Abstract
Background
Retinal vein thrombosis has in case reports been reported a clinical sign of cancer, especially hematological cancer. However, it is unclear whether retinal vein thrombosis is a marker of underlying cancer, as is the case for deep venous thrombosis and pulmonary embolism. We investigated the risk of occult cancer in patients with retinal vein thrombosis.
Methods
A nationwide population‐based cohort study in Denmark on all patients diagnosed with a retinal vein thrombosis during 1994 and 2013. The main outcome measures were any cancer and site‐specific cancers <6 months, 6‐12 months, and 5 years following a retinal vein thrombosis diagnosis, as registered in the Danish Cancer Registry and the National Pathology Registry. We calculated the absolute cancer risk and computed standardized incidence ratios (SIRs) with 95% confidence intervals (CIs) for cancer within <6 months, 6‐12 months, and 5 years following a retinal vein thrombosis diagnosis.
Results
Among 9589 patients with retinal vein thrombosis, we observed 1514 cancer cases. The risk of any cancer was 1.2% <6 months and 28.8% after 5 years. The <6 months SIR was 1.20 (95% CI 0.99‐1.44), 6‐12 months SIR was 1.15 (95% CI 0.94‐1.39), and the 5 years’ SIR was 1.08 (95% CI 1.03‐1.14). Stratification by age, gender, calendar year, and Charlson Comorbidity Index score did not change overall cancer risk estimates.
Conclusion
Retinal vein thrombosis was not an important clinical marker for occult cancer. An extensive diagnostic cancer workup does not appear warranted for retinal vein thrombosis patients.
Keywords: cohort study, neoplasm, retinal vein occlusion, risk, venous thromboembolism
1. INTRODUCTION
Retinal vein thrombosis is the second most common cause of vision loss after diabetic retinopathy.1 Risk factors include atherosclerosis, advanced age, hypertension, diabetes, and hyperlipidemia,1, 2, 3, 4 which also increase the propensity for deep venous thromboembolism and pulmonary embolism.
Cancer induces a systemic pro‐coagulant state, which increases the risk of venous thromboembolism.5, 6 Thus, venous thromboembolism in the lower extremities and the lungs are markers of cancer.7, 8, 9, 10, 11, 12, 13 Several case reports have suggested that retinal vein thrombosis may also be a clinical sign of cancer, especially hematological cancers,2, 3, 10, 14, 15, 16, 17 but firm epidemiological evidence is lacking. Putative mechanisms involve direct neoplastic infiltration leading to impaired drainage and obstruction of the retinal veins, as well as dehydration and hyperviscosity caused by the cancer.1, 2, 3, 16, 18 To investigate these suppositions, we examined the risk of cancer following a retinal vein thrombosis compared with cancer risk in the general population.
2. METHODS
2.1. Setting
Denmark has a tax‐supported health care system ensuring equal and income‐independent free access to health care services for all residents. Individuals born in or immigrating to Denmark receive a unique Civil Personal Registration Number, enabling accurate linkage among Danish registries at the individual level.19 In the present study, we used data from the Danish National Patient Registry (DNPR),20 the Danish Cancer Registry (DCR),21, 22 and the Civil Registration System (CRS).19 The DNPR has recorded data on all admissions and discharges from non‐psychiatric hospitals since 1977 and from emergency rooms and outpatient clinics since 1995.20 Each hospital discharge or outpatient visit has been coded according to the International Classification of Diseases, Eighth Revision (ICD‐8) from 1977 until the end of 1993 and the Tenth Revision (ICD‐10) thereafter. The DCR contains complete prospectively collected data on all incident cases of primary cancer diagnosed in Denmark since 1943.21, 22, 23 DCR data include information on tumor staging according to TNM and Ann Arbor classifications.22 Reporting to the DCR has been mandatory for all hospital departments since 1987 and for general practitioners since 2004, ensuring nationwide completeness of its data.21, 22, 23 DCR data are linked to the National Pathology Registry, ensuring high validity of cancer diagnoses; 89% of diagnoses in the DCR have histopathological verification.24 The high quality of DCR data also is ensured by continuous manual and electronic quality control.
2.2. Study population
We used the DNPR and DCR to identify all patients diagnosed with retinal vein thrombosis, on an inpatient or hospital outpatient basis, between 1 January 1994 and 30 November 2013. A history of a previous cancer diagnosis was an exclusion criteria. We defined date of discharge or outpatient contact as the retinal vein thrombosis diagnosis date. We obtained information from the DNPR on comorbid conditions, including a history of deep venous thrombosis, myocardial infarction, heart failure, atrial fibrillation or flutter, chronic pulmonary disease, chronic kidney disease, diabetes mellitus, obesity, and alcoholism‐related disorders, as well as provoking factors for deep venous thrombosis within 90 days of the retinal vein thrombosis event (fracture, trauma, surgery, pregnancy). We used the Charlson Comorbidity Index to categorize patients’ comorbidity burden at the time of retinal vein thrombosis (normal: score = 0; moderate: score = 1 or 2; or severe: score ≥ 3).25, 26
2.3. Cancer incidence
The primary outcome was the absolute risk of all cancers recorded in the DCR. We obtained data on tumor staging at the time of diagnosis for all cancer cases according to the TNM classification and Ann Arbor classification.22, 23
2.4. Statistical analyses
We followed patients from the date of retinal vein thrombosis diagnosis until a diagnosis of cancer recorded in the DCR, death, emigration, or end of the study period (30 November 2013), whichever came first. We tabulated patient characteristics as described above, and for improving specificity, we calculated the proportion of patients diagnosed with retinal vein thrombosis at hospital departments of ophthalmology. We calculated absolute cancer risks using the cumulative incidence risk function, accounting for death as a competing risk.27 As a measure of relative risks, we furthermore calculated standardized incidence ratios (SIRs) for cancer, using indirect standardization, as the ratio of the observed number of cancer cases to the number of cancer cases expected in the general population among persons of the same age and in the same calendar period (in one‐year intervals) based on data in the DCR.28 We investigated the risk of cancer overall, within <6 months, 6‐12 months, and more than one year following the retinal vein thrombosis diagnosis. Analyses were stratified by age, gender, calendar year, and comorbidity burden according to Charlson comorbidity scores.25, 26 Assuming that cancers diagnosed during the first year of follow‐up were present at the time of retinal vein thrombosis diagnosis, we computed the number of patients needed to screen at the time of diagnosis in order to detect one excess cancer as the reciprocal of the excess risk.
ICD codes used in the study are provided in Table 1. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Table 1.
Diagnosis codes for study exposures, outcomes, and covariates according to the International Classification of Diseases, Eighth and Tenth Revisions (ICD‐8 and ICD‐10)
| ICD‐8 codes | ICD‐10 codes | |
|---|---|---|
| Exposure | ||
| Retinal vein thrombosis | 377.08 | DH348 |
| Outcome | ||
| Cancer | 140‐209 | C00‐C99 |
| Covariates | ||
| Diabetes | 24900, 24906, 24907, 24909, 25000, 25006, 25007, 25009 | E10‐E14 (except E102, E112, E142). O24 (except O24.4), G63.2, H36.0, N08.3 |
| Atrial fibrillation or atrial flutter | 42793, 42794 | I48 |
| Myocardial infarction | 410 | I21 |
| Heart failure | 42709, 42710, 42711, 42719, 42899, 78249 | I50.0, I50.1, I50.2, I50.3, I50.8, I50.9, I11.0, I13.0, I13.2, I42.0, I42.7, I42.8, I42.9 |
| Lower‐extremity deep venous thrombosis or pulmonary embolism | 45100, 45099 | I80.1‐3, I26 |
| Chronic renal disease | 249.02, 250.02, 753.10‐753.19, 582, 583, 584, 590.09, 593.20, 792 | E10.2, E11.2, E14.2, N03, N05, N11.0, N14; N16, N18‐N19, N26.9, Q61.1‐Q61.4 |
| Chronic pulmonary disease | 490‐493; 515‐518 | J40‐J47; J60‐J67; J68.4; J70.1; J70.3; J84.1; J92.0; J96.1; J98.2; J98.3 |
| Obesity | 277 | E65‐E68 |
| Alcoholism‐related disorders | 980, 291.09‐291.99, 303.09‐303.99, 57109‐57111, 57710 | F10 (except F10.0), G31.2, G62.1, G72.1, I42.6, K29.2, K86.0, Z72.1 |
| Risk factors for venous thromboembolism | ||
| Fracture/trauma | 800‐929, 950‐959 | S00‐T14 |
| Surgery | N/A | Previous Danish classification until 1996:000000‐99960; NOMESCO classification after 1996: KA‐KQ, KX, KY |
| Pregnancy | 630‐680 | O00‐O99 |
The study was approved by the Danish Data Protection Agency (record number 1‐16‐02‐1‐08).
3. RESULTS
We followed 9589 patients diagnosed with a retinal vein thrombosis for a median of 5.1 years (25th–75th percentile: 2.2‐9.2 years; Table 2). Median age at the retinal vein thrombosis event was 70 years with equal gender distribution. Approximately 90% of patients were older than age 50 years when they experienced a retinal vein thrombosis. As expected, these patients had a higher comorbidity burden (including cardiovascular diseases) compared with patients aged below 50 years (Table 2). One‐third of the patients aged 50 years or more had undergone surgery within 90 days before the event. Nearly all retinal vein thrombosis patients (95%) were diagnosed at ophthalmologic departments.
Table 2.
Characteristics of retinal vein thrombosis patients according to age at diagnosis, Denmark, 1997‐2013
| All retinal vein thrombosis patients | 0‐50 y | 50+ y | |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| Total | 9589 (100.0) | 894 (100.0) | 8695 (100.0) |
| Male sex | 4714 (49.2) | 495 (55.4) | 4219 (48.5) |
| Calendar year of diagnosis | |||
| 1994‐2001 | 3411 (35.6) | 299 (33.4) | 3112 (35.8) |
| 2002‐2009 | 3801 (39.6) | 363 (40.6) | 3438 (39.5) |
| 2010‐2013 | 2377 (24.8) | 232 (26.0) | 2145 (24.7) |
| CCI comorbidity score | |||
| Normal | 6050 (63.1) | 722 (80.8) | 5328 (61.3) |
| Moderate | 2868 (29.9) | 149 (16.7) | 2719 (31.3) |
| Severe | 671 (7.0) | 23 (2.6) | 648 (7.5) |
| Medical history | |||
| Myocardial infarction | 552 (5.8) | 11 (1.2) | 541 (6.2) |
| Heart failure | 460 (4.8) | 9 (1.0) | 451 (5.2) |
| Lower‐extremity deep venous thrombosis or pulmonary embolism | 230 (2.4) | 13 (1.5) | 217 (2.5) |
| Atrial fibrillation or atrial flutter | 668 (7.0) | 11 (1.2) | 657 (7.6) |
| Chronic kidney disease | 217 (2.3) | 25 (2.8) | 192 (2.2) |
| Chronic pulmonary disease | 725 (7.6) | 25 (2.8) | 700 (8.1) |
| Diabetes mellitus | 973 (10.1) | 69 (7.7) | 904 (10.4) |
| Obesity | 322 (3.4) | 39 (4.4) | 283 (3.3) |
| Alcoholism‐related disorders | 189 (2.0) | 30 (3.4) | 159 (1.8) |
| Pregnancy within 90 d | 7 (0.1) | 7 (0.8) | 0 (0) |
| Surgery within 90 d | 3093 (32.3) | 159 (17.8) | 2934 (33.7) |
| Fracture/trauma within 90 d | 253 (2.6) | 22 (2.5) | 231 (2.7) |
CCI, Charlson Comorbidity Index.
Normal (score = 0), moderate (score = 1‐2), or severe (score ≥ 3),
Overall, we observed 1514 cancer events (Figure 1). During overall follow‐up, we observed a 20% increase in the risk of hematological cancer among patients with retinal vein thrombosis (overall SIR 1.20, 95% CI 0.97‐1.48, data not shown). The <6‐month risk of any cancer was 1.2% (95% CI 1.0%‐1.5%), increasing to 2.4% (95% CI 2.1%‐2.7%) one year after retinal vein thrombosis, and 28.8% (95% CI 26.9%‐30.8%) >5 years (end of follow‐up). The <6 months’ SIR was 1.20 (95% CI 0.99‐1.44; Figure 2) and remained unchanged 6‐12 months following retinal vein thrombosis (SIR 1.15, 95% CI 0.94‐1.39), >1 year after the event (SIR 1.07, 95% CI, 1.01‐1.13), and ≥5 years after the event (SIR 1.08, 95% CI 1.03‐1.14).
Figure 1.
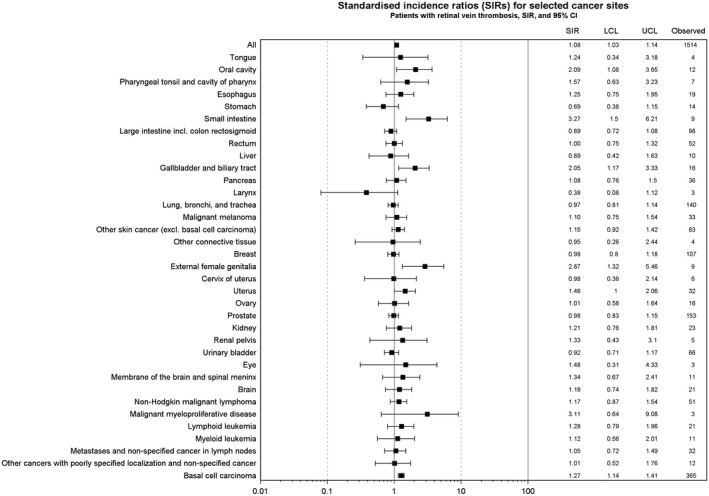
Overall and site‐specific cancer risk during entire follow‐up in patients with retinal vein thrombosis, Denmark, 1997‐2013, expressed as absolute numbers and standardized incidence rates (SIRs) with lower (LCL) upper confidence limits (UCL). Site‐specific cancers with at least three cases included in the figure
Figure 2.
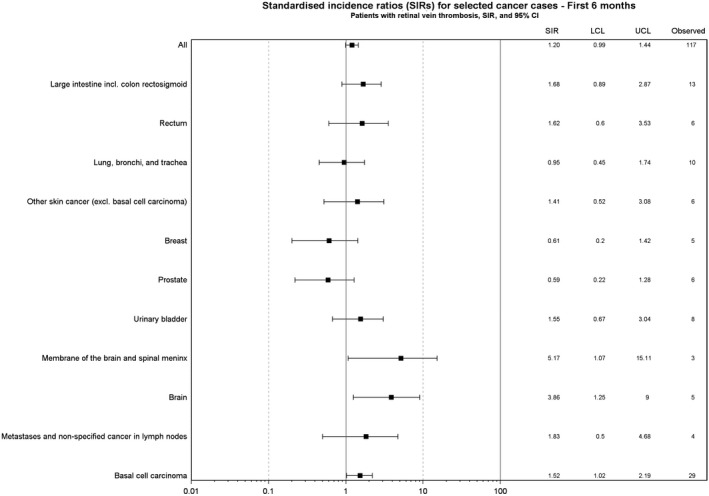
Overall and site‐specific cancer risk during first 6 mo following a retinal vein thrombosis, Denmark, 1997‐2013, expressed as absolute numbers and standardized incidence rates (SIRs) with lower (LCL) and upper confidence limits (UCL). Site‐specific cancers with at least three cases included in the figure
Among patients with retinal vein thrombosis, 53% of cancers were localized tumors, 12% were tumors with regional spread, 15% were tumors with distant metastases, and 20% had missing data on tumor staging. The overall SIR estimates were almost similar in subgroups of patients, according to age, gender, calendar year, and comorbidity burden, measured by the Charlson comorbidity score (Figure 3). Based on 33 excess cancers detected during a follow‐up time of 8969 person‐years (corresponding to the first year of follow‐up), the number of patients with retinal vein thrombosis needed to screen to detect one excess cancer per year was 271 for any cancer.
Figure 3.
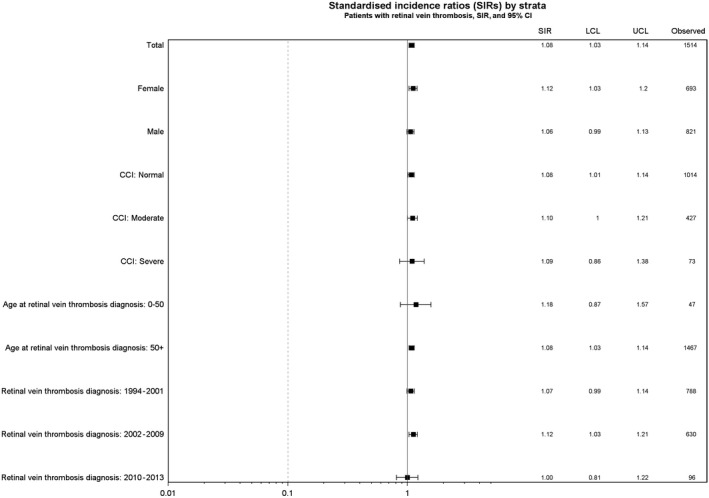
Overall cancer risk during entire follow‐up in patients with retinal vein thrombosis, Denmark, 1997‐2013, expressed as absolute numbers and standardized incidence rates (SIRs) with lower (LCL) upper confidence limits (UCL).Stratified on age and calendar year at retinal vein thrombosis, gender, and Charlson Comobidity Index (CCI)
4. DISCUSSION
In this nationwide population‐based cohort study, the absolute cancer risk within six months following a retinal vein thrombosis was low and only slightly increased compared to the general population. Thus, retinal vein thrombosis was not an important clinical marker for occult cancer. Interestingly, the relative cancer risk beyond the first year following retinal vein thromboses was comparable with the cancer risk reported for deep venous thrombosis.8 This is probably explained by the comorbidity burden or common risk factors. Previous case reports suggesting an association between retinal vein thrombosis and hematological cancers could not be confirmed.2, 3, 10, 14, 15, 16, 17
Although deep venous thrombosis and pulmonary embolism are strong markers for occult cancer,7, 8, 9, 11, 12, 13 this was not observed for retinal vein thrombosis. This suggests that different underlying mechanisms are likely to be involved in the pathophysiology of venous thrombosis at different vascular sites. Disturbances in one or more of the components of the Virchow's triad (stasis of blood flow, vessel wall injury, and hypercoagulability) usually explain deep venous thrombosis and pulmonary embolism. Activation of the coagulation cascade, a systemic hypercoagulability, is essential for the venous clot formation observed in the larger veins11, 13 and may be caused by cancer. In contrast, it seems likely that most cases of retinal vein thrombosis in our study were caused by shared risk factors for atherosclerosis, for example, stiffness in adjacent arteriosclerotic arteries leading to turbulence and retinal thrombus formation.1, 2, 3, 4 This was supported by the high prevalence of arteriosclerosis we observed, especially in the older age groups. By contrast, the subgroup of young patients had a low comorbidity burden and, not surprisingly, had an increased cancer risk within the first year following a retinal vein thrombosis. Other etiologies may have played a role for their thrombosis, for example, underlying cancer. Nevertheless, the estimates for this subgroup were based on few cancer cases with rather imprecise risk estimates. The present study was overall a negative study. Furthermore, we reported no P‐values and made no significance testing. Therefore, adjustments for multiple comparisons are not recommended.29, 30 However, in the present study on cancer risk with multiple comparisons, we cannot rule out by chance findings.
We based our study on prospectively collected nationwide data with complete population coverage in the setting of a universal tax‐supported health care system, which likely eliminated selection and recall bias. Confounding is generally not a problem in the reporting of absolute risks. As regards the relative SIR estimates, stratification by possible confounders did not substantially change the SIRs. The validity of the retinal vein thrombosis in the DNPR is assumed to be high, because nearly all retinal vein thrombosis patients were diagnosed at ophthalmologic departments. Nevertheless, we cannot rule out misclassification of some of the retinal vein thrombotic events. The validity of cancer diagnoses in the DCR is high.21, 22, 23
In conclusion, the absolute risk of occult cancer in retinal vein thrombosis was low, as reflected in the high number needed to screen. An extensive diagnostic cancer workup does not appear warranted for retinal vein thrombosis patients. The clinical impact of these negative findings is important. Furthermore, the results of our study may contribute to our understanding of the mechanisms of carcinogenesis. The occult cancer‐associated hypercoagulabilty contributing to an increased risk of venous thromboembolism does not seem to cause retinal vein thrombosis.11
CONFLICT OF INTEREST
The authors declare no conflict of interests.
Hansen AT, Veres K, Prandoni P, Adelborg K, Sørensen HT. Retinal vein thrombosis and risk of occult cancer: A nationwide cohort study. Cancer Med. 2018;7:5789–5795. 10.1002/cam4.1803
Funding information
The Program for Clinical Research Infrastructure (PROCRIN) supported by the Lundbeck Foundation and the Novo Nordisk Foundation. No grants from NIH.
REFERENCES
- 1. Jaulim A, Ahmed B, Khanam T, Chatziralli IP. Branch retinal vein occlusion: epidemiology, pathogenesis, risk factors, clinical features, diagnosis, and complications. An update of the literature. Retina. 2013;33(5):901‐910. [DOI] [PubMed] [Google Scholar]
- 2. Yoshida A, Watanabe M, Ohmine K, Kawashima H. Central retinal vein occlusion caused by hyperviscosity syndrome in a young patient with Sjogren's syndrome and MALT lymphoma. Int Ophthalmol. 2015;35(3):429‐432. [DOI] [PubMed] [Google Scholar]
- 3. Ratanam M, Ngim YS, Khalidin N, Subrayan V. Intravitreal bevacizumab: A viable treatment for bilateral central retinal vein occlusion with serous macular detachment secondary to Waldenstrom macroglobulinaemia. Br J Haematol. 2015;170(3):431‐434. [DOI] [PubMed] [Google Scholar]
- 4. Martinelli I, De Stefano V. Extra‐abdominal venous thromboses at unusual sites. Best Pract Res Clin Haematol. 2012;25(3):265‐274. [DOI] [PubMed] [Google Scholar]
- 5. Lip GY, Chin BS, Blann AD. Cancer and the prothrombotic state. Lancet Oncol. 2002;3(1):27‐34. [DOI] [PubMed] [Google Scholar]
- 6. Caine GJ, Stonelake PS, Lip GY, Kehoe ST. The hypercoagulable state of malignancy: pathogenesis and current debate. Neoplasia. 2002;4(6):465‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sorensen HT, Svaerke C, Farkas DK, et al. Superficial and deep venous thrombosis, pulmonary embolism and subsequent risk of cancer. Eur J Cancer. 2012;48(4):586‐593. [DOI] [PubMed] [Google Scholar]
- 8. Sorensen HT, Mellemkjaer L, Steffensen FH, Olsen JH, Nielsen GL. The risk of a diagnosis of cancer after primary deep venous thrombosis or pulmonary embolism. N Engl J Med. 1998;338(17):1169‐1173. [DOI] [PubMed] [Google Scholar]
- 9. Baron JA, Gridley G, Weiderpass E, Nyren O, Linet M. Venous thromboembolism and cancer. Lancet. 1998;351(9109):1077‐1080. [DOI] [PubMed] [Google Scholar]
- 10. Castro‐Navarro V, Odaibo SG, Ghodasra DH, Besirli CG. Bilateral BRVO in a patient with recurrent prostate cancer. BMJ Case Rep. 2015;2015:bcr2015212463. 10.1136/bcr-2015-212463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Falanga A, Russo L, Milesi V. The coagulopathy of cancer. Curr Opin Hematol. 2014;21(5):423‐429. [DOI] [PubMed] [Google Scholar]
- 12. Prandoni P, Lensing AW, Buller HR, et al. Deep‐vein thrombosis and the incidence of subsequent symptomatic cancer. N Engl J Med. 1992;327(16):1128‐1133. [DOI] [PubMed] [Google Scholar]
- 13. Plegmasia Alba Dolens. In: Trousseau A. lectures on clinical medicine, delivered at the Hotel‐Dieu, Paris. Cormack JR, trans. London: New Sydenham Society, 1872:281–295. [Google Scholar]
- 14. Golesic EA, Sheidow TG. An otherwise healthy young man presents with bilateral CRVO as the first sign of hyperviscosity syndrome in the setting of new multiple myeloma. Retin Cases Brief Rep. 2015;9(1):38–40. [DOI] [PubMed] [Google Scholar]
- 15. Tee J, Spratt A, Cwynarski K, Davey C. Central retinal vein occlusion heralding the relapse of haematodermic neoplasm. Br J Haematol. 2008;143(3):000–000. [DOI] [PubMed] [Google Scholar]
- 16. Moisseiev E, Ling J, Morse LS. Leukemic optic nerve infiltration complicated by retinal artery and vein occlusions. Retina. 2017;37(2):e10. [DOI] [PubMed] [Google Scholar]
- 17. Casares PZ, Gillet DS, Verity DH, Rowson NR. Bilateral simultaneous central retinal vein occlusion (CRVO) caused by Waldenstrom's macroglobulinaemia with acquired von Willebrand's disease. Br J Haematol. 2002;118(1):344–347. [DOI] [PubMed] [Google Scholar]
- 18. Talcott KE, Garg RJ, Garg SJ. Ophthalmic manifestations of leukemia. Curr Opin Ophthalmol. 2016;27(6):545–551. [DOI] [PubMed] [Google Scholar]
- 19. Schmidt M, Pedersen L, Sorensen HT. The Danish civil registration system as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541–549. [DOI] [PubMed] [Google Scholar]
- 20. Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sorensen HT. The Danish national patient registry: A review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Storm HH, Michelsen EV, Clemmensen IH, Pihl J. The Danish cancer registry–history, content, quality and use. Dan Med Bull. 1997;44(5):535–539. [PubMed] [Google Scholar]
- 22. Gjerstorff ML. The Danish cancer registry. Scand J Public Health. 2011;39(7 Suppl):42–45. [DOI] [PubMed] [Google Scholar]
- 23. Statens serum institut. det moderniserede cancerregister—metode og kvalitet. 2009.
- 24. Bjerregaard B, Larsen OB. The Danish pathology register. Scand J Public Health. 2011;39(7 Suppl):72–74. [DOI] [PubMed] [Google Scholar]
- 25. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 26. Ording AG, Sorensen HT. Concepts of comorbidities, multiple morbidities, complications, and their clinical epidemiologic analogs. Clin Epidemiol. 2013;5:199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rothman KJ, Greenland S, Lash TL. Modern Epidemiology, 3rd edn Philadelphia, PA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 28. Schmidt SA, Veres K, Ording AG, Farkas DK, Fogh K, Sorensen HT. Venous ulcers and risk of occult hematological or other cancers: A nationwide cohort study. Blood. 2016;128(6):874–877. [DOI] [PubMed] [Google Scholar]
- 29. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–46. [PubMed] [Google Scholar]
- 30. Rothman KJ. Six persistent research misconceptions. J Gen Intern Med. 2014;29(7):1060–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]


