Introduction
Stroke is an important disease that is prevalent worldwide [1–3]. Ischemic stroke accounts for 80% of stroke cases. Currently, evidence-based effective treatments for ischemic stroke are limited, and only intravenous thrombolysis with Alteplase (a commercially available thrombolytic agent) within 4.5 h of stroke onset and thrombectomy and arterial thrombolysis within 6–24 h of onset are effective [4, 5]. However, because these two treatments have strict indications and certain risks (reperfusion injury and bleeding) [5–8], there is an urgent need to develop new treatment methods. Thus, comprehensive elucidation of the molecular mechanisms underlying ischemic brain damage and the search for key signaling pathways and protein molecules are important for guiding the clinical treatment of ischemic stroke.
The inflammatory reaction is an important pathophysiological mechanism underlying cerebral ischemic injury. The inflammatory reaction after cerebral ischemia influences the development of ischemic injury and is considered to be a key element related to lesion progression. Both permanent ischemia and reperfusion after transient ischemia have been shown to induce inflammatory reactions in cerebral tissues [9]. However, the pathophysiological processes of these two ischemic conditions are different [10], and the severity of the inflammatory reactions also differ. An inflammatory reaction can be initiated within several hours of stroke onset and can last for several days or even longer [11]. Therefore, treatments targeting the inflammatory reactions have a longer time window and excellent prospects for clinical application.
Cells in the ischemic core undergo necrosis within a short time after cerebral artery occlusion, whereas cells in the surrounding area (ischemic penumbra) can survive for several hours [12]. Restoration of blood flow within this time window may allow cells to survive but may also result in irreversible cell death (reperfusion injury) [12]. For several minutes to several hours during cerebral ischemia, dead cells release danger signals, such as purines, high mobility group box 1 protein, heat shock proteins, and peroxiredoxin family proteins [13]. These danger signals bind to the receptors in the cell membrane or intracellular receptors of adjacent cells to initiate innate immune responses, resulting in inflammatory reactions [13]. These endogenous danger signaling molecules are known as damage-associated molecular patterns (DAMPs). The receptors that recognize DAMPs are known as pattern recognition receptors (PRRs) and mainly include families such as the Toll-like receptors and Nod-like receptors (NLRs) [14]. DAMPs released by necrotic cells in the ischemic core are the source of signals that initiate inflammatory reactions. However, the sources of the danger signals that promote further development of the inflammatory reactions and how they exert their functions are still unclear. Cells in the ischemic penumbra are thought to undergo apoptosis, which can last for several weeks [15]. However, during apoptosis, the cell membrane is intact, and there is no spillover of cytoplasmic components [16]. Hence, apoptosis is unlikely to be the primary instigator of inflammation during brain ischemia.
Pyroptosis is a special form of programmed cell death with strong pro-inflammatory effects. Previous research has shown that the upstream molecules of pyroptosis are highly expressed in the ischemic penumbra. Therefore, we speculated that pyroptosis might occur in the ischemic penumbra and play an important role in the inflammatory injury of cerebral ischemia. In this article, we discuss the possible development of pyroptosis after cerebral ischemia and hypothesize the possible molecular mechanisms.
Discovery of Cell Pyroptosis
For many years, the forms of cell death were divided into apoptosis and necrosis. Apoptosis is gene-regulated programmed cell death, whereas necrosis is accidental cell death. However, recent studies have shown that necrosis can also be gene-regulated programmed cell death which is called programmed necrosis [17]. Pyroptosis is a form of programmed necrosis in which significant research progress has been made in recent years. The phenomenon of pyroptosis was first discovered in 1992 during a study of macrophages infected with Shigella flexneri [18]. Subsequently, cytoplasmic vacuolization, DNA fragmentation, chromatin condensation, and caspase-1 activation were also observed in macrophages infected with the mouse Salmonella typhimurium strain [19, 20]. Researchers at that time considered caspase-dependent activation to be a specific feature of apoptosis and mistook this type of cell death as apoptosis. With in-depth studies and technological development, researchers have found that pyroptosis is mainly mediated by caspase-1 rather than the traditional apoptosis-related reactive molecule caspase-3 [21]. Therefore, this process was proposed as a form of programmed cell death that differs from apoptosis, and the concept of pyroptosis was formally proposed in 2000 [22]. Pyroptosis does not only occur during infectious diseases. Recent studies have shown that pyroptosis is also present in sterile inflammation, including diabetic atherosclerosis [23], acute liver injury [24], alcoholic hepatitis [25], diabetic cardiomyopathy [26], diabetic nephropathy [27], benign prostatic hyperplasia [28], Alzheimer’s disease [29], temporal lobe epilepsy [30], renal ischemia/reperfusion [31], traumatic brain injury [32], ethanol-induced brain injury [33], and myocardial ischemia/reperfusion [34].
Characteristics of Pyroptosis
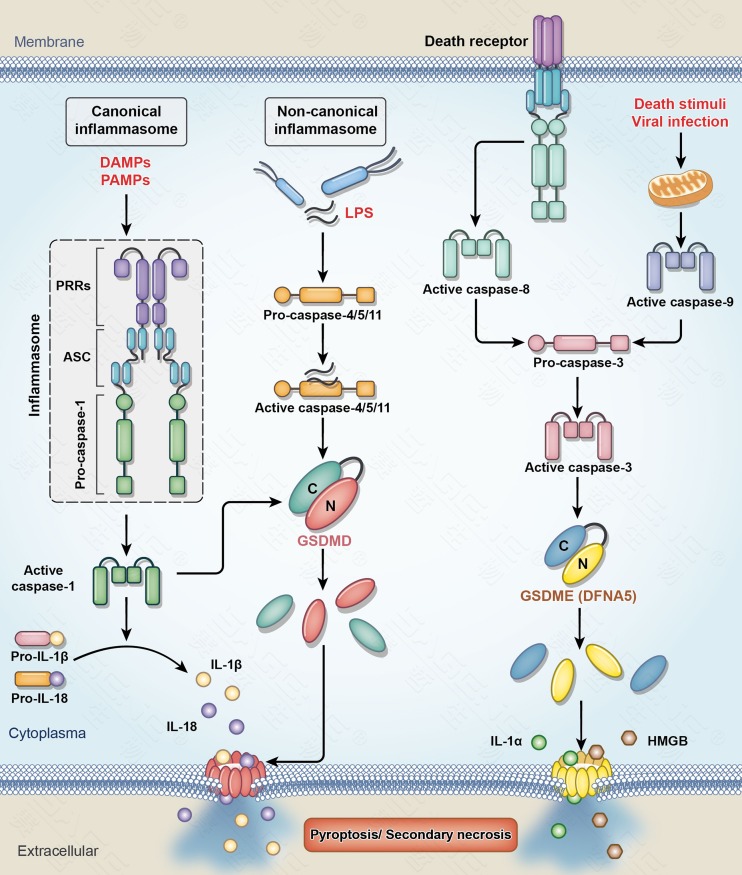
Pyroptosis differs from other forms of cell death. (1) The most distinctive morphological feature is that the cell membrane has pores with diameters of 10–20 nm during pyroptosis [35–40]. After the pores form in the plasma membrane, water influx is driven by intracellular non-ionic osmolytes and causes swelling, membrane rupture, and the release of cellular contents. Nuclear chromatin condensation and DNA cleavage and fragmentation also occur, similar to apoptosis. However, the nuclear membrane is intact, unlike apoptosis [41, 42]. (2) The most distinctive biochemical feature is that the development of pyroptosis mainly depends on the activation of inflammatory caspases (including caspase-1 or caspase-4/5/11). Caspase-1 is present in both humans and rodents, while caspase-11 is present only in rodents but is homologous with caspase-4 and caspase-5, which are present only in humans. Caspase-1 activation usually occurs in a multi-protein complex called the inflammasome. The inflammasome generally contains the following 3 components: receptors—PRRs in the cytoplasm that mainly include NLRs (such as NLRP1, NLRP3, and NLRC4) and absent in melanoma 2 (AIM2); effectors—the caspase-1 precursor (pro-caspase-1); and apoptosis-associated speck-like protein containing a CARD (ASC), which is an adaptor protein connecting PRRs and pro-caspase-1. Caspase-1 gene-knockout mice do not develop pyroptosis [43, 44], so the Nomenclature Committee on Cell Death 2012 recommended defining pyroptosis as a form of caspase-1-dependent cell death [44]. However, studies have shown that caspase-1 gene-knockout in mice also results in caspase-11 inactivation. In addition, studies on bacteria-induced cell death reported that the bacterial component lipopolysaccharide (LPS) directly activates caspase-11 (rodent-derived) or caspase-4/5 (human-derived), resulting in pyroptosis in infected cells [45]. The two different pyroptosis pathways (caspase-1 and caspase-4/5/11) are called the canonical and the non-canonical inflammasome pathways, respectively (Fig. 1).
Fig. 1.
Pyroptosis pathways. In the canonical inflammasome pathway, microbial pathogens (PAMPs) or sterile inflammatory substances (DAMPs) are detected by various cytosolic sensor proteins (PRRs) which leads to caspase-1 activation through an inflammasome complex. Active caspase-1 converts the precursors of IL-1β and IL-18 to their mature forms, which are released from cells by pyroptosis. In the non-canonical pathway, LPS in the cytosol of cells infected by bacteria binds to precursor caspase-4/5/11, which leads to the activation of caspase-4/5/11. Both caspase-1 and caspase-4/5/11 process GSDMD, which results in the release of the N-terminal fragment of GSDMD (GSDMD-N). GSDMD-N forms pores in the plasma membrane that induce pyroptosis. The other pathway of pyroptosis is caspase-3/GSDME (DFNA5). Caspase-3 can be activated by the mitochondrial pathway and the death receptor pathway. Activated caspase-3 in turn cleaves GSDME to generate the GSDME-N fragment that forms pores in the plasma membrane and induces secondary necrosis/pyroptosis [1]. PAMPs pathogen-associated molecular patterns; DAMPs danger-associated molecular patterns; PRRs pattern recognition receptors; ASC apoptosis-associated speck-like protein containing a CARD; LPS lipopolysaccharide; GSDMD gasdermin D; GSDME (DFNA5), gasdermin E (deafness autosomal dominant type 5).
Necroptosis is another form of programmed cell death with a common feature of plasma membrane rupture. Unlike pyroptosis, necroptosis is initiated by a ligand binding to death receptors, including tumor necrosis factor receptor 1 [46]. The rupture in necroptosis is explosion-like and it is known that the execution of necroptosis is mediated by mixed lineage kinase domain-like oligomers in the plasma membrane [17, 38, 46].
Pro-inflammatory Function of Pyroptosis
Necrotic cells release various cell components (DAMPs) under many pathological conditions. These DAMPs act on cell membranes or intracellular receptors (PRRs) in adjacent cells to cause inflammasome formation and caspase-1 or caspase-11 activation. Consequentially, pyroptosis occurs, and the inflammatory factors IL-1β and IL-18 are released. Further, these DAMPs induce PRR activation in other adjacent cells, resulting in pyroptosis and the release of inflammatory factors [47]. This cascade amplifies the inflammatory reactions to aggravate injury. Recent studies have shown that NLRP3 and ASC are released outside of the cells after pyroptosis. After extracellular NLRP3 and ASC undergo recombination and polymerization or ASC undergoes self-aggregation, they continue to activate caspase-1 and promote IL-1β maturation. In addition, after engulfment of NLRP3 and ASC by macrophages, the above process can occur in cells to amplify the inflammatory reactions [47, 48]. Therefore, pyroptosis not only participates in the initiation of inflammatory reactions but also plays a critical role in spreading inflammatory signals and amplifying inflammatory reactions.
Induction of Pyroptosis by Gasdermin Proteins
Studies on the mechanisms of pyroptosis have made major breakthroughs in the last two years. The gasdermin protein family has been found to be the direct executor of pyroptosis. This family includes gasdermins A (GSDMA), B (GSDMB), C (GSDMC), D (GSDMD), E (GSDME, also known as DFNA5), and DFNB59. GSDMD and GSDME are the proteins most studied in pyroptosis.
Three articles published in 2015 in journals such as Nature showed that GSDMD is the common substrate of all inflammatory caspases (caspase-1 and caspase-4/5/11) [49–51]. These activated caspases cleave GSDMD between the N-terminal and C-terminal domains. The N-terminal domain of GSDMD (GSDMD-N) has pyroptosis-inducing activity and plays a decisive role in IL-1β and IL-18 secretion. The C-terminal domain of GSDMD (GSDMD-C) does not have these functions but inhibits the N-terminal domain when cells are in a quiescent state to maintain GSDMD in an inactivate state. Five research articles published in 2016 elucidated the process of the direct involvement of GSDMD-N in membrane pore formation during pyroptosis [35–39]. GSDMD-N specifically interacts with phospholipid molecules on the cell membrane and results in pore formation to change the cellular osmotic pressure, eventually leading to cell membrane lysis and pyroptosis. Studies also showed that the N-terminal domains of almost all gasdermin family proteins have pyroptosis-inducing function. However, aside from GSDMD, the other family members are not substrates of the inflammatory caspases.
Recent studies have shown that caspase-3, which has long been considered an apoptosis marker, can also induce secondary necrosis (pyroptosis) through activation of GSDME [52–54]. These results broke with the classic concept that caspase-3 activation necessarily leads to apoptosis. One caspase-3 cleavage site is present between the N-terminal and C-terminal domains of GSDME. The N-terminal fragment of GSDME (GSDME-N) produced by activated caspase-3 cleavage is similar to GSDMD-N, leading to pore formation in the cell membrane and inducing pyroptosis. Therefore, GSDME is a key factor mediating the conversion of apoptosis (induced by the activation of caspase-3) into pyroptosis (Fig. 1).
Possibility of Pyroptosis After Cerebral Ischemia
Many studies using rodent models of focal cerebral ischemia/reperfusion have shown that the formation of the NLRP1, NLRP3, AIM2, and NLRC4 inflammasomes and caspase-1 activation occur in ischemic cerebral tissues. In addition, an increase in the NLRP1 or NLRP3 inflammasome or caspase-1 expression has been reported in neurons, microglia, astrocytes, vascular endothelial cells, and infiltrated white blood cells from the peripheral blood. Gene knockout of these inflammasomes or caspase-1, or inhibition of their activity, can relieve ischemic injury (see review by Allan et al. for details) [55]. Up-regulation of NLRP1, NLRP3, ASC, and caspase-1 expression has also been found in cortical neurons from human cerebral infarction tissues [56]. In addition, in vivo and in vitro studies in mice have shown that caspase-11 is activated in ischemic cortical neurons [56]. Therefore, the above studies confirm that the expression of pyroptosis-related proteins is up-regulated in neurovascular cells after cerebral ischemia and aggravates ischemic injury. These results strongly suggest that pyroptosis occurs after cerebral ischemia to aggravate tissue injury. However, these studies did not search for direct evidence of pyroptosis since these studies only observed the upstream molecules of pyroptosis in ischemic cells and did not further observe whether these cells possess the morphological feature or biochemical markers of pyroptosis.
As noted above, previous studies have confirmed that inflammasomes, caspase-1, and caspase-11 expression is up-regulated after cerebral ischemia. Since these proteins are all upstream molecules of the GSDMD protein [55, 56], we hypothesized that two inflammatory caspase-mediated signaling pathways of pyroptosis, the inflammasome/caspase-1/GSDMD pathway and the caspase-11/GSDMD pathway, can both be activated after cerebral ischemia, although the activation levels might differ. Caspase-11 expression is up-regulated in ischemic cortical neurons [56], but the current literature reports that the induction of pyroptosis by caspase-11 is limited in infectious diseases. Whether sterile inflammation has the same mechanisms requires further confirmation. We suggest that cerebral ischemia mainly activates the inflammasome/caspase-1/GSDMD pathway.
Many studies have shown that caspase-3 is activated in ischemic cerebral tissues and cells (generally considered to indicate apoptosis). Recent studies have shown that caspase-3 induces pyroptosis through activation of GSDME [52–54]. Previous studies on apoptosis in cerebral ischemia usually used terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) and Annexin V staining to show DNA damage and phosphatidylserine expression on the cell membrane, respectively [15]. However, these two staining methods can both be positive in pyroptotic cells. Therefore, part of the apoptosis observed might actually be pyroptosis. We speculate that the caspase-3/GSDME pathway might also be partially activated after cerebral ischemia to induce pyroptosis.
The cell type(s) undergoing pyroptosis after ischemia remains unclear. Neuronal, microglial, and astroglial pyroptosis have been reported in some pathological states without ischemia. However, all these cells undergo caspase-1-dependent pyroptosis [29, 30, 32, 33, 57]. Microglia are macrophages located in the brain and are the major executors of inflammatory reactions in the brain. Microglial activation is a key element of inflammation following ischemic stroke [9, 11]. Therefore, we speculate that microglia may be the major cell type involved in inflammatory caspase-mediated pyroptosis after cerebral ischemia and play a critical role in the pyroptosis-mediated spread of inflammation. Furthermore, since caspase-3 is mainly activated in neurons after cerebral ischemia [58], caspase-3-mediated pyroptosis may mainly occur in neurons.
The pathophysiological states differ during the different stages after cerebral ischemia. The presence of inflammation precedes apoptosis, and the time frame of apoptosis lasts longer. Therefore, we speculate that the signaling pathways that induce pyroptosis at different stages after cerebral ischemia might be different, with the inflammatory caspase/GSDMD pathway serving as the major pathway in the early stage, and the caspase-3/GSDME pathway serving as the major pathway in the late stage. Furthermore, the inflammatory reaction after cerebral ischemia/reperfusion is more severe than the reaction in sustained cerebral ischemia. Therefore, the development of pyroptosis and the activation of its signaling pathways may also be more intense after cerebral ischemia/reperfusion.
In summary, the GSDMD and GSDME signaling pathways (inflammasome/caspase-1/GSDMD, caspase-11/GSDMD, and caspase-3/GSDME) can be activated after cerebral ischemia to induce pyroptosis. However, the levels of activation of these pathways and the degree of pyroptosis may differ under different ischemic conditions (permanent ischemia or ischemia/reperfusion), during different ischemic stages (early or late stage), and in different cell types.
Conclusions
Previous studies on cerebral ischemia have shown up-regulation of the expression of pyroptosis-related signaling proteins, indicating the possible role of pyroptosis in neurovascular cells after cerebral ischemia. Recent studies have shown that GSDMD and GSDME are the executors of pyroptosis and that their upstream molecules are the inflammatory caspases and caspase-3, respectively. Therefore, we speculated that the microglial inflammasome/caspase-1/GSDMD pathway and the neuronal caspase-3/GSDME signaling pathway are the major molecular mechanisms underlying pyroptosis after cerebral ischemia. In-depth studies of pyroptosis and its mechanisms will further elucidate the mechanisms underlying the inflammatory injury of cerebral ischemia and provide new drug targets for the treatment of ischemic stroke.
Acknowledgements
This perspective was supported by the Natural Science Foundation of Guangdong Province, China (2017A030313869) and the Science and Technology Project of Guangzhou City, China (201607010325).
Compliance with Ethical Standards
Conflict of interest
The authors claim no conflicts of interest.
References
- 1.Wang W, Jiang B, Sun H, Ru X, Sun D, Wang L, et al. Prevalence, incidence, and mortality of stroke in China: results from a nationwide population-based survey of 480 687 adults. Circulation. 2017;135:759–771. doi: 10.1161/CIRCULATIONAHA.116.025250. [DOI] [PubMed] [Google Scholar]
- 2.Koton S, Schneider AL, Rosamond WD, Shahar E, Sang Y, Gottesman RF, et al. Stroke incidence and mortality trends in US communities, 1987 to 2011. JAMA. 2014;312:259–268. doi: 10.1001/jama.2014.7692. [DOI] [PubMed] [Google Scholar]
- 3.Norrving B, Kissela B. The global burden of stroke and need for a continuum of care. Neurology. 2013;80:S5–S12. doi: 10.1212/WNL.0b013e3182762397. [DOI] [PubMed] [Google Scholar]
- 4.Demaerschalk BM, Kleindorfer DO, Adeoye OM, Demchuk AM, Fugate JE, Grotta JC, et al. Scientific rationale for the inclusion and exclusion criteria for intravenous alteplase in acute ischemic stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2016;47:581–641. doi: 10.1161/STR.0000000000000086. [DOI] [PubMed] [Google Scholar]
- 5.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–e110. doi: 10.1161/STR.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 6.Alberts MJ. Stroke treatment with intravenous tissue-type plasminogen activator: more proof that time is brain. Circulation. 2017;135:140–142. doi: 10.1161/CIRCULATIONAHA.116.025724. [DOI] [PubMed] [Google Scholar]
- 7.Yang J, Liang H, Song Y, Shen L, Wang S. Analysis of the efficacy and safety of recombinant tissue plasminogen activator for chinese patients over 80 years of age with acute ischemic stroke: a pilot study. Neurosci Bull. 2016;32:202–203. doi: 10.1007/s12264-016-0020-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Q, Li X, Dong W, Ye M, Cao Y, Zhang M, et al. Factors associated with thrombolysis outcome in ischemic stroke patients with atrial fibrillation. Neurosci Bull. 2016;32:145–152. doi: 10.1007/s12264-016-0019-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawabori M, Yenari MA. Inflammatory responses in brain ischemia. Curr Med Chem. 2015;22:1258–1277. doi: 10.2174/0929867322666150209154036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hossmann KA. The two pathophysiologies of focal brain ischemia: implications for translational stroke research. J Cereb Blood Flow Metab. 2012;32:1310–1316. doi: 10.1038/jcbfm.2011.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vidale S, Consoli A, Arnaboldi M, Consoli D. Postischemic Inflammation in acute stroke. J Clin Neurol. 2017;13:1–9. doi: 10.3988/jcn.2017.13.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manning NW, Campbell BC, Oxley TJ, Chapot R. Acute ischemic stroke: time, penumbra, and reperfusion. Stroke. 2014;45:640–644. doi: 10.1161/STROKEAHA.113.003798. [DOI] [PubMed] [Google Scholar]
- 13.Gelderblom M, Sobey CG, Kleinschnitz C, Magnus T. Danger signals in stroke. Ageing Res Rev. 2015;24:77–82. doi: 10.1016/j.arr.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Xu X, Jiang Y. The Yin and Yang of innate immunity in stroke. Biomed Res Int. 2014;2014:807978. doi: 10.1155/2014/807978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zille M, Farr TD, Przesdzing I, Muller J, Sommer C, Dirnagl U, et al. Visualizing cell death in experimental focal cerebral ischemia: promises, problems, and perspectives. J Cereb Blood Flow Metab. 2012;32:213–231. doi: 10.1038/jcbfm.2011.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aachoui Y, Sagulenko V, Miao EA, Stacey KJ. Inflammasome-mediated pyroptotic and apoptotic cell death, and defense against infection. Curr Opin Microbiol. 2013;16:319–326. doi: 10.1016/j.mib.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wallach D, Kang TB, Dillon CP, Green DR. Programmed necrosis in inflammation: Toward identification of the effector molecules. Science. 2016;352:aaf2154. doi: 10.1126/science.aaf2154. [DOI] [PubMed] [Google Scholar]
- 18.Zychlinsky A, Prevost MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
- 19.Chen LM, Kaniga K, Galan JE. Salmonella spp. are cytotoxic for cultured macrophages. Mol Microbiol. 1996;21:1101–1115. doi: 10.1046/j.1365-2958.1996.471410.x. [DOI] [PubMed] [Google Scholar]
- 20.Monack DM, Raupach B, Hromockyj AE, Falkow S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc Natl Acad Sci U S A. 1996;93:9833–9838. doi: 10.1073/pnas.93.18.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porter AG, Janicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 22.Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9:113–114. doi: 10.1016/S0966-842X(00)01936-3. [DOI] [PubMed] [Google Scholar]
- 23.Han Y, Qiu H, Pei X, Fan Y, Tian H, Geng J. Low-dose sinapic acid abates the pyroptosis of macrophages via downregulation of lncRNA-MALAT1 in rats with diabetic atherosclerosis. J Cardiovasc Pharmacol. 2017;71:104–112. doi: 10.1097/FJC.0000000000000550. [DOI] [PubMed] [Google Scholar]
- 24.Chen YL, Xu G, Liang X, Wei J, Luo J, Chen GN, et al. Inhibition of hepatic cells pyroptosis attenuates CLP-induced acute liver injury. Am J Transl Res. 2016;8:5685–5695. [PMC free article] [PubMed] [Google Scholar]
- 25.Khanova E, Wu R, Wang W, Yan R, Chen Y, French SW, et al. Pyroptosis by caspase11/4-gasdermin-D pathway in alcoholic hepatitis. Hepatology. 2017;67:1737–1753. doi: 10.1002/hep.29645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, Du N, Zhang Q, Li J, Chen X, Liu X, et al. MicroRNA-30d regulates cardiomyocyte pyroptosis by directly targeting foxo3a in diabetic cardiomyopathy. Cell Death Dis. 2014;5:e1479. doi: 10.1038/cddis.2014.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Zeng L, Cao C, Lu C, Lian W, Han J, et al. Long noncoding RNA MALAT1 regulates renal tubular epithelial pyroptosis by modulated miR–23c targeting of ELAVL1 in diabetic nephropathy. Exp Cell Res. 2017;350:327–335. doi: 10.1016/j.yexcr.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Jiang MY, Han ZD, Li W, Yue F, Ye J, Li B, et al. Mitochondrion-associated protein peroxiredoxin 3 promotes benign prostatic hyperplasia through autophagy suppression and pyroptosis activation. Oncotarget. 2017;8:80295–80302. doi: 10.18632/oncotarget.17927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan MS, Tan L, Jiang T, Zhu XC, Wang HF, Jia CD, et al. Amyloid-beta induces NLRP1-dependent neuronal pyroptosis in models of Alzheimer’s disease. Cell Death Dis. 2014;5:e1382. doi: 10.1038/cddis.2014.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan CC, Zhang JG, Tan MS, Chen H, Meng DW, Jiang T, et al. NLRP1 inflammasome is activated in patients with medial temporal lobe epilepsy and contributes to neuronal pyroptosis in amygdala kindling-induced rat model. J Neuroinflammation. 2015;12:18. doi: 10.1186/s12974-014-0233-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang JR, Yao FH, Zhang JG, Ji ZY, Li KL, Zhan J, et al. Ischemia-reperfusion induces renal tubule pyroptosis via the CHOP-caspase-11 pathway. Am J Physiol Renal Physiol. 2014;306:F75–F84. doi: 10.1152/ajprenal.00117.2013. [DOI] [PubMed] [Google Scholar]
- 32.Adamczak SE, de Rivero Vaccari JP, Dale G, Brand FJ, Nonner D, Bullock M, et al. Pyroptotic neuronal cell death mediated by the AIM2 inflammasome. J Cereb Blood Flow Metab. 2014;34:621–629. doi: 10.1038/jcbfm.2013.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alfonso-Loeches S, Urena-Peralta JR, Morillo-Bargues MJ, Oliver-De La Cruz J, Guerri C. Role of mitochondria ROS generation in ethanol-induced NLRP3 inflammasome activation and cell death in astroglial cells. Front Cell Neurosci. 2014;8:216. doi: 10.3389/fncel.2014.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qiu Z, Lei S, Zhao B, Wu Y, Su W, Liu M, et al. NLRP3 Inflammasome activation-mediated pyroptosis aggravates myocardial ischemia/reperfusion injury in diabetic rats. Oxid Med Cell Longev. 2017;2017:9743280. doi: 10.1155/2017/9743280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sborgi L, Ruhl S, Mulvihill E, Pipercevic J, Heilig R, Stahlberg H, et al. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016;35:1766–1778. doi: 10.15252/embj.201694696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535:111–116. doi: 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- 37.Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–158. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen X, He WT, Hu L, Li J, Fang Y, Wang X, et al. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res. 2016;26:1007–1020. doi: 10.1038/cr.2016.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aglietti RA, Estevez A, Gupta A, Ramirez MG, Liu PS, Kayagaki N, et al. GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes. Proc Natl Acad Sci U S A. 2016;113:7858–7863. doi: 10.1073/pnas.1607769113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mulvihill E, Sborgi L, Mari SA, Pfreundschuh M, Hiller S, Muller DJ. Mechanism of membrane pore formation by human gasdermin-D. EMBO J 2018, 37(14). pii: e98321.. [DOI] [PMC free article] [PubMed]
- 41.Sanz AB, Sanchez-Nino MD, Izquierdo MC, Gonzalez-Espinoza L, Ucero AC, Poveda J, et al. Macrophages and recently identified forms of cell death. Int Rev Immunol. 2014;33:9–22. doi: 10.3109/08830185.2013.771183. [DOI] [PubMed] [Google Scholar]
- 42.Kepp O, Galluzzi L, Zitvogel L, Kroemer G. Pyroptosis - a cell death modality of its kind? Eur J Immunol. 2010;40:627–630. doi: 10.1002/eji.200940160. [DOI] [PubMed] [Google Scholar]
- 43.Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunol Rev. 2011;243:206–214. doi: 10.1111/j.1600-065X.2011.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19:107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 46.Fayaz SM, Suvanish Kumar VS, Rajanikant GK. Necroptosis: who knew there were so many interesting ways to die? CNS Neurol Disord Drug Targets. 2014;13:42–51. doi: 10.2174/18715273113126660189. [DOI] [PubMed] [Google Scholar]
- 47.Kono H, Kimura Y, Latz E. Inflammasome activation in response to dead cells and their metabolites. Curr Opin Immunol. 2014;30:91–98. doi: 10.1016/j.coi.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 48.Franklin BS, Bossaller L, De Nardo D, Ratter JM, Stutz A, Engels G, et al. The adaptor ASC has extracellular and ‘prionoid’ activities that propagate inflammation. Nat Immunol. 2014;15:727–737. doi: 10.1038/ni.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 50.Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 51.He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res. 2015;25:1285–1298. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rogers C, Fernandes-Alnemri T, Mayes L, Alnemri D, Cingolani G, Alnemri ES. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat Commun. 2017;8:14128. doi: 10.1038/ncomms14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Y, Gao W, Shi X, Ding J, Liu W, He H, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547:99–103. doi: 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
- 54.Wang Y, Yin B, Li D, Wang G, Han X, Sun X. GSDME mediates caspase-3-dependent pyroptosis in gastric cancer. Biochem Biophys Res Commun. 2018;495:1418–1425. doi: 10.1016/j.bbrc.2017.11.156. [DOI] [PubMed] [Google Scholar]
- 55.Barrington J, Lemarchand E, Allan SM. A brain in flame; do inflammasomes and pyroptosis influence stroke pathology? Brain Pathol. 2017;27:205–212. doi: 10.1111/bpa.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fann DY, Lee SY, Manzanero S, Tang SC, Gelderblom M, Chunduri P, et al. Intravenous immunoglobulin suppresses NLRP1 and NLRP3 inflammasome-mediated neuronal death in ischemic stroke. Cell Death Dis. 2013;4:e790. doi: 10.1038/cddis.2013.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jamilloux Y, Pierini R, Querenet M, Juruj C, Fauchais AL, Jauberteau MO, et al. Inflammasome activation restricts Legionella pneumophila replication in primary microglial cells through flagellin detection. Glia. 2013;61:539–549. doi: 10.1002/glia.22454. [DOI] [PubMed] [Google Scholar]
- 58.Love S. Apoptosis and brain ischaemia. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:267–282. doi: 10.1016/S0278-5846(03)00022-8. [DOI] [PubMed] [Google Scholar]