The Vibrio cholerae O1 serogroup is responsible for pandemic cholera and is divided into the classical and El Tor biotypes. Classical V. cholerae produces acid when using glucose as a carbon source, whereas El Tor V. cholerae produces the neutral product acetoin when using glucose as a carbon source.
KEYWORDS: Vibrio cholerae, cholera, probiotics, zebrafish
ABSTRACT
The Vibrio cholerae O1 serogroup is responsible for pandemic cholera and is divided into the classical and El Tor biotypes. Classical V. cholerae produces acid when using glucose as a carbon source, whereas El Tor V. cholerae produces the neutral product acetoin when using glucose as a carbon source. An earlier study demonstrated that Escherichia coli strains that metabolize glucose to acidic by-products drastically reduced the survival of V. cholerae strains in vitro. In the present study, zebrafish were fed 1% glucose and either inoculated with single V. cholerae or E. coli strains or coinfected with both V. cholerae and E. coli. A significant decrease in classical biotype colonization was observed after glucose feeding due to acid production in the zebrafish intestine. El Tor colonization was unaffected by glucose alone. However, the El Tor strain exhibited significantly lower colonization of the zebrafish when either of the acid-producing E. coli strains was coinoculated in the presence of glucose. An E. coli sugar transport mutant had no effect on V. cholerae colonization even in presence of glucose. Glucose and E. coli produced a prophylactic effect on El Tor colonization in zebrafish when E. coli was inoculated before V. cholerae infection. Thus, the probiotic feeding of E. coli inhibits V. cholerae colonization in a natural host. This suggests that a similar inhibitory effect could be seen in cholera patients, especially if a glucose-based oral rehydration solution (ORS) is administered in combination with probiotic E. coli during cholera treatment.
INTRODUCTION
Cholera is a severe acute watery diarrhea of humans that is caused by Vibrio cholerae and is a major problem in developing countries (1). More than 200 serogroups of Vibrio cholerae have been identified as causative agents of human diarrhea. Of these, serogroups O1 and O139 are the main causes of epidemic cholera (2). Despite large gains in collective understanding and decades of research, cholera remains a large burden on human health resources worldwide. For example, in 2010 and 2017, 700,000 Haitians and over a million Yemenis, respectively, were infected with cholera during particularly severe epidemics (3–5). WHO estimates that over 3 million people contract cholera annually, leading to over 100,000 deaths. According to the U.S. CDC, administration of oral rehydration solution (ORS) is the preferred treatment course for cholera (6). Vaccines may be of use during an outbreak to limit the spread of cholera but cannot aid patients who have already contracted the disease. The use of antibiotics to treat cholera is also common but is less preferable because rampant use of antibiotics leads to multidrug resistance (MDR) (7–9).
Every reported cholera pandemic has been caused by strains of the O1 serogroup of V. cholerae that can be classified into two major biotypes, classical and El Tor. In recent years, the O1 serogroup El Tor biotype has been the major disease-causing biotype, replacing the previously predominant classical biotype (10). The Ogawa serotype of the El Tor biotype was most common in past years, but recently the Inaba serotype was also reported to cause outbreaks in India (11). In the presence of exogenous sugars (such as glucose), the classical biotype strains (e.g., O395) produce organic acids, resulting in a sharp decrease in medium pH and drastic loss of viability. El Tor strains (e.g., N16961) have evolved to metabolize sugars to produce acetoin, a neutral fermentation end product that does not inhibit bacterial growth (12). It has been suggested that the ability to metabolize sugars without production of growth-inhibitory acidic products might account for the increased evolutionary fitness of the V. cholerae El Tor biotype, leading to displacement of the classical biotype as the predominant cause of epidemic cholera (12). In previous work, V. cholerae classical and El Tor biotype strains were cocultured in the presence of glucose with Escherichia coli strains that produce acidic by-products of glucose metabolism. The hypothesis was that this would circumvent the mechanisms used by El Tor V. cholerae to prevent acidification and loss of viability. The results of that study showed that E. coli strains that metabolize glucose to acidic compounds significantly reduced V. cholerae survival in vitro (13).
Previously, we described the use of zebrafish as a novel animal model for the study of V. cholerae colonization and transmission. There are many advantages to using zebrafish as a V. cholerae model. The natural habitat of zebrafish in Asia broadly overlaps areas of cholera endemicity, strongly suggesting that there is an interaction between zebrafish and V. cholerae in the wild (14). Fish are natural V. cholerae hosts, and V. cholerae rapidly colonizes the zebrafish intestine after exposure by immersion in inoculated water. This colonization occurs in the presence of intact, mature intestinal microbiota and results in production of diarrhea, which can transmit V. cholerae to naive fish. Thus, zebrafish provide a model in which the entire V. cholerae infectious cycle can be studied in a natural host (15). Here, we developed a glucose feeding model of zebrafish and studied the effect of acid-producing E. coli strains on V. cholerae colonization in the presence of glucose in the zebrafish intestine. Our results suggest that probiotic E. coli can indeed reduce the bacterial load of V. cholerae in a natural host.
RESULTS
Glucose content in the fish intestine.
To understand the effect of glucose on V. cholerae colonization in zebrafish intestine, it first had to be established that enough glucose would be present in the zebrafish intestine. To accomplish this, fish were fed glucose by addition to water. Glucose (1%) was dissolved in fish infection water (autoclaved fish housing system water), and fish were kept in the glucose-water for 6, 12, or 24 h. A semiquantitative assay with Benedict’s solution was done with the fish intestinal homogenate to roughly quantify the amount of glucose present in the fish gut. Depending on the color and appearance of the precipitate (ppt) after boiling intestinal homogenate with Benedict’s solution, we found 0 to 2 mg, 2 to 5 mg, and 5 to 9 mg of glucose per gut after 6 h, 12 h, and 24 h of glucose feeding, respectively (n = 8) (Table 1; Fig. 1). However, one out of eight fish died during the 24-h glucose feeding. Therefore, we did our subsequent colonization experiments using 12 h of glucose feeding.
TABLE 1.
Glucose content of fish intestine
| Sample | Color and appearance of ppt or solution | Glucose content |
|---|---|---|
| Negative control (1× PBS) | Blue transparent solution | 0 |
| Positive control (1% glucose solution) | Brick red ppt | 1% (wt/vol) |
| Negative working control (fish gut without glucose feeding) | Blue cloudy solution | 0 |
| Fish gut with glucose feeding for: | ||
| 6 h | Blue-green solution | 0–2 mg/gut |
| 12 h | Green ppt | 2–5 mg/gut |
| 24 h | Green to yellow-green ppt | 5–9 mg/gut |
FIG 1.
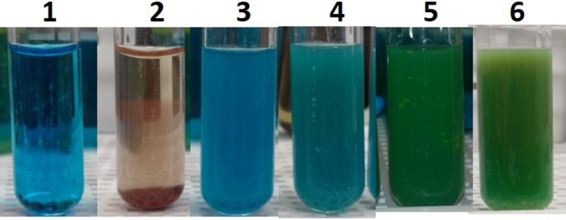
Glucose content in zebrafish intestine after 12 h of 1% glucose feeding. One percent glucose was dissolved in autoclaved fish tank water. Five fish were kept in the water for 6, 12, and 24 h of glucose feeding. Fish intestines were homogenized, heated with Benedict’s solution in boiling water, and observed for color formation. Samples were 1× PBS (tube 1), 1% glucose (tube 2), control fish intestine without glucose feeding (tube 3), and fish intestine with 6 h (tube 4), 12 h (tube 5), and 24 h (tube 6) of glucose feeding. The results shown are representative of multiple experiments conducted. The content of glucose in fish intestine is listed in Table 1.
Effect of glucose on V. cholerae colonization in zebrafish.
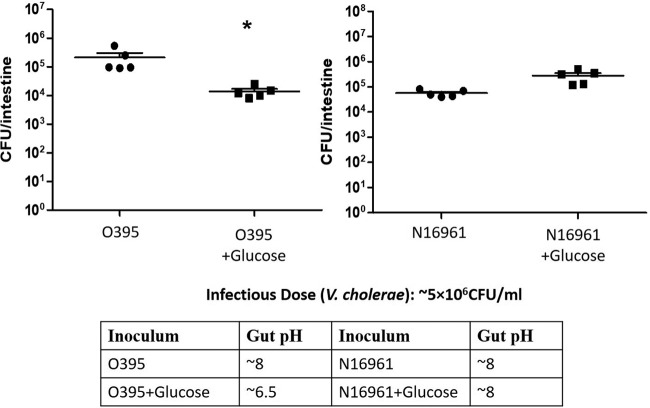
As observed previously when glucose was present in the medium in vitro, the growth of classical V. cholerae strain O395 was severely inhibited in Luria broth medium supplemented with 1% glucose (LBG) (references 12 and 13 and data not shown). Classical biotype V. cholerae produces acidic by-products of glucose metabolism, leading to a pH drop. V. cholerae El Tor biotype strain N16961, which metabolizes glucose to the neutral product acetoin, grew normally in LBG (12, 13). To determine whether these in vitro results could be recapitulated in a fish infection model, zebrafish were bathed in water containing glucose prior to exposure to V. cholerae. When zebrafish were infected with V. cholerae classical strain O395 after 12 h of glucose feeding, we observed a 30-fold decrease in colonization compared to O395 colonization of fish without glucose feeding (n = 5; P = 0.005). When zebrafish were infected with V. cholerae El Tor strain N16961 after 12 h of glucose feeding, we observed an ∼5-fold increase in colonization compared to N16961 colonization of fish without glucose feeding (n = 5; P = 0.0636) (Fig. 2). The pH of the fish intestinal homogenates was assessed using pH paper to obtain an approximate pH. We observed acidic intestinal homogenate (pH ∼6.5) in glucose-treated, O395-infected fish. We observed no differences in pH between glucose-treated, N16961-infected fish and control fish without glucose feeding that were infected with either O395 or N16961 (pH ∼8) (Fig. 2). These results suggested that glucose feeding reduced the colonization of classical biotype V. cholerae due to acid production in the fish intestine but did not hinder the colonization of El Tor V. cholerae because no acidic compounds were produced.
FIG 2.
Effect of glucose feeding on classical and El Tor V. cholerae colonization of zebrafish intestine. Zebrafish were fed 1% glucose for 12 h, and ∼5 × 106 CFU/ml of either V. cholerae O395 or N16961 was inoculated in zebrafish. V. cholerae levels were determined by plating of serial dilutions of the intestinal homogenates. *, P = 0.005.
Effect of E. coli strains on V. cholerae colonization in the presence or absence of glucose in the zebrafish intestine.
As observed above, glucose feeding to zebrafish can reduce the colonization of classical V. cholerae strains but not El Tor V. cholerae strains. El Tor strains have evolved to metabolize sugars to produce acetoin, a neutral fermentation end product. A previous study found two E. coli human isolate strains (see Table S1 in the supplemental material) that produce acids from glucose under in vitro culture conditions (13). In coculture with N16961 in LBG, E. coli strains produce acid and reduce N16961 growth. We hypothesized that this effect of E. coli plus glucose may be useful to reduce the V. cholerae load during infection.
To assess the effects of E. coli strains on V. cholerae colonization of zebrafish in the presence of glucose, the first step was to establish the colonization efficacy of these two E. coli strains in zebrafish intestine. Fish were inoculated for 6 h with E. coli by bath as described above for V. cholerae, and then the fish were washed to remove external bacteria and incubated in fresh water for 18 h. Both E. coli strains were found to abundantly colonize zebrafish intestines (n = 5) (see Fig. S1 in the supplemental material). E. coli colonization and acid production in the presence of glucose were determined by feeding fish 1% glucose for 12 h and then inoculating with E. coli for 6 h. Colonization was assessed at 6 h, 12 h, 24 h, 48 h, and 72 h after E. coli inoculation. We observed abundant colonization by all three E. coli strains tested, i.e., E. coli 40, E. coli N, and an E. coli ΔptsG (sugar transport) mutant, up to 72 h after inoculation (n = 5) (see Fig. S2A in the supplemental material). A low pH of fish intestinal homogenates was observed with colonization of E. coli 40 and E. coli N but not with the E. coli ΔptsG (see Table S2 in the supplemental material). If the fish were fed a single dose of glucose before E. coli inoculation, the intestinal pH was restored to normal levels after 24 h. If the fish were fed 1% glucose daily for 6 h, the low pH of fish intestinal homogenates was maintained throughout the colonization period up to 72 h (Table S2). The mucin level in excreted water, which is used as a measurement of diarrhea induced by V. cholerae colonization (16), was measured after 24 h of colonization with all three E. coli (40, N, and ΔptsG)-infected fish groups. There was a nonsignificant increase of mucin secretion by the three E. coli-infected groups compared to the uninfected control group (Fig. S2B). The effective optical density (OD) was calculated by subtraction of the OD of phosphate-buffered saline (PBS) alone from the OD of fish excreted water.
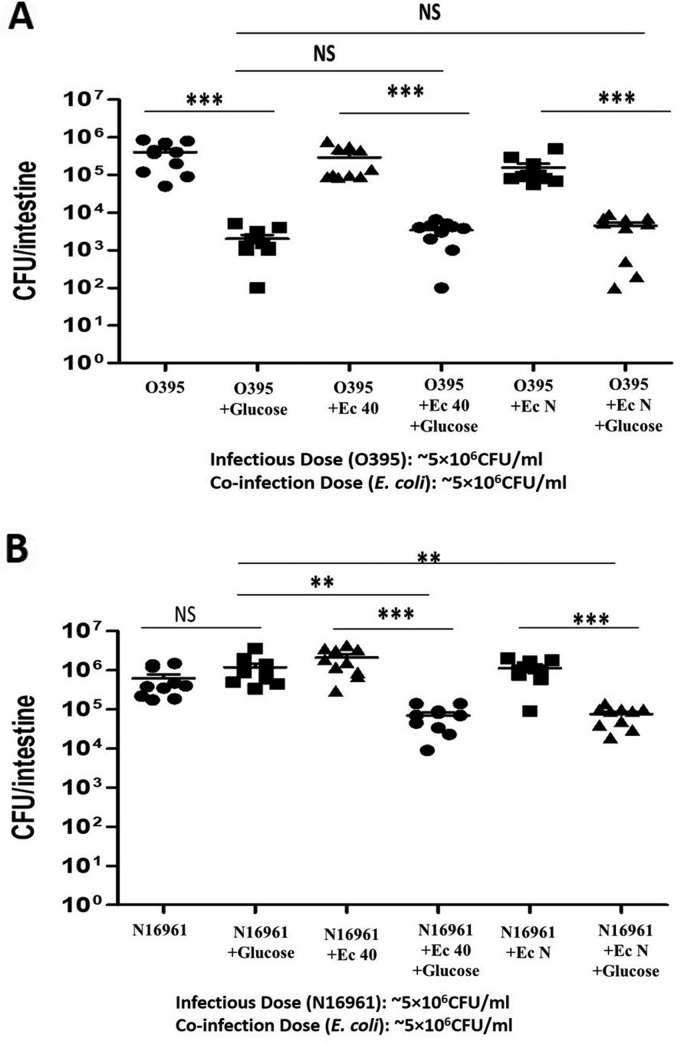
The next step was to determine if the colonizing E. coli would alter V. cholerae colonization in the presence of glucose. Zebrafish were fed 1% glucose in water for 12 h as described above. The fish were then inoculated with a 1:1 ratio of V. cholerae (O395 or N16961) and E. coli (40 or N) for 6 h. Finally, the fish were transferred to fresh sterile water twice to remove external bacteria and incubated for 18 h in fresh water. After the fish were euthanized, serial dilutions of intestinal homogenates were plated to assess the colonization of V. cholerae under the different experimental conditions. In the case of the classical strain (O395), we again observed significantly reduced colonization with 1% glucose and no E. coli (n = 10; P < 0.0001) (Fig. 3A). Coinfection with either E. coli strain had no significant effect on V. cholerae O395 colonization in the presence of glucose (n = 10) (Fig. 3A). In the case of the El Tor strain (N16961), glucose alone had no significant effect on V. cholerae colonization, as observed above. However, coinfection with either of the E. coli strains in the presence of glucose caused an ∼40-fold decrease in V. cholerae colonization compared to the coinfected group without glucose feeding (n = 10; P < 0.0001) and the glucose feeding group without E. coli coinfection (n = 10; P < 0.0001) (Fig. 3B). In both cases (O395 and N16961 infection groups), E. coli strains alone had no significant effect on V. cholerae colonization. However, both E. coli strains were found to colonize simultaneously with V. cholerae strains (see Fig. S3 in the supplemental material). A sugar transport mutant of E. coli strain MG1655 (ΔptsG), which cannot metabolize glucose to acid, was used to verify that reduced colonization of V. cholerae was caused by reduced pH and not simply the E. coli coinfection. This ΔptsG strain was unable to reduce the fish intestinal pH in the presence of glucose (Table S2). In a separate experiment, when N16961 was inoculated together with the E. coli ΔptsG mutant, no significant difference in N16961 colonization was observed (see Fig. S4 in the supplemental material).
FIG 3.
Effect of E. coli strains plus glucose on V. cholerae colonization of zebrafish intestine. Zebrafish were fed 1% glucose for 12 h, and ∼5 × 106 CFU/ml of E. coli 40 and N was coinoculated with ∼5 × 106 CFU/ml of either V. cholerae O395 or N16961. (A) Coinfection of E. coli with classical V. cholerae strain O395. (B) Coinfection of E. coli with El Tor V. cholerae strain N16961. V. cholerae levels were determined by plating of serial dilutions of the intestinal homogenates. Horizontal bars indicate the mean colonization level for each group, and individual symbols indicate the results for individual fish. ***, P < 0.0001; **, P < 0.001; NS, nonsignificant differences.
To verify that E. coli was reducing the pH in the presence of glucose, the pH of the fish intestinal homogenate was semiquantitatively analyzed at 6, 12, and 24 h after the coinfection using pH strips. An intestinal homogenate pH between 6 and 7 was observed during these time periods in coinfected fish that were also fed glucose, whereas a pH of ∼8 was observed in coinfected fish that were not fed glucose (Table 2).
TABLE 2.
Gut pH of fish after V. cholerae and E. coli coinoculation with or without glucose
| Inoculum | Approx gut pH |
|---|---|
| O395 plus: | |
| No addition | 8 |
| Glucose | 6.5 |
| E. coli 40 | 8 |
| E. coli 40 + glucose | 6.5 |
| E. coli N | 7.5 |
| E. coli N + glucose | 6 |
| N16961 plus: | |
| No addition | 8 |
| Glucose | 8 |
| E. coli 40 | 8 |
| E. coli 40 + glucose | 6.5 |
| E. coli N | 8 |
| E. coli N + glucose | 6.5 |
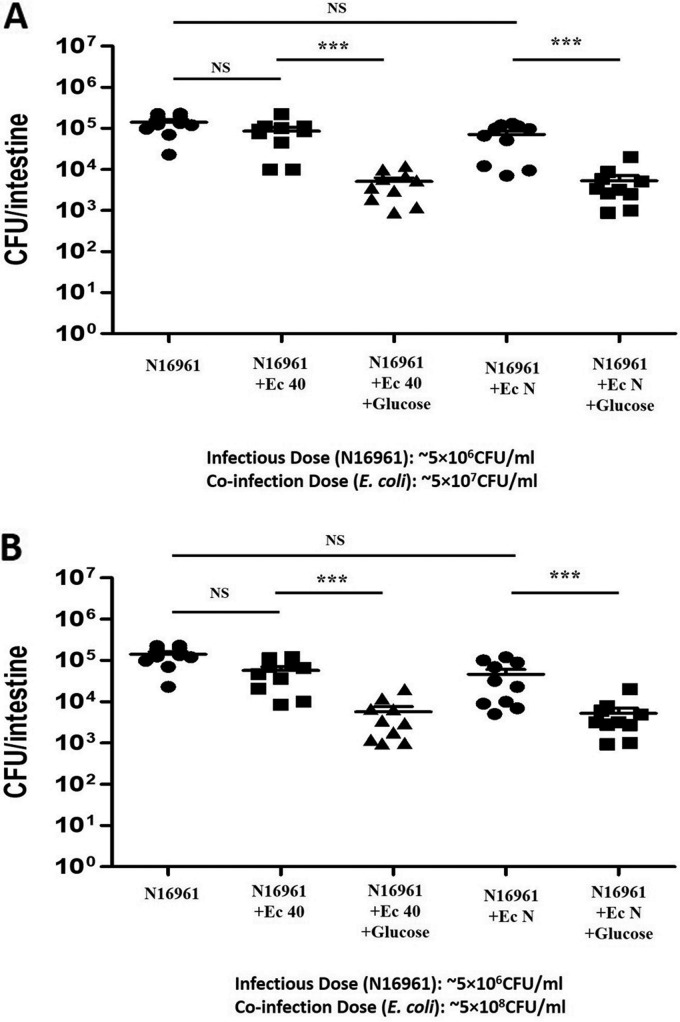
To assess whether a larger E. coli inoculum may have a greater effect on El Tor V. cholerae colonization, the ratio of E. coli to V. cholerae was increased to 10:1 or 100:1 and the same experiment assessing colonization in fish with 1% glucose feeding was conducted. Increasing the ratio of E. coli to V. cholerae to 10:1 in the presence of glucose did not reduce V. cholerae significantly below what was observed for a 1:1 ratio (Fig. 4A). Similarly, increasing the ratio of E. coli to V. cholerae to 100:1 in the presence of glucose did not reduce V. cholerae significantly below what was observed for a 1:1 or 10:1 ratio (Fig. 4B). This suggests that the number of E. coli organisms that colonized at the 1:1 ratio filled the available niche, so further addition of E. coli had little effect. However, a 100:1 E. coli-to-V. cholerae coinfection without glucose feeding did produce an ∼5-fold decrease in N16961 colonization due to competition of V. cholerae with the higher number of E. coli organisms (Fig. 4B).
FIG 4.
Effect of different doses of E. coli strains plus glucose on V. cholerae colonization of zebrafish intestine. Zebrafish were fed 1% glucose for 12 h, and ∼5 × 107 CFU/ml or ∼5 × 108 CFU/ml of E. coli 40 or N was coinoculated with ∼5 × 106 CFU/ml of either V. cholerae O395 or N16961. The ratio of the coinfection of E. coli to V. cholerae was 10:1 (A) or 100:1 (B). The same N16961 control group was used during both experiments, and these results are shown in both panels to facilitate comparisons. V. cholerae levels were determined by plating of serial dilutions of the intestinal homogenates. ***, P < 0.0001; NS, nonsignificant differences.
Prophylactic effects of glucose and E. coli on V. cholerae colonization in zebrafish.
The results of previous experiments suggest that E. coli coinfection in the presence of glucose significantly reduces the colonization of El Tor strain N16961 in zebrafish. To evaluate the prophylactic effect of glucose and E. coli on V. cholerae colonization, fish were fed 1% glucose for 12 h and during the final 6 h of glucose feeding were inoculated with either probiotic E. coli strain (E. coli 40 or N) for 6 h. Fish were then infected with V. cholerae N16961 for 6 h, washed as described above to remove external bacteria, and transferred to fresh water for 18 h of incubation. Fish that were inoculated with E. coli strains plus glucose before N16961 infection showed an ∼20-fold decrease in N16961 colonization compared to the fish inoculated with E. coli strains without glucose (E. coli 40-preinfected group [n = 10; P = 0.0017] and E. coli N-preinfected group [n = 10; P = 0.0055]) (Fig. 5). In the previous experiment we showed the E. coli strains can colonize well in the presence of glucose for up to 72 h. The prophylactic effect of E. coli and glucose on V. cholerae colonization requires a daily dose of glucose to maintain a low gut pH (Table S2).
FIG 5.
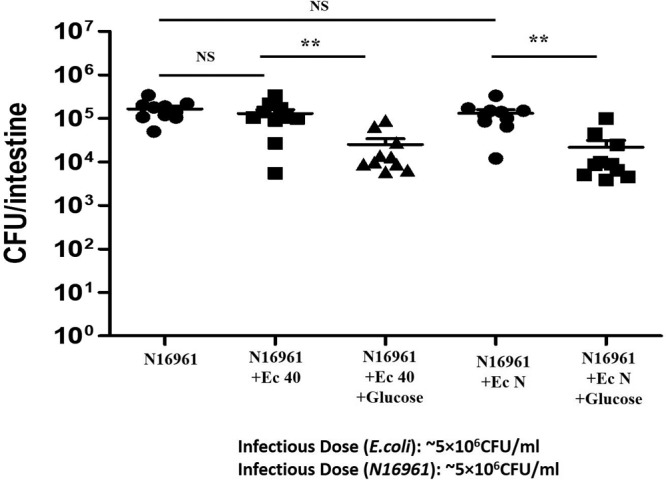
Effect of E. coli feeding plus glucose before V. cholerae infection on N16961 colonization in zebrafish. Fish were fed 1% glucose for 12 h, and during the last 6 h of glucose feeding fish were inoculated with ∼5 × 106 CFU/ml of either E. coli strain (E. coli 40 or N) for 6 h. Fish were then infected with ∼5 × 106 CFU/ml N16961 for 6 h. **, P < 0.001; NS, nonsignificant differences.
Excreted mucin levels in water.
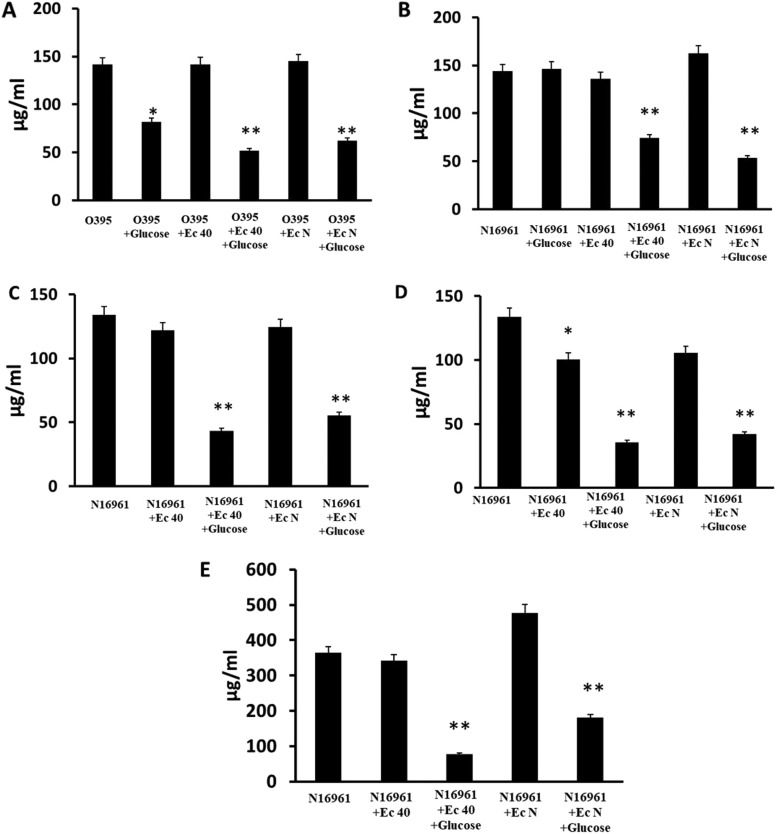
To determine whether E. coli could also reduce the diarrhea induced by V. cholerae colonization, we quantified the level of mucin secreted by zebrafish in the excretory water after colonization. Mucus is a major component of rice-water stool in human cholera diarrhea as well as in zebrafish diarrhea induced by V. cholerae colonization (16, 17). Higher numbers of colonizing V. cholerae organisms correlate with higher excretion of mucin into the water. Coinfection of V. cholerae and E. coli in the presence of glucose reduced both colonization of V. cholerae, as described above, and the mucin level in the excretory water. These observations were consistent in the three coinfection groups, with E. coli-to-V. cholerae ratios of 1:1 (Fig. 6A and B), 10:1 (Fig. 6C), and 100:1 (Fig. 6D). The mucin levels in the excretory water were also assessed in the prophylactic E. coli experiment described above, in which E. coli and glucose were administered to fish for 6 h before inoculation with V. cholerae (Fig. 6E). The mucin levels in excretory water following infection with only E. coli were not significantly higher than in the uninfected control group (Fig. S2B), indicating that mucin excretion is induced by V. cholerae and not by E. coli.
FIG 6.
Mucin assay to assess diarrhea. Different combinations of infection with V. cholerae, E. coli, and glucose were used as indicated under the x axes. Mean values of mucin detected in water by modified periodic acid-Schiff stain (PAS) assay are indicated by the black bars above each infectious dose. The bar graphs show the mucin levels in excreted water after 24 h of 1:1 (A and B), 1:10 (C), and 1:100 (D) coinfection with V. cholerae and E. coli with or without glucose feeding in zebrafish or the mucin levels in excreted water after the prophylactic E. coli and glucose administration followed by V. cholerae inoculation (E). Error bars indicate standard deviation. *, P < 0.05; **, P < 0.005.
DISCUSSION
The V. cholerae O1 serogroup classical biotype ferments glucose and produces a mixture of organic acids in its microenvironment, resulting in decreased pH and a drastic loss of viability (12). In contrast, the V. cholerae O1 serogroup El Tor biotype ferments glucose and produces acetoin, known as 3-hydroxy-2-butanone or acetyl methyl carbinol, converting two molecules of pyruvate to avoid the resulting acidic stress (18, 19). Because acetoin is a neutral fermentation end product and this biosynthetic reaction consumes intracellular protons, bacterial growth can occur on a glucose carbon source without a pH decrease (19, 20). Additionally, acetoin production offers V. cholerae cells a survival advantage during infection by downregulating the host innate immune responses (21). Another report showed that inhibiting acetoin production could be an antibacterial mechanism for V. cholerae elimination (22). Previous work explored another way to reduce the growth of El Tor strains in vitro by using a coculture of E. coli that is acid producing in the presence of glucose in the medium (13). In the present work, we showed the effect of acid-producing E. coli strains in the presence of glucose on V. cholerae colonization in the zebrafish model.
All the carbohydrates that can be metabolized by glycolysis in V. cholerae are fermented into either organic acids (classical strains) or neutral products (El Tor strains). Glucose is the primary substrate of glycolysis. All other carbohydrates must be converted into glucose before entering the glycolytic pathway. Based on this as well as the previous work showing the effects of glucose on V. cholerae growth in vitro (13, 19), we chose glucose to examine the effects of E. coli glycolysis on V. cholerae colonization. Glucose is also a crucial component of ORS, as glucose can induce Na+/K+ ion uptake in the intestine. This ion uptake is essential to replace the lost electrolytes and water caused by the voluminous diarrhea caused by cholera toxin (23). On the other hand, there are concerns that the glucose content in ORS might worsen the disease by increasing the expression of toxic genes in El Tor strains (22). Here we reversed the inductive effect of glucose on El Tor strains by using acid-producing E. coli. In the previous study, a human-isolated commensal E. coli strain, sample 40 (E. coli 40), which can produce acid from glucose, was identified (13, 24). In addition, another well-studied commercially available probiotic strain of E. coli, Nissle 1917 (E. coli N), which is also able to produce acid from glucose, was used (13, 25).
The commensal and probiotic E. coli N strain has been used clinically against ulcerative colitis and Crohn’s disease (25–27). Other than clinical use, E. coli N and other nonpathogenic E. coli strains have been evaluated for their potential as delivery vehicles (28), in reducing intestinal colonization of Salmonella enterica serovar Typhimurium (29), and in cancer therapy (30). As these strains are human isolates, they should colonize well in the human intestine despite the presence of a complex heterogeneous microbiome and should produce an acidic environment upon metabolism of glucose, which would accordingly reduce V. cholerae colonization. Thus, these nonpathogenic commensal and probiotic E. coli strains have tremendous potential for therapeutic use in combination with glucose against cholera.
Glucose or glucose-based ORS can be fed to humans orally. Therefore, an oral feeding model in zebrafish was used here by adding glucose to incubation water. We observed significant glucose content in fish intestines, which did not have any obvious effect on fish health unless incubation with glucose water was extended to 24 h. Zebrafish is an effective model for this study for three reasons. First, the development of cholera in humans is a complex, multifactorial process that can be reproduced in zebrafish very easily. Second, the animal models typically used in cholera research are not native cholera hosts, and the mechanisms of their colonization may be different; however, zebrafish naturally host V. cholerae (31, 32). Third, V. cholerae colonization in other animal models requires the disruption or absence of intestinal microbiota, whereas V. cholerae colonization in zebrafish occurs in the presence of intact intestinal microbiota (15, 17).
This is the first observation that glucose feeding to zebrafish can significantly reduce the colonization of classical strain O395 and increase the colonization of El Tor strain N16961. Other groups previously showed the effect of glucose on V. cholerae colonization in the infant mouse model, but the glucose treatment of V. cholerae was in vitro before the infection (22). In a clinical scenario, pathogenic bacteria cannot be treated before infection; glucose must be an effector of colonization during the treatment. Therefore, the new observation in a natural host model has implications for clinical treatments.
The colonization of classical strain O395 was hampered by glucose due to acid production by the strain itself. Acid-producing E. coli had no additional effect in reducing classical strain colonization. Conversely, the colonization of El Tor strain N16961 was enhanced by glucose due to production of acetoin. Acid-producing E. coli metabolized glucose in the gut, which lowered the pH and reduced the El Tor colonization. The two E. coli strains tested (E. coli 40 and N) colonized zebrafish intestines without any prior modification of gut microbiota, indicating their fitness in this environment and their ability to compete with the intestinal microbiota for colonization niches. In the presence of glucose, these E. coli strains produced an acidic pH, which was confirmed by testing the pH of the gut homogenate. This mirrors a previous study in which it was also shown that the ingestion of acid-producing bacteria in the presence of carbohydrate leads to decreased gut pH (33). When the sugar transport mutant of E. coli (ΔptsG) was tested in the presence of glucose, E. coli colonization was observed in fish intestine, but V. cholerae colonization was not hampered by this mutant E. coli strain, suggesting that the inhibition of N16961 colonization in other experiments was the result of acid production by E. coli and not its mere presence.
Coinfection of equal numbers of either E. coli strain with V. cholerae N16961 caused a significant decrease in N16961 colonization in the presence of glucose. Generally, V. cholerae competes with other bacteria for colonization of a niche using its type six secretion system (T6SS), resulting in fewer competitors (34, 35). Another recent observation was that the V. cholerae T6SS acts primarily to increase the strength of gut contractions to expel competitors, rather than directly killing the bacterial competitor (35). Here, E. coli strains colonized simultaneously with V. cholerae and produced an acidic pH, which killed V. cholerae. A possible reason behind this might be that at an early time point after coinfection, E. coli colonized faster than V. cholerae. Another possibility is that E. coli strains were able to produce acid within a short time period before either species colonized, which reduced the V. cholerae load in gut and thus reduced the ability of killing by T6SS. A third possibility is that E. coli and V. cholerae colonize different niches in the intestinal tract but that the pH-lowering effect of E. coli extends to the area colonized by V. cholerae. A slightly lower colonization of E. coli during coinfection in the absence of glucose was observed (see Fig. S2 in the supplemental material), which suggests that in the absence of glucose, V. cholerae can colonize and compete with E. coli to some degree. When the coinfection ratio was increased to 10 and 100 times E. coli to V. cholerae, effects similar to those with the equal coinfection were noted. A small decrease in N16961 colonization due to competition with the higher numbers of E. coli was observed in the second case (100:1).
One possibility for future clinical treatments would be prophylactic use of E. coli probiotics to reduce V. cholerae colonization of a vulnerable population. Administering glucose and E. coli before the onset of V. cholerae infection did have a prophylactic effect, but the prophylactic effect was lesser than the effect observed during coinfection experiments. Our results suggest that a single dose of E. coli can colonize in fish intestine for up to 72 h in the presence of glucose. To maintain the low pH during E. coli colonization, glucose should be fed periodically. As the tested E. coli strains are from a human source, it is possible that these strains may have a greater capacity to colonize the human intestine than the fish intestine. A daily feeding of glucose in ORS could be applied to maintain the low gut pH during rehydration therapy. Therefore, therapeutic treatment with probiotic E. coli as a supplement to ORS may be preferable to prophylactic treatment.
In summary, the results presented here suggest that use of probiotic E. coli strains plus glucose could be a successful therapeutic measure against V. cholerae infection. Administration of probiotic E. coli could also be used as a preventive measure to reduce the spread of disease during a cholera outbreak.
MATERIALS AND METHODS
Strains and culture condition.
V. cholerae El Tor strain N16961 (Smr [100 µg/ml]), V. cholerae classical strain O395 (Smr [100 µg/ml]), and probiotic E. coli strains Nissle (E. coli N), human commensal E. coli strain sample 40 (E. coli 40), and E. coli MG 1655 ΔptsG (E. coli ΔptsG) were used for this study (13). E. coli N and 40 were transformed with either pSMC21 (Ampr [100 µg/ml] Kanr [50 µg/ml]) or pKK-1773R1 (Ampr [100 µg/ml]), and E. coli ΔptsG was transformed with pBAD 18 (Kanr [50 µg/ml]), for this study. All strains were frozen in 15% glycerol in Luria-Bertani (LB) broth (Difco, NJ, USA) at −80°C. Prior to experimentation, each strain was grown in LB broth (Difco, NJ, USA) at 37°C under shaking conditions (100 rpm) or on plates in LB agar (Difco, NJ, USA) with the appropriate antibiotic(s). Thiosulfate-citrate-bile-sucrose (TCBS) agar (Difco, NJ, USA) and deoxycholate-citrate-lactose-sucrose (DCLS) agar (Sigma, St. Louis, MO, USA) were used as selective media for V. cholerae. LB agar with the desired antibiotic concentrations was prepared for the selection of strains during colonization study.
Animal model: zebrafish.
Adult, wild-type ZDR zebrafish were used in all experiments. The fish were housed in an automated recirculating tank system (Aquaneering, CA, USA) using water filtered by reverse osmosis and maintained at pH 7.0 to 7.5. The tank water was conditioned with Instant Ocean salt (Aquarium Systems, OH, USA) to a conductivity of 600 to 700 μS. The fish were fasted for at least 12 h prior to each experiment. Zebrafish were euthanized in 100 ml of 32-μg/ml Tricaine-S (tricaine methane sulfonate; MS-222 [Western Chemical, WA, USA]) for a minimum of 25 min. All animal protocols were approved by the Wayne State University IACUC.
Glucose feeding and detection of glucose in zebrafish intestine.
Five zebrafish per experimental group were placed into a 40-ml beaker with a perforated lid containing 200 ml of sterile infection water (autoclaved tank water), and then 1% glucose (Fisher Chemicals, NH, USA) was added to the infection water. Fish were kept in glucose water for 6, 12, and 24 h. The glucose content of fish intestine was measured by homogenizing the intestinal tract and using Benedict’s solution (Sigma, MO, USA). Briefly, after glucose feeding, the fish were euthanized and their intestines were homogenized in 1 ml 1× PBS (pH 7.4) (VWR, PA, USA) with 1.5 g of 1-mm glass beads (BioSpec Products Inc., OK, USA) in a bead beater homogenizer (BioSpec Products, Inc.). Three milliliters of Benedict’s reagent was added with 1 ml of intestinal homogenate. The mixture was heated in a boiling water bath until the color changed. The approximate concentration of the glucose in the solution was measured visually by the color and texture of any precipitate (ppt) formed (for example, brick-red ppt for greater than 10 mg/ml of glucose, green to yellow-green ppt for 5 to 9 mg/ml of glucose, green ppt for 2 to 5 mg/ml of glucose, and blue-green suspension for less than 2 mg/ml of glucose). In this experiment, 1 ml of 1% glucose solution was used as positive control, 1 ml of 1× PBS was used as negative control, and intestinal homogenate from control fish (without glucose feeding) was used as a working control.
Infection procedure in zebrafish.
Four or five zebrafish per experimental group were placed into a 400-ml beaker with a perforated lid containing 200 ml of sterile infection water (autoclaved tank water). Bacterial cultures were grown with aeration in LB broth at 37°C for 16 to 18 h. Cells were centrifuged at 10,000 × g for 10 min. The resulting pellet was washed twice with 1× PBS and resuspended in 1× PBS to an estimated concentration of 109 CFU/ml by measuring the OD at 600 nm. One milliliter of bacterial inoculum was added to the beaker containing the fish. The final V. cholerae cell density used was ∼5 × 106 CFU/ml (infection dose) for this study and was verified by plating serial dilutions of the inoculated infection water. The CFU of E. coli used was equal to, 10 times the number, or 100 times the number of V. cholerae CFU in the inoculum. The fish were infected with bacteria for 6 h, and then the fish were washed twice for removal of surface bacteria and kept in fresh sterile water for 18 h. The control group included fish that were exposed to 1 ml of 1× PBS only. E. coli was inoculated either with (coinfection) or before V. cholerae infection. Each beaker was placed into a glass-front incubator set at 28°C for the duration of the experiment.
Colonization assay.
After the specified time points, fish were euthanized, the intestine of each fish was aseptically dissected, placed into homogenization tubes (2.0-ml screw-cap tubes; Sarstedt, Nümbrecht, Germany) with 1.5 g of 1.0-mm glass beads (BioSpec Products, Inc., Bartlesville, OK) and 1 ml of 1× PBS, and held on ice. Homogenization tubes were loaded into a Mini-Beadbeater-24 (BioSpec Products, Inc.) and shaken at maximum speed for two 1-min cycles, with the samples being incubated for 1 min on ice after both the first and last cycles. Intestinal homogenates from each fish were diluted and plated for enumeration on appropriate LB agar plates with appropriate antibiotics. The plates were incubated overnight at 37°C. After glucose feeding and the inoculation with E. coli, the pH of the fish gut homogenates was measured at 6, 12, and 24 h after the first inoculation. The fish intestine was homogenized in 200 µl of normal saline water, and 20 µl of the suspension was dropped on Whatman pH measuring paper (GE Healthcare Bio-Sciences, PA, USA) to get a semiquantitative result.
Processing of infection water with fish excretion.
With a 60-ml syringe (BD 309653; BD, NJ, USA), 50 ml of fish infection water was extracted before the colonization assay, in duplicate, and put into two 50-ml conical tubes. For all assays, 50-ml conical tubes were centrifuged at 10,000 rpm for 15 min at 4°C and supernatant was decanted, with care not to disturb the pellet. Each pellet was resuspended in 2 ml of 1× PBS. Unprocessed water samples were stored at 4°C for up to 1 week before analysis.
Mucin assay.
The mucin content in excreted water was determined as described earlier (17, 36). Prior to the procedure, 1 ml of a 50% (wt/vol) periodic acid (Sigma-Aldrich) stock solution was made. A 96-well plate (Corning Costar; Corning, NY, USA) was loaded with 100 μl/well of the blank (1× PBS) and mucin standards, and samples were loaded in triplicate. A volume of 50 μl/well of fresh 0.1% periodic acid solution (10 μl of the 50% periodic acid stock added to 5 ml of 7% acetic acid, used immediately after making) was added and mixed by pipetting. The plate was covered in plastic wrap and incubated at 37°C for 1 to 1.5 h. After incubation, the plate was cooled to room temperature before adding 100 μl/well Schiff’s reagent (Sigma-Aldrich), and the contents were mixed with a pipette. The plate was again covered in plastic wrap and placed on a rocker or shaker for 15 to 40 min or until sufficient color developed. Absorbance was read at 560 nm using a plate reader (Tecan Spectra Fluor plus; Tecan, Männedorf, Switzerland). The effective ODs of test samples were calculated by subtraction of the OD of the PBS control (uninfected fish) excreted water from that of the test (infected) fish excreted water.
Statistical analysis.
Each experiment was performed a minimum of three times on separate occasions, unless otherwise specified in the figure legends. Analyzed data are presented as the mean ± standard deviation (SD). Significant frequencies were compared using the χ2 test, and continuous variables were compared using Student’s t test. A two-tailed t test was performed to test against a control as described in the figure legends. Analyses were performed using GraphPad Prism 7.0.
Supplementary Material
ACKNOWLEDGMENTS
The research reported here was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number R01AI127390 (to J.H.W.). This work is also a part of an ongoing institutional project grant (LCP 151) to S.R.
We gratefully acknowledge Rudolf Von Bunau Ardeypharm GmbH, Herdecke, Germany, for the generous gift of Escherichia coli Nissle 1917 and Roberto Kolter, Harvard University, USA, for generously providing plasmid pSMC21.
D.N., S.R., and J.H.W. designed the experiments, D.N. and P.B. carried out the experiments, D.N. and J.H.W. wrote the manuscript, and all of the authors gave editorial input.
We declare no conflict of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00486-18.
REFERENCES
- 1.Harris JB, LaRocque RC, Qadri F, Ryan ET, Calderwood SB. 2012. Cholera. Lancet 379:2466–2476. doi: 10.1016/S0140-6736(12)60436-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zmeter C, Tabaja H, Sharara A, Kanj S. 2018. Non-O1, non-O139 Vibrio cholera septicemia at a tertiary care center in Beirut, Lebanon; a case report and review. J Infect Public Health 11:601–604. doi: 10.1016/j.jiph.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Ali M, Nelson AR, Lopez AL, Sack DA. 2015. Updated global burden of cholera in endemic countries. PLoS Negl Trop Dis 9:e0003832. doi: 10.1371/journal.pntd.0003832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor DL, Kahawita TM, Cairncross S, Ensink JHJ. 2015. The impact of water, sanitation and hygiene interventions to control cholera: a systematic review. PLoS One 10:e0135676. doi: 10.1371/journal.pone.0135676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camacho A, Bouhenia M, Alyusfi R, Alkohlani A, Naji MAM, de Radiguès X, Abubakar AM, Almoalmi A, Seguin C, Sagrado MJ, Poncin M, McRae M, Musoke M, Rakesh A, Porten K, Haskew C, Atkins KE, Eggo RM, Azman AS, Broekhuijsen M, Saatcioglu MA, Pezzoli L, Quilici ML, Al-Mesbahy AR, Zagaria N, Luquero FJ. 2018. Cholera epidemic in Yemen, 2016-18: an analysis of surveillance data. Lancet Glob Health 6:e680–e690. doi: 10.1016/S2214-109X(18)30230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 2018. Cholera—Vibrio cholerae infection. CDC, Atlanta, GA. https://www.cdc.gov/cholera/treatment/index.html.
- 7.Gupta SS, Bharati K, Sur D, Khera A, Ganguly NK, Nair GB. 2016. Why is the oral cholera vaccine not considered an option for prevention of cholera in India? Analysis of possible reasons. Indian J Med Res 143:545–551. doi: 10.4103/0971-5916.187102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhattacharya D, Sayi DS, Thamizhmani R, Bhattacharjee H, Bharadwaj AP, Roy A, Sugunan AP. 2012. Emergence of multidrug-resistant Vibrio cholerae O1 biotype El Tor in Port Blair, India. Am J Trop Med Hyg 86:1015–1017. doi: 10.4269/ajtmh.2012.11-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thapa Shrestha U, Adhikari N, Maharjan R, Banjara MR, Rijal KR, Basnyat SR, Agrawal VP. 2015. Multidrug resistant Vibrio cholerae O1 from clinical and environmental samples in Kathmandu city. BMC Infect Dis 15:104. doi: 10.1186/s12879-015-0844-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siddique AK, Nair GB, Alam M, Sack DA, Huq A, Nizam A, Longini IM Jr, Qadri F, Faruque SM, Colwell RR, Ahmed S, Iqbal A, Bhuiyan NA, Sack RB. 2010. El Tor cholera with severe disease: a new threat to Asia and beyond. Epidemiol Infect 138:347–352. doi: 10.1017/S0950268809990550. [DOI] [PubMed] [Google Scholar]
- 11.Garg P, Nandy RK, Chaudhury P, Chowdhury NR, De K, Ramamurthy T, Yamasaki S, Bhattacharya SK, Takeda Y, Nair GB. 2000. Emergence of Vibrio cholerae O1 biotype El Tor serotype Inaba from the prevailing O1 Ogawa serotype strains in India. J Clin Microbiol 38:4249–4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoon SS, Mekalanos JJ. 2006. 2,3-Butanediol synthesis and the emergence of the Vibrio cholerae El Tor biotype. Infect Immun 74:6547–6556. doi: 10.1128/IAI.00695-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sengupta C, Ekka M, Arora S, Dhaware PD, Chowdhury R, Raychaudhuri S. 2017. Crossfeeding of glucose metabolism byproducts of Escherichia coli human gut isolate sand probiotic strains affect survival of Vibrio cholerae. Gut Pathog 17:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engeszer RE, Patterson LB, Rao AA, Parichy DM. 2007. Zebrafish in the wild: a review of natural history and new notes from the field. Zebrafish 4:21–40. doi: 10.1089/zeb.2006.9997. [DOI] [PubMed] [Google Scholar]
- 15.Runft DL, Mitchell KC, Abuaita BH, Allen JP, Bajer S, Ginsburg K, Neely MN, Withey JH. 2014. Zebrafish as a natural host model for Vibrio cholerae colonization and transmission. Appl Environ Microbiol 80:1710–1717.16. doi: 10.1128/AEM.03580-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell KC, Breen P, Britton S, Neely MN, Withey JH. 2017. Quantifying Vibrio cholerae enterotoxicity in a zebrafish infection model. Appl Environ Microbiol 83:e00783-17. doi: 10.1128/AEM.00783-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nag D, Mitchell K, Breen P, Withey JH. 2018. Quantifying Vibrio cholerae colonization and diarrhea in the adult zebrafish model. J Vis Exp doi: 10.3791/57767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao Z, Lu JR. 2014. Strategies for enhancing fermentative production of acetoin: a review. Biotechnol Adv 32:492–503. doi: 10.1016/j.biotechadv.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Thomas VC, Sadykov MR, Chaudhari SS, Jones J, Endres JL, Widhelm TJ, Ahn JS, Jawa RS, Zimmerman MC, Bayles KW. 2014. A central role for carbon-overflow pathways in the modulation of bacterial cell death. PLoS Pathog 10:e1004205. doi: 10.1371/journal.ppat.1004205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merrell DS, Camilli A. 2002. Acid tolerance of gastrointestinal pathogens. Curr Opin Microbiol 5:51–55. doi: 10.1016/S1369-5274(02)00285-0. [DOI] [PubMed] [Google Scholar]
- 21.Bari W, Song YJ, Yoon SS. 2011. Suppressed induction of proinflammatory cytokines by a unique metabolite produced by Vibrio cholerae O1 El Tor biotype in cultured host cells. Infect Immun 79:3149–3158. doi: 10.1128/IAI.01237-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh YT, Kim HY, Kim EJ, Go J, Hwang W, Kim HR, Kim DW, Yoon SS. 2016. Selective and efficient elimination of Vibrio cholerae with a chemical modulator that targets glucose metabolism. Front Cell Infect Microbiol 16:156. doi: 10.3389/fcimb.2016.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munos MK, Walker CL, Black RE. 2010. The effect of oral rehydration solution and recommended home fluids on diarrhoea mortality. Int J Epidemiol 39:i75–i87. doi: 10.1093/ije/dyq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dureja C, Mahajan S, Raychaudhuri S. 2014. Phylogenetic distribution and prevalence of genes encoding class I integrons and CTX-M-15 extended-spectrum β-lactamases in Escherichia coli isolates from healthy humans in Chandigarh, India. PLoS One 9:e112551. doi: 10.1371/journal.pone.0112551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kruis W, Fric P, Pokrotnieks J, Lukás M, Fixa B, Kascák M, Kamm MA, Weismueller J, Beglinger C, Stolte M, Wolff C, Schulze J. 2004. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut 53:1617–1623. doi: 10.1136/gut.2003.037747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ukena SN, Singh A, Dringenberg U, Engelhardt R, Seidler U, Hansen W, Bleich A, Bruder D, Franzke A, Rogler G, Suerbaum S, Buer J, Gunzer F, Westendorf AM. 2007. Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS One12 2:e1308. doi: 10.1371/journal.pone.0001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schultz M. 2008. Clinical use of E. coli Nissle 1917 in inflammatory bowel disease. Inflamm Bowel Dis 14:1012–1018. doi: 10.1002/ibd.20377. [DOI] [PubMed] [Google Scholar]
- 28.Duan F, Curtis KL, March JC. 2008. Secretion of insulinotropic proteins by commensal bacteria: rewiring the gut to treat diabetes. Appl Environ Microbiol 74:7437–7438. doi: 10.1128/AEM.01019-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deriu E, Liu JZ, Pezeshki M, Edwards RA, Ochoa RJ, Contreras H, Libby SJ, Fang FC, Raffatellu M. 2013. Probiotic bacteria reduces Salmonella typhimurium intestinal colonization by competing for iron. Cell Host Microbe 14:26–37. doi: 10.1016/j.chom.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson CJ, Clarke EJ, Arkin AP, Voigt CA. 2005. Environmentally controlled invasion of cancer cells by engineered bacteria. J Mol Biol 355:1–9. doi: 10.1016/j.jmb.2005.10.076. [DOI] [PubMed] [Google Scholar]
- 31.Ho BT, Fu Y, Dong TG, Mekalanos JJ. 2017. Vibrio cholerae type 6 secretion system effector trafficking in target bacterial cells. Proc Natl Acad Sci U S A 114:9427–9432. doi: 10.1073/pnas.1711219114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanther M, Rawls JF. 2010. Host-microbe interactions in the developing zebrafish. Curr Opin Immunol 22:10–19. doi: 10.1016/j.coi.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zoetendal EG, Raes J, van den Bogert B, Arumugam M, Booijink CC, Troost FJ, Bork P, Wels M, de Vos WM, Kleerebezem M. 2012. The human small intestinal microbiotas driven by rapid uptake and conversion of simple carbohydrates. ISME J 6:1415–1426. doi: 10.1038/ismej.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pukatzki S, Ma AT, Sturtevant D, Krastins B, Sarracino D, Nelson WC, Heidelberg JF, Mekalanos JJ. 2006. Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc Natl Acad Sci U S A 103:1528–1533. doi: 10.1073/pnas.0510322103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Logan SL, Thomas J, Yan J, Baker RP, Shields DS, Xavier JB, Hammer BK, Parthasarathy R. 2018. The Vibrio cholerae type VI secretion system can modulate host intestinal mechanics to displace gut bacterial symbionts. Proc Natl Acad Sci U S A 115:E3779–E3787. doi: 10.1073/pnas.1720133115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nelson EJ, Chowdhury A, Harris JB, Begum YA, Chowdhury F, Khan AI, Larocque RC, Bishop AL, Ryan ET, Camilli A, Qadri F, Calderwood SB. 2007. Complexity of rice-water stool from patients with Vibrio cholerae plays a role in the transmission of infectious diarrhea. Proc Natl Acad Sci U S A 104:19091–19096. doi: 10.1073/pnas.0706352104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.