Abstract
Post-transplant immunosuppression has reduced the incidence of T cell-mediated acute rejection, yet long-term cardiac graft survival rates remain a challenge. An important determinant of chronic solid organ allograft complication is accelerated vascular disease of the transplanted graft. In the case of cardiac allograft vasculopathy (CAV), the precise cellular etiology remains inadequately understood; however, histologic evidence hints at the accumulation and activation of innate phagocytes as a causal contributing factor. This includes monocytes, macrophages, and immature dendritic cell subsets. In addition to crosstalk with adaptive T and B immune cells, myeloid phagocytes secrete paracrine signals that directly activate fibroblasts and vascular smooth muscle cells, both of which contribute to fibrous intimal thickening. Though maladaptive phagocyte functions may promote CAV, directed modulation of myeloid cell function, at the molecular level, holds promise for tolerance and prolonged cardiac graft function.
Keywords: Transplant, Vasculopathy, Macrophage, Tolerance
Introduction
Clinical significance and introduction to innate immune cells during chronic heart rejection
Cardiac transplantation is now a well-established and accepted therapy for end-stage heart failure for patients of all ages. Numerous clinical advances have reduced acute organ transplant rejection. Beyond 1 year, however, immunologic-related complications are significant and morbid. Due to the chronic need for pharmacologic immunosuppression, or the side effects of these drugs, patients are at risk for opportunistic infections, oncologic, hematologic [78], metabolic, and nephrotoxic side effects [42]. Moreover, current broad-spectrum immunosuppressive approaches do not adequately prevent cardiac allograft vasculopathy (CAV). This phenomenon resembles an accelerated form of coronary artery disease [55] with similar non-resolving inflammation and eventual chronic or acute insufficiency of coronary blood flow. A result is both cardiomyocyte injury and predisposition to sudden cardiac events. Indeed, CAV is a major indication for retransplantation.
Unfortunately, current imaging technology and diagnostic modalities lack the ability to consistently detect at-risk and early signs of CAV. This includes invasive testing such as routine angiography, which is considered a “gold standard” for atherosclerotic coronary disease. Intravascular ultrasonography (IVUS), though more sensitive, can only evaluate larger, more proximal regions of coronary arterial arborization. IVUS is also not suitable for infants and many smaller patients due to the catheter dimensions. This difficulty with the diagnosis of early CAV is particularly problematic, as it is estimated that nearly one third of patients develop CAV within 5 years of transplant [10]. Earlier detection of CAV is therefore a significant focus of ongoing research. As it is recognized that this pathologic process is initiated well before clinical symptoms, one avenue of investigation is to look at the innate immune cell “signatures” for correlation with CAV [6]. The early identification of the immunologic activation instigating and perpetuating CAV requires an understanding of the cellular mechanisms underlying this pathologic process. This review will serve as an introduction to these mechanisms, especially those involving innate pathways.
While much of the past clinical and research activity in transplant immunology has focused on adaptive immunity, including the T cell response and donor-reactive antibodies, an increasing body of evidence implicates immune cells that are traditionally associated with the innate immune response. For example, in some patients, chronic allograft rejection is not correlated with detectable circulating alloantibodies [80]. In addition, donor-reactive antibodies are rarely detected after minor antigen-mismatched transplants, which may act through Batf3-transcription factor-dependent dendritic cells, to promote rejection [3]. Consistent with this point, experimental mice that are deficient for antibodies, but not B lymphoid cells, can still mount a chronic allograft vasculopathy response [86]. In the case of innate myeloid immune cells, macrophages may act as culprits in a wide spectrum of progressive and non-resolving diseases of chronic inflammation [60]. This may occur through the secretion of pro-inflammatory cytokines and growth factors that are induced independent of T cell or B cell alloreactivity [80]. A causal role for macrophages in fulminant allograft vasculopathy was suggested in early studies by experimental macrophage depletion [39]. Interpretation of data from depletion strategies such as this, though inventive at the time, should be cautious, as many depletion strategies utilizing chemical toxins may not be cell-subset specific and could also induce inflammation in their own right.
The contribution of phagocytes to acute cardiac allograft rejection
While the incidence of perioperative and acute rejection is relatively low, these events still occur and contribute to significant morbidity and even mortality. Contributing leukocytes include endogenous memory T cells that cross-react with donor major histocompatibility complex (MHC) molecules to promote acute rejection [77]. This is facilitated by suboptimal allograft cold ischemia, which also may activate innate immune cells. In humans, accumulation of intra-graft macrophage subsets has been directly correlated with acute allograft rejection, even in the setting of immunosuppression [57]. An initial event in acute graft inflammation is the recruitment of recipient monocytes, some of which may be derived from spleen [29], and infiltrate via donor endothelial integrin molecules. Integrin ligands play significant roles in this process as their deficiency attenuates allograft rejection [75]. After transplant-associated ischemia-reperfusion injury, release of injury-associated molecules, including the antioxidant protein haptoglobin, are causally linked to the recruitment of dendritic cells to the graft; this includes a heightened anti-donor T cell response, and eventually rejection [74]. Along these lines, targeted depletion of infiltrating dendritic cells reduces T cell proliferation, leading to significantly prolonged organ survival [88]. As eluded above, perioperative allograft inflammation may further be linked to the duration of solid organ cold ischemia, and therefore chronic rejection. Interestingly, donor brain death can exacerbate ischemia/reperfusion injury in transplanted mouse hearts, in what appears to be a complement-mediated fashion [4]. This may be associated with an immunologic priming of the donor organ and has been ameliorated with complement inhibition. In addition to recipient depots of inflammatory cells, tissue allografts may carry resident “passenger” immune cells, derived from the donor, which in turn hold the potential to become activated and also contribute to graft inflammation. For example, monocytes of the non-classical subset, and retained from donor grafts, appear to play a role in recruiting neutrophils to promote allograft dysfunction [87]. In this light, resident cardiac macrophages have been shown to promote neutrophil recruitment to the heart under ischemic stress [47]. Depletion of neutrophils has the potential to prolong graft survival in association with reduced T cell infiltration. This was observed after adoptive transfer of alloantigen-primed T cells, where grafts of animals treated with depleting antibodies for neutrophils exhibited a delay in T cell infiltration, relative to control [20]. Consistent with these activated phagocyte signatures, non-invasive detectors of allograft rejection employ molecular imaging reporters to detect phagocytic or protease activity [12]. Thus, perioperative and acute events have been associated with chronic rejection and could serve as early indicators of later susceptibility to graft complications.
Roles for hypoxia in the acute setting
Though post-procurement continuous perfusion transport devices are being evaluated, cold ischemia is still utilized for the vast majority of cardiac transplants. Prolonged graft ischemia during preservation and ischemia-reperfusion injury activates the hypoxiainducible factor (HIF) pathway in both rodent and human cardiac transplantation [24, 37]. The activated HIF consists of an oxygen-sensitive α subunit (HIF-1α or HIF-2α) and a constitutively expressed β subunit (HIF-1β). Accumulation of HIF-1α, for example in kidney allografts after reperfusion, was associated with earlier recovery of graft function in humans [63]. Under normoxic conditions, HIFα subunits are hydroxylated by oxygen-dependent prolyl hydroxylases (PHDs) leading to their ubiquitination and subsequent degradation. Pharmacological activation of HIF-1α without immunosuppression in the recipient, but not the donor heart, enhanced allograft survival [38]. Given the current data, a case may be made that activation of HIF in the immune cells of the recipient may lead to allograft tolerance. Further evidence of this stems from investigations in which loss of HIF-1α in myeloid cells accelerated rejection of tracheal allografts [67]. While there are limited studies on HIF-1α following heart transplantation, there has yet to be a study investigating a potential role for HIF-2α, which may exhibit non-overlapping function relative to HIF-1α [33]. Importantly, HIFs have been shown to be regulated by both mammalian target of rapamycin (mTOR) and calcineurin [31, 49], which are targets of current immunosuppressive drugs, indicating that the cardioprotective effects of HIFs may be compromised by chronic immunosuppression.
Acute alloimmune triggers
That vasculopathy predominantly affects donor arteries and not those of the recipient, is consistent with an alloimmune trigger and specificity. As introduced before, during surgery-associated inflammation, liberation of intracellular proteins, or so-called danger signals, leads to activation of the innate immune response. Although this response may not be sufficient in and of itself to completely trigger alloimmunity, these molecular events may lessen the activation threshold for alloimmune responsiveness. Relative to syngeneic hearts, and in the acute setting, allogenic heart transplants have been shown to exhibit heightened infiltration of monocyte-derived dendritic cells. For example, while studying immunocompromised recipients during allogeneic versus syngeneic cardiac transplant, investigators described persistent maturation of monocytes into antigen-presenting cells, and this occurred primarily in allogenic transplants. Although acute inflammation and maturation of monocyte-derived dendritic cells was still observed in syngeneic transplants, this response was undetectable weeks later and unable to induce inflammatory IFN-γ [61]. Circulating monocytes themselves may also contribute to the mounting of an adaptive immune response, for example after engulfing dead cells and indirect cross-presentation of cell-associated antigens [41]. In some cases, macrophages may act similar to natural killer (NK) cells to engage in an allospecific response with the help of CD4+ T cells and through costimulatory CD40/CD40L interactions [48].
Molecular mechanisms of innate allorecognition and the CD47/Sirp-α pathway
Even in the absence of T, B, and NK cells, experimental rodents respond uniquely to allogenic non-self [61]. Using recombination activating genes/Rag-deficient mice, which lack adaptive immune response B and T cells, investigators demonstrated that innate immune cells are capable of distinguishing allogeneic from syngeneic antigen, mounting an immune response in allogenic transplant [85]. Recent elegant positional cloning efforts identified donor factor SIRPα, or donor signal regulatory protein-α, to be recognized by recipient monocyte CD47 [17]. That is, polymorphisms of allograft SIRPα can promote activation of recipient phagocytes through modulation of recipient phagocyte CD47 signaling. It is important to note that MHC-II mismatch was not found to be the underlying mechanism of innate immune cell allorecognition, as donor mice with identical MHC-II alleles elicited divergent responses. Instead, allorecognition was facilitated by polymorphisms in SIRPα, which also acts as an important regulator of macrophage phagocytosis, and shown to regulate interactions with its ligand CD47 [17]. In the case of cross-species xenotransplantation, engagement of recipient phagocyte SIRP-α to donor CD47 (similar to during phagocytosis) regulates engraftment survival. Thus, CD47-SIRPα interactions are species specific and CD47/SIRPα incompatibility between species drives macrophage-associated rejection. In fact, soluble human CD47-Fc fusion proteins inhibit the phagocytic activity of human macrophages toward xenografts [32]. Moreover, earlier studies documented roles for CD47 on apoptotic self-cells, through SIRPα on host phagocytes. Specifically, CD47 on viable cells acts as a “don’t eat me” signal. As evidence for this, transplantation of Cd47-deficient cells into wild-type experimental animals leads to a failure to engraft the host. This is because graft cells lacking CD47 are cleared from wild-type hosts, due to phagocytosis. Continuing the example of non-self-cells, as above, tumor cells take advantage of the “don’t eat me” role of CD47 and elevate CD47 levels to increase their “selfness” and protect themselves from innate and adaptive immunity. Other studies have examined the SIRPα-CD47 axis in transplant. Administration of anti-CD47 monoclonal antibodies into harvested livers or kidneys suppressed ischemia-reperfusion injury and improved survival of animals receiving the treated organs [84]. On the therapeutic front, and given that SIRPα-CD47 interactions pose an impediment to allotransplantion and xenotransplantation, forthcoming strategies may include the development of humanized CD47 blocking monoclonal antibodies. Also in principle, soluble SIRPα could act as competitive inhibitors by blocking endogenous CD47. Though most studies have examined cancer, more studies are needed with allografts. Finally, the natural ligand of CD47 is TSP-1. Interestingly, blockade of the CD47 thrompospopndin-1 axis, prevents necrosis of full-thickness skin grafts [34]. It is unclear the role of natural TSP-1 in allograft rejection, and this deserves future study. Forthcoming studies may reveal other molecules involved in allorecognition.
Contribution of phagocytes to tolerance through efferocytosis
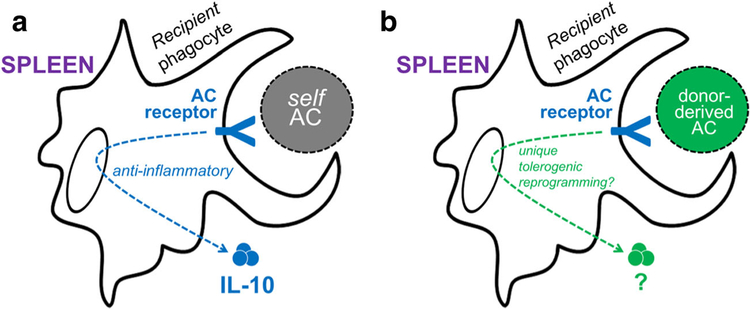
In the absence of alloantigen, macrophages naturally promote immune tolerance billions of times each day, per person, at steady state [2]. This tolerogenic property is stimulated by the regular turnover of apoptotic cells by the process of efferocytosis [81], or the phagocytic clearance of apoptotic cells. Efferocytosis promotes active signal transduction that modulates inflammation, without which may lead to autoimmune syndromes [58]. In the case of transplant, previous studies have shown that injection of apoptotic donor cells can tolerize the recipient immune system in a donor-specific manner [52, 56]. One rationale for the therapeutic potential of this approach stems from this understanding of the body’s daily non-phlogistic clearance during efferocytosis [2, 28, 50]. Harnessing innate potential for therapeutic transplantation may take advantage of pre-evolved immunosuppressive properties of apoptotic cells (Fig. 1). While this approach has recently shown efficacy in a phase I/IIa clinical trial for GVHD prophylaxis in patients receiving HLA-matched allogeneic bone marrow [54], it has not yet been successfully applied to HLA-mismatched allogeneic organ and/or tissue transplantation for tolerance induction. Interestingly, recent studies show that CD36, a key phagocytic receptor, is highly expressed on CD8α+ dendritic cells (DCs) and mediates transfer of self-antigen from thymic endothelial cells to DCs to promote tolerance [64]. Given that phagocytes are an early point of encounter between host and infused apoptotic donor cells [56], a precise understanding of these interactions will significantly enhance our ability to manipulate efferocytic machinery for therapeutic tolerance induction.
Fig. 1.
Hypothetical distinct roles for apoptotic cell receptors in the homeostatic anti-inflammatory response versus allogenic tolerance. a Clearance of self-apoptotic cells (ACs) is known to induce anti-inflammatory signaling in macrophages, including cytokine IL-10. b Tolerogenic macrophage function may be stimulated by injection of donor-derived ACs, such as ECDI-SPs (see text), and may induce unique signaling downstream of apoptotic cell receptors
Non-efferocytic tolerance mechanisms by phagocytes and myeloid derived suppressor subsets
Although evidence for myeloid suppression of T cell immunity to allografts has been reproduced by multiple groups [18] [23], the underlying mechanisms and specific identity of the casual cell populations remain unclear. Multiple reports have provided evidence that CD11b+ Gr-1 mononuclear cells exhibit immune-suppressive function [7]. However, this definition encompasses a broad swath of potential cell subsets. In one experimental scenario, recipient monocytic CD11b+CD115+Gr1+ cells were found to be necessary for the induction of tolerance by costimulatory blockade with CD40L-specific mAbs [23]. In this experimental scenario, Gr1+ monocytes migrated from the bone marrow into the transplanted organ, where they prevented the initiation of adaptive immune responses that lead to allograft rejection. In another scenario, DC-SIGN+ macrophages were shown to promote tolerance by suppression of CD8+ T cell proliferation and T regulatory cell expansion. This was mediated in part by fucosylated ligands and TLR4 signaling [15]. Similarly, macrophage-specific ablation of regulators of macrophage polarization, in this case mTOR, abrogated chronic rejection and was also associated with expansion of FOXP3+ T cells. These investigators implicated the upregulation of PD-L1, which acts to silence the T cell response, as the major contributor to long-term graft survival [91]. On the other hand, DCs have been shown to exhibit an interesting divergent role in early rejection versus allograft tolerance. Notably, in the acute stage, DCs from donor allografts rapidly (within hours) migrated to secondary lymphoid tissues of recipients. In a model of full MHCII mismatch, where donor hearts were deficient for the chemokine receptor Cx3cr1, fewer DCs accumulated, which prolonged transplant survival, in the absence of immunosuppression [92]. This effect was not observed when recipients received co-stimulatory blockade treatment (MR1 or CTLA4-Ig), as the absence of donor dendritic cells resulted in enhanced chronic inflammation and vasculopathy. These findings reinforced earlier literature describing MHCII-deficient animals in which blocking of CD4 signaling led to inefficient costimulatory blockade [93]. One explanation for these findings is that tolerance-promoting DCs triggered the generation of T-regulatory cells. Taken together, these implicated multi-modal and subset-specific contributions of phagocyte subsets necessitates more nuanced cellular targeting and the consideration of specific therapeutic windows.
Direct mechanisms of CAV by phagocytes.
Although facilitators of cardiac allograft vasculopathy may be a consequence of subclinical and allospecific stimulation that are set in motion early after cardiac transplant, CAV is not solely a delayed manifestation of acute triggers. Rather, activation events that occur after the first year of transplant can directly fuel the progression of CAV. Strategies that aim to modulate such non-acute innate immune reactivity may be clinically relevant as early as 5 years post-transplant, when overt signs of CAV are often detectable [10]. In reality, the subclinical genesis of transplant coronary artery disease may arise much earlier; early detection might be revealed in the circulation, in the form of an activated profile of blood monocyte subsets [94, 95]. Despite the clinical significance of CAV to cardiac allograft rejection, the underlying cellular and molecular mechanisms remain less defined in comparison to the immunology of acute graft rejection. During CAV, increasing evidence implicates cross-talk between innate immune cells, vascular smooth muscle cells, and fibroblasts (Fig. 2). This multifaceted cellular interplay is at the center of adverse tissue remodeling and ultimate graft failure, as discussed below.
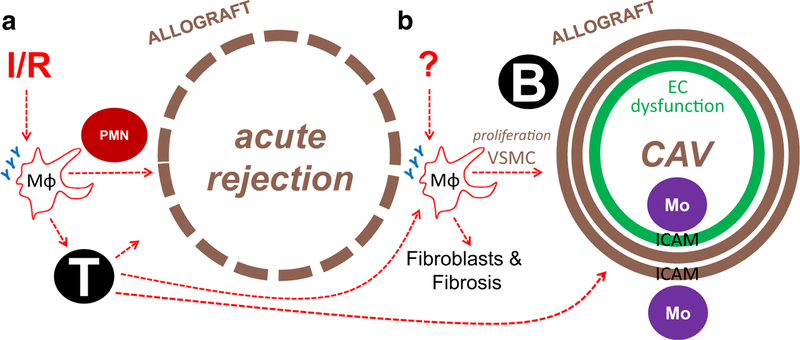
Fig. 2.
Unique stimuli and consequences for anti-inflammatory phagocyte receptors in acute rejection versus CAV. a Ischemia-reperfusion (I/R) of the allograft may stimulate shedding of anti-inflammatory receptors(Y) on macrophages (MΦs), contributing to activation of T cells and B cells and neutrophils (PMNs) and acute rejection and delayed CAV. b Unknown stimuli during chronic rejection, including from events triggered acutely after transplant, may similarly lead to shedding of anti-inflammatory receptors and activation of features of CAV. EC endothelial cell, Mo monocyte, ICAM intercellular adhesion molecule
Phagocyte crosstalk with VSMCs, fibroblasts, and endothelial cells
One of the cardinal features of CAV is concentric vascular thickening leading to luminal narrowing and impaired vascular function. This is characterized by proliferation of vascular smooth muscle cells (VSMCs), which may also represent a response to chronic inflammation [101]. Although graft SMCs comprise the majority of the expanded myointima, other cell types, including host B cells, memory T cells, and intra-graft subendothelial leukocytes, are also found, hinting at molecular crosstalk [73]. These immune cell collections may share residence within inflammation-associated elementary tertiary lymphoid nodules [82] and precede clinical indications of graft dysfunction. The arteriosclerosis pathology of CAValso shares many features with the maturation of atherosclerosis, which similarly triggers the recruitment and proliferation of VSMCs, as well as the secretion of extracellular matrix proteins [101]. Risk factors of atherosclerosis, including hyperlipidemia, also exacerbate transplant lesions, and lipid accumulation in allograft arteries may be found [71]. Nonetheless, atherosclerosis and CAV are distinct in their etiology, maturation, and at the cell and molecular level [101]. Besides the accelerated kinetics of disease progression in CAV, a key difference is the VSMC, which unlike macrophage-rich atherosclerotic plaque, is the dominant cell in transplant arteriosclerosis. VSMC proliferation is a key determinant of CAV progression, and inhibiting SMC proliferation may act to delay disease progression. For example, platelet-derived growth factor (PDGF) is a cytokine capable of activating VSMC proliferation, and its blockade greatly reduces transplant vasculopathy [51]. Evidence for PDGF expression may be found in mononuclear inflammatory cells during experimental cardiac transplant [44]. In addition to VSMC proliferation, VSMC migration contributes to vascular remodeling, which also requires permissive matrix remodeling to accommodate neointimal expansion. Phagocytes produce matrix metallo-proteinases, including their cognate inhibitors, to regulate the turnover of extracellular matrix [22, 101]. VSMCs may further act in a reciprocal fashion to affect phagocyte biology. For example, binding interactions between monocytes and VSMCs could contribute to subendothelial monocyte retention and a mechanism that perpetuates inflammation [8]. Another outstanding feature of late-stage CAVis graft fibrosis [13]. Macrophages have been found in close proximity to collagen-producing myofibroblasts in allografts, where they may secrete chemokines that recruit fibroblasts, and other soluble factors including TGF-beta and PDGF, which also are fibroblast-activating [13]. In the case of endothelial cells, inflammation is linked to microvascular endothelial dysfunction and injury. For example, antibodies directed against donor HLA can stimulate outside-in signal transduction of donor endothelial cells, culminating in clustering of intercellular adhesion molecules such as ICAM-1. This ICAM activation was permissive for elevated inflammation by inducing increased monocyte adhesion. Interestingly, mechanistic target of rapamycin (mTOR) inhibitors, which can attenuate allograft vasculopathy, also blocked ICAM clustering and monocyte adhesion [69].
Potential contributions of defective efferocytosis in the chronic allograft milieu
Chronic allograft inflammation is a type of non-resolving inflammation, which may be characterized by heightened cellular turnover. When combined with inefficiencies of cell clearance, for example by defective efferocytosis [79], this then leads to an environment that perpetuates chronic inflammation. At the molecular level, there are a number of candidate scenarios that could contribute to impaired efferocytosis in CAV. For example, inflammatory-induced destruction of the efferocytosis receptor MERTK has been associated with delayed inflammation resolution. In this context, expression of growth arrest-specific gene 6, a ligand of MERTK, has been connected with dysfunctional human renal allografts [101]. Defective efferocytosis prevents active inflammation resolution, including the failure to generate specialized pro-resolving mediators [102–104]. This is important as endogenous pro-resolving mediators, lipoxin A4 and resolving E1, have been shown to preserve organ function in allograft rejection [46]. Although one article suggested that inhibition of macrophage phagocytosis alone, with gadolinium, failed to prevent CAV [39], additional independent validation tests of the significance of efferocytosis to CAV remain to be investigated. Interestingly, recent data suggest divergence in expression pattern and activation states of MERTK and structurally homologous receptor AXL, on macrophages. Contrary to the pro-resolving profile of MERTK, AXL expression was markedly increased in association with pro-inflammatory stimuli [105]. Such specificity of action raises many questions as to the underlying mechanisms of dichotomous control that these tyrosine kinases may play during chronic transplant inflammation.
Additional notable molecular mediators of CAV and phagocyte function
Allograft inflammatory factor-1, as its name implies, has been implicated in transplant inflammation. The expression of AIF-1 correlates with inflammation, cardiac rejection, and CAV [5, 76]. Specifically, graft-infiltrating macrophages express heightened levels of AIF-1, leading to direct cellular crosstalk and activation of VSMC migration through enhanced cytoskeletal activation [106]. An interesting cytokine factor is IL-34. IL-34 is linked to regulation of macrophage function and allograft rejection. IL-34 shares functional similarity with M-CSF in that it can signal via the M-CSF receptor [107]. The extent of this mechanistic overlap is not clear; however, recent evidence suggests that like M-CSF, the effect of IL-34 on macrophage polarization trends toward a pro-resolving phenotype, associated with heightened levels of IL-10 production [108]. Another interleukin of interest is IL-33, a member of the IL-1 cytokine family. Toll-like receptor signaling can stimulate IL-33 expression, particularly in immune cells (macrophages and DCs). An alarmin, IL-33 is released in its active form from necrotic cells. Its cognate receptor, ST2, has long been implicated in inflammatory and autoimmune processes and is a marker of rejection [109]. IL-33 administration alters the immune cell repertoire in the graft, not only limiting T cell infiltration, but also promoting expansion of regulatory T cells [110]. Finally, and in humans, components of the hypoxia-responsive pathway have also been linked to chronic vasculopathy. For example, prolyl hydroxylase/PHD expression was increased during heart failure and also correlated with progressive reduction in HIF-1α, as well as the onset of fibrosis in cardiac allografts [24, 89]. Moreover, adenovirus-mediated HIF-1α overexpression in cardiomyocytes attenuated vasculopathy and promoted allograft survival in heart allografts [35, 36]. Such findings support the contention that HIFs may have a protective role in the human allograft; however, it will be important to tease out differences between HIF expression in myocytes, versus other cell types, including immune cells.
Cardiac lymphatics, inflammation, and CAV
Recent findings have implicated a role for lymphatics in CAV. This is significant from an inflammation perspective as lymph in general is an important conduit of inflammation resolution and antigen trafficking [111]. After heart transplant, chronic rejection of allografts is associated with increased lymphatic flow and cellular trafficking. Sufficient revascularization of transplanted organs is critical to successful outcomes. Re-establishment of lymphatic flow in organ transplant is subsequently important for tissue fluid hemostasis. Until recently, little was understood on the importance and origin of neo-lymphatics in grafted organs. In a recent study, investigators used non-invasive imaging techniques such as SPECT to measure the lymphatic flow index (LFI) after experimental mouse heterotopic heart transplant. Significant correlation was observed between heightened and sustained lymphatic flow in allogeneic hearts versus syngeneic controls. Interestingly, early measurements of LFI (after 1 week) were significantly greater in syngeneic hearts [112]. Such observations suggest a temporal regulation of lymphangiogensis that is defective with allograft vasculopathy and may be targeted for therapy. Taking advantage of these concepts, separate studies employed soluble VEGFR3 and blocking antibodies to inhibit VEGF-C/VEGFR3 signaling, thereby preventing lymphatic endothelial cell (LEC) stimulation. LECs produce CCL21, a chemoattractant of DCs, and this approach additionally limited DC recruitment, altogether improving graft survival [36]. While both of these studies point out that the majority of neolymphatic vessels were indeed donor derived, the underlying signaling mechanisms are still incompletely defined. In the case of immune cells, phagocytes may be significant contributors to lymphatic growth [113]. For example, phagocytes may secrete VEGF-C to promote LEC branching and proliferation. Examples of this mechanism have been found in various pathological conditions such as IBS and cancer [16] [72]. Additional suggestive evidence comes after clodronate macrophage depletion studies in corneal transplant, which was observed to inhibit lymphangiogenesis. Remarkably, macrophages themselves may transdifferentiate into LECs [26]. Morevover, as antigen-presenting cells, phagocytes utilize lymphatics to deliver alloantigen to lymph nodes, where additional cellular crosstalk may occur with Tcell subsets to regulate arms of adaptive immunity [62]. While enhanced lymphangiogensis during transplant is associated with poorer outcomes, other pathologies of sterile inflammation, such as myocardial infarction, suggest a protective role of lymphatics [27, 40]. Overall, the efficacious therapeutic targeting of lymphatics to affect inflammation and CAV remains an open question.
Phagocytes, B cells, and basophils during CAV
Interplay between innate immune cells and B cells may contribute to antibody-mediated rejection [11]. Episodes of graft inflammation that are associated with alloantibodies contribute to early and late graft rejection, and this is in part mediated by the fixation of complement that leads to graft injury [14]. Antibody Fc receptors on macrophages are triggered by alloantibodies, leading to macrophage pro-inflammatory activation. Independent of antibody secretion, B cells also regulate innate immunity. This includes triggering the mobilization of monocytes to the heart [90]. Innate immune cells communicate with B cells to modulate their activation, differentiation, and cell survival, and these interactions have the potential to act in shorter duration relative to with T cell-dependent help [9]. Phagocyte crosstalk also occurs with basophils, which are implicated in CAV. Basophils trigger fibro-blast activation during cardiac allograft fibrosis, and depletion of basophils markedly reduces the presence of interstitial α-smooth muscle actin, as well as collagen deposition [70]. In this example, grafts deficient in the cytokine receptor for IL4 exhibited decreased extracellular matrix deposition; basophil depletion did not further enhance this effect, implicating IL-4 as a key regulator of fibrosis on donor tissue. Lastly, basophils drive the differentiation of inflammatory monocytes into alternatively activated macrophages [19], which could further fuel fibrotic scarring.
Other potential relationships between phagocytes and CAV
Hyperglycemia, insulin resistance, the presence of baseline coronary artery disease in the graft donor or recipient, a donor history of hypertension, and increasing donor age may all contribute to CAV [59]. Hyperlipidemia frequently occurs after heart transplantation in association with immunosuppressive treatment [68]. Elevated circulating lipids fuel systemic inflammation, including in heart [66], and heart-transplant patients who receive statins exhibit reduced CAV [83]. Perturbations in metabolism, including from the microbiome [53] may perpetuate systemic inflammation, biasing macrophage polarization and increasing inflammatory cytokine production. Cytomegalovirus (CMV) seropositivity is also implicated in CAV [65], as well as immune cell activation. Ganciclovir anti-CMV prophylaxis after heart transplantation tracks with indications of reduced vasculopathy. Additionally, chlamydia pneumonia infection in heart transplant recipients is linked to CAV exacerbation. In pediatric heart transplant recipients, the presence of immune reactive adenovirus and other viral genomes in myocardial biopsies is correlated with early vasculopathy. Finally, loss of para- and sympathetic allograft innervation disturbs cardiac output [25]. Direct evidence that denervation contributes to long-term graft survival and/or CAV is quite limited. One study investigation showed that patients with observable vasculopathy exhibited lower degrees of re-innervation compared to those that did not [21]. Thus, CAV and associated inflammation likely also impede potential graft re-innervation, further limiting cardiac output.
Future research directions
Strategies to ameliorate transplant arteriosclerosis may consider multiple levels of cell and molecular intervention. This includes prior to transplantation during organ storage, as well as after transplantation. An emerging area of interest is the field of immune metabolism. For example, immune cell activation is now accepted to integrate closely with cellular metabolic reprogramming. Acutely activated macrophages are frequently glycolytic, whereas macrophages that orchestrate inflammation resolution, and thereby may dampen CAV, rely more on oxidative phosphorylation [30]. Thus, pharmacologically altering the leukocyte “diet” has potential to selectively polarize the immune response [45]. For instance, metformin, 2DG, and a third drug to block glutamine metabolism led to significantly prolonged survival of skin and heart grafts [43]. Yet, another thought-provoking area is the role of microbiota, whose signals may affect systemic alloimmunity [1]. Taken together, heightened sensitivity of diagnostic approaches, as well as therapeutic roadmaps, will hinge on future basic research.
Acknowledgments
Funding information This review was supported by NHLBI R01HL122309 to ET, R01HL139812–01 to XL, and ET, and an AHA post-doctoral award to KG.
Footnotes
This article is a contribution to the special issue on Professional and Nonprofessional Phagocytes and Diseases - Guest Editor: Toru Miyazaki
References
- 1.Alegre ML, Bartman C, Chong AS (2014) Microbes and allogeneic transplantation. Transplantation 97(1):5–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arandjelovic S, Ravichandran KS (2015) Phagocytosis of apoptotic cells in homeostasis. Nat Immunol 16(9):907–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atif SM, Nelsen MK, Gibbings SL, Desch AN, Kedl RM, Gill RG, Marrack P, Murphy KM, Grazia TJ, Henson PM, Jakubzick CV (2015) Cutting edge: roles for Batf3-dependent APCs in the rejection of minor histocompatibility antigen-mismatched grafts. J Immunol 195(1):46–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atkinson C, Floerchinger B, Qiao F, Casey S, Williamson T, Moseley E, Stoica S, Goddard M, Ge X, Tullius SG, Tomlinson S (2013) Donor brain death exacerbates complement-dependent ischemia/reperfusion injury in transplanted hearts. Circulation 127(12):1290–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Autieri MV, Kelemen S, Thomas BA, Feller ED, Goldman BI, Eisen HJ (2002) Allograft inflammatory factor-1 expression correlates with cardiac rejection and development of cardiac allograft vasculopathy. Circulation 106(17):2218–2223 [DOI] [PubMed] [Google Scholar]
- 6.Azad TD, Donato M, Heylen L, Liu AB, Shen-Orr SS, Sweeney TE, Maltzman JS, Naesens M, Khatri P (2018) Inflammatory macrophage-associated 3-gene signature predicts subclinical allo-graft injury and graft survival. JCI Insight 3(2):e95659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bronte V, Apolloni E, Cabrelle A, Ronca R, Serafini P, Zamboni P, Restifo NP, Zanovello P (2000) Identification of a CD11b(+)/Gr-1(+)/CD31(+) myeloid progenitor capable of activating or suppressing CD8(+) T cells. Blood 96(12):3838–3846 [PMC free article] [PubMed] [Google Scholar]
- 8.Cai Q, Lanting L, Natarajan R (2004) Interaction of monocytes with vascular smooth muscle cells regulates monocyte survival and differentiation through distinct pathways. Arterioscler Thromb Vasc Biol 24(12):2263–2270 [DOI] [PubMed] [Google Scholar]
- 9.Cerutti A, Puga I, Cols M (2011) Innate control of B cell responses. Trends Immunol 32(5):202–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chih S, Chong AY, Mielniczuk LM, Bhatt DL, Beanlands RS (2016) Allograft vasculopathy: the Achilles’ heel of heart transplantation. J Am Coll Cardiol 68(1):80–91 [DOI] [PubMed] [Google Scholar]
- 11.Chong AS, Khiew SH (2017) Transplantation tolerance: don’t forget about the B cells. Clin Exp Immunol 189(2):171–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christen T, Nahrendorf M, Wildgruber M, Swirski FK, Aikawa E, Waterman P, Shimizu K, Weissleder R, Libby P (2009) Molecular imaging of innate immune cell function in transplant rejection. Circulation 119(14):1925–1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clemmensen TS, Holm NR, Eiskjaer H, Logstrup BB, Christiansen EH, Dijkstra J, Barkholt TO, Terkelsen CJ, Maeng M, Poulsen SH (2017) Layered fibrotic plaques are the predominant component in cardiac allograft vasculopathy: systematic findings and risk stratification by OCT. JACC Cardiovasc Imaging 10(7):773–784 [DOI] [PubMed] [Google Scholar]
- 14.Colvin RB, Smith RN (2005) Antibody-mediated organ-allograft rejection. Nat Rev Immunol 5(10):807–817 [DOI] [PubMed] [Google Scholar]
- 15.Conde P, Rodriguez M, van der Touw W, Jimenez A, Burns M, Miller J, Brahmachary M, Chen HM, Boros P, Rausell-Palamos F, Yun TJ, Riquelme P, Rastrojo A, Aguado B, Stein-Streilein J, Tanaka M, Zhou L, Zhang J, Lowary TL, Ginhoux F, Park CG, Cheong C, Brody J, Turley SJ, Lira SA, Bronte V, Gordon S, Heeger PS, Merad M, Hutchinson J, Chen SH, Ochando J (2015) DC-SIGN(+) macrophages control the induction of transplantation tolerance. Immunity 42(6):1143–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Alessio S, Correale C, Tacconi C, Gandelli A, Pietrogrande G, Vetrano S, Genua M, Arena V, Spinelli A, Peyrin-Biroulet L, Fiocchi C, Danese S (2014) VEGF-C–dependent stimulation of lymphatic function ameliorates experimental inflammatory bowel disease. J Clin Invest 124(9):3863–3878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dai H, Friday AJ, Abou-Daya KI, Williams AL, Mortin-Toth S, Nicotra ML, Rothstein DM, Shlomchik WD, Matozaki T, Isenberg JS, Oberbarnscheidt MH, Danska JS, Lakkis FG (2017) Donor SIRPalpha polymorphism modulates the innate immune response to allogeneic grafts. Sci Immunol 2(12):eaam6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dugast AS, Haudebourg T, Coulon F, Heslan M, Haspot F, Poirier N, Vuillefroy de Silly R, Usal C, Smit H, Martinet B, Thebault P, Renaudin K, Vanhove B (2008) Myeloid-derived suppressor cells accumulate in kidney allograft tolerance and specifically suppress effector T cell expansion. J Immunol 180(12):7898–7906 [DOI] [PubMed] [Google Scholar]
- 19.Egawa M, Mukai K, Yoshikawa S, Iki M, Mukaida N, Kawano Y, Minegishi Y, Karasuyama H (2013) Inflammatory monocytes recruited to allergic skin acquire an anti-inflammatory M2 pheno-type via basophil-derived interleukin-4. Immunity 38(3):570–580 [DOI] [PubMed] [Google Scholar]
- 20.El-Sawy T, Belperio JA, Strieter RM, Remick DG, Fairchild RL (2005) Inhibition of polymorphonuclear leukocyte-mediated graft damage synergizes with short-term costimulatory blockade to prevent cardiac allograft rejection. Circulation 112(3):320–331 [DOI] [PubMed] [Google Scholar]
- 21.Estorch M, Camprecios M, Flotats A, Mari C, Berna L, Catafau AM, Ballester M, Narula J, Carrio I (1999) Sympathetic reinnervation of cardiac allografts evaluated by 123I-MIBG imaging. J Nucl Med 40(6):911–916 [PubMed] [Google Scholar]
- 22.Galis ZS, Sukhova GK, Kranzhofer R, Clark S, Libby P (1995) Macrophage foam cells from experimental atheroma constitutively produce matrix-degrading proteinases. Proc Natl Acad Sci U S A 92(2):402–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia MR, Ledgerwood L, Yang Y, Xu J, Lal G, Burrell B, Ma G, Hashimoto D, Li Y, Boros P, Grisotto M, van Rooijen N, Matesanz R, Tacke F, Ginhoux F, Ding Y, Chen SH, Randolph G, Merad M, Bromberg JS, Ochando JC (2010) Monocytic suppressive cells mediate cardiovascular transplantation tolerance in mice. J Clin Invest 120(7):2486–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gramley F, Lorenzen J, Pezzella F, Kettering K, Himmrich E, Plumhans C, Koellensperger E, Munzel T (2009) Hypoxia and myocardial remodeling in human cardiac allografts: a time-course study. J Heart Lung Transplant 28(11):1119–1126 [DOI] [PubMed] [Google Scholar]
- 25.Grupper A, Gewirtz H, Kushwaha S (2018) Reinnervation post-heart transplantation. Eur Heart J 39(20):1799–1806 [DOI] [PubMed] [Google Scholar]
- 26.Hall KL, Volk-Draper LD, Flister MJ, Ran S (2012) New model of macrophage acquisition of the lymphatic endothelial phenotype. PLoS One 7(3):e31794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henri O, Pouehe C, Houssari M, Galas L, Nicol L, Edwards-Lévy F, Henry J-P, Dumesnil A, Boukhalfa I, Banquet S, Schapman D, Thuillez C, Richard V, Mulder P, Brakenhielm E (2016) “<span hwp:id=“article-title-1″ class=“article-title”>Selective stimulation of cardiac lymphangiogenesis reduces myocardial edema and fibrosis leading to improved cardiac function following myocardial infarction<span hwp:id=“article-title-58” class=“sub-article-title”>CLINICAL PERSPECTIVE“. Circulation 133: 1484–1497 [DOI] [PubMed] [Google Scholar]
- 28.Henson PM, Bratton DL (2013) Antiinflammatory effects of apoptotic cells. J Clin Invest 123(7):2773–2774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsiao EC, Yoshinaga Y, Nguyen TD, Musone SL, Kim JE, Swinton P, Espineda I, Manalac C, deJong PJ, Conklin BR (2008) Marking embryonic stem cells with a 2A self-cleaving peptide: a NKX2–5 emerald GFP BAC reporter. PLoS One 3(7): e2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang SC, Smith AM, Everts B, Colonna M, Pearce EL, Schilling JD, Pearce EJ (2016) Metabolic reprogramming mediated by the mTORC2-IRF4 signaling axis is essential for macrophage alternative activation. Immunity 45(4):817–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F, Giaccia AJ, Abraham RT (2002) Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol Cell Biol 22(20):7004–7014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ide K, Wang H, Tahara H, Liu J, Wang X, Asahara T, Sykes M, Yang YG, Ohdan H (2007) Role for CD47-SIRPalpha signaling in xenograft rejection by macrophages. Proc Natl Acad Sci U S A 104(12):5062–5066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imtiyaz HZ, Williams EP, Hickey MM, Patel SA, Durham AC, Yuan LJ, Hammond R, Gimotty PA, Keith B, Simon MC (2010) Hypoxia-inducible factor 2alpha regulates macrophage function in mouse models of acute and tumor inflammation. J Clin Invest 120(8):2699–2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Isenberg JS, Pappan LK, Romeo MJ, Abu-Asab M, Tsokos M, Wink DA, Frazier WA, Roberts DD (2008) Blockade of thrombospondin-1-CD47 interactions prevents necrosis of full thickness skin grafts. Ann Surg 247(1):180–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang X, Khan MA, Tian W, Beilke J, Natarajan R, Kosek J, Yoder MC, Semenza GL, Nicolls MR (2011) Adenovirus-mediated HIF-1alpha gene transfer promotes repair of mouse airway allograft microvasculature and attenuates chronic rejection. J Clin Invest 121(6):2336–2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keranen MA, Nykanen AI, Krebs R, Pajusola K, Tuuminen R, Alitalo K, Lemstrom KB (2010) Cardiomyocyte-targeted HIF-1alpha gene therapy inhibits cardiomyocyte apoptosis and cardiac allograft vasculopathy in the rat. J Heart Lung Transplant 29(9): 1058–1066 [DOI] [PubMed] [Google Scholar]
- 37.Keranen MA, Nykanen AI, Krebs R, Tuuminen R, Sandelin H, Koskinen PK, Lemstrom KB (2006) Effect of graft preservation and acute rejection on hypoxia-inducible factor-1 in rat cardiac allografts. Transplant Proc 38(10):3372–3373 [DOI] [PubMed] [Google Scholar]
- 38.Keranen MA, Tuuminen R, Syrjala S, Krebs R, Walkinshaw G, Flippin LA, Arend M, Koskinen PK, Nykanen AI, Lemstrom KB (2013) Differential effects of pharmacological HIF preconditioning of donors versus recipients in rat cardiac allografts. Am J Transplant 13(3):600–610 [DOI] [PubMed] [Google Scholar]
- 39.Kitchens WH, Chase CM, Uehara S, Cornell LD, Colvin RB, Russell PS, Madsen JC (2007) Macrophage depletion suppresses cardiac allograft vasculopathy in mice. Am J Transplant 7(12): 2675–2682 [DOI] [PubMed] [Google Scholar]
- 40.Klotz L, Norman S, Vieira JM, Masters M, Rohling M, Dubé KN, Bollini S, Matsuzaki F, Carr CA, Riley PR (2015) Cardiac lymphatics are heterogeneous in origin and respond to injury. Nature 522:62–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larson SR, Atif SM, Gibbings SL, Thomas SM, Prabagar MG, Danhorn T, Leach SM, Henson PM, Jakubzick CV (2016) Ly6C(+) monocyte efferocytosis and cross-presentation of cell-associated antigens. Cell Death Differ 23(6):997–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lechler RI, Sykes M, Thomson AW, Turka LA (2005) Organ transplantation—how much of the promise has been realized? Nat Med 11(6):605–613 [DOI] [PubMed] [Google Scholar]
- 43.Lee CF, Lo YC, Cheng CH, Furtmuller GJ, Oh B, Andrade-Oliveira V, Thomas AG, Bowman CE, Slusher BS, Wolfgang MJ, Brandacher G, Powell JD (2015) Preventing allograft rejection by targeting immune metabolism. Cell Rep 13(4):760–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lemstrom KB, Koskinen PK (1997) Expression and localization of platelet-derived growth factor ligand and receptor protein during acute and chronic rejection of rat cardiac allografts. Circulation 96(4):1240–1249 [DOI] [PubMed] [Google Scholar]
- 45.Leslie M (2018) Putting immune cells on a diet. Science 359(6383):1454–1456 [DOI] [PubMed] [Google Scholar]
- 46.Levy BD, Zhang QY, Bonnans C, Primo V, Reilly JJ, Perkins DL, Liang Y, Amin Arnaout M, Nikolic B, Serhan CN (2011) The endogenous pro-resolving mediators lipoxin A4 and resolvin E1 preserve organ function in allograft rejection. Prostaglandins Leukot Essent Fatty Acids 84(1–2):43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li W, Hsiao HM, Higashikubo R, Saunders BT, Bharat A, Goldstein DR, Krupnick AS, Gelman AE, Lavine KJ, Kreisel D (2016) Heart-resident CCR2(+) macrophages promote neutrophil extravasation through TLR9/MyD88/CXCL5 signaling. JCI Insight 1(12):e87315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu W, Xiao X, Demirci G, Madsen J, Li XC (2012) Innate NK cells and macrophages recognize and reject allogeneic nonself in vivo via different mechanisms. J Immunol 188:2703–2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu YV, Hubbi ME, Pan F, McDonald KR, Mansharamani M, Cole RN, Liu JO, Semenza GL (2007) Calcineurin promotes hypoxia-inducible factor 1alpha expression by dephosphorylating RACK1 and blocking RACK1 dimerization. J Biol Chem 282(51):37064–37073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luo X, Pothoven KL, McCarthy D, DeGutes M, Martin A, Getts DR, Xia G, He J, Zhang X, Kaufman DB, Miller SD (2008) ECDI-fixed allogeneic splenocytes induce donor-specific tolerance for long-term survival of islet transplants via two distinct mechanisms. Proc Natl Acad Sci U S A 105(38):14527–14532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mancini MC, Evans JT (2000) Role of platelet-derived growth factor in allograft vasculopathy. Ann Surg 231(5):682–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCarthy DP, Bryant J, Galvin JP, Miller SD, Luo X (2015) Tempering allorecognition to induce transplant tolerance with chemically modified apoptotic donor cells. Am J Transplant 15(6):1475–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McIntosh CM, Chen L, Shaiber A, Eren AM, Alegre ML (2018) Gut microbes contribute to variation in solid organ transplant outcomes in mice. Microbiome 6(1):96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mevorach D, Zuckerman T, Reiner I, Shimoni A, Samuel S, Nagler A, Rowe JM, Or R (2014) Single infusion of donor mono-nuclear early apoptotic cells as prophylaxis for graft-versus-host disease in myeloablative HLA-matched allogeneic bone marrow transplantation: a phase I/IIa clinical trial. Biol Blood Marrow Transplant 20(1):58–65 [DOI] [PubMed] [Google Scholar]
- 55.Mitchell RN, Libby P (2007) Vascular remodeling in transplant vasculopathy. Circ Res 100(7):967–978 [DOI] [PubMed] [Google Scholar]
- 56.Morelli AE, Larregina AT (2016) “Concise review: mechanisms behind apoptotic cell-based therapies against transplant rejection and graft versus host disease. Stem Cells 34(5): 1142–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mues B, Brisse B, Steinhoff G, Lynn T, Hewett T, Sorg C, Zuhdi N, Robbins G (1991) Diagnostic assessment of macrophage phenotypes in cardiac transplant biopsies. Eur Heart J 12(Suppl D): 32–35 [DOI] [PubMed] [Google Scholar]
- 58.N AG, Bensinger SJ, Hong C, Beceiro S, Bradley MN, Zelcer N, Deniz J, Ramirez C, Diaz M, Gallardo G, de Galarreta CR, Salazar J, Lopez F, Edwards P, Parks J, Andujar M, Tontonoz P, Castrillo A (2009) Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity 31(2):245–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nagji AS, Hranjec T, Swenson BR, Kern JA, Bergin JD, Jones DR, Kron IL, Lau CL, Ailawadi G (2010) Donor age is associated with chronic allograft vasculopathy after adult heart transplantation: implications for donor allocation. Ann Thorac Surg 90(1): 168–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nathan C, Ding A (2010) Nonresolving inflammation. Cell 140(6):871–882 [DOI] [PubMed] [Google Scholar]
- 61.Oberbarnscheidt MH, Zeng Q, Li Q, Dai H, Williams AL, Shlomchik WD, Rothstein DM, Lakkis FG (2014) Non-self recognition by monocytes initiates allograft rejection. J Clin Invest 124(8):3579–3589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ochando JC, Homma C, Yang Y, Hidalgo A, Garin A, Tacke F, Angeli V, Li Y, Boros P, Ding Y, Jessberger R, Trinchieri G, Lira SA, Randolph GJ, Bromberg JS (2006) Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol 7(6):652–662 [DOI] [PubMed] [Google Scholar]
- 63.Oda T, Ishimura T, Yokoyama N, Ogawa S, Miyake H, Fujisaw M (2017) Hypoxia-inducible factor-1alpha expression in kidney transplant biopsy specimens after reperfusion is associated with early recovery of graft function after cadaveric kidney transplantation. Transplant Proc 49(1):68–72 [DOI] [PubMed] [Google Scholar]
- 64.Perry JSA, Russler-Germain EV, Zhou YW, Purtha W, Cooper ML, Choi J, Schroeder MA, Salazar V, Egawa T, Lee B-C, Abumrad NA, Kim BS, Anderson MS, DiPersio JF, Hsieh C-S (2018) CD36 mediates cell-surface antigens to promote thymic development of the regulatory t cell receptor repertoire and allo-tolerance. Immunity 48(5):923–936.e924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Petrakopoulou P, Kubrich M, Pehlivanli S, Meiser B, Reichart B, von Scheidt W, Weis M (2004) Cytomegalovirus infection in heart transplant recipients is associated with impaired endothelial function. Circulation 110(11 Suppl 1):Ii207–Ii212 [DOI] [PubMed] [Google Scholar]
- 66.Raichlin ER, McConnell JP, Lerman A, Kremers WK, Edwards BS, Kushwaha SS, Clavell AL, Rodeheffer RJ, Frantz RP (2007) Systemic inflammation and metabolic syndrome in cardiac allograft vasculopathy. J Heart Lung Transplant 26(8):826–833 [DOI] [PubMed] [Google Scholar]
- 67.Ropponen JO, Keranen MA, Raissadati A, Nykanen AI, Krebs R, Lemstrom KB, Tikkanen JM (2016) Increased myeloid cell hypoxia-inducible factor-1 delays obliterative airway disease in the mouse. J Heart Lung Transplant 35(5):671–678 [DOI] [PubMed] [Google Scholar]
- 68.Rudas L, Pflugfelder PW, McKenzie FN, Menkis AH, Novick RJ, Kostuk WJ (1990) Serial evaluation of lipid profiles and risk factors for development of hyperlipidemia after cardiac transplantation. Am J Cardiol 66(15):1135–1138 [DOI] [PubMed] [Google Scholar]
- 69.Salehi S, Sosa RA, Jin YP, Kageyama S, Fishbein MC, Rozengurt E, Kupiec-Weglinski JW, Reed EF (2018) Outside-in HLA class I signaling regulates ICAM-1 clustering and endothelial cell-monocyte interactions via mTOR in transplant antibody-mediated rejection. Am J Transplant 18(5):1096–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schiechl G, Hermann FJ, Rodriguez Gomez M, Kutzi S, Schmidbauer K, Talke Y, Neumayer S, Goebel N, Renner K, Bruhl H, Karasuyama H, Obata-Ninomiya K, Utpatel K, Evert M, Hirt SW, Geissler EK, Fichtner-Feigl S, Mack M (2016) Basophils trigger fibroblast activation in cardiac allograft fibrosis development. Am J Transplant 16(9):2574–2588 [DOI] [PubMed] [Google Scholar]
- 71.Schiopu A, Nadig SN, Cotoi OS, Hester J, van Rooijen N, Wood KJ (2012) Inflammatory Ly-6C(hi) monocytes play an important role in the development of severe transplant arteriosclerosis in hyperlipidemic recipients. Atherosclerosis 223(2):291–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schoppmann SF, Birner P, Stöckl J, Kalt R, Ullrich R, Caucig C, Kriehuber E, Nagy K, Alitalo K, Kerjaschki D (2002) Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol 161(3):947–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seipelt IM, Pahl E, Seipelt RG, Mavroudis C, Backer CL, Stellmach V, Cornwell M, Crawford SE (2005) Neointimal inflammation and adventitial angiogenesis correlate with severity of cardiac allograft vasculopathy in pediatric recipients. J Heart Lung Transplant 24(8):1039–1045 [DOI] [PubMed] [Google Scholar]
- 74.Shen H, Heuzey E, Mori DN, Wong CK, Colangelo CM, Chung LM, Bruce C, Slizovskiy IB, Booth CJ, Kreisel D, Goldstein DR (2015) Haptoglobin enhances cardiac transplant rejection. Circ Res 116(10):1670–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shimizu K, Libby P, Shubiki R, Sakuma M, Wang Y, Asano K, Mitchell RN, Simon DI (2008) Leukocyte integrin Mac-1 promotes acute cardiac allograft rejection. Circulation 117(15): 1997–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sibinga NE, Feinberg MW, Yang H, Werner F, Jain MK (2002) Macrophage-restricted and interferon gamma-inducible expression of the allograft inflammatory factor-1 gene requires Pu.1. J Biol Chem 277(18):16202–16210 [DOI] [PubMed] [Google Scholar]
- 77.Su CA, Iida S, Abe T, Fairchild RL (2014) Endogenous memory CD8 T cells directly mediate cardiac allograft rejection. Am J Transplant 14(3):568–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sykes M, Levy G (2011) Advances in transplantation. Semin Immunol 23(4):222–223 [DOI] [PubMed] [Google Scholar]
- 79.I Tabas (2017) 2016 Russell Ross memorial lecture in vascular biology: molecular-cellular mechanisms in the progression of atherosclerosis. Arterioscler Thromb Vasc Biol 37(2):183–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Uehara S, Chase CM, Cornell LD, Madsen JC, Russell PS, Colvin RB (2007) Chronic cardiac transplant arteriopathy in mice: relationship of alloantibody, C4d deposition and neointimal fibrosis. Am J Transplant 7(1):57–65 [DOI] [PubMed] [Google Scholar]
- 81.Vandivier RW, Henson PM, Douglas IS (2006) Burying the dead: the impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease. Chest 129(6):1673–1682 [DOI] [PubMed] [Google Scholar]
- 82.Wehner JR, Fox-Talbot K, Halushka MK, Ellis C, Zachary AA, Baldwin WM 3rd (2010) B cells and plasma cells in coronaries of chronically rejected cardiac transplants. Transplantation 89(9): 1141–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu AH, Ballantyne CM, Short BC, Torre-Amione G, Young JB, Ventura HO, Eisen HJ, Radovancevic B, Rayburn BK, Lake KD, Yancy CW, Taylor DO, Mehra MR, Kubo SH, Fishbein DP, Zhao X-Q, O’Brien KD (2005) Statin use and risks of death or fatal rejection in the Heart Transplant Lipid Registry. Am J Cardiol 95(3):367–372 [DOI] [PubMed] [Google Scholar]
- 84.Xu M, Wang X, Banan B, Chirumbole DL, Garcia-Aroz S, Balakrishnan A, Nayak DK, Zhang Z, Jia J, Upadhya GA, Gaut JP, Hiebsch R, Manning PT, Wu N, Lin Y, Chapman WC (2018) Anti-CD47 monoclonal antibody therapy reduces ischemiareperfusion injury of renal allografts in a porcine model of donation after cardiac death. Am J Transplant 18(4):855–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zecher D, van Rooijen N, Rothstein DM, Shlomchik WD, Lakkis FG (2009) An innate response to allogeneic nonself mediated by monocytes. J Immunol 183(12):7810–7816 [DOI] [PubMed] [Google Scholar]
- 86.Zeng Q, Ng YH, Singh T, Jiang K, Sheriff KA, Ippolito R, Zahalka S, Li Q, Randhawa P, Hoffman RA, Ramaswami B, Lund FE, Chalasani G (2014) B cells mediate chronic allograft rejection independently of antibody production. J Clin Invest 124(3):1052–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zheng Z, Chiu S, Akbarpour M, Sun H, Reyfman PA, Anekalla KR, Abdala-Valencia H, Edgren D, Li W, Kreisel D, Korobova FV, Fernandez R, McQuattie-Pimentel A, Zhang ZJ, Perlman H, Misharin AV, Scott Budinger GR, Bharat A (2017) Donor pulmonary intravascular nonclassical monocytes recruit recipient neutrophils and mediate primary lung allograft dysfunction. Sci Transl Med 9(394):eaal4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhuang Q, Liu Q, Divito SJ, Zeng Q, Yatim KM, Hughes AD, Rojas-Canales DM, Nakao A, Shufesky WJ, Williams AL, Humar R, Hoffman RA, Shlomchik WD, Oberbarnscheidt MH, Lakkis FG, Morelli AE (2016) Graft-infiltrating host dendritic cells play a key role in organ transplant rejection. Nat Commun 7:12623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zolk O, Solbach TF, Eschenhagen T, Weidemann A, Fromm MF (2008) Activation of negative regulators of the hypoxia-inducible factor (HIF) pathway in human end-stage heart failure. Biochem Biophys Res Commun 376(2):315–320 [DOI] [PubMed] [Google Scholar]
- 90.Zouggari Y, Ait-Oufella H, Bonnin P, Simon T, Sage AP, Guerin C, Vilar J, Caligiuri G, Tsiantoulas D, Laurans L, Dumeau E, Kotti S, Bruneval P, Charo IF, Binder CJ, Danchin N, Tedgui A, Tedder TF, Silvestre JS, Mallat Z (2013) B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat Med 19(10):1273–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhao Y, Chen S, Lan P, Wu C, Dou Y, Xiao X, Zhang Z, Minze L, He X, Chen W, Li XC (2018) Macrophage subpopulations and their impact on chronic allograft rejection versus graft acceptance in a mouse heart transplant model. Am J Transplant 18(3):604–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ueno T, Tanaka K, Jurewicz M, Murayama T, Guleria I, Fiorina P, Paez JC, Augello A, Vergani A, Wong M, Smith RN, Abdi R (2009) Divergent Role of Donor Dendritic Cells in Rejection versus Tolerance of Allografts. J Am Soc Nephrol 20(3):535–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yamada A, Chandraker A, Laufer TM, Gerth AJ, Sayegh MH, Auchincloss H (2001) Cutting Edge: Recipient MHC Class II Expression Is Required to Achieve Long-Term Survival of Murine Cardiac Allografts After Costimulatory Blockade. J Immunol 167(10):5522–5526 [DOI] [PubMed] [Google Scholar]
- 94.Salama M, Andrukhova O, Roedler S, Zuckermann A, Laufer G, Aharinejad S (2011) Association of CD14+ monocyte-derived progenitor cells with cardiac allograft vasculopathy. J Thorac Cardiovasc Surg 142(5):1246–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Holzhauser L, Arnold KA, Schroeder A, Imamura T, Nguyen A, Chung B, Narang N, Costanzo M, Jeevanandam V, Murks C, Riley T, Powers J, Sarswat N, Kalantari S, Raikhelkar J, Sayer G, Kim G, Uriel N, Alenghat FJ (2018) Circulating Monocyte Subtypes Correlate with Cardiac Allograft Vasculopathy and Differ from Atherosclerotic Disease: A Tool for Monitoring? J Heart Lung Transplant 37(4):S174–S175 [Google Scholar]
- 96.Yin Q, Jiang D, Li L, Yang Y, wu P, Luo Y, Yang R, Li D (2018) LPS Promotes Vascular Smooth Muscle Cells Proliferation Through the TLR4/Rac1/Akt Signalling Pathway. Cell Physiol Biochem 44(6):2189–2200 [DOI] [PubMed] [Google Scholar]
- 97.Pilmore HL, Painter DM, Bishop GA, McCaughan GW, Eris JM (2000) Early up-regulation of macrophages and myofibroblasts: a new marker for development of chronic renal allograft rejection. Transplantation 69(12):2658–2662 [DOI] [PubMed] [Google Scholar]
- 98.Bennett MR, Sinha S, Owens GK (2016) Vascular Smooth Muscle Cells in Atherosclerosis. Circ Res 118(4):692–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pober JS, Jane-wit D, Qin L, Tellides G (2014) Interacting Mechanisms in the Pathogenesis of Cardiac Allograft Vasculopathy. Arterioscler Thromb Vasc Biol 34(8):1609–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mason DP, Kenagy RD, Hasenstab D, Bowen-Pope DF, Seifert RA, Coats S, Hawkins SM, Clowes AW (1999) Matrix Metalloproteinase-9 Overexpression Enhances Vascular Smooth Muscle Cell Migration and Alters Remodeling in the Injured Rat Carotid Artery. Circ Res 85:1179–1185 [DOI] [PubMed] [Google Scholar]
- 101.Yin JL, Hambly BD, Bao SS, Dorothy P, Alex Bishop G, Eris JM (2003) Expression of growth arrest-specific gene 6 and its receptors in dysfunctional human renal allografts. Transpl Int 16(9): 681–688 [DOI] [PubMed] [Google Scholar]
- 102.Schif-Zuck S, Gross N, Assi S, Rostoker R, Serhan CN, Ariel A (2011) Saturated-efferocytosis generates pro-resolving CD11blow macrophages: Modulation by resolvins and glucocorticoids. Eur J Immunol 41(2):366–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cai B, Thorp EB, Doran AC, Sansbury BE, Daemen MJAP, Dorweiler B, Spite M, Fredman G, Tabas I (2017) MerTK receptor cleavage promotes plaque necrosis and defective resolution in atherosclerosis. J Clin Investig 127(2):564–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wan E, Yeap XY, Dehn S, Terry R, Novak M, Zhang S, Iwata S, Han X, Homma S, Drosatos K, Lomasney J, Engman DM, Miller SD, Vaughan DE, Morrow JP, Kishore R, Thorp EB (2013) Enhanced Efferocytosis of Apoptotic Cardiomyocytes Through Myeloid-Epithelial-Reproductive Tyrosine Kinase Links Acute Inflammation Resolution to Cardiac Repair After Infarction. Circ Res 113(8):1004–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zagórska A, Través PG, Lew ED, Dransfield I, Lemke G (2014) Diversification of TAM receptor tyrosine kinase function. Nat Immunol 15(10):920–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Autieri MV, Kelemen SE, Wendt KW (2003) AIF-1 Is an Actin-Polymerizing and Rac1-Activating Protein That Promotes Vascular Smooth Muscle Cell Migration. Circ Res 92:1107–1114 [DOI] [PubMed] [Google Scholar]
- 107.Bézie S, Picarda E, Ossart J, Tesson L, Usal C, Renaudin K, Anegon I, Guillonneau C (2015) IL-34 is a Treg-specific cytokine and mediates transplant tolerance. J Clin Investig 125(10):3952–3964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Foucher ED, Blanchard S, Preisser L, Garo E, Ifrah N, Guardiola P, Delneste Y, Jeannin P, Nataf S (2013) IL-34 Induces the Differentiation of Human Monocytes into Immunosuppressive Macrophages. Antagonistic Effects of GM-CSF and IFNγ. PLoS One 8(2):e56045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pascual-Figal DA, Garrido IP, Blanco R, Minguela A, Lax A, Ordoñez-Llanos J, Bayes-Genis A, Valdés M, Moore SA, Januzzi JL (2011) Soluble ST2 Is a Marker for Acute Cardiac Allograft Rejection. Ann Thorac Surg 92(6):2118–2124 [DOI] [PubMed] [Google Scholar]
- 110.Turnquist HR, Zhao Z, Rosborough BR, Liu Q, Castellaneta A, Isse K, Wang Z, Lang M, Beer Stolz D, Zheng XX, Demetris AJ, Liew FY, Wood KJ, Thomson AW (2011) IL-33 Expands Suppressive CD11b+ Gr-1int and Regulatory T Cells, including ST2L+ Foxp3+ Cells, and Mediates Regulatory T Cell-Dependent Promotion of Cardiac Allograft Survival. J Immunol 187(9): 4598–4610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Martel C, Li W, Fulp B, Platt AM, Gautier EL, Westerterp M, Bittman R, Tall AR, Chen S-H, Thomas MJ, Kreisel D, Swartz MA, Sorci-Thomas MG, Randolph GJ (2013) Lymphatic vasculature mediates macrophage reverse cholesterol transport in mice. J Clin Investig 123(4):1571–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Edwards LA, Nowocin AK, Jafari NV, Meader LL, Brown K, Sarde A, Lam C, Murray A, Wong W (2018) Chronic Rejection of Cardiac Allografts Is Associated With Increased Lymphatic Flow and Cellular Trafficking. Circulation 137(5):488–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Aspelund A, Robciuc MR, Karaman S, Makinen T, Alitalo K (2016) Lymphatic System in Cardiovascular Medicine. Circ Res 118(3):515–530 [DOI] [PubMed] [Google Scholar]