Abstract
Astrocytes are closely associated with Alzheimer’s disease (AD). However, their precise roles in AD pathogenesis remain controversial. One of the reasons behind the different results reported by different groups might be that astrocytes were targeted at different stages of disease progression. In this study, by crossing hAPP (human amyloid precursor protein)-J20 mice with a line of GFAP-TK mice, we found that astrocytes were activated specifically at an early stage of AD before the occurrence of amyloid plaques, while microglia were not affected by this crossing. Activation of astrocytes at the age of 3–5 months did not affect the proteolytic processing of hAPP and amyloid plaque loads in the brains of hAPP-J20 mice. Our data suggest that early activation of astrocytes does not affect the deposition of amyloid β in an animal model of AD.
Electronic supplementary material
The online version of this article (10.1007/s12264-018-0262-2) contains supplementary material, which is available to authorized users.
Keywords: Astrocyte, Alzheimer’s disease, Amyloid plaque, Mouse
Introduction
Alzheimer’s disease (AD) is the most common neurodegenerative disease. It is characterized by extracellular deposits of amyloid β (Aβ) peptides and intracellular neurofibrillary tangles composed of hyperphosphorylated tau in the brain [1–3]. Numerous studies have demonstrated that the pathogenesis of AD is often accompanied by neuroinflammatory responses [4–7]. Microglia and astrocytes are the main categories of cells involved in inflammation in the central nervous system. While extensive efforts have been made to investigate the roles of microglia in AD [4], the association between AD and astrocytes has received relatively little attention.
Astrocytes are the most abundant cells in the central nervous system. Under normal conditions, astrocytes are key players in several physiological functions such as neurogenesis, synaptogenesis, synaptic pruning, neurotransmitter homeostasis, formation of the blood–brain barrier, and the regulation of local blood flow [5, 8–13]. Under pathological circumstances, reactive astrocytes are either protective or detrimental, depending on the specific type of neurological disorder and different stages of the disease progress [4–6]. In AD brains, reactive astrocytes are frequently observed surrounding amyloid plaques [14], but we do not know what the astrocytes do there. Previous in vitro studies have shown that astrocytes degrade Aβ and amyloid plaques [15, 16]. These results have been supported by a recent in vivo analysis showing that inhibiting the activity of astrocytes by deleting GFAP (glial fibrillary acidic protein) and Vim (vimentin) results in increased amyloid plaques in APP/PS1 mice [17]. A subsequent study, however, did not confirm these results in the same AD models [18]. On the other hand, selectively inhibiting the inflammatory signaling of astrocytes by AAV-delivery of VIVIT to disrupt the interaction between calcineurin and NFAT (nuclear factor of activated T cells) results in a reduced amyloid plaque load and improved learning and memory in APP/PS1 mice [19]. Reactive astrocytes in the hippocampus of APP/PS1 mice have also been suggested to account for the impaired memory by abnormally expressing and releasing γ-aminobutyric acid (GABA) [20]. Furthermore, some in vitro studies have shown that exogenous Aβ stimulates astrocytes to express pro-inflammatory mediators such as interleukin (IL)-1β, IL-6, tumor necrosis factor-α, interferon-γ, and inducible nitric oxide synthase, which might promote neurodegeneration [21]. Astrocytes are also involved in Aβ clearance [15, 22, 23]. Therefore, the precise roles of astrocytes in AD pathogenesis are far from clear.
In the present study, we found that astrocytes in the cortex and hippocampus of hAPP (human amyloid precursor protein)-J20 mice were activated by crossing hAPP-J20 with GFAP-TK (thymidine kinase) mice. This activation of astrocytes was restricted to 3–5 month-old mice. Interestingly, activation of astrocytes at this stage did not affect the amyloid plaque load or the enzymatic processing of hAPP in the brains of hAPP-J20 mice.
Materials and Methods
Animals
The hAPP-J20 mice were from the Mutant Mouse Resource and Research Center (Stock No. 034836, Davis, CA). This line of mice expresses hAPP with the Swedish (K670 N and M671L) and Indiana (V717F) mutations under the control of the platelet-derived growth factor β-chain promoter [30]. GFAP-TK mice were from the Jackson Laboratory (Stock No. 005698, Bar Harbor, ME). Transgene-derived HSV-TK in these mice is present exclusively in cells expressing endogenous Gfap [10, 24]. hAPP-J20 mice were crossed with GFAP-TK mice, and male and female hAPP+/GFAP-TK+, hAPP+/GFAP-TK−, hAPP−/GFAP-TK+, and hAPP−/GFAP-TK− mice were used in experiments. All mice were maintained on a C5BL/6 J background. Animals were housed in a pathogen-free barrier facility with a 12-h light/dark cycle and ad libitum access to food and water. All experiments were approved by the Animal Care and Use Committee of Zhejiang University.
Immunohistochemical Staining
All immunohistochemical analyses were done blindly. The expression of GFAP and the plaque loads were assessed as described previously with modifications [32, 33]. Briefly, animals were perfused transcardially with 0.9% saline, and the brain was immediately removed and immersed in 4% paraformaldehyde. Coronal sections (30 μm thick, every 10th section) were cut on a sliding microtome (Leica,Wetzlar, DE) after the brain was saturated in 30% sucrose in phosphate-buffered saline (PBS). After quenching endogenous peroxidase activity by incubation with 3% H2O2 in methanol, the sections were incubated with rabbit anti-GFAP (1:1000, Sigma, Temecula, CA), rabbit anti-vimentin (1:500, Abcam, Cambridge, UK), mouse anti-S100β (1:500, Sigma, Temecula, CA), rabbit anti-Iba1 (1:200, Wako, Osaka, JP), or 3D6 (5 μg/mL, Janssen Research and Development, South San Francisco, CA) followed by biotinylated goat or mouse anti-rabbit antibodies (1:250, Vector Laboratories, Burlingame, CA). Binding of the antibody was detected using Elite kits (Vector Laboratories, Burlingame, CA) with diaminobenzidine (Sigma, Temecula, CA) and H2O2 for development. Images were captured by a DEI-470 digital camera (Optronics, Goleta, CA). The relative numbers of GFAP-, vimentin- and S100β-positive neurons were determined by counting positive cells in the cortex in every 10th section. At least five coronal sections were analyzed per mouse, and the average of the individual measurements was used to calculate group means. Total plaque load was calculated as the percentage area of the hippocampus covered by 3D6-immunoreactive material.
Immunofluorescence Staining
Free-floating sections prepared as above were first blocked with blocking buffer (10% serum, 1% nonfat milk, and 0.2% gelatin in PBS containing 0.5% Triton X-100) and then incubated with the primary antibodies rabbit anti-GFAP (1:1000, Sigma, Temecula, CA), rabbit anti-Iba1 (1:200, Wako, Osaka, JP), or mouse anti-BrdU (1:200, Roche, Basel, CH) followed by incubation with appropriate secondary antibodies conjugated with fluorescein isothiocyanate (1:150, Vector Laboratories, Burlingame, CA) or Cy3 or 594 (1:250, Jackson, Lancaster, PA). For BrdU staining, sections were pretreated with 2N HCl at 37 °C for 30 min, washed in 0.1 mol/L borate buffer (pH 8.5) for 10 min, and then washed in TBST-Triton (10 mmol/L Tris–HCl [pH 7.4], with 150 mmol/L NaCl, 0.05% Tween-20, and 1% Triton X-100) for 30 min at room temperature before incubation with blocking buffer (10% serum in 0.05% TBST). The morphology of GFAP-expressing astrocytes was analyzed from images captured on a confocal microscope (FV1000, Olympus, Tokyo, Japan).
Western Blot
Cortical and hippocampal samples were homogenized and sonicated at 4 °C in RIPA buffer containing 10 mmol/L HEPES (pH 7.4), 150 mmol/L NaCl, 50 mmol/L NaF, 1 mmol/L EDTA, 1 mmol/L dithiothreitol, 1 mmol/L phenylmethylsulfonyl fluoride, 1 mmol/L Na3VO, 10 μg/mL leupeptin, 10 μg/mL aprotinin, and 1% SDS. Equal amounts of protein (by BCA assay, Thermo, Waltham, MA) were resolved by SDS-PAGE and transferred to nitrocellulose membranes. After blocking, the membranes were labeled with rabbit anti-CT15 antibody (1:1000, a kind gift from Dr. E. H. Koo, University of California San Diego, La Jolla, CA), 6E10 (1:1000, Covance, Princeton, NJ), rabbit anti-BACE1 (1:5000, Abcam, Cambridge, UK), rabbit anti-PSD95 (1:3000, CST, Danvers, MA), rabbit anti-GFAP (1:2000, Sigma, Temecula, CA), rabbit anti-Iba1 (1:1000, Wako, Osaka, JP), rabbit anti-vimentin (1:1000, Abcam, Cambridge, UK), or mouse anti-GAPDH (1:10000, Millipore, Billerica, MA), rabbit anti-ALDH1L1 (1:1000 Abcam, Cambridge, UK), mouse anti-Glutamine (1:5000, Millipore, Billerica, MA), rabbit anti- GLT1 (1:5000, Calbiochem, Darmstadt, DE), rabbit anti-GAPDH (1:10000, CST, Danvers, MA), and incubated with horseradish peroxidase-conjugated goat anti-rabbit antibody (1:2000, CST, Danvers, MA) or goat anti-mouse antibody (1:2000, Millipore, Billerica, MA). Bands were visualized by enhanced chemiluminescence, and the densitometry measurements of the bands were acquired from scanned images with Quantity One software (Bio-Rad, Hercules, CA).
Statistical Analyses
Statistical analyses were performed with Graphpad Prism 5 (San Diego, CA). Differences between two means were assessed using the unpaired, two-tailed t test. Differences among three means were assessed by one-way ANOVA. Only values that had P < 0.05 were accepted as significant.
Results
Expression of GFAP and Vimentin was Increased in the Cortex and Hippocampus of hAPP-J20 Mice After Crossing with GFAP-TK Mice
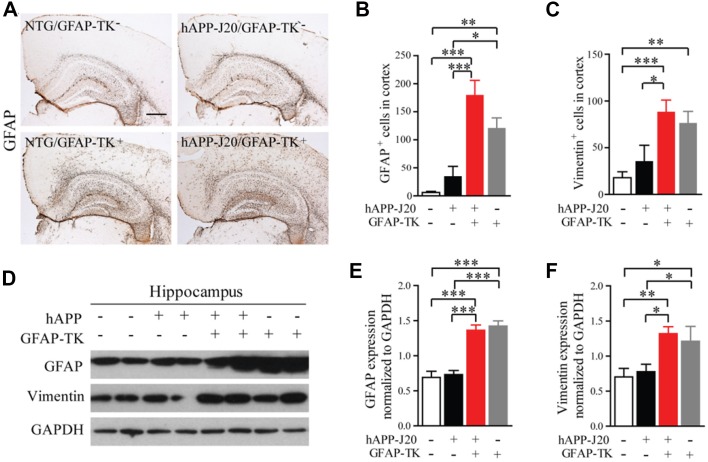
GFAP-TK mice are widely used to manipulate dividing astrocytes and neural stem cells with ganciclovir (GCV) treatment. Transgene-derived TK in these mice is present exclusively in cells expressing endogenous Gfap [24], and GCV kills dividing cells expressing GFA [10]. Unexpectedly, however, we found that immunohistochemical staining showed that the number of GFAP+ astrocytes was dramatically increased in the cortex of non-transgenic (NTG)/GFAP-TK+ and hAPP-J20/GFAP-TK+ mice without GCV treatment (Fig. 1A, B). Interestingly, the increased expression of GFAP in the cortex of hAPP-J20/GFAP-TK+ mice was only found at 3–5 months of age (Fig. S1). Western blotting analysis showed that the GFAP expression was greatly increased in the hippocampus of NTG/GFAP-TK+ and hAPP-J20/GFAP-TK+ mice compared to that in NTG/GFAP-TK− and hAPP-J20/GFAP-TK− mice (Fig. 1D, E). Similarly, immunostaining and western blot analysis revealed that the number of vimentin+ astrocytes in the cortex and the expression of vimentin in the hippocampus were also significantly higher in NTG/GFAP-TK+ and hAPP-J20/GFAP-TK+ mice than in NTG/GFAP-TK− and hAPP-J20/GFAP-TK− mice (Fig. 1C, D, F).
Fig. 1.
Expression of GFAP and vimentin was increased in the cortex and hippocampus of 3-month-old hAPP-J20 mice after crossing with GFAP-TK mice. A Brain sections stained with an antibody against GFAP. NTG/GFAP-TK+ and hAPP-J20/GFAP-TK+ mice showed significantly higher GFAP expression in the cortex and hippocampus than in NTG/GFAP-TK− and hAPP-J20/GFAP-TK− mice (scale bar, 500 μm). B Quantification of the number of GFAP+ cells in the cortex of NTG/GFAP-TK−, hAPP-J20/GFAP-TK−, NTG/GFAP-TK+, and hAPP-J20/GFAP-TK+ mice (n = 6–13 per genotype; data represent mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001). C Quantification of the number of vimentin+ cells in the cortex of NTG/GFAP-TK−, hAPP-J20/GFAP-TK−, NTG/GFAP-TK+, and hAPP-J20/GFAP-TK+ mice (n = 9–15 per genotype; data represent mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001). D Protein bands of GFAP and vimentin in the hippocampus. GAPDH served as the loading control. E Quantification of the levels of GFAP in the hippocampus of 3-month-old NTG/GFAP-TK−, hAPP-J20/GFAP-TK−, NTG/GFAP-TK+, and hAPP-J20/GFAP-TK+ mice (n = 6–10 per genotype; data represent mean ± SEM; ***P < 0.001). F Quantification of the levels of vimentin in the hippocampus of 3-month-old NTG/GFAP-TK−, hAPP-J20/GFAP-TK−, NTG/GFAP-TK+, and hAPP-J20/GFAP-TK+ mice (n = 6–10 per genotype; data represent mean ± SEM; *P < 0.05, **P < 0.01).
Increased Expression of GFAP and Vimentin in the Cortex and Hippocampus of hAPP-J20/GFAP-TK+ Mice was not Due to Proliferation of Astrocytes
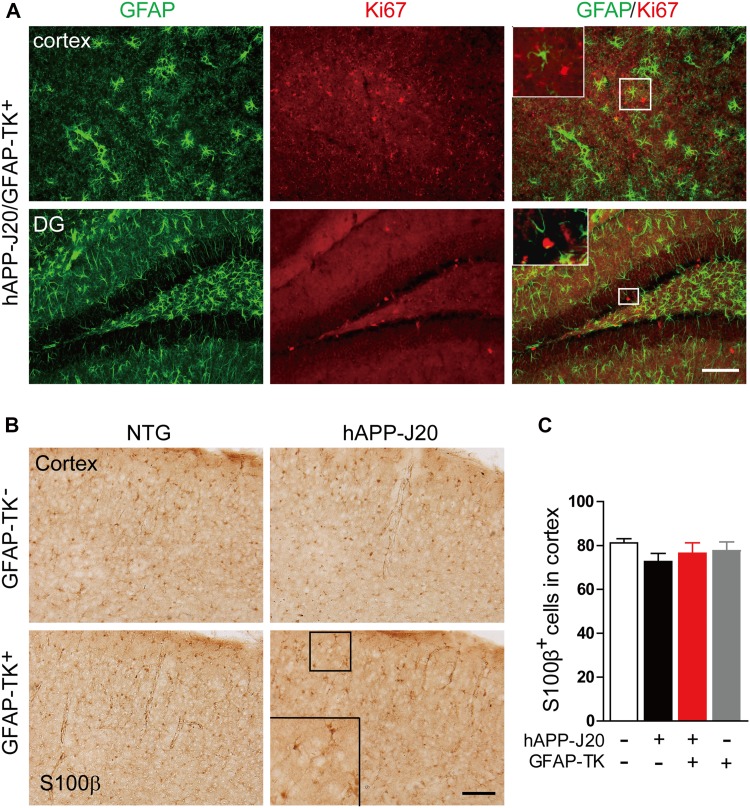
Under normal conditions, astrocytes in the middle layers of the cerebral cortex do not express GFAP [11]. To determine why the GFAP expression was increased in the cortex of hAPP-J20 mice after crossing with GFAP-TK mice, the sections were stained with an antibody against S100β, another marker of astrocytes. The numbers of S100β-positive astrocytes in the cortex were similar in the hAPP-J20/GFAP-TK− and hAPP-J20/GFAP-TK+ mice (Fig. 2B, C), suggesting that the increased GFAP expression was not due to increased numbers of astrocytes. Sections were then stained with an antibody against Ki67, a marker of cell proliferation. In the hippocampus, Ki67+ cells were mainly found in the subgranular zone of the DG (Fig. 2A). However, no Ki67+/GFAP+ cells were observed in the hippocampus and cortex (Fig. 2A), suggesting that astrocytes were not proliferating in these areas. The BrdU labeling results confirmed the absence of proliferating astrocytes in the cortex and hippocampus of each group of mice (data not shown), further indicating that the number of astrocytes was not increased.
Fig. 2.
Increased expression of GFAP and vimentin in the cortex and hippocampus of hAPP-J20/GFAP-TK+ mice was not due to proliferation of astrocytes. A Photomicrographs of immunofluorescence staining for GFAP and Ki67 in hAPP-J20/GFAP-TK+ mice (scale bar, 100 μm). B Photomicrographs of immunohistochemical staining for S100β in NTG/GFAP-TK−, hAPP-J20/GFAP-TK−, NTG/GFAP-TK+, and hAPP-J20/GFAP-TK+ mice (scale bar, 100 μm). C Quantification of the number of S100β+ cells in the cortex of NTG/GFAP-TK−, hAPP-J20/GFAP-TK−, NTG/GFAP-TK+, and hAPP-J20/GFAP-TK+ mice (n = 20–28 per genotype; data represent mean ± SEM).
Expression of Several Astrocyte Markers was not Changed in the Brain of hAPP-J20 Mice After Crossing with GFAP-TK Mice
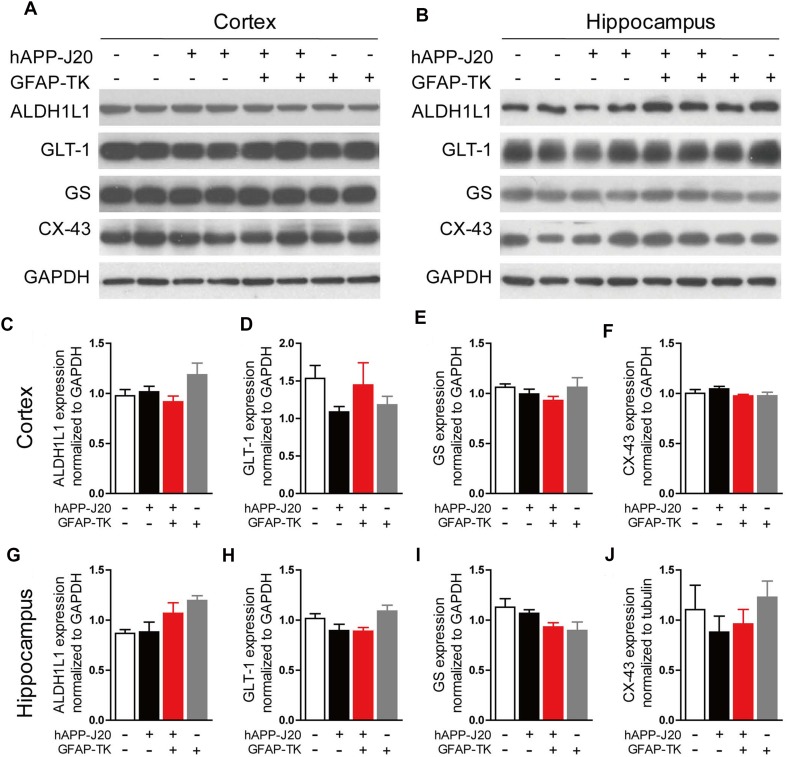
Aldh1l1, GLT-1, GS, and CX-43 are abundantly expressed in astrocytes and play important roles in regulating their physiological functions [25–29]. To determine whether these factors were affected in the cortex and hippocampus of hAPP-J20 mice after crossing with GFAP-TK mice, we performed western blots to measure their expression in the hippocampus and cortex. The results showed no difference between hAPP-J20/GFAP-TK− and hAPP-J20/GFAP-TK+ mice in the expression of Aldh1l1, GLT-1, GS, and CX-43 in either the hippocampus or the cortex (Fig. 3). Again, these data suggested that the number of astrocytes was not affected in the brain of hAPP-J20 mice after crossing with GFAP-TK mice.
Fig. 3.
Expression of several astrocyte markers did not change in the brain of hAPP-J20 mice after crossing with GFAP-TK mice. A, B Protein bands of ALDH1L1, GLT-1, GS, and CX-43 in cortex (A) and hippocampus (B); GAPDH served as the loading control. C–J Quantification of the levels of ALDH1L1, GLT-1, GS, and CX-43 in cortex (C–F) and hippocampus (G–J) of 3-month-old NTG/GFAP-TK−, hAPP-J20/GFAP-TK−, NTG/GFAP-TK+, and hAPP-J20/GFAP-TK+ mice (n = 6–10 per genotype; data represent mean ± SEM).
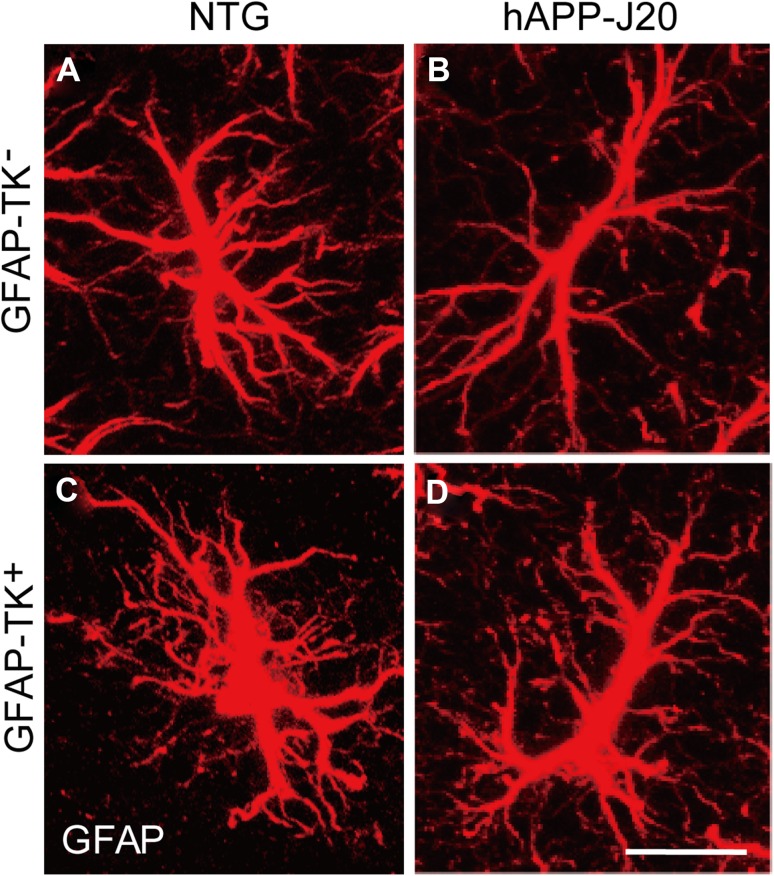
We then analyzed the morphology of GFAP-expressing astrocytes by confocal microscopy, and frequently found hypertrophy of processes in the astrocytes of hAPP-J20/GFAP-TK+ mice (Fig. 4).
Fig. 4.
Hypertrophy of processes was frequently observed in the astrocytes of hAPP-J20/GFAP-TK+ mice. A–D Confocal micrographs of immunohistochemical staining for GFAP in NTG/GFAP-TK−, hAPP-J20/GFAP-TK−, NTG/GFAP-TK+, and hAPP-J20/GFAP-TK+ mice (n = 3 per genotype; scale bar, 10 μm).
Taken together, our results suggested that the increased expression of GFAP and vimentin in the cortex and hippocampus of hAPP-J20/GFAP-TK+ mice was caused by the activation but not the proliferation of astrocytes.
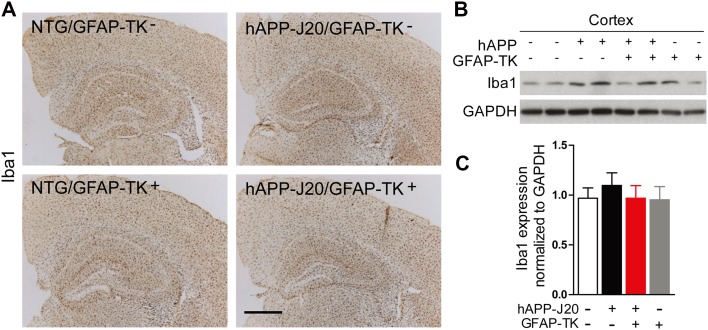
Iba1 Expression was not Affected in the Hippocampus and Cortex of hAPP-J20 Mice After Crossing with GFAP-TK Mice
We also checked the expression of Iba1, a marker of microglia, by immunostaining and western blotting in the cortex and hippocampus of hAPP-J20 mice after crossing with GFAP-TK mice and found no difference between hAPP-J20/GFAP-TK− and hAPP-J20/GFAP-TK+ mice (Fig. 5), suggesting that microglia were not affected in the hippocampus and cortex of hAPP-J20 mice after crossing with GFAP-TK mice.
Fig. 5.
Iba1 expression was not affected in the hippocampus and cortex of hAPP-J20 mice after crossing with GFAP-TK mice. A Brain sections stained with an antibody against Iba1. Compared with NTG/GFAP-TK− and hAPP-J20/GFAP-TK− mice, NTG/GFAP-TK+ and hAPP-J20/GFAP-TK+ mice did not show different Iba1 expression in the cortex and hippocampus (scale bar, 500 μm). B Protein bands of Iba1 in cortex; GAPDH served as the loading control. C Quantification of the levels of Iba1 in the cortex of 3-month-old NTG/GFAP-TK−, hAPP-J20/GFAP-TK−, NTG/GFAP-TK+, and hAPP-J20/GFAP-TK+ mice (n = 6–13 per genotype; data represent mean ± SEM).
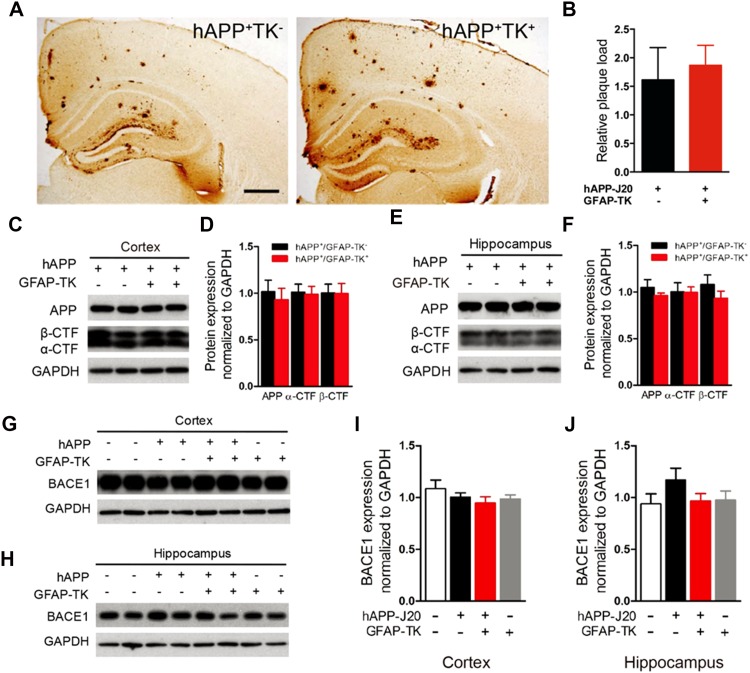
Activation of Astrocytes did not Affect the Amyloid Plaque Load and the Processing of hAPP in hAPP-J20 Mice
Astrocyte activation is closely associated with AD, and previous studies have shown that astrocytes affect the deposits of Aβ [17, 19]. To test whether the activation of astrocytes in the early stages of AD affects the amyloid plaque load in the brain, we performed the free-floating immunohistochemical staining with 3D6, a monoclonal antibody to human Aβ. Consistent with previous studies [30], abundant amyloid plaques were observed in the cortex and hippocampus of 8–10 month-old hAPP-J20 mice. A similar amyloid plaque load was found in the cortex and hippocampus of hAPP-J20/GFAP-TK+ mice in which astrocytes were dramatically activated (Fig. 6A, B), suggesting that activation of astrocytes at 3–5 months of age did not affect the deposits of Aβ in hAPP-J20 mice.
Fig. 6.
Activation of astrocytes did not affect the amyloid plaque load and the processing of hAPP in hAPP-J20 mice. A Photomicrographs of 3D6 immunostaining of 9-month-old hAPP-J20/GFAP-TK− and hAPP-J20/GFAP-TK+ mice (scale bar, 500 μm). B Percentage of area covered by plaques in the hippocampus (n = 5 mice per genotype). C Protein bands of APP and APP carboxyl-terminal fragments (CTFs) in cortex; GAPDH served as the loading control. D Quantification of APP and APP-CTFs in cortex. E Protein bands of APP and APP-CTFs in hippocampus. F Quantification of the levels of APP and APP-CTFs in the hippocampus of 3-month-old hAPP-J20/GFAP-TK−, and hAPP-J20/GFAP-TK+ mice (n = 8–10 per group) assessed by western blot. G, H Protein bands of BACE1 in cortex and hippocampus. I, J Quantification of the levels of BACE1 in cortex and hippocampus of 3-month-old NTG/GFAP-TK−, hAPP-J20/GFAP-TK−, NTG/GFAP-TK+ and hAPP-J20/GFAP-TK+ mice (n = 4–9 per genotype; data represent mean ± SEM).
To investigate the proteolytic processing of hAPP after the activation of astrocytes, we did western blot analysis with 6E10 and CT-15 to assess the hAPP and CTFs of hAPP, respectively. Similar levels of full-length hAPP, α-CTF, and β-CTF were found in the cortex and hippocampus in hAPP-J20/GFAP-TK− and hAPP-J20/GFAP-TK+ mice at both 3 (Fig. 6C–F) and 10 months of age (data not shown), suggesting that activation of astrocytes in the early stages did not affect the processing of hAPP. The BACE1 level was not affected by the activation of astrocytes either (Fig. 6G–J).
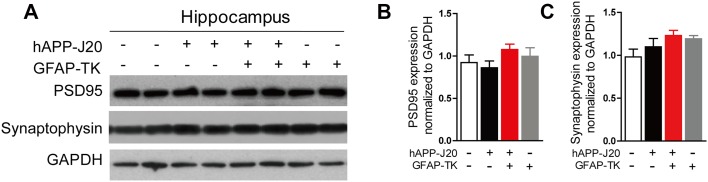
Activation of Astrocytes did not Affect the Levels of PSD-95 and Synaptophysin in the Cortex and Hippocampus of hAPP-J20 Mice
Hippocampal lysates from hAPP-J20/GFAP-TK− and hAPP-J20/GFAP-TK+ mice at 3 months of age were analyzed for PSD-95 and synaptophysin expression by western blotting (Fig. 7). Our results revealed that the levels were comparable to those of hAPP-J20/GFAP-TK− mice, suggesting that early activation of astrocytes did not affect the levels of PSD-95 and synaptophysin in the cortex and hippocampus of hAPP-J20 mice.
Fig. 7.
Activation of astrocytes did not affect the levels of PSD-95 and synaptophysin in the cortex and hippocampus of hAPP-J20 mice. A Protein bands of PSD-95 and synaptophysin in the hippocampus; GAPDH served as the loading control. B, C Quantification of the levels of PSD-95 and synaptophysin in the hippocampus of 3-month-old NTG/GFAP-TK−, hAPP-J20/GFAP-TK−, NTG/GFAP-TK+ and hAPP-J20/GFAP-TK+ mice (n = 6 to 10 per genotype; data are mean ± SEM).
Discussion
Astrocytes are closely associated with AD [5, 14]. However, their roles in its pathogenesis are still controversial. There is evidence that reactive astrocytes secrete pro-inflammatory mediators to promote neurodegeneration in AD [21]. Reactive astrocytes have also been shown to disturb synaptic plasticity and cognitive functions by excessive release of glutamate [31] and GABA [20] in mouse models of AD. Consistent with these reports, downregulation of astrocyte activation decreases the amyloid plaque load and improves memory in AD mice [19]. In contrast, other studies have suggested that astrocytes are protective by facilitating Aβ clearance [22, 23] and degrading Aβ and amyloid plaques [5, 15, 16], while attenuating astrocyte activity worsens the amyloid pathology in AD mice [17]. Although there are multiple reasons behind the discrepancies regarding the roles of astrocytes in AD, one possible explanation could be that astrocytes are targeted in different stages of progress of the disease under different experimental circumstances. For example, astrocytes in the brain of AD mice before or after amyloid plaques appear exhibit dramatic differences in morphological features and functions [11].
Here, we unexpectedly found that astrocytes in the cortex and hippocampus of hAPP-J20 mice were specifically activated between 3 and 5 months of age (before amyloid plaques appear) by crossing with a line of GFAP-TK mice [10, 24]. Meanwhile, microglia were not affected. We compared the amyloid deposits in the brains of 8-10 month-old hAPP-J20 mice with or without astrocyte activation and found no difference. Similarly, a recent report showed that inhibiting astrocyte activity in APP/PS1 mice by crossing them with GFAP−/− Vim−/− mice does not affect the amyloid plaque load in their brains [18]. These data suggested that the activity of astrocytes in early stages of the disease has no effects on Aβ deposits in the brain of older mice. However, different stimuli may affect astrocytes in different ways, and different factors could be released or different signaling pathways could be activated ultimately [11]. Therefore, the possibility that other types of manipulation of astrocytes affect plaque load cannot be excluded at the moment.
Neuroinflammation is a prominent feature of AD [4, 6]. We did find GFAP-expressing astrocytes and Iba1-expressing microglia surrounding the amyloid plaques in the brains of 8–10 month-old hAPP-J20 mice (data not shown). However, GFAP expression in the hippocampus and cortex was similar in hAPP-J20 and non-transgenic mice before plaques appear, although the levels of soluble Aβ are already very high [30]. Our data suggested that fibril Aβ but not soluble Aβ induces the activation of astrocytes in hAPP mice.
Taken together, we have discovered a specific method to activate astrocytes in the cortex and hippocampus of hAPP mice at an early stage of the disease. Our results indicated that early activation of astrocytes does not affect the proteolytic processing of hAPP and the amyloid plaque load in the brains of hAPP mice.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Dr. Edward Koo (Department of Neuroscience, UCSD) for providing the CT-15 antibody. The 3D6 was provided by Janssen Research & Development (South San Francisco, CA). We are grateful to the Core Facilities of Zhejiang University Institute of Neuroscience for technical assistance. This work was supported by grants from the National Basic Research Development Program of China (2014CB964602), the National Natural Science Foundation of China (91132713 and 81400878), the Zhejiang Provincial Natural Science Foundation of China (LR13H090001), the Science and Technology Planning Project of Zhejiang Province, China (2017C03011), the ‘Double First-Rate’ Project Initiative, and Chinese Ministry of Education Project 111 Program (B13026).
Compliance with Ethical Standards
Conflict of interest
The authors disclosed that there was no conflict of interest.
References
- 1.Musiek ES, Holtzman DM. Three dimensions of the amyloid hypothesis: time, space and ‘wingmen’. Nat Neurosci. 2015;18:800–806. doi: 10.1038/nn.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Y, Mandelkow E. Tau in physiology and pathology. Nat Rev Neurosci. 2016;17:5–21. doi: 10.1038/nrn.2015.1. [DOI] [PubMed] [Google Scholar]
- 3.Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148:1204–1222. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pekny M, Pekna M, Messing A, Steinhauser C, Lee JM, Parpura V, et al. Astrocytes: a central element in neurological diseases. Acta Neuropathol. 2016;131:323–345. doi: 10.1007/s00401-015-1513-1. [DOI] [PubMed] [Google Scholar]
- 6.Su F, Bai F, Zhang Z. Inflammatory cytokines and Alzheimer’s disease: a review from the perspective of genetic polymorphisms. Neurosci Bull. 2016;32:469–480. doi: 10.1007/s12264-016-0055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He L, Chen L, Li L. The TBK1-OPTN axis mediates crosstalk between mitophagy and the innate immune response: a potential therapeutic target for neurodegenerative diseases. Neurosci Bull. 2017;33:354–356. doi: 10.1007/s12264-017-0116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 2013;504:394–400. doi: 10.1038/nature12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/S0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 10.Garcia AD, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- 11.Khakh BS, Sofroniew MV. Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci. 2015;18:942–952. doi: 10.1038/nn.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sofroniew MV. Astrocyte barriers to neurotoxic inflammation. Nat Rev Neurosci. 2015;16:249–263. doi: 10.1038/nrn3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zuchero JB, Barres BA. Glia in mammalian development and disease. Development. 2015;142:3805–3809. doi: 10.1242/dev.129304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osborn LM, Kamphuis W, Wadman WJ, Hol EM. Astrogliosis: an integral player in the pathogenesis of Alzheimer’s disease. Prog Neurobiol. 2016;144:121–141. doi: 10.1016/j.pneurobio.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Wyss-Coray T, Loike JD, Brionne TC, Lu E, Anankov R, Yan F, et al. Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat Med. 2003;9:453–457. doi: 10.1038/nm838. [DOI] [PubMed] [Google Scholar]
- 16.Yin KJ, Cirrito JR, Yan P, Hu X, Xiao Q, Pan X, et al. Matrix metalloproteinases expressed by astrocytes mediate extracellular amyloid-beta peptide catabolism. J Neurosci. 2006;26:10939–10948. doi: 10.1523/JNEUROSCI.2085-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kraft AW, Hu X, Yoon H, Yan P, Xiao Q, Wang Y, et al. Attenuating astrocyte activation accelerates plaque pathogenesis in APP/PS1 mice. FASEB J. 2013;27:187–198. doi: 10.1096/fj.12-208660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamphuis W, Kooijman L, Orre M, Stassen O, Pekny M, Hol EM. GFAP and vimentin deficiency alters gene expression in astrocytes and microglia in wild-type mice and changes the transcriptional response of reactive glia in mouse model for Alzheimer’s disease. Glia. 2015;63:1036–1056. doi: 10.1002/glia.22800. [DOI] [PubMed] [Google Scholar]
- 19.Furman JL, Sama DM, Gant JC, Beckett TL, Murphy MP, Bachstetter AD, et al. Targeting astrocytes ameliorates neurologic changes in a mouse model of Alzheimer’s disease. J Neurosci. 2012;32:16129–16140. doi: 10.1523/JNEUROSCI.2323-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jo S, Yarishkin O, Hwang YJ, Chun YE, Park M, Woo DH, et al. GABA from reactive astrocytes impairs memory in mouse models of Alzheimer’s disease. Nat Med. 2014;20:886–896. doi: 10.1038/nm.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li C, Zhao R, Gao K, Wei Z, Yin MY, Lau LT, et al. Astrocytes: implications for neuroinflammatory pathogenesis of Alzheimer’s disease. Curr Alzheimer Res. 2011;8:67–80. doi: 10.2174/156720511794604543. [DOI] [PubMed] [Google Scholar]
- 22.Iliff J. J., Wang M., Liao Y., Plogg B. A., Peng W., Gundersen G. A., Benveniste H., Vates G. E., Deane R., Goldman S. A., Nagelhus E. A., Nedergaard M. A Paravascular Pathway Facilitates CSF Flow Through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid . Science Translational Medicine. 2012;4(147):147ra111–147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanekiyo T, Xu H, Bu G. ApoE and Abeta in Alzheimer’s disease: accidental encounters or partners? Neuron. 2014;81:740–754. doi: 10.1016/j.neuron.2014.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, et al. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998;93:189–201. doi: 10.1016/S0092-8674(00)81571-8. [DOI] [PubMed] [Google Scholar]
- 25.Houades V, Koulakoff A, Ezan P, Seif I, Giaume C. Gap junction-mediated astrocytic networks in the mouse barrel cortex. J Neurosci. 2008;28:5207–5217. doi: 10.1523/JNEUROSCI.5100-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pannasch U, Freche D, Dallerac G, Ghezali G, Escartin C, Ezan P, et al. Connexin 30 sets synaptic strength by controlling astroglial synapse invasion. Nat Neurosci. 2014;17:549–558. doi: 10.1038/nn.3662. [DOI] [PubMed] [Google Scholar]
- 27.Chai H, Diaz-Castro B, Shigetomi E, Monte E, Octeau JC, Yu X, et al. Neural Circuit-Specialized Astrocytes: transcriptomic, Proteomic, Morphological, and Functional Evidence. Neuron. 2017;95(531–549):e539. doi: 10.1016/j.neuron.2017.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Y, Vidensky S, Jin L, Jie C, Lorenzini I, Frankl M, et al. Molecular comparison of GLT1 + and ALDH1L1 + astrocytes in vivo in astroglial reporter mice. Glia. 2011;59:200–207. doi: 10.1002/glia.21089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suarez I, Bodega G, Fernandez B. Glutamine synthetase in brain: effect of ammonia. Neurochem Int. 2002;41:123–142. doi: 10.1016/S0197-0186(02)00033-5. [DOI] [PubMed] [Google Scholar]
- 30.Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, et al. High-level neuronal expression of abeta 1-42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Talantova M, Sanz-Blasco S, Zhang X, Xia P, Akhtar MW, Okamoto S, et al. Abeta induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proc Natl Acad Sci U S A. 2013;110:E2518–E2527. doi: 10.1073/pnas.1306832110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palop JJ, Jones B, Kekonius L, Chin J, Yu GQ, Raber J, et al. Neuronal depletion of calcium-dependent proteins in the dentate gyrus is tightly linked to Alzheimer’s disease-related cognitive deficits. Proc Natl Acad Sci U S A. 2003;100:9572–9577. doi: 10.1073/pnas.1133381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun B, Zhou Y, Halabisky B, Lo I, Cho SH, Mueller-Steiner S, et al. Cystatin C-cathepsin B axis regulates amyloid beta levels and associated neuronal deficits in an animal model of Alzheimer’s disease. Neuron. 2008;60:247–257. doi: 10.1016/j.neuron.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.