Abstract
Chromosome microarray analysis (CMA) is a cost-effective molecular cytogenetic technique that has been used as a first-line diagnostic test in neurodevelopmental disorders in the USA since 2011. The impact of CMA results on clinical practice in China is not yet well studied, so we aimed to better evaluate this phenomenon. We analyzed the CMA results from 434 patients in our clinic, and characterized their molecular diagnoses, clinical features, and follow-up clinical actions based on these results. The overall diagnostic yield for our patients was 13.6% (59 out of 434). This gave a detection rate of 14.7% for developmental delay/intellectual disability (DD/ID, 38/259) and 12% for autism spectrum disorders (ASDs, 21/175). Thirty-three recurrent (n ≥ 2) variants were found, distributed at six chromosomal loci involving known chromosome syndromes (such as DiGeorge, Williams Beuren, and Angelman/Prader-Willi syndromes). The spectrum of positive copy number variants in our study was comparable to that reported in Caucasian populations, but with specific characteristics. Parental origin tests indicated an effect involving a significant maternal transmission bias to sons. The majority of patients with positive results (94.9%) had benefits, allowing earlier diagnosis (36/59), prioritized full clinical management (28/59), medication changes (7/59), a changed prognosis (30/59), and prenatal genetic counseling (15/59). Our results provide information on de novo mutations in Chinese children with DD/ID and/or ASDs. Our data showed that microarray testing provides immediate clinical utility for patients. It is expected that the personalized medical care of children with developmental disabilities will lead to improved outcomes in long-term developmental potential. We advocate using the diagnostic yield of clinically actionable results to evaluate CMA as it provides information of both clinical validity and clinical utility.
Keywords: Chromosome microarray analysis, Neurodevelopmental disorder, Autism spectrum disorder, Chromosome syndrome, Clinical management
Introduction
Neurodevelopmental disorders (NDDs), including but not limited to intellectual disability (ID), global developmental delay, and autism spectrum disorders (ASDs), affect >15% of children [1]. The prevalence estimates of developmental delay (DD)/ID range from 1% to 3% [2], and the estimated prevalence of ASDs is 1 in 68 [3]. Based on the worldwide prevalence and considering the population of China, 770,000–2,310,000 children in China suffer from DD/ID. NDDs are of great concern for public health and society since they require expensive care throughout the whole life of a patient.
Chromosome microarray analysis (CMA) is a molecular cytogenetic technique which allows genome-wide scanning to detect clinically significant copy number variants (CNVs), and deletions and duplications as small as 10 kb as reported recently [4]. CMA holds promise as an efficient and cost-effective approach to genetic testing because of its high throughput, high resolution, and affordability. In 2010, The American College of Medical Genetics and Genomics published guidelines for CMA as a first-line diagnostic test in the following groups of patients: (1) multiple anomalies not specific to a well-delineated genetic syndrome, (2) apparently nonsyndromic DD/ID, and (3) ASDs [4, 5] In the same year, the CMA test was further supported for pediatric practice by the International Collaboration for Clinical Genomics [4] and the American Academy of Neurology. Although the diagnostic yields vary [patients with global developmental delay/ID and/or ASD (median, 13.6%; interquartile range IQR, 9.5%–17.2%), and primarily ASD (median, 8.4%; IQR, 7.2%–17.3%)] [6], this test has been used in developed countries for several years to help clinicians improve the medical service, and to understand the prognosis and future monitoring.
Large-scale whole-genome CNV studies have established the importance of de novo CNVs in NDDs, especially in Caucasian populations of European ancestry [7–9]. Importantly, the CMA results efficiently affect the treatment plan. Retrospective studies have shown that the change rate of clinical management based on CMA findings is 34.0%–75.7% [10–13] in all reviewed abnormal CMA cases including DD/ID, ASDs, seizures, dysmorphic features, and congenital anomalies. Application of the CMA test in China started relatively late. According to several genome-wide studies during the past three years [14–17], the CMA-based diagnostic rate of NDDs in Chinese children is comparable to that reported worldwide. However, all these studies have focused on significant CNVs and new disease genes, while the impact of CMA results on clinical practice in China is not well studied.
Therefore, in this study, we analyzed 434 patients in our clinic using CMA, and we characterized their molecular diagnoses, clinical features, and follow-up clinical action based on the CMA results. The objective of this study was to investigate the abnormal CNVs in a Chinese NDD cohort and evaluate the impact of CMA results on the medical management of developmental behavioral pediatric patients, in order to advance personalized treatment in China.
Methods
Patients
The clinical cohort that underwent chromosomal testing was recruited from July 2014 to December 2016 in two clinics of the Department of Developmental Behavioral Pediatrics of Shanghai Children’s Medical Center and Xinhua Hospital Affiliated to Shanghai Jiaotong University School of Medicine. A total of 434 patients with different degrees of DD/ID and/or ASDs (371 males and 63 females; average age 5.63 years, ranging from 4 months to 17 years) were enrolled. Among them, 175 (1.6–9.8 years, 81.14% male) were diagnosed with ASDs based on the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, and confirmed with the Chinese version of the Autism Diagnostic Observation Schedule and/or the Autism Diagnosis Interview-Revised. Patients diagnosed with DD/ID using the intellectual assessment DQ <75 assessed with the Gesell development scales, or IQ <70 assessed with the Wechsler Intelligence Scale for Children-Revised or the Wechsler Preschool and Primary Scale of Intelligence [17], were included. The exclusion criteria were: (1) neurological disorders, such as cerebral palsy; and (2) known chromosomal/genetic disorders, mainly trisomy 21, 18, or 13 syndromes. This study was approved by the Ethics Committee of both Shanghai Children’s Medical Center (SCMCIRB-K2014051) and Xinhua Hospital (XHEC-C-2017-062). Informed written consent was given by parents.
CMA
Genomic DNA was extracted from the peripheral blood of patients and their parents. CMA was performed using the CytoScan™ HD system (Affymetrix, Thermo fisher, Santa Clara, CA) following the manufacturer’s instructions. Data were visualized and analyzed with the Chromosome Analysis Suite software package (Affymetrix). The CNV calling threshold was set at 25 consecutive probes encompassing 25 kb or more in length.
Genetic Analyses
A qualified cytogeneticist made a clinical laboratory interpretation for each sample and assessed each of the chromosomal CNV regions reported in every sample, classifying them as benign, likely benign, pathogenic, likely pathogenic, or variant of uncertain significance.
Data Analysis
Data were analyzed using SPSS version 18.0 software (SPSS Inc., Chicago, IL). A one-way ANOVA test was used when comparing three groups and rates. P < 0.05 was considered significant.
Results
Demographics
Of the 434 patients, 59 were found to have pathogenic/likely pathogenic variants, accounting for 13.6% of all patients. The general demographic features of the patients are listed in Table 1. There was a higher percentage with pathogenic variants under 2 years of age (70%) than in older patients (6.4% at 2–5 years and 12.7% at >5 years, P < 0.05).
Table 1.
Demographics of patients and CMA diagnostic yield.
| Total no. | Patients with pathogenic CNV (%) | Patients with likely pathogenic CNV (%) | Diagnostic yield (%) | |
|---|---|---|---|---|
| Total | 434 | 47 (10.8) | 12 (2.8) | 59 (13.6) |
| Sex | ||||
| Male | 371 | 32 (8.6) | 12 (3.2) | 44 (11.8) |
| Female | 63 | 15 (23.8) | 0 | 15 (23.8) |
| Age (years) | ||||
| <2 | 20 | 14 (70) | 1 (5) | 15 (75) |
| 2-5 | 312 | 20 (6.4) | 7 (2.2) | 27 (8.7) |
| >5 | 102 | 13 (12.7) | 4 (3.9) | 17 (16.7) |
| Clinical features | ||||
| DD/ID | 259 | 31 (12.0) | 7 (2.7) | 38 (14.7) |
| ASD | 175 | 16 (9.1) | 5 (2.9) | 21 (12) |
Molecular Diagnoses
We detected 51 pathogenic CNVs in 47 patients (10.8%) and 12 likely pathogenic CNVs in 12 patients (2.8%) (Table 2). The overall diagnostic yield of CMA testing for patients with DD/ID and/or ASDs was 13.6% when considering pathogenic and likely pathogenic CNVs as positive findings. This gave a detection rate of 14.7% for DD/ID (38/259) and 12% for ASDs (21/175) (Table 1). The ages of the probands with positive results ranged from 4 months to 17 years.
Table 2.
Summary of the CNVs identified in the proband.
| No. | Proband | Coordinate | Copy number (CN) | Size (Kb) | Note |
|---|---|---|---|---|---|
| 1 | 2q37.3 | chr2:241494455-242340252 | Loss | 846 | |
| 2 | 16p13.11 | chr16:15420069-16514368 | Loss | 1094 | |
| 3 | 5p15.33-p14.1 | chr5:113576-25944592 | Loss | 25831 | Cri du chat syndrome |
| 4 | 15q26.3 | chr15:100,384,057-102,429,112 | Loss | 2045 | |
| 5 | 1q21.1-q21.2 | chr1:146043713-148513854 | Loss | 2470 | |
| 6 | 7q11.23 | chr7:72718277-74142190 | Loss | 1424 | Williams syndrome |
| 7 | 7q11.23 | chr7:72718277-74143240 | Loss | 1425 | Williams syndrome |
| 8 | 20q13.33 | chr20:61579927-62915555 | Loss | 1336 | |
| 9 | 17q11.2 | chr17: 28,464,942-30,528,569 | Loss | 2063 | Neurofibromatosis type 1 |
| 10 | 7q11.23 | chr7:72718277-74143240 | Loss | 1425 | Williams syndrome |
| 11 | 22q11.21 | chr22:18916842-21465659 | Loss | 2549 | DiGeorge syndrome |
| 12 | 1q21.2 | chr1:146498298-147823369 | Gain | 1325 | |
| 13 | 7q11.23 | chr7:72700524-74147166 | Gain | 1447 | 7q11.23 duplication |
| 14 | 18q22.2-q23 | chr18:66840930-78014123 | Loss | 11173 | |
| 15 | 1q21.1-q21.2 | chr1:145885645-147926347 | Loss | 2041 | |
| 3q29 | chr3:195703615-197344176 | Loss | 1641 | ||
| 16 | 7q11.23 | chr7:72718277-74142256 | Loss | 1424 | Williams syndrome |
| 17 | 5p15.33-p12 | chr5:113,576-50,101,846 | Mosaic gain (CN = 2.2) | 49988 | Cri du chat syndrome |
| 18 | 2p16.3 | chr2:50749598-50880967 | Loss | 131 | NRXN1 (exons 4-10) |
| 19 | 10q11.22-q11.23 | chr10:46206775-51812795 | Gain | 5606 | |
| 20 | 1q21.1-q21.2 | chr1:145786360-147897962 | Loss | 2112 | |
| 21 | 11q22.1-q22.3 | chr11:101484717-108657329 | Gain | 7173 | |
| 22 | 15q11.2-q26.3 | chr15:22752398-102429049 | Uniparental disomy (UPD) | 79,000 | Angelman/Prader-Willi syndrome |
| 23 | 7q11.23 | chr7: 72691242-74142190 | Loss | 1450 | Williams syndrome |
| 24 | 7q11.23 | chr7:72718277-74143240 | Loss | 1425 | Williams syndrome |
| 25 | 7q11.23 | chr7:72848720-74143060 | Loss | 1294 | Williams syndrome |
| 26 | 15q11.2-q13.1 | chr15:22770421-28545355 | Gain | 5775 | |
| 27 | 22q11.21 | chr22:18916842-21798907 | Loss | 2882 | DiGeorge syndrome |
| 28 | Xp22.31 | chrX:6458939-8135645 | Loss | 1677 | |
| 29 | 10q26.12-q26.3 | chr10: 121,508,975 - 135,534,747 | Mosaic copy-neutral loss of heterozygosity (LOH) | ||
| 30 | 7q11.23 | chr7:72650240-74142190 | Loss | 1492 | Williams syndrome |
| 31 | 7q11.23 | chr7:72611954-74286977 | Loss | 1675 | Williams syndrome |
| 32 | 1q21.1-q21.2 | chr1:146043713-147926347 | Loss | 1883 | |
| 33 | 1p21.3-p21.1 | chr1:96378051-103052282 | Loss | 6674 | |
| 34 | 17q11.2 | chr17: 27386433-30622025 | Mosaic loss (CN = 2.3) | 3236 | Neurofibromatosis type 1 |
| 35 | 8q13.3 | chr8:71556059-73521359 | Loss | 1965 | |
| 9p24.1-p23 | chr9:7849231-12101640 | Loss | 4252 | ||
| 36 | 7q11.23 | chr7:72718277-74143240 | Loss | 1425 | Williams syndrome |
| 37 | 3p26.3-p24.1 | chr3:285805-28206877 | Gain | 27596 | Parental balanced translocation |
| 6p25.3 | chr6:380684-883245 | Loss | 503 | ||
| 38 | 15q11.2-q13.1 | chr15:22,770,421-28,560,269 | Loss | 5789 | Angelman syndrome |
| 39 | 22q11.21 | chr22:18644790-21798907 | Loss | 3154 | DiGeorge syndrome |
| 40 | 7q11.23 | chr7:72718277-74143240 | Loss | 1425 | Williams syndrome |
| 41 | 15q11.2-q13.1 | chr15:22770421-28534245 | Gain | 5764 | |
| 42 | 3q22.1-q22.3 | chr3:133679690-136971562 | Loss | 3292 | |
| 43 | 22q11.21 | chr22:18644790-21465662 | Loss | 2821 | DiGeorge syndrome |
| 44 | 16p11.2 | chr16:29412891-30191848 | Loss | 779 | |
| 45 | 15q11.2-q13.1 | chr15:22770421-28545601 | Loss | 5775 | Angelman/Prader-Willi syndrome |
| 46 | 15q11.2-q13.1 | chr15:22770421-28915864 | Loss | 6145 | Angelman/Prader-Willi syndrome |
| 47 | 9pter-9q21.13 | chr9:203861-75444559 | Gain | 75600 | Partial chromosome 9 trisomy |
| 48 | 1p33-p32.2 | chr1:47831383-58364913 | Loss | 10533 | |
| 49 | 15q11.2-q13.1 | chr15: 22714985-28559437 | Gain | 5844 | |
| 50 | 17p11.2 | chr17:16761814-20318253 | Gain | 3556 | Potocki-Lupski syndrome |
| 51 | 15q11.2-q13.1 | chr15:22770421-28534245 | Gain | 5764 | |
| 52 | 3p26.1p11.1 | chr3:5,464,640-90,485,635 | LOH | 23297 | |
| 3q11.1q29 | chr3:93,536,053-197,851,260 | LOH | 26247 | ||
| 53 | 22q13.33 | chr22:50,990,475-51,115,526 | Loss | 125 | SHANK3 gene |
| 54 | 15q11.2 | chr15:22,770,421-23,282,799 | Loss | 512 | |
| 55 | 17q13.1p11.2 | chr17:8,235,947-18,992,506 | Gain | 10687 | |
| 56 | 4q31.21q33 | chr4:145149737-170141221 | Gain | 24991 | |
| 57 | 13q34 | chr13:112352171-115107733 | Loss | 2755 | |
| 58 | 14q31.1q32.12 | chr14:82876271-92988797 | Loss | 10113 | |
| 18q21.2q22.3 | chr18:53637282-71890740 | Loss | 18253 | ||
| 59 | 16q22.2 | Gain | 739 |
We found 33 recurrent (n ≥ 2) variants distributed at six chromosomal loci (Table 3); they included imbalances involved in known chromosomal syndromes like DiGeorge syndrome, Williams Beuren syndrome (WBS) and Angelman/Prader-Willi syndrome (AS/PWS). Notably, some classical syndromic loci were found with both deletions and duplications, including 7q11.23, 15q11-q13, and 1q21.2. We found 12 imbalances (11 deletions and 1 duplication) at 7q11.23 and five aberrations (4 deletions and 1 duplication) at 1q21.2 involving the GJA5 and GJA8 genes. We also identified 8 patients with a 15q11-q13 abnormality (4 duplications, 3 deletions, and 1 uniparental disomy), which ranged in length from 5764 kb to 79000 kb. Furthermore, mosaicism was found for both 17q11.2 deletion and 5p terminal deletion.
Table 3.
Recurrent CNVs identified in this study. UPD, uniparental disomy.
| Loci | CN | Size range (Kb) | No. of patients | Syndrome involved |
|---|---|---|---|---|
| 7q11.23 | Loss | 1294–1675 | 11 | Williams syndrome |
| Gain | 1 | |||
| 15q11.2-q13.1 | Gain | 5764–79000 | 4 | Angelman/Prader-Willi syndrome |
| Loss | 3 | |||
| UPD | 1 | |||
| 22q11.21 | Loss | 2821–3154 | 4 | DiGeorge syndrome |
| 1q21.2 | Loss | 1325–2470 | 4 | 1q21.2 microdeletion syndrome |
| Gain | 1 | |||
| 17q11.2 | Loss | 2063–3236 | 2 | Neurofibromatosis type 1 |
| 5pter | Loss | 25831–49988 | 2 | Cri du chat syndrome |
In addition, parents were tested for patients carrying non-recurrent pathogenic/likely pathogenic CNVs smaller than 10 Mb, when samples were available. Finally, the parental origin was determined in 12 affected individuals (Table 4). CNVs carried by 8 patients were identified as de novo, and four were inherited. Specifically, in one patient, two de novo CNVs were verified to be caused by imbalanced DNA translocation between chromosomes 8 and 9.
Table 4.
Parental testing for 12 patients carrying non-recurrent pathogenic/likely pathogenic CNVs.
| No. | Gender | Loci | CN | Size (Kb) | Inheritance | Note |
|---|---|---|---|---|---|---|
| 1 | Female | 20q13.33 | Loss | 1336 | de novo | |
| 2 | Male | 1q21.2 | Gain | 1325 | Maternal | |
| 3 | Male | 2p16.3 | Loss | 131 | de novo | NRXN1 (exons 4-10) |
| 4 | Male | 10q11.22-q11.23 | Gain | 5606 | Maternal | |
| 5 | Male | 11q22.1-q22.3 | Gain | 7173 | de novo | |
| 6 | Female | 1p21.3-p21.1 | Loss | 6674 | de novo | |
| 7 | Male | 8q13.3 | Loss | 1965 | de novo | Imbalanced translocation |
| 9p24.1-p23 | Loss | 4252 | ||||
| 8 | Female | 3q22.1-q22.3 | Loss | 3292 | de novo | |
| 9 | Male | 15q11.2-q13.1 | Gain | 5775 | Maternal | |
| 10 | Female | 22q13.33 | Loss | 125 | de novo | SHANK3 gene |
| 11 | Male | 15q11.2 | Loss | 512 | Maternal | |
| 12 | Male | 16q22.2 | Gain | 739 | de novo |
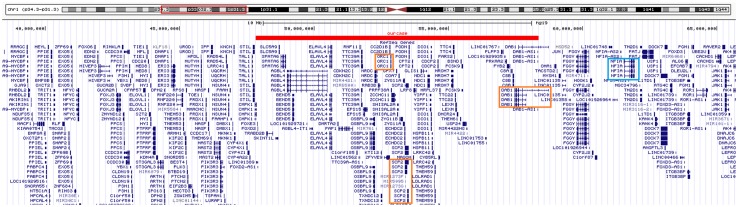
We identified a 10-Mb microdeletion at 1p33-p32.2 (Fig. 1) in a 3.6-year-old boy with global developmental delay and congenital anomalies. This interstitial microdeletion on the short arm of chromosome 1 is a rare aberration, which has not been reported elsewhere. This deletion region overlaps with the 1p32-p31 deletion syndrome, of which critical gene is NFIA. Of note, the NFIA gene was not included in the CNV region of this case, and three possible candidate genes were further pinpointed: ORC1, SCP2, and DAB1. These three genes may be responsible for the main phenotypes observed in the patient: microcephaly, growth retardation, short stature, leukoencephalopathy, and DD/ID. The spectrum of phenotypes caused by this 1p33-p32.2 deletion may represent a new microdeletion syndrome.
Fig. 1.
Genotype comparison between our patient and the 1p31.3p32.2 deletion syndrome. Red bar: the 1p31.1-p32.2 deletion region detected in our case. Three possible candidate genes (red boxes), including ORC1, SCP2, and DAB1, were pinpointed in this case. However, the NFIA gene (blue box), which is the core gene for 1p32-p31 deletion syndrome, was not involved in our case.
Management Changes
Based on the previous reports [9] and our clinical practice, we applied five categories of clinical action after a positive CMA result: (i) allowing an earlier diagnosis, (ii) prioritizing full clinical assessment, including referral to other specialists and further diagnostic testing, (iii) changing medication, such as discontinuation, starting a new medication, and a clinical trial, (iv) changing surveillance and prognosis, and (v) recommending prenatal genetic counseling. Our CMA results affected the clinical management in the above ways for 94.9% (56 out of 59) of the patients with abnormal CNVs (Table 5).
Table 5.
Clinical action for patients with pathogenic CMA results.
| Case no. | Loci | Allowing earlier diagnosis | Prioritizing full clinical management | Changing medication | Changing prognosis | Parental genetic counselling |
|---|---|---|---|---|---|---|
| 1 | 2q37.3 | |||||
| 2 | 16p13.11 | + | ||||
| 3 | 5p15.33-p14.1 | + | + | + | ||
| 4 | 15q26.3 | + | ||||
| 5 | 1q21.1-q21.2 | + | ||||
| 6 | 7q11.23 | + | + | |||
| 7 | 7q11.23 | + | + | |||
| 8 | 20q13.33 | + | + | |||
| 9 | 17q11.2 | + | + | |||
| 10 | 7q11.23 | + | + | |||
| 11 | 22q11.21 | + | + | |||
| 12 | 1q21.2 | + | ||||
| 13 | 7q11.23 | + | + | |||
| 14 | 18q22.2-q23 | + | + | + | + | |
| 15 | 1q21.1-q21.2 | + | ||||
| 3q29 | ||||||
| 16 | 7q11.23 | + | + | |||
| 17 | 5p15.33-p12 | + | ||||
| 18 | 2p16.3 | + | + | + | + | |
| 19 | 10q11.22-q11.23 | + | + | |||
| 20 | 1q21.1-q21.2 | + | ||||
| 21 | 11q22.1-q22.3 | + | ||||
| 22 | 15q11.2-q26.3 | + | + | + | ||
| 23 | 7q11.23 | + | + | + | ||
| 24 | 7q11.23 | + | + | + | ||
| 25 | 7q11.23 | + | + | + | ||
| 26 | 15q11.2-q13.1 | + | + | |||
| 27 | 22q11.21 | + | + | |||
| 28 | Xp22.31 | + | + | |||
| 29 | 10q26.12-q26.3 | |||||
| 30 | 7q11.23 | + | + | |||
| 31 | 7q11.23 | + | + | |||
| 32 | 1q21.1-q21.2 | + | ||||
| 33 | 1p21.3-p21.1 | + | + | |||
| 34 | 17q11.2 | + | + | + | + | |
| 35 | 8q13.3 | + | + | + | + | |
| 9p24.1-p23 | ||||||
| 36 | 7q11.23 | + | + | |||
| 37 | 3p26.3-p24.1 | + | + | |||
| 6p25.3 | ||||||
| 38 | 15q11.2-q13.1 | + | + | |||
| 39 | 22q11.21 | + | + | |||
| 40 | 7q11.23 | + | + | + | ||
| 41 | 15q11.2-q13.1 | + | ||||
| 42 | 3q22.1-q22.3 | + | ||||
| 43 | 22q11.21 | + | + | |||
| 44 | 16p11.2 | + | + | |||
| 45 | 15q11.2-q13.1 | + | + | + | + | |
| 46 | 15q11.2-q13.1 | + | + | + | + | |
| 47 | 9pter-9q21.13 | + | ||||
| 48 | 1p33-p32.2 | + | ||||
| 49 | 15q11.2-q13.1 | + | + | + | ||
| 50 | 17p11.2 | + | + | + | ||
| 51 | 15q11.2-q13.1 | + | ||||
| 52 | 3p26.1-p11.1 | |||||
| 3q11.1-q29 | ||||||
| 53 | 22q13.33 | + | + | + | ||
| 54 | 15q11.2 | + | + | |||
| 55 | 17q13.1-p11.2 | + | ||||
| 56 | 4q31.21-q33 | + | ||||
| 57 | 13q34 | + | ||||
| 58 | 14q31.1-q32.12 | + | + | + | + | |
| 18q21.2-q22.3 | ||||||
| 59 | 16q22.2 | + |
For 36 patients (61%), the results allowed us to give an earlier diagnosis: one for ASD (mosaic 17q11.2 deletion); 18 for known syndromes (including Cri du chat syndrome, Williams syndrome, AS/PWS, Neurofibromatosis type 1and 1q21 microdeletion syndromes), and 17 for other microdeletion/microduplication syndromes caused by rare CNVs.
For patients with known symptoms presenting with atypical phenotypes, the CMA results allowed not only earlier diagnosis but also full clinical management and/or intervention changes. With these clinical actions, 30 patients (50.8%) had an improved prognosis. Referral and further clinical evaluation was done for almost half of the patients with pathogenic CNVs (n = 29; 49.2%). Eleven patients (18.6%) with WBS underwent full clinical assessment, such as serum calcium and thyroid function tests, and were further referred to cardiology and/or endocrinology clinics. In 4 patients (6.8%) with 15q11.2-q13 loss or uniparental disomy, the diagnosis was first confirmed (AS/PWS) and then clinical action was taken, including referral to a neurologist and conducting an EEG and pituitary hormone test. Four patients (6.8%) with DiGeorge syndrome who visited our department were recommended by a cardiologist and/or an endocrinologist. They received full behavioral evaluation in addition to echocardiography, and serum calcium, immunology, and pituitary hormone tests. Two patients (3.4%) with 17q11.2 deletions involving the NF1 gene linked to neurofibromatosis risk were given further evaluation and lifelong follow-up. Another two patients (3.4%) were recommended for a hearing test. Based on the molecular diagnoses, medication or intervention were changed in 7 patients (11.9%). In the patient with a 2p16.3 deletion involving NRXN1 exons 4-10, who had mild autism symptoms and epileptic discharges, methylphenidate was replaced by atomoxetine [18]. Another patient with a CMA result of SHANK3 loss (22q13.3 deletion syndrome) prompted a further clinical therapeutic trial with insulin-like growth factor. Two patients with 18q22 loss were confirmed with growth hormone deficiency and were given growth hormone treatment.
In 15 of these patients (25.4%), the results were essential for prenatal counseling to help the patients’ parents to determine reproductive planning, especially in four patients who had abnormalities of maternal origin.
Discussion
The use of CMA to detect pathogenic CNVs underlying DD/ID or ASDs has improved a lot recently. At least two cohort studies have found that a CMA-first strategy may be the most cost-effective [19, 20]. In our study, the diagnostic yield is comparable to those in previous publications on similar patient populations [21, 22]. The yield for patients under 2 years of age was significantly higher than that of older children, partly due to most of the younger patients having a medical history of other problems such as asphyxia of the newborn, neonatal jaundice, or nutritional diseases like malnutrition and anemia in addition to neurological abnormalities (such as microcephaly, macrocephaly, or hypotonia). These clinical findings suggest that NDD patients, especially at younger ages, may have comorbidity with other systemic abnormalities, reminding clinicians to consider genetic evaluation with CMA for young patients.
In our cohort, there were more male than female patients; the ratio was 5.89:1, which is higher than in studies from other countries [23]. There are three possible reasons for the difference. One is that the prevalence of DD/ID and ASDs is much higher in boys than in girls; the sex differentiation is 4:1 for ASDs in western countries [23]. According to statistics from the Institute of Mental Health, Peking University School of Medicine [24], the occurrence of autism is ~5–9 times higher in boys than in girls in China. This figure indicates that Asian males may be more susceptible to autism than Caucasians. Further statistical and epidemiological data are needed to clarify this. The second explanation may be a referral bias caused by the culture in China. Traditionally, Chinese parents value boys over girls [25]. The third possible cause comes from genetics, in that there is an effect of a higher female tolerance for additional mutations [26]. Generally, most pathogenic variations have been shown to be de novo. However, some recurrent genomic disorders like 15q11-q13 deletion/duplication and 22q11 deletion, represent a maternal bias [27]. This bias has been replicated and confirmed, revealing a highly significant maternal bias in the origin of the 22q11.2 deletion [28]. Krumm et al. [29] found that inherited truncating SNVs may be associated with an effect involving significant maternal transmission bias to sons. In the parental testing, we identified four inherited pathogenic CNVs which were all inherited from seemingly healthy mothers of male patients. This indicates that females are more tolerant to pathogenic/likely pathogenic CNVs, and tend to transmit them to male offspring.
Our results confirm the importance of CNVs underlying DD/ID and ASD, as reported in previous studies [30]. The profiles of abnormal CNVs in our study are mostly comparable to those reported previously [9, 31]. However, we did find some differences in the CNV spectrum between Chinese and Caucasian cohorts. Many studies have reported that the proximal 16p11.2 deletion, which has a population frequency of ~0.03% world-wide [32], is among the most frequent genetic etiologies of ASDs in Caucasian populations [33, 34] and accounts for ~1% of autism cases [35]. But there was only one 16p11.2 deletion in our cohort. Zhang et al. have demonstrated the potential involvement of the proximal 16p11.2 deletion in congenital scoliosis in a Han Chinese population [36], indicating a different genotype-phenotype association in this population. Our results highlight an urgent need for investment and extensive studies to establish a database and nation-wide guidelines covering the Chinese population, considering low overall clinical application of CMA in China. Interestingly, we also found variability in the relationships between phenotypes and genotypes in the affected probands. For example, for 15q11.2-q13.1, the phenotypic spectrum of both deletions and duplications appeared to be primarily neurological and included developmental and speech delays, and the deletion was recognized as classic AS/PWS, comparable to previously published work [4]. In this study, we also found that the phenotypes seen in patients with 7q11.23 microduplications were quite unlike those seen with the common microdeletion [5]. Those with WBS (7q11.23 microdeletion) are prone to have hyperverbal speech, a lack of stranger anxiety, and supravalvular aortic stenosis, while those with the 7q11.23 microduplication have speech delay, selective mutism (SM), and social anxiety, and are prone to aortic dilatation [37]. This variability of phenotypes caused by loss or gain mutation in the same region not only provides additional information on the interpretation of the effect of CNVs for counseling patient families but also sheds light on the underlying mechanism.
To date, all the CNV analysis studies on Chinese populations have focused on delineating the relationship between genotype and phenotype and explaining the pathogenic mechanism [14, 15, 17, 38]. But no studies have revealed the effects of positive CMA results on clinical practice in China. As developmental behavioral pediatricians, we are concerned about how to combine CMA results with clinical practice and how to use the positive results to optimize clinical management. Our study is the first report on using CNV analysis to guide the clinical management of a DD/ID and/or ASD cohort in a Chinese population. Based on the CMA tests, almost all patients (94.9%) with abnormal CMA results benefited from changed or optimized clinical actions, such as referral to specialists, further diagnostic tests, medication changes, and genetic consulting. Since the social challenges arising from the “one couple one child” policy, the Government of China announced that the policy was changed to encourage couples to have 2 children from January 1, 2016 [39]. Thus, there will be a baby boom in the coming years. Moreover, it seems probable that this population will include high-risk pregnancies associated with advanced paternal age and the use of assisted reproduction. Genetic consulting allows clinicians to estimate the recurrence risk and enable parents to make appropriate decisions on the second child or future pregnancy.
The clinical application of CMA in China is overall far behind, but increasing attention is being paid to this technology. In 2016, the Chinese expert consensus on clinical application of chromosomal microarray analysis in pediatric inherited disorders was released [40], and this has accelerated the spread of CMA in clinical practice.
A potential limitation of this study is the relatively limited follow-up time, which may underestimate the benefits of clinical management. Because of our study design, patients recruited more recently had a shorter follow-up than those who had been tested earlier. This limitation may affect the effective rate of the clinical management in our study. Another limitation is that the sample size was not large enough, and this may undervalue the power of CMA in clinical management. Our next step is to carry out a larger study to determine recommendations or guidelines for proper medical management based on CMA results and follow up the health outcomes of affected patients.
Acknowledgements
We thank all of the families who participated in this project. This work was supported by grants from the National Natural Science Foundation of China (81761128035 and 81781220701), the Shanghai Municipal Science and Technology Committee (17XD1403200 and 18dz2313505), the Research Physician Project of Shanghai Municipal Education Commission (20152234), the Shanghai Municipal Health and Family Planning Commission (GDEK201709, 2017ZZ02026, and 2017EKHWYX-02), and the Scientific Program of Shanghai Shenkang Hospital Development Center (16CR2025B) of China.
Compliance with Ethical Standards
Conflict of interest
All authors claim that there are no conflicts of interest.
Contributor Information
Juan Geng, Email: gjuan@jionstar.cn.
Fei Li, Email: feili@shsmu.edu.cn.
References
- 1.Boyle CA, Boulet S, Schieve LA, Cohen RA, Blumberg SJ, Yeargin-Allsopp M, et al. Trends in the prevalence of developmental disabilities in US children, 1997-2008. Pediatrics. 2011;127:1034–1042. doi: 10.1542/peds.2010-2989. [DOI] [PubMed] [Google Scholar]
- 2.Michelson DJ, Shevell MI, Sherr EH, Moeschler JB, Gropman AL, Ashwal S. Evidence report: Genetic and metabolic testing on children with global developmental delay: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2011;77:1629–1635. doi: 10.1212/WNL.0b013e3182345896. [DOI] [PubMed] [Google Scholar]
- 3.Christensen DL, Baio J, Van Naarden Braun K, Bilder D, Charles J, Constantino JN, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years–Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2012. MMWR Surveill Summ. 2016;65:1–23. doi: 10.15585/mmwr.ss6503a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller DT, Adam MP, Aradhya S, Biesecker LG, Brothman AR, Carter NP, et al. Consensus statement: chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies. Am J Hum Genet. 2010;86:749–764. doi: 10.1016/j.ajhg.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manning M, Hudgins L. Array-based technology and recommendations for utilization in medical genetics practice for detection of chromosomal abnormalities. Genet Med. 2010;12:742–745. doi: 10.1097/GIM.0b013e3181f8baad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blue Cross Blue Shield Asssociation Special report: Chromosomal microarray for the genetic evaluation of patients with global developmental delay, intellectual disability, and autism spectrum disorder. Technol Eval Cent Assess Program Exec Summ. 2015;30:1–4. [PubMed] [Google Scholar]
- 7.Marshall CR, Noor A, Vincent JB, Lionel AC, Feuk L, Skaug J, et al. Structural variation of chromosomes in autism spectrum disorder. Am J Hum Genet. 2008;82:477–488. doi: 10.1016/j.ajhg.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinto D, Pagnamenta AT, Klei L, Anney R, Merico D, Regan R, et al. Functional impact of global rare copy number variation in autism spectrum disorders. Nature. 2010;466:368–372. doi: 10.1038/nature09146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coe BP, Witherspoon K, Rosenfeld JA, van Bon BW, Vulto-van Silfhout AT, Bosco P, et al. Refining analyses of copy number variation identifies specific genes associated with developmental delay. Nat Genet. 2014;46:1063–1071. doi: 10.1038/ng.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coulter ME, Miller DT, Harris DJ, Hawley P, Picker J, Roberts AE, et al. Chromosomal microarray testing influences medical management. Genet Med. 2011;13:770–776. doi: 10.1097/GIM.0b013e31821dd54a. [DOI] [PubMed] [Google Scholar]
- 11.Riggs ER, Wain KE, Riethmaier D, Smith-Packard B, Faucett WA, Hoppman N, et al. Chromosomal microarray impacts clinical management. Clin Genet. 2014;85:147–153. doi: 10.1111/cge.12107. [DOI] [PubMed] [Google Scholar]
- 12.Henderson LB, Applegate CD, Wohler E, Sheridan MB, Hoover-Fong J, Batista DA. The impact of chromosomal microarray on clinical management: a retrospective analysis. Genet Med. 2014;16:657–664. doi: 10.1038/gim.2014.18. [DOI] [PubMed] [Google Scholar]
- 13.Tao VQ, Chan KY, Chu YW, Mok GT, Tan TY, Yang W, et al. The clinical impact of chromosomal microarray on paediatric care in Hong Kong. PLoS One. 2014;9:e109629. doi: 10.1371/journal.pone.0109629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yin CL, Chen HI, Li LH, Chien YL, Liao HM, Chou MC, et al. Genome-wide analysis of copy number variations identifies PARK2 as a candidate gene for autism spectrum disorder. Mol Autism. 2016;7:23. doi: 10.1186/s13229-016-0087-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gazzellone MJ, Zhou X, Lionel AC, Uddin M, Thiruvahindrapuram B, Liang S, et al. Copy number variation in Han Chinese individuals with autism spectrum disorder. J Neurodev Disord. 2014;6:34. doi: 10.1186/1866-1955-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chong WW, Lo IF, Lam ST, Wang CC, Luk HM, Leung TY, et al. Performance of chromosomal microarray for patients with intellectual disabilities/developmental delay, autism, and multiple congenital anomalies in a Chinese cohort. Mol Cytogenet. 2014;7:34. doi: 10.1186/1755-8166-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang B, Ji T, Zhou X, Wang J, Wang X, Wang J, et al. CNV analysis in Chinese children of mental retardation highlights a sex differentiation in parental contribution to de novo and inherited mutational burdens. Sci Rep. 2016;6:25954. doi: 10.1038/srep25954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harfterkamp M, Buitelaar JK, Minderaa RB, van de Loo-Neus G, van der Gaag RJ, Hoekstra PJ. Long-term treatment with atomoxetine for attention-deficit/hyperactivity disorder symptoms in children and adolescents with autism spectrum disorder: an open-label extension study. J Child Adolesc Psychopharmacol. 2013;23:194–199. doi: 10.1089/cap.2012.0012. [DOI] [PubMed] [Google Scholar]
- 19.Sagoo GS, Mohammed S, Barton G, Norbury G, Ahn JW, Ogilvie CM, et al. Cost effectiveness of using array-CGH for diagnosing learning disability. Appl Health Econ Health Policy. 2015;13:421–432. doi: 10.1007/s40258-015-0172-7. [DOI] [PubMed] [Google Scholar]
- 20.Trakadis Y, Shevell M. Microarray as a first genetic test in global developmental delay: a cost-effectiveness analysis. Dev Med Child Neurol. 2011;53:994–999. doi: 10.1111/j.1469-8749.2011.04080.x. [DOI] [PubMed] [Google Scholar]
- 21.Morrow EM. Genomic copy number variation in disorders of cognitive development. J Am Acad Child Adolesc Psychiatry. 2010;49:1091–1104. doi: 10.1016/j.jaac.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tammimies K, Marshall CR, Walker S, Kaur G, Thiruvahindrapuram B, Lionel AC, et al. Molecular diagnostic yield of chromosomal microarray analysis and whole-exome sequencing in children with autism spectrum disorder. JAMA. 2015;314:895–903. doi: 10.1001/jama.2015.10078. [DOI] [PubMed] [Google Scholar]
- 23.Xu LM, Li JR, Huang Y, Zhao M, Tang X, Wei L. AutismKB: an evidence-based knowledgebase of autism genetics. Nucleic Acids Res. 2012;40:D1016–1022. doi: 10.1093/nar/gkr1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang AX, Wheeler JJ. Children with autism in the People’s Republic of China: diagnosis, legal issues, and educational services. J Autism Dev Disord. 2013;43:1991–2001. doi: 10.1007/s10803-012-1722-6. [DOI] [PubMed] [Google Scholar]
- 25.Zeng Y, Hesketh T. The effects of China’s universal two-child policy. Lancet. 2016;388:1930–1938. doi: 10.1016/S0140-6736(16)31405-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duyzend MH, Nuttle X, Coe BP, Baker C, Nickerson DA, Bernier R, et al. Maternal modifiers and parent-of-origin bias of the autism-associated 16p11.2 CNV. Am J Hum Genet. 2016;98:45–57. doi: 10.1016/j.ajhg.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas NS, Durkie M, Potts G, Sandford R, Van Zyl B, Youings S, et al. Parental and chromosomal origins of microdeletion and duplication syndromes involving 7q11.23, 15q11-q13 and 22q11. Eur J Hum Genet. 2006;14:831–837. doi: 10.1038/sj.ejhg.5201617. [DOI] [PubMed] [Google Scholar]
- 28.Delio M, Guo T, McDonald-McGinn DM, Zackai E, Herman S, Kaminetzky M, et al. Enhanced maternal origin of the 22q11.2 deletion in velocardiofacial and DiGeorge syndromes. Am J Hum Genet. 2013;92:439–447. doi: 10.1016/j.ajhg.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krumm N, Turner TN, Baker C, Vives L, Mohajeri K, Witherspoon K, et al. Excess of rare, inherited truncating mutations in autism. Nat Genet. 2015;47:582–588. doi: 10.1038/ng.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beaudet AL. Reaching a CNV milestone. Nat Genet. 2014;46:1046–1048. doi: 10.1038/ng.3106. [DOI] [PubMed] [Google Scholar]
- 31.Shishido E, Aleksic B, Ozaki N. Copy-number variation in the pathogenesis of autism spectrum disorder. Psychiatry Clin Neurosci. 2014;68:85–95. doi: 10.1111/pcn.12128. [DOI] [PubMed] [Google Scholar]
- 32.Rosenfeld Jill A., Coppinger Justine, Bejjani Bassem A., Girirajan Santhosh, Eichler Evan E., Shaffer Lisa G., Ballif Blake C. Speech delays and behavioral problems are the predominant features in individuals with developmental delays and 16p11.2 microdeletions and microduplications. Journal of Neurodevelopmental Disorders. 2009;2(1):26–38. doi: 10.1007/s11689-009-9037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anguera JA, Brandes-Aitken AN, Rolle CE, Skinner SN, Desai SS, Bower JD, et al. Characterizing cognitive control abilities in children with 16p11.2 deletion using adaptive ‘video game’ technology: a pilot study. Transl. Psychiatry. 2016;6:e893. doi: 10.1038/tp.2016.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shinawi M, Liu P, Kang SH, Shen J, Belmont JW, Scott DA, et al. Recurrent reciprocal 16p11.2 rearrangements associated with global developmental delay, behavioural problems, dysmorphism, epilepsy, and abnormal head size. J Med Genet. 2010;47:332–341. doi: 10.1136/jmg.2009.073015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar RA, KaraMohamed S, Sudi J, Conrad DF, Brune C, Badner JA, et al. Recurrent 16p11.2 microdeletions in autism. Hum Mol Genet. 2008;17:628–638. doi: 10.1093/hmg/ddm376. [DOI] [PubMed] [Google Scholar]
- 36.Wu N, Ming X, Xiao J, Wu Z, Chen X, Shinawi M, et al. TBX6 null variants and a common hypomorphic allele in congenital scoliosis. N Engl J Med. 2015;372:341–350. doi: 10.1056/NEJMoa1406829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abbas Elham, Cox Devin, Smith Teri, Butler Merlin. The 7q11.23 Microduplication Syndrome: A Clinical Report with Review of Literature. Journal of Pediatric Genetics. 2016;05(03):129–140. doi: 10.1055/s-0036-1584361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siu WK, Lam CW, Mak CM, Lau ET, Tang MH, Tang WF, et al. Diagnostic yield of array CGH in patients with autism spectrum disorder in Hong Kong. Clin Transl Med. 2016;5:18. doi: 10.1186/s40169-016-0098-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ke Q, Zhang L, He C, Zhao Z, Qi M, Griggs RC, et al. China’s shift from population control to population quality: Implications for neurology. Neurology. 2016;87:e85–88. doi: 10.1212/WNL.0000000000003010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Subspecialty Group of Clinical Genetics Society of Adolescent Medicine, Chinese Medical Doctor Association; Society of Medical Genetics, Chinese Medical Doctor Association; Subspecialty Group of Endocrinologic, Hereditary and Metabolic Diseases, The Society of Pediatrics, Chinese Medical Association. Expert consensus on the clinical application of chromosomal microarray analysis in pediatric genetic diseases. Chin. J Pediatr. 2016;54:410–413. doi: 10.3760/cma.j.issn.0578-1310.2016.06.004. [DOI] [PubMed] [Google Scholar]