Abstract
Purpose: to assess synovial inflammation using musculoskeletal ultrasonography, in a cohort of patients with early rheumatoid arthritis, and establish the correlations with disease activity score. Material and methods: We enrolled 29 patients diagnosed with early RA, according to ACR-EULAR 2010 classification criteria, in Rheumatology Clinic, Emergency County Hospital Craiova, between September 2013-August 2014. We performed clinical evaluation, assessed laboratory tests and performed US for all the patients. Musculoskeletal ultrasonography was performed using an ESAOTE MyLab 25 ultrasound, by the same examiner, with multi-frequency linear array transducers (7-12Mh). The presence of synovitis was assessed both in grey scale (GSUS) and power-Doppler (PDUS), according to OMERACT-EULAR consent. Results: Assessment of synovial inflammatory activity by ultrasound examination, using both grey scale and PDUS, has shown active synovitis in all patients, with a mean number of joints with active synovitis of 5. Evaluating the grade, using PDUS, we found grade 1 in all patients, 2 in 14 and grade 3 in 3 patients. Inflammatory markers correlated significant with both GSUS and PDUS. Analysis of correlation between GSUS examination parameters and disease activity score, found a positive, moderate inter-relation, results found also for PD score. Conclusion: our results sustain the role of US for detecting inflammatory activity in early RA patients, and, in addition with clinical and biological assessment, represents a sensitive, reliable and reproducible method, easily approached, that should be included in our routine evaluation.
Keywords: early rheumatoid arthritis, ultrasonography, disease activity score
Introduction
Rheumatoid arthritis (RA) a chronic, autoimmune, inflammatory disease, characterized by progressive joint damage, event that begins from the first weeks of evolution [1]. The primary site of inflammation is represented by synovial membrane, and in early stages, the first alteration is constituted by synovial inflammation, followed by consecutive synovial proliferation, associated with increased vascularization and angiogenesis, start point in pathogenesis of rheumatoid arthritis [2, 3]. Therefore, assessment of synovial inflammation is essential in order to enable the therapeutic measures, appreciate the outcome, evaluate response to treatment and achieve remission, the current aim of RA treatment [4].
Synovitis has been traditionally assessed, indirectly, by evaluating inflammatory markers and clinical examination [3]. Also, in daily practice, composite indices are used to assess disease activity, considering tender and swollen joints, visual analog scale by physician and patient, erythrocyte sedimentation rate and/or C-reactive protein [5,6,7]. These indices however, may have some disadvantages and miss accuracy due to increase subjectivity in patients with associated depression or fibromyalgia, joint deformities that can interfere with tender and swollen joint count etc. [8,9,10,11]. Imaging techniques, such as magnetic resonance imaging (MRI) and musculoskeletal ultrasonography (US) have an increasingly important contribution in evaluation and monitoring such patients [3]. Although it has been proven that has a strong correlation with histological data and provides a predictive value in structural joint damage, MRI is rather expensive, time consuming, not always available for routine examinations and difficult to reproduce [12, 13]. US, by its increased degree of resolution due to high-frequency transducers, constitutes a reliable and compulsory method to diagnose and monitor RA patients. Unlike MRI is relatively cheap, available and can be used as many times as necessary during patient examination, improving the exactitude of clinical examination [14,15,16]. Both grey-scale and power Doppler ultrasonography are extensively used to detect synovial inflammation and appreciate disease activity, in addition to clinical and laboratory data, in order to apply the proper therapeutic measures, monitor the patients and assess the outcome [15, 17].
The aim of the study was to assess synovial inflammation using musculoskeletal ultrasonography, in a cohort of patients with early rheumatoid arthritis, and establish the correlations with disease activity score.
Material and Methods
We enrolled 29 patients diagnosed with early RA, according to ACR-EULAR 2010 classification criteria, in Rheumatology Clinic, Emergency County Hospital Craiova, between September 2013-August 2014, . All the patients signed the informed consent and the study was approved by the Local Ethic Committee.
We performed clinical evaluation, assessed laboratory tests and performed US for all the patients. Evaluation included recording of age, sex, body mass index, medical history and symptoms duration. Clinical examination assessed 28 joints (glenohumeral, elbow, wrist, metacarophalangeal-MCP, proximal interphalangeal-PIP of the hands and knees) for tenderness and swelling. Global pain intensity was assessed by the pateint and doctor, using visual analog scale score, range 0-100mm. Functional status was evaluated using Health Assessment Questionnaire (HAQ) [18] and disease activity by calculating 28-Disease Activity Score (DAS) [19], with 4 variables and simplified disease activity score (SDAI)[20]. Laboratory assessment included serum inflammatory markers (erythrocyte sedimentation rate-ESR and C-reactive protein-CRP), rheumatoid factor-RF, anti-cyclic citrullinated peptide antibodies-CCP and usual biological analysis.
Musculoskeletal ultrasonography was performed using an ESAOTE MyLab 25 ultrasound, by the same examiner, with multi-frequency linear array transducers (7-12Mh). The presence of synovitis was assessed both in grey scale (GSUS) and power-Doppler (PDUS), according to OMERACT-EULAR consent [21].
GraphPad Prism 5.5 program was used for statistical analysis. Data were presented as mean ±standard deviation (mean±SD). We compared groups using T-test and calculated Pearson coefficient for establishing correlations. A p value less than 0.05 was considered statistically significant.
Results
Our cohort included 29 consecutive patients, diagnosed with early rheumatoid arthritis, with a duration of the symptoms <1 year, diagnosed with early RA, with a duration of the symptoms under 12 months, 25 (86.20%) women and 4 men, with a mean age of 48.97±9.72 years. Analysis of the inflammatory profile, recorded a mean ESR of 30.41±10.88 mm/h and a CRP that ranged from 0.53 to 3.1 mg/dl, with a mean of 1.180± 0.4933. RF was present in 72.41% of the cases (21) and anti-CCP antibodies in 58.62% (17 cases). All the data for the patients are exposed in Table 1.
Table 1.
General characteristics of the patients
| N | Mean | CI 95% | SD | Median | Min | Max | |
| Sex(women)(N; %) | 25 (86.20) | - | - | - | - | - | - |
| Sex (men)(N; %) | 4 (13.80) | - | - | - | - | - | - |
| Age (years) | 29 | 48.97 | 44.78-53.15 | 10.99 | 51 | 21 | 65 |
| Weight (kg) | 29 | 59.21 | 61.2-82.9 | 14.65 | 47 | 43 | 92 |
| Height (cm) | 29 | 156.81 | 161.0-171.2 | 11.21 | 167 | 153 | 172 |
| ESR (mm/h) | 29 | 14.28 | 11.74-16.82 | 6.154 | 28.00 | 20.00 | 70.00 |
| CRP (mg/dl) | 29 | 1.180 | 0.39-0.42 | 0.49 | 1.100 | 0.5300 | 3.10 |
| RF (UI/ml) | 29 | 40.12 | 26.69-53.54 | 33.24 | 32.00 | 5 | 23 |
| Anti-CCP | 29 | 118.4 | 67.90-236.6 | 129.0 | 45.00 | 3.000 | 375 |
| NAD | 29 | 7.93 | 2.70-3.98 | 2.28 | 8.00 | 4.00 | 15.00 |
| NAT | 29 | 3.75 | 2.83-4.68 | 2.43 | 4.00 | 0 | 10.00 |
| VAS | 29 | 52.76 | 47.89-57.62 | 12.79 | 50.00 | 30.00 | 100.00 |
| DAS28(4v) | 29 | 5.11 | 4.87-5.34 | 0.61 | 5.12 | 3.81 | 6.02 |
| HAQ | 29 | 1.24 | 1.05-1.43 | 0.49 | 1.00 | 0.70 | 2.80 |
Evaluating disease activity, we found a mean DAS28 (4v) of 5.11± 0.61; 15 patients (51.72%) had a high disease activity and 14 (48.27%) patients a moderate disease activity. The disability index, calculated using health assessment questionnaire (HAQ) had a mean value of 1.31±0.53, limits 0.7-2.8.
Assessment of synovial inflammatory activity by ultrasound examination, using both grey scale and PDUS, has shown active synovitis in all patients (Fig. 1a), with a mean number of joints with active synovitis of 5 (limits between 1 and 12). Evaluating the grade, using PDUS, we found grade I in all patients, II in 14 and grade 3 in 3 patients (Fig. 1b).
Figure 1.
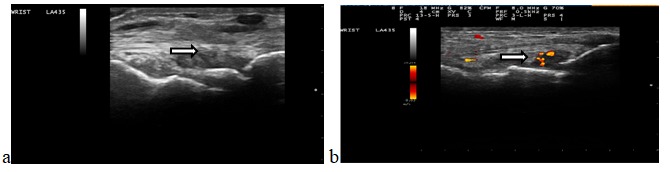
a.GSUS synovitis grade 3; b. PDUS synovitis grade 2, PD grade 2
Analysis of these findings depending on disease activity, we found a mean number of 6.93±1.33 joints with active synovitis in patients with a high disease activity score, whereas for patients with a moderate disease activity the mean was 5.21±1.85, difference statistically significant, p=0.007 (Fig. 2). Also, regarding PDUS, for the 15 patients with high disease activity, the mean number of joints with PD grade 1 synovits was 6.26±1.28, grade 2 0.66±0.69; grade 3 was found in 3 patients, for 2 in one joint and for one in 2 joints. The 14 patients with a moderate disease activity had 4.57±2.02 joints with PD grade 1 synovitis and 0.50±0.62 with PD grade 2 synovitis.
Figure 2.
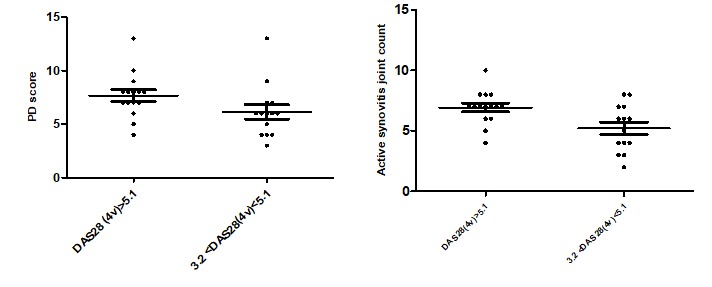
GSUS/PDUS findings depending on DAS28(4v)
Inflammatory markers correlated significant with both GSUS and PDUS, with a Pearson correlation coefficient of 0.598, p=0.0006 for ESR/GSUS; 0.5917, p= 0.0007 ESR/PDUS and 0.654, p=0.0001 CRP/GSUS; 0.5995, p= 0.0006 CRP/PDUS (Table 2).
Table 2.
Correlations between GSUS/PDUS and inflammatory markers
| ESR | CRP | |||||
| r | p | 95% CI | r | p | 95% CI | |
| GSUS | 0.598 | 0.0006 | 0.29-0.79 | 0.6549 | 0.0001 | 0.37-0.82 |
| PDUS | 0.591 | 0.0007 | 0.28-0.78 | 0.599 | 0.0006 | 0.29-0.79 |
Analysis of correlation between GSUS examination parameters and disease activity score, found a positive, moderate inter-relation, p=0.453, CI 95% 0.104 to 0.703, statistically significant, p=0.013. For PD score, we also found a positive correlation, r= 0.427, p= 0.020 (Table 3, Fig.3). Regarding disability index, HAQ, the correlation coefficient was 0.32 (p=0.03)/0.41 (p=0.10) (GSUS/PDUS).
Table 3.
Correlations between GSUS/PDUS and DAS28(4v)
| DAS 28(4v) | |||
| r | p | 95% CI | |
| GSUS | 0.453 | 0.013 | 0.10-0.70 |
| PDUS | 0.427 | 0.020 | 0.07-0.68 |
Figure 3.
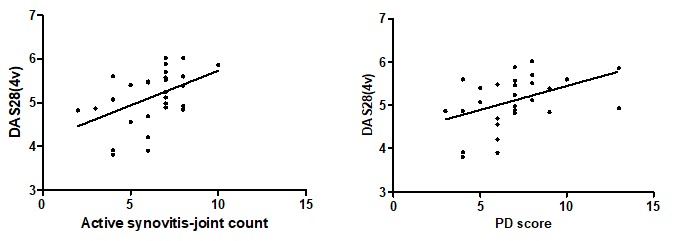
Correlations between GSUS/PDUS-DAS28 (4v)
Discussion
Rheumatoid arthritis is a chronic, autoimmune disease, characterized by systemic inflammation, with progressive joint damage, events that begin from the first weeks of evolution. The primary site of inflammation is represented by synovial membrane, and, in early stages, the first alteration is constituted by synovitis, followed by consecutive synovial proliferation, associated with increased vascularization and angiogenesis, the start point in pathogenesis of rheumatoid arthritis [1,2,3]. These findings constituted the start point of assessing the direct relationship between synovial vascularization and joint inflammatory activity [4]. In daily practice, several composite indices are used to evaluate disease activity, considering tender and swollen joint count, visual analog scales by patient and physician, inflammation markers, but, sometimes, they can have a lower accuracy due to associated fibromyalgia, depression, deformities that influence joint count etc [8,9,10,11]. Imaging methods, such as musculoskeletal ultrasonography or magnetic resonance, are precise, more sensitive and reproducible than clinical evaluation in assessing joint inflammation [14,15,16,17]. Moreover, US can be used as often as required during patient examination, improving the exactitude of clinical data.
We studied a cohort of 29 patients, diagnosed with early RA, using both GSUS and PDUS, as US variables that indicate disease activity. US examination has shown active synovitis in all patients, with a mean number of joints with active synovitis of 5 (limits between 1 and 12). Evaluating the grade, using PDUS, we found grade 1 in all patients, 2 in 14 and grade 3 in 3 patients. Similar US findings, of estimating local inflammation and activity, have been reported by several studies [3, 15, 22, 23, 24]. We found a significant correlation between inflammatory markers, ESR (with a mean value of 30.41+ 10.88 mm/h) and CRP (mean value 1.180+ 0.493 mg/dl), and both GSUS (moderate r=0.598, p= 0.0006 for ESR and r= 0.654, p=0.0001 for CRP) and PDUS (moderate p= 0.5917, p= 0.0007 for ESR and r= 0.5995, p= 0.0006 for CRP). Also Scire et al [22] reported, in 2009, a positive, moderate correlation, statistically significant, between inflammatory markers and both active synovitis count and the synovial vascularization index, obtained by PDUS, on a cohort of 106 early RA. Moreover, our findings are in consistency with several previous reports, as the one published Naredo et al, in 2007 [3], on a group of 42 early RA patients, Naredo et al in 2005 [15], Terslev et al [25] and Hameed et al in 2008 [26]. This results sustain the general accepted concept that PD is a marker of active synovitis, directly inter-related with inflammatory activity [15, 22, 26].
The report published by Scire et al [22], also stated a significant correlation between DAS 28(4v) and US examination, both GS and PD, results reported by several other recent trials, as the one published in Arthritis&Rheum, in 2009, by Bachaus et al [27], Watanabe et al in 2012 [28], in Clinical Rheumatology, Naredo et al in 2007, Arthritis&rRheum [3], etc. [14, 29, 30, 31]. Our results show a positive, moderate inter-relation, for both GSUS (p=0.47, statistically significant, p=0.009), and PDUS score (r= 0.4279, p= 0.0206), in agreement with previous studies.
Several scientific reports have concluded that angiogenesis, reflected by vascularization in early RA synovial proliferation, has a pathogenic destructive role [1, 2, 3], reflecting the importance of PDUS as a strong predictor of disease evolution and an important tool for therapeutic decisions.
Conclusions
Our results sustain the role of US for detecting inflammatory activity in early RA patients, and, in addition with clinical and biological assessment, represents a sensitive, reliable and reproducible method, easily approached, that should be included in our routine evaluation, in order to enable therapeutic decisions in early RA patients and obtain remission, the current aim of the treatment.
Acknowledgement
”This paper was published under the frame of European Social Fund, Human Resources Development Operational Program 2007-2013, Parteneriat strategic pentru creșterea calității cercetării științifice din universitățile medicale prin acordarea de burse doctorale și postdoctorale, project no. POSDRU/159/1.5/136893
References
- 1.Conaghna P, O'Connor P, McGonagle D, et al. Elucidation of the relationship between synovitis and bone damage: a randomized magnetic resonance study of individual joints in patients with early rheumatoid arthritis. Arthritis & Rheumatism. 2003;48:64–71. doi: 10.1002/art.10747. [DOI] [PubMed] [Google Scholar]
- 2.Pap T , Distler O. Linking angiogenesis to bone destruction in arthritis. Arthritis & Rheumatism. 2005;52:1346–1348. doi: 10.1002/art.21015. [DOI] [PubMed] [Google Scholar]
- 3.Naredo E, Collado P, Cruz A, et al. Longitudinal Power Doppler Ultrasonographic Assessment of Joint Inflammatory Activity in Early Rheumatoid Arthritis: Predictive value in disease activity and radiologic progresssion. Arthritis & Rheumatism. 2007;57:116–124. doi: 10.1002/art.22461. [DOI] [PubMed] [Google Scholar]
- 4.Monteccuco C. Remission, a therapeutic goal in inflammatory artropathies?, Clinical data from adalimumab studies. Drugs. 2006;66:1783–1795. doi: 10.2165/00003495-200666140-00001. [DOI] [PubMed] [Google Scholar]
- 5.Anderson J, Caplan L, Yazdany Y, et al. Rheumatoid arthritis disease activity measures: American College Rheumatology recommendations for use in clinical practice. Arthritis Care Res. 2012;64(5):640–647. doi: 10.1002/acr.21649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gajoux-Vilaia C, Mouterde G, Smolen J, et al. Evaluating disease activity in rheumatoid arthritis: which composite index is best? a systematic literature analysis of studies comparing the psychometric properties of the DAS, DAS28, SDAI and CDAI. Joint Bone Spine. 2012;79(2):149–155. doi: 10.1016/j.jbspin.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Felson DT, Smolen JS, Wells G, Zhang B, van Tuyl LH, Funovits J, et al. American College of Rheumatology/European League against rheumatism provisional definition of remission in rheuma- toid arthritis for clinical trials. Ann Rheum Dis. 2011;70(3):404–413. doi: 10.1136/ard.2011.149765. [DOI] [PubMed] [Google Scholar]
- 8.Rathbun AM, Reed GW, Harrold LR. The temporal relationship between depression and rheumatoid arthritis disease activity, treatment persistence, and response: a systematic review. Rheumatology Oxford. 2013;52(10):1785–1794. doi: 10.1093/rheumatology/kes356. [DOI] [PubMed] [Google Scholar]
- 9.Ranzolin A, Brenol JC, Bredemeier M, Guarienti J, Rizzatti M, Feldman D, et al. Association of concomitant fibromyalgia with worse disease activity score in 28 joints, health assessment questionnaire, and short form 36 scores in patients with rheuma- toid arthritis. Arthritis Rheum. 2009;61(6):794–800. doi: 10.1002/art.24430. [DOI] [PubMed] [Google Scholar]
- 10.Jousse-Joulin S, d'Agostino MA, Marhadour T, Albert JD, Bentin J, Chary Valckenaere I, et al. Reproducibility of joint swelling assessment by sonography in patients with long-lasting rheumatoid arthritis (SEA-Repro study part II) J Rheumatol. 2010;37(5):938–945. doi: 10.3899/jrheum.090881. [DOI] [PubMed] [Google Scholar]
- 11.Le Boedec M, Jousse-Joulin S, Ferlet JF, Marhadour T, Chales G, Grange L. Factors influencing concordance between clinical and ultrasound findings in rheumatoid arthritis. J Rheumatol. 2013;40(3):244–252. doi: 10.3899/jrheum.120843. [DOI] [PubMed] [Google Scholar]
- 12.Ostendorf B, Peters R, Dann P, et al. Magnetic resonance imaging and miniarthroscopy of metacarpophalangeal joints: sensitive detection of morphologic changes in rheumatoid arthritis. Arthritis & Rheumatism. 2001;44:2492–2502. doi: 10.1002/1529-0131(200111)44:11<2492::aid-art429>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 13.McGonagle D, Conaghan PG, O'Connor P, et al. The relationship between synovitis and bone changes in early untreated rheumatoid arthritis: a cotrolled magnetic resonance imaging study. Arthritis & Rheumatism. 1999;42:1232–1245. doi: 10.1002/1529-0131(199908)42:8<1706::AID-ANR20>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 14.Damjanov N, Radunovic G, Prodanovic S, Vukovic V, Milic V, Simic Pasalic K, et al. Construct validity and reliability of ultrasound disease activity score in assessing joint inflammation in RA: comparison with DAS-28. Rheumatology (Oxford) 2012;51(1):120–128. doi: 10.1093/rheumatology/ker255. [DOI] [PubMed] [Google Scholar]
- 15.Naredo E, Bonilla G, Gamero F, Uson J, Carmona L, Laffon A. Assessment of inflammatory activity in rheumatoid arthritis: a comparative study of clinical evaluation with gray scale and power Doppler ultrasonography. Ann Rheum Dis. 2005;64(3):375–381. doi: 10.1136/ard.2004.023929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mendonca R, Mendonca J, Viegas Brenol C, et al. Assessing rheumatoid arthritis disease activity with ultrasound. Clin Rheumatol. 2013;32:1249–1254. doi: 10.1007/s10067-013-2291-6. [DOI] [PubMed] [Google Scholar]
- 17.Farrant JM, O'Connor PJ, Grainger AJ. Advanced imaging in rheumatoid arthritis. Part 1: synovitis. Skeletal Radiol. 2007;36:269–279. doi: 10.1007/s00256-006-0219-9. [DOI] [PubMed] [Google Scholar]
- 18.Naredo E1, Wakefield RJ, Iagnocco A, et al. The OMERACT ultrasound task force-status and perspectives. J Rheumatol. 2011;38(9):2063–2067. doi: 10.3899/jrheum.110425. [DOI] [PubMed] [Google Scholar]
- 19.Wells G, Becker JC, Teng J, Dougados M, Schiff M, Smolen J, et al. Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis. 2009;68(6):954–960. doi: 10.1136/ard.2007.084459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smolen JS, Aletaha D. Activity assessments in rheumatoid arthritis. Curr Opin Rheumatolo. 2008;57:440–447. doi: 10.1097/BOR.0b013e3282fbd382. [DOI] [PubMed] [Google Scholar]
- 21.Naredo E1, Wakefield RJ, Iagnocco A, et al. The OMERACT ultrasound task force--status and perspectives. J Rheumatol. 2011;38(9):2063–2067. doi: 10.3899/jrheum.110425. [DOI] [PubMed] [Google Scholar]
- 22.Scire CA, Montecucco C, Codullo V, et al. Ultrasonographic evaluation of joint involvement in early rheumatoid arthritis in clinical remission: power Doppler signal predicts short-term relapse. Rheumatology (Oxford) 2009;48(9):1092–1097. doi: 10.1093/rheumatology/kep171. [DOI] [PubMed] [Google Scholar]
- 23.Funck-Brentano T, Gandjbakhch F, Etchepare F, Jousse-Joulin S, et al. Sonographic erosions and power-Doppler signal predict radiographic damage in early arthritis: The ESPOIR ultrasonography longitudinal study. Arthritis Care Res(Hoboken) 2013;65(6):896–902. doi: 10.1002/acr.21912. [DOI] [PubMed] [Google Scholar]
- 24.Saleem B, Brown AK, Quinn M, Karim Z, Hensor EM, Conaghan P, et al. Can flare be predicted in DMARD-treated RA patients in remission, and is it important? A cohort study. Ann Rheum Dis. 2012;1(8):1316–1321. doi: 10.1136/annrheumdis-2011-200548. [DOI] [PubMed] [Google Scholar]
- 25.Terslev L1, von der Recke P, Torp-Pedersen S, et al. Diagnostic sensitivity and specificity of Doppler ultrasound in rheumatoid arthritis. J Rheumatol. 2008;35(1):49–53. [PubMed] [Google Scholar]
- 26.Hameed B, Pilcher J, Heron C, et al. The relation between composite ultrasound measures and the DAS 28 score, its components and acute phase markers in adult RA. Rheumatolog. 2008;47:460–480. doi: 10.1093/rheumatology/kem383. [DOI] [PubMed] [Google Scholar]
- 27.Backhaus M, Ohrndorf S, Kellner H, et al. Evaluation of a novel 7-joint ultrasound score in daily rheumatologic practice: a pilot project. Arthritis Rheum. 2009;61(9):1194–1201. doi: 10.1002/art.24646. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe T, Takemura M, Sato M, et al. Quantitative analysis of vascularization in the finger joints in patients with rheumatoid arthritis using three- dimensional volumetric ultrasonography with power Doppler. Clin Rheumatol. 2012;31(2):299–307. doi: 10.1007/s10067-011-1811-5. [DOI] [PubMed] [Google Scholar]
- 29.Ellegaard K, Torp-Pedersen S, Terslev L, Danneskiold-Samsoe B, Henriksen M, Bliddal H. Ultrasound color Doppler mea- surements in a single joint as measure of disease activity in patients with rheumatoid arthritis-assessment of concurrent validity. Rheumatology (Oxford) 2009;48(3):254–257. doi: 10.1093/rheumatology/ken459. [DOI] [PubMed] [Google Scholar]
- 30.Hammer HB, Sveinsson M, Kongtorp AK, Kvien TK. A 78-joints ultrasonographic assessment is associated with clinical as- sessments and is highly responsive to improvement in a longitudinal study of patients with rheumatoid arthritis starting adalimumab treatment. Ann Rheum Dis. 2010;69(7):1349–1351. doi: 10.1136/ard.2009.126995. [DOI] [PubMed] [Google Scholar]
- 31.Larche MJ, Seymour M, Lim A, Eckersley RJ, Petavy F, Chiesa F, et al. Quantitative power Doppler ultrasonography is a sensitive measure of metacarpophalangeal joint synovial vascularity in rheumatoid arthritis and declines significantly following a 2-week course of oral low dose corticosteroids. J Rheumatol. 2010;37(12):2493–2501. doi: 10.3899/jrheum.100322. [DOI] [PubMed] [Google Scholar]


