Abstract
AIM
To investigate the effects of tumor localization on disease free survival (DFS) and overall survival (OS) in patients with stage II-III colon cancer.
METHODS
This retrospective study included 942 patients with stage II and III colon cancer, which were followed up in our clinics between 1995 and 2017. The tumors from the caecum to splenic flexure were defined as right colon cancer (RCC) and those from splenic flexure to the sigmoid colon as left colon cancer (LCC).
RESULTS
The median age of the patients was 58 years (range: 19-94 years). Male patients constituted 54.2%. The rates of RCC and LCC were 48.4% (n = 456) and 51.6% (n = 486), respectively. During the median follow-up of 90 mo (range: 6-252 mo), 14.6% of patients developed recurrence and 9.1% of patients died. In patients with stage II and III disease with or without adjuvant therapy, DFS was similar in terms of primary tumor localization (stage II; P = 0.547 and P = 0.481, respectively; stage III; P = 0.976 and P = 0.978, respectively). In patients with stage II and III disease with or without adjuvant therapy, OS was not statistically significant with respect to primary tumor localization (stage II; P = 0.381 and P = 0.947, respectively; stage III; P = 0.378 and P = 0.904, respectively). The difference between median OS of recurrent RCC (26 ± 6.2 mo) and LCC (34 ± 4.9 mo) cases was eight months (P = 0.092).
CONCLUSION
Our study showed no association of tumor localization with either DFS or OS in patients with stage II or III colon cancer managed with or without adjuvant therapy. However, post-recurrence OS appeared to be worse in RCC patients.
Keywords: Colon cancer, Tumor localization, Adjuvant treatment, Overall survival, Disease free survival
Core tip: It is well known that metastatic right colon cancer is more aggressive than left colon cancer. However, the effects of tumor location on the decision of adjuvant therapy and survival are not clearly known in early stage disease. In this retrospective study, we investigated the effects of tumor location on disease free survival and overall survival in patients with and without adjuvant therapy for stage II-III colon cancer. There was no difference for disease free survival or overall survival between patients with right or left localized colon cancer, but we established that right localized tumors were more aggressive than left side after recurrence.
INTRODUCTION
Colon cancer (CC) is a common and fatal disease. It is estimated that about 95520 CC cases are diagnosed annually in the United States. CC is the third most common cancer in men and the second most common cancer in women. Despite a declining mortality since 1990, it ranked the third in women and the second in men in cancer-related deaths. From 1992 to 2012, the incidence of men and women under the age of 50 diagnosed with CC increased by 2.1% per year. These increases were primarily seen in left-sided cancers, and particularly in rectal cancer (3.9% per year). Approximately 39% of the cases are local, and 37% are locoregional at diagnosis. Seventy to 80% of patients with locoregional disease at diagnosis are suitable for curative surgery. While surgery is essential for curative treatment, some patients have recurrence even after curative surgery. The prognosis is worse after recurrence. For this reason, it is important to identify reliable factors for identification of patients at high risk of recurrence[1,2].
The proximal and distal segments of the colon possess different embryological origins. The segment extending from the caecum to the proximal two-thirds of the transverse colon develops from the midgut. The part from the distal third of the transverse colon to the rectum develops from the hindgut. While the right colon consists of the caecum, ascending colon, hepatic flexure, and transverse colon, the left colon consists of the splenic flexure, descending colon, and sigmoid colon. Blood supply, innervation, and lymphatic drainage anatomically differ between the right and left colon. Considering these differences in anatomy and embryological origin, variation in clinical features may be identified for the same disease of the colon[2].
It has been known for many years that right CC (RCC) and left CC (LCC) represent dissimilar tumors with differences in epidemiology, biology, pathology, and clinical outcomes. Recently, the relationship between tumor localization and prognosis in metastatic disease has been investigated. These studies, however, primarily focused on responses to chemo- or targeted therapy[3,4]. For this reason, it is still not clear for patients and clinicians whether tumor localization is an important additional risk factor in locoregional disease.
In our study, we aimed to examine the association of tumor localization to disease free survival (DFS) and overall survival (OS) in patients who underwent curative surgery for stage II and III CC.
MATERIALS AND METHODS
Patients
This retrospective study included patients who were followed up in the oncology outpatient clinic of Okmeydani Training and Research Hospital between 1995 and 2017. Clinical and pathological data were obtained from medical patient records. Those with rectal cancer, another malignancy distinct from CC, multiple primary tumors, metastatic disease, patients under 18 years and those without sufficient data were not included in the study. A total of 942 patients with full medical records and a pathological diagnosis of stage II-III CC were identified. The study was approved by the institutional ethics committee.
Data collection
Data obtained from medical records included the age, gender, alcohol or tobacco use, type of surgery (emergent or elective), presence of diabetes mellitus (DM) or hypertension (HT), histological characteristic (adenocarcinoma, mucinous adenocarcinoma), grade, primary tumor localization, stage, pathological tumor stage (pT), pathological node stage (pN), lymph node status (≥ 12 or < 12), numbers of excised and involved lymph nodes, presence of perineural invasion (PNI) or lymphovascular invasion (LVI), surgical margin positivity, use of adjuvant therapy, adjuvant therapeutic regimen, recurrence, and most recent status (exitus-alive). Patients were re-staged according to the 8th tumor, node, and metastasis staging manual 2017 of the American Joint Committee on Cancer/Union for International Cancer Control. Patients were divided into two groups, right colon and left colon. Tumors extending from the caecum to the splenic flexure were classified as RCC, those from the splenic flexure to the sigmoid colon as LCC. Age was grouped as < 65 and ≥ 65 years. Grades were grouped as 1 + 2 and 3. pT was grouped as 1 + 2, 3 and 4. DFS was estimated as the time elapsed from diagnosis to local recurrence or systemic metastasis. OS was estimated as the time from diagnosis to death. OS2 was defined as the time from recurrence to death.
Statistical analysis
SPSS 15.0 for Windows software package was used for statistical analysis. Descriptive variables were expressed with mean, standard deviation, minimum, and maximum values for numerical parameters, and with number and percentage values for categorical parameters. Numeric variables in two independent groups were analyzed by a Student’s t-test when the data were normally distributed and by Mann Whitney U test when the normal distribution condition was not met. Comparisons of rates in groups were made with chi-square. Monte Carlo simulation was applied when conditions were not met. The survival analyses were performed with Kaplan Meier. Determinants were analyzed by Cox regression. In univariate analysis, a forward stepwise model was used for values with P < 0.250. An overall 5% alpha error level was used to infer statistical significance.
RESULTS
The rates of RCC and LCC were 48.4% (n = 456) and 51.6% (n = 486), respectively. Male patients constituted 54.2%. The median patient age was 58 years (range: 19-94 years). Nearly one-third of patients (32.5%) were equal to or above 65 years old (Table 1).
Table 1.
Comparison of clinical and pathological data according to tumor localization
| All patients | RCC | LCC | ||||||
| (n = 942) | (n = 456) | (n = 486) | ||||||
| n | % | n | % | n | % | P | ||
| Age (yr) | < 65 | 636 | 67.5 | 304 | 66.7 | 332 | 68.3 | 0.590 |
| ≥ 65 | 306 | 32.5 | 152 | 33.3 | 154 | 31.7 | ||
| Gender | Male | 511 | 54.2 | 250 | 54.8 | 261 | 53.7 | 0.730 |
| Female | 431 | 45.8 | 206 | 45.2 | 225 | 46.3 | ||
| Family history | No | 916 | 97.2 | 439 | 96.3 | 477 | 98.1 | 0.790 |
| Yes | 26 | 2.8 | 17 | 3.7 | 9 | 1.9 | ||
| Smoking status | No | 592 | 62.8 | 277 | 60.7 | 315 | 64.8 | 0.192 |
| Yes | 350 | 37.2 | 179 | 39.3 | 171 | 35.2 | ||
| Alcohol use status | No | 893 | 94.8 | 434 | 95.2 | 459 | 94.4 | 0.614 |
| Yes | 49 | 5.2 | 22 | 4.8 | 27 | 5.6 | ||
| Mode of surgery | Elective | 791 | 84 | 400 | 87.7 | 391 | 80.5 | 0.002 |
| Emergent | 151 | 16 | 56 | 12.3 | 95 | 19.5 | ||
| DM | No | 845 | 89.7 | 407 | 89.3 | 438 | 90.1 | 0.527 |
| Yes | 93 | 9.9 | 48 | 10.5 | 45 | 9.3 | ||
| HT | No | 717 | 76.1 | 344 | 75.4 | 373 | 76.7 | 0.329 |
| Yes | 223 | 23.7 | 112 | 24.6 | 111 | 22.8 | ||
| Histology | Adenocarcinoma | 779 | 82.7 | 356 | 78.1 | 423 | 87 | < 0.001 |
| Mucinous adenocarcinoma | 163 | 17.3 | 100 | 21.9 | 63 | 13 | ||
| Tumor grade | Well and moderately | 879 | 93.3 | 420 | 92.1 | 459 | 94.4 | 0.151 |
| Poorly | 63 | 6.7 | 36 | 7.9 | 27 | 5.6 | ||
| Tumor stage | II | 567 | 60.2 | 271 | 59.4 | 296 | 60.9 | 0.644 |
| III | 375 | 39.8 | 185 | 40.6 | 190 | 39.1 | ||
| pT stage | T1-2 | 133 | 14.1 | 57 | 12.5 | 76 | 15.6 | 0.267 |
| T3 | 752 | 79.8 | 374 | 82 | 378 | 77.8 | ||
| T4 | 57 | 6.1 | 25 | 5.5 | 32 | 6.6 | ||
| The number of removed lymph nodes | < 12 | 296 | 31.4 | 102 | 22.4 | 194 | 39.9 | < 0.001 |
| ≥ 12 | 646 | 68.6 | 354 | 77.6 | 292 | 60.1 | ||
| pN | N0 | 567 | 60.2 | 269 | 59 | 298 | 61.3 | 0.589 |
| N1 | 273 | 29 | 133 | 29.2 | 140 | 28.8 | ||
| N2 | 102 | 10.8 | 54 | 11.8 | 48 | 9.9 | ||
| PNI | Negative | 728 | 78.3 | 354 | 78.5 | 374 | 78.1 | 0.879 |
| Positive | 202 | 21.7 | 97 | 21.5 | 105 | 21.9 | ||
| LVI | Negative | 629 | 67.8 | 303 | 67.3 | 326 | 68.2 | 0.777 |
| Positive | 299 | 32.2 | 147 | 32.7 | 152 | 31.8 | ||
| Surgical margin | Negative | 928 | 98.5 | 449 | 98.5 | 479 | 98.6 | 0.096 |
| Positive | 8 | 0.8 | 6 | 1.3 | 2 | 0.4 | ||
| Adjuvant treatment | No | 208 | 22.1 | 94 | 20.6 | 114 | 23.5 | 0.293 |
| Yes | 734 | 77.9 | 362 | 79.4 | 372 | 76.5 | ||
| Adjuvant treatment regimen | 5-FU-based | 493 | 67.2 | 243 | 67.1 | 250 | 67.2 | 0.978 |
| Oxaliplatin-based | 241 | 32.8 | 119 | 32.9 | 122 | 32.8 | ||
| Completion rate of adjuvant treatment | 695 | 94.7 | 344 | 95 | 351 | 94.4 | 0.685 | |
| Tumor recurrence | No | 804 | 85.4 | 389 | 85.3 | 415 | 85.4 | 0.971 |
| Yes | 138 | 14.6 | 67 | 14.7 | 71 | 14.6 | ||
| Locoregional recurrence | 40 | 29 | 21 | 31.3 | 19 | 26.8 | 0.553 | |
| Systemic recurrence | 98 | 71 | 46 | 68.7 | 52 | 73.2 | ||
| Metastasectomy | 48 | 34.8 | 24 | 35.8 | 24 | 33.8 | 0.804 | |
| Status | Exitus | 95 | 9.1 | 51 | 11.2 | 44 | 9.1 | 0.278 |
| Alive | 847 | 90.9 | 405 | 88.8 | 486 | 90.9 | ||
| Median | Min-Max | Median | Min-Max | Median | Min-Max | |||
| Age (yr) | 58 | 19-94 | 57 | 19-89 | 58 | 21-94 | 0.141 | |
| Follow-up (mo) | 90 | 1-252 | 90 | 1-252 | 90 | 5-235 | ||
| mean | SD | mean | SD | mean | SD | |||
| Number of removed lymph nodes | 17.57 | 10.843 | 19.78 | 11.059 | 15.5 | 10.223 | < 0.001 | |
| Number of metastatic lymph nodes | 1.46 | 4.068 | 1.41 | 2.86 | 1.5 | 4.944 | 0.743 | |
DM: Diabetes mellitus; HT: Hypertension; Max: Maximum; Min: Minimum; LCC: Left colon cancer; LVI: Lymphovascular invasion; n: Number of patients; pN: Pathological lymph node stage; PNI: Perineural invasion; pT: Pathological tumor stage; RCC: Right colon cancer.
Twenty-six patients (2.8%) had a family history of CC in their first-degree relatives. The history of smoking and regular alcohol use was present in 45.8% (n = 350) and 5.2% (n = 49) of patients, respectively. Emergency surgery was performed in 151 patients (16%). DM and HT were present in 9.9% and 23.7% of the study population, respectively (Table 1).
Analysis of tumor histology showed mucinous adenocarcinoma in 17.3% of patients, grade III tumor in 6.7% of patients, and stage II disease in the majority of patients (60.2%). The rates of pT3 and pT4 were 79.8% and 6.1%, respectively. The mean number of lymph node dissections performed was 17.57 ± 10.8, where lymph node involvement was 1.48 ± 4.0. The rate of lymph node dissection below 12 was 31.4%. The number of patients with pN2 and pN1 were 102 (10.8%) and 273 (29%), respectively. PNI and LVI positivity was found in 21.7 and 32.2% of patients, respectively. Eight patients (0.8%) had positive surgical margins (Table 1).
Postoperative systemic therapy was initiated in 734 patients (77.9%), 67.2% (n = 493) of which received 5-FU-based (5-fluorouracil + leucovorin, capecitabine) and 32.8% (n = 241) received oxaliplatin-based (capecitabine + oxaliplatin, 5-fluorouracil + leucovorin + oxaliplatin) regimens. A total of 695 patients (94.7%) completed planned adjuvant chemotherapy regimens (Table 1).
During the median follow-up of 90 mo (range: 6-252 mo), 138 (14.6%) patients developed recurrence, and 40 (29.0%) of recurrences were locoregional and 98 (71.0%) were distant and 95 (9.1%) of patients died. Metastasectomy was performed for 48 of patients with recurrence (Table 1).
No statistical difference existed between RCC and LCC in terms of gender, smoking and alcohol use, history of DM and HT, tumor grade, stage, pT stage, pN stage, LVI and PNI positivity, positive surgical margins, adjuvant therapy use, the regimen used for adjuvant therapy, rates for recurrence (locoregional or distant), metastasectomy and death. Rate of mucinous adenocarcinoma histology, rate of LN number of ≥ 12, and the mean number of LNs dissected were significantly higher in the RCC group (P = 0.002, P < 0.001, and P < 0.001, respectively) (Table 1).
At all stages, 1, 3, 5, 10, and 15-year DFS and OS rates were 97.9%, 89.8%, 87.0%, 84.4%, 82.7% and 99.8%, 96.7%, 92.4%, 86.7%, 86.6%, respectively. In stage II RCC and LCC, rates of DFS at 1, 3, 5, 10, and 15 years were 98.9%, 93.9%, 93.1%, 92.0%, 90.3% and 98.0%, 94.5%, 91.8%, 90.5%, 90.5%, respectively. In stage III RCC and LCC, rates of DFS at 1, 3, 5, 10, and 15 years were 96.2%, 83.6%, 79.4%, 75.0%, 73.2% and 96.8%, 81.9%, 78.2%, 74.4%, 72.2%, respectively (Table 2).
Table 2.
Disease free survival and overall survival rates (%) at 12, 36, 60, 90, 120 and 180 mo according to tumor localization
| All patients (%) |
RCC (%) |
LCC (%) |
|||
| DFS (mo) | Stage II | Stage III | Stage II | Stage III | |
| 12 | 97.9 | 98.9 | 96.2 | 98.0 | 96.8 |
| 36 | 89.8 | 93.9 | 83.6 | 94.5 | 81.9 |
| 60 | 87.0 | 93.1 | 79.4 | 91.8 | 78.2 |
| 90 | 84.9 | 92.6 | 75.9 | 91.3 | 76.7 |
| 120 | 84.4 | 92.0 | 75.0 | 90.5 | 74.4 |
| 180 | 82.7 | 90.3 | 73.2 | 90.5 | 72.2 |
| OS (mo) | |||||
| 12 | 99.8 | 99.3 | 100.0 | 99.7 | 100.0 |
| 36 | 96.7 | 96.2 | 95.5 | 99.3 | 94.4 |
| 60 | 92.4 | 94.5 | 86.2 | 97.0 | 87.9 |
| 90 | 89.5 | 94.0 | 82.5 | 94.4 | 86.4 |
| 120 | 87.6 | 92.7 | 78.9 | 93.8 | 82.9 |
| 180 | 86.6 | 92.7 | 78.9 | 92.1 | 82.9 |
LCC: Left colon cancer; OS: Overall survival; RCC: Right colon cancer; DFS: Disease free survival.
In stage II RCC and LCC, rates of OS at 1, 3, 5, 10, and 15 years were 99.3%, 96.2%, 94.5%, 92.7%, 92.7% and 99.7%, 99.3%, 97.0%, 93.8%, 92.1%, respectively. In stage III RCC and LCC, rates of OS at 1, 3, 5, 10, 15 years were 100.0%, 95.5%, 86.2%, 78.9%, 78.9% and 100.0%, 94.4%, 87.9%, 82.9%, 82.9%, respectively (Table 2).
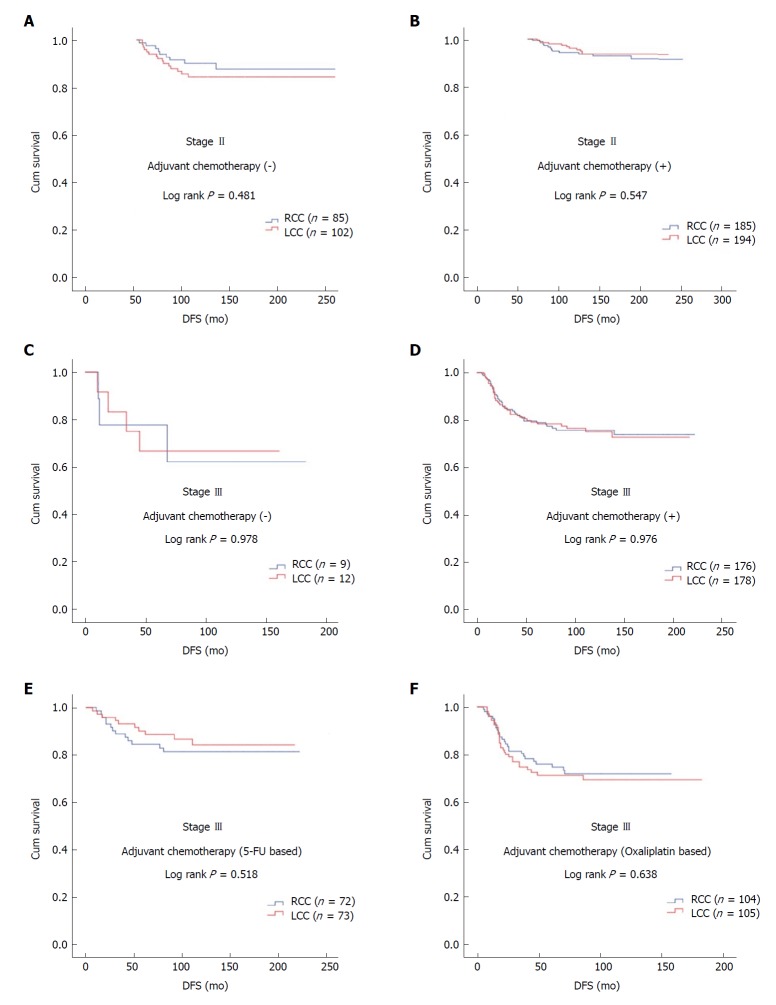
In patients with stage II and III disease with or without adjuvant therapy, DFS was similar in terms of primary tumor localization (stage II; log rank P = 0.547 and log rank P = 0.481, respectively; stage III; log rank P = 0.976 and log rank P = 0.978, respectively). In stage III disease, there was no statistically significant difference for DFS in patients receiving 5-FU-based or oxaliplatin-based regimens according to tumor location (log rank P = 0.518 and log rank P = 0.638, respectively) (Figure 1).
Figure 1.
Disease free survival by primary tumor localization in Kaplan-Meier analysis. A: Stage II patients not receiving adjuvant therapy; B: Stage II patients receiving adjuvant therapy; C: Stage III patients not receiving adjuvant therapy; D: Stage III patients receiving adjuvant therapy; E: Stage III patients receiving adjuvant 5-fluorouracil based therapy; F: Stage III patients receiving adjuvant oxaliplatin based therapy. 5-FU: 5-Fluorouracil; DFS: Disease free survival; LCC: Left colon cancer; n: Number of patients; RCC: Right colon cancer.
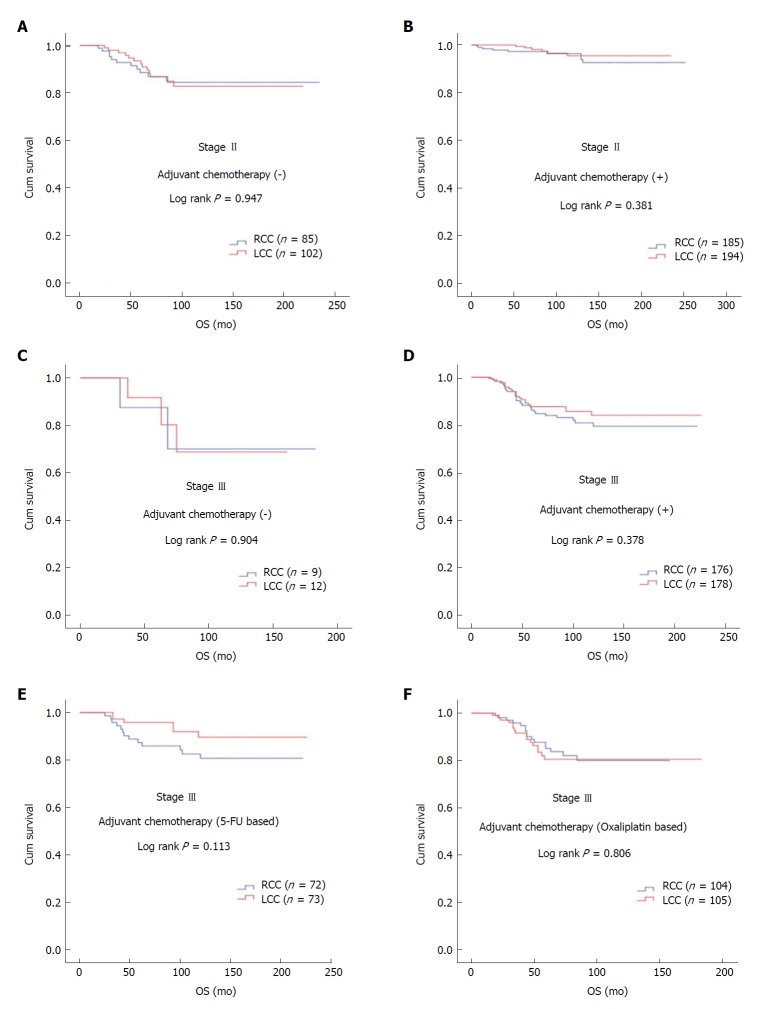
In patients with stage II and III disease with or without adjuvant therapy, OS was not statistically significant with respect to primary tumor localization (stage II; log rank P = 0.381 and log rank P = 0.947, respectively; stage III; log rank P = 0.378 and log rank P = 0.904, respectively). In stage III disease, there was no statistically significant difference for OS in patients receiving 5-FU-based or oxaliplatin-based regimens according to tumor location (log rank P = 0.113 and log rank P = 0.806, respectively) (Figure 2). No statistically significant difference was detected between median survival after recurrent/metastatic (OS2) RCC (26 ± 6.2 mo) and LCC (34 ± 4.9 mo) cases (log rank P = 0.092) (Figure 3).
Figure 2.
Overall survival by primary tumor localization in Kaplan-Meier analysis. A: Stage II patients not receiving adjuvant therapy; B: Stage II patients receiving adjuvant therapy; C: Stage III patients not receiving adjuvant therapy; D: Stage III patients receiving adjuvant therapy; E: Stage III patients receiving adjuvant 5-fluorouracil based therapy; F: Stage III patients receiving adjuvant oxaliplatin based therapy. 5-FU: 5-Fluorouracil; LCC: Left colon cancer; n: Number of patients; OS: Overall survival; RCC: Right colon cancer.
Figure 3.
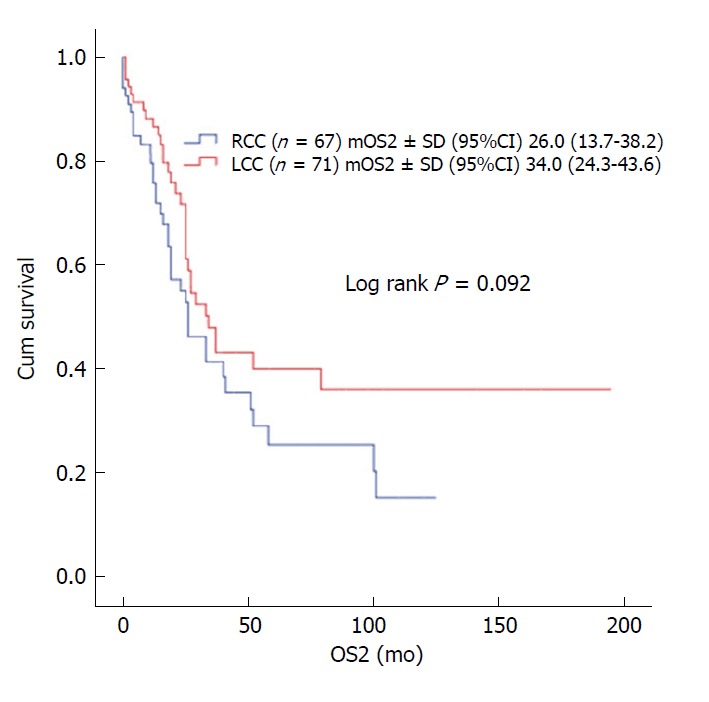
The overall survival effect of tumor localization after recurrence. LCC: Left colon cancer; OS2: Overall survival after recurrence; RCC: Right colon cancer; n: number of patients.
Univariate analysis for DFS showed statistically significant factors as age ≥ 65 years, presentation with ileus, stage, pT stage, pN stage, dissected LN < 12, PNI, LVI, surgical margin positivity, and adjuvant therapy (P = 0.001, P = 0.003, P < 0.001, P < 0.001, P < 0.001, P < 0.001, P < 0.001, P < 0.001, P = 0.008, and P = 0.041, respectively). In multivariate analysis, age ≥ 65 years, presentation with ileus, stage, dissected LN < 12, PNI, LVI, and adjuvant therapy were detected as statistically significant factors (P = 0.001, P = 0.011, P < 0.001, P = 0.012, P < 0.001, P = 0.003, and P = 0.005, respectively) (Table 3).
Table 3.
Factors affecting disease free survival
|
Univariate analysis |
Multivariate analysis |
||||||||||||
| HR | 95%CI | P | HR | 95%CI | P | ||||||||
| Age (yr) | < 65 | 1 | 1 | ||||||||||
| ≥ 65 | 1.779 | 1.268 | 2.496 | 0.001 | 1.88 | 1.305 | 2.708 | 0.001 | |||||
| Gender | Male | 1 | |||||||||||
| Female | 0.96 | 0.686 | 1.343 | 0.812 | |||||||||
| Family history | No | 1 | |||||||||||
| Yes | 1.195 | 0.489 | 2.919 | 0.696 | |||||||||
| Smoking status | No | 1 | |||||||||||
| Yes | 0.908 | 0.641 | 1.287 | 0.587 | |||||||||
| Alcohol using status | No | 1 | |||||||||||
| Yes | 0.372 | 0.118 | 1.167 | 0.09 | |||||||||
| Mode of surgery | Elective | 1 | 1 | ||||||||||
| Emergent | 1.796 | 1.22 | 2.646 | 0.003 | 1.718 | 1.131 | 2.611 | 0.011 | |||||
| DM | No | 1 | |||||||||||
| Yes | 0.973 | 0.549 | 1.724 | 0.925 | |||||||||
| HT | No | 1 | |||||||||||
| Yes | 1.541 | 0.967 | 2.224 | 0.067 | |||||||||
| Histology | Adenocarcinoma | 1 | |||||||||||
| Mucinous adenocarcinoma | 1.207 | 0.793 | 1.839 | 0.38 | |||||||||
| Tumor grade | Well and moderately | 1 | |||||||||||
| Poorly | 1.574 | 0.889 | 2.787 | 0.119 | |||||||||
| Tumor location | RCC | 1 | |||||||||||
| LCC | 0.997 | 0.714 | 1.392 | 0.984 | |||||||||
| Tumor stage | II | 1 | 1 | ||||||||||
| III | 2.99 | 2.109 | 4.238 | < 0.001 | 2.281 | 1.485 | 3.505 | < 0.001 | |||||
| pT stage | T1 + 2 | 1 | < 0.001 | ||||||||||
| T2 | 1.912 | 0.999 | 3.662 | 0.05 | |||||||||
| T4 | 9.308 | 4.478 | 19.348 | < 0.001 | |||||||||
| Number of removed lymph nodes | ≥ 12 | 1 | 1 | ||||||||||
| < 12 | 2.166 | 1.421 | 3.301 | < 0.001 | 1.751 | 1.13 | 2.712 | 0.012 | |||||
| pN | N0 | 1 | < 0.001 | ||||||||||
| N1 | 2.779 | 1.908 | 4.047 | < 0.001 | |||||||||
| N2 | 3.56 | 2.237 | 5.664 | < 0.001 | |||||||||
| PNI | Negative | 1 | 1 | ||||||||||
| Positive | 3.953 | 2.801 | 5.578 | < 0.001 | 2.277 | 1.549 | 3.347 | < 0.001 | |||||
| LVI | Negative | 1 | 1 | ||||||||||
| Positive | 3.372 | 2.382 | 4.774 | < 0.001 | 1.825 | 1.221 | 2.728 | 0.003 | |||||
| Surgical margin | Negative | 1 | |||||||||||
| Positive | 3.884 | 1.436 | 10.505 | 0.008 | |||||||||
| Adjuvant treatment | No | 1 | 1 | ||||||||||
| Yes | 0.591 | 0.346 | 0.954 | 0.041 | 0.514 | 0.323 | 0.82 | 0.005 | |||||
DM: Diabetes mellitus; HT: Hypertension; Max: Maximum; Min: Minimum; LCC: Left colon cancer; LVI: Lymphovascular invasion; pN: Pathological lymph node stage; PNI: Perineural invasion; pT: Pathological tumor stage; RCC: Right colon cancer.
Univariate analysis for OS revealed statistically significant factors as age ≥ 65 years, HT, stage, pT stage, pN stage, PNI, LVI, and adjuvant therapy (P < 0.001, P < 0.001, P < 0.001, P < 0.001, P < 0.001, P < 0.001, P < 0.001, and P = 0.017, respectively). In multivariate analysis, age ≥ 65 years, stage, PNI, LVI, and adjuvant therapy were found to be statistically significant factors (P < 0.001, P = 0.036, P = 0.001, P < 0.001, and P = 0.011, respectively) (Table 4).
Table 4.
Factors affecting overall survival
|
Univariate analysis |
Multivariate analysis |
||||||||||||
| HR | 95%CI | P | HR | 95%CI | P | ||||||||
| Age (yr) | < 65 | 1 | 1 | ||||||||||
| ≥ 65 | 4.136 | 2.731 | 6.263 | < 0.001 | 4.049 | 2.578 | 6.358 | < 0.001 | |||||
| Gender | Male | 1 | |||||||||||
| Female | 0.951 | 0.636 | 1.423 | 0.808 | |||||||||
| Family history | No | 1 | |||||||||||
| Yes | 0.306 | 0.043 | 2.196 | 0.239 | |||||||||
| Smoking status | No | 1 | |||||||||||
| Yes | 0.815 | 0.533 | 1.247 | 0.346 | |||||||||
| Alcohol using status | No | 1 | |||||||||||
| Yes | 0.348 | 0.086 | 1.411 | 0.139 | |||||||||
| Mode of surgery | Elective | 1 | |||||||||||
| Emergent | 1.342 | 0.812 | 2.219 | 0.252 | |||||||||
| DM | No | 1 | |||||||||||
| Yes | 1.683 | 0.953 | 2.972 | 0.073 | |||||||||
| HT | No | 1 | |||||||||||
| Yes | 3.067 | 2.035 | 4.623 | < 0.001 | |||||||||
| Histology | Adenocarcinoma | 1 | |||||||||||
| Mucinous adenocarcinoma | 1.213 | 0.733 | 2.006 | 0.452 | |||||||||
| Tumor grade | Well and moderately | 1 | |||||||||||
| Poorly | 1.036 | 0.453 | 2.369 | 0.933 | |||||||||
| Tumor location | RCC | 1 | |||||||||||
| LCC | 0.807 | 0.539 | 1.208 | 0.297 | |||||||||
| Tumor stage | II | 1 | 1 | ||||||||||
| III | 2.363 | 1.57 | 3.557 | < 0.001 | 1.723 | 1.037 | 2.863 | 0.036 | |||||
| pT stage | T1 + 2 | 1 | < 0.001 | ||||||||||
| T2 | 4.836 | 1.526 | 15.326 | 0.007 | |||||||||
| T4 | 21.34 | 6.162 | 73.897 | < 0.001 | |||||||||
| Number of removed lymph nodes | ≥ 12 | 1 | |||||||||||
| < 12 | 1.402 | 0.897 | 2.192 | 0.138 | |||||||||
| pN | N0 | 1 | < 0.001 | ||||||||||
| N1 | 2.122 | 1.353 | 3.327 | 0.001 | |||||||||
| N2 | 3.015 | 1.742 | 5.219 | < 0.001 | |||||||||
| PNI | Negative | 1 | 1 | ||||||||||
| Positive | 3.653 | 2.4 | 5.562 | < 0.001 | 2.198 | 1.374 | 3.517 | 0.001 | |||||
| LVI | Negative | 1 | 1 | ||||||||||
| Positive | 3.735 | 2.445 | 5.707 | < 0.001 | 2.523 | 1.543 | 4.127 | < 0.001 | |||||
| Surgical margin | Negative | 1 | |||||||||||
| Positive | 2.57 | 0.633 | 10.435 | 0.187 | |||||||||
| Adjuvant treatment | No | 1 | 1 | ||||||||||
| Yes | 0.587 | 0.379 | 0.91 | 0.017 | 0.517 | 0.311 | 0.86 | 0.011 | |||||
DM: Diabetes mellitus; HT: Hypertension; LCC: Left colon cancer; LVI: Lymphovascular invasion; pN: Pathological lymph node stage; PNI: Perineural invasion; pT: Pathological tumor stage; RCC: Right colon cancer.
DISCUSSION
In this trial, we aimed to investigate whether tumor location had prognostic significance in patients who underwent curative surgery for stage II or III CC with or without adjuvant therapy. In our study, we found that primary tumor localization had no effect on DFS and OS. A number of studies have been conducted in different regions of the world to describe the differences between RCC and LCC[5-10]. The data related to the prognosis of RCC and LCC are contradictory in recent studies[5-9,11]. Most studies reported patients with RCC as likely to be older, often female, in advanced stages, and poorly differentiated[6-12].
In their study of 1224 patients, Mik et al[5] reported that RCC patients were older than LCC patients, with a median age of 67.8 years. LCC patients were likely to have operations for emergent indications. The number of dissected lymph nodes were reported to be higher in RCC (11.7 ± 6 vs 8.3 ± 5, P = 0.0001)[5]. In another study, the likelihood of RCC was associated with increased age. In addition, T4 tumor, poor differentiation rate, and presence of venous invasion were detected to be significantly higher in RCC[6]. In our study, the median age was 58 years (range: 19-94 years). Similarly, in our study, LCC patients were more likely to have operations for emergent indications. Likewise, mucinous type was significantly more common in RCC. Unlike other studies, we did not detect significant differences between RCC and LCC in terms of age, gender, pT stage, stage, LVI, and PNI[5-9,11-13].
Lim et al[7] followed 414 patients with stage I-III CC with a median duration of 66.7 mo, during which the 5-year DFS was significantly higher in LCC (88.3%) than in RCC (81.4%). In multivariate analysis, pT3-4, pN1-2, and histologic grades were reported to be prognostic factors for DFS[7]. Moritani et al[8] recruited 820 stage I to III patients with a median follow-up of 55.8 ± 34.9 mo. No statistically significant difference was reported between RCC and LCC in five-year DFS (RCC 88.6%, LCC 89.4%, P = 0.231)[8]. Another study had 4029 stage I to III patients, for which the median follow-up was five years. While three- and five-year DFS rates of patients with RCC were 79.8% and 76.7%, it was 82.0% and 77.6% for LCC, respectively, with no statistically significant difference (P = 0.35) [9].
Five, ten, and 15-year DFS were 87.5%, 84.0%, and 82.1% for RCC and 86.7%, 84.2%, and 83.4% for LCC, respectively. In patients with stage II and III disease with or without adjuvant therapy, DFS was similar in terms of primary tumor localization. Independent risk factors for recurrence included age ≥ 65 years, presentation with ileus, advanced stage, dissected number of LNs < 12, and presence of PNI and LVI.
In the study by Aoyama et al[9], three and five-year median OS rates were 87.6% and 81.6% for RCC and 91.5% and 84.5% for LCC, where the difference was statistically significant (P < 0.009). Investigators have emphasized that this difference might originate from the fact that RCC patients were more likely to be older and to have poorly differentiated and mucinous histology[9]. A Far East study performed with 4426 RCC, LCC and rectal cancer patients in all stages reported significantly longer DFS and OS in LCC than those in RCC in univariate analysis, yet survival failed to show significant difference by localization in multivariate analysis. The authors concluded that primary tumor localization was not an independent prognostic factor in Chinese patients with stage I-III colorectal cancer (CRC)[10]. Patel et al[6] recruited stage II-III CRC patients, 40% of which were RCC and 31% of which had rectal cancer. Merely 45% of stage III CRC cases had received adjuvant therapy. No correlation was found between survival and tumor localization in patients receiving and not receiving adjuvant treatment[6].
Weis et al[12] reported no difference in 5-year mortality between RCC and LCC of any stage with stage I to III. Analysis by stage indicated lower mortality at stage II of LCC than RCC and higher mortality at stage III of LCC than RCC[12]. Warschkow et al[13] reported 5-year OS rate for patients with RCC as 65.1% (95%CI: 64.6-65.6) and LCC as 72.1% (95%CI: 71.5-72.6). The prognosis of RCC in stages I and II was reported as better overall. RCC and LCC had a similar prognosis at stage III. In multivariate analysis, there was no difference between RCC and LCC in terms of 5-year OS[13]. In another study by Huang et al[14], with 1095 patients at all stages and at all sites including the rectum, only in stage 3 disease were right colon localized tumors worse for survival.
In our study, OS rates at five, ten, and 15 years were found as 91.2%, 87.1%, and 85.2% in RCC compared to 93.8%, 88.1%, 88.1% in LCC. There was no significant difference between stage 2 and stage 3 RCC and LCC patients without adjuvant treatment. Despite having a slightly higher mortality in RCC, especially in stage III patients receiving 5-FU-based regimens, but this difference did not reach statistical significance in terms of primary tumor localization in stage II and III patients. Age ≥ 65 years, advanced stage, PNI, and LVI were found to be the most statistically significant factors for mortality in multivariate analysis.
The relationship between tumor localization and prognosis in metastatic disease has been investigated, and studies reported worse prognosis of the right colon than the left colon[3,4,15]. In a study of 1947 patients with metastatic disease, the median OS was 14 mo (95%CI: 12.7-15.3 mo) in RCC and 20.5 mo (95%CI: 18.5-22.5) in LCC, and this difference was statistically significant (P < 0.001)[15]. In another study by Lee et al[16] using Australian CRC registry data, the post-recurrence survival in early stage patients was worse in right CC. In a study by Kerr et al[17], after recurrence, the median OS was 1.25 years and 2.25 years in RCC and LCC, respectively. In the subgroup analysis of 138 patients with recurrence in our study, median OS was 26 mo (95%CI: 13.7-38.2) in RCC and 34 mo (95%CI: 24.3-43.6) in LCC, where the difference did not reach statistical significance, possibly due to the small number of cases (P = 0.092).
It is known that in recent years, the incidence of CC at younger ages has increased[1]. Surveillance, Epidemiology, and End Results (SEER) trials usually involve elderly patients, and data on comorbidities and family history are not available in the SEER database[11,12]. It is not clear how much these parameters may have affected the analyses. In our study, patients from all age groups (19-94 years) were included, and the median age was lower than that in other studies. In addition, the duration of median follow-up in our study was 90 mo (6-252 mo), which was longer than that in all other studies[15-12,14-16]. Besides, our study only included stage II and III patients, unlike other studies[4,5,8,15-18]. In our study, family history and comorbidities were added to the analysis, where those receiving and not receiving adjuvant therapies were assessed separately.
The causes of the inconsistent relationship between mortality and tumor localization are most likely related to tumor biology. Microsatellite instability (MSI) and BRAF mutations are more likely to be found in RCC than in LCC. BRAF mutations have been reported to be associated with poor prognosis[13,18]. On the other hand, MSI was reported to have a positive effect on the prognosis of stage II CRC[13]. Perhaps the most important limitation of our study is the absence of BRAF and MSI data of patients. It is not known how the MSI and BRAF situation affects the results of the study. In our study, the number of dissected LNs was lower than that in RCC, and the percentage of patients with < 12 dissected LN number were higher in LCC. This might have affected DFS and OS in LCC. In addition, our study did not analyze disease-specific survival; therefore, some of the mortal events might have occurred for non-cancer reasons during the long follow-up period.
In conclusion, tumor localization was not found to be associated with DFS or OS in stage II and III CC patients who were treated with or without adjuvant therapy. However, it was observed that OS was worse in RCC patients after recurrence. Further large and prospective studies also involving MSI and BRAF status are warranted.
ARTICLE HIGHLIGHTS
Research background
It is well known that metastatic right colon cancer (RCC) is more aggressive than left colon cancer (LCC). However, the effects of tumor location on the decision of adjuvant therapy and survival are not clearly known in early stage disease.
Research motivation
In recent trials, prognosis data of early stage RCC and LCC are conflicting. The uncertainty of whether tumor localization is functioning as an important additional risk factor for patients and clinicians in locoregional disease is still present.
Research objectives
In our study, we examined the effect of tumor localization on survival in patients who received or did not receive adjuvant therapy for stage II and III colon cancer. We also investigated the effects of chemotherapy regimens in stage III disease on survival in terms of tumor site.
Research methods
In the study, a total of 942 patients with stage II-III colon cancer, excluding rectal cancer, were included. Comorbidities (diabetes mellitus, hypertension), family histories, adjuvant therapy status and chemotherapy regimens were added to the analysis. The tumors from the caecum to the splenic flexure were defined as RCC and those from the splenic flexure to the sigmoid colon as LCC.
Research results
There was no difference for age and gender in the groups. Mucinous adenocarcinoma rate and the number of removed lymph nodes was higher in the RCC group. Recurrence and mortality risk was lower in patients with adjuvant treatment for all stages. In patients with stage II and III disease with or without adjuvant therapy, disease free survival and overall survival were similar in terms of primary tumor localization. In stage III disease, there was no statistically significant difference for disease free survival and overall survival in patients receiving 5-Fluorouracil (commonly known as 5-FU)-based or oxaliplatin-based regimens according to tumor location. After recurrence, RCC was more aggressive.
Research conclusions
In conclusion, our study showed no association of tumor localization with either disease free survival or overall survival in patients with stage II or III colon cancer managed with or without adjuvant therapy. However, after recurrence, RCC was more aggressive.
Research perspectives
Further large and prospective studies also involving microsatellite instability and BRAF status are needed to determine the effectiveness of tumor location on decision of adjuvant therapy in patients with stage II-III colon cancer.
Footnotes
Institutional review board statement: This study was reviewed and approved by the Ethics Committee of the University of Health Sciences, Okmeydani Training and Research Hospital.
Informed consent statement: Patients were not required to give informed consent to the study because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent.
Conflict-of-interest statement: All authors declare no conflicts-of-interest related to this article.
Data sharing statement: No additional data are available.
Manuscript source: Unsolicited manuscript
Peer-review started: August 13, 2018
First decision: August 24, 2018
Article in press: October 17, 2018
Specialty type: Oncology
Country of origin: Turkey
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Alkan A, Aykan NF, Shu X, Sunakawa Y S- Editor: Wang JL L- Editor: Filipodia E- Editor: Tan WW
Contributor Information
Abdullah Sakin, Department of Medical Oncology, Yuzuncu Yil University Medical School, Van 65090, Turkey. drsakin@hotmail.com.
Serdar Arici, Department of Medical Oncology, University of Health Sciences, Okmeydani Training and Research Hospital, Istanbul 34384, Turkey.
Saban Secmeler, Department of Medical Oncology, University of Health Sciences, Okmeydani Training and Research Hospital, Istanbul 34384, Turkey.
Orcun Can, Department of Medical Oncology, University of Health Sciences, Okmeydani Training and Research Hospital, Istanbul 34384, Turkey.
Caglayan Geredeli, Department of Medical Oncology, University of Health Sciences, Okmeydani Training and Research Hospital, Istanbul 34384, Turkey.
Nurgul Yasar, Department of Medical Oncology, University of Health Sciences, Okmeydani Training and Research Hospital, Istanbul 34384, Turkey.
Cumhur Demir, Department of Medical Oncology, University of Health Sciences, Okmeydani Training and Research Hospital, Istanbul 34384, Turkey.
Osman Gokhan Demir, Department of Medical Oncology, Acıbadem University, Istanbul 34396, Turkey.
Sener Cihan, Department of Medical Oncology, University of Health Sciences, Okmeydani Training and Research Hospital, Istanbul 34384, Turkey.
References
- 1.Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson B, Mariotto A, Lake AJ, Wilson R, Sherman RL, et al. Annual Report to the Nation on the Status of Cancer, 1975-2014, Featuring Survival. J Natl Cancer Inst. 2017;109:djx030. doi: 10.1093/jnci/djx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bufill JA. Colorectal cancer: evidence for distinct genetic categories based on proximal or distal tumor location. Ann Intern Med. 1990;113:779–788. doi: 10.7326/0003-4819-113-10-779. [DOI] [PubMed] [Google Scholar]
- 3.Modest DP, Schulz C, von Weikersthal LF, Quietzsch D, von Einem JC, Schalhorn A, Vehling-Kaiser U, Laubender RP, Giessen C, Stintzing S, et al. Outcome of patients with metastatic colorectal cancer depends on the primary tumor site (midgut vs. hindgut): analysis of the FIRE1-trial (FuFIRI or mIROX as first-line treatment) Anticancer Drugs. 2014;25:212–218. doi: 10.1097/CAD.0000000000000041. [DOI] [PubMed] [Google Scholar]
- 4.Brulé SY, Jonker DJ, Karapetis CS, O’Callaghan CJ, Moore MJ, Wong R, Tebbutt NC, Underhill C, Yip D, Zalcberg JR, et al. Location of colon cancer (right-sided versus left-sided) as a prognostic factor and a predictor of benefit from cetuximab in NCIC CO.17. Eur J Cancer. 2015;51:1405–1414. doi: 10.1016/j.ejca.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 5.Mik M, Berut M, Dziki L, Trzcinski R, Dziki A. Right- and left-sided colon cancer - clinical and pathological differences of the disease entity in one organ. Arch Med Sci. 2017;13:157–162. doi: 10.5114/aoms.2016.58596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel M, McSorley ST, Park JH, Roxburgh CSD, Edwards J, Horgan PG, McMillan DC. The relationship between right-sided tumour location, tumour microenvironment, systemic inflammation, adjuvant therapy and survival in patients undergoing surgery for colon and rectal cancer. Br J Cancer. 2018;118:705–712. doi: 10.1038/bjc.2017.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim DR, Kuk JK, Kim T, Shin EJ. Comparison of oncological outcomes of right-sided colon cancer versus left-sided colon cancer after curative resection: Which side is better outcome? Medicine (Baltimore) 2017;96:e8241. doi: 10.1097/MD.0000000000008241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moritani K, Hasegawa H, Okabayashi K, Ishii Y, Endo T, Kitagawa Y. Difference in the recurrence rate between right- and left-sided colon cancer: a 17-year experience at a single institution. Surg Today. 2014;44:1685–1691. doi: 10.1007/s00595-013-0748-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aoyama T, Kashiwabara K, Oba K, Honda M, Sadahiro S, Hamada C, Maeda H, Mayanagi S, Kanda M, Sakamoto J, et al. Clinical impact of tumor location on the colon cancer survival and recurrence: analyses of pooled data from three large phase III randomized clinical trials. Cancer Med. 2017;6:2523–2530. doi: 10.1002/cam4.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu F, Li C, Jia H, Yang L, Wu Y, Zhao J, Cai S, Zhu J, Xu Y. Is there a prognostic value of tumor location among Chinese patients with colorectal cancer? Oncotarget. 2017;8:38682–38692. doi: 10.18632/oncotarget.16305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warschkow R, Sulz MC, Marti L, Tarantino I, Schmied BM, Cerny T, Güller U. Better survival in right-sided versus left-sided stage I - III colon cancer patients. BMC Cancer. 2016;16:554. doi: 10.1186/s12885-016-2412-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiss JM, Pfau PR, O’Connor ES, King J, LoConte N, Kennedy G, Smith MA. Mortality by stage for right- versus left-sided colon cancer: analysis of surveillance, epidemiology, and end results--Medicare data. J Clin Oncol. 2011;29:4401–4409. doi: 10.1200/JCO.2011.36.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merok MA, Ahlquist T, Røyrvik EC, Tufteland KF, Hektoen M, Sjo OH, Mala T, Svindland A, Lothe RA, Nesbakken A. Microsatellite instability has a positive prognostic impact on stage II colorectal cancer after complete resection: results from a large, consecutive Norwegian series. Ann Oncol. 2013;24:1274–1282. doi: 10.1093/annonc/mds614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang CW, Tsai HL, Huang MY, Huang CM, Yeh YS, Ma CJ, Wang JY. Different clinicopathologic features and favorable outcomes of patients with stage III left-sided colon cancer. World J Surg Oncol. 2015;13:257. doi: 10.1186/s12957-015-0640-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed S, Pahwa P, Le D, Chalchal H, Chandra-Kanthan S, Iqbal N, Fields A. Primary Tumor Location and Survival in the General Population With Metastatic Colorectal Cancer. Clin Colorectal Cancer. 2018;17:e201–e206. doi: 10.1016/j.clcc.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Lee MM, MacKinlay A, Semira C, Schieber C, Jimeno Yepes AJ, Lee B, Wong R, Hettiarachchige CKH, Gunn N, Tie J, et al. Stage-based Variation in the Effect of Primary Tumor Side on All Stages of Colorectal Cancer Recurrence and Survival. Clin Colorectal Cancer. 2018;17:e569–e577. doi: 10.1016/j.clcc.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Kerr DJ, Domingo E, Kerr R. Is sidedness prognostically important across all stages of colorectal cancer? Lancet Oncol. 2016;17:1480–1482. doi: 10.1016/S1470-2045(16)30431-4. [DOI] [PubMed] [Google Scholar]
- 18.Kalady MF, Dejulius KL, Sanchez JA, Jarrar A, Liu X, Manilich E, Skacel M, Church JM. BRAF mutations in colorectal cancer are associated with distinct clinical characteristics and worse prognosis. Dis Colon Rectum. 2012;55:128–133. doi: 10.1097/DCR.0b013e31823c08b3. [DOI] [PubMed] [Google Scholar]