Abstract
Background
To compare the predictive value of the current AJCC stage grouping for renal cell carcinoma (RCC) to our modifications.
Patients and methods
A total of 2120 patients with RCC from Fudan University Shanghai Cancer Center (FUSCC) database and 74 506 counterparts from SEER database were included. Cox regression was used to calculate the relative impacts between prognostic groups. The predictive accuracy of overall survival (OS) was assessed using the concordance index (C‐index), which was compared by likelihood ratio test.
Results
In FUSCC cohort, the 5‐year‐OS rate for T3N0M0 patients was higher than T1‐3N1M0 (72.7% vs 38.1%). The 5‐year‐OS rate for T4N0M0 was 36.2%, which was close to T1‐3N1M0 but not to T4N1M0 (0%) and TanyNanyM1 (12.6%). The elements of AJCC groups were regrouped according to the ranks of hazard ratios. The modified stages II (T3N0M0), III (T1‐3N1M0, T4N0M0), and IV (T4N1M0, TanyNanyM1) exhibited greater survival stratification than AJCC groups. The modifications were validated in SEER cohort and yielded similar survival outcomes. The predictive accuracy of OS in modified prognostic groups was significantly higher than AJCC groups in stages II‐IV subgroups in both FUSCC (C‐index: 0.801 vs 0.779, P < 0.001) and SEER cohort (C‐index: 0.770 vs 0.764, P < 0.001).
Conclusions
The modified AJCC prognostic groups for RCC provided significantly improved survival prediction compared with the 8th AJCC edition. A precise risk stratification of modified stages II‐IV disease provides an important basis for risk‐equivalent treatment recommendation.
Keywords: AJCC staging system, overall survival, renal cell carcinoma
1. INTRODUCTION
Nearly 63 990 new cases of kidney and renal pelvis cancer were expected to be diagnosed in the USA in 2017, accounting for about 5% of all new cases in males and 3% in females.1, 2 Tumor stage is considered as the most important prognostic parameter for the clinical behavior and outcome of renal cell carcinoma (RCC).3
The American Joint Committee on Cancer (AJCC) tumor‐node‐metastasis stage grouping (TNM) is the most commonly used cancer staging system.4, 5, 6 The eighth edition (8th) of the AJCC stage grouping was the latest version. Despite some minor revisions in comparison with the seventh (7th) system, there were no changes in the AJCC prognostic stage grouping. It means that the system of old prognostic stage grouping has no changes in nearly ten years and will continue to be used.
Stage grouping (TNM) plays an important role in treatments decisions according to the National Comprehensive Cancer Network (NCCN) guidelines, especially in the selection criteria for adjuvant therapy.7 High‐risk patients with RCC were recommended for clinical trials and adjuvant therapy. Therefore, precise stage grouping is critical for accurate risk stratification in RCC.8 However, problems arose in the selection of suitable patients for adjuvant therapy with the application of AJCC stage grouping in various populations.9, 10, 11
Given the need for more precise stage grouping and treatment stratification,12 we investigated the overall survival (OS) of each subgroup and refined prognostic stage grouping in a large population of patients. Hence, the purpose of our study was to validate the predictive value and feasibility of our modifications in AJCC prognostic stage grouping.
2. METHODS
2.1. Patients
2.1.1. FUSCC cohort
The Fudan University Shanghai Cancer Center (FUSCC) cohort group (training set) of patients with RCC was obtained from the FUSCC (2000‐2015). Our study was approved by the Ethics Committee of FUSCC. All patients with RCC have been histologically confirmed by surgery or biopsy in our department. In addition, abdominal/pelvic computed tomography (CT) scan and Magnetic Resonance Imaging (MRI) were used when needed. Patients included in this study were staged according to the definitions of the 8th AJCC stage grouping. After informed consent was obtained, patients were well informed of the importance of follow‐up. Patients were regularly followed up every 3 months for the first 3 years, then every 6 months up to 5 years, then annually thereafter.
2.1.2. SEER cohort
The Surveillance, Epidemiology, and End Results (SEER) cohort was used as the test set because of its large sample size. From the SEER database, the test cohort data were retrieved from 2004 to 2014. Only patients with microscopically confirmed RCC (using ICD‐O‐3 histology/behavior codes: 8260/3, 8270/3, 8290/3, 8310/3, 8312/3, 8316/3, 8317/3, 8319/3, 8320/3, 8323/3, 8480/3, and 8510/3) were included. The other variables such as year of diagnosis, age at diagnosis, race/ethnicity, and sex were also collected. For staging information, the following codes were obtained from SEER: (a) Derived AJCC Stage Group, 6th ed (2004+), Derived AJCC Stage Group, 7th ed (2010+). (b) Derived AJCC T, 6th ed (2004+), Derived AJCC T, 7th ed (2010+). (c) Derived AJCC N, 6th ed (2004+), Derived AJCC N, 7th ed (2010+). (d) Derived AJCC M, 6th ed (2004+), Derived AJCC M, 7th ed (2010+). (e) OS and follow‐up data. In addition, TxNanyM0, TxNanyMx, TanyNxM0, and TanyNxMx patients were excluded. If patients had both 6th and 7th AJCC Stage Group information, they were restaged according to the definitions of TNM the 7th AJCC staging system. Patients with tumor that directly invaded the ipsilateral adrenal gland were classified into T3a in 6th AJCC Stage Group but T4 in 7th AJCC Stage Group. The patients classified into T3a only by 6th AJCC Stage System information were excluded. Flow diagram of selection is outlined in Figure S1.
2.2. Statistical analyses
OS is defined as the months from the date of diagnosis to the date of death or last follow‐up. Patients were censored if they were lost to follow‐up or died. To assess the associations between each group in stage grouping and outcome, Kaplan‐Meier curves and log‐rank tests were used. Cox proportional hazards analysis was used to assess the relative impacts of different stages on OS.
The concordance index (C‐index) and heatmaps were performed to evaluate the discriminatory powers of the two staging systems. To assess predictive ability, likelihood ratio test was used to compare the C‐index of both staging system. All statistical analyses were performed using R (version 3.4.2, http://www.r-project.org). All statistical tests were 2‐sided, and P value < 0.05 was considered statistically significant.
3. RESULTS
3.1. Cohort characteristics
FUSCC cohort of 2120 RCC patients with 68.4% of males were included. The median age of the patients at diagnosis was 55.0 years. Additionally, 74 506 patients from SEER cohort were included. Of these, 46 928 (63%) were male and 27 578 (37%) were female. The median age at diagnosis was 60.3 years. Table 1 summarizes the demographic and clinical characteristics of the patients in both cohort.
Table 1.
The demographic and clinical characteristics of SEER and FUSCC cohort
| SEER cohort | FUSCC cohort | |
|---|---|---|
| Characteristics | n = 74 506 | n = 2120 |
| Age, years | ||
| <65 | 45 933 (61.65) | 1633 (77.03) |
| ≥ 65 | 28 573 (38.35) | 487 (22.97) |
| Sex | ||
| Male | 46 928 (62.99) | 1450 (68.40) |
| Female | 27 578 (37.01) | 670 (31.60) |
| 8th AJCC TNM stage | ||
| T1N0M0 | 47 954 (64.36) | 1493 (70.42) |
| T1N1M0 | 122 (0.16) | 19 (0.90) |
| T2N0M0 | 7753 (10.41) | 190 (8.96) |
| T2N1M0 | 192 (0.26) | 19 (0.90) |
| T3N0M0 | 7162 (9.61) | 164 (7.74) |
| T3N1M0 | 531 (0.71) | 31 (1.46) |
| T4N0M0 | 325 (0.44) | 16 (0.75) |
| T4N1M0 | 85 (0.11) | 9 (0.42) |
| TanyNanyM1 | 10 382 (13.93) | 179 (8.44) |
| 8th AJCC prognostic stage | ||
| I | 47 954 (64.36) | 1493 (70.42) |
| II | 7753 (10.41) | 190 (8.96) |
| III | 8007 (10.75) | 233 (10.99) |
| IV | 10 792 (14.48) | 204 (9.62) |
| Modified 8th AJCC prognostic stage | ||
| I | 55 707 (74.77) | 1683 (79.39) |
| II | 7162 (9.61) | 164 (7.74) |
| III | 1170 (1.57) | 85 (4.01) |
| IV | 10 467 (14.05) | 188 (8.87) |
| Histopathologic type | ||
| Clear cell renal cell carcinoma | 43 959 (59.00) | 1740 (82.08) |
| Papillary renal cell carcinoma | 8644 (11.60) | 89 (4.20) |
| Chromophobe renal cell carcinoma | 4160 (5.58) | 82 (3.87) |
| Collecting duct renal cell carcinoma | 201 (0.27) | 6 (0.28) |
| Renal medullary carcinoma | 48 (0.06) | 15 (0.71) |
| Other renal cell carcinoma | 17 494 (23.48) | 188 (8.87) |
AJCC, American Joint Committee on Cancer; FUSCC, Fudan University Shanghai Cancer Center; SEER, Surveillance, epidemiology, and end results.
Data are presented as number (percentage) unless otherwise indicated.
3.2. Modification of the 8th AJCC Staging System based on OS
The predictive value of the 8th AJCC staging system for RCC in the Chinese cohort using Kaplan‐Meier survival analysis based on the splitting of the TNM subgroups (T1N0M0, T1N1M0, T2N0M0, T2N1M0, T3N0M0, T3N1M0, T4N0M0, T4N1M0, TanyNanyM1) was performed in the FUSCC cohort. Figure S2A shows that the OS of the subgroup of T3N0M0 (stage III) and T4N0M0 (stage IV) were much better than the other subgroups in the same 8th AJCC stage grouping, respectively (stage III: T1N1M0, T2N1M0, and T3N1M0; stage IV: T4N1M0 and TanyNanyM1). The number of patients in T1N1M0, T2N1M0, or T3N1M0 was so small in our cohort, and these patients were amalgamated into one subgroup (T1‐3N1M0). Furthermore, the survival curve of T4N0M0 was very close to the curve of T1‐3N1M0, while the survival curve of T3N0M0 was much higher than that of T1‐3N1M0 (Figure 1A). In addition, the 5‐year‐OS rate for T3N0M0 was significantly higher than T1‐3N1M0 (72.7% vs 38.1%). The 5‐year‐OS rate for T4N0M0 was 36.2% (Table S1), which was close to T1‐3N1M0 but not to T4N1M0 (0%) and TanyNanyM1 (12.6%).
Figure 1.
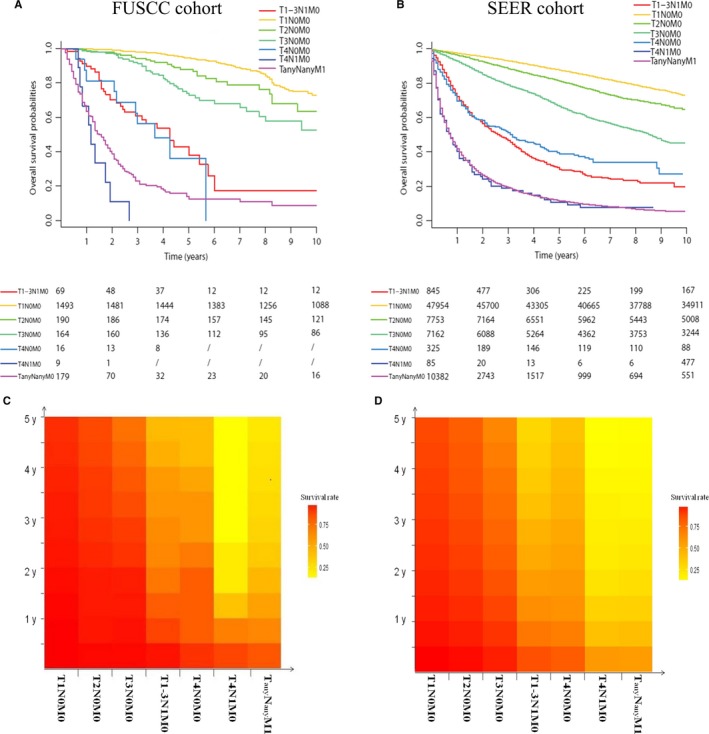
Kaplan‐Meier survival curves of the patients in T1‐3N1M0, T1N0M0, T2N0M0, T3N0M0, T4N0M0, T4N1M0, and TanyNanyM1 from (A) the FUSCC cohort and (B) the SEER cohort. Color variation on the Y axis of the heatmaps reflected the 5‐year‐OS rates variation of these patients from (C) the FUSCC cohort and (D) the SEER cohort
Hence, the current 8th AJCC staging system may not be accurate and appropriate. We regrouped the AJCC prognostic stage grouping according to the OS of each subgroup without changing the definition of TNM. T1N0M0 and T2N0M0 were classified as stage I, and this was subdivided into IA (T1N0M0) and IB (T1N0M0). T3N0M0 was classified as stage II. T1‐3N1M0 and T4N0M0 were classified as stage III. T4N1M0 and TanyNanyM1 were classified as stage IV (Figure 2B).
Figure 2.
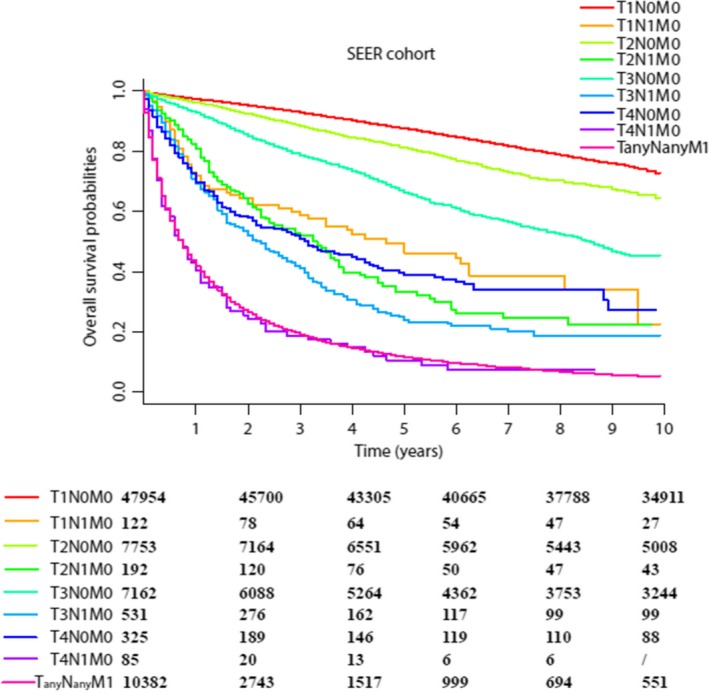
(A) Kaplan‐Meier survival curves for the patients of different TNM subgroup from the SEER cohort. (B) The 8th Editions of the AJCC Staging Definitions and the Modified 8th Staging Definitions for RCC
The modified AJCC stage grouping was better suitable for the outcomes of patients. Similar results were obtained for the hazard ratios (HRs) compared with T1N0M0 disease. The HRs of T4N0M0 (13.07) were closer to T1‐3N1M0 (12.46) rather than T4N1M0 (69.14) and TanyNanyM1 (29.16). Additionally, the HRs of T3N0M0 (3.21) were significantly lower than T1‐3N1M0 (Table 2).
Table 2.
HRs of different staging group and C‐indexes in SEER and FUSCC cohort
| Staging system | SEER cohort | FUSCC cohort | |
|---|---|---|---|
| 8th AJCC staging system | HR (95% CI) | HR 95% CI | |
| Stage I | T1N0M0 | / | / |
| Stage II | T2N0M0 | 1.53 (1.44‐1.63) | 1.89 (1.29‐2.77) |
| Stage III | T1‐3N1M0 + T3N0M0 | 3.23 (3.08‐3.40) | 4.82 (3.64‐6.39) |
| Stage IV | TanyNanyM1+T4N0‐1M0 | 18.36 (17.73‐19.03) | 27.66 (21.77‐35.14) |
| Modified 8th AJCC staging system | |||
| Stage Ia | T1N0M0 | / | / |
| Stage Ib | T2N0M0 | 1.53 (1.44‐1.63) | 1.89 (1.29‐2.77) |
| Stage II | T3N0M0 | 2.76 (2.61‐2.92) | 3.21 (2.27‐4.55) |
| Stage III | T1‐3N1M0 + T4N0M0 | 7.80 (7.19‐8.46) | 12.82 (9.08‐18.10) |
| Stage IV | TanyNanyM1 + T4N1M0 | 19.11 (18.44‐19.80) | 30.02 (23.54‐38.27) |
| T1N0M0 | / | / | |
| T2N0M0 | 1.53 (1.44‐1.63) | 1.89 (1.29‐2.77) | |
| T3N0M0 | 2.76 (2.61‐2.92) | 3.21 (2.27‐4.55) | |
| T1N1M0 | 5.68 (4.41‐7.33) | 8.29 (4.05‐16.96) | |
| T2N1M0 | 7.15 (5.93‐8.62) | 17.53 (9.67‐31.78) | |
| T3N1M0 | 9.44 (8.41‐10.60) | 12.93 (7.52‐22.23) | |
| T1‐3N1M0 | 8.14 (7.41‐8.94) | 12.46 (8.54‐18.19) | |
| T4N0M0 | 6.96 (5.97‐8.12) | 13.07 (6.84‐24.98) | |
| T4N1M0 | 18.84 (14.95‐23.74) | 69.14 (34.56‐138.33) | |
| TanyNanyM1 | 19.12 (18.46‐19.82) | 29.16 (22.81‐37.29) | |
| C‐indexes (stages II and IV patients) | |||
| 8th AJCC staging system | 0.764 (0.758‐0.769) | 0.779 (0.743‐0.815) | |
| Modified 8th AJCC staging system | 0.770 (0.765‐0.776) | 0.801 (0.765‐0.838) | |
CI, confidence interval; C‐index, concordance index; HRs, hazard ratios compared with T1N0M0.
3.3. Verification of modified 8th AJCC Staging System based on SEER cohort
The trends of survival curve in each subgroup in SEER cohort strengthened the need and appropriateness for the modification of stage grouping. As shown in Figure 1B, T4N0M0 and T1‐3N1M0 were more suitable in one stage than T3N0M0. Besides, each subgroup of T1‐3N1M0 (such as T1N1M0, T2N1M0, and T3N1M0) showed worsened prognostic rates significantly than T3N0M0 (Figure 2A).
As the patients in T1‐3N1M0 were far more than the FUSCC cohort, the improved changes in this classification were more visible and reliable. The 5‐year‐OS rates for T3N0M0, T1‐3N1M0, and T4N0M0 patients were 66.4%, 30.0%, and 39.0%, respectively (Table S1). The HRs of T4N0M0, T1‐3N1M0, and T3N0M0 compared with T1N0M0 were 6.96, 8.14, and 2.76, respectively (Table 2). We found that T1‐3N1M0 and T3N0M0 were arranged in the same stage III (HR = 3.23) in the 8th AJCC stage grouping was unreasonable. Besides, the HRs of T4N0M0 were much lower than T4N1M0 (18.84) and TanyNanyM1 (19.12).
The heatmaps (Figure 1C,D) reflected the modified stage more intuitively. The subgroup with similar color variation on the Y axis should be assigned to the same stage. It was not hard to find that the modified stage grouping was more in line with color variation than the 8th AJCC stage grouping. As the huge number of T1N0M0 patients could diminish the better discrimination in stages II‐IV patients, C‐indexes of two types of staging systems were only calculated for patients in stages II‐IV (Table 2). C‐indexes for the modified 8th stage grouping were improved significantly (0.770, 95% CI: 0.765‐0.776 vs 0.764, 95% CI: 0.758‐0.769) than the 8th stage grouping in SEER cohort. The P‐value of likelihood ratio test was <0.001. Similar results could be found in the FUSCC cohort. Based on the above analysis, our results validated significant improvements of the modified 8th AJCC stage grouping. (Figure 3)
Figure 3.
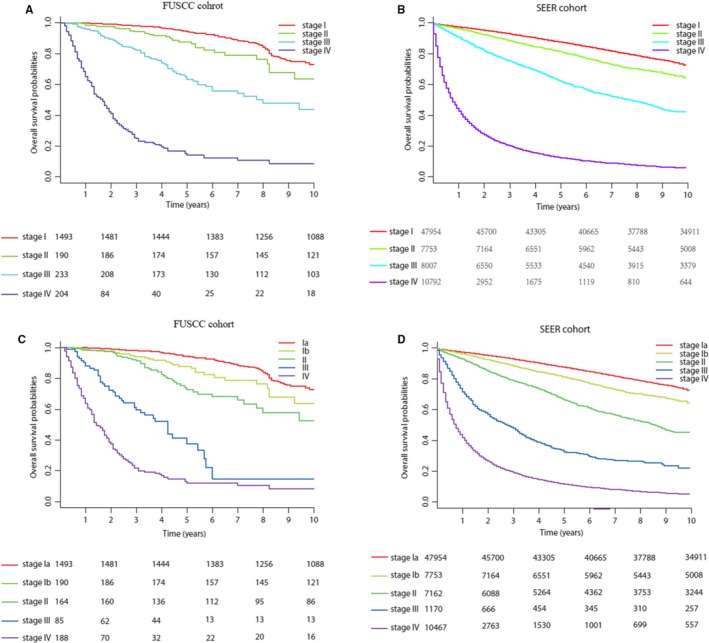
Kaplan‐Meier survival curves of the patients from (A) the FUSCC cohort and (B) the SEER cohort according to the 8th AJCC staging system. Kaplan‐Meier survival curves for the patients from (C) the FUSCC cohort and (D) the SEER cohort according to the modified 8th staging system
3.4. Sensitivity analysis
Because the SEER cohort was much larger than the FUSCC cohort, especially in the T1‐3N1M0 group, we used SEER cohort to conduct a sensitivity analysis. To identify different independent variables that may impact our modified stage grouping, stratified analysis was performed based on the histopathological types (ccRCC, papillary RCC, chromophobe carcinoma, and other RCC), race, and years at diagnosis (2004‐2010, after 2010). As shown in Figure S3, both ccRCC and non‐ccRCC (including papillary RCC, chromophobe carcinoma, and other RCC) demonstrated similar results. The OS of T3N0M0 was much better than T1‐3N1M0, and T4N0M0 was more suitable in the same stage with T1‐3N1M0. Then, according to the race recode variables (White, Black, Other) in SEER database, KM‐survival analysis was also performed (Figure S2B‐D). The results indicated that the outcome of T3N0M0 and T4N0M0 was much better than other subgroups in the same stage. Finally, the patients with diagnosis years between 2004 and 2010 and after 2010 also showed similar trends (Figures S3 and S4), meaning that our modified 8th AJCC stage grouping was feasible and credible.
4. DISCUSSION
The most important function of stage grouping is to predict outcomes as accurately as possible.13 Interestingly, our results indicated that 8th AJCC Staging System had much room for improvement. This is because both stages III and IV included two types of subgroups with significantly different prognosis. T3N0M0, with much better outcome, was classified into stage III with T1‐3N1M0. Similarly, T4N0M0 was classified into stage IV inappropriately with T4N1M0 and TanyNanyM1. These findings suggested that 8th AJCC stage grouping underestimated the prognosis of T3or4N0M0. Indeed, appropriate and minor modifications of AJCC stage grouping that can bring more precise prediction of prognosis are necessary.14, 15
Our modifications without changing the definition of TNM may be considered as a good choice. The proposed system furthermore partitions risk over a great spectrum: patients in stages II, III, and IV in the proposed system have 2.76, 7.80, and 19.11 times risk of death, respectively, compared with T1N0M0 patients. In comparison, the HR for 8th AJCC stages II, III, and IV patients is 1.53, 3.23, and 18.36, respectively. Approximately 10% RCC patients (T3N0M0: 9.6%, T4N0M0: 0.4% according to SEER cohort) were regrouped according to our modifications, which made the survival curves separated accurately between the stages. The greatest impact of this modifications is not the subtle and appropriate reclassification, but in the characterization of real high‐risk subgroup.
The other important function of the AJCC stage grouping is to help clinical treatment decision‐making process, evaluate treatment efficacy, and determine the selection criteria for clinical trials.16, 17 As TNM stage grouping is the most widely used staging system, it also reflects the treatment changing paradigm.18, 19 The definition of stage Ia and Ib reflects better outcome of both T1N0M0 and T2N0M0, which means a good outcome for these patients in nowadays management. Similarly, the upgrading of T3N0M0 and T4N0M0 indicates that surgical consolidation in localized massive disease is more feasible in current surgical treatments. Only high‐risk patients with ccRCC are recommended for the use of adjuvant therapy (Sunitinib) as an option according to the NCCN guidelines unlike others in stages II and III.7 The intension of adjuvant trials was to delay or diminish recurrence in high‐risk patients. However, there were two famous randomized controlled trials (RCTs) with contrary results. The HR of S‐TRAC RCT was 0.76 (95% CI: 0.59‐0.98) with 90.6% and 90.8% T3N0M0 patients in Sunitinib and Placebo groups, respectively.20 The HR (AJCC stages III‐IV) of ASSURE RCT was 1.01 (95% CI: 0.81‐1.25) with 97.9% and 99.0% stage III patients (there was no details data in stage III) in Sunitinib and Placebo groups, respectively.21 The 5‐year‐OS rates were nearly 60% in Sunitinib and 46% in Placebo in high‐risk patients in S‐TRAC RCT. The 5‐year‐OS rates were nearly 51% in both Sunitinib and Placebo groups in high‐risk patients in ASSURE RCT. Our results showed that the 5‐year‐OS rates of T3N0M0 patients were 66.4% in SEER cohort and 72.7% in FUSCC cohort, both of which were much higher than that of patients in the placebo/Sunitinib in RCTs. It indicated that T3N0M0 may not be considered as high‐risk stage as considered before. These patients may not benefit from the adjuvant therapy significantly. Importantly, our modified stage grouping emphasizes the better prognostic of T3N0M0 patients and suggests that these patients should not be treated as equivalent to T1‐3N1M0 patients. Given the poor outcomes in stages III and IV patients in our modified stage grouping, these patients may derive great benefit from adjuvant treatments. Additionally, they may also be excellent candidates for novel treatments, such as immunotherapy. Besides, overestimating the risks of T3N0M0 (9.6% of RCC patients) implied a higher disease burden with overtreatment of adjuvant therapy.22, 23, 24 Evaluation of the efficacy and excessive treatment could be solved better if the future adjuvant therapy RCTs adopted the modified AJCC staging system and performed subgroup analysis accordingly. Collectively, our modified AJCC staging system may have some impact on the adjuvant therapy trials setting which is highly controversial according to recent trials.
The strengths of this study were larger sample size, an adequate number of death events, external validation in SEER database, and reproducible test. Additionally, modifications were only in staging group without changing the definition of TNM. There are also some limitations to our study. Firstly, the study was limited by its retrospective nature. The SEER database may still have the possibility of coding errors or erroneous data. Secondly, FUSCC cohort and SEER cohort did not reach all the people. Hence, future studies with larger number of participants from worldwide could validate our conclusions.
5. CONCLUSIONS
The present large study suggested that the current 8th AJCC stage grouping for RCC had much room for improvement. According to the OS of each subgroup, we mainly modified the AJCC prognostic stage groups, especially of stages III and IV. T3N0M0 was classified as an independent stage. T1‐3N1M0 and T4N0M0 were classified as stage III. T4N1M0 and TanyNanyM1 were classified as stage IV. This modified AJCC stage grouping is proved to be a more precise prediction of prognosis. Additionally, it is feasible and credible. Hence, the modification of 8th AJCC stage grouping brings new insights on the next version of the AJCC stage grouping and adjuvant therapies in the future RCTs.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Our study was approved by the Ethics Committee of FUSCC. Additionally, informed consent was obtained.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
Supporting information
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (Grant No.81370073) and Shanghai Rising star Program (Grant No.16QA1401100).
Shao N, Wang H‐K, Zhu Y, Ye D‐W. Modification of American Joint Committee on cancer prognostic groups for renal cell carcinoma. Cancer Med. 2018;7:5431–5438. 10.1002/cam4.1790
Ning Shao and Hong‐Kai Wang are contributed equally to the present work and each is considered first author.
Contributor Information
Yao Zhu, Email: mailzhuyao@163.com.
Ding‐Wei Ye, Email: dwye.shca@gmail.com.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017:67:7‐30. [DOI] [PubMed] [Google Scholar]
- 2. Larkin J, Paine A, Tumur I, et al. Second‐line treatments for the management of advanced renal cell carcinoma: systematic review and meta‐analysis. Expert Opin Pharmacother. 2013;14:27‐39. [DOI] [PubMed] [Google Scholar]
- 3. Moch H, Artibani W, Delahunt B, et al. Reassessing the current UICC/AJCC TNM staging for renal cell carcinoma. Eur Urol. 2009;56:636‐643. [DOI] [PubMed] [Google Scholar]
- 4. Howard GE, Wood CG. Staging refinements in renal cell carcinoma. Curr Opin Urol. 2006;16:317‐320. [DOI] [PubMed] [Google Scholar]
- 5. Li ZS, Yao K, Chen P, et al. Modification of N staging systems for penile cancer: a more precise prediction of prognosis. Br J Cancer. 2015;112:1766‐1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tong LL, Gao P, Wang ZN, et al. Is the seventh edition of the UICC/AJCC TNM staging system reasonable for patients with tumor deposits in colorectal cancer? Ann Surg. 2012;255:208–213. [DOI] [PubMed] [Google Scholar]
- 7. Motzer RJ, Jonasch E, Agarwal N, et al. Version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15:804–834. [DOI] [PubMed] [Google Scholar]
- 8. Novara G, Ficarra V, Antonelli A, et al. Validation of the 2009 TNM version in a large multi‐institutional cohort of patients treated for renal cell carcinoma: are further improvements needed? Eur Urol. 2010;58:588–595. [DOI] [PubMed] [Google Scholar]
- 9. Edwards SJ, Wakefield V, Cain P, et al. Axitinib, cabozantinib, everolimus, nivolumab, sunitinib and best supportive care in previously treated renal cell carcinoma: a systematic review and economic evaluation. Health Technol Assess. 2018;22:1–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meskawi M, Sun M, Trinh QD, et al. A review of integrated staging systems for renal cell carcinoma. Eur Urol. 2012;62:303–314. [DOI] [PubMed] [Google Scholar]
- 11. Escudier B, Albiges L, Massard C, Loriot Y, Fizazi K. How to select amongst available options for the treatment of advanced RCC? Ann Oncol. 2012;23(Suppl 10):x309–x312. [DOI] [PubMed] [Google Scholar]
- 12. Ficarra V, Galfano A, Mancini M, Martignoni G, Artibani W. TNM staging system for renal‐cell carcinoma: current status and future perspectives. Lancet Oncol. 2007;8:554–558. [DOI] [PubMed] [Google Scholar]
- 13. Adam MA, Thomas S, Roman SA, Hyslop T, Sosa JA. Rethinking the current American Joint Committee on cancer TNM staging system for medullary thyroid cancer. JAMA Surg. 2017;1(152):869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leibovich BC, Blute ML, Cheville JC, et al. Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer. 2003;1(97):1663–1671. [DOI] [PubMed] [Google Scholar]
- 15. Zisman A, Pantuck AJ, Wieder J, et al. Risk group assessment and clinical outcome algorithm to predict the natural history of patients with surgically resected renal cell carcinoma. J Clin Oncol. 2002;1(20):4559–4566. [DOI] [PubMed] [Google Scholar]
- 16. Miller LA, Stemkowski S, Saverno K, et al. Patterns of care in patients with metastatic renal cell carcinoma among a U.S. payer population with commercial or medicare. Advantage Membership. J Manag Care Spec Pharm. 2016;22:219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Plimack ER, Hudes GR. Selecting targeted therapies for patients with renal cell carcinoma. J Natl Compr Canc Netw. 2011;1(9): 997–1006; quiz 7. [DOI] [PubMed] [Google Scholar]
- 18. Lobo JM, Nelson M, Nandanan N, Krupski TL. Comparison of renal cell carcinoma surveillance guidelines: competing trade‐offs. J Urol. 2016;195:1664–1670. [DOI] [PubMed] [Google Scholar]
- 19. Molina AM, Motzer RJ. Clinical practice guidelines for the treatment of metastatic renal cell carcinoma: today and tomorrow. Oncologist. 2011;16(Suppl 2):45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Motzer RJ, Ravaud A, Patard JJ, et al. Adjuvant sunitinib for high‐risk renal cell carcinoma after nephrectomy: subgroup analyses and updated overall survival results. Eur Urol. 2018;73:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haas NB, Manola J, Uzzo RG, et al. Adjuvant sunitinib or sorafenib for high‐risk, non‐metastatic renal‐cell carcinoma (ECOG‐ACRIN E2805): a double‐blind, placebo‐controlled, randomised, phase 3 trial. Lancet. 2016;14(387):2008–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Virgo KS, Basch E, Loblaw DA, et al. Second‐line hormonal therapy for men with chemotherapy‐naive, castration‐resistant prostate cancer: American Society of Clinical Oncology Provisional Clinical Opinion. J Clin Oncol. 2017;35:1952–1964. [DOI] [PubMed] [Google Scholar]
- 23. Fernandez‐Pello S, Hofmann F, Tahbaz R, et al. A systematic review and meta‐analysis comparing the effectiveness and adverse effects of different systemic treatments for non‐clear cell renal cell carcinoma. Eur Urol. 2017;71:426–436. [DOI] [PubMed] [Google Scholar]
- 24. Shore ND, Chowdhury S, Villers A, et al. Efficacy and safety of enzalutamide versus bicalutamide for patients with metastatic prostate cancer (TERRAIN): a randomised, double‐blind, phase 2 study. Lancet Oncol. 2016;17:153–163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


