Abstract
We report the case of a 52-year-old gentleman, admitted to the medical intensive care unit with multiple organ system dysfunction due to acute severe pancreatitis. He was found to have severe hypocalcemia, bradycardia and an electrocardiogram (EKG) showing ST-segment elevation in infero-lateral leads. The patient was treated with intravenous calcium gluconate with prompt improvement of heart rate and reversal of EKG changes. Subsequent evaluation for myocardial ischemia was negative. We believe the EKG changes mimicking acute ST-segment elevation myocardial infarction were due to severe hypocalcemia. To our knowledge this is very rare occurrence.
Introduction
Prompt recognition and treatment of patients with ST-segment elevation myocardial infarction (STEMI) has a significant morbidity and mortality benefit. In the absence of contraindications, STEMI patients have improved outcomes following coronary angiography and percutaneous coronary intervention [1]. Occlusive coronary lesions are identified in the vast majority of cases. However, up to 3% of patients are found to have normal coronary vessels at angiography [2]. Electrolyte abnormalities such as hypo- or hyper-calcemia [3–5] and hyperkalemia [6–8] are recognized causes of a pseudo-infarct pattern on electrocardiogram (EKG). In patients with critical illness or significant comorbidities, electrolyte abnormalities are common. In this patient population, clinicians need to be cautious in the interpretation of EKG changes. These patients are high-risk candidates for invasive procedures, and unnecessary interventions should be avoided.
We present a case of acute pancreatitis with severe hypocalcemia mimicking an acute inferior ST-segment elevation myocardial infarction.
CASE PRESENTATION
A 52-year-old male presented to the emergency department (ED) with complaints of lethargy, weakness, poor intake and non-bloody diarrhea. The patient’s past medical history was significant for coronary artery disease (with previous drug-eluting stent placement to the right coronary and left anterior descending arteries 7 years prior), hypertension, hypothyroidism and alcohol-associated hepatic cirrhosis. On examination, he appeared unwell, with dry mucous membranes and a non-tender abdomen. The patient was drowsy but easily aroused. The remainder of his physical examinations was unremarkable. Initial vitals were: blood pressure of 88/59 mmHg, heart rate of 95 bpm and oral temperature of 101.3°F (38.5°C). Following intravenous fluid volume resuscitation with 4 L of crystalloid, his blood pressure improved to 122/73 mmHg with a heart rate of 84 bpm.
Laboratory evaluation showed an acute metabolic acidosis (PH 7.34, Bicarbonate of 14.6, lactate of 4.5 mmol/l), acute kidney injury (Cr 3.46 mg/dl, BUN 81 mg/dl), normal white cell count of (8.9 × 1000/μl), elevated lipase (3026 U/l, normal 23–300 U/L), elevated troponin (3.2 ng/ml, normal <0.012 ng/ml) and hypocalcemia (5.1 mg/dl, normal 8.4–10.2 mg/dl) with low ionized calcium (2.49 mg/d, normal 4.40–5.30 mg/dl). Initial EKG performed in the ED showed normal sinus rhythm, low voltage QRS complexes and non-specific ST and T-wave abnormalities with a QTc interval of 518 ms.
In the ED, intravenous vancomycin and piperacillin–tazobactam were started empirically given concern for sepsis. He was given intravenous magnesium sulfate and calcium gluconate repletion before being admitted to the medical ICU with severe alcohol-induced pancreatitis with multi-organ dysfunction (APACHE II-17) [9]. He was also managed for alcohol withdrawal with symptom triggered benzodiazepine as per Clinical Institute Withdrawal Assessment-Alcohol, Revised (CIWA-AR) protocol [10].
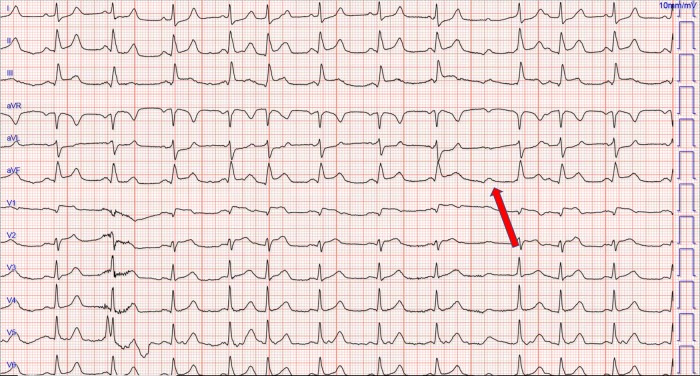
On the day of admission, the patient started having abnormal dystonic body movements with associated confusion, difficulty breathing, bradycardia (range: 40–50 bpm) and relative hypotension (acute drop from systolic range: 130–140 to 106 mmHg). The patient remained alert and denied any chest pain at this stage. He subsequently became progressively confused and agitated and was endotracheally intubated for airway protection. EKG at this time showed sinus rhythm with prolongation of AV conduction, ST elevation in leads II, III, aVF, and V1, V2 with ST depressions in lateral leads (I and aVL) suggestive of the acute infero-lateral myocardial infarct. QTc was 481ms (Fig. 1).
Figure 1:
An electrocardiogram showing normal sinus rhythm with occasional prolongation of AV conduction, ST elevation in the inferior leads and V1, ST depressions in lateral leads. Eventual non-conducted atrial impulse indicated by red arrow
An urgent transthoracic echocardiogram showed normal left ventricular cavity size and function (systolic and diastolic). Estimated LVEF (left ventricular ejection fraction) was 50–55% with no regional wall motion abnormalities. Repeat laboratory evaluation showed severe hypocalcemia (5.2 mg/dl, with ionized calcium 2.90 mg/dl) with a normal serum magnesium and potassium (2.2 mg/dl and 3.5 mmol/l, respectively).
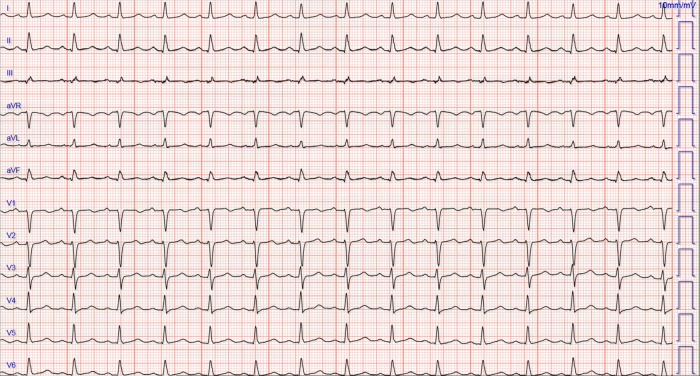
The patient was treated for his severe hypocalcemia with intravenous calcium gluconate. EKG performed 4 h later when the ionized calcium had improved to 4 mg/dl, showed complete resolution of the ST-segment abnormalities; however, QTc interval remained prolonged at 515 ms (Fig. 2.). Troponin I measured at the time of both EKGs were 0.629 and 0.559 ng/ml, respectively.
Figure 2:
Repeat electrocardiogram after infusion of intravenous calcium showing resolution of inferior ST-segment changes
The patient was successfully extubated within 24 h. The remainder of his hospital course was significant for management of severe acute pancreatitis including percutaneous drainage of the peri-pancreatic fluid collection. He had no further acute cardiovascular issues during this admission and made an excellent recovery from his critical illness.
Given the absence of chest pain, the presence of severe metabolic derangements, prompt reversal of EKG changes with correction of hypocalcemia and absence of wall motion abnormality on echocardiogram and low clinical suspicion, the patient was not treated for acute coronary syndrome.
Interval nuclear medicine perfusion single-photon emission computed tomography (SPECT) testing showed negative EKG response to pharmacological stress with no symptoms of ischemia. Perfusion SPECT after pharmacological stress showed no new area of ischemia or perfusion defect. The decision to defer coronary angiography for non-invasive nuclear testing was a joint decision between the physician and patient request.
DISCUSSION
Abnormalities of the ST-segment are common electrocardiographic findings, and the list of ischemic and non-ischemic etiologies is extensive. Some of the non-ischemic causes are due to electrolyte derangements. Causes such as hyper- and hypocalcemia [3–5] as well as hyperkalemia [6–8] can present with ST-segment elevation and a pseudo-infarction pattern on the EKG.
Of the known list of electrolyte derangements affecting the ST segment, hypocalcemia is a very rare etiology [11–14]. Hypocalcemia prolongs the duration of phase 2 of the action potential of cardiac muscle. This results in elevation of the ST-segment and prolongation of the QT interval (due to prolongation of the ST-segment). Hypocalcemia has no effect on the QRS complex or T wave. Low serum calcium increases the permeability of the cell membrane to sodium ions, causing progressive depolarization [15]. Progressive depolarization (and subsequent repolarization) from an epi to endocardial region likely generates the injury current which is represented by deflection in the ST-segment on the EKG tracing. The exact mechanism by which hypocalcemia results in a pseudo-infarct pattern on EKG remains unclear.
Lehmann et al. [11] presented a case of severe hypocalcemia with an acute anterior wall injury pattern on the EKG resembling that of an acute anterior wall myocardial infarction with reciprocal ST-segment depression in the inferior leads which resolved on correction of serum calcium. The authors did, however, note that the case was compounded by the presence of hypomagnesemia, hypokalemia and hyperphosphatemia which often co-exist with derangements in serum calcium. The conclusion was that the EKG changes were due to resultant coronary artery vasospasm. Two cases of hypocalcemia related pseudo-infarction were reported by Reddy et al. [12] and Khardori et al. [13], each describing a patient with an acute anteroseptal injury pattern on the EKG with subsequent exclusion of infarction. These patients had significant comorbidities in addition to other electrolyte abnormalities.
It is of interest to note that our patient exhibited these ST-segment and QTc changes in the presence of normal serum magnesium and potassium levels. This distinguishes our case from previous reports and removes these previous compounding factors. A similar case of isolated hypocalcemia simulating myocardial infarction was reported by Ilveskoski et al. [14]. The authors made an argument against coronary vasospasm being the sole mechanism behind the pseudo-infarct pattern. Their patient’s EKG changes persisted for days until calcium correction; without serum evidence of ongoing ischemia. Also, there was no evidence of coronary artery vasospasm noted at angiography. Interestingly, however, the EKG changes were representative of the territory perfused by the left anterior descending coronary artery—thus, supporting a vasospastic mechanism. The alternative mechanism suggested in their report was that hypocalcemia-induced action potential differences between myocardial regions or transmural heterogeneity of action potential duration might play a role in regional ST-segment elevation.
Acute pancreatitis of itself has been reported in the literature as a very rare cause of myocardial infarct pattern on EKG [16]. Some of the hypothesized relationships between acute pancreatitis and infarct EKG changes are: metabolic and electrolyte derangements, direct toxic effects on the myocardium, coronary artery vasospasm, and prothrombotic and inflammatory states [17–20]. Interestingly, a few of these mechanisms are seen in patients with hypocalcemia from other causes (e.g. hypoparathyroidism) [11]. Unfortunately, serum electrolytes- in particular serum calcium, have been inconsistently reported across the literature, making it unclear to what degree derangements in electrolytes could have resulted in the EKG changes in some cases of pancreatitis.
Our patient likely had increased vagal tone at the time of his acute decompensation. This is supported by his relative hypotension and bradycardia noted on telemetry. His retroperitoneal inflammation from his severe pancreatitis likely resulted in increased vagus nerve stimulation or sensitivity. Heightened vagal output manifested in atrioventricular (AV) conduction slowing noted in Fig. 1. There is variability in the PR interval, with premature atrial complexes and eventual block when the AV node is left refractory (indicated by red arrow). The effect of vagal stimulation on AV conduction has been well established. Early studies in animal models found a linear response in PR interval and vagal stimulation [21].
There is apparent beat to beat variation in ST-segment elevation in the rhythm strips-particularly the second–fourth beats in the inferior leads II, III, aVF. This is due to baseline wander causing dampening of the degree of elevation from the baseline. The concave ST-segment elevation in the contiguous inferior leads with reciprocal ST-segment depression in I, aVL is characteristic of an inferior STEMI and distinguishes it from other causes of inferior lead ST elevation. The reciprocal ST depression in leads away from the site of an acute infarct is a highly sensitive indicator of acute MI—seen in up to 30% of inferior MIs [22]. When signs of inferior infarction are associated with ST-segment elevation in leads V1 and V2, it is often indicative of right ventricular infarction; which is often overlooked [23, 24].
The etiology of our patient’s EKG pseudo-infarct pattern was due to a transient cardiac metabolic state. The insult was enough to result in myocardial cell injury (as indicated by his troponin release), but insufficient to result in cell death. This was later confirmed by SPECT imaging. The events leading up to his acute deterioration likely resulted in shifts in his acid-base balance and calcium availability. Hyper-ventilation in the setting of an already profoundly low ionized calcium resulting in transient coronary artery vasospasm is a reasonable hypothesis.
CONCLUSION
Severe electrolyte abnormalities can result in ST-segment elevation and a pseudo-infarct pattern on EKG. In patients with risk factors for derangements in serum calcium (e.g. sepsis, hypoparathyroidism, acute pancreatitis, vitamin D deficiency, massive transfusion of citrate containing blood products and hemodialysis) serum chemistry values should be used to aide in the interpretation of EKG abnormalities. The decision to proceed with urgent coronary angiography should be made in the context of the entire clinical scenario, including accounting for the presence of culprit electrolyte abnormalities.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. American College of Emergency Physicians Society for Cardiovascular Angiography and Interventions O’Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;61:485–510. [DOI] [PubMed] [Google Scholar]
- 2. Widimsky P, Stellova B, Groch L, Aschermann M, Branny M, Zelizko M, et al. Prevalence of normal coronary angiography in the acute phase of suspected ST-elevation myocardial infarction: experience from the PRAGUE studies. Can J Cardiol 2006;22:1147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wesson LC, Suresh V, Parry RG. Severe hypercalcaemia mimicking acute myocardial infarction. Clin Med (Northfield Il) 2009;9:186–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nishi SP, Barbagelata NA, Atar S, Birnbaum Y, Tuero E. Hypercalcemia-induced ST-segment elevation mimicking acute myocardial infarction. J Electrocardiol 2006;39:298–300. [DOI] [PubMed] [Google Scholar]
- 5. Fang CF, Xu G, Chen YX. Acute myocardial infarction mimicking squamous cell lung cancer with bone metastases due to hypercalcemia: a case report. Chin Med J (Engl) 2010;123:369–71. [PubMed] [Google Scholar]
- 6. Levine HD, Merrill JP, Somerville W. Advanced disturbances of the cardiac mechanism in potassium intoxication in man. Circulation 1951;3:889–905. [DOI] [PubMed] [Google Scholar]
- 7. Levine HD, Wanzer SH, Merrill JP. Dialyzable currents of injury in potassium intoxication resembling acute myocardial infarction or pericarditis. Circulation 1956;13:29–36. [DOI] [PubMed] [Google Scholar]
- 8. Pastor JA, Castellanos A, Moleiro F, Myerburg RJ. Patterns of acute inferior wall myocardial infarction caused by hyperkalemia. J Electrocardiol 2001;34:53–8. [DOI] [PubMed] [Google Scholar]
- 9. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818–29. [PubMed] [Google Scholar]
- 10. Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict 1989;84:1353–7. [DOI] [PubMed] [Google Scholar]
- 11. Lehmann G, Deisenhofer I, Ndrepepa G, Schmitt C. ECG changes in a 25-year-old woman with hypocalcemia due to hypoparathyroidism. Chest 2000;118:260–2. [DOI] [PubMed] [Google Scholar]
- 12. Reddy CV, Gould L, Gomprecht RF. Unusual electrocardiographic manifestations of hypocalcemia. Angiology 1974;25:764–8. [DOI] [PubMed] [Google Scholar]
- 13. Khardori R, Cohen B, Taylor D, Soler NG. Electrocardiographic finding simulating acute myocardial infarction in a compound metabolic aberration. Am J Med 1985;78:529–32. [DOI] [PubMed] [Google Scholar]
- 14. Ilveskoski E, Sclarovsky S, Nikus K. Severe hypocalcemia simulating ST-elevation myocardial infarction. Am J Emerg Med 2012;30:256.e3–6. [DOI] [PubMed] [Google Scholar]
- 15. Surawicz BGL. Effect of electrolyte abnormalities on the heart and circulation. Cardiac and Vascular Diseases Philadelphia 1971. pp. 539–76.
- 16. Ralapanawa U, Jayalath T, Senadhira D. A case of acute necrotizing pancreatitis complicated with non ST elevation myocardial infarction. BMC Res Notes 2018;11:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hsu PC, Lin TH, Su HM, Lin ZY, Lai WT, Sheu SH. Acute necrotizing pancreatitis complicated with ST elevation acute myocardial infarction: a case report and literature review. Kaohsiung J Med Sci 2010;26:200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lieberman JS, Taylor A, Wright IS. The effect of intravenous trypsin administration on the electrocardiogram of the rabbit. Circulation 1954;10:338–42. [DOI] [PubMed] [Google Scholar]
- 19. Kellner A. Selective necrosis of cardiac and skeletal muscle induced experimentally by means of proteolytic enzyme solutions given intravenously. J Exp Med 1954;99:387–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cafri C, Basok A, Katz A, Abuful A, Gilutz H, Battler A. Thrombolytic therapy in acute pancreatitis presenting as acute myocardial infarction. Int J Cardiol 1995;49:279–81. [DOI] [PubMed] [Google Scholar]
- 21. Pirola FT, Potter EK. Vagal action on atrioventricular conduction and its inhibition by sympathetic stimulation and neuropeptide Y in anaesthetised dogs. J Auton Nerv Syst 1990;31:1–12. [DOI] [PubMed] [Google Scholar]
- 22. Channer K, Morris F. ABC of clinical electrocardiography: myocardial ischaemia. Br Med J 2002;324:1023–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morris F. ABC of clinical electrocardiography: acute myocardial infarction—Part I. Br Med J 2002;324:831–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Finn AV, Antman EM. Images in clinical medicine. Isolated right ventricular infarction. N Engl J Med 2003;349:1636. [DOI] [PubMed] [Google Scholar]