Abstract
Objective
To outline the association between the National Institutes of Health Stroke Scale (NIHSS) in the acute stage and the Functional Independence Measure (FIM) of motor items several months later.
Methods
Seventy-nine infarct cases with middle-cerebral-artery region transferred to long-term rehabilitation facilities were analyzed. Patients were allocated to either the model-development group or the confirmatory group at a 2:1 ratio. Independent variables were based on the NIHSS during the acute care and on demographic factors such as age and modified Rankin Scale (mRS) before onset. Multivariate logistic analyses were performed to predict the independence of each FIM motor item. These models were evaluated in the confirmatory group.
Results
Multivariate logistic analyses in the model-development group (n=53) indicated that at least one NIHSS item was statistically significantly associated with the functional independence of a single FIM motor item. Of the NIHSS items, the affected lower extremity item was the most widely associated with 11 of the FIM motor items, except for eating and shower transfer. The affected upper extremity function was the second widely involved factor associated with 7 of the FIM motor items including eating, grooming, bathing, toileting, bed transfer, toilet transfer, and shower transfer. Age and mRS were also statistically significant contributing factors. The obtained predictive models were assessed in the confirmatory group (n=26); these were successful except for the stairs climb item.
Conclusion
In combination with age and pre-stroke status, the NIHSS items (especially the affected extremity items) may be useful for the prediction of long-term outcome in terms of activities in daily living.
Keywords: Rehabilitation, NIHSS, FIM, Prediction, Middle cerebral artery infarction
INTRODUCTION
As the elderly population is increasing worldwide [1], the number of stroke patients is also rapidly rising; the number of first time stroke patients worldwide was 16.9 million in 2010 [2]. These patients often suffer from neural sequelae such as hemiparesis, which results in disabilities whereby the patient requires assistance for their activities in daily living (ADL) [3]. This aspect imposes a heavy burden on both medical and social welfare costs. The goals of rehabilitative treatment for stroke patients are to alleviate patients’ disabilities and to thus reduce social costs. To design an effective rehabilitative treatment, outcome prediction is critically important.
Several test scales have been used in daily clinical practices for stroke patients. In the acute stage, the National Institute of Health Stroke Scale (NIHSS) is the most commonly used because of its practical applicability at the bedside [4]. However, this impairment assessment may undermine its applicability for the long-term outcome prediction when disability in ADL is often considered to be the most serious clinical concern.
The Functional Independence Measure (FIM) [5] is also a frequently used assessment tool for stroke patients. FIM calculates 13 motor and 5 cognitive items in reference to ADL, and directly reflects the burdens of care. Nevertheless, scoring FIM is often difficult within a few days after the onset at the bedside, where activities of FIM items are usually not allowed (e.g., bathing and/or stairs climb). In contrast to NIHSS, which is often assessed during the acute stage, FIM is often used in long-term rehabilitation settings, several months after onset.
As mentioned above, a long-term outcome prediction is needed in terms of ADL from the acute stage, when only impairment level assessment is currently possible. However, only a few studies have been carried out that outline the association between NIHSS in the acute stage and FIM motor items several months later. In this study, we assessed the relationships between NIHSS and FIM motor assessment items with adjustments made to demographic and clinical data such as age and lesion size in order to address this gap. To minimize variability of stroke symptoms, we focused on cerebral infarct in the middle cerebral artery (MCA) areas, the most common type of stroke [6].
MATERIALS AND METHODS
This is a retrospective cohort study. The Ethics and Hospital Committees of Goshi Hospital approved this study.
Patients
We sampled patients with MCA infarct who were admitted to Goshi Hospital from October 2011 to April 2014. The diagnosis of infarct was made using a diffusionweighted magnetic resonance imaging (MRI) scanner (Intera 1.5T Pulsar; Philips, Eindhoven, The Netherlands) at the Department of Emergency of Goshi Hospital. According to the Japanese medical insurance system, a stroke patient typically receives inpatient medical treatments of a few weeks in an acute medical service hospital and is then transferred to a long-term rehabilitative facility. Therefore, to focus on the long-term outcomes that were accurately assessed by FIM, we sampled the patients who were transferred to 13 long-term rehabilitation facilities allied with Goshi Hospital via the local healthcare network system. Their maximal therapy sessions were 3 hours per day, 7 days a week.
Exclusion criteria for patients were worsening clinical courses by primary disease or severe comorbidity (e.g., neurological deterioration due to branch atheromatous disease, fracture, etc.) in acute care and/or long-term rehabilitative facilities, as well as refusal of rehabilitative treatment and/or incomplete dataset for NIHSS or FIM.
From the medical records during hospitalization, we collected the patients’ data including age, gender, mRS [7] before the onset, lesion laterality, diffusion-weighted MRI imaging-Alberta Stroke Program Early CT Score +deep white matter lesions (DWI-ASPECTS+W) [8], infarction type, use of recombinant tissue plasminogen activator (rt-PA), removal of thrombosis of internal carotid artery, carotid artery stenting, botulinum toxin injection, length of stay of acute care hospital and rehabilitation facilities, and day of initiation of rehabilitative treatment.
NIHSS and FIM measurements
NIHSS is a scale used to measure the patients’ level of stroke impairment by scoring the following 15 items: consciousness (3 subclasses), gaze, visual-field loss, facial palsy, right and left upper extremity motor strength, right and left lower extremity motor strength, limb ataxia, sensory loss, language, dysarthria, and extinction/attention items. The total score (ranging from 0 to 42) is often used as an index of stroke severity. NIHSS data obtained on the first day of rehabilitative treatment were entered into the analytical database for the current study. In this study, the right or left sides of the NIHSS items were categorized as ‘affected’ or ‘unaffected’ depending on the lesion side.
FIM consists of 13 motor items and 5 cognitive items. In this study, we focused on the FIM motor assessment items of eating, grooming, bathing, dressing upper body, dressing lower body, toileting, bowel management, bladder management, transfers to bed/chair/wheelchair, transfers to toilet, transfers to tub/shower, walking or wheelchair propulsion, and stair climb. Each item was scored on a 7-point scale: (1) total assistance; (2) maximal assistance; (3) moderate assistance; (4) minimal contact assistance; (5) supervision or set-up; (6) modified independence; and (7) complete independence. FIM motor scores recorded at discharge from the long-term rehabilitative facilities were entered into the analytical database for the current study.
Statistics
First, we allocated the participating patients to either the model-development group or the c onfirmatory group in a 2:1 ratio according to a computer-generated schedule. To determine the relationships between NIHSS and FIM motor items, we then employed multivariate logistic modeling analyses for the model-development group. In the analyses, the NIHSS items were set as explanatory variables and the FIM motor items were set as target values. In an attempt to adjust the logistic models between NIHSS and FIM, we added demographic and clinical data such as age, gender, and mRS before the onset. To ensure the dataset was fit for logistic modeling, we organized our raw data as follows.
For explanatory values, ordinal variables such as NIHSS items and mRS were converted into multiple categorical dichotomous variables [9]. The method used to generate multiple categorical dichotomous variables depended on the number of classification levels (2 to 5 levels) for the items. Therefore, we set four levels of classification for the 5-level assessment of upper extremity and lower extremity functions (Fig. 1). The nominal variables of the explanatory variables such as gender and lesion laterality were converted to a dichotomous variable (man=1; woman=0; right=1; left=0).
Fig. 1.
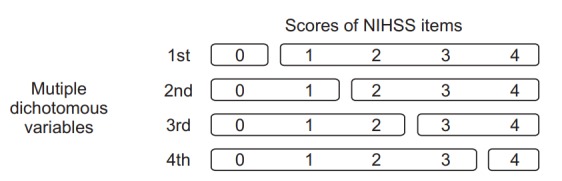
Method of changing ordinary variables of the National Institutes of Health Stroke Scale (NIHSS) item into multiple dichotomous variables.
For target values, we converted the outcome variables of each FIM item to dichotomous variables, such as ‘independence’ or ‘dependence’. Previous FIM studies [5,10] defined FIM values greater than 5 as the patient not requiring any help, implying ‘independence’, while a value equal to or less than 5 implies ‘dependence’. According to the previous literature, the level of independence in bathing, shower transfer, and stairs climb have been shown to be more difficult than other items [10,11]. Taking this into consideration, retaining the supervision level (FIM 5) is a realistic goal for MCA infarct patients for these difficult items. Accordingly, for the two FIM items of ‘dependence’ and ‘independence’, we defined FIM values equal to or greater than 5 (supervision) as ‘independence’ while FIM values less than 5 were defined as ‘dependence’.
Multiple logistic analyses were performed as follows. Variables showing a value of p<0.20 in the forward selection method were included in the multivariate model, provided that only one variable of each NIHSS item was selected. For example, even if the probability of the 2nd affected lower extremity is under 0.20, it was not considered when the 1st affected lower extremity had already been entered into the model. Statistically useless variables such as those that showed a 95% confidence of unit odds ratio interval including 1 were also excluded. A multivariate logistic model with p<0.05 was considered as statistically significant. Additionally, we assessed the regression coefficient of the predictive model obtained from each multivariate logistic regression equation.
Finally, we confirmed the predictive accuracy of the obtained logistic models by applying them to the confirmatory group. We used the proportion by chance accuracy criteria, whereby the accuracy rates were sufficiently high when the models showed 25% higher accuracy than the chance rate [12]. In a dichotomous logistic regression, this rate of chance, which occurs accidentally, can be calculated by squaring and summing the proportion of two real FIM outcome variables such as independence and dependence. For example, in the case of 70% independence and 30% dependence, the chance accuracy rate can be calculated as 0.72+0.32=0.49+0.09=0.58. This shows that this prediction meets accidentally with a probability of 58%. Therefore, we can judge this regression equation as excellent when the accuracy rate is higher than 72.5%, calculated as 0.58×1.25=0.725.
All statistical analyses in the present study were performed using the JMP software version 10.0.2 (SAS Institute Inc., Cary, NC, USA).
RESULTS
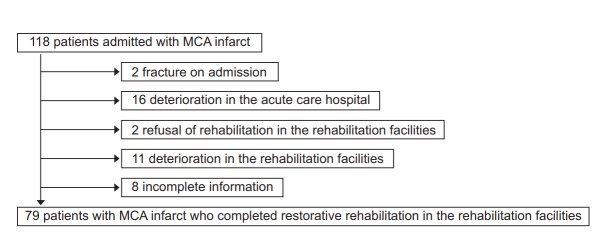
Fig. 2 presents the flow diagram of patients. Of the total 118 patients, 79 MCA region infarcted patients fulfilled the criteria, while 29 were excluded due to comorbidities or complications, 2 were excluded due to rehabilitation refusal, and 8 were excluded due to missing information. Hence, we allocated these 79 patients into two groups at a 2:1 ratio (53 in the model-development group and 26 in the confirmatory group). Table 1 shows the demographic profiles of these two groups. No statistically significant differences were found in age, gender, mRS, lesion laterality, DWI-ASPECTS+W, infarct type, contents of infarct therapy, length of stay of acute and rehabilitation facilities, and hospital day when the rehabilitative treatment began. Fig. 3 presents the characteristics of the 53 patients of the model-development group. The scatter diagrams show the relevance between the total score of NIHSS and the independence/dependence of each motor item of FIM.
Fig. 2.
Flow diagram of patients included and excluded in the study. MCA, middle cerebral artery.
Table 1.
Baseline characteristics of the two groups
| Model-development group (n=53) | Confirmatory group (n=26) | p-value | |
|---|---|---|---|
| Age (yr) | 74 (67–80) | 74 (61.25–78.5) | 0.75a) |
| Sex, male | 33 | 19 | 0.45b) |
| Modified Rankin Scale | 0 (0-1) | 0 (0–0.75) | 0.09a) |
| Laterality, right | 23 | 14 | 0.47b) |
| DWI–ASPECTS+W | 9 (9-10) | 9 (8.25–10) | 0.84a) |
| Infarction type | 0.99c) | ||
| Lacunar | 21 | 10 | |
| Atherome | 22 | 11 | |
| Cardiac emboli | 10 | 5 | |
| Recombinant tissue plasminogen activator therapy | 8 | 3 | 1.0b) |
| Removal of thrombosis of internal carotid artery | 1 | 0 | 1.0b) |
| Carotid artery stenting | 0 | 2 | 0.11b) |
| Botulinum toxin injection | 5 | 1 | 0.66b) |
| Length of stay in hospital (day) | |||
| Acute | 32 (26–38) | 30 (25–34.5) | 0.33a) |
| Rehabilitation | 105 (65–137) | 87.5 (45–141.5) | 0.50a) |
| Initial rehabilitation | 2 (1–2) | 2 (1–2) | 0.99a) |
| NIHSS total score | 9 (5–14) | 6 (4.25–11.25) | 0.06a) |
| FIM motor total score | 80 (51–87) | 78 (60–85.5) | 0.82a) |
| FIM cognition total score | 30 (19–35) | 27.5 (24–35) | 0.57a) |
| FIM total score | 110 (70–119) | 107.5 (84.5–118.75) | 0.80a) |
Values are presented as median (25%–75% interquartile range).
DWI–ASPECTS+W, diffusion-weighted magnetic resonance imaging–Alberta Stroke Program Early CT score+deep white matter lesion; NIHSS, National Institutes of Health Stroke Scale; FIM, Functional Independence Measure.
Wilcoxon rank sum test,
Fisher exact test,
chi-square test.
Fig. 3.
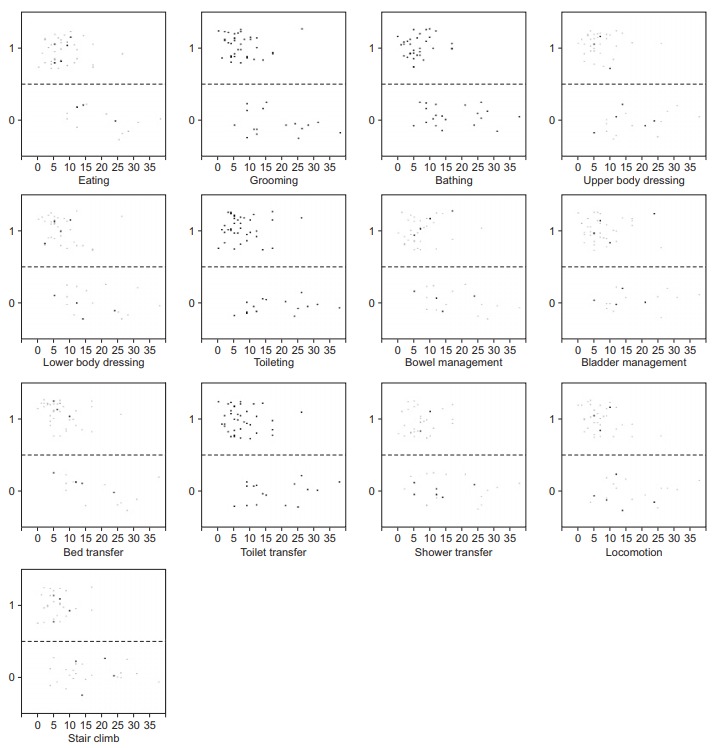
Scatter diagrams showing the total score of the National Institutes of Health Stroke Scale on the x-axis, and independence or dependence of Functional Independence Measure defined in the text on the y-axis in 53 patients of the model-development group (1=independence, 0=dependence).
Tables 2 and 3 indicate the results obtained from the multiple logistic regression analyses performed in the model-development group. A statistically significant correlation was found for at least one single NIHSS item for each FIM motor item. Of the NIHSS items, the most commonly involved factor was the affected lower extremity item. Among the 13 FIM motor items, affected lower extremity item contributed to 11 regression equations, but did not contribute to the eating and shower transfer items, indicating the importance of the lower extremity function of the affected side for the long-term ADL outcome. The second commonly involved NIHSS item was the affected upper extremity item, which accounted for 7 regression equations for the items of eating, grooming, bathing, toileting, bed transfer, toilet transfer, and shower transfer.
Table 2.
Multivariate logistic regression models
| FIM item | Multivariate logistic regression equation | Coefficient of determination | p-value | Predictive model |
|
|---|---|---|---|---|---|
| Regression coefficient | Standard error | ||||
| Eating | 2.14-(2.55×Affected upper extremity 3rd) | 0.23 | 0.0003 | 15.06 | 1734.98 |
| Grooming | 19.63-(0.20×Age)-(3.39×Affected upper extremity 2nd)-(3.91×Affected lower extremity 2nd) | 0.62 | <0.0001 | 1.00 | 0.30 |
| Bathing | 3.81-(2.82×Affected upper extremity 3rd)-(2.97×Affected lower extremity 2nd)-(3.39×Sensory 2nd) | 0.57 | <0.0001 | 0.40 | 0.13 |
| Upper body dressing | 4.85-(3.09×mRS 4th)-(3.79×Affected lower extremity 2nd)-(2.89×Extinction and inattention 2nd) | 0.55 | <0.0001 | 0.57 | 0.15 |
| Lower body dressing | 4.85-(3.09×mRS 4th)-(3.79×Affected lower extremity 2nd)-(2.89×Extinction and inattention 2nd) | 0.55 | <0.0001 | 0.57 | 0.15 |
| Toileting | 19.63-(0.20×Age)-(3.39×Affected upper extremity 3rd)-(3.91×Affected lower extremity 2nd) | 0.62 | <0.0001 | 1.00 | 0.30 |
| Bowel management | 4.85-(3.09×mRS 4th)-(3.79×Affected lower extremity 2nd)-(2.89×Extinction and inattention 2nd) | 0.55 | <0.0001 | 0.96 | 0.26 |
| Bladder management | 87.41-(0.91×Age)-(5.54×mRS 4th)-(9.68×LOC questions 2nd)-(19.15×Affected lower extremity 2nd) | 0.80 | <0.0001 | 1.00 | 0.49 |
| Bed transfer | 19.63-(0.20×Age)-(3.39×Affected upper extremity 3rd)-(3.91×Affected lower extremity 2nd) | 0.62 | <0.0001 | 1.00 | 0.30 |
| Toilet transfer | 19.63-(0.20×Age)-(3.39×Affected upper extremity 3rd)-(3.91×Affected lower extremity 2nd) | 0.62 | <0.0001 | 1.00 | 0.30 |
| Shower transfer | 6.73-(0.08×Age)-(1.84×Affected upper extremity 3rd)-(2.58×Sensory 2nd) | 0.29 | 0.0001 | 1.00 | 0.35 |
| Locomotion | 4.23-(2.60×LOC questions 2nd)-(3.99×Affected lower extremity 2nd)-(2.92×Sensory 2nd) | 0.48 | <0.0001 | 0.75 | 0.22 |
| Stair climb | 2.75-(2.67×mRS 2nd)-(2.51×LOC 1st)-(2.58×Affected lower extremity 2nd) | 0.39 | <0.0001 | 0.62 | 0.20 |
The plus score of multivariate logistic regression analyses means independence or supervision.
Predictive model was assessed using the logit of each multiple logistic regression equation and the real outcome.
FIM, Functional Independence Measure; mRS, modified Rankin Scale; LOC, level of consciousness.
Affected upper extremity, Affected lower extremity, mRS, Extinction and inattention, and Sensory 2nd: when these values are <2, they can be converted to ‘0’; ≥2, converted to ‘1’.
Affected upper extremity 3rd: when this value is <3, it can be converted to ‘0’; ≥3, converted to ‘1’.
mRS 4th: when this value is <4, it can be converted to ‘0’; ≥4, converted to ‘1’.
Table 3.
Unit and range ORs of multiple logistic regression models
| FIM motor item | Affected lower extremity | Affected upper extremity | Age |
mRS | Sensory | Extinction and inattention | LOC question | LOC | |
|---|---|---|---|---|---|---|---|---|---|
| Unit OR | Range OR | ||||||||
| Eating | - | 0.08 (0.02, 0.32) | - | - | - | - | - | - | |
| Grooming | 0.02 (0.0005, 0.22) | 0.03 (0.002, 0.29) | 0.82 (0.66, 0.94) | 4.93e-5 (1.69e-9;0.05) | - | - | - | - | - |
| Bathing | 0.05 (0.002, 0.39) | 0.06 (0.006, 0.38) | - | - | - | 0.03 (0.0008, 0.38) | - | - | - |
| Upper body dressing | 0.02 (0.0008, 0.19) | - | - | - | 0.05 (0.002, 0.43) | - | 0.06 (0.005, 0.40) | - | - |
| Lower body dressing | 0.02 (0.0008, 0.19) | - | - | - | 0.05 (0.002, 0.43) | - | 0.06 (0.005, 0.43) | - | - |
| Toileting | 0.02 (0.0005, 0.22) | 0.03 (0.002, 0.29) | 0.82 (0.66, 0.94) | 4.93e-5 (1.69e-9;0.05) | - | - | - | - | - |
| Bowel management | 0.02 (0.0008, 0.19) | - | - | - | 0.05 (0.002, 0.43) | - | 0.06 (0.005, 0.40) | - | - |
| Bladder management | 4.86e-9 (9.0e-22, 0.002) | - | 0.4 (0.11,0.76) | 4.63e-20 (0, 1.20e-6) | 0.004 (2.91e-6, 0.31) | - | - | 6.27e-5 (6.22e-22, 0.002) | - |
| Bed transfer | 0.02 (0.0005, 0.22) | 0.03 (0.002, 0.29) | 0.82 (0.66, 0.94) | 4.93e-5 (1.69e-9; 0.05) | - | - | - | - | - |
| Toilet transfer | 0.02 (0.0005, 0.94) | 0.03 (0.002, 0.29) | 0.82 (0.66, 0.94) | 4.93e-5 (1.69e-9;0.05) | - | - | - | - | - |
| Shower transfer | - | 0.16 (0.03, 0.70) | 0.93 (0.85; 0.99) | 0.02 (0.0003, 0.75) | - | 0.08 (0.003, 0.58) | - | - | - |
| Locomotion | 0.02 (0.0005, 0.15) | - | - | - | - | 0.05 (0.001, 0.62) | - | 0.07 (0.002, 0.76) | - |
| Stair climb | 0.08 (0.01, 0.34) | - | - | - | 0.07 (0.007, 0.44) | - | - | - | 0.08 (0.009, 0.45) |
Values are presented as (lower confidence limit, upper confidence limit).
OR, odds ratio; FIM, Functional Independence Measure; mRS, modified Rankin Scale; LOC, level of consciousness.
To adjust the correlation analyses between the NIHSS and FIM motor items, we added parameters from demographic and clinical data. Of these items, in combination with several NIHSS items, age showed statistically significant relationships for six items including grooming, toileting, bladder management, bed transfer, toilet transfer, and shower transfer. For example, the result for the FIM item for bed transfer produces the prediction equation of 19.63-(0.20×Age)-(3.39×Affected upper extremity 3rd)-(3.91×Affected lower extremity 2nd) (Table 2). Fig. 4 shows the logistic curve obtained from this formula. When the value of the affected upper extremity is under 3 and that of the affected lower extremity is under 2, these values can be converted to 0 (referring to Fig. 1). The positive number of this equation indicates independence: . This means that a patient under 98 years old who can show anti-gravitational upper extremity movement and hold the affected lower extremity at 30° in the supine position (NIHSS definition) during the acute phase, is predicted to regain the ability to transfer between a bed and a wheelchair referring to Fig. 4. Apart from age, mRS before stroke onset was also detected as having statistically significant relationships for 5 items including upper body dressing, lower body dressing, bowel management, bladder management, and stairs climb (Tables 2 and 3).
Fig. 4.
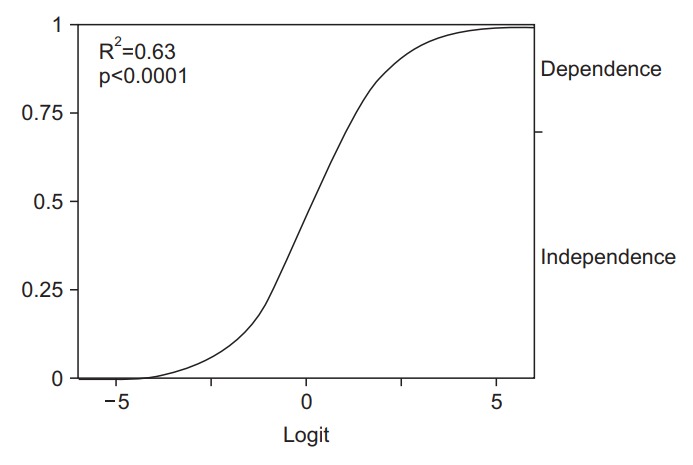
Example of logistic curve obtained from multivariate logistic model (bed transfer). The model equation is as follows: 19.63-(0.20×Age)-(3.39×Affected Upper Extremity 3rd)-(3.91×Affected Lower Extremity 2nd). Zero in the horizontal axis indicates a logistic probability 0.50. The curve showed the probability of bed transfer independence. The distance from the bottom to the top of the graph curve is the probability of independence.
Table 4 presents the accuracy rates of the model-development and confirmatory groups.
Table 4.
Accuracy of multiple logistic regression models
| FIM motor item | Model-development group |
Confirmatory group |
||||||
|---|---|---|---|---|---|---|---|---|
| Independence/Supervision | Dependence/Physically assistance | Total | PCR×1.25 (%) | Independence/Supervision | Dependence/Physically assistance | Total | PCR×1.25 | |
| Eating (%) | 34/38 (89) | 9/15 (60) | 43/53 (81) | >78.7 | 17/19 (89) | 4/7 (57) | 21/26 (81) | >80.6 |
| Grooming | 34/37 (92) | 13/16 (81) | 47/53 (89) | >72.5 | 18/21 (86) | 5/5 (100) | 23/26 (88) | >71.7 |
| Bathinga) | 32/36 (89) | 16/17 (94) | 48/53 (91) | >66.3 | 16/21 (76) | 3/5 (60) | 19/26 (73) | >71.7 |
| Upper body dressing | 34/38 (89) | 13/15 (87) | 47/53 (80) | >70.6 | 18/24 (75) | 2/2 (100) | 20/26 (77) | >71.7 |
| Lower body dressing | 34/38 (89) | 13/15 (87) | 47/53 (80) | >70.6 | 18/24 (75) | 2/2 (100) | 20/26 (77) | >71.7 |
| Toileting | 34/37 (92) | 13/16 (81) | 47/53 (80) | >72.3 | 18/21 (86) | 4/5 (80) | 22/26 (85) | >75.8 |
| Bowel management | 34/38 (89) | 13/15 (87) | 47/53 (80) | >70.6 | 19/24 (79) | 2/2 (100) | 21/26 (81) | >75.8 |
| Bladder management | 37/39 (95) | 13/14 (93) | 50/53 (94) | >74.3 | 19/21 (90) | 4/5 (80) | 23/26 (88) | >80.6 |
| Bed transfer | 34/37 (92) | 13/16 (81) | 47/53 (89) | >72.3 | 17/21 (81) | 3/5 (60) | 20/26 (77) | >75.8 |
| Toilet transfer | 34/37 (92) | 13/16 (81) | 47/53 (89) | >72.3 | 17/21 (81) | 4/5 (80) | 21/26 (81) | >71.7 |
| Shower transfera) | 29/37 (78) | 14/16 (88) | 43/53 (81) | >64.3 | 15/21 (71) | 4/5 (80) | 19/26 (73) | >65.8 |
| Locomotion | 33/38 (87) | 13/15 (87) | 46/53 (87) | >72.3 | 17/21 (81) | 4/5 (80) | 21/26 (81) | >71.7 |
| Stair climba) | 28/38 (74) | 15/15 (100) | 43/53 (81) | >62.7 | 10/21 (48) | 5/5 (100) | 15/26 (58) | <65.8 |
Values are presented as number (%).
FIM, Functional Independence Measure; PCR, proportion by chance rate.
Outcome variable is supervision or physically assistance.
We employed the criteria using an accuracy rates 25% higher than the value based on the chance rate. According to that, our confirmatory group items were excellent except a stairs climb item. Thus, the prediction models derived from the multiple logistic models were confirmed for within-institutional validity for FIM motor items except stairs climb.
DISCUSSION
By using multivariate logistic regression statistics employing NIHSS assessed within a few days after stroke and adjusting factors such as age and pre-stroke independence level, we were able to develop prediction models for the long-term outcomes of FIM motor items. At least one single NIHSS item correlated with each FIM motor item, implying the applicability of acute phase NIHSS for the long-term outcome prediction in terms of disability. The accuracy rates in the confirmatory group were as high as those for the model-development group.
Of all NIHSS items, the degrees of paralysis in the affected upper and lower extremities most evidently affected the long-term FIM outcomes. In particular, the score for affected lower extremity was predictive for the FIM motor items, except for eating and shower transfer, implying that gross assessment of hemiparesis may be predictive for long-term ADL outcomes. In the daily clinical practice for stroke treatment, clinicians often use the manual muscle test (MMT/MRC) [13,14] to assess the gross levels of hemiparesis in the upper extremity and lower extremity. This method may be more widely used than NIHSS because NIHSS is an evaluation used specifically by stroke specialists. In reality, some stroke patients who are treated by general physicians are unfamiliar with NIHSS. The present findings suggest that the gross assessment of hemiparesis such as MMT/MRC in the acute stage may be partially predictive for long-term ADL.
In addition to NIHSS, age was shown to be a statistically significant contributing factor for the prediction of the long-term outcome assessed by FIM motor items. The previous studies indicated a correlation of age with general independence at a few months after the onset [15,16] and with walking independence at 6 months [17]. In this study, among the explanatory variables, statistics revealed that age had the greatest effect on the outcomes. However, age is only one explanatory variable that was assessed on an interval scale. Such an aspect might contribute to the greatest predictive power. Careful consideration should be given to the difference of the scales (nominal, ordinal, or interval) when the relative importance among explanatory variables is to be discussed.
In contrast to the previous studies on this topic [18,19], we included several patients who were not fully independent before the onset. Indeed, some patients in our database were recurrent stroke cases who were affected by previous episodes. To address this concern, in this study, we assessed ADL before the onset by using mRS. Analysis results showed that ADL status before stroke was also a powerful predictive factor for the long-term outcomes assessed by FIM motor items. In actual clinics, stroke cases often show recurrence and are accompanied with disability in ADL. The methodology in the present study may be more practical than that used in the previous studies on stroke outcome, in which only data on firstever stroke cases who were fully independent before the onset were collected [20-22].
In this study, to assess the within-institutional validity, we employed the confirmatory group. The results obtained from the confirmatory group were very similar to those obtained from the model-development group (Table 4), indicating the obtained prediction models were sufficient. However, the results for stairs climb were negative. Previous studies indicated that the stairs climb item is the most difficult among the FIM motor items [10,11]. The negative finding for this item could be partially attributed to the ceiling effect, under which most of patients can gain a goal for its easiness. In addition, cognitive factors, one of which is considered to be falling risks [23], may influence the lower accuracy rate of stairs climb because NIHSS is not sufficient for the assessment of cognitive functions in terms of ADL [24].
This study has several limitations. First, the sample size was small, with only 53 cases in the model-development group and 26 cases in the confirmatory group. However, we detected statistically significant models for all 13 FIM motor items in the model-development group, and the validity of the success of all models except stair climb was confirmed. Despite the small sample size, the results from our analyses were relatively positive. Second, due to the nature of our sample, the findings need careful consideration when applying the models to wider stroke populations. The study samples were collected from patients who were transferred to long-term rehabilitative facilities after acute medical services. Therefore, mild or severe cases were omitted in the study sample; mild cases are usually discharged because of their minimum impairment, and severe ones are transferred to nursing facilities. Third, inter-facility validation was not assessed. For the study, to avoid variability of the treatment methodology during the acute medical service, we collected patients who were treated in a single acute hospital. The obtained results were positive. To further confirm the validity of the present study, a well-designed study for inter-facility validation is needed. Fourth, in this study, to simplify our analytical procedures, we defined ‘independence’ for the bathing, shower transfer, and stairs climb items as including supervision levels (FIM 5). Original FIM terminology does not include supervision as independence. However, as the patients with MCA infarct often suffer from severe hemiparesis, retaining supervision level (FIM 5) could be a realistic goal for these difficult items. We therefore employed a modified terminology for this study.
Footnotes
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.UN Department of Economic and Social Affairs . World population ageing 2015. New York: United Nations; 2015. [Google Scholar]
- 2.Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383:245–54. doi: 10.1016/s0140-6736(13)61953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 4.Jauch EC, Saver JL, Adams HP, Jr, Bruno A, Connors JJ, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 5.Linacre JM, Heinemann AW, Wright BD, Granger CV, Hamilton BB. The structure and stability of the Functional Independence Measure. Arch Phys Med Rehabil. 1994;75:127–32. [PubMed] [Google Scholar]
- 6.Chung JW, Park SH, Kim N, Kim WJ, Park JH, Ko Y, et al. Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification and vascular territory of ischemic stroke lesions diagnosed by diffusion-weighted imaging. J Am Heart Assoc. 2014;3:e001119. doi: 10.1161/JAHA.114.001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bloch RF. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:1448. doi: 10.1161/01.str.19.11.1448. [DOI] [PubMed] [Google Scholar]
- 8.Kawano H, Hirano T, Inatomi Y, Terasaki T, Yonehara T, Uchino M. Presence of deep white matter lesions on diffusion-weighted imaging is a negative predictor of early dramatic improvement after intravenous tissue plasminogen activator thrombolysis. Cerebrovasc Dis. 2010;30:230–6. doi: 10.1159/000317183. [DOI] [PubMed] [Google Scholar]
- 9.Katz MH. Multivariable analysis: a practical guide for clinicians and public health researchers. 3rd ed. Cambridge: Cambridge University Press; 2011. [Google Scholar]
- 10.Tsuji T, Sonoda S, Domen K, Saitoh E, Liu M, Chino N. ADL structure for stroke patients in Japan based on the functional independence measure. Am J Phys Med Rehabil. 1995;74:432–8. doi: 10.1097/00002060-199511000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Koyama T, Matsumoto K, Okuno T, Domen K. Relationships between independence level of single motor-FIM items and FIM-motor scores in patients with hemiplegia after stroke: an ordinal logistic modelling study. J Rehabil Med. 2006;38:280–6. doi: 10.1080/16501970600731420. [DOI] [PubMed] [Google Scholar]
- 12.White JL. Logistic regression model effectiveness: proportional chance criteria and proportional reduction in error. J Contemp Res Educ. 2013;2:4–10. [Google Scholar]
- 13.Logigian MK, Samuels MA, Falconer J, Zagar R. Clinical exercise trial for stroke patients. Arch Phys Med Rehabil. 1983;64:364–7. [PubMed] [Google Scholar]
- 14.Gregson JM, Leathley MJ, Moore AP, Smith TL, Sharma AK, Watkins CL. Reliability of measurements of muscle tone and muscle power in stroke patients. Age Ageing. 2000;29:223–8. doi: 10.1093/ageing/29.3.223. [DOI] [PubMed] [Google Scholar]
- 15.Lei C, Wu B, Liu M, Chen Y, Yang H, Wang D, et al. Totaled health risks in vascular events score predicts clinical outcomes in patients with cardioembolic and other subtypes of ischemic stroke. Stroke. 2014;45:1689–94. doi: 10.1161/STROKEAHA.113.004352. [DOI] [PubMed] [Google Scholar]
- 16.Weimar C, Konig IR, Kraywinkel K, Ziegler A, Diener HC; German Stroke Study Collaboration. Age and National Institutes of Health Stroke Scale Score within 6 hours after onset are accurate predictors of outcome after cerebral ischemia: development and external validation of prognostic models. Stroke. 2004;35:158–62. doi: 10.1161/01.STR.0000106761.94985.8B. [DOI] [PubMed] [Google Scholar]
- 17.Kwah LK, Harvey LA, Diong J, Herbert RD. Models containing age and NIHSS predict recovery of ambulation and upper limb function six months after stroke: an observational study. J Physiother. 2013;59:189–97. doi: 10.1016/S1836-9553(13)70183-8. [DOI] [PubMed] [Google Scholar]
- 18.Koyama T, Matsumoto K, Okuno T, Domen K. A new method for predicting functional recovery of stroke patients with hemiplegia: logarithmic modelling. Clin Rehabil. 2005;19:779–89. doi: 10.1191/0269215505cr876oa. [DOI] [PubMed] [Google Scholar]
- 19.Koyama T, Tsuji M, Miyake H, Ohmura T, Domen K. Motor outcome for patients with acute intracerebral hemorrhage predicted using diffusion tensor imaging: an application of ordinal logistic modeling. J Stroke Cerebrovasc Dis. 2012;21:704–11. doi: 10.1016/j.jstrokecerebrovasdis.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Kwakkel G, Veerbeek JM, van Wegen EE, Nijland R, Harmeling-van der Wel BC, Dippel DW, et al. Predictive value of the NIHSS for ADL outcome after ischemic hemispheric stroke: does timing of early assessment matter? J Neurol Sci. 2010;294:57–61. doi: 10.1016/j.jns.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Veerbeek JM, Van Wegen EE, Harmeling-Van der Wel BC, Kwakkel G, EPOS Investigators Is accurate prediction of gait in nonambulatory stroke patients possible within 72 hours poststroke? The EPOS study. Neurorehabil Neural Repair. 2011;25:268–74. doi: 10.1177/1545968310384271. [DOI] [PubMed] [Google Scholar]
- 22.Vora NA, Shook SJ, Schumacher HC, Tievsky AL, Albers GW, Wechsler LR, et al. A 5-item scale to predict stroke outcome after cortical middle cerebral artery territory infarction: validation from results of the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE) Study. Stroke. 2011;42:645–9. doi: 10.1161/STROKEAHA.110.596312. [DOI] [PubMed] [Google Scholar]
- 23.Vogt L, Wieland K, Bach M, Himmelreich H, Banzer W. Cognitive status and ambulatory rehabilitation outcome in geriatric patients. J Rehabil Med. 2008;40:876–8. doi: 10.2340/16501977-0260. [DOI] [PubMed] [Google Scholar]
- 24.Gottesman RF, Kleinman JT, Davis C, Heidler-Gary J, Newhart M, Hillis AE. The NIHSS-plus: improving cognitive assessment with the NIHSS. Behav Neurol. 2010;22:11–5. doi: 10.3233/BEN-2009-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]