Ascaris lumbricoides (roundworm) is the most common helminth infection globally and a cause of lifelong morbidity that may include allergic airway disease, an asthma phenotype. We hypothesize that Ascaris larval migration through the lungs leads to persistent airway hyperresponsiveness (AHR) and type 2 inflammatory lung pathology despite resolution of infection that resembles allergic airway disease.
KEYWORDS: Ascaris, airway hyperreactivity, allergic airway disease, asthma, hygiene hypothesis
ABSTRACT
Ascaris lumbricoides (roundworm) is the most common helminth infection globally and a cause of lifelong morbidity that may include allergic airway disease, an asthma phenotype. We hypothesize that Ascaris larval migration through the lungs leads to persistent airway hyperresponsiveness (AHR) and type 2 inflammatory lung pathology despite resolution of infection that resembles allergic airway disease. Mice were infected with Ascaris by oral gavage. Lung AHR was measured by plethysmography and histopathology with hematoxylin and eosin (H&E) and periodic acid-Schiff (PAS) stains, and cytokine concentrations were measured by using Luminex Magpix. Ascaris-infected mice were compared to controls or mice with allergic airway disease induced by ovalbumin (OVA) sensitization and challenge (OVA/OVA). Ascaris-infected mice developed profound AHR starting at day 8 postinfection (p.i.), peaking at day 12 p.i. and persisting through day 21 p.i., despite resolution of infection, which was significantly increased compared to controls and OVA/OVA mice. Ascaris-infected mice had a robust type 2 cytokine response in both the bronchoalveolar lavage (BAL) fluid and lung tissue, similar to that of the OVA/OVA mice, including interleukin-4 (IL-4) (P < 0.01 and P < 0.01, respectively), IL-5 (P < 0.001 and P < 0.001), and IL-13 (P < 0.001 and P < 0.01), compared to controls. By histopathology, Ascaris-infected mice demonstrated early airway remodeling similar to, but more profound than, that in OVA/OVA mice. We found that Ascaris larval migration causes significant pulmonary damage, including AHR and type 2 inflammatory lung pathology that resembles an extreme form of allergic airway disease. Our findings indicate that ascariasis may be an important cause of allergic airway disease in regions of endemicity.
INTRODUCTION
Ascaris lumbricoides (roundworm) is the most common intestinal helminth infection worldwide, infecting 761.9 million people in impoverished areas of Africa, Asia, and Central and South America (1, 2). In regions of endemicity, children are often infected soon after birth, enduring recurrent infection throughout childhood and reaching maximum worm burden by preschool and school age (3, 4). Ascariasis is associated with a significant level of global morbidity, leading to 1.46 million disability-adjusted life years (DALYs), an impact similar to those of other well-known childhood illnesses, such as meningitis (1.52 million DALYs) (1, 5). Ascariasis due to the presence of adult worms in the gut specifically results in growth stunting, cognitive delays, malnutrition (vitamin A deficiency), abdominal pain, and obstruction (3, 6–8). Beyond the intestinal form of ascariasis due to the presence of adult worms, larval migration through lung tissue following oral ingestion of Ascaris eggs has been clinically linked to a transient inflammatory lung disease termed Loeffler’s syndrome (7). Additionally, some investigators have suggested that larval Ascaris infection represents a major environmental cause of allergic airway disease, an asthma phenotype, and inflammatory lung disease in resource-poor countries (3, 6–12). These epidemiological studies suggest that children with Ascaris-induced allergic airway disease have more-severe allergic airway disease as well as increased cross-reactivity to bystander antigens like house dust mites (12–15). However, it is not clear how ascariasis induces prolonged allergic airway disease in children. As a result of the profound impact on morbidity, there is an urgent need to gain an understanding of end-organ damage resulting from ascariasis and to develop preventative and therapeutic interventions.
Other helminths, such as Nippostrongylus, known as the rat hookworm, that have a larval lung stage have been linked to lung damage, type 2 immune responses, and long-term changes in lung function and structure in nonhuman hosts consistent with allergic airway disease (16). Migration of Nippostrongylus larvae through the lungs causes hemorrhage and damage to the epithelium, promoting the release of damage-associated molecular patterns (DAMPs) and alarmins from airway epithelial cells and type II alveolar macrophages (16–18). The release of DAMPs and alarmins, including interleukin-33 (IL-33) and IL-25, promotes the activation of innate immune cells, such as antigen-presenting cells (dendritic cells) and innate lymphoid cells (ILC2), leading to an increase in the release of the type 2 cytokines IL-4, IL-5, and IL-13 (16, 18). IL-4, IL-5, and IL-13 are required for the protective cellular and humoral responses generated during helminth infection (18, 19). IL-4 and IL-13, signaling through the IL-4Rα/Stat6 pathway, have been shown to be part of an important pathway in both the innate and adaptive responses to lung larval migration, allowing for infection control as well as eventual tissue healing and fibrosis in Nippostrongylus-infected mice (17, 18, 20, 21).
This study used an Ascaris-infected mouse model to gain further knowledge on the impact of pulmonary ascariasis on lung physiology and histopathology. We found that Ascaris larval migration causes significant airway hyperresponsiveness (AHR), type 2 inflammatory infiltration, and early airway remodeling, which resembles an extreme form of allergic airway disease that is significantly greater than the classical mouse allergic airway disease model induced by ovalbumin (OVA) sensitization and challenge. We propose that human and animal Ascaris infections will induce a persistent allergic airway disease that resembles human asthma.
(Data in this article were presented in November 2016 and 2017 at the American Society of Tropical Medicine and Hygiene annual meeting in Atlanta, GA.)
RESULTS
Ascaris-infected mice have increased AHR and marked lung allergic inflammatory infiltrates throughout the life cycle of the larvae.
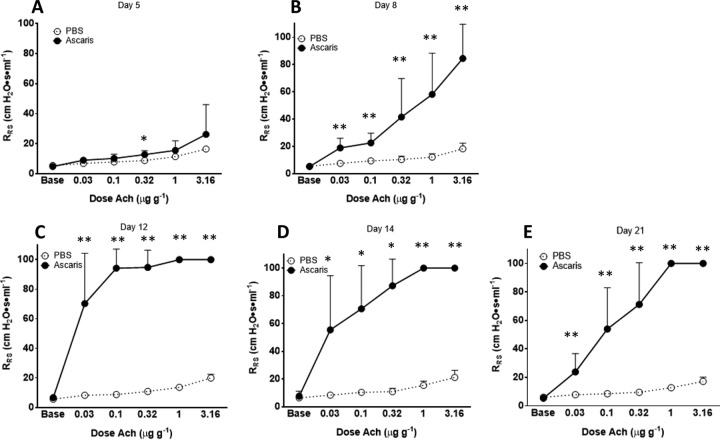
To evaluate the impact of ascariasis on the host lung during the life cycle of the parasite, 25 mice were each infected with 2,500 embryonated eggs in 200 μl phosphate-buffered saline (PBS) by oral gavage on day 0, while 25 mice received 200 μl PBS by oral gavage at day 0 (negative control). AHR was measured by using whole-body plethysmography with acetylcholine (Ach) provocation challenges at day 5, day 8, day 12, day 14, and day 21 postinfection (p.i.) in both the Ascaris-infected and negative-control groups (n = 5 per group on each day of the life cycle) (Fig. 1A to E).
FIG 1.
A. suum-infected mice develop prolonged, exaggerated AHR. AHR was assessed by plotting respiratory system resistance (RRS) against increasing concentrations of acetylcholine chloride (Ach) injected intravenously into anesthetized, Ascaris-infected mice on day 5 (A), day 8 (B), day 12 (C), day 14 (D), and day 21 (E) postinfection (n = 5 mice per group per life cycle day). *, P < 0.05; **, P < 0.01 (means ± SD, as determined by a Mann-Whitney test in two different experiments).
Ascaris-infected mice were found to have increasing Ach-induced AHR starting at day 8 p.i., at which time point larvae are at the maximum number in the lungs (Fig. 1B). By day 12 p.i., Ascaris infection caused severe AHR at very low concentrations of Ach compared to PBS-treated negative controls, leading to complete bronchoconstriction (airway narrowing) and restriction of airflow (Fig. 1C). Interestingly, AHR persisted at high levels at day 21 p.i. (Fig. 1E), despite resolution of infection, suggesting that ascariasis not only causes increased AHR during the life cycle of the larvae but also has persistent effects without evidence of active infection.
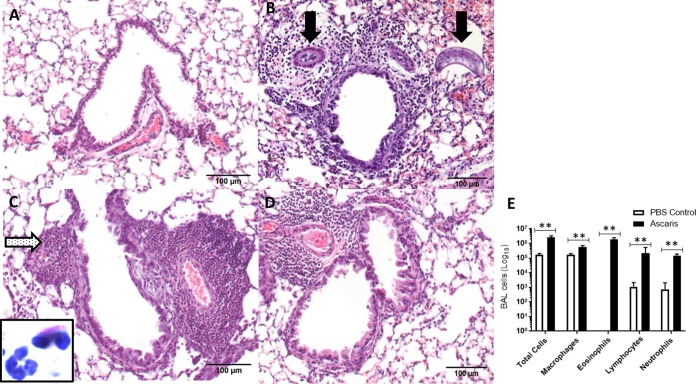
In comparison to PBS-treated negative controls (Fig. 2A), histopathological evaluation of the lung tissue using hematoxylin and eosin (H&E) staining showed marked perivascular and peribronchial inflammatory infiltrates with alveolitis (peripheral lung inflammation) from day 8 p.i. through day 21 p.i. in Ascaris-infected mice (Fig. 2B to D), consisting of neutrophils and eosinophils (Fig. 2C, inset). On day 8 p.i., there was evidence of larvae in the parenchyma as well as in the vasculature, which resolved by day 12. Ascaris-infected mice also had increased total cell counts in bronchoalveolar lavage (BAL) fluid, with a predominance of eosinophils, compared to control mice (Fig. 2E).
FIG 2.
Temporal assessment of histopathological and airway cellular changes following Ascaris lung infection. (A to D) Histological images of lung showing representative bronchovascular bundles. (A) PBS negative control; (B) after Ascaris infection on day 8; (C) after Ascaris infection on day 12; (D) after Ascaris infection on day 21. Black arrows, Ascaris larvae within the lung parenchyma and vasculature; dotted arrow, perivasculature and peribronchial granulocytic infiltration; inset, eosinophil with bilobed nucleus and granular eosinophilic cytoplasm adjacent to a neutrophil with a segmented nucleus and pale cytoplasm. Magnification, ×200. (E) Mean total BAL fluid cells and mean absolute numbers of macrophages, eosinophils, lymphocytes, and neutrophils enumerated from Ascaris-infected or PBS control mice at day 12 p.i. **, P < 0.01 (means ± SD, as determined by a Mann-Whitney test in two different experiments).
Ascaris-infected mice have similar but profound AHR and inflammatory infiltrates compared to a known allergic airway disease mouse model.
Ascaris-infected mice were compared to a known murine allergic airway disease model of OVA-sensitized and OVA-challenged (OVA/OVA) mice. Ten mice were each infected with 2,500 embryonated eggs in 200 μl PBS by oral gavage, 10 mice were given 200 μl PBS by oral gavage (negative controls), and 10 mice were sensitized and challenged with OVA (allergic airway disease positive control). On day 12 p.i., Ascaris-infected mice and negative controls were anesthetized using etomidate for physiological evaluation of airway resistance using plethysmography. Day 12 was chosen based on the predicted maximum inflammatory infiltrate in the lungs during Ascaris infection (determined from the above-described experiment) and the high likelihood of larvae not being present within the lung tissue. On day 24 of the OVA/OVA protocol, OVA/OVA mice were anesthetized using etomidate for physiological evaluation of AHR using plethysmography. Evaluation of AHR demonstrated that Ascaris-infected mice had increased peak airflow resistance compared to both OVA/OVA mice and PBS-treated negative controls (Fig. 3).
FIG 3.
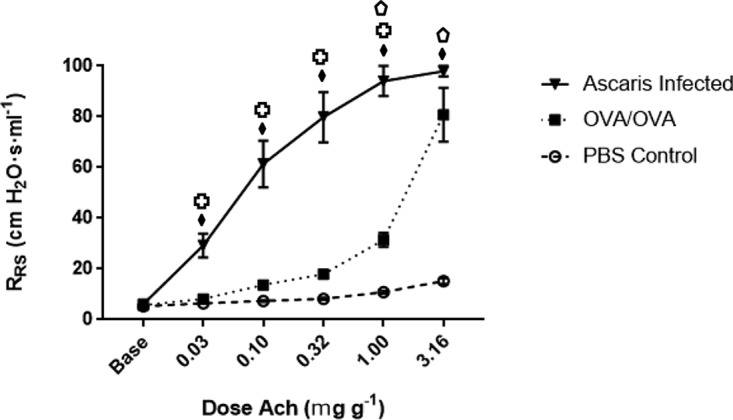
AHR for Ascaris-infected and OVA/OVA asthmatic mice and PBS negative controls. Ascaris-infected mice had profound AHR at all doses of Ach (n = 10 mice per group). Statistical analysis was performed by Kruskal-Wallis and post hoc Dunn’s multiple-comparison tests, with group comparisons represented by symbols. Diamonds represent differences between the PBS control and Ascaris-infected mice, the crosses represent differences between Ascaris-infected and OVA/OVA asthmatic mice, and the pentagons represent differences between PBS control and OVA/OVA asthmatic mice. No fill, P < 0.05; striped, P < 0.01; filled, P < 0.001 (means ± SD in two different experiments).
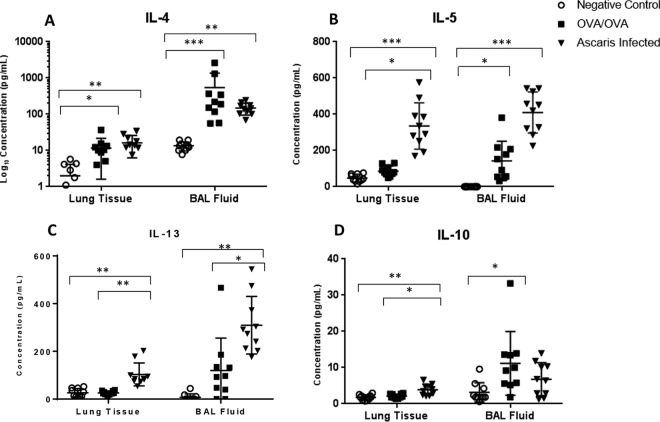
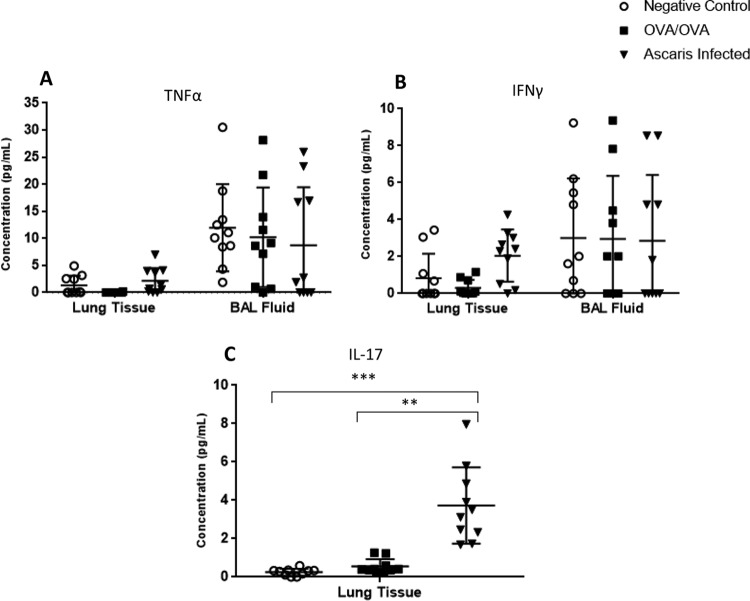
Levels of inflammatory cytokines in the BAL fluid and lung tissue were quantitated in all three groups. Ascaris-infected mice had a robust inflammatory response dominated by type 2 cytokines in both the BAL fluid and lung tissue supernatant, including IL-4 (P < 0.01 and P < 0.01, respectively), IL-5 (P < 0.001 and P < 0.001), and IL-13 (P < 0.001 and P < 0.01), compared to negative controls (Fig. 4A to C). In addition, levels of type 2-specific cytokines in Ascaris-infected mice were similar to or higher than those in OVA/OVA mice. The levels of IL-5 and IL-13 were noted to be especially high, exceeding a 10-fold difference relative to those in OVA/OVA mice. The level of IL-10 was slightly increased in Ascaris-infected mouse lung tissue compared to those in negative controls (P < 0.01) and in OVA/OVA mice (P < 0.05), but the levels were low, and there was no difference in BAL fluid (Fig. 4D). In contrast, there was no difference in the type 1-specific cytokine tumor necrosis factor alpha (TNF-α) or gamma interferon (IFN-γ) in the BAL fluid or in the lung tissue (Fig. 5A and B). Interestingly, a small amount of IL-17 was detected in lung tissue at higher concentrations in the Ascaris-infected group than in both the OVA/OVA mice (P < 0.01) and negative controls (P < 0.001), but IL-17 was not detected in the BAL fluid (Fig. 5C).
FIG 4.
Cytokine concentrations in homogenized lung tissue supernatants and BAL fluid from Ascaris-infected mice at day 12 p.i. Shown are data for PBS negative controls and OVA/OVA asthmatic mice, including IL-4 (A), IL-5 (B), IL-13 (C), and IL-10 (D) (n = 10 mice per group). *, P < 0.05; **, P < 0.01; ***, P < 0.001 (means ± SD, as determined by a Kruskal-Wallis test with post hoc Dunn’s multiple-comparison test in two different experiments, with each sample in duplicate).
FIG 5.
Cytokine concentrations in lung tissue and BAL fluid from Ascaris-infected mice at day 12 p.i. Shown are data for negative controls and OVA/OVA asthmatic mice, including TNF-α (A), IFN-γ (B), and IL-17 in lung tissue only, as IL-17 was unable to be detected in BAL fluid (C) (n = 10 mice per group). **, P < 0.01; ***, P < 0.001 (means ± SD, as determined by a Kruskal-Wallis test with post hoc Dunn’s multiple-comparison test in two different experiments, with each sample in duplicate).
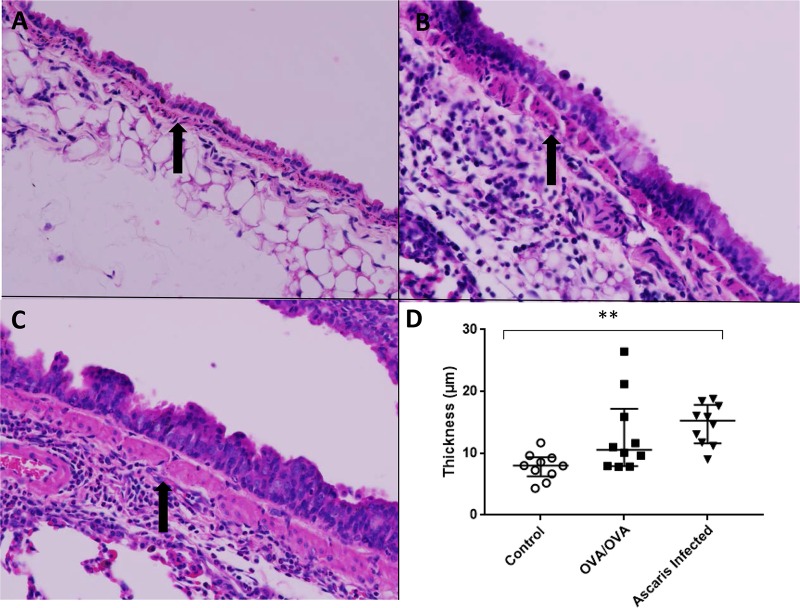
Histopathological differences were compared in negative controls, OVA/OVA mice, and Ascaris-infected mice (22). Ascaris-infected mice demonstrated the greatest inflammatory infiltrate within lung tissue, consisting mostly of neutrophils and eosinophils found in the perivascular and pleural spaces. Compared to negative controls (Fig. 6A), both OVA/OVA lung tissue (Fig. 6B) and Ascaris-infected lung tissue after a single infection (Fig. 6C) showed signs of early airway remodeling with siginificant goblet cell metaplasia. However, Ascaris-infected mice also had evidence of smooth muscle hypertrophy (7.85 ± 2.15 μm for negative controls, 12.94 ± 6.33 μm for OVA/OVA mice, and 14.64 ± 3.31 μm for Ascaris-infected mice; P < 0.01) (Fig. 7A to D), further demonstrating the profound impact of Ascaris infection on the lung tissue and suggesting lasting effects of a single infection on the host lung.
FIG 6.
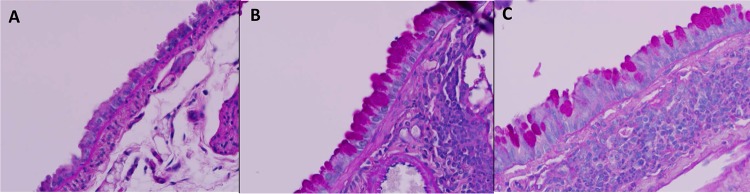
Goblet cell metaplasia and intracellular mucin production demonstrated by PAS staining in negative controls (A), OVA/OVA asthmatic mice (B), and Ascaris-infected mice (C) (magnification, ×500 under oil).
FIG 7.
(A to C) Increased bronchial muscular layer thickness in control mice (A) and OVA/OVA (B) and Ascaris-infected (C) mouse models (H&E; magnification, ×400). (D) Measurement of increased bronchial muscular layer thickness in Ascaris-infected mice compared to negative controls (n = 10 mice per group). **, P < 0.01 (means ± SD, as determined by a Kruskal-Wallis test in two different experiments).
DISCUSSION
The establishment of a mouse model of Ascaris infection has provided significant insight into end-organ damage endured during Ascaris infection. This study details for the first time that Ascaris infection, with resultant larval migration, can cause an extreme form of allergic airway disease compared to a standard disease model using OVA. Moreover, we have shown that after a single Ascaris infection, the resulting allergic airway disease extends weeks past the duration of the actual infection. Our study thus supports the clinical observations that children with Ascaris-induced asthma have more-profound allergic disease-related symptoms than children with conventional, non-parasite-associated type 2 asthmatic disease. This model will be useful in further dissecting the pathogenesis of Ascaris-induced allergic airway disease and the relationship between environmental parasites and allergen sensitization.
Asthma is a heterogeneous syndrome of intermittent wheezing, airway obstruction and remodeling, and type 2 inflammatory infiltration, affecting more than 300 million individuals, and is associated with significant mortality, morbidity, and economic consequences globally (23–26). Over the past 2 decades, allergic airway disease, an asthma phenotype, has become a major health threat in low- and middle-income countries, which had previously reported low levels of diseases (23, 27). A robust type 2 cytokine milieu comprising IL-4, IL-5, and IL-13 is central to the immune response in allergic airway disease (28). In conventional allergic airway disease phenotypes, IL-4 drives TH2 differentiation, immunoglobulin isotype switching to IgE in B cells, and activation of alveolar macrophages, while IL-5 promotes the growth, differentiation, and survival of eosinophils (28, 29). IL-13 has many effector functions in the pathogenesis of allergic airway disease, including goblet cell metaplasia, smooth muscle hypertrophy, and AHR (30). Additionally, inflammation, goblet cell metaplasia, and smooth muscle hypertrophy are evidence of airway remodeling in allergic airway disease (31). Similar to conventional allergic airway disease, helminth infections, like ascariasis, are known to induce a type 2 polarized immunological response (30, 32).
In the present study, levels of IL-4, IL-5, and IL-13 were elevated in the BAL fluid and lung tissue of Ascaris-infected mice, supporting the critical role of the type 2 inflammatory cascade during Ascaris larval migration in the lungs, similar to the cytokine environment observed in allergic airway disease. The elevation of IL-5 level was particularly notable and exceeded the levels in OVA/OVA-induced allergic airway disease by 10-fold or more. Likewise, the level of IL-13, a main driver in the pathogenesis of allergic airway disease, was significantly elevated compared to that in the OVA/OVA model. These type 2 cytokines, IL-5 and IL-13, are likely a major link to the severe allergic airway disease observed in Ascaris-infected mice. Conversely, the level of IL-10, produced largely by regulatory T cells (Tregs) and aiding immunomodulation during chronic Ascaris infection, was elevated only minimally in Ascaris-infected mice (32). As expected based on the polarized type 2 response in allergic airway disease and Ascaris-infected mice, the levels of type 1-specific cytokines were not elevated in any group. Other cell populations, including TH17 cells, may play an important role in the pathogenesis of allergic airway disease through the production and propagation of a neutrophilic response, the induction of Muc5ac hypersecretion, and the promotion of airway remodeling involving the activation of fibroblasts (33, 34). Interestingly, the level of IL-17, a key TH17 cytokine that has been correlated with the severity of allergic airway disease (34), was mildly elevated in Ascaris-infected mice. Given the role of IL-17 in enhancing the neutrophil response and promoting mucosal integrity, the presence of IL-17 may be a sign of tissue remodeling after Ascaris infection in the lungs.
Our studies also found a dramatic level of AHR in response to provocative challenge with Ach, a marker of clinically significant allergic airway disease (35), in Ascaris-infected mice that greatly exceeded that seen in the OVA/OVA model. In addition, Ascaris-infected mice had exaggerated goblet cell metaplasia and smooth muscle hypertrophy, suggesting early airway remodeling. Studies are under way to evaluate the pathogenesis of these persistent physiological and anatomical findings in the lung and whether they reflect the marked increases in IL-5 and IL-13 or some other pathogenic mechanism. It is possible that the etiology of this severe allergic airway phenotype is multifactorial, including both the host immune response to the larvae and retained larval proteins in the lung tissue in combination with significant tissue destruction secondary to larval migration and protease secretion.
In some clinical studies, ascariasis has been noted to be an independent risk factor for the development of childhood asthma in areas of endemicity (10, 12–14, 36). These clinical studies suggest that Ascaris-induced allergic airway disease in children is associated with more-severe disease as well as increased cross-reactivity to bystander antigens, like house dust mites, compared to conventional allergic airway disease (12–15, 36). The type 2 cytokine response as a result of larval migration, as described in this study, may have evolved to protect against helminth reinfection but simultaneously makes the host more susceptible to allergic airway disease (13, 15, 36, 37). Alternatively, other clinical studies suggest that Ascaris infection may protect against allergic disease (38–40). It has been postulated that ascariasis may inhibit allergic reactivity through the induction of high levels of polyclonal IgE, which saturate effector cells and suppress the response to specific environmental allergens, or through excretory and secretory immunomodulatory molecules, such as PAS-1, that exhibit anti-inflammatory properties (38, 41, 42). Direct comparisons of data from clinical studies evaluating the impact of ascariasis on the host lungs remain challenging secondary to inconsistent diagnostic capabilities in areas of endemicity as well as variation in study populations, such as rural versus urban environments, seasonality and frequency of infection, genetic predisposition, age of infection, and intensity of infection (10, 11, 40, 42, 43).
In response to conflicting clinical data, the use of animal models continues to provide significant insight into disease pathogenesis. Mouse models have demonstrated that repetitive infection with Ascaris causes significant inflammatory infiltrates, thickening of the intra-alveolar septa, and parenchyma destruction in the lung on day 8 p.i. (44). Likewise, after inhalation of an Ascaris suum extract, dogs have dyspnea, increased airway secretions, and enhanced bronchoconstriction (45), and nonhuman primates sensitized to Ascaris through natural exposure and subsequently administered an aerosolized A. suum antigen have increased airway resistance (46). The findings in these animal models, along with the evidence presented in the present study, further support the detrimental impact of ascariasis on the host lung.
The use of a mouse model inhibits the ability to fully understand the impact of helminths outside the larval migration stage. However, as this study was focused primarily on the impact of infection on the lung environment, the mouse model is equipped to evaluate the impact of larva migration through the lungs. However, it should be noted that there are differences in lung size as well as lung structure between mouse and human lungs, and thus, changes in the lung parenchyma as a result of larva migration might be enhanced in the mouse model. Additionally, A. suum was used in this study instead of the human pathogen A. lumbricoides. It is possible that A. suum may produce a more exaggerated response than what occurs in the highly adapted parasite-host relationship between humans and A. lumbricoides. However, both species are morphologically and biologically indistinguishable, considered to be distinct valid species based only on epidemiological observations and minor anatomical and genomic variances (47, 48). Furthermore, both Ascaris species have been found to be infective in human hosts (47) and thus would likely reproduce similar findings during larval migration in the host lung.
Diverse microbes have been linked to the development of allergic airway disease. Viruses causing early childhood bronchiolitis, such as respiratory syncytial virus (RSV), are independent risk factors for abnormal lung function and wheezing (49). Additionally, the impacts of other helminths on the host type 2 immune response leading to allergic airway disease have been evaluated. Toxocariasis, caused by a zoonotic helminth, is associated with human asthmatic disease globally (50). Mouse models of toxocariasis have demonstrated that infection leads to increased AHR and type 2 inflammatory infiltration similar to what was observed in the present study (51). The helminth Anisakis simplex is also a known inducer of human asthma. Anisakis-induced asthma is considered an occupational hazard for workers in the seafood-processing industry, in which the ingestion of larvae can cause severe allergic disease (52). Mouse models of Anisakis have shown that multiple Anisakis L3 allergens can induce type 2 allergic airway inflammation (53). While ascariasis, toxocariasis, and anisakiasis may be major drivers of childhood asthma in their respective regions of endemicity, it is important to recognize the possible variability among different helminth species. Other helminths, such as hookworms, may in fact have an inverse relationship with allergic airway disease (9, 10, 40, 43). Further studies dissecting the immunological mechanism of Ascaris-induced allergic airway disease may clarify why only subsets of migratory helminths cause allergic airway disease.
Conclusion.
In accordance with data from many epidemiological studies, our results add experimental support to suggest that ascariasis may be an important cause of allergic airway diseases in regions of endemicity around the world. Currently, the mechanism of Ascaris-induced allergic airway disease is unknown; however, the studies here indicate that larval invasion causes profound direct damage to the lung mucosa while at the same time activating a type 2 inflammatory cascade within the lung tissue (44). Such changes are associated with high levels of host IL-4, IL-5, and IL-13 production, early airway remodeling, and marked AHR that persists after the resolution of a single infection. While the inflammatory infiltrate and degree of AHR may decrease gradually with time, it is important to highlight that children living in regions of endemicity are continuously reinfected with Ascaris eggs, leading to successive waves of migrating larvae throughout their lifetimes. In the setting of this permanent exposure, children will continue to mount an allergic inflammatory response with each subsequent wave of larval migration. Future studies aimed at understanding the mechanism of Ascaris-induced allergic airway disease as well as the impact of successive infections will aid in uncovering the clinical impact of pulmonary ascariasis in children and identifying small molecules and antigenic targets for the development of preventative and therapeutic interventions that are urgently needed. Furthermore, understanding the mechanism of exaggerated microbe-induced allergic airway disease, as seen with ascariasis, may not only answer key questions regarding type 2 immune responses that drive allergic airway phenotypes but also provide insight into other microbe-induced lung diseases that extract profound global morbidity.
MATERIALS AND METHODS
Mice.
Wild-type BALB/c mice (female, 6 weeks of age) were purchased from Taconic (Hudson, NY, USA). Mouse models of allergic airway disease using an ovalbumin (OVA) sensitization and OVA challenge regimen, designated OVA/OVA, commonly use female mice to ensure adequate and consistent airway responsiveness (54, 55). For consistency between the models, female mice were used in all groups throughout the study. Mice were housed in groups of 5, in standard bedded cages, under biosafety level 2 (BSL-2) conditions, in accordance with recommendations of the Center for Comparative Medicine at Baylor College of Medicine. This study was carried out in accordance with the recommendations and approval of the Baylor College of Medicine Institutional Animal Care and Use Committee (approvals AN-6297 and AN-1819).
Experimental model of Ascaris infection.
Embryonated A. suum eggs were obtained from the Departamento de Parasitologia, Universidade Federal de Minas Gerais, Brazil, during peak infectivity between the 100th and 200th days of incubation (56). A mouse model for ascariasis using Ascaris suum, the etiological agent of porcine ascariasis, was described in detail previously (56). Mice are nonpermissive hosts for chronic ascariasis in which adult worm maturation does not occur in the gut as it does in humans and pigs. However, A. suum completes a 14-day life cycle in the mouse model, with larval migration after oral ingestion through the intestines, liver, and lungs prior to fecal elimination. Ascaris larvae initially penetrate lung tissue on day 3 postinfection (p.i.), reaching peak penetration on days 7 to 8 p.i., with peak lung inflammatory infiltration on day 12 p.i. (44, 56, 57). The larvae ascend the bronchial tree and are swallowed and subsequently eliminated from the mouse without developing into adult worms by day 14 p.i. (56). To determine extent of infection, larvae are counted within the lung tissue, commonly at days 7 to 8 p.i., with approximately 3.2% of the initial embryonated egg inoculum being recovered from the lungs in BALB/c mice (57). A standard 2,500 fully embryonated A. suum eggs, incubated in 0.2 N H2SO4 for approximately 150 days, were administered by oral gavage to allow the most consistent larva recovery from lungs (56). On day 8 p.i., the mean number of larvae recovered from the lungs of each mouse was 56.3, approximately 2.2% of the initial inoculum of 2,500 A. suum eggs.
Experimental model of allergic airway disease.
A standard chicken egg OVA (grade V; Sigma Chemical Company, St. Louis, MO)-sensitized, OVA-challenged mouse model was used as a positive control to evaluate allergic airway disease. Mice in the OVA/OVA allergic airway disease control groups received 50 μl OVA (2.5 mg/5 ml) adsorbed to a 10% alum solution in pyrogen-free distilled water (0.5 g/5 ml) intraperitoneally weekly for 3 weeks (on days 0, 7, and 14). Mice were intranasally challenged with OVA at 50 μl (500 μg/ml) under isoflurane sedation for three consecutive days (on days 21, 22, and 23). Subsequent studies of OVA/OVA mice, including plethysmography, euthanasia, and necropsy, were completed on day 24. The time course was based on the standard OVA/OVA model protocol (58) and thus would be a reasonable comparison to the Ascaris model.
Measurement of AHR.
Mice were anesthetized using etomidate for physiological evaluation of AHR using whole-body plethysmography. The trachea was cannulated with an angiocatheter and connected to a rodent ventilator. A venous catheter was placed in the lateral tail vein, and intravenous placement was confirmed. Five serial doses of acetylcholine (Ach), the natural agonist for airway smooth muscle M3 muscarinic receptors, were administered intravenously through the tail vein catheter for provocation challenge. Airway resistance was measured with in-line pressure and flow transducers (35). Respiratory system resistance (RRS) was determined through the quantitation of the change in the tracheal pressure divided by airflow (DPt/V) (35). A baseline RRS value was established, followed by increasing serial doses of Ach at 0.058, 0.18, 0.59, 1.58, and 5.8 mg/kg of body weight in saline. After each administered dose, the RRS returned to baseline prior to the administration of the next dose. The mice were subsequently euthanized, and bronchoalveolar lavage (BAL) fluid and lung tissue were collected during necropsy.
Collection and analysis of bronchoalveolar lavage fluid and lung tissue.
The angiocatheter was maintained in the airway, and 800 μl of PBS was flushed into the lung via a syringe and retracted for the removal of BAL fluid while maintaining airway patency (35). The procedure was repeated once, followed by the removal of the angiocatheter. BAL fluid was centrifuged, and the supernatant was collected and placed in a −80°C freezer until further use.
The right lung from each mouse was removed and placed into a strainer in complete RPMI (cRPMI) (cell culture medium consisting of RPMI 1640 plus l-glutamine, 10% fetal bovine serum [FBS], and 10 mM penicillin-streptomycin) in a 6-well culture plate. For each sample, the lung tissue was macerated, the strainer was removed and placed on a 50-ml conical tube, and the cell culture plate was washed with cRPMI. The collected wash specimen from the 6-well culture plate was placed back through the strainer on the 50-ml conical tube, which was centrifuged. The resultant supernatant was discarded, and the pellet was resuspended in cRPMI. Resuspended samples were incubated at 37°C in 5% CO2 for 24 h. The supernatant was then collected and placed in a −80°C freezer until further use for cytokine quantitation.
BAL fluid and lung tissue supernatants were thawed, and cytokine levels were quantitated by using a Bio-Rad (Hercules, CA) Pro mouse cytokine TH1/TH2 (IFN-γ, IL-4, IL-5, IL-10, IL-13, IL-17, and TNF-α) kit with 96-well DropArray DA-bead plates and a DropArray LT210 washing station MX instrument from Curiox (San Carlos, CA), as previously described (59). The results were acquired on a Luminex Magpix instrument using Bio-Plex MP and Bio-Plex Manager software.
Histopathological evaluation.
After removal of the right lung, the left lung was inflated and fixed in a 10% neutral buffered formalin solution. The tissue was subsequently embedded in paraffin and stained with hematoxylin and eosin (H&E) for evaluation of cellular infiltration and epithelium damage and with periodic acid-Schiff (PAS) stain to evaluate goblet cell metaplasia. The grading scale used to compare groups was adapted from the one described previously by Wachtel et al. (22).
Statistical analysis.
Differences between 2 groups were assessed by the Mann-Whitney test. Differences between 3 groups were assessed by a Kruskal-Wallis test. If statistical significance was achieved using the Kruskal-Wallis test, post hoc Dunn’s multiple-comparison test was performed, and results are presented in the representative figures. Data are represented as means ± standard deviations (SD). Tests were considered statistically significant if the P value was <0.05. Data analyses were performed by the use of GraphPad Prism software version 7.00 (GraphPad, La Jolla, CA).
ACKNOWLEDGMENTS
This study was supported by the Michelson Medical Research Foundation, NIH grant T32 AI055413, and Baylor College of Medicine Pathology and Histology Cancer Center grant P30 CA125123. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Angela Major assisted with preparation and staining of histopathology samples for all studies.
Several of the authors are involved in the development of a vaccine to prevent ascariasis.
REFERENCES
- 1.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. 2016. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388:1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pullan RL, Smith JL, Jasrasaria R, Brooker SJ. 2014. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasit Vectors 7:37. doi: 10.1186/1756-3305-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weatherhead JE, Hotez PJ. 2015. Worm infections in children. Pediatr Rev 36:341–352. doi: 10.1542/pir.36-8-341. [DOI] [PubMed] [Google Scholar]
- 4.Hotez PJ, Bundy DAP, Beegle K, Brooker S, Drake L, de Silva N, Montresor A, Engels D, Jukes M, Chitsulo L, Chow J, Laxminarayan R, Michaud C, Bethony J, Correa-Oliveira R, Shuhua X, Fenwick A, Savioli L. 2006. Helminth infections: soil-transmitted helminth infections and schistosomiasis, p 467–482. In Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, Musgrove P (ed), Disease control priorities in developing countries, 2nd ed Oxford University Press, New York, NY. [Google Scholar]
- 5.GBD 2015 Mortality and Causes of Death Collaborators. 2016. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388:1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miguel E, Kremer M. 2004. Worms: identifying impacts on education and health in the presence of treatment externalities. Econometrica 72:159–217. doi: 10.1111/j.1468-0262.2004.00481.x. [DOI] [Google Scholar]
- 7.Akuthota P, Weller PF. 2012. Eosinophilic pneumonias. Clin Microbiol Rev 25:649–660. doi: 10.1128/CMR.00025-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahalanabis D, Jalan KN, Maitra TK, Agarwal SK. 1976. Vitamin A absorption in ascariasis. Am J Clin Nutr 29:1372–1375. doi: 10.1093/ajcn/29.12.1372. [DOI] [PubMed] [Google Scholar]
- 9.Briggs N, Weatherhead J, Sastry KJ, Hotez PJ. 2016. The hygiene hypothesis and its inconvenient truths about helminth infections. PLoS Negl Trop Dis 10:e0004944. doi: 10.1371/journal.pntd.0004944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leonardi-Bee J, Pritchard D, Britton J. 2006. Asthma and current intestinal parasite infection: systematic review and meta-analysis. Am J Respir Crit Care Med 174:514–523. doi: 10.1164/rccm.200603-331OC. [DOI] [PubMed] [Google Scholar]
- 11.Gelpi AP, Mustafa A. 1967. Seasonal pneumonitis with eosinophilia. A study of larval ascariasis in Saudi Arabs. Am J Trop Med Hyg 16:646–657. doi: 10.4269/ajtmh.1967.16.646. [DOI] [PubMed] [Google Scholar]
- 12.Buendía E, Zakzuk J, Mercado D, Alvarez A, Caraballo L. 2015. The IgE response to Ascaris molecular components is associated with clinical indicators of asthma severity. World Allergy Organ J 8:8. doi: 10.1186/s40413-015-0058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takeuchi H, Khan AF, Yunus M, Hasan MI, Delwer M, Hawlader H, Takanashi S, Kano H, Zaman K, Chowdhury HR, Wagatsuma Y, Nakahara S, Iwata T. 2016. Anti-Ascaris immunoglobulin E associated with bronchial hyper-reactivity in 9-year-old rural Bangladeshi children. Allergol Int 65:141–146. doi: 10.1016/j.alit.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Da Silva ER, Sly PD, De Pereira MU, Pinto LA, Jones MH, Pitre PM, Stein RT. 2008. Intestinal helminth infestation is associated with increased bronchial responsiveness in children. Pediatr Pulmonol 43:662–665. doi: 10.1002/ppul.20833. [DOI] [PubMed] [Google Scholar]
- 15.Hagel I, Cabrera M, Hurtado MA, Sanchez P, Puccio F, Di Prisco MC, Palenque M. 2007. Infection by Ascaris lumbricoides and bronchial hyper reactivity: an outstanding association in Venezuelan school children from endemic areas. Acta Trop 103:231–241. doi: 10.1016/j.actatropica.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz C, Hams E, Fallon PG. 2018. Helminth modulation of lung inflammation. Trends Parasitol 34:388–403. doi: 10.1016/j.pt.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Chen F, Liu Z, Wu W, Rozo C, Bowdridge S, Millman A, Van Rooijen N, Urban JF, Wynn TA, Gause WC. 2012. An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nat Med 18:260–266. doi: 10.1038/nm.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen JE, Sutherland TE. 2014. Host protective roles of type 2 immunity: parasite killing and tissue repair, flip sides of the same coin. Semin Immunol 26:329–340. doi: 10.1016/j.smim.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonne-Anneé S, Hess JA, Abraham D. 2011. Innate and adaptive immunity to the nematode Strongyloides stercoralis in a mouse model. Immunol Res 51:205–214. doi: 10.1007/s12026-011-8258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeda H, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura SI, Nakanishi K, Yoshida N, Kishimoto T, Akira S. 1996. Essential role of Stat6 in IL-4 signalling. Nature 380:627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 21.Urban JF Jr, Noben-Trauth N, Donaldson DD, Madden KB, Morris SC, Collins M, Finkelman FD. 1998. IL-13, IL-4Rα, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity 8:255–264. doi: 10.1016/S1074-7613(00)80477-X. [DOI] [PubMed] [Google Scholar]
- 22.Wachtel MS, Shome G, Sutherland M, McGlone JJ. 2009. Derivation and validation of murine histologic alterations resembling asthma, with two proposed histologic grade parameters. BMC Immunol 10:58. doi: 10.1186/1471-2172-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marks G, Pearch N, Strachan IAD. 2014. The global asthma report 2014. Global Asthma Network, Auckland, New Zealand. [Google Scholar]
- 24.Porter P, Susarla SC, Polikepahad S, Qian Y, Hampton J, Kiss A, Vaidya S, Sur S, Ongeri V, Yang T, Delclos GL, Abramson S, Kheradmand F, Corry DB. 2009. Link between allergic asthma and airway mucosal infection suggested by proteinase-secreting household fungi. Mucosal Immunol 2:504–517. doi: 10.1038/mi.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Millien VO, Lu W, Shaw J, Yuan X, Mak G, Roberts L, Song L-Z, Knight JM, Creighton CJ, Luong A, Kheradmand F, Corry DB. 2013. Cleavage of fibrinogen by proteinases elicits allergic responses through Toll-like receptor 4. Science 341:792–796. doi: 10.1126/science.1240342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez-Gonzalez I, Steer CA, Takei F. 2015. Lung ILC2s link innate and adaptive responses in allergic inflammation. Trends Immunol 36:189–195. doi: 10.1016/j.it.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Hawlader MDH, Ma E, Noguchi E, Itoh M, Arifeen SE, Persson LÅ, Moore SE, Raqib R, Wagatsuma Y. 2014. Ascaris lumbricoids [sic] infection as a risk factor for asthma and atopy in rural Bangladeshi children. Trop Med Health 42:77–85. doi: 10.2149/tmh.2013-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu J. 2015. T helper 2 (Th2) cell differentiation, type 2 innate lymphoid cell (ILC2) development and regulation of interleukin-4 (IL-4) and IL-13 production. Cytokine 75:14–24. doi: 10.1016/j.cyto.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li BWS, Beerens DMJM, Brem MD, Hendriks RW. 2017. Characterization of group 2 innate lymphoid cells in allergic airway inflammation models in the mouse. Methods Mol Biol 1559:169–183. doi: 10.1007/978-1-4939-6786-5_12. [DOI] [PubMed] [Google Scholar]
- 30.Robinson DM, Humbert R, Buhl AA, Cruz H, Inoue S, Korom NA, Hanania PN. 2017. Revisiting type 2-high and type 2-low airway inflammation in asthma: current knowledge and therapeutic implications. Clin Exp Allergy 47:161–175. doi: 10.1111/cea.12880. [DOI] [PubMed] [Google Scholar]
- 31.Bergeron C, Al-Ramli W, Hamid Q. 2009. Remodeling in asthma. Proc Am Thorac Soc 6:301–305. doi: 10.1513/pats.200808-089RM. [DOI] [PubMed] [Google Scholar]
- 32.Nutman TB. 2015. Looking beyond the induction of Th2 responses to explain immunomodulation by helminths. Parasite Immunol 37:304–313. doi: 10.1111/pim.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cosmi L, Liotta F, Annunziato F. 2016. Th17 regulating lower airway disease. Curr Opin Allergy Clin Immunol 16:1–6. doi: 10.1097/ACI.0000000000000227. [DOI] [PubMed] [Google Scholar]
- 34.Borish L. 2016. The immunology of asthma: asthma phenotypes and their implications for personalized treatment. Ann Allergy Asthma Immunol 117:108–114. doi: 10.1016/j.anai.2016.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Polikepahad S, Barranco WT, Porter P, Anderson B, Kheradmand FCD. 2010. A reversible, non-invasive method for airway resistance measurements and bronchoalveolar lavage fluid sampling in mice. J Vis Exp 2010:1720. doi: 10.3791/1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palmer LJ, Celedón JC, Weiss ST, Wang B, Fang Z, Xu X. 2002. Ascaris lumbricoides infection is associated with increased risk of childhood asthma and atopy in rural China. Am J Respir Crit Care Med 165:1489–1493. doi: 10.1164/rccm.2107020. [DOI] [PubMed] [Google Scholar]
- 37.Caraballo L, Acevedo N, Buendía E. 2015. Human ascariasis increases the allergic response and allergic symptoms. Curr Trop Med Rep 2:224–232. doi: 10.1007/s40475-015-0058-7. [DOI] [Google Scholar]
- 38.Di Lorenzo G, Pacor ML, Mansueto P, Esposito-Pellitteri M, Scichilone N, Ditta V, Lo Bianco C, Leto-Barone MS, Di Fede G, Corrocher R, Mansueto S, Rini GB. 2006. Relationship between specific serum IGE to Ascaris lumbricoides and onset of respiratory symptoms in Bangladesh immigrants. Int J Immunopathol Pharmacol 19:629–638. doi: 10.1177/039463200601900319. [DOI] [PubMed] [Google Scholar]
- 39.Cardoso LS, Costa DM, Almeida MCF, Souza RP, Carvalho EM, Araujo MI, Oliveira RR. 2012. Risk factors for asthma in a helminth endemic area in Bahia, Brazil. J Parasitol Res 2012:796820. doi: 10.1155/2012/796820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zakzuk J, Casadiego S, Mercado A, Alvis-Guzman N, Caraballo L. 2018. Ascaris lumbricoides infection induces both, reduction and increase of asthma symptoms in a rural community. Acta Trop 187:1–4. doi: 10.1016/j.actatropica.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 41.Hagel I, Cabrera M, Puccio F, Santaella C, Buvat E, Infante B, Zabala M, Cordero R, Di Prisco MC. 2011. Co-infection with Ascaris lumbricoides modulates protective immune responses against Giardia duodenalis in school Venezuelan rural children. Acta Trop 117:189–195. doi: 10.1016/j.actatropica.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 42.Caraballo L. 2018. The tropics, helminth infections and hygiene hypotheses. Expert Rev Clin Immunol 14:99–102. doi: 10.1080/1744666X.2018.1424543. [DOI] [PubMed] [Google Scholar]
- 43.Cruz AA, Cooper PJ, Figueiredo CA, Alcantara-Neves NM, Rodrigues LC, Barreto ML. 2017. Global issues in allergy and immunology: parasitic infections and allergy. J Allergy Clin Immunol 140:1217–1228. doi: 10.1016/j.jaci.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 44.Nogueira DS, Gazzinelli-Guimarães PH, Barbosa FS, Resende NM, Silva CC, de Oliveira LM, Amorim CCO, Oliveira FMS, Mattos MS, Kraemer LR, Caliari MV, Gaze S, Bueno LL, Russo RC, Fujiwara RT. 2016. Multiple exposures to Ascaris suum induce tissue injury and mixed Th2/Th17 immune response in mice. PLoS Negl Trop Dis 10:e0004382. doi: 10.1371/journal.pntd.0004382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamatake Y, Sasagawa S, Yanaura S, Kobayashi N. 1977. Allergy induced asthma with Ascaris suum administration to dogs. Jpn J Pharmacol 27:285–293. doi: 10.1254/jjp.27.285. [DOI] [PubMed] [Google Scholar]
- 46.Bree A, Schlerman FJ, Wadanoli M, Tchistiakova L, Marquette K, Tan X-Y, Jacobson BA, Widom A, Cook TA, Wood N, Vunnum S, Krykbaev R, Xu X, Donaldson DD, Goldman SJ, Sypek J, Kasaian MT. 2007. IL-13 blockade reduces lung inflammation after Ascaris suum challenge in cynomolgus monkeys. J Allergy Clin Immunol 119:1251–1257. doi: 10.1016/j.jaci.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 47.de Silva Alves EB, Conceição MJ, Leles D. 2016. Ascaris lumbricoides, Ascaris suum, or “Ascaris lumbrisuum”? J Infect Dis 213:1355. doi: 10.1093/infdis/jiw027. [DOI] [PubMed] [Google Scholar]
- 48.Liu G-H, Wu C-Y, Song H-Q, Wei S-J, Xu M-J, Lin R-Q, Zhao G-H, Huang S-Y, Zhu X-Q. 2012. Comparative analyses of the complete mitochondrial genomes of Ascaris lumbricoides and Ascaris suum from humans and pigs. Gene 492:110–116. doi: 10.1016/j.gene.2011.10.043. [DOI] [PubMed] [Google Scholar]
- 49.Wu P, Hartert TV. 2012. Evidence for a causal relationship between respiratory syncytial virus infection and asthma. Expert Rev Anti Infect Ther 9:731–745. doi: 10.1586/eri.11.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walsh MG. 2011. Toxocara infection and diminished lung function in a nationally representative sample from the United States population. Int J Parasitol 41:243–247. doi: 10.1016/j.ijpara.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 51.Pinelli E, Withagen C, Fonville M, Verlaan A, Dormans J, van Loveren H, Nicoll G, Maizels RM, van der Giessen J. 2005. Persistent airway hyper-responsiveness and inflammation in Toxocara canis-infected BALB/c mice. Clin Exp Allergy 35:826–832. doi: 10.1111/j.1365-2222.2005.02250.x. [DOI] [PubMed] [Google Scholar]
- 52.Audicana MT, Kennedy MW. 2008. Anisakis simplex: from obscure infectious worm to inducer of immune hypersensitivity. Clin Microbiol Rev 21:360–379. doi: 10.1128/CMR.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cho MK, Park MK, Kang SA, Caballero ML, Perez-Pinar T, Rodriguez-Perez R, Ock MS, Cha HJ, Hong YC, Yu HS. 2014. Allergenicity of two Anisakis simplex allergens evaluated in vivo using an experimental mouse model. Exp Parasitol 146:71–77. doi: 10.1016/j.exppara.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 54.Takeda M, Tanabe M, Ito W, Ueki S, Konnno Y, Chihara M, Itoga M, Kobayashi Y, Moritoki Y, Kayaba HCJ. 2013. Gender difference in allergic airway remodelling and immunoglobulin production in mouse model of asthma. Respirology 18:797–806. doi: 10.1111/resp.12078. [DOI] [PubMed] [Google Scholar]
- 55.Bonnegarde-Bernard A, Jee J, Fial MJ, Steiner H, DiBartola S, Davis IC, Cormet-Boyaka E, Tomé DBP. 2014. Routes of allergic sensitization and myeloid cell IKKβ differentially regulate antibody responses and allergic airway inflammation in male and female mice. PLoS One 9:e392307. doi: 10.1371/journal.pone.0092307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gazzinelli-Guimarães PH, Gazzinelli-Guimarães AC, Silva FN, Mati VLT, Dhom-Lemos LDC, Barbosa FS, Passos LSA, Gaze S, Carneiro CM, Bartholomeu DC, Bueno LL, Fujiwara RT. 2013. Parasitological and immunological aspects of early Ascaris spp. infection in mice. Int J Parasitol 43:697–706. doi: 10.1016/j.ijpara.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 57.Lewis R, Behnke JM, Cassidy JP, Stafford P, Murray N, Holland CV. 2007. The migration of Ascaris suum larvae, and the associated pulmonary inflammatory response in susceptible C57BL/6j and resistant CBA/Ca mice. Parasitology 134:1301. doi: 10.1017/S0031182007002582. [DOI] [PubMed] [Google Scholar]
- 58.Evans KLJ, Bond RA, Corry DB, Shardonofsky FR. 2003. Frequency dependence of respiratory system mechanics during induced constriction in a murine model of asthma. J Appl Physiol 94:245–252. doi: 10.1152/japplphysiol.00593.2002. [DOI] [PubMed] [Google Scholar]
- 59.Versteeg L, Le Guezennec X, Zhan B, Liu Z, Angagaw M, Woodhouse JD, Biswas S, Beaumier CM. 2017. Transferring Luminex cytokine assays to a wall-less plate technology: validation and comparison study with plasma and cell culture supernatants. J Immunol Methods 440:74–82. doi: 10.1016/j.jim.2016.11.003. [DOI] [PubMed] [Google Scholar]