Mucosal-associated invariant T cells (MAITs) are positioned in airways and may be important in the pulmonary cellular immune response against Mycobacterium tuberculosis infection, particularly prior to priming of peptide-specific T cells. Accordingly, there is interest in the possibility that boosting MAITs through tuberculosis (TB) vaccination may enhance protection, but MAIT responses in the lungs during tuberculosis are poorly understood.
KEYWORDS: MAIT cells, Mycobacterium tuberculosis, T cells
ABSTRACT
Mucosal-associated invariant T cells (MAITs) are positioned in airways and may be important in the pulmonary cellular immune response against Mycobacterium tuberculosis infection, particularly prior to priming of peptide-specific T cells. Accordingly, there is interest in the possibility that boosting MAITs through tuberculosis (TB) vaccination may enhance protection, but MAIT responses in the lungs during tuberculosis are poorly understood. In this study, we compared pulmonary MAIT and peptide-specific CD4 T cell responses in M. tuberculosis-infected rhesus macaques using 5-OP-RU-loaded MR-1 tetramers and intracellular cytokine staining of CD4 T cells following restimulation with an M. tuberculosis-derived epitope megapool (MTB300), respectively. Two of four animals showed a detectable increase in the number of MAIT cells in airways at later time points following infection, but by ∼3 weeks postexposure, MTB300-specific CD4 T cells arrived in the airways and greatly outnumbered MAITs thereafter. In granulomas, MTB300-specific CD4 T cells were ∼20-fold more abundant than MAITs. CD69 expression on MAITs correlated with tissue residency rather than bacterial loads, and the few MAITs found in granulomas poorly expressed granzyme B and Ki67. Thus, MAIT accumulation in the airways is variable and late, and MAITs display little evidence of activation in granulomas during tuberculosis in rhesus macaques.
INTRODUCTION
Mycobacterium tuberculosis is the leading cause of death due to a single infectious agent (1). Approximately one-fourth of the global population harbors latent infection, and 1.4 million individuals succumbed to tuberculosis (TB) in 2015 despite the availability of effective chemotherapy (1, 2). The development of a highly protective vaccine for tuberculosis would have a dramatic impact on global public health. To date, vaccines eliciting conventional T cells have failed to provide high levels of durable protection against M. tuberculosis infection (3). This has led to a renewed interest in the possibility that other lymphocytes, such as B cells and donor-unrestricted T cells (DURTs), may be able to provide vaccine-mediated protection against M. tuberculosis infection (4–7).
Mucosal-associated invariant T cells (MAITs) are innate lymphocytes of particular interest in the setting of pulmonary tuberculosis due to their abundance in the airways and demonstrated ability to respond to cells infected with mycobacteria (4, 5, 8–12). MAITs are CD8+ lymphocytes which express a semi-invariant T cell receptor restricted by the highly conserved, nonclassical class I molecule MR-1, which can present microbe-derived riboflavin metabolites (8, 13–15). MAITs can produce inflammatory cytokines, have been shown to drive the differentiation of monocyte-derived dendritic cells in pulmonary infection (16), and can kill infected cells (17). Patients with active tuberculosis have been shown to have lower frequencies of circulating MAITs (18–20), and simian immunodeficiency virus (SIV) infection is associated with systemic loss of MAITs (21). Furthermore, polymorphisms in the MR-1 gene have been shown to be associated with susceptibility to tuberculosis in humans (15). Finally, peripheral blood MAITs in rhesus macaques have been found to upregulate granzyme B and Ki67 after both Mycobacterium bovis BCG vaccination and M. tuberculosis infection (4), indicating that at least the MAITs in circulation respond following mycobacterial exposure. Therefore, MAITs can potentially provide an early defense against M. tuberculosis infection, which may be especially important before the arrival of conventional effector Th1 cells in the lungs. However, pulmonary MAIT responses after M. tuberculosis infection of macaques have not been examined, and it is not known if MAITs are able to accumulate at the site of infection following M. tuberculosis infection or enter granulomas.
In this study, we compared the kinetics of MAIT and conventional peptide-specific CD4 T cell responses in bronchoalveolar lavage fluid (BAL) following M. tuberculosis infection and used the intravascular stain approach to quantify MAIT responses in individual granulomas and other infected tissue sites upon necropsy at 7 to 8 weeks postinfection. We demonstrate that MR-1–5-OP-RU tetramer-restricted MAITs show little evidence of granzyme B or Ki67 expression following M. tuberculosis infection and are quickly outnumbered by Th1 cells. Moreover, we found that very few MAITs are present within M. tuberculosis granulomas relative to Th1 cells. Unexpectedly, these data indicate that pulmonary MAITs may not significantly contribute to the lymphocyte influx into pulmonary lesions during M. tuberculosis infection.
RESULTS
Kinetics of MAIT versus M. tuberculosis-specific CD4 T cell expansion in the airways during M. tuberculosis infection in rhesus macaques.
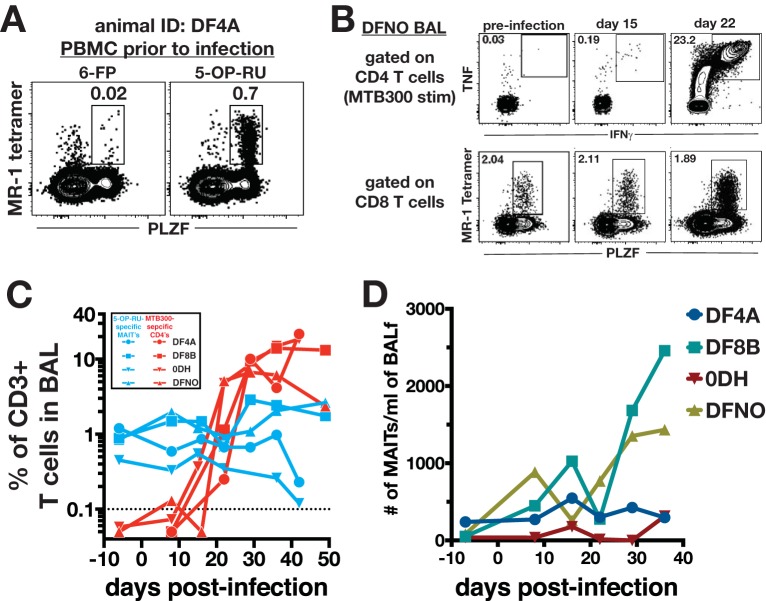
To test the hypothesis that airway MAITs will respond to M. tuberculosis infection prior to the development of peptide-specific CD4 T cells, we infected four rhesus macaques with ∼40 to 80 CFU of M. tuberculosis Erdman-mCherry and obtained BAL and peripheral blood mononuclear cell (PBMC) samples every week. To identify MAITs, we used rhesus macaque MR-1 tetramers loaded with the ligand 5-OP-RU or the negative control 6-FP. A population of MR-1 5–OP-RU+ cells was observed in PLZF+ CD8+ cells and very few cells stained with 6-FP-loaded tetramers, confirming the specificity of the staining (Fig. 1A). To measure the M. tuberculosis-peptide specific CD4 T cell response, cells were stimulated with a pool of 300 peptides previously shown to account for the majority of the M. tuberculosis-specific T cell response in both humans and macaques (22, 23), and M. tuberculosis-specific CD4 T cells were detected as tumor necrosis factor-positive (TNF+), gamma interferon-positive (IFN-γ+) responding CD4 T cells (Fig. 1B).
FIG 1.
Kinetics of MAIT versus conventional M. tuberculosis-specific CD4 T cell responses in the airways following M. tuberculosis infection. (A) Sample fluorescence-activated cell sorting (FACS) plots of MR-1 tetramer staining specificity. PBMCs from an animal prior to infection were stained with 5-OP-RU-loaded rhesus macaque MR-1 tetramers. 6-FP-loaded tetramers served as a negative control. MAITs were identified as CD3+ CD8+ PLZF+ tetramer-binding T cells. (B) Sample FACS plots of MAITs and peptide-specific Th1 cells following infection with ∼40 to 80 CFU of M. tuberculosis Erdman-mCherry. M. tuberculosis-specific CD4 T cells were identified by intracellular cytokine staining for IFN-γ and TNF after a 6-h stimulation with the MTB300 peptide megapool. CD4 T cells were identified as CD3+ CD4+ CD8− cells. MAITs were identified as for panel A. (C) Summary graphs of the kinetics of MAIT and MTB300-specific CD4 T cells in the airways following M. tuberculosis infection. In order to allow for direct comparison of their relative abundances, the frequencies of both populations are expressed as a percentage of total live CD3+ cells. (D) Summary graph of the number of MAIT cells per milliliter of bronchoalveolar lavage fluid.
To directly compare responses of MAITs with peptide-specific CD4 T cells, we calculated the frequency of either T cell subset among total CD3+ cells. MAITs were present in the BAL at preinfection time points in all 4 animals we studied, although at lower frequencies than in a previously studied group of macaques (4). In contrast, MTB300-specific CD4 T cells were first detected between 2 and 3 weeks postinfection (Fig. 1B and C), and by day 28 postinfection, ∼10% of all CD3+ T cells in the BAL were MTB300-specific CD4 T cells. In contrast, 5-OP-RU-specific MAITs comprised ∼1% of the total number of T cells at this time point (Fig. 1C). Animals DFNO and DF8B did show an increase in number MAITs per milliliter of BAL collected by day 36 postinfection, while DF4A and 0DH remained steady in the number of MAITs throughout the experiment (Fig. 1D).
Ki67 and granzyme B expression in airway MAITs following M. tuberculosis infection.
We next examined the activation of MAITs in the airways after infection. Previously, it has been shown that MAITs express Ki67 and granzyme B following bacterial stimulation (4, 17), so we used these molecules as markers of activation. There was no change in Ki67 expression between baseline and day 14 postinfection. After arrival of Th1 cells into the airways on day 22 postinfection, MAITs showed a small increase in Ki67 expression that decreased back to baseline by day 28 (Fig. 2A). Prior to infection, granzyme B expression was undetectable in tetramer+ MAITs in the BAL of all animals (Fig. 2B). Following infection, 2 animals showed transient expression of granzyme B in airway MAITs, but the proportion of tetramer+ MAITs that expressed granzyme B never exceeded ∼20% (Fig. 2B). We did not measure the ability of airway MAITs to produce cytokines, so it is possible that other effector functions were activated in MAITs following M. tuberculosis infection. Nonetheless, we were unable to detect significant MAIT activation based on these two markers.
FIG 2.
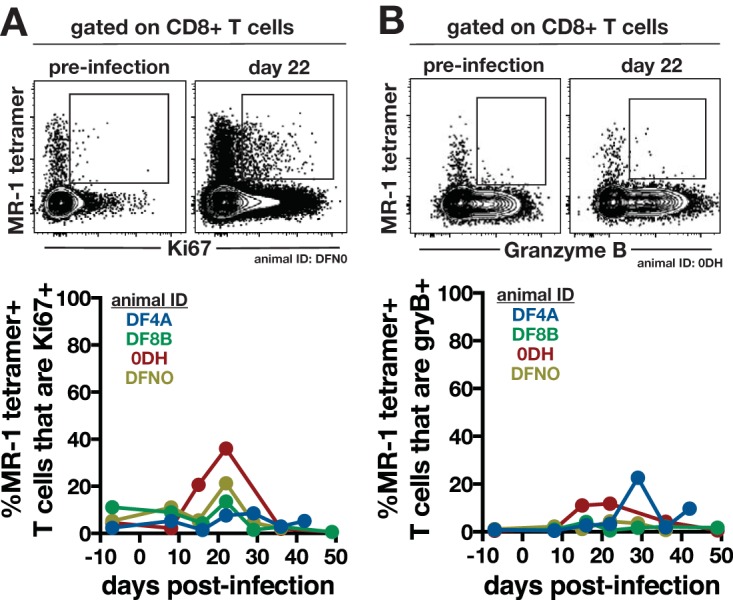
Expression of Ki67 and granzyme B by airway MAITs following M. tuberculosis infection. MR-1–5-OP-RU-specific MAITs in the airways were analyzed for Ki67 (A) and granzyme B (B) expression following M. tuberculosis infection. Sample FACS plots from preinfection and day 22 postinfection (top) and summary graphs of entire infection kinetics (bottom) are shown.
Comparison of MAIT and M. tuberculosis-specific CD4 T cell expansion in tissues of M. tuberculosis-infected rhesus macaques.
We did not detect appreciable MAIT activation in the airways following infection, so we next compared the accumulations of MAIT and peptide-specific CD4 T cells in the circulation, lymphoid tissues, and individual granulomas. At weeks 6 to 7 postinfection, the animals were humanely euthanized and T cell responses measured by MR-1 tetramer staining and MTB300 restimulation as before (Fig. 3A). In order to carefully identify T cells that had migrated into the tissue parenchyma, we used the intravascular staining approach (24) and gated on intravascular stain-negative cells. As a fraction of total CD3+ cells, MAITs and Th1 cells were present at similar frequencies in pulmonary and peripheral lymph nodes, as well as in the circulation (Fig. 3B). In contrast, MTB300-specific CD4 T cells were ∼20-fold more abundant in individual granulomas. At this time point, animals 0DH and DF4A had developed large lesions at the site of bacterial instillation. In these consolidation-like lesions, peptide-specific CD4 T cells were ∼100-fold more abundant than MAITs.
FIG 3.
Abundance of MAIT versus peptide-specific Th1 cells in M. tuberculosis-infected tissues of rhesus macaques. Animals were euthanized 6 to 7 weeks postinfection for analysis of MAIT and M. tuberculosis-specific CD4 T cells in infected tissues. Animals were intravenously injected with biotinylated anti-CD45 just prior to euthanasia to allow for identification of parenchymal T cells. (A) Sample FACS plots of MAITs and MTB300-specific CD4 T cells in different tissues of a rhesus macaque 6 weeks postinfection. Both MAITs and peptide-specific CD4 T cells are gated on intravascular stain-negative cells. (B) The frequencies of MAIT and MTB300-specific CD4 T cells among total CD3+ cells were compared in various tissues. Connecting lines indicate that both MAITs and MTB300-specific CD4 T cells were measured in the same samples from spleen, peripheral lymph nodes, pulmonary lymph nodes, PBMCs, BAL, and large instillation site lesions. Paired t tests were used to calculate statistical difference for these tissues. For individual granulomas, either MAITs or MTB300-specific T cells were measured in different lesions, so an unpaired t test was used to calculate statistical difference. (C and D) Bacterial burdens in individual granulomas (C) or lymph nodes (D) from each animal in this study. (E and F) Correlation between bacterial burdens and MAITs (E) or MTB300-specific CD4 T cells (F) in individual granulomas.
Bacterial loads in individual granulomas ranged from 2 to 3 logs in each animal, were undetectable in all peripheral lymph nodes, and were detectable in 10 of 16 pulmonary lymph nodes (Fig. 3C and D). When results for all animals were pooled, the fraction of total CD3+ T cells that were MTB300-specific CD4 T cells positively correlated with individual bacterial loads, while MAITs showed no such correlation (Fig. 3E and F). This is consistent with higher antigen burdens driving larger CD4 T cell responses but not MAITs. Thus, although we expected to find a relative enrichment of MAITs in the lungs, we found that MAITs and conventional MTB300-specific CD4 T cells were present at similar frequencies in blood and lymphoid tissues, while MTB300-specific CD4 T cells greatly outnumbered MAITs in pulmonary tuberculous lesions.
MAIT activation in lymphoid tissue and pulmonary lesions of M. tuberculosis-infected rhesus macaques.
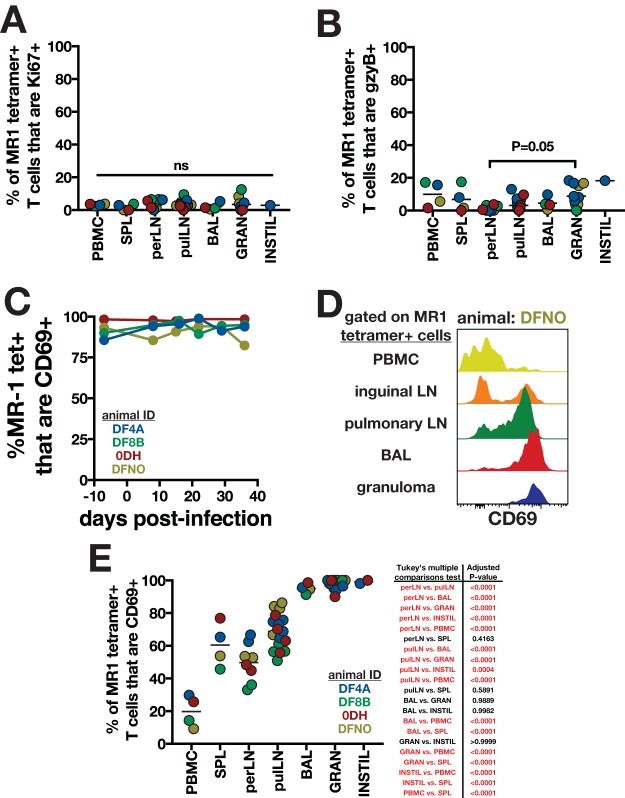
In order to determine if MAITs in tissues become activated in M. tuberculosis infection, we analyzed Ki67 and granzyme B expression in lymphoid tissues and pulmonary lesions at necropsy 6 to 7 weeks postinfection. We found that <10% of intravascular stain-negative tetramer+ MAITs expressed Ki67 in any tissue examined, and there was no difference between tissues (Fig. 4A). Similarly, <20% of MAITs expressed granzyme B, and the only statistical difference in granzyme B expression was between peripheral lymph nodes and granulomas (Fig. 4B). Therefore, we were unable to detect significant local upregulation or enrichment of these activation markers in MAITs at sites of bacterial replication.
FIG 4.
MAIT activation in tissues of rhesus macaques following M. tuberculosis infection. The percentages of MR-1 tetramer+ T cells in various tissues of M. tuberculosis-infected rhesus macaques that were positive for Ki67 (A) and granzyme B (B) are shown. P values are for one-way ANOVA with Fisher's LSD test for multiple comparisons. (C) CD69 expression on tetramer+ MAITs in BAL. (D and E) Sample FACS plots (D) and summary graphs (E) of CD69 expression on tetramer+ MAITs in various tissues. MAITs in lymph nodes, BAL, granulomas, and instillation site lesions are gated on intravascular stain-negative cells. P values for one-way ANOVA with Fisher's LSD test for multiple comparisons are shown to the right of the graph, with significant differences highlighted in red.
In contrast to Ki67 and granzyme B, we found that the majority of MAITs in the BAL expressed CD69 (Fig. 4C). Prior to infection, more than 80% of airway MAITs expressed CD69, and this did not change following M. tuberculosis exposure. In fact, we found a hierarchical expression of CD69 on tetramer+ MAITs in tissues, with the lowest levels in the PBMCs, intermediate levels in the lymph nodes, and the highest levels in the BAL, granulomas, and large instillation site lesions (Fig. 4D and E). Interestingly, more MAITs in pulmonary nodes expressed CD69 than in peripheral lymph nodes. This may indicate that MAITs are more activated at sites of infection. However, we do not prefer this hypothesis, because CD69 was uniformly expressed on MAITs in the BAL prior to infection, and CD69 was uniformly expressed on MAITs in granulomas despite a 3- to 4-log range in bacterial loads. Therefore, CD69 expression on MAITs is most likely reflective of a tissue residency program, similar to Trm cells, rather than recent antigen recognition.
DISCUSSION
MAITs are known to respond to mycobacteria, and previous reports have shown that circulating MAITs become activated following mycobacterial exposure in macaques (4). In this study, we examined pulmonary MAIT responses during tuberculosis, with the hypothesis that these cells would be enhanced at sites of bacterial replication. Surprisingly, we found little evidence of MAIT activation in the pulmonary compartment, and MAITs were relatively rare in lung granulomas.
There are several caveats to our study. We did not measure cytokine production by MAITs, so it is possible that MAITs in the lungs upregulate other effector functions following infection. It is also possible that 5-OP-RU-loaded tetramers fail to detect the population of MAITs that respond to M. tuberculosis infection in the lungs. If MAIT activation in the airways is very local and occurs only proximal to sites of infection, we may have diluted out the locally responding MAITs with MAITs from distant airways during the BAL collection. Moreover, we cannot make conclusions about MAIT responses early in granuloma formation. It is also possible that MAIT responses may be actively repressed in the setting of M. tuberculosis infection in the lungs. Most importantly, we have not directly interfered with MAIT function in vivo, so we cannot conclude that MAITs play no role in M. tuberculosis infection of rhesus macaques. In fact, in our data there may be an association with accumulation of BAL MAITs and disease progression. By later time points postinfection, BAL MAITs in animals DF4A and 0DH had not increased in number and these animals had more severe disease (indicated by the development of large lesions at the instillation site). In contrast, DF8B and DFNO showed some increase in MAITs in the BAL and did not develop significant instillation site lesions. The 2 animals that did develop instillation site lesions not only had MAITs fail to accumulate in the airways but also mounted larger Th1 cell responses, so the ratio of MAITs to Th1 cells was dramatically lower in the sicker animals. However, due to the small number of study animals, the correlation between MAIT/Th1 ratios and disease severity will remain anecdotal until more carefully explored in a larger study.
These data also have implications for TB vaccination. The inability of Th1-generating vaccines to thus far deliver on their promise of robust and durable protection against M. tuberculosis infection has led to increased interest in the possibility of targeting other innate-like and adaptive lymphocytes. MAITs are potentially important effector cells against M. tuberculosis infection, but our data highlight a potential barrier to the development of a MAIT-targeting vaccine for tuberculosis, given that we did not observe significant MAIT activation in primary M. tuberculosis infection. A tuberculosis MAIT vaccine would need to induce a long-lived population of expanded MAITs able to mount amnestic responses against M. tuberculosis challenge that are quantitatively or qualitatively enhanced compared to primary responses. Therefore, while we cannot discount the possibility that MAIT responses play a pivotal role in host defense against M. tuberculosis infection, targeting MAITs to generate a highly protective TB vaccine may prove challenging.
MATERIALS AND METHODS
Animals and M. tuberculosis infections.
Four rhesus macaques originally from the Morgan Island NIH breeding colony were selected for this study and were housed in an animal biological safety level 3 (ABSL3) vivarium which is fully accredited by AAALAC International. For M. tuberculosis infection, animals were sedated and ∼40 to 80 CFU of an mCherry-expressing Erdman strain of M. tuberculosis were instilled via bronchoscope into the lower right lobe of the lung. The mCherry-expressing strain of M. tuberculosis used in this study was able to stimulate MAITs similarly to M. tuberculosis Erdman, as measured by the induction of granzyme B and CD69 expression after 24 h of stimulation (see Fig. S1 in the supplemental material). All CFU measurements were serial dilutions on 7H11 agar plates supplemented with OADC (Difco, Detroit, MI).
Animals were contained in nonhuman primate biocontainment racks and were given a high-fiber commercial diet (2050 Harlan Teklad global primate diet) twice per day. Animals were given fruit supplements 3 times per week and also received daily enrichment. All housing of and procedures involving animals were done according to the Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals (25), the AVMA Guidelines on Euthanasia (26), and other federal statutes. All procedures were approved by the NIAID DIR Animal Care and Use Committee under approved animal study proposal LPD-25E.
Bronchoalveolar lavage fluid collection.
BAL collections were taken by inserting a flexible tubing into the trachea of an anesthetized animal. Twenty milliliters of sterile saline was slowly inserted and withdrawn using a 30-ml syringe, and this process was repeated two more times. Recovery was usually between 40 and 50 ml of total fluid. The BAL was then passed through a 100-μm cell strainer and centrifuged for the collection of cells.
Intravascular staining.
Intravascular straining of blood-borne leukocytes was performed as previously described (24). Briefly, prior to euthanasia, animals were anesthetized, a BAL sample collected, and animals were injected with 50 μg/kg of body weight of anti-nonhuman primate CD45 (clone MB4-6D6; Miltenyi Biotec, San Diego, CA). After 10 min, animals were exsanguinated and humanely euthanized, at which time tissues were collected and granulomas were excised.
In vitro stimulations and flow cytometry.
Lungs were resected and then minced and enzymatically digested using a gentleMACS dissociator (Miltenyi Biotec) in RPMI 1640 medium supplemented with 1 mg/ml of collagenase D (Roche Diagnostics, Indianapolis, IN), 1 mg/ml of hyaluronidase, and 50 U/ml of DNase 1 (both from Sigma-Aldrich, St. Louis, MO). Lymph nodes and spleens were homogenized also using the gentleMACs dissociator, while granulomas were simply mashed through a 100-μm cell strainer and cells collected for analysis.
For T cell stimulations, cells were incubated with 2 μg/ml of MTB300 peptide pool (23) (A&A Labs, San Diego, CA) in the presence of brefeldin A and monensin (Thermo Fisher Scientific, Waltham, MA). For MR-1 tetramer stains, cells were incubated for 40 min at 37°C in the presence of monensin with either 5-OP-RU (MR-1 ligand) or 6-FP (negative control) MR-1 tetramer at a concentration of 75 μg/ml. Tetramers were produced by the NIAID Tetramer Core Facility (Emory University, Atlanta, GA). Following stimulations or tetramer staining, cells were stained with various combinations of the following fluorochrome-labeled antibodies: CD3 (SP34-2), CD4 (OKT4), CD8 (RPA-T8), TNF (MAB11), IFN-γ (4S.B3), CD69 (FN50), Ki67 (B56), granzyme B (GB11), CD161 (HP-3G10), and PLZF (Mags.21F7) and fixable viability dye eFluor 780, all purchased from BioLegend, eBioscience (San Diego, CA), or BD Biosciences (San Jose, CA). All samples were acquired on a BD LSRfortessa and analyzed using FlowJo software (version 10.4, Tree Star, Ashland, OR).
Statistical analysis.
All statistical analysis was performed in Prism (GraphPad Software, La Jolla, CA). A two-tailed t test, paired t test, and one-way analysis of variance (ANOVA) with Fisher's least significant difference (LSD) test for multiple comparisons were used in the analysis.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Nannan Zhu and the NIAID ABSL3 facility technicians for laboratory and animal technical support. We thank Alan Sher and Jason Brenchley for their comments on the manuscript.
This work was supported by the Intramural Research Program of NIAID/NIH.
K.D.K. and D.L.B. conceived the study, analyzed data, and wrote the manuscript. K.D.K., M.A.S., S.G.H., and S.S. performed experiments. R.M., T.W.-K., and I.N.M. performed necropsies. A.S. and C.S.L.A. provided necessary reagents to perform experiments. R.M. and T.W.-K. performed bronchoscopic M. tuberculosis infections and provided veterinary care for infected animals under ABSL3 conditions.
We declare no financial conflicts of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00431-18.
REFERENCES
- 1.World Health Organization. 2016. Global tuberculosis report. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Houben RM, Dodd PJ. 2016. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med 13:e1002152. doi: 10.1371/journal.pmed.1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, Shea JE, McClain JB, Hussey GD, Hanekom WA, Mahomed H, McShane H, the MVA85A 020 Trial Study Team. 2013. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet 381:1021–1028. doi: 10.1016/S0140-6736(13)60177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greene JM, Dash P, Roy S, McMurtrey C, Awad W, Reed JS, Hammond KB, Abdulhaqq S, Wu HL, Burwitz BJ, Roth BF, Morrow DW, Ford JC, Xu G, Bae JY, Crank H, Legasse AW, Dang TH, Greenaway HY, Kurniawan M, Gold MC, Harriff MJ, Lewinsohn DA, Park BS, Axthelm MK, Stanton JJ, Hansen SG, Picker LJ, Venturi V, Hildebrand W, Thomas PG, Lewinsohn DM, Adams EJ, Sacha JB. 2017. MR1-restricted mucosal-associated invariant T (MAIT) cells respond to mycobacterial vaccination and infection in nonhuman primates. Mucosal Immunol 10:802–813. doi: 10.1038/mi.2016.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gold MC, Napier RJ, Lewinsohn DM. 2015. MR1-restricted mucosal associated invariant T (MAIT) cells in the immune response to Mycobacterium tuberculosis. Immunol Rev 264:154–166. doi: 10.1111/imr.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Godfrey DI, Uldrich AP, McCluskey J, Rossjohn J, Moody DB. 2015. The burgeoning family of unconventional T cells. Nat Immunol 16:1114–1123. doi: 10.1038/ni.3298. [DOI] [PubMed] [Google Scholar]
- 7.Kaufmann SH. 2013. Tuberculosis vaccines: time to think about the next generation. Semin Immunol 25:172–181. doi: 10.1016/j.smim.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Gold MC, Cerri S, Smyk-Pearson S, Cansler ME, Vogt TM, Delepine J, Winata E, Swarbrick GM, Chua WJ, Yu YY, Lantz O, Cook MS, Null MD, Jacoby DB, Harriff MJ, Lewinsohn DA, Hansen TH, Lewinsohn DM. 2010. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol 8:e1000407. doi: 10.1371/journal.pbio.1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Bourhis L, Martin E, Peguillet I, Guihot A, Froux N, Core M, Levy E, Dusseaux M, Meyssonnier V, Premel V, Ngo C, Riteau B, Duban L, Robert D, Huang S, Rottman M, Soudais C, Lantz O. 2010. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol 11:701–708. doi: 10.1038/ni.1890. [DOI] [PubMed] [Google Scholar]
- 10.Harriff MJ, Karamooz E, Burr A, Grant WF, Canfield ET, Sorensen ML, Moita LF, Lewinsohn DM. 2016. Endosomal MR1 trafficking plays a key role in presentation of Mycobacterium tuberculosis ligands to MAIT cells. PLoS Pathog 12:e1005524. doi: 10.1371/journal.ppat.1005524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang J, Wang X, An H, Yang B, Cao Z, Liu Y, Su J, Zhai F, Wang R, Zhang G, Cheng X. 2014. Mucosal-associated invariant T-cell function is modulated by programmed death-1 signaling in patients with active tuberculosis. Am J Respir Crit Care Med 190:329–339. [DOI] [PubMed] [Google Scholar]
- 12.Jiang J, Chen X, An H, Yang B, Zhang F, Cheng X. 2016. Enhanced immune response of MAIT cells in tuberculous pleural effusions depends on cytokine signaling. Sci Rep 6:32320. doi: 10.1038/srep32320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gold MC, Lewinsohn DM. 2011. Mucosal associated invariant T cells and the immune response to infection. Microbes Infect 13:742–748. doi: 10.1016/j.micinf.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Rhijn I, Godfrey DI, Rossjohn J, Moody DB. 2015. Lipid and small-molecule display by CD1 and MR1. Nat Rev Immunol 15:643–654. doi: 10.1038/nri3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seshadri C, Thuong NT, Mai NT, Bang ND, Chau TT, Lewinsohn DM, Thwaites GE, Dunstan SJ, Hawn TR. 2017. A polymorphism in human MR1 is associated with mRNA expression and susceptibility to tuberculosis. Genes Immun 18:8–14. doi: 10.1038/gene.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meierovics AI, Cowley SC. 2016. MAIT cells promote inflammatory monocyte differentiation into dendritic cells during pulmonary intracellular infection. J Exp Med 213:2793–2809. doi: 10.1084/jem.20160637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurioka A, Ussher JE, Cosgrove C, Clough C, Fergusson JR, Smith K, Kang YH, Walker LJ, Hansen TH, Willberg CB, Klenerman P. 2015. MAIT cells are licensed through granzyme exchange to kill bacterially sensitized targets. Mucosal Immunol 8:429–440. doi: 10.1038/mi.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saeidi A, Tien Tien VL, Al-Batran R, Al-Darraji HA, Tan HY, Yong YK, Ponnampalavanar S, Barathan M, Rukumani DV, Ansari AW, Velu V, Kamarulzaman A, Larsson M, Shankar EM. 2015. Attrition of TCR Valpha7.2+ CD161++ MAIT cells in HIV-tuberculosis co-infection is associated with elevated levels of PD-1 expression. PLoS One 10:e0124659. doi: 10.1371/journal.pone.0124659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwon YS, Cho YN, Kim MJ, Jin HM, Jung HJ, Kang JH, Park KJ, Kim TJ, Kee HJ, Kim N, Kee SJ, Park YW. 2015. Mucosal-associated invariant T cells are numerically and functionally deficient in patients with mycobacterial infection and reflect disease activity. Tuberculosis (Edinb) 95:267–274. doi: 10.1016/j.tube.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Wong EB, Akilimali NA, Govender P, Sullivan ZA, Cosgrove C, Pillay M, Lewinsohn DM, Bishai WR, Walker BD, Ndung'u T, Klenerman P, Kasprowicz VO. 2013. Low levels of peripheral CD161++CD8+ mucosal associated invariant T (MAIT) cells are found in HIV and HIV/TB co-infection. PLoS One 8:e83474. doi: 10.1371/journal.pone.0083474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vinton C, Wu F, Rossjohn J, Matsuda K, McCluskey J, Hirsch V, Price DA, Brenchley JM. 2016. Mucosa-associated invariant T cells are systemically depleted in simian immunodeficiency virus-infected rhesus macaques. J Virol 90:4520–4529. doi: 10.1128/JVI.02876-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mothe BR, Lindestam Arlehamn CS, Dow C, Dillon MBC, Wiseman RW, Bohn P, Karl J, Golden NA, Gilpin T, Foreman TW, Rodgers MA, Mehra S, Scriba TJ, Flynn JL, Kaushal D, O'Connor DH, Sette A. 2015. The TB-specific CD4(+) T cell immune repertoire in both cynomolgus and rhesus macaques largely overlap with humans. Tuberculosis (Edinb) 95:722–735. doi: 10.1016/j.tube.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindestam Arlehamn CS, McKinney DM, Carpenter C, Paul S, Rozot V, Makgotlho E, Gregg Y, van Rooyen M, Ernst JD, Hatherill M, Hanekom WA, Peters B, Scriba TJ, Sette A. 2016. A quantitative analysis of complexity of human pathogen-specific CD4 T cell responses in healthy M. tuberculosis infected South Africans. PLoS Pathog 12:e1005760. doi: 10.1371/journal.ppat.1005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kauffman KD, Sallin MA, Sakai S, Kamenyeva O, Kabat J, Weiner D, Sutphin M, Schimel D, Via L, Barry CE III, Wilder-Kofie T, Moore I, Moore R, Barber DL. 2018. Defective positioning in granulomas but not lung-homing limits CD4 T-cell interactions with Mycobacterium tuberculosis-infected macrophages in rhesus macaques. Mucosal Immunol 11:462–473. doi: 10.1038/mi.2017.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 26.American Veterinary Medical Association. 2013. AVMA guidelines for the euthanasia of animals: 2013 edition. American Veterinary Medical Association, Schaumburg, IL. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.