Abstract
Laparoscopic and endoscopic cooperative surgery (LECS) is a surgical technique that combines laparoscopic partial gastrectomy and endoscopic submucosal dissection. LECS requires close collaboration between skilled laparoscopic surgeons and experienced endoscopists. For successful LECS, experience alone is not sufficient. Instead, familiarity with the characteristics of both laparoscopic surgery and endoscopic intervention is necessary to overcome various technical problems. LECS was developed mainly as a treatment for gastric submucosal tumors without epithelial lesions, including gastrointestinal stromal tumors (GISTs). Local gastric wall dissection without lymphadenectomy is adequate for the treatment of gastric GISTs. Compared with conventional simple wedge resection with a linear stapler, LECS can provide both optimal surgical margins and oncological benefit that result in functional preservation of the residual stomach. As technical characteristics, however, classic LECS involves intentional opening of the gastric wall, resulting in a risk of tumor dissemination with contamination by gastric juice. Therefore, several modified LECS techniques have been developed to avoid even subtle tumor exposure. Furthermore, LECS for early gastric cancer has been attempted according to the concept of sentinel lymph node dissection. LECS is a prospective treatment for GISTs and might become a future therapeutic option even for early gastric cancer. Interventional endoscopists and laparoscopic surgeons collaboratively explore curative resection. Simultaneous intraluminal approach with endoscopy allows surgeons to optimizes the resection area. LECS, not simple wedge resection, achieves minimally invasive treatment and allows for oncologically precise resection. We herein present detailed tips and pitfalls of LECS and discuss various technical considerations.
Keywords: Minimally invasive surgery, Laparoscopic and endoscopic cooperative surgery, Facility-based, Gastrointestinal stromal tumor, Early gastric cancer
Core tip: Laparoscopic and endoscopic cooperative surgery (LECS) was first described as a treatment of gastric submucosal tumors in 2008, although a similar concept had been developed before that time. Thereafter, many researchers described LECS as a feasible technique for gastric resection, regardless of tumor location. LECS is a novel procedure that minimizes invasive damage to patients and preserves physiologic function of the residual stomach while securing oncological benefit. Currently, many physicians can fully utilize the advantages of LECS for gastric submucosal tumors located even at the esophagogastric junction by avoiding conventional total gastrectomy or proximal gastrectomy. This technique requires close cooperation between skilled surgeons and experienced endoscopists. Therefore, many tips and pitfalls should be discussed to accelerate this collaboration during LECS. We hope that the herein-described tips will benefit laparoscopic surgeons and interventional endoscopists who are interested in LECS.
INTRODUCTION
Minimally invasive surgery is currently available for benign and borderline malignant tumors of the stomach[1-3]. Resection is a curative treatment for submucosal tumors (SMTs) and early gastric cancer (EGC)[4]. Many endoscopic physicians and general surgeons focus on the invention of novel tools and innovation of technical procedures[3,5,6]. Various therapeutic options have become well developed[2,3,5,7,8]. Interventional endoscopists continue to search for techniques with curative resectability [e.g., endoscopic submucosal dissection (ESD)][9-11], and it was previously considered that endoscopic full-thickness resection is possible only by a surgical approach[12,13]. Since laparoscopy-assisted gastrectomy was first reported in 1994[14], a drastic evolution of laparoscopic surgery has occurred in parallel, and skilled laparoscopic surgeons now precisely perform minimally invasive segmental resection[15-18]. A smooth postoperative course, good functional outcome, and rapid recovery after such procedures have been established[15-17].
Each approach has its own strengths and limitations[3,10]. Hence, a hybrid approach (i.e., cooperation between endoscopic intervention and laparoscopic surgery) was developed[3]. This technique aims to accumulate the strong points of intraluminal and intraperitoneal procedures and negate the technical limitations[3]. This novel concept has been described using different names (e.g., hybrid laparoscopic, combined laparoscopic and endoscopic, laparoscopic-endoscopic rendezvous, and cooperative laparoscopicendoscopic procedures)[3,19-21]; however, use of these multiple terms might confuse endoscopic physicians and general surgeons. Despite the differing names, this hybrid concept focuses on a simultaneous approach via intraluminal and intraperitoneal pathways, subsequent precise resection with oncologic principles, and physiological closure of the defect[3,22,23].
Optimal resection techniques for gastric SMTs and EGC have been established based on the oncologic behaviors of these lesions[22,23]. Laparoscopic and endoscopic cooperative surgery (LECS), not simple wedge resection, achieves minimally invasive treatment and allows for precise resection of these tumors[3]. We herein focus on LECS with a review of previous literature and describe the actual procedures, including technical tips and pitfalls. Moreover, this hybrid approach is discussed with respect to extended indications, oncological benefits, and technical developments.
HISTORY
From an oncological viewpoint, the clinical and pathological behaviors of EGC and SMTs, including gastrointestinal stromal tumors (GISTs), have been well investigated[22,23]. Partial or segmental resection is considered acceptable based on oncologic principles[3,22,23]. General surgeons have an interest in minimally invasive treatment by laparoscopic local resection for SMTs and EGC[24-26]. Simple wedge resection is very easy to perform for most SMTs with extraluminal growth[27]; however, a laparoscopic approach is often difficult with respect to accessing the posterior wall, and postoperative stenosis may occur near the esophagogastric junction (EGJ) or pyloric ring.
Gastric cancer originates from the mucosa, and some SMTs are accompanied by intraluminal growth. A dilemma faced by interventional endoscopists is that endoscopic full-thickness resection is impossible without surgical assistance[3,12,13]. In Japan, laparoscopic wedge resection using a lesion-lifting method was reported for treatment of SMTs with intraluminal growth and EGC[28-31], and a stabbing tool with a T-shaped bar was developed for partial lifting of the target wall[31,32]. However, this lesion-lifting method cannot minimize the resected area because the staple line cannot be determined by an intraluminal approach, and use of this method may increase the rate of positive surgical margins[21].
LECS has long been attempted for treatment of EGC and SMTs[21,33-35]. Interventional endoscopists and laparoscopic surgeons collaboratively explore the potential for curative resection (i.e., a facility-based method) based on the abilities of the physicians at each individual institution[36]. In laparoscopy-assisted endoscopic resection, laparoscopic surgeons assist in resolution of accidental perforation or control of blood loss[37]. In endoscopic-assisted wedge resection, the target gastric wall is resected by linear staplers under intraluminal observation after laparoscopic mobilization of the stomach[37,38]. This combined resection procedure is the most commonly performed because of its technical simplicity[37,39]. Simple wedge resection and the lesion-lifting method are associated with difficulty in resection of tumors located in the posterior wall; thus, surgeons have also developed laparoscopic transluminal or intraluminal surgeries (i.e., endoscope-assisted laparoscopic intraluminal surgery[32,40,41], endoscope-assisted laparoscopic transluminal surgery[42,43], and endoscope-assisted laparoscopic intragastric stapling[44-46])[3]. The resection line can be determined during transluminal or intraluminal surgeries, although these surgeries involve a gastric incision for creation of an intraluminal pathway and require advanced skills[3,21,32].
Novel cooperative laparoscopic and endoscopic techniques for gastric tumors (EGC and SMTs) have been developed mainly in Asian regions[34,47-50]. Procedures of both ESD and LECS originate in Japan, and this may be the reason why LECS is mainly developed in Asian countries so far. The term “LECS” was first reported in 2008[50]; thereafter, this combined procedure was commonly referred to as LECS. Previously established procedures (e.g., the lesion-lifting method[31] and laparoscopy-assisted endoscopic resection[37]) might retrospectively be included as types of LECS procedures. Many physicians have demonstrated that LECS for gastric tumors (mainly SMTs) is feasible and safe.
LECS as described above involves intentional opening of the gastric wall and thus has a risk of tumor dissemination via gastric juice and contamination of the peritoneal cavity by enterobacteria[3,48,51]. LECS is therefore performed for gastric SMTs (mainly GIST), and the indications for LECS have been limited to cases without epithelial lesions including depressed lesions and/or ulcers[3,48]. To overcome this limitation and expand the indications for LECS, several modified LECS procedures have been developed (e.g., inverted LECS[47], laparoscopy-assisted endoscopic full-thickness resection[52], nonexposed endoscopic wallinversion surgery[53-57], clean non-exposure technique[58], closed LECS[51], and lift-and-cut method[59]) and are currently applied to patients even with epithelial lesions. These novel LECS procedures are based on a clear concept of fullthickness resection without intentional perforation (i.e., no exposure of gastric juice) for tumors accompanied by epithelial lesions.
SIMPLE WEDGE RESECTION BY A LINEAR STAPLER
Until LECS became well developed, simple wedge resection was generally conducted as a curative treatment for gastric SMTs. Wedge resection by a linear stapler has the advantage of avoiding the risk of intraoperative dissemination during laparoscopic surgery[60]. Another advantage of wedge resection is its technical simplicity and lack of requirement for advanced skills[3]. However, this simplicity easily results in rough resection and oncological inadequacy[61]. The simple wedge resection technique is associated with both excessive and inadequate resection of the gastric wall, which may lead to postoperative gastric stenosis, gastric dysfunction, and local recurrence[62,63]. Hence, simple wedge resection by a linear stapler is considered a technically easy but high-risk procedure[3,62].
CLASSIC LECS
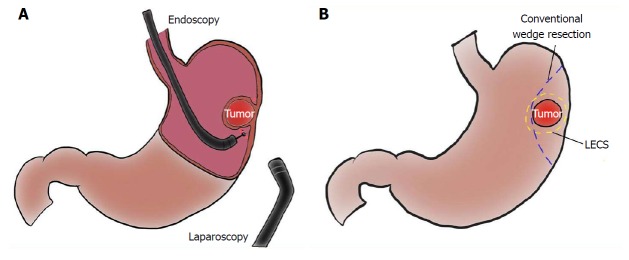
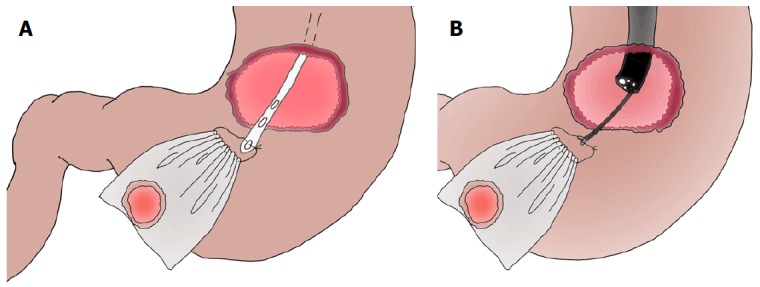
LECS is a surgical technique that combines laparoscopic partial gastrectomy and ESD (Figure 1A). This combined technique is used mainly for gastric SMTs, such as GISTs. The simultaneous intraluminal approach with endoscopy allows surgeons to resect the gastric wall according to the appropriate cutting line without excessive or inadequate margins[63]. From an oncologic viewpoint, LECS optimizes the resection area by providing sufficient margins as a curative resection for gastric SMTs (Figure 1B). This is the most advantageous point of LECS compared with other approaches. Even if an SMT is located near the EGJ, optimal and precise resection by LECS may avoid the need for proximal gastrectomy.
Figure 1.
Schema of laparoscopic and endoscopic cooperative surgery, and comparison of resection line between laparoscopic and endoscopic cooperative surgery and conventional wedge resection. A: Laparoscopic and endoscopic cooperative surgery (LECS) is a combined procedure involving laparoscopy and endoscopy; B: The resection line of LECS minimizes the surgical margin, securing an adequate distance from the tumor. Conventional wedge resection is too close to the tumor and involves excessive wall dissection.
As described above, modified LECS procedures using the concept of “no exposure” have been established for tumors accompanied by epithelial lesions[47,51-58]. The first documented version of LECS[50] has been categorized as “classic LECS” to distinguish it from other modified LECS procedures[48].
INDICATIONS
The indications for LECS should be considered based on the patient’s disease, institutional ability, and individual skills[3,36]. Hence, the indications for LECS may be affected by both tumor- and facility-related factors[36]. Indication and contraindication for LECS are mainly considered based on three factors (i.e., the tumor’s characteristics, institutional ability and individual skills). Other clinical factors (e.g., age, gender, body mass index and comorbidity) never affect the indication for LECS, and these factors in previous documents are summarized in Table 1. In our institution, all patients with a suspicious diagnosis of a gastric GIST routinely undergo gastrointestinal endoscopy, an upper gastrointestinal series, endoscopic ultrasound, and enhanced computed tomography to identify the size and location of the tumor. Moreover, a preoperative pathological diagnosis is made by ultrasound-guided fine-needle aspiration because the therapeutic strategy will be affected by the pathological assessment. For example, although lymph node dissection is not required for surgical treatment of GISTs[64], some SMTs (e.g., carcinoid or submucosal carcinoma and submucosal adenocarcinoma) require lymph node dissection during surgery[65]. In our institution, patients diagnosed with EGC are treated by robot-assisted gastrectomy with lymph node dissection[66].
Table 1.
Clinical outcomes of laparoscopic endoscopic cooperative surgery
| Author | Ref.1 | Published | Patient | Age2 | Gender | BMI2 | Procedures | Diagnosis | Size2 | Conversion rate to | Positive | Complications | Mortality | Recurrence | Folow-up |
| year | number | (male/female) | (kg/m2) | (mm) | gastrectomy or laparotomy | surgical margin | (treatment and case number) | rate | period2 | ||||||
| (case) | (%) | (%) | (%) | (mo) | |||||||||||
| Hiki et al | [50] | 2008 | 7 | 53 ± 6 | 0/7 | 22.0 ± 1 | Classic LECS | SMT | 46 | 0 | 0 | None | 0 | - | - |
| Kikuchi et al | [51] | 2017 | 10 | 62 | 5/5 | - | Closed LECS | SMT | 24.1 | 0 | - | Intra-abdominal abscess (n = 1) | 0 | 0 | 12 |
| Mitsui et al | [56] | 2014 | 6 | 60 | 4/2 | - | NEWS | SMT | 34 | 0 | 0 | None | 0 | 0 | 8 |
| Inoue et al | [58] | 2012 | 24 | 66.2 | - | - | Clean-NET | EGC | - | - | - | Gastric deformity | 0 | 0 | - |
| (Reoperation, n=1) | |||||||||||||||
| Okumura et al | [59] | 2017 | 28 | 67.6 | 8/20 | - | Lift-and-cut method | GIST | 33 | 0 | 0 | None | 0 | 0 | 26.6 |
| Matsuda et al | [71] | 2016 | 100 | 59.8 | 47/53 | 22.7 ± 3.3 | Classic LECS | SMT | 30.9 | 5 | 0 | Leakage (n = 1) | 0 | 0 | 25.3 |
| Postoperative stenossis (n = 2) | |||||||||||||||
| Postoperative bleeding (n = 1) | |||||||||||||||
| Tsujimoto et al | [72] | 2012 | 20 | 59.3 ± 11.9 | 10/10 | 21.8 ± 2.7 | NEWS | SMT | 37.9 ± 11 | 0 | 0 | None | 0 | 0 | 20.7 |
| Kawahira et al | [73] | 2012 | 16 | 61 | 4/12 | 22.1 | Classic LECS | SMT | 27.5 | 0 | 0 | Lymphorrhea (n = 1) | 0 | 0 | - |
| Hoteya et al | [74] | 2014 | 25 | 60 | 10/15 | - | LECS | SMT in EGJ | 32.3 | 0 | 0 | None | 0 | 0 | 18 |
See the reference list;
Data were given as mean ± SD, or the median. BMI: Body mass index; Clean-NET: Clean non-exposure technique; EGC: Early gastric cancer; EGJ: Esophagogastric junction; GIST: Gastrointestinal stromal tumor; LECS: Laparoscopic endoscopic cooperative surgery; NEWS: Non-exposed endoscopic wall-inversion surgery; SMT: Submucosal tumor.
Classic LECS is mainly employed for gastric SMTs, and a GIST is a common target tumor. As described above, opening the gastric wall is associated with a risk of tumor dissemination via gastric juice[3,48,51], and classic LECS has limitations in the treatment of epithelial lesions[3,48]. From the viewpoint of tumor size, however, laparoscopic surgery for larger gastric GISTs is thought to carry a higher risk of tumor capsule injury[67]. The National Comprehensive Cancer Network and European Society for Medical Oncology argue that there is no good evidence in support of laparoscopic surgery for GISTs of > 5 cm[68], although skilled physicians emphasize that laparoscopic surgery for gastric GISTs is safe and feasible regardless of tumor size[69,70]. In our institution, we generally apply laparoscopic surgery to gastric GISTs of ≤ 5 cm in diameter, and we employ LECS only to intraluminal types without epithelial lesions. As a prerequisite, we routinely have detailed preoperative discussions with the patients and obtain adequate informed consent.
Skilled physicians have demonstrated that laparoscopic surgery can be applied to gastric GISTs of larger size and/or epithelial lesions if surgical and oncological safety (e.g., tumor location, layers involved/occupied, expected malignancy of the tumor, institutional ability, and individual skills) are guaranteed[3,33,36,47,51-58,69,70]. Skilled physicians have also documented that LECS is feasible and safe for gastric SMTs in any location[37,50,71-74]. LECS was recently applied to duodenal SMTs[75]. However, application of LECS to SMTs near the EGJ should be carefully considered because laparoscopic suturing in this region requires advanced skill to avoid postoperative stenosis and leakage[34,76-78]. In fact, when the tumor covers more than one-third of the whole circumference of the EGJ, patients have a high rate of conversion to open surgery or proximal gastrectomy[71]. Tumor occupation of more than one-third of the whole circumference of the EGJ should be a contraindication for LECS. Although no definitive risk factors for anastomotic stenosis and postoperative leakage have been established, surgeons should not hesitate to convert to open surgery or proximal gastrectomy during laparoscopic surgery if surgical and oncological safety cannot be guaranteed.
INITIAL SET-UP FOR INTERVENTIONAL ENDOSCOPY AND LAPAROSCOPIC SURGERY
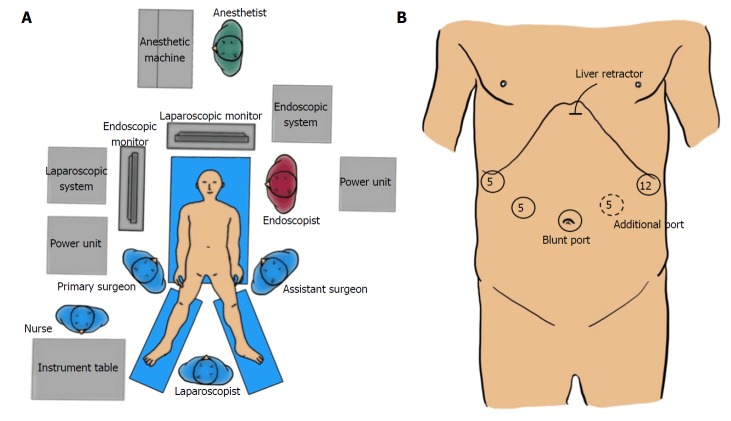
LECS is performed under general anesthesia in the leg-open position. Both arms of the patient are fixed along the body to avoid interference with the procedures performed by the interventional endoscopists. The primary surgeon stands on the right side of the patient, and the assistant surgeon stands on the opposite side. The laparoscopist stands between the patient’s legs. Both the interventional and assistant endoscopists stand beside the patient’s head. The arrangement of various apparatuses and medical staff members in the operation room is shown in Figure 2A.
Figure 2.
Set-up of staffs and devices in the operation theater and port placement. A: Apparatus position and staff placement in the operation room; B: Port placement.
The patient is placed in the supine position with the head directed straight. The tracheal intubation tube has already been inserted through the mouth. Even if the patient’s face can be slightly turned toward the left for endoscope insertion, the interventional endoscopists are repeatedly forced to handle the endoscope under unfamiliar situations (i.e., supine body position, straight face direction, and competitive oral tube). Endoscopists must continuously perform very careful handling of the devices and patient, and placement of a flexible overtube (ST-SB1S; Olympus Medical Systems Corporation, Tokyo, Japan) is a solution for stress-free endoscopic maneuvers. Moreover, as described later, an overtube is a powerful tool for tumor removal via the mouth.
For the endoscopic intervention, an endoscopic system with fine vision and advanced apparatuses, including energy devices, is set up as for ESD. An insulation-tipped diathermic knife (ITknife2, KD-611L; Olympus Medical Systems Corporation) and soft coagulation system (VIO 300 D; Erbe, Tubingen, Germany) are prepared.
A camera port is placed on the umbilicus. Three additional ports (two 5-mm ports and one 12-mm port) are inserted into the left upper, left lower, and right upper quadrants, respectively, under pneumoperitoneum of 12 mmHg with a laparoscopic view. One additional 5- mm port in the right lower quadrant is acceptable, if necessary (Figure 2B).
During LECS, the laparoscopic surgeon should never forget that both the pneumoperitoneal pressure and light intensity are higher on the laparoscopic than endoscopic side. Under the conventional settings of usual laparoscopic surgery, interventional endoscopists cannot secure an adequate field because the stomach would collapse by pneumoperitoneal pressure and cannot obtain fine vision because the laparoscopic light would be too dazzling. The laparoscopic settings of these two factors should be optimally adjusted as necessary during LECS. In our institution, we adjust the light intensity manually as needed and downregulate the pneumoperitoneal pressure to 4 to 6 mm Hg while the interventional endoscope is being operated. However, the endoscopic setting is the same as or similar to that of usual ESD, according to the physician’s preference.
ANATOMICAL RECOGNITION
The stomach is fixed by ligaments and tendons that surround organs and structures such as the hepatoduodenal ligament, celiac axis, pancreatic capsule, crura of the diaphragm, and spleen. The target gastric wall should be mobilized ventrally with a free space made by carbon dioxide gas to ensure the safety of the interventional endoscopic procedure. Even subtle injury to the surrounding organs (e.g., pancreas and aorta) during the endoscopic intervention should be avoided. Especially for SMTs at the posterior wall or EGJ, adequate dissection of the posterior side is key to good mobilization of the target stomach wall. In patients with GISTs, the target gastric wall is directly exposed because of rare metastasis to the regional lymph nodes[64].
PERITONEAL APPROACH BY A LAPAROSCOPIC VIEW
First, the tumor location is identified. Although gastric tumors are intraluminal, the tumor location can often be found from the extraluminal view because the gastric wall is slightly depressed or elevated. If the tumor location cannot be detected via the laparoscopic view, it should be confirmed by the endoscopic view. Excessive dilatation of the digestive tract by endoscopic insufflation of carbon dioxide should be prevented before the start of the intraluminal endoscopic investigation. Clamping of the antrum or jejunum should be performed using clamp forceps (PL541S; B. Braun Aesculap, Tokyo, Japan). Technically, placement of a jejunal clamp at about 10 cm on the anal side of the Treitz ligament is easier than placement of an antral clamp (Figure 3A and B), although an antral clamp provides a better surgical field by prevention of duodenal dilatation (Figure 3A). Notably, endoscopic insufflation into the intestines will remarkably disturb the laparoscopic field. In contrast, the stomach is well expanded by insufflation and clamping, providing an intraluminal working field for the endoscopic intervention.
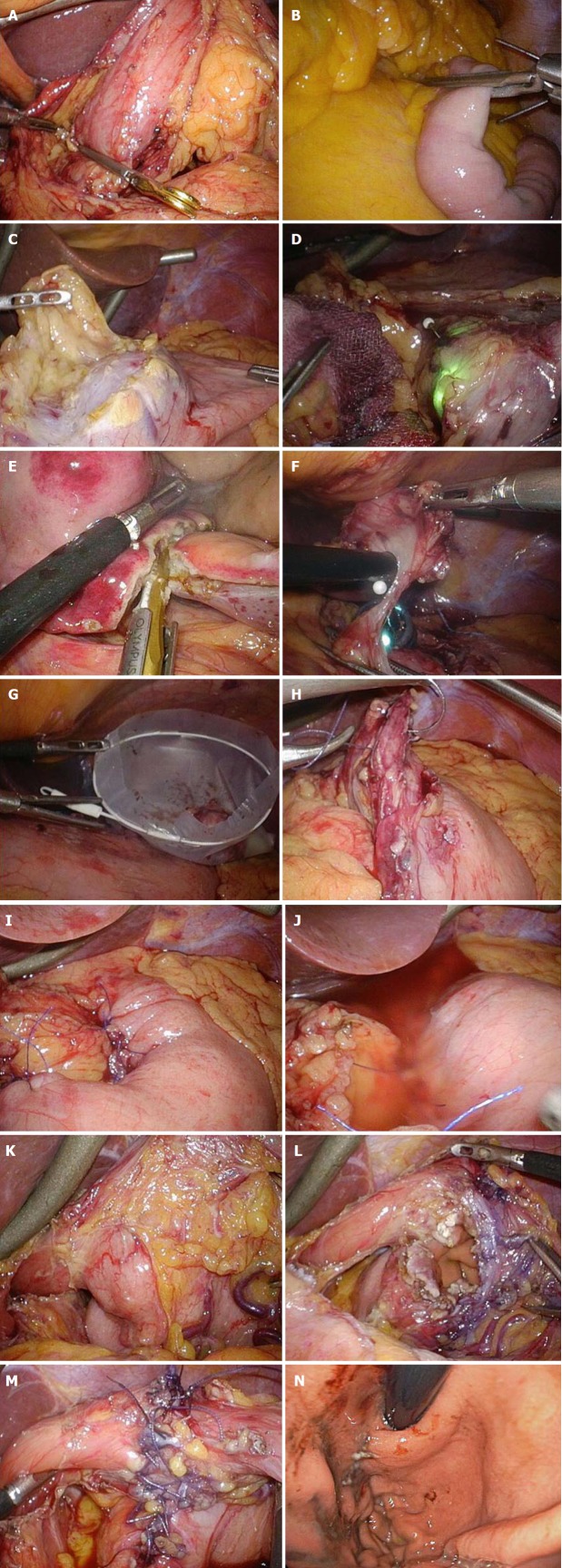
Figure 3.
Intraoperative laparoscopic view of laparoscopic and endoscopic cooperative surgery. A and B: Clamping of the (A) antrum or (B) jejunum should be performed using clamp forceps. This allows for adequate gastric expansion that provides an intraluminal working field for the endoscopic intervention; C: The surrounding fat tissue and vessels of the gastric wall are dissected, and the target wall is then mobilized to the ventral side; D: The laparoscopic surgeon should mobilize the gastric wall and prevent it from touching any surrounding organs for a safe intraluminal intervention. The pneumoperitoneal pressure and light intensity of laparoscopy are decreased to avoid disturbing the endoscopist; E: The laparoscopic surgeon can dissect the proximal gastric wall on behalf of the interventional endoscopist, if necessary; F: The surgeon and the endoscopist cooperate to complete the operation while avoiding injury to the adjacent organs; G: The resected specimen is placed in a plastic bag and removed intraluminally using endoscopy; H: The mucosal layer is closed with a running 4-0 absorbable suture thread; I: The seromuscular layer is closed with interrupted 3-0 absorbable sutures; J: A leak test is performed after suturing. K: This image depicts a case involving a tumor located in the posterior wall near the EGJ; The target gastric wall is turned as much as possible with a marginal free space established by carbon dioxide gas. The right side of the EGJ has enough working space laparoscopically; L: The defect of the gastric wall tends to become larger than many physicians expect; M: The defect in the gastric wall is closed with the laparoscopic hand-sewn technique in a layer-to-layer fashion; N: Intraluminal view after suturing. The absence of stenosis and malformation is confirmed. EGJ: Esophagogastric junction.
The surrounding fat tissue and vessels of the gastric wall are confirmed. To mobilize the stomach, omental fat tissue is cut while preserving the vessels coursing into the stomach (mainly gastroepiploic vessels). When excising the lesser omentum, the gastric branch of the vagus nerve should be maximally preserved to prevent postoperative gastroparesis. After the stomach mobilization, the stomach should be twisted until the target wall faces the ventral side to ensure the safety of the gastric wall during the endoscopic intervention (Figure 3C). Briefly, the target gastric wall never touches any surrounding organs (e.g., pancreas and aorta) (Figure 3D). The ventrally mobilized target wall should then be exposed with a marginal free space established by carbon dioxide gas. Adequate dissection is performed near the tumor and traced to the stomach, and the gastric wall around the tumor is exposed and mobilized to the ventral side. This process is very important to prevent unexpected injury to adjacent organs (e.g., pancreas, liver, aorta, and spleen). Laparoscopic surgeons can dissect the proximal gastric wall with the assistance of interventional endoscopists, if necessary (Figure 3E). The surgeon and the endoscopist cooperate to complete the operation without injuring the adjacent organs (Figure 3F).
Determination of the cutting line with optimal margins based on the endoscopic findings is an oncological benefit. Although the cutting line is set by the interventional endoscopist, resection of the seromuscular layers can be performed with either the interventional endoscopists’ insulation-tipped diathermic knife or the laparoscopic surgeon’s ultrasonic coagulation shears. The resected specimen is placed in a plastic bag and removed intraluminally using endoscopy (Figure 3G).
The defect in the gastric wall is closed with a layer-to-layer laparoscopic hand-sewn technique. The mucosal layer is closed with a running suture using 4-0 absorbable suture thread (4-0 VICRYL, SH-1; Ethicon, Cincinnati, OH, United States). To prevent laxity of the running suture, an assistant surgeon holds the end of the last suture with a needle forceps, which has a strong grip force without any slip. The seromuscular layer is then closed with interrupted sutures using 3-0 absorbable suture thread (3-0 VICRYL, SH-1; Ethicon) (Figure 3H and I). When suturing is completed, a leakage test should be performed. The absence of air leakage should be confirmed by excessively inflating the stomach with endoscopy under adequate saline accumulation using a laparoscopic irrigation device (Figure 3J). The clamp forceps must be removed when the laparoscopic surgery is finished.
The upper stomach is a common site of SMTs, especially GISTs[4,79]. GISTs are frequently located at the fornix/fundus and/or near the EGJ[76,79]. When tumors are located in the posterior wall near the EGJ or in the antrum near the pylorus, ventral mobilization of the stomach wall around the tumor is generally left incomplete. Two solutions are available in such cases. If the SMT has no epithelial lesion, one solution is utilization of the concept of transluminal and intraluminal surgeries, as described above. The gastric wall can be incised to approach the tumor in patients without a possibility of tumor dissemination. The other solution is endoscopic intervention performed under incomplete mobilization but secure surgical fixation of the stomach wall. Mobilization of the stomach is performed, and the target gastric wall is then turned as much as possible with a marginal free space created by carbon dioxide gas. The right side of the EGJ has enough laparoscopic working space[17]. In our institution, the stomach wall around the tumor is securely fixed by laparoscopic forceps, with a marginal free space even if this space is not located ventrally (Figure 3K). When the incision extends to the EGJ, the defect of the gastric wall tends to become larger than many physicians expect (Figure 3L). In such cases, closure of the larger defect should be started at the far side from the laparoscopic surgeons because the surgical field is unclear if the open defect remains on the far side (Figure 3H). Laparoscopic hand-sewn suturing is completed in a layer-to-layer fashion (Figure 3M). To avoid postoperative anastomotic stenosis, esophageal patency and gastric passage are endoscopically confirmed after suturing (Figure 3N). If the endoscope is set through the EGJ as a guide to prevent anastomotic stenosis, the EGJ caliber will be sustained during suturing. Notably, any damage or injury induced by the suture needles should be carefully avoided.
ORAL APPROACH BY ENDOSCOPIC VISUALIZATION
For an oral approach by endoscopic visualization, the location of the tumor is first confirmed (Figure 4A). The periphery of the tumor is then marked using argon plasma coagulation as close as possible to the tumor edge (Figure 4B). After injection of 10% glycerin mixed with indigo blue into the submucosal layer (Figure 4C), a small initial incision is made with a dual knife (Dual knife, KD-650L; Olympus Medical Systems Corporation), and the tip of an insulation-tipped diathermic knife is inserted into the submucosal layer. The whole circumference of the marked area is then cut using the insulation-tipped diathermic knife (Figure 4D). Finally, an intentional perforation is made (Figure 4E), and seromuscular dissection is circumferentially performed according to the determined line of the submucosal layer. The laparoscopic light is too dazzling for the endoscopic side (Figure 4F). The stomach rapidly collapses after gastric perforation, and thereafter, maintenance of an adequate intragastric field for endoscopic manipulation becomes difficult. Laparoscopic surgeons must help the endoscopist to appropriately perform these procedures, avoiding injury to the adjacent organs. According to determined cutting line with optimal margins, resection of the seromuscular layers can be performed by either the interventional endoscopist’s insulation-tipped diathermic knife or the laparoscopic surgeon’s ultrasonic coagulation shears. Especially when cutting the proximal side of the ventrally mobilized gastric wall, the interventional endoscopist may encounter some difficulties because of the reversed endoscopic image (Figure 5). Laparoscopic vision from the umbilicus may be a good solution to this problem. If necessary, the laparoscopic surgeon can dissect the proximal gastric wall on behalf of the interventional endoscopist. The absence of stenosis or malformation should be confirmed after suturing (Figure 4G).
Figure 4.
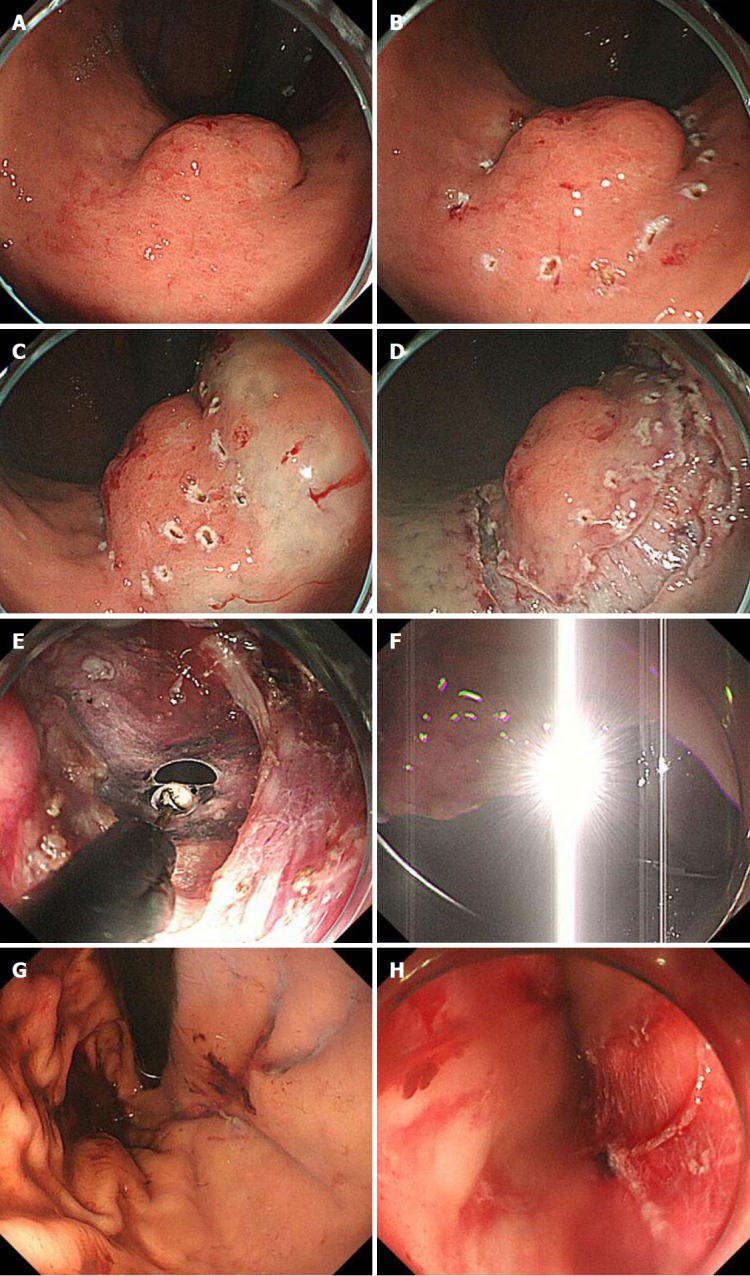
Intraoperative endoscopic view of laparoscopic and endoscopic cooperative surgery. A: First, the location of the tumor is confirmed; B: The periphery of the tumor is marked using argon plasma coagulation as close as possible to the tumor edge; C: Glycerin mixed with indigo blue is injected into the submucosal layer; D: The whole circumference of the marked area is cut using an insulation-tipped diathermic knife; E: An intentional perforation is made; F: The laparoscopic light is too dazzling for the endoscopic side; G: Intraluminal view after suturing. The absence of stenosis and malformation is confirmed; H: Esophageal mucosa injury by the plastic bag during specimen removal.
Figure 5.
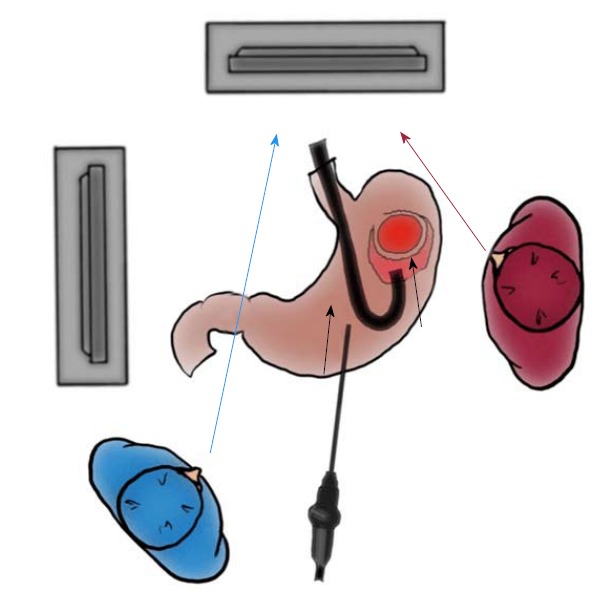
Importance of interventional endoscopist’s line of vision while cutting the proximal side. The interventional endoscopist may experience some difficulties while cutting the proximal side of the gastric wall because of the reversed endoscopic image. If such difficulties are encountered, the endoscopist should turn his or her eyes to the laparoscopic monitor instead of the endoscopic monitor.
The resected specimen is placed in a plastic bag (Rusch MemoBag; Teleflex, Tokyo, Japan) and removed intraluminally using endoscopy if the size of the tumor is ≤ 5 cm[20,80]. Larger tumors of > 5 cm are removed trough the umbilicus with a plastic bag. The thread of the bag is ligated to the nasogastric tube (Figure 6A) or held by a strong grasper (Figure 6B). The stored tumor is then removed through the mouth with utilization of the overtube.
Figure 6.
Options of specimen removal with plastic bag. A: Specimen removal with a nasogastric tube; B: Specimen removal with an endoscopic forcep.
The endoscope is inserted through the overtube. The overtube is used to protect the mucosal wall during the procedure and specimen removal. Appropriate use of an overtube is essential for successful LECS. The stored tumor in the bag is conically set in the overtube (Figure 7), and the overtube is removed with the tumor bag. Hence, injury to the esophageal mucosa can be avoided during specimen removal (Figure 4H).
Figure 7.
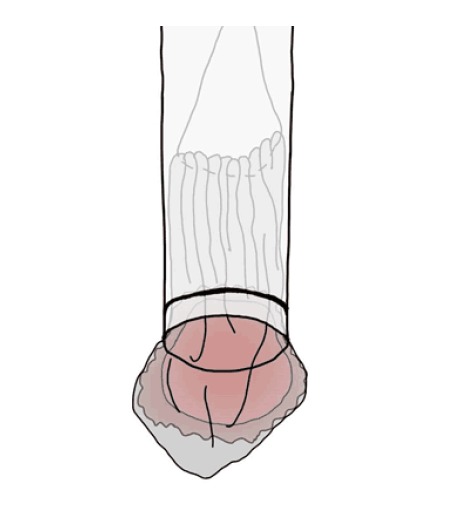
Effective use of an overtube when removing the specimen. The tumor encased in the bag should be sheathed as much as possible in the overtube and removed through the mouth along with the overtube. Hence, injury to the esophageal mucosa by the plastic bag during specimen removal can be avoided.
KEY POINTS AND TECHNICAL PITFALLS
Placement of an overtube has some advantages for repeated endoscopic insertion and tumor removal through the mouth. The cutting line is determined with an optimal circular margin according to the intraluminal findings. This is an oncological benefit of LECS. Laparoscopic pressure and light are stronger than those of endoscopy. Hence, laparoscopic surgeons must pay closer attention to avoid disturbances during endoscopic interventions. The stomach is dissected from related ligaments and omentum, and the target gastric wall is ventrally mobilized. The target gastric wall should be exposed with a marginal free space by carbon dioxide gas and should never touch any surrounding organs for safe intraluminal intervention. To cut the proximal side of the ventrally mobilized gastric wall, laparoscopic vision from the umbilicus may be adequate for endoscopic maneuvers. The laparoscopic surgeon can dissect the proximal gastric wall on behalf of the interventional endoscopist if the interventional endoscopist experiences some difficulties. After tumor removal, the defect is closed in a layer-to-layer fashion. Because laxity of running suture results in leakage, an assistant surgeon holds the end of the last suture with a needle forceps, which has a strong grip force. A leak test can be performed with enough air pressure. To avoid excessive dilatation of the small intestine due to insufflation of carbon dioxide gas from endoscopy, clamp forceps are placed on the antrum or jejunum. This clamp should be removed at the end of surgery.
POSTOPERATIVE COURSE
Patients begin drinking on postoperative day 1 and eating on postoperative day 2. If the postoperative course is uneventful, the patients can be discharged around postoperative day 7. In previous studies, the postoperative hospital stay was 4.6 to 10.5 d[37,71-74,81]. The postoperative hospital stay tends to be prolonged in patients with tumors involving the EGJ[74], and postoperative obstruction due to stenosis is a major concern in patients with lesions near the cardia.
ONCOLOGICAL ADVANTAGES
In LECS, the tumor is resected with careful observation from both the intraluminal and extraluminal side. Consequently, the surgical margins from the tumor are guaranteed, and excessive gastric wall resection is minimized (Figure 8A)[50,81]. Previous important studies reported no recurrent cases (Table 1). Conventional simple wedge resection with only an extraluminal approach results in excessive and unnecessary resection of the gastric wall (Figure 8B-D). It may also have a risk of unexpected crushing of the tumor with the stapler because it is an intraluminally blinded procedure.
Figure 8.
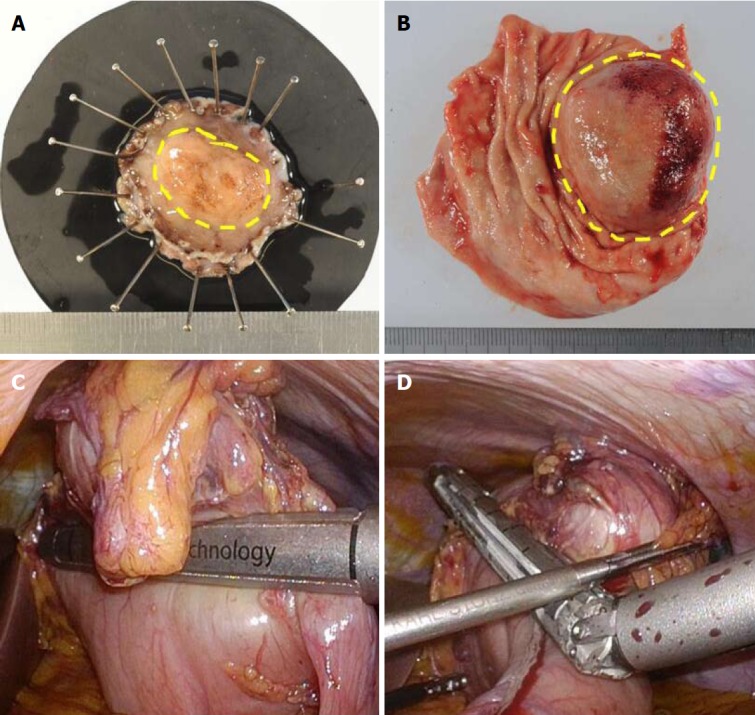
Comparison of surgical margins between laparoscopic and endoscopic cooperative surgery and conventional wedge resection. A: Specimen of Laparoscopic and endoscopic cooperative surgery (LECS). The surgical margin from the tumor is kept at the proper distance; B: Specimen of conventional wedge resection. Simple wedge resection causes both excessive and inadequate resection of the gastric wall, which may lead to postoperative gastric stenosis, gastric dysfunction, and local recurrence; C and D: Intraoperative view of conventional wedge resection with a linear stapler. The resection line is as shown in Figure 1B. The specimen has a portion too close to the tumor and a portion far from the tumor.
LIMITATIONS OF LECS
Many researchers have reported that LECS is feasible and safe for the treatment of gastric SMTs[37,71-74,81]. The main limitation of LECS is the possibility of tumor dissemination during opening of the gastric wall, and contamination with gastric juice into the abdominal cavity may occur. This is why LECS can only be applied to gastric SMTs without epithelial lesions. To overcome this weakness, several procedures based on the concept of “no exposure” have been developed, such as inverted LECS[47], laparoscopy-assisted endoscopic full-thickness resection[52], nonexposed endoscopic wallinversion surgery[53-57], the clean non-exposure technique[58], closed LECS[51], and the lift-and-cut method[59]. Closed LECS, endoscopic resection after plate statement under seromuscular layers, is an effective technique[51].
FACILITY-BASED PRIORITY BETWEEN SURGEONS AND PHYSICIANS
LECS is a combined procedure involving laparoscopic surgery and endoscopic intervention performed in an institution-based manner[36]. However, the balance between the surgeons’ technique and the endoscopists’ skill will vary depending on each facility. Although close cooperation is essential, and collaboration of skilled surgeons and experienced endoscopists is ideal. Skills are set within each institution, and the best facility-based service should be considered on an individual basis[36]. Whether the surgeons or endoscopists will take the initiative and proceed with the operation differs among individual facilities. This does not mean that if a skilled doctor is on one side, the other doctor can be unskilled. Of course, both must be skilled.
From a surgical viewpoint, experience alone is not enough for reliable laparoscopic surgery[16]. Laparoscopic surgeries without reconstructive procedures (e.g., cholecystectomy and appendectomy) do not require advanced techniques, and these surgeries have therefore rapidly spread worldwide. In contrast, complicated laparoscopic surgeries (e.g., gastrectomy and proctectomy) have not yet become typical procedures because of the need for skilled surgeons. LECS is not a markedly difficult procedure, although special skills of laparoscopic suturing are required. The laparoscopic closure is technically challenging. Minimally educated and poorly experienced surgeons who are not familiar with suturing in the abdominal cavity under laparoscopy and have no choice except to use staplers should not pursue this procedure. Ironically, simple wedge resection with linear staplers may accomplish the concept of “no exposure”[60], and employment of a linear stapler itself is actually an effective option to avoid tumor dissemination[60]. This is a critical issue; i.e., that the oncological benefits of LECS are ignored by misuse of simple wedge resection.
MORTALITY AND MORBIDITY
Clinical outcomes (e.g., oncological resectability, mortality, morbidity and follow-up term) in previous important documents were summarized in Table 1. LECS has demonstrated no mortality and a low incidence of postoperative complications[48,81], and we speculate that strict performance of the leakage test may play an important role to avoid leakage.
Even subtle stenosis or obstruction of the upper digestive tract will easily result in refractory symptoms after surgery, and the risk factors for stenosis or obstruction remain undefined. There is no evidence of a lower frequency of postoperative stenosis or obstruction in LECS, conversions to proximal gastrectomy and open surgery have been reported, and a good operative course after double-flap technique anastomosis during proximal gastrectomy has been documented[82].
FUTURE POTENTIAL OF LECS
Although LECS has a risk of tumor dissemination, its application for treatment of EGC has been reported by some researchers[47,57]. Laparoscopic-assisted endoscopic full-thickness resection is also an established procedure[83]. LECS without lymph node dissection for EGC has been applied to limited cases involving technical difficulties when performing ESD such as severe ulcer-related scarring, an unfavorable tumor location, and a large tumor size. However, patients with lymph node metastasis have not been included. LECS for EGC has also been attempted according to the concept of sentinel lymph node dissection[84]. Sentinel lymph node biopsy for EGC is reportedly useful when deciding whether to perform lymph node dissection[85]. If the sentinel lymph node concept is established in the surgical treatment for gastric cancer, the indications for LECS for EGC could be expanded in the future, which could result in increasingly successful gastric cancer treatment. Gastrectomy with lymph node dissection for older patients with gastric cancer, especially those aged ≥ 85 years, has been highly associated with mortality during the postoperative course[86]. To prevent postoperative morbidity and mortality, maintaining an appropriate balance in the surgical procedure and range of lymph node dissection is very important based on the patient’s general condition, comorbidities, and assumed risk. For selected patients, LECS may be useful as a palliative or symptom-alleviating measure.
ADVANCED TECHNIQUES AND COSMETIC ADVANTAGES
Stab and incisional wounds should be considered as distinct from each other[16,87]. The tumor cased in the bag can be sheathed as much as possible in the overtube (Figure 7), and tumor removal through the mouth can omit the need for an incisional wound. To reduce the need for incisional wounds, natural orifice transluminal endoscopic surgery is currently challenged[88,89].
Robot-assisted excision (da Vinci Surgical System; Intuitive Surgical, Inc., Sunnyvale, CA, United States) regardless of tumor size and location has been reported[90]. Additionally, single-port robotic surgery (Single Port Robotic Surgical System, da Vinci Sp; Intuitive Surgical, Inc.) is currently available.
CONCLUSION
LECS can be safely introduced in a facility-based manner by either surgeons or endoscopists with advanced skills. LECS is a function-preserving surgery with oncological safety and is mainly indicated for gastric SMTs if educated, experienced, and skilled physicians are available. LECS has various possibilities for further developments.
Footnotes
Conflict-of-interest statement: No author has potential conflicts of interest.
Manuscript source: Invited manuscript
Peer-review started: August 22, 2018
First decision: August 31, 2018
Article in press: October 12, 2018
Specialty type: Oncology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Huerta-Franco MR, Kopljar M S- Editor: Ma RY L- Editor: A E- Editor: Tan WW
Contributor Information
Yuki Aisu, Department of Digestive Surgery, Tenri Hospital, Tenri 632-8552, Nara, Japan.
Daiki Yasukawa, Department of Surgery, Shiga University of Medical Science, Otsu 520-2192, Japan.
Yusuke Kimura, Department of Hepato-Biliary-Pancreatic Surgery and Transplantation, Graduate School of Medicine, Kyoto University, Kyoto 606-8507, Japan.
Tomohide Hori, Department of Surgery, Shiga General Hospital, Moriyama 524-8524, Shiga, Japan. horitomo55office@yahoo.co.jp.
References
- 1.Liang H, Liang W, Lei Z, Liu Z, Wang W, He J, Zeng Y, Huang W, Wang M, Chen Y, et al. Three-Dimensional Versus Two-Dimensional Video-Assisted Endoscopic Surgery: A Meta-analysis of Clinical Data. World J Surg. 2018 doi: 10.1007/s00268-018-4681-z. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 2.Abe N, Takeuchi H, Ohki A, Hashimoto Y, Mori T, Sugiyama M. Comparison between endoscopic and laparoscopic removal of gastric submucosal tumor. Dig Endosc. 2018;30 Suppl 1:7–16. doi: 10.1111/den.13010. [DOI] [PubMed] [Google Scholar]
- 3.Ntourakis D, Mavrogenis G. Cooperative laparoscopic endoscopic and hybrid laparoscopic surgery for upper gastrointestinal tumors: Current status. World J Gastroenterol. 2015;21:12482–12497. doi: 10.3748/wjg.v21.i43.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDonnell MJ, Punnoose S, Viswanath YKS, Wadd NJ, Dhar A. Gastrointestinal stromal tumours (GISTs): an insight into clinical practice with review of literature. Frontline Gastroenterol. 2017;8:19–25. doi: 10.1136/flgastro-2015-100670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin X, Yin Y, Chen H, Shen C, Tang S, Cai Z, Zhang B, Chen Z. Comparison Analysis of Three Different Types of Minimally Invasive Procedures for Gastrointestinal Stromal Tumors ≤5 cm. J Laparoendosc Adv Surg Tech A. 2018;28:58–64. doi: 10.1089/lap.2017.0305. [DOI] [PubMed] [Google Scholar]
- 6.Kim CG. Endoscopic Full-Thickness Resection Combined with Laparoscopic Surgery. Clin Endosc. 2018;51:33–36. doi: 10.5946/ce.2017.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh HJ, Kim CY. Non-Exposure Endoscopic-Laparoscopic Cooperative Surgery for Stomach Tumors. Clin Endosc. 2018;51:113–114. doi: 10.5946/ce.2018.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shoji Y, Takeuchi H, Goto O, Tokizawa K, Nakamura R, Takahashi T, Wada N, Kawakubo H, Yahagi N, Kitagawa Y. Optimal minimally invasive surgical procedure for gastric submucosal tumors. Gastric Cancer. 2018;21:508–515. doi: 10.1007/s10120-017-0750-5. [DOI] [PubMed] [Google Scholar]
- 9.Tan Y, Tan L, Lu J, Huo J, Liu D. Endoscopic resection of gastric gastrointestinal stromal tumors. Transl Gastroenterol Hepatol. 2017;2:115. doi: 10.21037/tgh.2017.12.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goto O, Takeuchi H, Yahagi N. Laparoscopic endoscopic cooperative surgery: from the view of endoscopists. Nihon Shokakibyo Gakkai Zasshi. 2017;114:209–217. doi: 10.11405/nisshoshi.114.209. [DOI] [PubMed] [Google Scholar]
- 11.Andalib I, Yeoun D, Reddy R, Xie S, Iqbal S. Endoscopic resection of gastric gastrointestinal stromal tumors originating from the muscularis propria layer in North America: methods and feasibility data. Surg Endosc. 2018;32:1787–1792. doi: 10.1007/s00464-017-5862-9. [DOI] [PubMed] [Google Scholar]
- 12.Shim CN, Lee SK. Endoscopic submucosal dissection for undifferentiated-type early gastric cancer: do we have enough data to support this? World J Gastroenterol. 2014;20:3938–3949. doi: 10.3748/wjg.v20.i14.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abe N, Takeuchi H, Ooki A, Nagao G, Masaki T, Mori T, Sugiyama M. Recent developments in gastric endoscopic submucosal dissection: towards the era of endoscopic resection of layers deeper than the submucosa. Dig Endosc. 2013;25 Suppl 1:64–70. doi: 10.1111/j.1443-1661.2012.01387.x. [DOI] [PubMed] [Google Scholar]
- 14.Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4:146–148. [PubMed] [Google Scholar]
- 15.Yasukawa D, Kadokawa Y, Kato S, Aisu Y, Hori T. Safety and feasibility of laparoscopic gastrectomy accompanied by D1+ lymph node dissection for early gastric cancer in elderly patients. Asian J Endosc Surg. 2018 doi: 10.1111/ases.12480. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 16.Hori T, Oike F, Furuyama H, Machimoto T, Kadokawa Y, Hata T, Kato S, Yasukawa D, Aisu Y, Sasaki M, et al. Protocol for laparoscopic cholecystectomy: Is it rocket science? World J Gastroenterol. 2016;22:10287–10303. doi: 10.3748/wjg.v22.i47.10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hori T, Kaido T, Iida T, Yagi S, Uemoto S. Comprehensive guide to laparoscope-assisted graft harvesting in live donors for living-donor liver transplantation: perspective of laparoscopic vision. Ann Gastroenterol. 2017;30:118–126. doi: 10.20524/aog.2016.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee HH, Hur H, Jung H, Park CH, Jeon HM, Song KY. Laparoscopic wedge resection for gastric submucosal tumors: a size-location matched case-control study. J Am Coll Surg. 2011;212:195–199. doi: 10.1016/j.jamcollsurg.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Caron PH, Martins MI, Bertevello PL. Preliminary analysis of hybrid laparoscopic procedure for resection of gastric submucosal tumors. Rev Col Bras Cir. 2016;43:129–135. doi: 10.1590/0100-69912016002010. [DOI] [PubMed] [Google Scholar]
- 20.Mino JS, Guerron AD, Monteiro R, El-Hayek K, Ponsky JL, Patil DT, Walsh RM. Long-term outcomes of combined endoscopic/laparoscopic intragastric enucleation of presumed gastric stromal tumors. Surg Endosc. 2016;30:1747–1753. doi: 10.1007/s00464-015-4416-2. [DOI] [PubMed] [Google Scholar]
- 21.Ludwig K, Wilhelm L, Scharlau U, Amtsberg G, Bernhardt J. Laparoscopic-endoscopic rendezvous resection of gastric tumors. Surg Endosc. 2002;16:1561–1565. doi: 10.1007/s00464-001-9224-1. [DOI] [PubMed] [Google Scholar]
- 22.ESMO/European Sarcoma Network Working Group. Gastrointestinal stromal tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 Suppl 7:vii49–vii55. doi: 10.1093/annonc/mds252. [DOI] [PubMed] [Google Scholar]
- 23.Yada T, Yokoi C, Uemura N. The current state of diagnosis and treatment for early gastric cancer. Diagn Ther Endosc. 2013;2013:241320. doi: 10.1155/2013/241320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishikawa K, Inomata M, Etoh T, Shiromizu A, Shiraishi N, Arita T, Kitano S. Long-term outcome of laparoscopic wedge resection for gastric submucosal tumor compared with open wedge resection. Surg Laparosc Endosc Percutan Tech. 2006;16:82–85. doi: 10.1097/00129689-200604000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Kitano S, Shiraishi N. Minimally invasive surgery for gastric tumors. Surg Clin North Am. 2005;85:151–164, xi. doi: 10.1016/j.suc.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Seto Y, Yamaguchi H, Shimoyama S, Shimizu N, Aoki F, Kaminishi M. Results of local resection with regional lymphadenectomy for early gastric cancer. Am J Surg. 2001;182:498–501. doi: 10.1016/s0002-9610(01)00747-4. [DOI] [PubMed] [Google Scholar]
- 27.Choi SM, Kim MC, Jung GJ, Kim HH, Kwon HC, Choi SR, Jang JS, Jeong JS. Laparoscopic wedge resection for gastric GIST: long-term follow-up results. Eur J Surg Oncol. 2007;33:444–447. doi: 10.1016/j.ejso.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Ohgami M, Otani Y, Kumai K, Kubota T, Kitajima M. [Laparoscopic surgery for early gastric cancer] Nihon Geka Gakkai Zasshi. 1996;97:279–285. [PubMed] [Google Scholar]
- 29.Ohgami M, Otani Y, Kubota T, Kumai K, Kitajima M. [Laparoscopic curative surgery for early gastric cancer] Nihon Rinsho. 1996;54:1307–1311. [PubMed] [Google Scholar]
- 30.Ohgami M, Otani Y, Kumai K, Kubota T, Kitajima M. [Laparoscopic wedge resection of the stomach for early gastric cancer using a lesion-lifting-method: curative and minimally invasive treatment] Zentralbl Chir. 1998;123:465–468. [PubMed] [Google Scholar]
- 31.Ohgami M, Otani Y, Kumai K, Kubota T, Kim YI, Kitajima M. Curative laparoscopic surgery for early gastric cancer: five years experience. World J Surg. 1999;23:187–192; discussion 192-193. doi: 10.1007/pl00013167. [DOI] [PubMed] [Google Scholar]
- 32.Ohashi S. Laparoscopic intraluminal (intragastric) surgery for early gastric cancer. A new concept in laparoscopic surgery. Surg Endosc. 1995;9:169–171. doi: 10.1007/BF00191960. [DOI] [PubMed] [Google Scholar]
- 33.Aoki M, Tokioka S, Narabayashi K, Hakoda A, Inoue Y, Yorifuji N, Chino Y, Sato I, Egashira Y, Takeuchi T, et al. Laparoscopic and endoscopic cooperative surgery for intra-mucosal gastric carcinoma adjacent to the ulcer scars. World J Surg Oncol. 2018;16:53. doi: 10.1186/s12957-018-1355-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsuda T, Nunobe S, Kosuga T, Kawahira H, Inaki N, Kitashiro S, Abe N, Miyashiro I, Nagao S, Nishizaki M, et al. Laparoscopic and luminal endoscopic cooperative surgery can be a standard treatment for submucosal tumors of the stomach: a retrospective multicenter study. Endoscopy. 2017;49:476–483. doi: 10.1055/s-0043-104526. [DOI] [PubMed] [Google Scholar]
- 35.Balde AI, Chen T, Hu Y, Redondo N JD, Liu H, Gong W, Yu J, Zhen L, Li G. Safety analysis of laparoscopic endoscopic cooperative surgery versus endoscopic submucosal dissection for selected gastric gastrointestinal stromal tumors: a propensity score-matched study. Surg Endosc. 2017;31:843–851. doi: 10.1007/s00464-016-5042-3. [DOI] [PubMed] [Google Scholar]
- 36.Kozarek RA. The society for gastrointestinal intervention. Are we, as an organization of disparate disciplines, cooperative or competitive? Gut Liver. 2010;4 Suppl 1:S1–S8. doi: 10.5009/gnl.2010.4.S1.S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qiu WQ, Zhuang J, Wang M, Liu H, Shen ZY, Xue HB, Shen L, Ge ZZ, Cao H. Minimally invasive treatment of laparoscopic and endoscopic cooperative surgery for patients with gastric gastrointestinal stromal tumors. J Dig Dis. 2013;14:469–473. doi: 10.1111/1751-2980.12076. [DOI] [PubMed] [Google Scholar]
- 38.Kang WM, Yu JC, Ma ZQ, Zhao ZR, Meng QB, Ye X. Laparoscopic-endoscopic cooperative surgery for gastric submucosal tumors. World J Gastroenterol. 2013;19:5720–5726. doi: 10.3748/wjg.v19.i34.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Novitsky YW, Kercher KW, Sing RF, Heniford BT. Long-term outcomes of laparoscopic resection of gastric gastrointestinal stromal tumors. Ann Surg. 2006;243:738–745; discussion 745-747. doi: 10.1097/01.sla.0000219739.11758.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schubert D, Kuhn R, Nestler G, Kahl S, Ebert MP, Malfertheiner P, Lippert H, Pross M. Laparoscopic-endoscopic rendezvous resection of upper gastrointestinal tumors. Dig Dis. 2005;23:106–112. doi: 10.1159/000088591. [DOI] [PubMed] [Google Scholar]
- 41.Dong HY, Wang YL, Jia XY, Li J, Li GD, Li YQ. Modified laparoscopic intragastric surgery and endoscopic full-thickness resection for gastric stromal tumor originating from the muscularis propria. Surg Endosc. 2014;28:1447–1453. doi: 10.1007/s00464-013-3375-8. [DOI] [PubMed] [Google Scholar]
- 42.Huguet KL, Rush RM Jr, Tessier DJ, Schlinkert RT, Hinder RA, Grinberg GG, Kendrick ML, Harold KL. Laparoscopic gastric gastrointestinal stromal tumor resection: the mayo clinic experience. Arch Surg. 2008;143:587–590; discussion 591. doi: 10.1001/archsurg.143.6.587. [DOI] [PubMed] [Google Scholar]
- 43.Sasaki A, Koeda K, Obuchi T, Nakajima J, Nishizuka S, Terashima M, Wakabayashi G. Tailored laparoscopic resection for suspected gastric gastrointestinal stromal tumors. Surgery. 2010;147:516–520. doi: 10.1016/j.surg.2009.10.035. [DOI] [PubMed] [Google Scholar]
- 44.Ridwelski K, Pross M, Schubert S, Wolff S, Günther T, Kahl S, Lippert H. Combined endoscopic intragastral resection of a posterior stromal gastric tumor using an original technique. Surg Endosc. 2002;16:537. doi: 10.1007/s004640042014. [DOI] [PubMed] [Google Scholar]
- 45.Pross M, Wolff S, Nestler G, Schubert D, Kahl S, Lippert H. A technique for endo-organ resection of gastric wall tumors using one intragastric trocar. Endoscopy. 2003;35:613–615. doi: 10.1055/s-2003-40239. [DOI] [PubMed] [Google Scholar]
- 46.Sahm M, Pross M, Lippert H. Intraluminal resection of gastric tumors using intragastric trocar technique. Surg Laparosc Endosc Percutan Tech. 2011;21:e169–e172. doi: 10.1097/SLE.0b013e318221749c. [DOI] [PubMed] [Google Scholar]
- 47.Nunobe S, Hiki N, Gotoda T, Murao T, Haruma K, Matsumoto H, Hirai T, Tanimura S, Sano T, Yamaguchi T. Successful application of laparoscopic and endoscopic cooperative surgery (LECS) for a lateral-spreading mucosal gastric cancer. Gastric Cancer. 2012;15:338–342. doi: 10.1007/s10120-012-0146-5. [DOI] [PubMed] [Google Scholar]
- 48.Hiki N, Nunobe S, Matsuda T, Hirasawa T, Yamamoto Y, Yamaguchi T. Laparoscopic endoscopic cooperative surgery. Dig Endosc. 2015;27:197–204. doi: 10.1111/den.12404. [DOI] [PubMed] [Google Scholar]
- 49.Hiki N, Yamamoto Y, Hirasawa T. Current status and prospect of laparoscopy endoscopy cooperative surgery (LECS) Nihon Shokakibyo Gakkai Zasshi. 2017;114:205–208. doi: 10.11405/nisshoshi.114.205. [DOI] [PubMed] [Google Scholar]
- 50.Hiki N, Yamamoto Y, Fukunaga T, Yamaguchi T, Nunobe S, Tokunaga M, Miki A, Ohyama S, Seto Y. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc. 2008;22:1729–1735. doi: 10.1007/s00464-007-9696-8. [DOI] [PubMed] [Google Scholar]
- 51.Kikuchi S, Nishizaki M, Kuroda S, Tanabe S, Noma K, Kagawa S, Shirakawa Y, Kato H, Okada H, Fujiwara T. Nonexposure laparoscopic and endoscopic cooperative surgery (closed laparoscopic and endoscopic cooperative surgery) for gastric submucosal tumor. Gastric Cancer. 2017;20:553–557. doi: 10.1007/s10120-016-0641-1. [DOI] [PubMed] [Google Scholar]
- 52.Abe N, Takeuchi H, Yanagida O, Masaki T, Mori T, Sugiyama M, Atomi Y. Endoscopic full-thickness resection with laparoscopic assistance as hybrid NOTES for gastric submucosal tumor. Surg Endosc. 2009;23:1908–1913. doi: 10.1007/s00464-008-0317-y. [DOI] [PubMed] [Google Scholar]
- 53.Goto O, Mitsui T, Fujishiro M, Wada I, Shimizu N, Seto Y, Koike K. New method of endoscopic full-thickness resection: a pilot study of non-exposed endoscopic wall-inversion surgery in an ex vivo porcine model. Gastric Cancer. 2011;14:183–187. doi: 10.1007/s10120-011-0014-8. [DOI] [PubMed] [Google Scholar]
- 54.Goto O, Takeuchi H, Kawakubo H, Sasaki M, Matsuda T, Matsuda S, Kigasawa Y, Kadota Y, Fujimoto A, Ochiai Y, et al. First case of non-exposed endoscopic wall-inversion surgery with sentinel node basin dissection for early gastric cancer. Gastric Cancer. 2015;18:434–439. doi: 10.1007/s10120-014-0406-7. [DOI] [PubMed] [Google Scholar]
- 55.Mitsui T, Goto O, Shimizu N, Hatao F, Wada I, Niimi K, Asada-Hirayama I, Fujishiro M, Koike K, Seto Y. Novel technique for full-thickness resection of gastric malignancy: feasibility of nonexposed endoscopic wall-inversion surgery (news) in porcine models. Surg Laparosc Endosc Percutan Tech. 2013;23:e217–e221. doi: 10.1097/SLE.0b013e31828e3f94. [DOI] [PubMed] [Google Scholar]
- 56.Mitsui T, Niimi K, Yamashita H, Goto O, Aikou S, Hatao F, Wada I, Shimizu N, Fujishiro M, Koike K, et al. Non-exposed endoscopic wall-inversion surgery as a novel partial gastrectomy technique. Gastric Cancer. 2014;17:594–599. doi: 10.1007/s10120-013-0291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goto O, Takeuchi H, Kawakubo H, Matsuda S, Kato F, Sasaki M, Fujimoto A, Ochiai Y, Horii J, Uraoka T, et al. Feasibility of non-exposed endoscopic wall-inversion surgery with sentinel node basin dissection as a new surgical method for early gastric cancer: a porcine survival study. Gastric Cancer. 2015;18:440–445. doi: 10.1007/s10120-014-0358-y. [DOI] [PubMed] [Google Scholar]
- 58.Inoue H, Ikeda H, Hosoya T, Yoshida A, Onimaru M, Suzuki M, Kudo SE. Endoscopic mucosal resection, endoscopic submucosal dissection, and beyond: full-layer resection for gastric cancer with nonexposure technique (CLEAN-NET) Surg Oncol Clin N Am. 2012;21:129–140. doi: 10.1016/j.soc.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 59.Okumura S, Kanaya S, Hosogi H, Ito T, Miura S, Okada T, Shimoike N, Akagawa S, Kawada H, Arimoto A. Our experience with laparoscopic partial gastrectomy by the ‘lift-and-cut method’ for gastric gastrointestinal stromal tumor with maximal preservation of the remnant stomach. Surg Endosc. 2017;31:3398–3404. doi: 10.1007/s00464-016-5367-y. [DOI] [PubMed] [Google Scholar]
- 60.Kiyozaki H, Saito M, Chiba H, Takata O, Rikiyama T. Laparoscopic wedge resection of the stomach for gastrointestinal stromal tumor (GIST): non-touch lesion lifting method. Gastric Cancer. 2014;17:337–340. doi: 10.1007/s10120-013-0272-8. [DOI] [PubMed] [Google Scholar]
- 61.Willingham FF, Reynolds P, Lewis M, Ross A, Maithel SK, Rocha FG. Hybrid push-pull endoscopic and laparoscopic full thickness resection for the minimally invasive management of gastrointestinal stromal tumors: a pilot clinical study. Gastroenterol Res Pract. 2015;2015:618756. doi: 10.1155/2015/618756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hu J, Or BH, Hu K, Wang ML. Comparison of the post-operative outcomes and survival of laparoscopic versus open resections for gastric gastrointestinal stromal tumors: A multi-center prospective cohort study. Int J Surg. 2016;33 Pt A:65–71. doi: 10.1016/j.ijsu.2016.07.064. [DOI] [PubMed] [Google Scholar]
- 63.Couch RS. Radiology of the urinary system. Lond Clin Med J. 1966;7:47–53. [PubMed] [Google Scholar]
- 64.Fong Y, Coit DG, Woodruff JM, Brennan MF. Lymph node metastasis from soft tissue sarcoma in adults. Analysis of data from a prospective database of 1772 sarcoma patients. Ann Surg. 1993;217:72–77. doi: 10.1097/00000658-199301000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saund MS, Al Natour RH, Sharma AM, Huang Q, Boosalis VA, Gold JS. Tumor size and depth predict rate of lymph node metastasis and utilization of lymph node sampling in surgically managed gastric carcinoids. Ann Surg Oncol. 2011;18:2826–2832. doi: 10.1245/s10434-011-1652-0. [DOI] [PubMed] [Google Scholar]
- 66.Aisu Y, Kadokawa Y, Kato S, Yasukawa D, Kimura Y, Hori T. Robot-assisted distal gastrectomy with lymph node dissection for gastric cancer in a patient with situs inversus partialis: a case report with video file. Surg Case Rep. 2018;4:16. doi: 10.1186/s40792-018-0422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mochizuki Y, Kodera Y, Fujiwara M, Ito S, Yamamura Y, Sawaki A, Yamao K, Kato T. Laparoscopic wedge resection for gastrointestinal stromal tumors of the stomach: initial experience. Surg Today. 2006;36:341–347. doi: 10.1007/s00595-005-3164-7. [DOI] [PubMed] [Google Scholar]
- 68.Nishida T, Blay JY, Hirota S, Kitagawa Y, Kang YK. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer. 2016;19:3–14. doi: 10.1007/s10120-015-0526-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lin J, Huang C, Zheng C, Li P, Xie J, Wang J, Lu J. Laparoscopic versus open gastric resection for larger than 5 cm primary gastric gastrointestinal stromal tumors (GIST): a size-matched comparison. Surg Endosc. 2014;28:2577–2583. doi: 10.1007/s00464-014-3506-x. [DOI] [PubMed] [Google Scholar]
- 70.Karakousis GC, Singer S, Zheng J, Gonen M, Coit D, DeMatteo RP, Strong VE. Laparoscopic versus open gastric resections for primary gastrointestinal stromal tumors (GISTs): a size-matched comparison. Ann Surg Oncol. 2011;18:1599–1605. doi: 10.1245/s10434-010-1517-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Matsuda T, Hiki N, Nunobe S, Aikou S, Hirasawa T, Yamamoto Y, Kumagai K, Ohashi M, Sano T, Yamaguchi T. Feasibility of laparoscopic and endoscopic cooperative surgery for gastric submucosal tumors (with video) Gastrointest Endosc. 2016;84:47–52. doi: 10.1016/j.gie.2015.11.040. [DOI] [PubMed] [Google Scholar]
- 72.Tsujimoto H, Yaguchi Y, Kumano I, Takahata R, Ono S, Hase K. Successful gastric submucosal tumor resection using laparoscopic and endoscopic cooperative surgery. World J Surg. 2012;36:327–330. doi: 10.1007/s00268-011-1387-x. [DOI] [PubMed] [Google Scholar]
- 73.Kawahira H, Hayashi H, Natsume T, Akai T, Uesato M, Horibe D, Mori M, Hanari N, Aoyama H, Nabeya Y, et al. Surgical advantages of gastric SMTs by laparoscopy and endoscopy cooperative surgery. Hepatogastroenterology. 2012;59:415–417. doi: 10.5754/hge11456. [DOI] [PubMed] [Google Scholar]
- 74.Hoteya S, Haruta S, Shinohara H, Yamada A, Furuhata T, Yamashita S, Kikuchi D, Mitani T, Ogawa O, Matsui A, et al. Feasibility and safety of laparoscopic and endoscopic cooperative surgery for gastric submucosal tumors, including esophagogastric junction tumors. Dig Endosc. 2014;26:538–544. doi: 10.1111/den.12215. [DOI] [PubMed] [Google Scholar]
- 75.Ojima T, Nakamori M, Nakamura M, Hayata K, Katsuda M, Takifuji K, Yamaue H. Laparoscopic and Endoscopic Cooperative Surgery Versus Endoscopic Submucosal Dissection for the Treatment of Low-Risk Tumors of the Duodenum. J Gastrointest Surg. 2018;22:935–940. doi: 10.1007/s11605-018-3680-6. [DOI] [PubMed] [Google Scholar]
- 76.Xiong W, Zhu J, Zheng Y, Luo L, He Y, Li H, Diao D, Zou L, Wan J, Wang W. Laparoscopic resection for gastrointestinal stromal tumors in esophagogastric junction (EGJ): how to protect the EGJ. Surg Endosc. 2018;32:983–989. doi: 10.1007/s00464-017-5776-6. [DOI] [PubMed] [Google Scholar]
- 77.Tanaka Y, Kosuga T, Komatsu S, Okamoto K, Shoda K, Arita T, Konishi H, Morimura R, Murayama Y, Shiozaki A, et al. [Laparoscopic Local Resection for a Gastric GIST with Ulcer Locating Near to the Esophagogastric Junction - A Case Report] Gan To Kagaku Ryoho. 2017;44:1308–1310. [PubMed] [Google Scholar]
- 78.Obuchi T, Sasaki A, Baba S, Nitta H, Otsuka K, Wakabayashi G. Single-port laparoscopic and endoscopic cooperative surgery for a gastric gastrointestinal stromal tumor: report of a case. Surg Today. 2015;45:641–646. doi: 10.1007/s00595-014-0870-z. [DOI] [PubMed] [Google Scholar]
- 79.An W, Sun PB, Gao J, Jiang F, Liu F, Chen J, Wang D, Li ZS, Shi XG. Endoscopic submucosal dissection for gastric gastrointestinal stromal tumors: a retrospective cohort study. Surg Endosc. 2017;31:4522–4531. doi: 10.1007/s00464-017-5511-3. [DOI] [PubMed] [Google Scholar]
- 80.Ojima T, Nakamura M, Nakamori M, Takifuji K, Hayata K, Katsuda M, Takei Y, Yamaue H. Laparoscopic and endoscopic cooperative surgery is a feasible treatment procedure for intraluminal gastric gastrointestinal stromal tumors compared to endoscopic intragastric surgery. Surg Endosc. 2018;32:351–357. doi: 10.1007/s00464-017-5683-x. [DOI] [PubMed] [Google Scholar]
- 81.Waseda Y, Doyama H, Inaki N, Nakanishi H, Yoshida N, Tsuji S, Takemura K, Yamada S, Okada T. Does laparoscopic and endoscopic cooperative surgery for gastric submucosal tumors preserve residual gastric motility? Results of a retrospective single-center study. PLoS One. 2014;9:e101337. doi: 10.1371/journal.pone.0101337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hayami M, Hiki N, Nunobe S, Mine S, Ohashi M, Kumagai K, Ida S, Watanabe M, Sano T, Yamaguchi T. Clinical Outcomes and Evaluation of Laparoscopic Proximal Gastrectomy with Double-Flap Technique for Early Gastric Cancer in the Upper Third of the Stomach. Ann Surg Oncol. 2017;24:1635–1642. doi: 10.1245/s10434-017-5782-x. [DOI] [PubMed] [Google Scholar]
- 83.Aslani N, Alkhamesi NA, Schlachta CM. Hybrid Laparoendoscopic Approaches to Endoscopically Unresectable Colon Polyps. J Laparoendosc Adv Surg Tech A. 2016;26:581–590. doi: 10.1089/lap.2015.0290. [DOI] [PubMed] [Google Scholar]
- 84.Zhang C, Hu X. [Function-preserving gastrectomy for early gastric cancer based on Japanese researches] Zhonghua Weichang Waike Zazhi. 2018;21:148–153. [PubMed] [Google Scholar]
- 85.Kitagawa Y, Takeuchi H, Takagi Y, Natsugoe S, Terashima M, Murakami N, Fujimura T, Tsujimoto H, Hayashi H, Yoshimizu N, et al. Sentinel node mapping for gastric cancer: a prospective multicenter trial in Japan. J Clin Oncol. 2013;31:3704–3710. doi: 10.1200/JCO.2013.50.3789. [DOI] [PubMed] [Google Scholar]
- 86.Kiyokawa T, Hiki N, Nunobe S, Honda M, Ohashi M, Sano T, Yamaguchi T. Feasibility of Gastrectomy with Standard Lymphadenectomy for Patients Over 85 Years Old with Gastric Cancer. Ann Surg Oncol. 2015;22:3962–3969. doi: 10.1245/s10434-015-4489-0. [DOI] [PubMed] [Google Scholar]
- 87.Hori T, Machimoto T, Kadokawa Y, Hata T, Ito T, Kato S, Yasukawa D, Aisu Y, Kimura Y, Sasaki M, et al. Laparoscopic appendectomy for acute appendicitis: How to discourage surgeons using inadequate therapy. World J Gastroenterol. 2017;23:5849–5859. doi: 10.3748/wjg.v23.i32.5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sanchez-Ocana R, Penas-Herrero I, Gil-Simon P, de la Serna-Higuera C, Perez-Miranda M. Natural orifice transluminal endoscopic surgery salvage of direct EUS-guided gastrojejunostomy. VideoGIE. 2017;2:346–348. doi: 10.1016/j.vgie.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu J, Bardawil E, Lin Q, Liang B, Wang W, Wu C, Guan X. Transvaginal natural orifice transluminal endoscopic surgery tubal reanastomosis: a novel route for tubal surgery. Fertil Steril. 2018;110:182. doi: 10.1016/j.fertnstert.2018.02.139. [DOI] [PubMed] [Google Scholar]
- 90.Furbetta N, Palmeri M, Guadagni S, Di Franco G, Gianardi D, Latteri S, Marciano E, Moglia A, Cuschieri A, Di Candio G, et al. Gastrointestinal stromal tumours of stomach: Robot-assisted excision with the da Vinci Surgical System regardless of size and location site. J Minim Access Surg. 2018 doi: 10.4103/jmas.JMAS_260_17. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]