Abstract
Purpose of review
This article is aimed to provide readers with an updated review on the applicability, efficacy, and challenges of employing donor apoptotic cell-based therapies to promote transplantation tolerance in various experimental and clinical settings.
Recent findings
Recently, donor apoptotic cell-based therapies have been employed in various models of cell (including pancreatic islets and bone marrow hematopoietic stem cells) and solid organ (heart and kidney) transplantation to promote donor-specific tolerance. Published data, thus far, have revealed a high potential of this approach in inducing robust transplantation tolerance. Recent clinical trials have also underscored the safety and potential efficacy of this approach in alleviating graft-versus-host disease (GVHD) in bone marrow transplantation (BMT). Host factors including prior allo-sensitization and opportunistic infections pose major obstacles in establishing transplantation tolerance employing this strategy. However, emerging data provide strategies for overcoming such obstacles in these clinically relevant settings.
Summary
Donor apoptotic cell therapy is an emerging strategy in promoting transplantation tolerance, with recent data emphasizing its efficacy and applicability for transplantation tolerance in the clinic.
Keywords: apoptotic cells, cytomegalovirus infection, sensitization, tolerance, transplantation
INTRODUCTION
Apoptosis (programmed cell death) of senescent or injured cells occurs on a daily basis during normal development, tissue-repair, and diverse pathologic conditions. Release of intracellular contents of dying cells into local milieu triggers inflammatory responses, therefore, it is necessary for apoptotic debris to be promptly cleared by tissue-resident macrophages and dendritic cells to avoid inflammation. This process is called efferocytosis [1■]. Cells undergoing apoptosis express several ‘find me’ and ‘eat me’ signals. Diverse receptors on phagocytes sense these signals, facilitating recognition and engulfment of apoptotic bodies [2,3]. Furthermore, engagement of these receptors stimulates SOCS1/3 signaling in phagocytes, which in turn suppresses Toll-like receptor and NFkB signaling, resulting in the inhibition of inflammatory cytokines [4]. Efferocytosis further stimulates macrophages and dendritic cells to express anti-inflammatory cytokines (IL-10 and TGF-β1) and restrains their activation [5–9]. Therefore, efferocytosis is an active biochemical process that exerts a profound impact on innate and adaptive immunity. Consequently, impaired or delayed clearance of apoptotic cells results in activation of both antigen-presenting cells (APCs) and T cells [10]. For example, genetic deficiency of the Mer receptor tyrosine kinase, a phagocytic receptor facilitating efferocytosis, leads to delayed clearance of apoptotic cells and lupus-like autoimmunity in mice [2,11]. Similarly, impaired identification and clearance of apoptotic cells in humans have also been reported in systemic lupus erythematosus patients [12–14]. Thus, efficient removal of apoptotic cells by macrophages and dendritic cells are critical for tissue homoeostasis and maintenance of self-tolerance.
Organ transplantation is often the only curative treatment for patients with end-stage organ diseases. Despite significant advances in new immunosuppressive drugs, chronic rejection is still common and side effects of life-long immunosuppression are inevitable [15]. Therefore, induction of donor-specific tolerance has been the ‘holy grail’ in transplantation that could potentially obviate these complications. The profound immunomodulatory effects of apoptotic cells on immunity have motivated several laboratories to investigate the potential of donor apoptotic cells in promoting transplantation tolerance. Intravenous infusions of donor apoptotic dendritic cells or recipient dendritic cells loaded with donor apoptotic cells provide protection to murine cardiac allografts [16,17]. Similarly, infusions of donor apoptotic cells generated by γ or ultraviolet B (UVB) irradiation have been shown to promote bone marrow engraftment without graft-versus-host disease (GVHD) in allogeneic BMT [18]. Our laboratory has demonstrated that donor splenocytes pretreated with a chemical cross-linker ethylenecarbodiimide (ECDI) rapidly undergo apoptosis, and that infusions of such apoptotic donor splenocytes (ECDI-SP) effectively induce donor-specific tolerance in various mouse models of allogeneic and xenogeneic transplantation [19–23]. These data collectively highlight the potential of using donor apoptotic cell-based therapies for inducing robust donor-specific tolerance for clinical applications.
In this review, we will discuss the efficacy of this approach in various transplant scenarios and potential obstacles for its successful implementation in clinically relevant settings.
ORGAN-SPECIFIC HETEROGENEITY IN TRANSPLANTATION TOLERANCE BY DONOR APOPTOTIC CELLS
Different organs display varying propensities for tolerance [24]. Using a mouse model of pancreatic islet transplantation, we have previously shown that peri-transplant intravenous infusions of donor ECDI-SP induce robust donor-specific tolerance to the allogeneic islets. In this setting, APCs play a crucial role in establishing and maintaining tolerance posttransplantation [25]. We and others have shown that efferocytosis by recipient APCs is critical in engaging several parallel tolerance mechanisms, including induction of Foxp3+ regulatory T cells (Tregs), anergy and depletion of allo-reactive T cells, and programmed-death (PD)-1/PD-ligand 1 signaling. In concert, these mechanisms promote indefinite survival of the transplanted allogeneic islets [19,21,26].
In contrast to allogeneic islet transplantation where infusion of donor ECDI-SP provides indefinite protection [19,27], the same tolerance regimen is less efficacious in allogeneic heart transplantation in providing long-term graft protection, but instead requires the addition of a short course of rapamycin[20]. Such a difference in tolerance efficacy by donor ECDI-SP may be attributed to the difference in allogeneic endothelial targets present in the allogeneic heart, but not in islets. Interestingly, even among solid organs (lung versus heart), efficacy of tolerance varies dramatically [28,29■]. In full-mismatched miniature swine cardiac and lung transplant models, Sommer et al. [29■] show that a tolerance protocol constituted of nonmyeloablative irradiation with donor-specific transfusion (DST) and immuno-suppression, while providing prolonged protection to the lung allografts, fails to promote heart allograft survival. Such organ-specific heterogeneity has also been observed in other nonapoptotic cell-based tolerance regimens [28]. For example, Madariaga et al.[28] demonstrate that tacrolimus induces tolerance to lung allografts, whereas cardiac allografts are readily rejected unless they are co-transplanted with the same donor kidney, suggesting the tolerogeneicity of kidneys possibly owing to Treg induction by kidney tubular epithelial cells. These data thus underscore the need for a better understanding of organ-specific factors underlying tolerance susceptibility for the design of apoptotic cell-based organ-specific tolerance protocols.
In allogeneic BMT, donor T cells co-infused with the hematopoietic stem cells readily proliferate and differentiate in response to host APCs and initiate GVHD [30], resulting in high morbidity and mortality [31]. Employing a mouse model of allogeneic BMT, Bittencourt et al. [18] report that infusion of apoptotic cells of either donor or recipient origin effectively promotes donor bone marrow engraftment without GVHD. In a mouse model of lethal GVHD, extracorporeal photopheresis that induces autologous apoptotic cells has also been shown to promote recipient survival without GVHD [32]. In these studies, bone marrow engraftment and improvement of GVHD following apoptotic cells infusions were mediated by TGF-β modulating several cell populations, including conventional dendritic cells, plasmacytoid dendritic cells, natural killer cells, and Tregs [32–35]. These findings are highly clinically relevant as allogeneic BMT is now being used to promote transplantation tolerance for solid organs [36,37]. Recently, a phase I/IIa multi-center clinical trial evaluated the safety, tolerability, and efficacy of apoptotic cell administration in 13 patients undergoing human leukocyte antigen-matched allogeneic BMT [38]. The study demonstrated safety and tolerability of a single infusion of donor apoptotic cells in these recipients, as well as a potential efficacy in reducing acute GVHD. This represents a pioneering step in translating the use of apoptotic cells to clinical practice for BMT, and possibly for other cell or solid organ transplantation.
CONSEQUENCES OF INFECTIONS ON TRANSPLANTATION TOLERANCE BY DONOR APOPTOTIC CELLS
Although induction of donor-specific tolerance by apoptotic cells in immunologically quiescent hosts promotes long-term graft function, opportunistic infections may negatively affect tolerance induction and/or its stability by enhancing host alloreactivity, and ultimately compromise graft function [39]. In this section, we will discuss how microbial infections may affect the efficacy of tolerance by apoptotic cells, and reciprocally how tolerance by apoptotic cells may affect antimicrobial immunity and viral latency activation.
Effects of acute infections on tolerance induction and maintenance
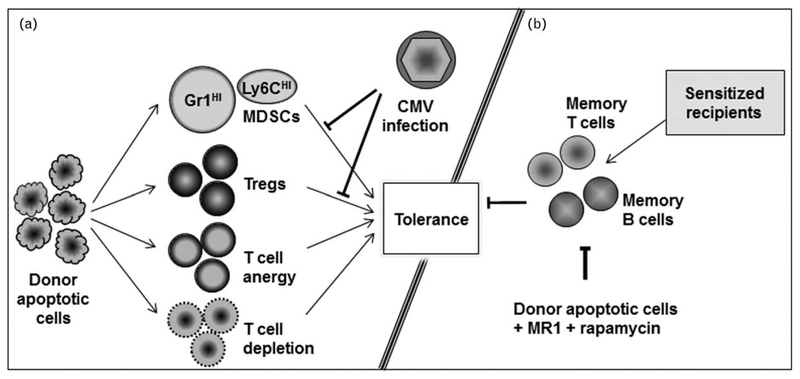
Cytomegalovirus (CMV) is a highly prevalent and clinically relevant pathogen in transplantation [40]. However, its impact on transplantation tolerance is not well defined. Employing murine CMV (MCMV) and an allogeneic islet transplantation model, we have recently reported that acute MCMV infection abrogates transplantation tolerance induction by donor ECDI-SP [41■■]. This tolerance impairment correlates with an alteration in the differentiation and function of myeloid-derived suppressor cells (MDSCs), a heterogeneous population of immature myeloid cells implicated in transplantation tolerance [42]. Our study reveals that acute MCMV infection depresses the number of the highly immunosuppressive Gr1HI granulocytic-MDSCs, whereas promotes the loss of MDSC-like suppressive function of the accumulating Ly6CHI-monocytic cells and supports their gain of an immunostimulatory phenotype that now enhances T-cell alloreactivity. Consequently, the islet allograft exhibits an altered effector to regulatory T-cell ratio, which correlates with the ultimate graft demise (Fig. 1a). Previously, it has been demonstrated that type 1 interferon signaling negatively affects tolerance induction during bacterial infection [43]. Supporting a role of type 1 interferon in mediating tolerance impairment in our model, we found that blocking type 1 interferon signaling during MCMV infection rescues MDSCs and restores transplantation tolerance [41■■].
FIGURE 1.
Transplantation tolerance induced by donor apoptotic cells. (a) Mechanisms of tolerance induction and its impairment by cytomegalovirus infection. (b) In sensitized recipients, donor apoptotic cell-based regimen suppresses memory T and B cells to induce tolerance.
Although these data highlight that acute microbial infections may be a potential obstacle in the induction of transplantation tolerance employing donor apoptotic cells, an equally important question is how such infections may affect the maintenance of tolerance once it is established. Using Listeria monocytogenes infection in an anti-CD154 antibody (MR1) with DST allogeneic heart transplant tolerance model, Young et al. [44■■] report that following an L. monocytogenes infection, majority of transplant recipients with established tolerance in fact experience an episode of rejection. They further demonstrate that though ~60% of such recipients are able to recover partial graft function once L. monocytogenes infection has abated, tolerance-associated gene signature is nevertheless significantly altered, showing an ‘erosion’ of the previously established tolerance [44■■]. Similarly, in a murine allogeneic islet transplantation model, we found that acute MCMV infection in previously tolerized recipients results into loss of islet allograft to acute rejection in ~50% recipients (Dangi et al.; unpublished data). These data underscore the need for seeking effective therapies for maintaining and/or restoring full transplantation tolerance in settings of inadvertent microbial infections. The roles of type 1 interferon and MDSCs in this process provide potential targets for successful interventions.
Reciprocal effects between latent cytomegalovirus reactivation and transplantation tolerance
CMV reactivation from latency is common in transplant recipients because of transplant-induced inflammatory cytokines (e.g. IL-1β, IL-6, and TNFα), which initiate viral early gene expression [45] and chronic immunosuppression, which impairs anti-CMV immunity and facilitates viral replication [46–48]. Latent CMV reactivation has long been associated with graft dysfunction [40]. Transplantation tolerance can both minimize alloimmunity-induced inflammation and eliminate the need for chronic immunosuppression [36,37], therefore can theoretically prevent CMV reactivation from latency. We examined this hypothesis in our donor apoptotic ECDI-SP tolerance model of allogeneic kidney transplantation. In clinical kidney transplantation, CMV seronegative recipients (R−) receiving a kidney allograft from seropositive donors (D+) are at the highest risk for CMV reactivation [49]. Creating an experimental setting using seropositive donors to seronegative recipients (D+/R−) in a mouse allogeneic kidney transplantation model, we observe that transplantation tolerance induced by donor apoptotic ECDI-SP is able to effectively prevent CMV reactivation by both inhibiting alloimmunity-induced inflammation and at the same time preserving host anti-CMV immunity (Dangi et al., manuscript in preparation). In contrast, in recipients chronically treated with immunosuppression, viral reactivation and dissemination from the latently infected kidney allograft are readily observed, together with a profoundly impaired host anti-CMV immunity. Therefore, our data support the notion that donor apoptotic cells as a relatively simple but robust strategy for tolerance induction can indeed prevent CMV reactivation and dissemination while preserving long-term graft survival.
On the other hand, CMV reactivation from latency is common when more aggressive regimens for tolerance induction, namely conditioning regimens in preparation for donor BMT, are used; and in some cases, lead to failure of tolerance itself. Currently, clinical experimental protocols for transplantation tolerance require BMT and chimerism induction [36,37]. In a recent study using a BMT-based tolerance protocol in Cynomolgus macaques, CMV reactivation was reported in five out of eight recipients, all of whom experienced a subsequent loss of chimerism and tolerance [50■]. The authors conclude that early CMV reactivation and possibly anti-CMV therapy (ganciclovir and foscarnet) impair bone marrow engraftment, and therefore are major impediments to inducing chimerism and tolerance [50■]. Employing a protocol combining low-dose irradiation and costimulation-blockade in Rhesus macaques, Zheng et al. also report that sustained chimerism and tolerance can be readily achieved. However, this protocol was also accompanied by a significant risk for CMV reactivation and end-organ CMV diseases because of impaired host anti-CMV immunity [51■]. Therefore, these data suggest that aggressive conditioning protocols needed for BMT and chimerism for tolerance induction frequently promote CMV reactivation and may in fact hinder the establishment of transplantation tolerance. Alternatively, apoptotic cell-based tolerance protocols may be a more attractive approach as they do not require recipient bone marrow ablation, therefore minimize risks for CMV reactivation and its associated complications.
CONSEQUENCES OF PRIOR SENSITIZATION ON TRANSPLANTATION TOLERANCE BY DONOR APOPTOTIC CELLS
In addition to infections, another prominent factor known to modulate the outcome of tolerance attempts is preexisting alloreactive T-cell and B-cell memories generated during previous blood transfusions, prior transplantations, and pregnancies[52]. Owing to a low-activation threshold, memory cells mount quicker and more vigorous immune responses compared with their naı¨ve counterparts. Therefore, sensitized recipients are highly challenging to transplant because of a high risk for accelerated graft rejection from memory immune responses [53,54]. It has been shown that preexisting donor-specific antibodies (DSAs) in sensitized recipients can readily bind to graft endothelium and induce graft destruction by complement activation[55]. Thus, presence of DSAs may also pose a major barrier in inducing transplantation tolerance. In this context, using a DST transplantation tolerance model, Burns and Chong [56] demonstrate that preexisting DSAs opsonize the transfused donor cells, which in turn activate recipient APCs to stimulate, rather than inhibit, alloreactive T cells, and consequently accelerate allograft rejection. Using a rat islet transplant model, de Kort et al. [57] also demonstrate that pretransplant infusion of dexamethasone-treated donor dendritic cells intended for inducing tolerance in fact results in generation of alloreactive antibodies, which in turn precipitate accelerated islet allograft rejection.
DSAs have similarly been associated with loss of islet graft function in sensitized human islet transplant recipients [58], although recent data suggest that if at low levels, they may be well tolerated without a consequence on the transplanted islets [59,60]. In a sensitized murine model of allogeneic islet transplantation, we have observed that infusion of donor ECDI-SP indeed results in an accelerated loss of islets in comparison with unsensitized recipients, similar to that observed by Burns and de Kort (Dangi et al., manuscript in preparation). However, when additionally combined with a short course of rapamycin and MR1, ECDI-SP in fact significantly prolong islet allograft survival beyond that seen with rapamycin and MR1 alone. In our model, low level DSAs are comparable in both groups and do not correlate with graft outcome. These data suggest that for allogeneic islet transplantation in sensitized recipients with low levels of DSAs, cellular alloimmune memory responses may be a more critical factor for determining graft outcomes but are readily modifiable by donor apoptotic cells. Indeed, we observe that ECDI-SP, in combination with rapamycin and MR1, efficiently regulate alloimmune memory T-cell and B-cell responses in sensitized recipients (Dangi et al., manuscript in preparation). Thus, donor apoptotic cells can be combined with additional targeting strategies to control alloimmune memory responses in sensitized recipients, and therefore also have a promising role in promoting tolerance even in such recipients (Fig. 1b).
CONCLUSION
Apoptotic cells exert profound immunosuppressive effects and promote immune homoeostasis and tolerance. Employing this strategy in various transplant models, several laboratories have demonstrated that robust transplantation tolerance and prolonged graft survival can be achieved. Recent data highlight that CMV infection and previous allosensitization constitute major obstacles for tolerance induction by this approach. These studies now form the basis for further investigations seeking effective strategies for promoting transplantation tolerance in such settings. Encouragingly, a recent clinical trial employing donor apoptotic cells in bone marrow transplantation has highlighted the safety of this approach and its efficacy in reducing GVHD, underscoring its clinical applicability and potentials for organ transplantation tolerance.
KEY POINTS.
Apoptotic cell-based therapies are effective in inducing transplantation tolerance in various experimental transplant models.
Organ-specific factors determine variable efficacies of tolerance by apoptotic cell-based therapies.
Donor apoptotic cell-based tolerance therapies may effectively prevent CMV reactivation while promoting transplantation tolerance.
Combining donor apoptotic cell-based therapies with transient immunosuppression controls alloimmune memory responses and promotes tolerance in previously sensitized recipients.
Acknowledgements
Financial support and sponsorship
This work was supported by grants from the National Institutes of Health P01 AI112522 (A.D. and X.L.), and the Chinese Scholarship Council (S.Y.).
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1■.Martinez J. Prix fixe: efferocytosis as a four-course meal. Curr Top Microbiol Immunol 2017; 403:1–36.This review article comprehensively describe the process of ‘efferocytosis’ and abnormalities in various pathological conditions.
- 2.Lemke G, Burstyn-Cohen T. TAM receptors and the clearance of apoptotic cells. Ann N Y Acad Sci 2010; 1209:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poon IK, Lucas CD, Rossi AG, Ravichandran KS. Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol 2014; 14: 166–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rothlin CV, Ghosh S, Zuniga EI, et al. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell 2007; 131:1124–1136. [DOI] [PubMed] [Google Scholar]
- 5.Sauter B, Albert ML, Francisco L, et al. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med 2000; 191:423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stuart LM, Lucas M, Simpson C, et al. Inhibitory effects of apoptotic cell ingestion upon endotoxin-driven myeloid dendritic cell maturation. J Immunol 2002; 168:1627–1635. [DOI] [PubMed] [Google Scholar]
- 7.Verbovetski I, Bychkov H, Trahtemberg U, et al. Opsonization of apoptotic cells by autologous iC3b facilitates clearance by immature dendritic cells, down-regulates DR and CD86, and up-regulates CC chemokine receptor 7. J Exp Med 2002; 196:1553–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morelli AE, Larregina AT, Shufesky WJ, et al. Internalization of circulating apoptotic cells by splenic marginal zone dendritic cells: dependence on complement receptors and effect on cytokine production. Blood 2003; 101:611–620. [DOI] [PubMed] [Google Scholar]
- 9.Voll RE, Herrmann M, Roth EA, et al. Immunosuppressive effects of apoptotic cells. Nature 1997; 390:350–351. [DOI] [PubMed] [Google Scholar]
- 10.Rovere P, Vallinoto C, Bondanza A, et al. Bystander apoptosis triggers dendritic cell maturation and antigen-presenting function. J Immunol 1998; 161:4467–4471. [PubMed] [Google Scholar]
- 11.Cohen PL, Caricchio R, Abraham V, et al. Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J Exp Med 2002; 196:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munoz LE, Lauber K, Schiller M, et al. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat Rev Rheumatol 2010; 6:280–289. [DOI] [PubMed] [Google Scholar]
- 13.Donnelly S, Roake W, Brown S, et al. Impaired recognition of apoptotic neutrophils by the C1q/calreticulin and CD91 pathway in systemic lupus erythematosus. Arthritis Rheum 2006; 54:1543–1556. [DOI] [PubMed] [Google Scholar]
- 14.Ren Y, Tang J, Mok MY, et al. Increased apoptotic neutrophils and macrophages and impaired macrophage phagocytic clearance of apoptotic neutrophils in systemic lupus erythematosus. Arthritis Rheum 2003; 48: 2888–2897. [DOI] [PubMed] [Google Scholar]
- 15.Becker LE, Morath C, Suesal C. Immune mechanisms of acute and chronic rejection. Clin Biochem 2016; 49(4–5):320–323. [DOI] [PubMed] [Google Scholar]
- 16.Kaneko K, Morelli AE, Wang Z, Thomson AW. Alloantigen presentation by ethylcarbodiimide-treated dendritic cells induces T cell hyporesponsiveness, and prolongs organ graft survival. Clin Immunol 2003; 108:190–198. [DOI] [PubMed] [Google Scholar]
- 17.Xu DL, Liu Y, Tan JM, et al. Marked prolongation of murine cardiac allograft survival using recipient immature dendritic cells loaded with donor-derived apoptotic cells. Scand J Immunol 2004; 59:536–544. [DOI] [PubMed] [Google Scholar]
- 18.Bittencourt MC, Perruche S, Contassot E, et al. Intravenous injection of apoptotic leukocytes enhances bone marrow engraftment across major histocompatibility barriers. Blood 2001; 98:224–230. [DOI] [PubMed] [Google Scholar]
- 19.Luo X, Pothoven KL, McCarthy D, et al. ECDI-fixed allogeneic splenocytes induce donor-specific tolerance for long-term survival of islet transplants via two distinct mechanisms. Proc Natl Acad Sci U S A 2008; 105: 14527–14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen G, Kheradmand T, Bryant J, et al. Intragraft CD11b(+) IDO(+) cells mediate cardiac allograft tolerance by ECDI-fixed donor splenocyte infusions. Am J Transplant 2012; 12:2920–2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kheradmand T, Wang S, Bryant J, et al. Ethylenecarbodiimide-fixed donor splenocyte infusions differentially target direct and indirect pathways of allorecognition for induction of transplant tolerance. J Immunol 2012; 189:804–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang S, Tasch J, Kheradmand T, et al. Transient B-cell depletion combined with apoptotic donor splenocytes induces xeno-specific T- and B-cell tolerance to islet xenografts. Diabetes 2013; 62:3143–3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bryant J, Lerret NM, Wang JJ, et al. Preemptive donor apoptotic cell infusions induce IFN-gamma-producing myeloid-derived suppressor cells for cardiac allograft protection. J Immunol 2014; 192:6092–6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chandrasekharan D, Issa F, Wood KJ. Achieving operational tolerance in transplantation: how can lessons from the clinic inform research directions? Transpl Int 2013; 26:576–589. [DOI] [PubMed] [Google Scholar]
- 25.Thomson AW, Humar A, Lakkis FG, Metes DM. Regulatory dendritic cells for promotion of liver transplant operational tolerance: Rationale for a clinical trial and accompanying mechanistic studies. Hum Immunol 2017; 79:314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu C, Zhang Y, Jiang Y, et al. Apoptotic cell administration enhances pancreatic islet engraftment by induction of regulatory T cells and tolerogenic dendritic cells. Cell Mol Immunol 2013; 10:393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang S, Zhang X, Zhang L, et al. Preemptive tolerogenic delivery of donor antigens for permanent allogeneic islet graft protection. Cell Transplant 2015; 24:1155–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madariaga ML, Spencer PJ, Michel SG, et al. Effects of lung cotransplantation on cardiac allograft tolerance across a full major histocompatibility complex barrier in miniature swine. Am J Transplant 2016; 16:979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29■.Sommer W, Buechler G, Jansson K, et al. Irradiation before and donor splenocyte infusion immediately after transplantation induce tolerance to lung, but not heart allografts in miniature swine. Transpl Int 2017; 30:420–431.This study describes the heterogeneity in tolerance induction in fully mismatch swine model of lung and cardiac transplantation.
- 30.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet (London, England) 2009; 373:1550–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mosaad YM. Immunology of hematopoietic stem cell transplant. Immunol Invest 2014; 43:858–887. [DOI] [PubMed] [Google Scholar]
- 32.Florek M, Sega EI, Leveson-Gower DB, et al. Autologous apoptotic cells preceding transplantation enhance survival in lethal murine graft-versus-host models. Blood 2014; 124:1832–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kleinclauss F, Perruche S, Masson E, et al. Intravenous apoptotic spleen cell infusion induces a TGF-beta-dependent regulatory T-cell expansion. Cell Death Differ 2006; 13:41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonnefoy F, Perruche S, Couturier M, et al. Plasmacytoid dendritic cells play a major role in apoptotic leukocyte-induced immune modulation. J Immunol 2011; 186:5696–5705. [DOI] [PubMed] [Google Scholar]
- 35.Chong WP, Zhou J, Law HK, et al. Natural killer cells become tolerogenic after interaction with apoptotic cells. Eur J Immunol 2010; 40:1718–1727. [DOI] [PubMed] [Google Scholar]
- 36.Scandling JD, Busque S, Dejbakhsh-Jones S, et al. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N Engl J Med 2008; 358:362–368. [DOI] [PubMed] [Google Scholar]
- 37.Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med 2008; 358: 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mevorach D, Zuckerman T, Reiner I, et al. Single infusion of donor mono-nuclear early apoptotic cells as prophylaxis for graft-versus-host disease in myeloablative HLA-matched allogeneic bone marrow transplantation: a phase I/IIa clinical trial. Biol Blood Marrow Transplant 2014; 20:58–65. [DOI] [PubMed] [Google Scholar]
- 39.Chong AS, Alegre ML. The impact of infection and tissue damage in solid-organ transplantation. Nat Rev Immunol 2012; 12:459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Azevedo LS, Pierrotti LC, Abdala E, et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo, Brazil) 2015; 70:515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41■■.Dangi A, Zhang L, Zhang X, Luo X. Murine CMV induces type 1 IFN that impairs differentiation of MDSCs critical for transplantation tolerance. BloodAdv 2018; 2:669–680.This study highlights that acute MCMV infection impairs induction of transplantation tolerance by donor apoptotic cells. This study demonstrated that it is not the direct cytopathic effect of MCMV infection, but heightened alloreactivity that caused graft dysfunction because of an interference in tolerance-inducing MDSCs differentiation and function.
- 42.Zhang C, Wang S, Yang C, Rong R. The crosstalk between myeloid derived suppressor cells and immune cells: to establish immune tolerance in transplantation. J Immunol Res 2016; 2016:4986797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang T, Chen L, Ahmed E, et al. Prevention of allograft tolerance by bacterial infection with Listeria monocytogenes. J Immunol 2008; 180: 5991–5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44■■.Young JS, Daniels MD, Miller ML, et al. Erosion of transplantation tolerance after infection. Am J Transplant 2017; 17:81–90.This study nicely demonstrated that bacterial infection during tolerance maintenance phase weakens the tolerance promoting mechanisms using Listeria as a model in a murine cardiac transplantation.
- 45.Liu XF, Jie C, Zhang Z, et al. Transplant-induced reactivation of murine cytomegalovirus immediate early gene expression is associated with recruitment of NF-kappaB and AP-1 to the major immediate early promoter. J Gen Virol 2016; 97:941–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Issa NC, Fishman JA. Infectious complications of antilymphocyte therapies in solid organ transplantation. Clin Infect Dis 2009; 48:772–786. [DOI] [PubMed] [Google Scholar]
- 47.Fisher RA. Cytomegalovirus infection and disease in the new era of immunosuppression following solid organ transplantation. Transpl Infect Dis 2009; 11:195–202. [DOI] [PubMed] [Google Scholar]
- 48.Xu H, Perez SD, Cheeseman J, et al. The allo- and viral-specific immunosuppressive effect of belatacept, but not tacrolimus, attenuates with progressive T cell maturation. Am J Transplant 2014; 14:319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hasegawa J, Hatakeyama S, Wakai S, et al. Preemptive anticytomegalovirus therapy in high-risk (donor-positive, recipient-negative cytomegalovirus serostatus) kidney transplant recipients. Int J Infect Dis 2017; 65:50–56. [DOI] [PubMed] [Google Scholar]
- 50■.Duran-Struuck R, Sondermeijer HP, Buhler L, et al. Effect of ex vivo-expanded recipient regulatory t cells on hematopoietic chimerism and kidney allograft tolerance across MHC barriers in Cynomolgus macaques. Transplantation 2017; 101:274–283.This study highlights that in nonhuman primate infusion of Tregs can promote durable mixed chimerism and kidney graft survival, however, early CMV reactivation is a potential problem achieving chimerism and tolerance.
- 51■.Zheng HB, Watkins B, Tkachev V, et al. The knife’s edge of tolerance: inducing stable multilineage mixed chimerism but with a significant risk of CMV reactivation and disease in rhesus macaques. Am J Transplant 2017; 17:657–670.This study demonstrated that in nonhuman primates, a prolonged mixed chimerism can be achieved to promote transplantation using a lengthy condition regimen but it is associated with a significant risk of CMV reactivation in these recipients.
- 52.D’Orsogna L, van den Heuvel H, van Kooten C, et al. Infectious pathogens may trigger specific allo-HLA reactivity via multiple mechanisms. Immunogenetics 2017; 69:631–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Avila CL, Zimmerer JM, Elzein SM, et al. mTOR inhibition suppresses post-transplant alloantibody production through direct inhibition of alloprimed B cells and sparing of CD8+antibody-suppressing T cells. Transplantation 2016; 100:1898–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Benichou G, Gonzalez B, Marino J, et al. Role of memory T cells in allograft rejection and tolerance. Front Immunol 2017; 8:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roux A, Thomas KA, Sage E, et al. Donor specific HLA antibody-mediated complement activation is a significant indicator of antibody-mediated rejection and poor long-term graft outcome during lung transplantation: a single center cohort study. Transpl Int 2018; 31: 761–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burns AM, Chong AS. Alloantibodies prevent the induction of transplantation tolerance by enhancing alloreactive T cell priming. J Immunol 2011; 186:214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Kort H, Crul C, van der Wal AM, et al. Accelerated antibody-mediated graft loss of rodent pancreatic islets after pretreatment with dexamethasone-treated immature donor dendritic cells. Transplantation 2012; 94:903–910. [DOI] [PubMed] [Google Scholar]
- 58.Holmes-Walker DJ, Kay TW. Long-term effects of islet transplantation. Curr Opin Organ Transplant 2016; 21:497–502. [DOI] [PubMed] [Google Scholar]
- 59.Chaigne B, Geneugelijk K, Bedat B,et al. Immunogenicityof anti-HLA antibodies in pancreas and islet transplantation. Cell Transplant 2016; 25:2041–2050. [DOI] [PubMed] [Google Scholar]
- 60.Pouliquen E, Baltzinger P, Lemle A, et al. Anti-donor HLA antibody response after pancreatic islet grafting: characteristics, risk factors, and impact on graft function. Am J Transplant 2017; 17:462–473. [DOI] [PubMed] [Google Scholar]