Abstract
Alzheimer’s disease (AD), the most common type of dementia, is becoming a major challenge for global health and social care. However, the current understanding of AD pathogenesis is limited, and no early diagnosis and disease-modifying therapy are currently available. During the past year, significant progress has been made in clinical research on the diagnosis, prevention, and treatment of AD. In this review, we summarize the latest achievements, including diagnostic biomarkers, polygenic hazard score, amyloid and tau PET imaging, clinical trials targeting amyloid-beta (Aβ), tau, and neurotransmitters, early intervention, and primary prevention and systemic intervention approaches, and provide novel perspectives for further efforts to understand and cure the disease.
Keywords: Alzheimer’s disease, Amyloid-beta, Tau, Immunotherapy, BACE1 inhibitor, 5-HT6 receptor antagonist, Primary prevention, Positron emission tomographic imaging, Biomarker
Introduction
Alzheimer’s disease (AD) is a chronic neurodegenerative disease featuring progressive memory loss and cognitive performance deficits. Globally, after the age of 65 years, the incidence rate of AD doubles every 5 years. Moreover, the number of cases is projected to reach > 115 million by 2050 [1], and the estimated cost imposed by AD was $818 billion in 2015 alone [2]. In addition, AD is associated with high comorbidity burdens [3, 4]. Given the heavy economic and social burdens of AD, major emphasis is being placed on understanding its pathogenesis and developing early diagnosis and effective intervention.
AD is pathologically characterized by extracellular senile plaques composed of amyloid-beta (Aβ) peptide, intracellular neurofibrillary tangles containing hyperphosphorylated tau protein, cerebral amyloid angiopathy due to the deposition of Aβ on vessel walls, and neuronal loss. Reduction of the production and clearance of the accumulation of Aβ and hyperphosphorylated tau in the brain are crucial therapeutic strategies for AD. Despite the fact that many advances in pathogenesis and clinical practice have been achieved over the past decades, the factors triggering AD onset and progression remain unclear. Current medicines approved for AD only improve patients’ symptoms without modifying the disease progression. Clinical trials to halt or slow the disease have nearly all ended in failure. Thus, we need to re-think the current therapeutic strategies for AD. Recently, researchers have reached a consensus on early diagnosis, early intervention, and multi-targeted therapeutic strategies for AD [5–7]. In this article we summarize the latest progress in clinical AD research to reflect the trend toward successful management of the disease.
New Trends in Biomarker Research
Therapeutic trials for AD have not succeeded so far, partially because of the advanced neurodegenerative stage of individuals typically targeted in clinical trials. Considering that the efficacy of potential AD treatments likely depend on early intervention, there appears to be an urgent need for accurate identification of preclinical individuals with underlying pathology. While cerebrospinal fluid (CSF) biomarkers have high accuracy and sensitivity for AD diagnosis, blood-based biomarkers are more desirable because the collection of blood is less invasive than that of CSF. Despite much effort, no studies to date have validated the clinical utility of any single blood-based biomarker, such as Aβ40, Aβ42, or tau [8, 9].
Previous studies have consistently found that the ratio of Aβ40 to Aβ42 is more valuable than either of the component biomarkers alone [10, 11]. The Alzheimer’s Disease Neuroimaging Initiative cohort studies demonstrated that the total-tau/Aβ42 ratio can be used to predict the progression from mild cognitive impairment (MCI) to AD with high sensitivity and specificity [12], suggesting that assays measuring the ratios of biomarkers may improve the accuracy of AD diagnosis. The inverse association between total plasma Aβ42/40 and the cerebral Aβ burden determined by positron emission tomography (PET) showed that patients with low plasma Aβ42/40 ratios are at risk of greater cognitive decline. In cognitively normal individuals, total plasma Aβ42/40 reached a positive predictive value of 81% for a high cortical Aβ burden, which represented a 110% increase over the population prevalence of cortical Aβ positivity [13], supporting the use of the plasma Aβ42/40 ratio as a surrogate biomarker of cortical Aβ deposition and as an enrichment tool. An average of 14.3% drop in absolute concentrations of Aβ42/40 has been demonstrated in the blood of amyloid-positive individuals, similar to the findings in CSF, suggesting that plasma Aβ kinetics reflect the central nervous system pathology of amyloidosis in a similar fashion to CSF [14]. A receiver operating characteristic (ROC) curve analysis demonstrated an area under the curve (AUC) of 0.8865, showing the inherent validity of using this plasma biomarker as a metric for predicting brain amyloid status.
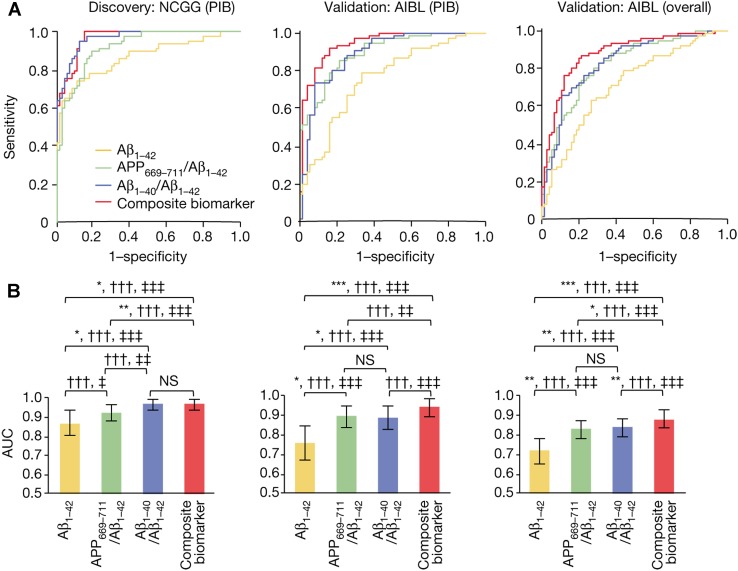
Recently, Nakamura et al. [15] proposed a set of plasma biomarkers, the APP669–711/Aβ42 and Aβ40/42 ratios and their composites, for AD diagnosis with high sensitivity and specificity; these markers are measured by immunoprecipitation coupled with mass spectrometry to predict the individual brain amyloid burden. Notably, the composite biomarker showed an accuracy of ~ 90% in two independent cohort studies using Pittsburgh compound B (PIB)-PET as a standard of truth (Fig. 1). These results suggest that plasma biomarkers can be helpful for the differential diagnosis of AD and the development of an appropriate therapeutic strategy.
Fig. 1.
High performance of different plasma biomarkers. A ROC analyses of different biomarkers to predict the states of Aβ+/Aβ− for the discovery and validation data sets. Unadjusted analyses of the National Center for Geriatrics and Gerontology from the Japanese (NCGG) PIB-PET discovery data (left), the Australian Imaging, Biomarker and Lifestyle Study of Ageing (AIBL) PIB-PET data (middle), and AIBL overall (right) validation data. B Comparisons of the performances of the above biomarkers corresponding to the ROC curves. Statistically significant differences between two AUCs are indicated by asterisks. *,†,‡P < 0.05; **,††,‡‡P < 0.01; ***,†††,‡‡‡P < 0.001.
(Figures adapted from Nakamura et al. [15] with permission)
In the brain, tau accumulates more quickly than amyloid. It takes only 5 years for tau to increase by one standard deviation, while it takes 20 years for amyloid to increase by a similar margin [16]. Buckley et al. [16] also found that tau PET is a more consistent measure than Aβ. Moreover, the rate of increase in tau PET in the temporal (entorhinal) cortex predicts subjective cognitive decline more effectively than amyloid or even baseline tau, showing that the dynamic change of tau levels during a period in PET is a promising marker for monitoring disease progression and drug efficacy in AD trials.
In the future, identification of blood-based biomarkers, as a non-invasive and easily accessible method, will be a major strategy for early diagnosis and monitoring the disease progression. Such an approach tends to directly measure brain-derived biomarkers such as Aβ and tau in blood. One challenge is that the levels of brain-derived Aβ and tau are very low due to dilution and degradation in blood [17–19]. Novel methods using immunomagnetic reduction, mass spectrometry, and immunoprecipitation technologies are expected to detect the indicator molecules in the blood with high sensitivity and specificity [15, 20, 21]. The identification of molecules that can easily efflux from the brain into blood and reflect the disease pathology is another important direction of biomarker search. One promising biomarker is plasma neurofilament light (NFL), which shows high diagnostic accuracy in AD patients, with a sensitivity comparable to the established CSF Aβ42 and phosphorylated-tau in a prospective case–control study [22], and also predicts the progression of AD [23].
Polygenic Hazard Score
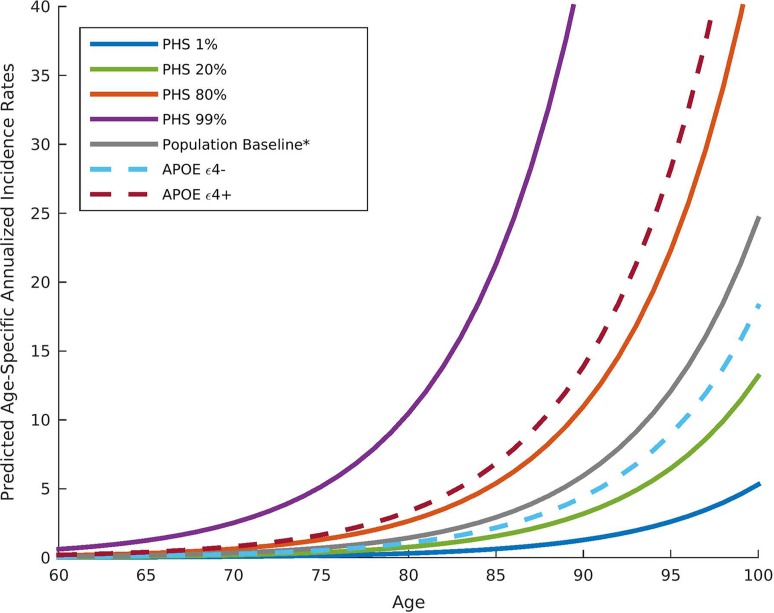
Identifying individuals at risk for AD is of the utmost importance. Although genetic studies have identified AD-associated single nucleotide polymorphisms in APOE (apolipoprotein E) and other genes, genetic information still has not been integrated into a clinical framework for risk prediction. Genotype data from 6409 AD patients and 9386 older controls from the International Genomics of Alzheimer’s Project (IGAP) have been analyzed in order to provide for each participant a polygenic hazard score (PHS), quantify the age at onset of AD dementia from a combination of APOE and 31 other genetic variants, and derive estimates of the instantaneous risk for developing AD (Fig. 2). In another independent cohort, the PHS strongly predicted the empirical age at AD onset (r = 0.90, P = 1.1 × 10−26) and longitudinal progression from normal ageing to AD, and was associated with neuropathology (Braak stage of neurofibrillary tangles) [24]. Importantly, PHS has been associated with in vivo biomarkers of AD pathology, such as reduced CSF Aβ42 and elevated CSF total-tau. In a large prospective cohort study, results have shown that PHS significantly predicts the time to AD dementia and steeper longitudinal cognitive decline, even after APOE status is controlled for. In cognitively normal individuals and those with MCI, a positive systematic relationship between PHS and cognitive decline has also been confirmed [25]. Overall, PHS may be useful in MCI studies and preclinical AD therapeutic trials to enrich the participant pool for biomarker-positive individuals at the highest risk for short-term clinical progression.
Fig. 2.
Annualized incidence rates showing the instantaneous hazard as a function of polygenic hazard score percentile and age. PHS, polygenic hazard score.
(Image adapted from Desikan et al. [24] with permission)
Positron Emission Tomographic Imaging
Abnormal accumulation of Aβ protein and hyperphosphorylation of tau protein in the brain are pathological hallmarks of AD. As the pathological processes start decades before the onset of clinical symptoms, it is crucial to study the cortical distribution of early pathological alterations in order to understand the underpinnings of AD. Recently, brain amyloid load has become a critical index of AD, but this biomarker has not yet demonstrated value in AD treatment. Owing to the development of molecular imaging, especially for PET-Aβ and tau, brain amyloid imaging has had a major impact on clinical diagnosis and treatment. Imaging Dementia-Evidence for Amyloid Scanning (IDEAS), an ongoing study assessing the impact of amyloid PET on the management of patients with dementia or MCI of uncertain cause, has shown that physicians change medications or recommendations for 67.8% of MCI patients and 65.9% of people with dementia in response to PET data on the extent of amyloid deposition (https://www.ideas-study.org/2018/01/09/ideas-study-reaches-recruitment-goal-demonstrates-value-of-pet-scans/). Importantly, diagnoses shift dramatically in accordance with the scans, particularly for people who had been wrongly diagnosed with AD. For clinical trials, molecular imaging will be a necessary means of screening cases and improving the reliability of the trials, thus reducing the number of participants needed and, consequently, recruitment costs in clinical trials, suggesting that PET may have a substantial impact on patient management and clinical trials.
Although AD-related pathological changes seem to spread along interconnected neuronal systems, there are limited neuroimaging approaches able to characterize the putative spreading pathways. A cross-sectional study of 88 elderly, cognitively normal individuals showed that tau and Aβ deposits in the elderly brain display well-defined hierarchical cortical relationships [26]. Moreover, the characterization of hierarchically organized maps of tau and Aβ may have crucial value in upcoming efforts to improve early individual staging and monitor the cognitive functional effect of tau accumulation on memory decline in the preclinical and clinical stages of AD.
AD Drug Clinical Trials
Although many factors contribute to AD pathogenesis, Aβ dyshomeostasis has emerged as the most compelling therapeutic target, as an imbalance between the production and clearance of Aβ is often a very early and initiating factor among the pathophysiological features of AD [27]. Thus, reducing the production and/or increasing the clearance of Aβ are pivotal therapeutic strategies for AD treatment [27, 28]. β-Site APP cleaving enzyme 1 (BACE1) plays a central role in the production of toxic Aβ peptide and is thus a noteworthy therapeutic target for AD. In 2016, Merck reported that verubecestat (MK-8931), a BACE1 inhibitor, causes a dose-dependent reduction of CSF Aβ40 in mild-to-moderate AD patients [29]. Supporters of the Aβ hypothesis had high hopes for the drug because of its marked efficacy. Unfortunately, in February 2017, Merck announced that the phase 3 study of verubecestat would end due to lack of efficacy. Despite the frustrations of BACE inhibitor research in the past, new ongoing clinical trials such as AMARANTH (NCT02783573), MissionAD2 (NCT03036280 and NCT02956486), and Generation S1 have shown encouraging data. Inhibition of Aβ production is regarded as a major strategy for the primary prevention of AD.
Antagonists of the 5-HT6 receptor have been proposed as options for the development of cognitive enhancers owing to the unique localization and pharmacology of that receptor. Phase 2 trials have shown that intepirdine, a 5-HT6 antagonist, improves cognition and function in patients with mild-to-moderate AD when given in conjunction with donepezil [30]. In 2017, however, Axovant announced that the phase 3 trials of intepirdine (NCT02585934) did not meet their primary efficacy endpoint. Recently, Pfizer announced that it is pulling out of research into drugs to treat AD. This decision predicts a “cold winter” for the whole field of AD research. Fortunately, Lilly, Merck, Biogen, and AstraZeneca have announced the continuation of pivotal clinical trials for AD.
Immunotherapy is a vital research direction that aims to clear out existing Aβ in AD patients; however, the results of clinical trials are discouraging. EXPEDITION 1 and EXPEDITION 2 were two phase 3 clinical trials of solanezumab, a monoclonal antibody that recognizes soluble monomeric Aβ; in those trials, however, the treatment failed to improve the cognition of patients with mild-to-moderate AD. Lilly decided to move forward with another phase 3 study, EXPEDITION 3, to investigate the effects on prodromal AD patients, as in pre-specified pooled secondary analyses, patients with mild AD had less cognitive decline by ~ 34% and less functional decline by ~ 18% on solanezumab than on the control treatment [31]. However, the trial ended in failure [32]. Although solanezumab has been proposed to reduce peripheral free plasma Aβ concentrations by > 90%, the total Aβ40 and Aβ42 levels remained significantly elevated and were maintained at high concentrations in both plasma and CSF, and there was no reduction of brain Aβ accumulation in patients treated with the antibody compared with those treated with placebo, indicating that solanezumab is not a suitable antibody for the removal of brain Aβ. This study also suggests that it is too late to rescue cognition at the stage of mild AD, by which time brain Aβ accumulation has already reached a plateau.
Aducanumab, a monoclonal antibody that selectively targets aggregated Aβ, has been investigated in patients with prodromal or mild AD. A 54-week course of monthly intravenous infusions of aducanumab reduced brain Aβ in a dose- and time-dependent manner. At the highest dose, 10 mg/kg, brain amyloid deposition in AD patients dropped below the threshold of positivity and remained as low as this at 166 weeks [33]. As aducanumab actually clears Aβ deposition in the brain, the results of the phase 3 trial are eagerly anticipated.
Although the above results are not sufficient to negate the “Aβ toxicity” hypothesis, the fact that anti-Aβ treatment does not slow the cognitive decline in patients with AD reminds us to rethink the current therapeutic strategies. Thorough removal of brain Aβ is expected to have a beneficial effect and improve cognition. In this regard, increasing the dosage of antibodies has become a realistic choice for current trials to meet their end points. In the ongoing A4 trial (anti-amyloid treatment in asymptomatic Alzheimer’s disease trial, NCT02008357), Lilly raised the dosage of solanezumab from 400 mg/day to 1600 mg/day for individuals not yet showing symptoms of AD-related cognitive impairment or dementia. This study will also be extended by 72 weeks to 4.5 years in hopes that these changes will reveal what is expected to be a small treatment benefit on the basis of previous trials. Similarly, Genentech quadrupled the dose of crenezumab to 60 mg/kg in the phase 3 CREAD trial (NCT02670083), informed by the predictions of a new drug-disease progression model [34]. Roche reported that high doses of gantenerumab reduced Aβ in the brains of people with AD [35], and at the Clinical Trials on Amyloid Disease meeting in 2017, they reported that their ongoing phase 3 trials indicate that monthly subcutaneous injections of 900 mg and 1200 mg gantenerumab reduce brain amyloid by up to 15% over 6 months–9 months. Overall, the major antibodies in these ongoing clinical trials are being pushed in the same direction of high doses for long periods of time.
Recently, immunotherapies in ongoing clinical trials have predominantly targeted the N-terminal domain of the Aβ peptide; however, there is a concern that antibodies against this domain can induce amyloid-related imaging abnormalities (including brain edema and cerebral micro-hemorrhage), increase neuronal hyperactivity, and promote Aβ production [36, 37]. Therefore, the optimal antibody for immunotherapy should be based on Aβ clearance and characterized by high safety. The appropriate time for AD patients to receive anti-Aβ therapeutics remains to be explored. A theoretical model of AD biomarkers has demonstrated that Aβ levels in CSF change in a sigmoidal pattern with time [38]. Therefore, treatments that target Aβ should be administered before the plateau stage or even before the period when Aβ rises and causes neuronal loss.
Neurofibrillary tangles composed of aggregated tau protein are another hallmark of AD. Given the lack of efficacy of Aβ-targeted therapies to date, targeting tau aggregation is, therefore, thought to be a rational therapeutic approach for AD. Unfortunately, tau-targeting therapies have also failed so far. The use of leuco-methylthioninium bis (hydromethanesulfonate) (LMTM), a second-generation tau protein aggregation inhibitor, is one such effort. In a phase 2 study, a significant benefit has been seen in both mild and moderate AD participants after 50 weeks of treatment with LMTM [39]. Unfortunately, LMTM failed to slow cognitive or functional decline in participants with mild-to-moderate AD in a phase 3 clinical trial [40]. Nevertheless, further trials are needed as LMTM seems to be effective as a monotherapy for AD [41]. Compared with Aβ, tau is a much larger antigen and thus has more immunogenic epitopes. Choosing the right epitope for immunization is critical for tau immunization. AADvac1, an active vaccine against pathological tau proteins, was used in the first-in-man vaccine study, which showed favorable safety and satisfied immunogenicity in patients with AD in a phase 1 clinical trial [42], and the phase 2 clinical trial (NCT02579252) is ongoing. Similarly, for passive immunotherapy, ABBV-8E12, a vaccine targeting extracellular aggregated tau, has also started a phase 2 clinical trial (NCT02880956) based on its acceptable safety and the tolerability profile of single doses [43]. Targeting tau is clearly more challenging than Aβ, as this protein mainly accumulates intracellularly, while Aβ aggregates occur in the extracellular space where it is accessible to therapeutic antibodies [4]. Currently, most of the anti-tau protein immunotherapies are still in preclinical trials (ACI-35, RG6100, BMS-986168, TPI-287, ANAVEX 2-73 and AZD0530), and their efficacy remains to be verified.
Early Intervention
Pathological evidence shows that Aβ is deposited in the brain 2–3 decades before the first symptoms of AD become manifest [44]. It is essential to target the early period of decline in therapeutic trials and to combine them with the lessons from the previous failure of immunotherapy trials. Recently, researchers reached an agreement on early intervention at the 10th Clinical Trials of AD, and the focus of clinical trials shifted from secondary to primary prevention.
The A4 study is designed to test whether treatment with an anti-amyloid antibody can slow the memory loss caused by AD. Older participants aged 65 years–85 years who had normal memory and cognitive function at the moment but might be at risk for memory loss were enrolled. One of the major interpretations for the failure of previous immunotherapy for AD is that the interventions may have begun too late for anti-Aβ antibodies to be beneficial. The A5 trial (NCT02569398), EARLY, is evaluating the BACE-1 inhibitor JNJ-54861911 in a slightly younger cognitively normal population, starting at age 60 years (instead of 65 in A4), who are asymptomatic but with amyloid accumulation confirmed by either CSF or PET. However, even a 60-year-old patient with established amyloid deposition is in a relatively late stage in the long pathogenic process of the disease.
Thus, the next trial, A3, or Ante-Amyloid Prevention of AD, will recruit participants aged 50 years–75 years who are at risk of accumulating amyloid and developing the subsequent biomarker changes. Unlike the A4 and A5 trials, the A3 trial will use biomarkers but not cognition as primary outcomes. Therefore, studies are pushing drug evaluation into ever-earlier disease stages.
Primary Prevention
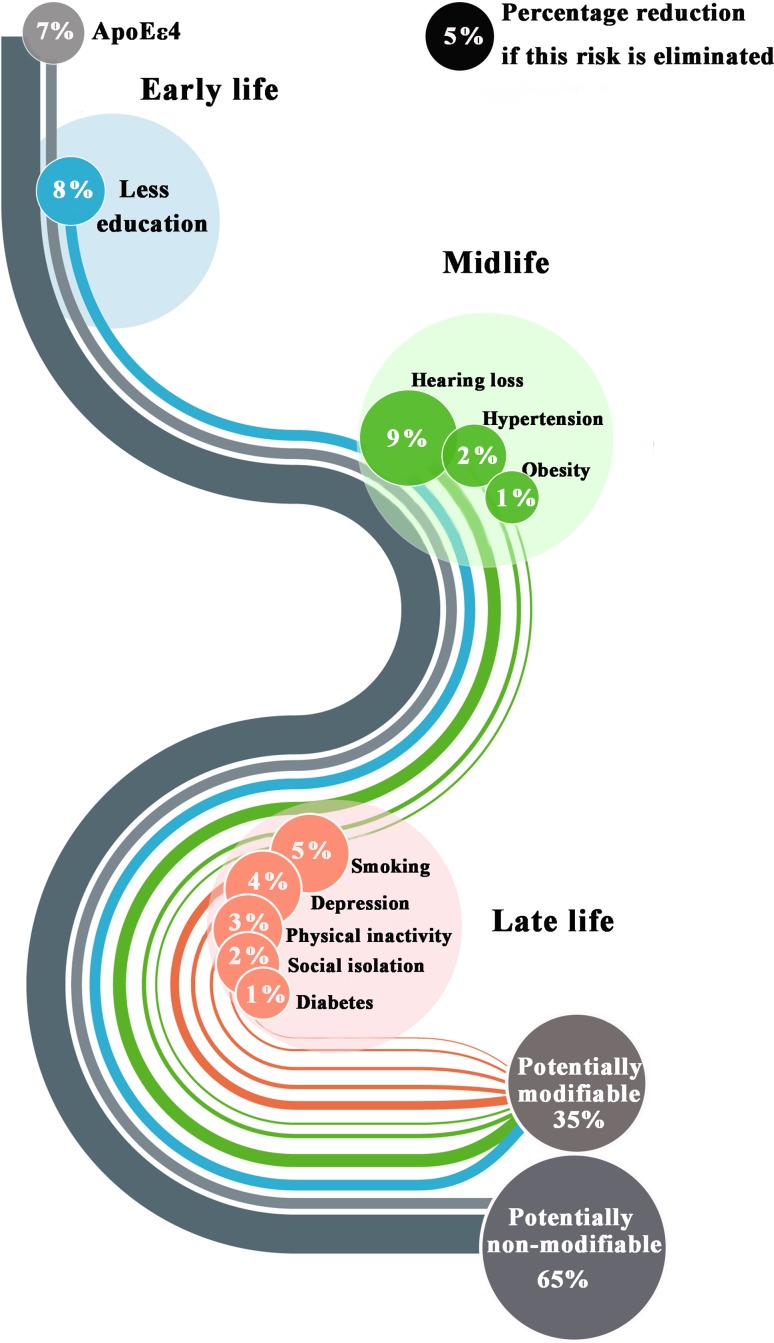
At the 2017 Alzheimer’s Association International Conference, scientists described new studies to test whether multi-domain lifestyle interventions can slow cognitive decline. The Lancet Commission claimed that one-third of dementia cases are preventable, being attributable to a combination of the following 9 risk factors: education to a maximum age of 11 years–12 years, midlife hypertension, midlife obesity, hearing loss, late-life depression, diabetes, physical inactivity, smoking, and social isolation [2]. Remarkably, it is estimated that complete elimination of the ApoE ε4 allele, the major genetic risk factor for AD, reduces AD incidence by 7% (Fig. 3) [2]. In line with the trend of early intervention, a two-year study called US POINTER will test the combined effects of physical and mental exercises, a healthful diet, and careful management of heart health to reduce AD risk. Healthy people at risk for dementia and people in the early stages of MCI will be recruited in this planned study. The trial is part of a larger, international effort that comprises studies in the UK, Singapore, and China. A similar trial will soon enroll participants in Australia as well. These trials all focus on clinically asymptomatic people who perform at or slightly below average on neuropsychological tests.
Fig. 3.
Life-course model of contributions of modifiable risk factors to dementia. Percentages are presented as potentially modifiable or non-modifiable risk factors.
(Image adapted from Livingston et al. [2] with permission)
Systemic Approaches to AD Intervention
Previously, it was found that exposure of aged mice to young blood late in life is capable of rejuvenating synaptic plasticity and improving cognitive function [45]. In 2016, it was found that some factors in young blood have the potential to ameliorate disease in an AD mouse model through intravenous injections of young plasma into adult mice [46], suggesting that anti-aging is feasible from a systemic approach. Similarly, it was found that the Aβ40 and Aβ42 levels were reduced by ~ 68% in the brains of APPswe/PS1dE9 transgenic mice when they were joined in parabiosis with wild-type mice. The levels of phosphorylated tau were also significantly reduced in the brain [47], suggesting that peripheral clearance is a potent approach for both brain Aβ and phosphorylated tau clearance. Recently, it was confirmed that human cord plasma treatment revitalizes the hippocampus and improves cognitive function in aged mice [48]. On the basis of the above research, a small trial was conducted to test whether plasma from the young helps patients with AD. The treated participants performed no better overall on objective cognitive tests, but their scores improved slightly on a scale reflecting whether they needed help with daily activities, as well as on a survey that asked caregivers how well the patients performed simple tasks (http://www.sciencemag.org/news/2017/11/blood-young-people-does-little-reverse-alzheimer-s-first-test. 10.1126/science.aar3723). Now, the researchers behind that study plan to launch another trial using only the plasma fraction that contains growth factors, excluding coagulation factors and other components that may cause adverse reactions; such refined plasma is more effective than whole plasma at improving cognition in a mouse model of AD, suggesting that developing a systemic strategy may provide a novel perspective for AD treatment.
In fact, the central and peripheral pathways of Aβ and tau metabolism are connected and work synergistically to clear Aβ and tau from the brain [47, 49–51], suggesting that treatment strategies for AD should have a corresponding focus not only on pathological changes in the brain but also on peripheral abnormalities of metabolism. Given that the emerging evidence shows that peripheral tissues and organs play important roles in regulating Aβ and tau metabolism in the brain, a systemic view of AD has been proposed to understand the disease pathogenesis and develop therapies from a systemic approach [28]. This systemic view also provides a novel perspective for understanding AD pathogenesis and optimizing therapeutic schedules [28].
Conclusions
Taking into account the challenges faced and the achievements up to the present, early intervention and primary prevention will be crucial turning points for AD research. Plasma biomarkers will no doubt provide a substantial foundation for this intervention and start a new upsurge of biomarker research. Moreover, antibodies should be developed based on the molecular structures of Aβ and tau, which are thought to be the main causes of neuronal death in AD. Considering that AD might not only be a brain disorder but also a systemic disease, developing a specific treatment strategy from a systemic viewpoint may provide a novel approach to AD prevention and treatment.
Acknowledgements
This review was supported by the the Chinese Ministry of Science and Technology (2016YFC1306401).
Compliance with Ethical Standards
Conflict of interest
All authors claim that there are no conflicts of interest.
Contributor Information
Xiu-Qing Yao, Email: dryaoxq@163.com.
Yan-Jiang Wang, Email: yanjiang_wang@tmmu.edu.cn.
References
- 1.Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- 2.Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. Lancet. 2017;390:2673–2734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 3.Wang QH, Wang X, Bu XL, Lian Y, Xiang Y, Luo HB, et al. Comorbidity burden of dementia: a hospital-based retrospective study from 2003 to 2012 in seven cities in China. Neurosci Bull. 2017;33:703–710. doi: 10.1007/s12264-017-0193-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li C, Gotz J. Tau-based therapies in neurodegeneration: opportunities and challenges. Nat Rev Drug Discov. 2017;16:863–883. doi: 10.1038/nrd.2017.155. [DOI] [PubMed] [Google Scholar]
- 5.Chetelat G. Multimodal neuroimaging in Alzheimer’s disease: Early diagnosis, physiopathological mechanisms, and impact of lifestyle. J Alzheimers Dis. 2018 doi: 10.3233/JAD-179920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Misra MK, Damotte V, Hollenbach JA. The immunogenetics of neurological disease. Immunology. 2018;153:399–414. doi: 10.1111/imm.12869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frisoni GB, Boccardi M, Barkhof F, Blennow K, Cappa S, Chiotis K, et al. Strategic roadmap for an early diagnosis of Alzheimer’s disease based on biomarkers. Lancet Neurol. 2017;16:661–676. doi: 10.1016/S1474-4422(17)30159-X. [DOI] [PubMed] [Google Scholar]
- 8.O’Bryant SE, Mielke MM, Rissman RA, Lista S, Vanderstichele H, Zetterberg H, et al. Blood-based biomarkers in Alzheimer disease: current state of the science and a novel collaborative paradigm for advancing from discovery to clinic. Alzheimers Dement. 2017;13:45–58. doi: 10.1016/j.jalz.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snyder HM, Carrillo MC, Grodstein F, Henriksen K, Jeromin A, Lovestone S, et al. Developing novel blood-based biomarkers for Alzheimer’s disease. Alzheimers Dement. 2014;10:109–114. doi: 10.1016/j.jalz.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim HJ, Park KW, Kim TE, Im JY, Shin HS, Kim S, et al. Elevation of the plasma Abeta40/Abeta42 ratio as a diagnostic marker of sporadic early-onset Alzheimer’s disease. J Alzheimers Dis. 2015;48:1043–1050. doi: 10.3233/JAD-143018. [DOI] [PubMed] [Google Scholar]
- 11.Koyama A, Okereke OI, Yang T, Blacker D, Selkoe DJ, Grodstein F. Plasma amyloid-beta as a predictor of dementia and cognitive decline: a systematic review and meta-analysis. Arch Neurol. 2012;69:824–831. doi: 10.1001/archneurol.2011.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74:201–209. doi: 10.1212/WNL.0b013e3181cb3e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fandos N, Perez-Grijalba V, Pesini P, Olmos S, Bossa M, Villemagne VL, et al. Plasma amyloid beta 42/40 ratios as biomarkers for amyloid beta cerebral deposition in cognitively normal individuals. Alzheimers Dement (Amst) 2017;8:179–187. doi: 10.1016/j.dadm.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ovod V, Ramsey KN, Mawuenyega KG, Bollinger JG, Hicks T, Schneider T, et al. Amyloid beta concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimers Dement. 2017;13:841–849. doi: 10.1016/j.jalz.2017.06.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamura A, Kaneko N, Villemagne VL, Kato T, Doecke J, Doré V, et al. High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature. 2018;554:249–254. doi: 10.1038/nature25456. [DOI] [PubMed] [Google Scholar]
- 16.Buckley RF, Hanseeuw B, Schultz AP, Vannini P, Aghjayan SL, Properzi MJ, et al. Region-specific association of subjective cognitive decline with tauopathy independent of global beta-amyloid burden. JAMA Neurol. 2017;74:1455–1463. doi: 10.1001/jamaneurol.2017.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varma VR, Oommen AM, Varma S, Casanova R, An Y, Andrews RM, et al. Brain and blood metabolite signatures of pathology and progression in Alzheimer disease: a targeted metabolomics study. PLoS Med. 2018;15:e1002482. doi: 10.1371/journal.pmed.1002482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sagare A, Deane R, Bell RD, Johnson B, Hamm K, Pendu R, et al. Clearance of amyloid-beta by circulating lipoprotein receptors. Nat Med. 2007;13:1029–1031. doi: 10.1038/nm1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mortberg E, Zetterberg H, Nordmark J, Blennow K, Catry C, Decraemer H, et al. Plasma tau protein in comatose patients after cardiac arrest treated with therapeutic hypothermia. Acta Anaesthesiol Scand. 2011;55:1132–1138. doi: 10.1111/j.1399-6576.2011.02505.x. [DOI] [PubMed] [Google Scholar]
- 20.Yang SY, Chiu MJ, Chen TF, Horng HE. Detection of plasma biomarkers using immunomagnetic reduction: a promising method for the early diagnosis of Alzheimer’s disease. Neurol Ther. 2017;6:37–56. doi: 10.1007/s40120-017-0075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lue LF, Sabbagh MN, Chiu MJ, Jing N, Snyder NL, Schmitz C, et al. Plasma levels of Abeta42 and Tau identified probable Alzheimer’s dementia: findings in two cohorts. Front Aging Neurosci. 2017;9:226. doi: 10.3389/fnagi.2017.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mattsson Niklas, Andreasson Ulf, Zetterberg Henrik, Blennow Kaj. Association of Plasma Neurofilament Light With Neurodegeneration in Patients With Alzheimer Disease. JAMA Neurology. 2017;74(5):557. doi: 10.1001/jamaneurol.2016.6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zetterberg H, Skillback T, Mattsson N, Trojanowski JQ, Portelius E, Shaw LM, et al. Association of cerebrospinal fluid neurofilament light concentration With Alzheimer disease progression. JAMA Neurol. 2016;73:60–67. doi: 10.1001/jamaneurol.2015.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desikan RS, Fan CC, Wang Y, Schork AJ, Cabral HJ, Cupples LA, et al. Genetic assessment of age-associated Alzheimer disease risk: development and validation of a polygenic hazard score. PLoS Med. 2017;14:e1002258. doi: 10.1371/journal.pmed.1002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan CH, Fan CC, Mormino EC, Sugrue LP, Broce IJ, Hess CP, et al. Polygenic hazard score: an enrichment marker for Alzheimer’s associated amyloid and tau deposition. Acta Neuropathol. 2018;135:85–93. doi: 10.1007/s00401-017-1789-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sepulcre J, Grothe MJ, Sabuncu M, Chhatwal J, Schultz AP, Hanseeuw B, et al. Hierarchical organization of Tau and amyloid deposits in the cerebral cortex. JAMA Neurol. 2017;74:813–820. doi: 10.1001/jamaneurol.2017.0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J, Gu BJ, Masters CL, Wang YJ. A systemic view of Alzheimer disease - insights from amyloid-beta metabolism beyond the brain. Nat Rev Neurol. 2017;13:612–623. doi: 10.1038/nrneurol.2017.111. [DOI] [PubMed] [Google Scholar]
- 29.Kennedy M. E., Stamford A. W., Chen X., Cox K., Cumming J. N., Dockendorf M. F., Egan M., Ereshefsky L., Hodgson R. A., Hyde L. A., Jhee S., Kleijn H. J., Kuvelkar R., Li W., Mattson B. A., Mei H., Palcza J., Scott J. D., Tanen M., Troyer M. D., Tseng J. L., Stone J. A., Parker E. M., Forman M. S. The BACE1 inhibitor verubecestat (MK-8931) reduces CNS -amyloid in animal models and in Alzheimers disease patients. Science Translational Medicine. 2016;8(363):363ra150–363ra150. doi: 10.1126/scitranslmed.aad9704. [DOI] [PubMed] [Google Scholar]
- 30.Maher-Edwards G, Dixon R, Hunter J, Gold M, Hopton G, Jacobs G, et al. SB-742457 and donepezil in Alzheimer disease: a randomized, placebo-controlled study. Int J Geriatr Psychiatry. 2011;26:536–544. doi: 10.1002/gps.2562. [DOI] [PubMed] [Google Scholar]
- 31.Siemers ER, Sundell KL, Carlson C, Case M, Sethuraman G, Liu-Seifert H, et al. Phase 3 solanezumab trials: secondary outcomes in mild Alzheimer’s disease patients. Alzheimers Dement. 2016;12:110–120. doi: 10.1016/j.jalz.2015.06.1893. [DOI] [PubMed] [Google Scholar]
- 32.Honig LS, Vellas B, Woodward M, Boada M, Bullock R, Borrie M, et al. Trial of Solanezumab for mild dementia due to Alzheimer’s disease. N Engl J Med. 2018;378:321–330. doi: 10.1056/NEJMoa1705971. [DOI] [PubMed] [Google Scholar]
- 33.Sevigny J, Chiao P, Bussiere T, Weinreb PH, Williams L, Maier M, et al. The antibody aducanumab reduces Abeta plaques in Alzheimer’s disease. Nature. 2016;537:50–56. doi: 10.1038/nature19323. [DOI] [PubMed] [Google Scholar]
- 34.Adolfsson O, Pihlgren M, Toni N, Varisco Y, Buccarello AL, Antoniello K, et al. An effector-reduced anti-beta-amyloid (Abeta) antibody with unique abeta binding properties promotes neuroprotection and glial engulfment of Abeta. J Neurosci. 2012;32:9677–9689. doi: 10.1523/JNEUROSCI.4742-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ostrowitzki S, Deptula D, Thurfjell L, Barkhof F, Bohrmann B, Brooks DJ, et al. Mechanism of amyloid removal in patients with Alzheimer disease treated with gantenerumab. Arch Neurol. 2012;69:198–207. doi: 10.1001/archneurol.2011.1538. [DOI] [PubMed] [Google Scholar]
- 36.Liu YH, Giunta B, Zhou HD, Tan J, Wang YJ. Immunotherapy for Alzheimer disease: the challenge of adverse effects. Nat Rev Neurol. 2012;8:465–469. doi: 10.1038/nrneurol.2012.118. [DOI] [PubMed] [Google Scholar]
- 37.Busche MA, Grienberger C, Keskin AD, Song B, Neumann U, Staufenbiel M, et al. Decreased amyloid-beta and increased neuronal hyperactivity by immunotherapy in Alzheimer’s models. Nat Neurosci. 2015;18:1725–1727. doi: 10.1038/nn.4163. [DOI] [PubMed] [Google Scholar]
- 38.Jack CR, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wischik CM, Staff RT, Wischik DJ, Bentham P, Murray AD, Storey JM, et al. Tau aggregation inhibitor therapy: an exploratory phase 2 study in mild or moderate Alzheimer’s disease. J Alzheimers Dis. 2015;44:705–720. doi: 10.3233/JAD-142874. [DOI] [PubMed] [Google Scholar]
- 40.Mullard A. Pharma pumps up anti-tau Alzheimer pipeline despite first Phase III failure. Nat Rev Drug Discov. 2016;15:591–592. doi: 10.1038/nrd.2016.176. [DOI] [PubMed] [Google Scholar]
- 41.Wilcock GK, Gauthier S, Frisoni GB, Jia J, Hardlund JH, Moebius HJ, et al. Potential of low dose leuco-methylthioninium bis(hydromethanesulphonate) (LMTM) monotherapy for treatment of mild Alzheimer’s disease: cohort analysis as modified primary outcome in a phase III clinical trial. J Alzheimers Dis. 2018;61:435–457. doi: 10.3233/JAD-170560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Novak P, Schmidt R, Kontsekova E, Zilka N, Kovacech B, Skrabana R, et al. Safety and immunogenicity of the tau vaccine AADvac1 in patients with Alzheimer’s disease: a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Neurol. 2017;16:123–134. doi: 10.1016/S1474-4422(16)30331-3. [DOI] [PubMed] [Google Scholar]
- 43.West T, Hu Y, Verghese PB, Bateman RJ, Braunstein JB, Fogelman I, et al. Preclinical and clinical development of ABBV-8E12, a humanized anti-Tau antibody, for treatment of Alzheimer’s disease and other tauopathies. J Prev Alzheimers Dis. 2017;4:236–241. doi: 10.14283/jpad.2017.36. [DOI] [PubMed] [Google Scholar]
- 44.Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Villeda SA, Plambeck KE, Middeldorp J, Castellano JM, Mosher KI, Luo J, et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med. 2014;20:659–663. doi: 10.1038/nm.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Middeldorp J, Lehallier B, Villeda SA, Miedema SS, Evans E, Czirr E, et al. Preclinical assessment of young blood plasma for Alzheimer disease. JAMA Neurol. 2016;73:1325–1333. doi: 10.1001/jamaneurol.2016.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiang Y, Bu XL, Liu YH, Zhu C, Shen LL, Jiao SS, et al. Physiological amyloid-beta clearance in the periphery and its therapeutic potential for Alzheimer’s disease. Acta Neuropathol. 2015;130:487–499. doi: 10.1007/s00401-015-1477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castellano JM, Mosher KI, Abbey RJ, McBride AA, James ML, Berdnik D, et al. Human umbilical cord plasma proteins revitalize hippocampal function in aged mice. Nature. 2017;544:488–492. doi: 10.1038/nature22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bu XL, Xiang Y, Jin WS, Wang J, Shen LL, Huang ZL, et al. Blood-derived amyloid-beta protein induces Alzheimer’s disease pathologies. Mol Psychiatry. 2017 doi: 10.1038/mp.2017.204. [DOI] [PubMed] [Google Scholar]
- 50.Jin WS, Shen LL, Bu XL, Zhang WW, Chen SH, Huang ZL, et al. Peritoneal dialysis reduces amyloid-beta plasma levels in humans and attenuates Alzheimer-associated phenotypes in an APP/PS1 mouse model. Acta Neuropathol. 2017;134:207–220. doi: 10.1007/s00401-017-1721-y. [DOI] [PubMed] [Google Scholar]
- 51.Pan X, Chen Z, Fei G, Pan S, Bao W, Ren S, et al. Long-term cognitive improvement after benfotiamine administration in patients with Alzheimer’s disease. Neurosci Bull. 2016;32:591–596. doi: 10.1007/s12264-016-0067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]