, Japan) overnight at 4°C before incubation with secondary antibody (Alexa Fluor 594, 1:500, Thermo, Waltham, MA) for 120 min at 37°C. Images were captured using a scanning confocal microscope (Nikon, Tokyo, Japan).
Western Blot
Whole protein extracts were prepared from mouse cortex and hippocampus, and the total protein content was quantified. A total of 20 μg protein from each sample was loaded onto an SDS-PAGE gel and separated by electrophoresis. Then, the proteins were transferred to nitrocellulose membranes, followed by blocking in 5% non-fat dry milk in PBST for 2 h at room temperature. The specific primary antibodies (zonulae occludentes, ZO-1, 1:2000, Invitrogen, CA; occludin, 1:2000, Invitrogen; claudin-5, 1:2000, Thermo; caspase3, 1:1000, Abcam, Cambridge, UK; β-actin, 1:10000, Sigma) were applied overnight at 4°C. After washing, the membranes were incubated with HRP-conjugated goat-anti-rabbit (Bio-Rad, Hercules, CA) or goat-anti-mouse secondary antibody (Bio-Rad) for 2 h at room temperature. The specific bands were revealed using an ECL detection kit (Bio-Rad).
Determination of BBB Permeability
Mice were given a caudal vein injection of Evans blue dissolved in saline (4 mg/kg body weight) 4 h before sacrifice. All mice were anesthetized with 1% sodium pentobarbital through intraperitoneal injection and perfused; the samples were dissolved in formamide and bathed in water (55°C, 24 h), and then the samples were centrifuged (12000 g, 30 min). Measurements were made at 620 nm.
Hematoxylin-Eosin (HE) Staining
Mice were anesthetized with 1% pentobarbital sodium (40 mg/kg. i.p.) and sacrificed before the colon was isolated and sectioned. The intestines were washed in ice-cold PBS and soaked overnight in paraformaldehyde (4% in 0.1 mol/L phosphate buffer, pH 7.4). After immersion in absolute ethyl alcohol through a graded alcohol series, the tissues were embedded in paraffin, sectioned, and stained with HE. Then, the sections were imaged under a microscope (Olympus, Tokyo, Japan).
Statistical Analysis
All experiments were repeated at least 3 times. The data are presented as the group mean values with standard errors of the mean (SEM), and were analyzed with one-way ANOVA followed by Dunnett’s test, two-way ANOVA followed by Bonferroni’s post hoc test for multiple comparisons, or t-tests. For all analyses, P < 0.05 was considered statistically significant.
Results
Intestinal Inflammation in DSS-Treated Mice
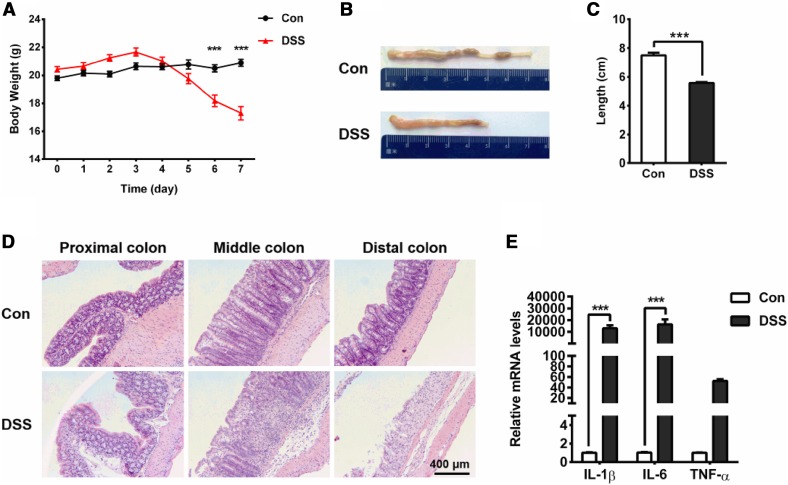
The acute colitis mouse model was induced by treatment with 5% DSS in the drinking water for 7 days, as previously described [21]. The mice exhibited bloody diarrhea (data not shown) and weight loss, as well as decreased colon length, as the colitis progressed (Fig. 1A–C). Histological analysis of the colons from the DSS-treated mice revealed cellularity and disorganization as well as infiltration of granulocytes in the mucosa (Fig. 1D). In addition, the pro-inflammatory cytokines IL-6, IL-1β, and TNF-α, were detected in the colonic tissues. The mRNA levels of IL-1β and IL-6 were significantly increased in the distal colon of mice administered with DSS (Fig. 1E). Thus, the mice exposed to 5% DSS for 7 days developed acute colitis.
Fig. 1.
Mouse model of colitis induced by 5% DSS after 7 days. A Cumulative changes in body weight in the course of DSS treatment. Note that severe weight loss was seen in the DSS-treated mice on days 6 and 7 (***P < 0.001; n = 10/group). B Representative photographs of intestinal mucosal congestion and shortened colon length. C Shrinkage of the colon in the DSS-treated mice occurred on day 7 (***P < 0.001; n = 5/group). D Pathological changes in HE-stained proximal, middle, and distal colon sections. Leukocyte infiltration into the mucosa and damage to the bowel wall are evident in the DSS-induced group. E Real-time PCR analysis of changes in the inflammatory cytokines IL-1β, IL-6, and TNF-α in the distal colon tissue in the mice. The increases in IL-1β (***P < 0.001; n = 5/group) and IL-6 (***P < 0.001; n = 5/group) were significant.
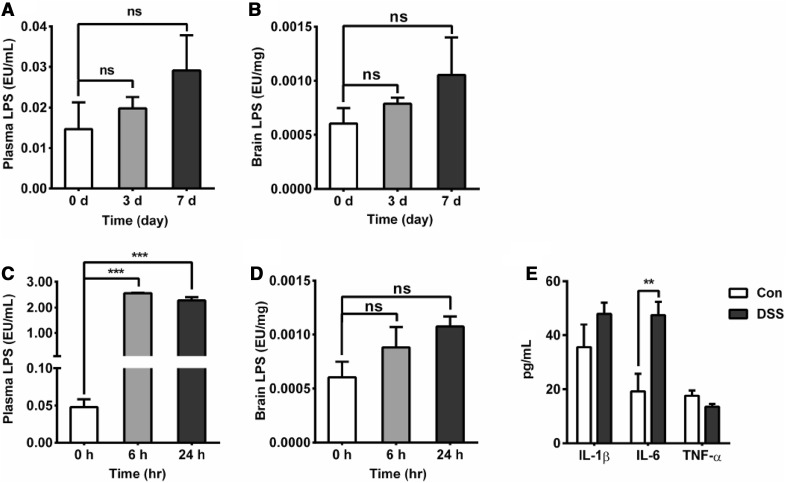
Concentrations of Endotoxin and Pro-Inflammatory Cytokines in the Plasma of Mice with DSS-Induced Colitis
Colitis can disturb the composition of gut microbiota and increase their endotoxin levels [22]. Therefore, it has been hypothesized that bacterial translocation occurs due to an impaired colonic barrier, leading to endotoxemia [23], However, the changes in endotoxin are still unknown in the colitis model. To explore whether endotoxin plays a direct or indirect role in DSS-induced nervous system inflammation, mice were sacrificed on days 3 and 7 after DSS administration. To our surprise, we did not find a significant increase in endotoxin in the plasma or in the brain on days 3 and 7 (Fig. 2A, B). In another independent experiment, the mice were injected with LPS (0.5 mg/kg i.p., 6 h and 24 h), which causes marked systemic inflammation, and then we measured the endotoxin content in the brain and plasma. The results showed that the LPS concentrations at both 6 h and 24 h were significantly increased, and the endotoxin content in the plasma was increased 25-fold (Fig. 2C). However, the levels of endotoxin in the brain did not increase after LPS injection (Fig. 2D). We concluded that plasma endotoxin translocation does not easily cause an elevation in the endotoxin levels in brain tissue under such conditions.
Fig. 2.
Effect of colitis on the endotoxin concentration in plasma and brain. A Plasma endotoxin levels after DSS treatment (n = 6/group); the endotoxin content in the plasma was not significantly increased on day 3 or 7. B Brain endotoxin levels after DSS treatment (n = 6/group); the endotoxin content in the brain showed a rising trend, but no significant difference was found on either day 3 or day 7 (n = 6/group). C Plasma endotoxin levels measured using a Limulus assay (LAL). LPS (0.5 mg/kg i.p.) increased plasma endotoxin levels at 6 h and 24 h (***P < 0.001; n = 5/group for both). D LAL analysis of brain endotoxin after LPS injection, showing that the LPS-treated mice had a rising trend, but no significant difference was found at either 6 h or 24 h (n = 5/group). E Changes in the serum levels of the pro-inflammatory cytokines IL-1β, IL-6, and TNF-α, showing that IL-6 (**P < 0.01; n = 5/group) was higher in the DSS-induced group on day 7 after DSS treatment.
We also analyzed the activation of systemic inflammation in the colitis mouse model. On day 7 after DSS treatment, the level of IL-6 in the serum was markedly elevated, while the levels of IL-1β and TNF-α did not change (Fig. 2E). This suggested that acute colitis induced the activation of systemic inflammation.
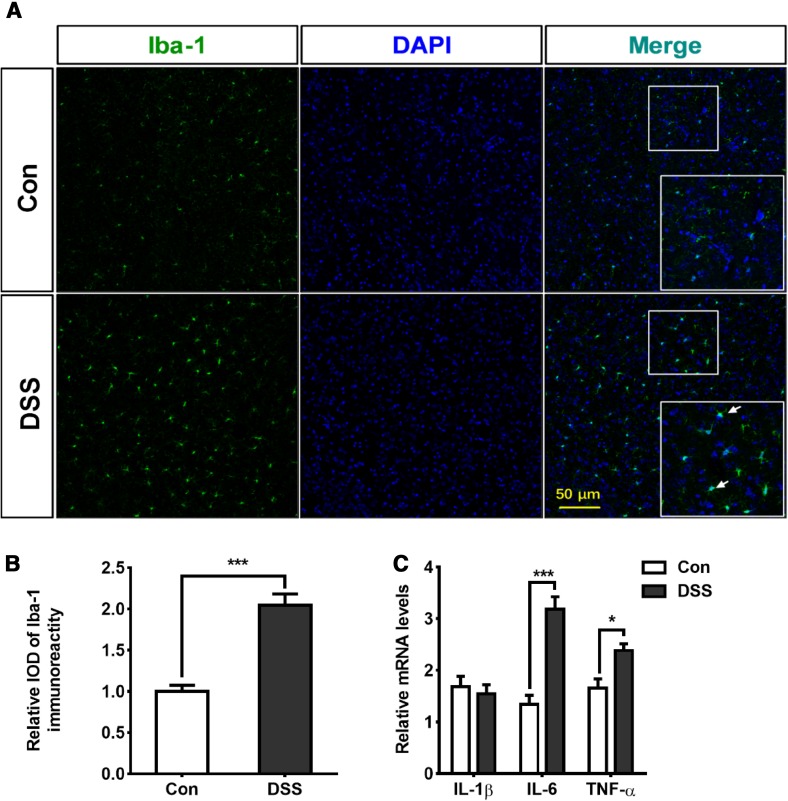
Activation of Microglia and Increased Pro-Inflammatory Cytokines in Cortical Tissue in DSS-Induced Colitis Model
The above results indicated that the endotoxin levels in both the plasma and the brain did not increase in the colitis mouse model. We then investigated whether a rise in plasma pro-inflammatory cytokines would result in neuroinflammation and brain injury after DSS-induced colitis. To evaluate brain inflammation in this model, we performed immunofluorescence staining for Iba-1 and analyzed the microglial population in the cortex. This population increased significantly after DSS-induced colitis (Fig. 3A, B). Next, we measured the expression of pro-inflammatory cytokines in the brain on day 7 of DSS-induced colitis. The level of IL-6 increased ~2-fold on day 7 compared with the control (Fig. 3C). In addition, the level of TNF-α mRNA in the brain also increased in the DSS group, while the level of IL-1β did not change as much (Fig. 3C). These results demonstrated that the peripheral inflammation involved in the colitis model triggered cortical inflammation.
Fig. 3.
Activation of microglia and increased pro-inflammatory cytokines in cortical tissue in mice with DSS-induced colitis. A, B Representative images (A) and statistics (B) of microglial activation in cortex on day 7 after DSS treatment (***P < 0.001; n = 4/group). C Effects of DSS-induced colitis on the mRNA levels of IL-1β, IL-6, and TNF-α in cortex. Increases in IL-6 (***P < 0.001; n = 5/group) and TNF-α (*P < 0.05; n = 5/group) occurred on day 7 after DSS treatment.
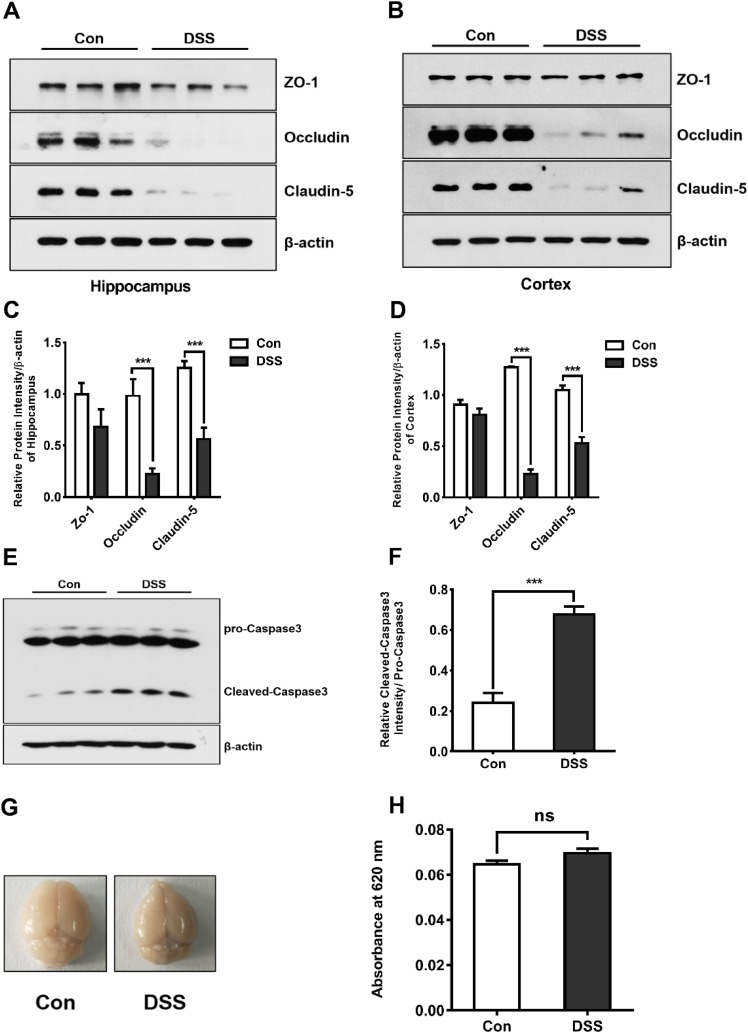
Reduction in Occludin and Claudin-5 Expression in Brain After DSS-Induced Colitis
It has been reported that peripheral inflammation can induce neuroinflammation in the CNS via disruption of the BBB [22], The unique characteristics of this barrier include tight intercellular junctions. We therefore isolated hippocampal and cortical tissues and analyzed the expression of TJ proteins. We found that the expression of occludin and claudin-5 was markedly downregulated in the cortex and the hippocampus of the group treated with DSS (Fig. 4A–D). The expression of cleaved caspase3 in the cortex was also up-regulated, suggesting the occurrence of apoptosis in the brain after the DSS treatment (Fig. 4E, F). To further describe alterations in the permeability of the BBB after DSS treatment in mice, we quantified the permeability using Evans blue dye. but did not find clear BBB disruption based on leakage and extravasation of Evans blue into the parenchyma (Fig. 4G, H). These results suggested that although DSS-induced colitis can generate mild brain injury by reducing TJ-related protein expression, it is not due to a severe increase in BBB permeability.
Fig. 4.
Reduction of occludin and claudin-5 expression, while the BBB permeability was not increased in the DSS-induced colitis model. A–D Western blots (A, B) and analysis (C, D) showing the down-regulation of occludin (***P < 0.001; n = 3/group) and claudin-5 (***P < 0.001; n = 3/group) in the hippocampus (A, C) and cortex (B, D) in the DSS-induced group. E, F Western blots (E) and analysis (F) showing up-regulation of cleaved caspase-3 in the cortex after DSS treatment (***P < 0.001; n = 3/group). G, H Photographs of brains (G) and statistics (H) showing no increase in the concentration of Evans blue in the brain after DSS treatment on day 7.
Discussion
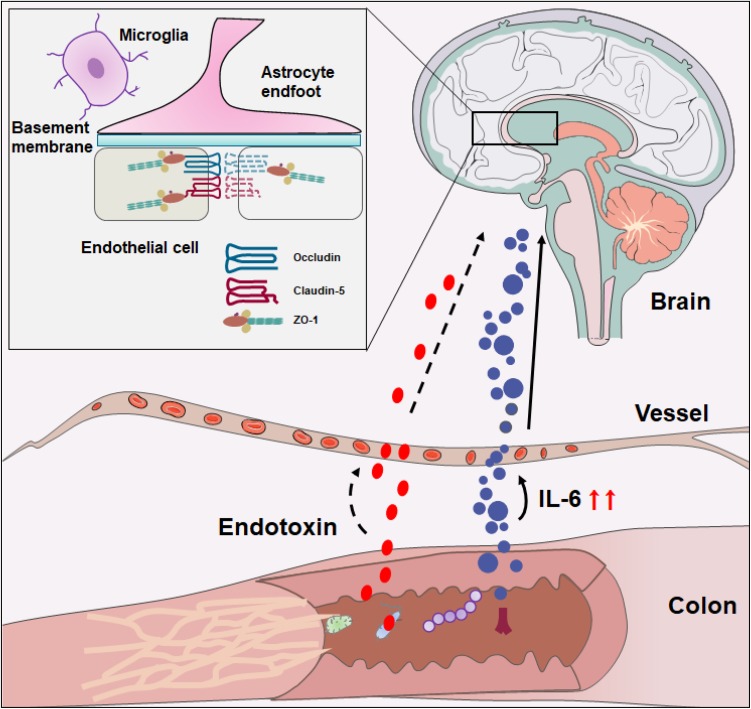
Here, we provide new information regarding the impact of DSS-induced colitis on the outcomes of neuroinflammation. The data showed that the endotoxin content was not increased in the plasma or in the brain on either day 3 or day 7 of the DSS-induced colitis model, whereas the serum levels of IL-6 increased and were accompanied by activation of microglia and an elevation in IL-6 and TNF-α expression levels in brain tissue during the DSS-induced colitis. Furthermore, DSS-induced colitis could generate a mild brain injury by reducing the TJ-related protein expression but not by severely increasing BBB permeability. In the light of this, the DSS-induced colitis model activated the population of microglia and changed the levels of pro-inflammatory cytokines, in addition to causing TJ injury, which may result in nonspecific CNS damage (Fig. 5).
Fig. 5.
Model of DSS-induced intestinal inflammation and triggering of cortical inflammation. The DSS-induced colitis increases the pro-inflammatory cytokines and triggers the activation of microglia as well as reduction of occludin and claudin-5 expression.
Peripheral inflammation can affect neuroinflammation in several ways. Peripheral inflammation signals the brain through the vagus nerve [24] and through cytokines in the systemic circulation that access the brain [25]. Engagement of the immune system-to-brain communication ultimately leads to the activation of resident microglia. By producing a variety of pro-inflammatory and neurotoxic factors [26], activated microglia can cause CNS damage [27, 28], but the mechanisms underlying the link between IBD and CNS changes are largely unknown. One way for peripheral inflammation to alter function in the CNS is through disruption of the BBB [29]. Such BBB disruption has been reported in 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced colitis [14]. IL-6 has been implicated in inflammatory processes and its elevation is associated with BBB disruption [30, 31]. In our study, we also found that elevated IL-6 levels induced a decrease of occludin and claudin-5 expression in mice with colitis. However, in this model, we did not find BBB disruption based on extravasation of Evans blue into the brain parenchyma, and staining for endogenous IgG proteins (Fig. S1). Several reports have emphasized that the BBB changes are transient; the extent of fluorescein leakage is limited to the first 2 days and only then in restricted regions despite the ongoing colitis. However, in our experiment, mice were treated with DSS for 7 days, so no leakage of Evans blue was observed in the brain. Importantly, the disruption of the BBB is always mild, which may explain why the leakage of Evans blue was not markedly changed under our experimental conditions [5, 14]. Though we found a decrease in occludin and claudin-5 expression, we cannot exclude the possibility that other TJ molecules that may play decisive roles in maintaining the integrity of the BBB were also affected. These data extend our knowledge regarding the disruption of the BBB during colitis and provide evidence that peripheral inflammation-induced neuroinflammation results in a mild disruption of the BBB. Colitis inducers, such as TNBS, DSS, and a high-fat diet, can increase gastrointestinal permeability, disturb the composition of gut microbiota, and increase their LPS levels [32, 33]. Previous studies have shown that treatment with LPS isolated from feces significantly increases nuclear factor-kappa B activation and TNF-alpha levels in TNBS-induced colitis [33]. In our study, at day 7, we also found that the serum levels of IL-6 were elevated in the DSS-treated animals, indicating the presence of systemic inflammation, together with the increased population of microglia as well as IL-6 and TNF-α mRNA levels in the brain. We hypothesized that endotoxin may play a direct role in DSS-induced nervous system inflammation. Then we measured the concentrations of endotoxin in the brain and plasma produced by gut microbiota in mice with DSS-induced colitis, but did not find a significant increase in blood endotoxin production – on the other hand, TNBS-induced colitis leads to endotoxin and bacterial translocation – this difference may have been due to the animal model used. In our experiments, DSS was administered orally, while TNBS is administered intrarectally [34]. In addition, in accord with previous reports, DSS-induced colitis is characterized by low circulating endotoxin levels [35]. This could be explained by the disruption degree of BBB, which prevents endotoxin from entering the brain. In another independent experiment, we injected LPS intraperitoneally then investigated the endotoxin content in the brain and plasma. The results showed that intraperitoneal LPS significantly increased the endotoxin content in the plasma of mice, while there was no change in brain endotoxin. We conclude that blood endotoxin translocation, under certain conditions, does not easily cause an elevation in brain endotoxin level, while the role of brain endotoxin cannot be excluded completely. Our present data did not support the conclusion that the presence of systemic endotoxin plays a major role in the outcome of neuroinflammation in the DSS mouse model. Nevertheless, it is hard to exclude a role of endotoxin in DSS-induced neuroinflammation.
In conclusion, 5% DSS administration for 7 days increases gastrointestinal inflammation, resulting in cortical inflammation in mice and leading to mild disruption of the BBB. Colitis may have an impact on the formation of blood and brain endotoxin related to the outcomes of cortical inflammation. However, further work is required to identify whether other colitis models, such as TNBS can cause a marked increase in blood and brain endotoxin.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Prof. TM Zhang of Academy of Military Medical Sciences for helpful comments. This work was supported by grants from the National Natural Science Foundation of China (81430044) and the National Basic Research Development Program of China (2012CB518200 and 2011CB910800).
Compliance with Ethical Standards
Conflict of interest
The authors have no conflict of interest to declare
Contributor Information
Ming Fan, Email: fanmingchina@126.com.
Ling-Ling Zhu, Email: linglingzhuamms@126.com.
References
- 1.Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis. 2006;12:S3–S9. doi: 10.1097/01.MIB.0000195385.19268.68. [DOI] [PubMed] [Google Scholar]
- 2.Sandler RS, Stewart WF, Liberman JN, Ricci JA, Zorich NL. Abdominal pain, bloating, and diarrheain the United States. Dig Dis Sci. 2000;45:1166–1171. doi: 10.1023/A:1005554103531. [DOI] [PubMed] [Google Scholar]
- 3.Zonis S, Pechnick RN, Ljubimov VA, Mahgerefteh M, Wawrowsky K, Michelsen KS, et al. Chronic intestinal inflammation alters hippocampal neurogenesis. J Neuroinflammation. 2015;12:65. doi: 10.1186/s12974-015-0281-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurina LM, Goldacre MJ, Yeates D, Gill LE. Depression and anxiety in people with inflammatory bowel disease. J Epidemiol Community Health. 2001;55:716. doi: 10.1136/jech.55.10.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villaran RF, Espinosa-Oliva AM, Sarmiento M, De Pablos RM, Arguelles S, Delgado-Cortes MJ, et al. Ulcerative colitis exacerbates lipopolysaccharide-induced damage to the nigral dopaminergic system: potential risk factor in Parkinson`s disease. J Neurochem. 2010;114:1687–1700. doi: 10.1111/j.1471-4159.2010.06879.x. [DOI] [PubMed] [Google Scholar]
- 6.Yokoyama JS, Wang Y, Schork AJ, Thompson WK, Karch CM, Cruchaga C, et al. Association between genetic traits for immune-mediated diseases and Alzheimer disease. JAMA Neurol. 2016;73:691. doi: 10.1001/jamaneurol.2016.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen B, Girgis S, El-Matary W. Childhood autism and eosinophilic colitis. Digestion. 2010;81:127–129. doi: 10.1159/000252765. [DOI] [PubMed] [Google Scholar]
- 8.Heberling CA, Dhurjati PS, Sasser M. Hypothesis for a systems connectivity model of Autism Spectrum Disorder pathogenesis: links to gut bacteria, oxidative stress, and intestinal permeability. Med Hypotheses. 2013;80:264. doi: 10.1016/j.mehy.2012.11.044. [DOI] [PubMed] [Google Scholar]
- 9.Riazi K, Galic MA, Kuzmiski JB, Ho W, Sharkey Ka, Pittman QJ. Microglial activation and TNFalpha production mediate altered CNS excitability following peripheral inflammation. Proc Natl Acad Sci U S A. 2008;105:17151–17153. doi: 10.1073/pnas.0806682105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raghavendra V, Tanga FY, Deleo JA. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur J Neurosci. 2004;20:467. doi: 10.1111/j.1460-9568.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- 11.Hathaway CA, Appleyard CB, Percy WH, Williams JL. Experimental colitis increases blood-brain barrier permeability in rabbits. Am J Physiol. 1999;276:G1174. doi: 10.1152/ajpgi.1999.276.5.G1174. [DOI] [PubMed] [Google Scholar]
- 12.Engelhardt Britta, Sorokin Lydia. The blood–brain and the blood–cerebrospinal fluid barriers: function and dysfunction. Seminars in Immunopathology. 2009;31(4):497–511. doi: 10.1007/s00281-009-0177-0. [DOI] [PubMed] [Google Scholar]
- 13.Sans M, Kawachi S, Soriano A, Palacin A, Morise Z, Granger D, et al. Brain endothelial adhesion molecule expression in experimental colitis. Microcirculation. 2001;8:105–114. doi: 10.1080/713774022. [DOI] [PubMed] [Google Scholar]
- 14.Natah SS, Mouihate A, Pittman QJ, Sharkey KA. Disruption of the blood-brain barrier during TNBS colitis. Neurogastroenterol Motil. 2005;17:433–446. doi: 10.1111/j.1365-2982.2005.00654.x. [DOI] [PubMed] [Google Scholar]
- 15.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104:13780. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jang SE, Lim SM, Jeong JJ, Jang HM, Lee HJ, Han MJ, et al. Gastrointestinal inflammation by gut microbiota disturbance induces memory impairment in mice. Mucosal Immunol 2017. [DOI] [PubMed]
- 17.Caradonna L, Amati L, Magrone T, Pellegrino NM, Jirillo E, Caccavo D. Enteric bacteria, lipopolysaccharides and related cytokines in inflammatory bowel disease: biological and clinical significance. J Endotoxin Res. 2000;6:205–214. [PubMed] [Google Scholar]
- 18.Yao YM, Xu CL, Yao FH, Yu Y, Sheng ZY. The pattern of nuclear factor-kappaB activation in rats with endotoxin shock and its role in biopterin-mediated nitric oxide induction. Zhonghua Shao Shang Za Zhi. 2006;22:405–410. [PubMed] [Google Scholar]
- 19.Kremlev SG, Palmer C. Interleukin-10 inhibits endotoxin-induced pro-inflammatory cytokines in microglial cell cultures. J Neuroimmunol. 2005;162:71–80. doi: 10.1016/j.jneuroim.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Y, Huang X, Zhao T, Qiao M, Zhao X, Zhao M, et al. Hypoxia augments LPS-induced inflammation and triggers high altitude cerebral edema in mice. Brain Behav Immunity. 2017;64:266–275. doi: 10.1016/j.bbi.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 21.Sainathan SK, Bishnupuri KS, Aden K, Luo Q, Houchen CW, Anant S, et al. Toll-like receptor-7 ligand imiquimod induces type I interferon and antimicrobial peptides to ameliorate dextran sodium sulfate-induced acute colitis. Inflamm Bowel Dis. 2012;18:955–967. doi: 10.1002/ibd.21867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Håkansson Å1, Tormo-Badia N, Baridi A, Xu J, Molin G, Hagslätt ML, et al. Immunological alteration and changes of gut microbiota after dextran sulfate sodium (DSS) administration in mice. Clin Exp Med. 2015;15:107–120. doi: 10.1007/s10238-013-0270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neilly MPJD, Gardiner KR, Kirk SJ, Jennings G, Anderson NH, Elia M, et al. Endotoxaemia and cytokine production in experimental colitis. Br J Surgery. 1995;82:1479. doi: 10.1002/bjs.1800821110. [DOI] [PubMed] [Google Scholar]
- 24.Ghia JE, Blennerhassett P, Kumar-Ondiveeran H, Verdu EF, Collins SM. The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology. 2006;131:1122–1130. doi: 10.1053/j.gastro.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 25.Dammann O, Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res. 1997;42:1. doi: 10.1203/00006450-199707000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Fang X, Sun D, Wang Z, Yu Z, Liu W, Pu Y, et al. MiR-30a positively regulates the inflammatory response of microglia in experimental autoimmune encephalomyelitis. Neurosci Bull. 2017;33:603–615. doi: 10.1007/s12264-017-0153-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buttini M, Limonta S, Boddeke HW. Peripheral administration of lipopolysaccharide induces activation of microglial cells in rat brain. Neurochem Int. 1996;29:25. doi: 10.1016/0197-0186(95)00141-7. [DOI] [PubMed] [Google Scholar]
- 28.Carson MJ. Microglia as liaisons between the immune and central nervous systems: Functional implications for multiple sclerosis. Glia. 2002;40:218–231. doi: 10.1002/glia.10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alvarez JI, Cayrol R, Prat A. Disruption of central nervous system barriers in multiple sclerosis. Biochim Biophys Acta. 2011;1812:252–264. doi: 10.1016/j.bbadis.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 30.Krizanac-Bengez L, Kapural M, Parkinson F, Cucullo L, Hossain M, Mayberg MR, et al. Effects of transient loss of shear stress on blood-brain barrier endothelium: role of nitric oxide and IL-6. Brain Res. 2003;977:239–246. doi: 10.1016/S0006-8993(03)02689-1. [DOI] [PubMed] [Google Scholar]
- 31.Blecharzlang KG, Wagner J, Fries A, Nieminenkelhä M, Rösner J, Schneider UC, et al. Interleukin 6-mediated endothelial barrier disturbances can be attenuated by blockade of the IL6 receptor expressed in brain microvascular endothelial cells. Transl Stroke Res 2018: 1–12. [DOI] [PubMed]
- 32.Moreira AP, Texeira TF, Ferreira AB, Peluzio MC, Alfenas RC. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br J Nutr. 2012;108:801. doi: 10.1017/S0007114512001213. [DOI] [PubMed] [Google Scholar]
- 33.Lee IA, Bae EA, Hyun YJ, Kim DH. Dextran sulfate sodium and 2,4,6-trinitrobenzene sulfonic acid induce lipid peroxidation by the proliferation of intestinal gram-negative bacteria in mice. J Inflamm. 2010;7:7. doi: 10.1186/1476-9255-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito H, Tanabe H, Kawagishi H, Tadashi W, Yasuhiko T, Sugiyama K, et al. Short-chain inulin-like fructans reduce endotoxin and bacterial translocations and attenuate development of TNBS-induced colitis in rats. Dig Dis Sci. 2009;54:2100–2108. doi: 10.1007/s10620-008-0599-x. [DOI] [PubMed] [Google Scholar]
- 35.Axelsson LG, Midtvedt T, Bylundfellenius AC. The role of intestinal bacteria, bacterial translocation and endotoxin in dextran sodium sulphate-induced colitis in the mouse. Microb Ecol Health Dis. 1996;9:225–237. doi: 10.3109/08910609609166463. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.