ABSTRACT
The recommendation to eat breakfast has received scrutiny due to insufficient causal evidence for improvements in weight management. Despite the limited number of randomized controlled trials examining the effects of breakfast consumption compared with skipping breakfast on weight loss, an increasing number of studies target the hormonal and behavioral mechanisms underlying weight management. This review provides a comprehensive examination of the intervention-based clinical trials that test whether breakfast consumption improves appetite control and satiety as well as energy expenditure compared with skipping breakfast. Several factors were considered when interpreting the body of evidence. These include, but were not limited to, the following: the composition of breakfast, with a specific focus on dietary protein; meal size and form; and habitual breakfast behaviors. The evidence within this review shows positive to neutral support for the inclusion of breakfast for improvements in appetite control, satiety, and postprandial energy expenditure. The protein content, energy content, and form of the meal (i.e., beverages compared with foods) are key modulating factors for ingestive behavior and energy expenditure mechanisms. Specifically, breakfast meals containing a larger amount of protein (≥30 g protein/meal) and energy (≥350 kcal/meal) and provided as solid foods increased the magnitude of the appetite and satiety response compared with breakfast skipping. Longer-term randomized controlled trials including the measurement of ingestive behavior and weight management are needed to identify the role of breakfast for health promotion.
Keywords: breakfast, appetite, breakfast skipping, energy expenditure, circadian, sleep
Introduction
Demand for dietary recommendations to improve health and mitigate lifestyle-related chronic diseases is increasing at both the individual and population levels (1). Breakfast continues to be touted as an important part of a healthy dietary pattern, potentially as a result of the myriad of observational studies documenting strong associations between breakfast consumption and the promotion of weight management (2). Despite the fact that breakfast recommendations have recently come under scrutiny due to a postulated lack of causal evidence supporting breakfast for weight and fat loss (3, 4), increasing consumer demand and sales of breakfast foods are climbing in the United States (5). Thus, it is imperative to examine the strength of evidence concerning the consumption of breakfast on health-related outcomes.
Our previous review (6) evaluated intervention-based studies comparing habitual breakfast consumption with breakfast skipping on outcomes related to weight management. Given the paucity of data from long-term randomized controlled breakfast trials, we were unable to support (or refute) the usefulness of daily breakfast consumption to promote weight loss, changes in body composition, or reductions in daily food intake (6). An extension to answering the breakfast–weight management question includes the evaluation of shorter-term, acute trials to assess whether breakfast alters the hormonal and behavioral mechanisms underlying weight management. Therefore, the purpose of this review was to critically evaluate the intervention-based literature examining the effects of breakfast consumption compared with breakfast skipping on appetite, satiety, energy expenditure, and circadian health because each contribute to the regulation of energy balance and thus modulate weight management.
Several dietary factors known to affect the hormonal and behavioral mechanisms will also be explored. These include breakfast meal composition, food form (beverages compared with foods), and breakfast size (i.e., energy content).
Methodology for the Comprehensive Review
Search strategies and terms
Breakfast was defined as the first eating occasion of the day before 1000, whereas skipping breakfast was the omission of any food or beverage besides water until 1000 (6, 7). The search terms included “breakfast,” “breakfast skipping,” and “morning meal” along with the following outcomes: appetite, hunger, satiety, fullness, ghrelin, glucagon-like peptide 1 (GLP-1), peptide YY (PYY), energy expenditure, physical activity, resting metabolism, resting metabolic rate (RMR), thermic effect of food, and postprandial energy expenditure.
Searches in PubMed encompassed all articles published before and including 31 August 2017. References from existing reviews and select articles were examined to supplement the electronic search.
Selection criteria and outcomes
This review was limited to articles published in English and peer-reviewed journals and included the following: 1) human clinical trials [i.e., randomized controlled trials (RCTs), randomized crossover designs, and parallel designs], 2) all age groups, 3) all diseases/conditions, 4) breakfast and breakfast skipping comparisons, and 5) studies including any of the following: a visual analog scale (VAS) for postprandial perceived hunger and fullness; postprandial ghrelin, GLP-1, and PYY concentrations; daily energy expenditure, physical activity, resting metabolism, and RMR; postprandial energy expenditure; and circadian phasing outcomes [including dim-light melatonin onset, circulating melatonin, core body temperature, heart rate (HR), and HR variability]. Circadian phasing refers to the periodicity and timing of circadian rhythms and is used to quantify changes (8). For a more extensive methodologic discussion, see the reviews in references 9–12.
We sought to assess whether breakfast composition, specifically protein quantity at breakfast, affects the outcomes of interest. A higher-protein breakfast was defined as a breakfast that included ≥30 g protein because this was the quantity shown to achieve elevated satiety (13). In addition to protein content, the food form of the breakfast was examined. Beverage breakfasts included only fluids that were consumed by drinking; a solid breakfast included only food items that were eaten; and the mixed breakfast contained beverages, solid foods, and liquids added to solid foods (i.e., milk on cereal). Last, breakfast meal size, based on energy content, as well as habitual breakfast habits were assessed.
Acute trials were defined as ranging from 1 d to 6 wk. Epidemiologic, observational, and cross-sectional studies were excluded. For the perceived and hormonal responses, postprandial individual time points, averages, and AUC analyses were included. The search flow diagrams are presented in Figures 1–3. Details of the study designs and breakfast characteristics were extracted from all included studies.
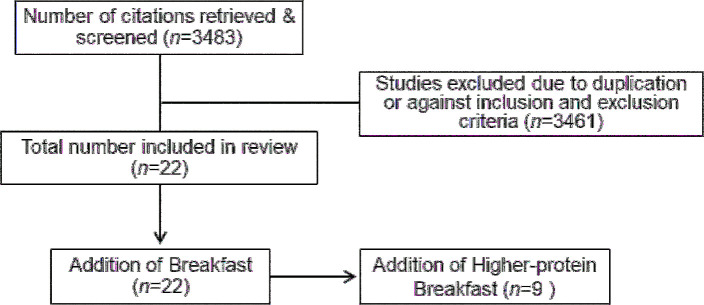
FIGURE 1.
Flow diagram of the article selection process for appetite control and satiety.
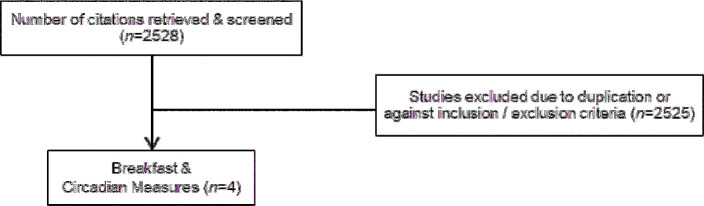
FIGURE 3.
Flow diagram of the article selection process for circadian rhythms.
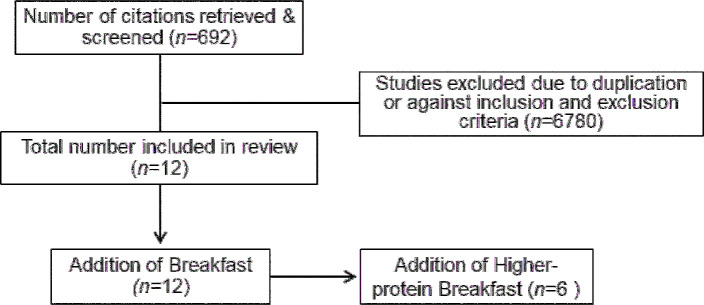
FIGURE 2.
Flow diagram of the article selection process for energy expenditure.
Appetite control and satiety
The majority of data assessing the effects of breakfast on indexes of appetite control and satiety originated from single-meal, acute, crossover-design trials that included pre- and postprandial VAS questionnaires for perceived hunger and fullness and/or serum or plasma ghrelin, PYY, or GLP-1 responses typically collected over 4 h. Twenty-two studies comprised this section and included 49 breakfast and breakfast skipping comparisons. Twenty-one studies reported VAS responses and 8 studies examined ≥1 hormonal response (Table 1, extended detail provided in Supplemental Table 1).
TABLE 1.
Acute and longer-term trials investigating the effects of breakfast consumption on appetite and satiety outcomes1
| Breakfast elicited (↑, ↓, or Ø) response vs. breakfast skipping | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| First author (reference) | Trial type (duration oftreatment) | Habitual breakfast habit | Breakfast composition, kcal (protein/CHO/fat, g) | Breakfast form | Hunger/appetite | Fullness/satiety | Ghrelin | PYY | GLP-1 |
| Allerton (14) | R-Cross (1 d) | Unknown | 430 (9/93/2) | Mixed | ↓ | ↑ | — | — | — |
| 520 (22/95/2) | ↓ | ↑ | — | — | — | ||||
| Astbury (15) | R-Cross (1 d) | Consumers | 260 (9/21/4) | Mixed | ↓ | ↑ | — | — | — |
| Blom (16) | R-Cross (1 d) | Unknown | 180 (15/29/1) | Beverages | Ø | Ø | Ø | — | — |
| 640 (13/146/1) | ↓ | ↑ | ↓ | — | — | ||||
| 640 (13/146/1) | ↓ | ↑ | ↓ | — | — | ||||
| Chowdhury (17) | R-Cross (1 d) | Consumers and skippers | 520 (7/91/10) | Mixed | ↓ | — | ↓ | ↑ | — |
| Clayton (18) | R-Cross (1 d) | Consumers | 740 (20/130/14) | Mixed | ↓ | ↑ | Ø | — | ↑ |
| de Graaf (19) | R-Cross (1 d) | Unknown | 400 (1/99/1) | Beverages | Ø | — | — | — | — |
| 400 (3/5/92) | Ø | — | — | — | — | ||||
| 400 (70/27/3) | ↓ | — | — | — | — | ||||
| 250 (1/62/0) | Ø | — | — | — | — | ||||
| 250 (2/3/58) | Ø | — | — | — | — | ||||
| 250 (44/17/2) | ↓ | — | — | — | — | ||||
| 100 (0/25/0) | Ø | — | — | — | — | ||||
| 100 (1/1/23) | Ø | — | — | — | — | ||||
| 100 (18/7/1) | Ø | — | — | — | — | ||||
| Defeyter (20) | R-Cross (1 d) | Skippers | 180 (9/32/2) | Mixed | ↓ | ↑ | — | — | — |
| Farshchi (21) | R-Cross (14 d) | Consumers | 250 (11/41/5) | Beverages | Ø | Ø | — | — | — |
| Gottero (22) | R-Cross (1 d) | Unknown | 400 (13/45/19) | Mixed | — | Ø | — | — | |
| 400 (0/100/0) | Beverages | — | Ø | — | — | ||||
| Hutchison (23) | R-Cross (1 d) | Unknown | 130 (30/0/0) | Beverages | ↓ | Ø | ↓ | — | ↑ |
| 280 (70/0/0) | ↓ | Ø | ↓ | — | ↑ | ||||
| Irvine (24) | R-Cross (1 d) | Unknown | 250 (4/39/9) | Beverages | Ø | Ø | — | — | — |
| 250 (20/39/2) | ↓ | Ø | — | — | — | ||||
| Kral (25) | R-Cross (1 d) | Consumers | 350 (11/69/4) | Mixed | ↓ | ↑ | — | — | — |
| 350 (10/68/5) | ↓ | ↑ | — | — | — | ||||
| 350 (12/69/4) | ↓ | ↑ | — | — | — | ||||
| Leidy (26) | R-Cross (1 d) | Skippers | 510 (18/95/8) | Foods | ↓ | Ø | Ø | ↑ | — |
| 510 (49/63/8) | ↓ | ↑ | Ø | ↑ | — | ||||
| Leidy (3) | R-Cross (7 d) | Skippers | 350 (13/57/8) | Mixed | ↓ | ↑ | Ø | Ø | — |
| 350 (35/35/8) | Foods | ↓ | ↑ | ↓ | ↑ | — | |||
| Leidy (27) | RCT (12 wk) | Skippers | 350 (13/57/8) | Mixed | Ø | Ø | — | — | — |
| 350 (35/35/8) | Foods | ↓ | Ø | — | — | — | |||
| Levitsky (28) | R-Cross (1 d) | Consumers and skippers | 340 (9/74/<1) | Mixed | ↓ | — | — | — | — |
| 340 (15/64/2) | ↓ | — | — | — | — | ||||
| 620 (18/121/12) | ↓ | — | — | — | — | ||||
| Neumann (29) | Parallel (8 d) | Skippers | 350 (10/59/8) | Foods | Ø | ↑ | — | — | — |
| 350 (30/39/8) | Ø | ↑ | — | — | — | ||||
| Rains (30) | R-Cross (1 d) | Consumers | 288 (3/44/11) | Foods | ↓ | ↑ | — | — | — |
| 280 (30/13/12) | ↓ | ↑ | — | — | — | ||||
| 294 (38/3/14) | ↓ | ↑ | — | — | — | ||||
| Stewart (31) | R-Cross (1 d) | Consumers | 340 (10/77/2) | Mixed | ↓ | ↑ | — | — | — |
| Thomas (32) | R-Cross (1 d) | Consumers and skippers | 500 (29/75/18) | Mixed | Ø | Ø | Ø | ↑ | ↑ |
| Veasey (33) | R-Cross (1 d) | Unknown | 240 (9/38/4) | Mixed | ↓ | ↑ | — | — | — |
| 120 (6/30/3) | ↓ | ↑ | — | — | — | ||||
| Vozzo (34) | R-Cross (1 d) | Unknown | 700 (25/107/20) | Foods | Ø | — | — | — | — |
| 700 (25/73/31) | Ø | — | — | — | — | ||||
| 700 (51/78/18) | Ø | — | — | — | — | ||||
1CHO, carbohydrate; GLP-1, glucagon-like peptide 1; PYY, peptide YY; R-Cross, randomized crossover trial; RCT, randomized controlled trial; ↑, increased response; ↓, decreased response; Ø, no difference in response.
The majority (66%) of the study comparisons showed postprandial reductions in hunger when breakfast was consumed compared with when it was skipped. Similarly, postprandial fullness increased in 68% of the study comparisons when breakfast was consumed instead of skipped. Although postprandial ghrelin was assessed in 14 comparisons, postprandial PYY and/or GLP-1 measures were only included in 6 and 4 comparisons, respectively. Forty-three percent of the comparisons reported greater reductions in postprandial ghrelin after breakfast compared with after skipping breakfast, whereas ≥80% showed increased postprandial PYY and/or GLP-1 concentrations. These findings suggest that breakfast consumption modulates ingestive behavior through enhanced satiety as evidenced by increased fullness and associated satiety hormones.
Experimental design and duration are key factors when determining the strength of evidence. To date, only 1 long-term RCT and only 2 subchronic (i.e., ≥1 wk) randomized crossover trials have examined the effects of breakfast compared with breakfast skipping on these outcomes. The remaining studies included single-day, acute randomized crossover trials.
Farshchi et al. (21) conducted a randomized crossover trial in which 10 healthy, breakfast-consuming women [mean ± SD age: 25.5 ± 5.7 y; mean ± SD BMI (kg/m2): 23.2 ± 1.6] consumed 250-kcal breakfasts or skipped breakfast for 14 d/pattern (pattern is defined as the dietary treatment and is the pattern of consumption within the study design). At the end of each pattern, the participants completed repeated VAS measures of hunger and fullness collected every 30 min for 3 h. No differences in postprandial hunger and fullness were observed after breakfast consumption compared with skipping breakfast. However, the small sample size and relatively small breakfast energy content may have limited the ability to detect differences. Furthermore, the participants were given a midmorning snack (∼200 kcal) each testing day. It is possible, although speculative, that the snack differentially influenced the postprandial responses between breakfast patterns because the snack initiated “breaking the fast” during the breakfast skipping trial.
In a contrasting population, Leidy et al. (3) completed a randomized crossover study examining the effects of breakfast consumption in 20 overweight, breakfast-skipping, adolescent girls (age: 19 ± 1 y; BMI: 28.6 ± 0.7). The participants consumed 2 breakfast meals, similar in energy (i.e., 350 kcal) but varying in macronutrient content, for 6 d/pattern or continued to skip breakfast. At the end of each period, a 10-h testing day was conducted and included repeated VAS questionnaires and plasma blood sampling across the day. Daily hunger and ghrelin responses were reduced, whereas daily fullness and PYY responses were increased after breakfast compared with breakfast skipping; however, these responses were dependent on the breakfast composition. Although both breakfast meals increased fullness compared with breakfast skipping, the higher-protein breakfast treatment elicited greater increases in fullness than the normal-protein version. In addition, only the higher-protein breakfast elicited improvements in PYY and ghrelin responses compared with skipping breakfast.
Last, Leidy et al. (27) completed a 12-wk RCT in 57 breakfast-skipping adolescents (age: 19 ± 1 y; BMI: 29.7 ± 4.6) who were provided with higher-protein breakfasts or normal-protein breakfasts or continued skipping breakfast. Baseline and poststudy 3-d free-living assessments of hourly hunger and fullness were collected. The consumption of breakfast, particularly the higher-protein version, led to reductions in daily hunger compared with breakfast skipping; however, the normal-protein breakfast did not. No differences in fullness were detected between the breakfast groups.
Collectively, the majority of study comparisons (67%) showed ≥1 improvement in select indexes of appetite and satiety after the consumption of breakfast compared with skipping. Moreover, none of the comparisons reported deleterious effects of breakfast consumption, such that all findings indicated either positive or neutral effects.
Point of consideration: breakfast meal protein content
Increased dietary protein at breakfast is of interest given the documented satiety effects of consuming ≥30 g protein (35). Of the 22 studies within this review, 8 studies (3, 19, 23, 26, 29, 30, 34, 36) included higher-protein breakfast and breakfast skipping comparisons. All but one study (34) reported improvements in ≥1 of the outcomes of interest after the higher-protein breakfast compared with breakfast skipping. The 5 studies that included a normal-protein breakfast comparison reported greater improvements in ≥1 of the appetitive and/or hormonal responses after a higher-protein breakfast compared with normal-protein versions (3, 19, 26, 30, 36). Regardless, the limited number of studies reduce the overall strength of evidence and suggest that further investigation is warranted.
Point of consideration: breakfast food form
Meal replacement beverages are frequently promoted as part of a reduced-calorie diet for weight loss and weight management (37). However, when compared with solid foods, beverages elicit a weaker satiety effect, reduced dietary compensation, and greater subsequent energy intake, possibly due to the faster digestive and absorptive rates of beverages (38–41). Of the 22 studies reviewed, 5 included beverage breakfasts (16, 19, 21, 23, 34). The beverage meals led to minimal to no effects on postprandial hunger and fullness compared with the solid or mixed meals. When the studies containing beverage meals were removed, the impact of breakfast consumption on appetite and satiety strengthened compared with breakfast skipping. Collectively, the evidence supports the inclusion of solid foods at breakfast to elicit improvements in appetite control and satiety.
Point of consideration: breakfast meal size
Meal size may also be a factor to consider. Work from Lombardo et al. (42) showed that consuming larger meals earlier in the day led to greater weight loss after a 3-mo intervention than consuming larger meals later in the day. Two studies within the current review directly compared lower-calorie with higher-calorie meals (16, 19). Blom et al. (16) examined lower-calorie (i.e., 180 kcal) and higher-calorie (i.e., 640 kcal), carbohydrate-rich breakfast meals in 20 healthy men. The larger breakfast led to greater reductions in postprandial hunger and ghrelin responses and greater increases in postprandial fullness than the lower-calorie version. de Graaf et al. (19) compared 100-, 200-, and 450-kcal breakfasts containing only carbohydrates, fats, or proteins in 29 healthy females. No differences in postprandial hunger among meals varying in energy content were detected. Because beverage breakfasts not mixed meals were utilized, identification of meal-size effects is problematic. Regardless, in comparing the appetite and satiety responses after lower-calorie breakfasts (i.e., <200 kcal) and breakfast skipping, half of the studies showed improvements in the appetite and satiety outcomes, whereas the remaining half reported no differences.
Further examination of the energy and macronutrient content as well as food form indicated that breakfast meals containing a larger amount of energy (≥350 kcal/meal) and protein (≥30 g protein/meal) and provided as solid foods increased the magnitude of the appetite and satiety response compared with breakfast skipping (3, 26, 27). However, due to the small number of studies including these comparisons, the recommendation to consume larger, higher-protein solid breakfasts should be viewed as preliminary.
Point of consideration: breakfast consumption habits
Last, habitual breakfast patterns might be an important moderating factor often left unaddressed. To our knowledge, only Thomas et al. (32) has directly compared appetite and satiety responses between habitual breakfast consumers with breakfast skippers. The breakfast consumers reported greater hunger and lower fullness during breakfast skipping compared with when the breakfast skippers continued to skip. Although these data are from a single study and have not been replicated in the literature, this work suggests a need to consider breakfast habits when determining the appetitive effects of breakfast. Schlundt et al. (43) also showed that individuals with the most substantial change in their breakfast habits lost the greatest weight over 3 mo, lending further support for the potential moderation of breakfast effects by habitual dietary behaviors.
In the current review, nearly half of the studies included habitual breakfast consumption as subject inclusion criteria. Five studies targeted habitual breakfast skippers (i.e., consumed breakfast ≤2 d/wk) (3, 20, 26, 29, 36) and 6 included breakfast consumers (i.e., consumed breakfast ≥5 d/wk) (15, 18, 21, 25, 30, 31). The remaining studies either documented or did not report habitual breakfast habits. It is possible, albeit untested, that the effects of breakfast consumption are more robust in breakfast skippers simply due to the significant change in eating behavior upon waking. When comparing all postprandial responses on the basis of breakfast habit, no differences were detected. We conclude that additional work directly comparing the responses of breakfast consumers with breakfast skippers is required.
In summary, the majority of studies reported improvements in appetite control and satiety indexes after the consumption of breakfast compared with skipping breakfast. However, the limited number of RCTs and variability in breakfast composition, size, and food form across studies might contribute to discrepant findings.
Energy expenditure
Another key component of energy balance and weight management includes the assessment of energy expenditure. Table 2 (expanded detail provided in Supplemental Table 2) includes the energy expenditure-related outcomes when breakfast is consumed compared with when it is skipped.
TABLE 2.
Acute and long-term trials investigating the effects of breakfast consumption on energy expenditure1
| Breakfast elicited (↑, ↓, or Ø) response vs. breakfast skipping | |||||||
|---|---|---|---|---|---|---|---|
| First author (reference) | Trial type (duration oftreatment) | Habitual breakfast habit | Breakfast composition, kcal (protein/CHO/fat, g) | Food form | Resting energy expenditure | Postprandial energy expenditure | Physical activity |
| Betts (44) | R-Cross (6 wk) | Consumers and skippers | ≥700 (varied) | Mixed | Ø | ↑ | ↑ |
| Bo (45) | R-Cross (7 d) | Unknown | 1168 (88/114/40) | Foods | Ø | ↑ | — |
| Chowdhury (17) | RCT (6 wk) | Consumers and skippers | ≥700 (varied) | Mixed | Ø | ↑ | Ø |
| Clayton (18) | R-Cross (1 d) | Consumers | 724 (19.5/130/14) | Mixed | ↑ | ↑ | Ø |
| Halsey (46) | R-Cross (3 d) | Consumers | Ad libitum | Mixed | — | — | Ø |
| Karst (47) | R-Cross (1 d) | Unknown | 239 (0/60/0) | Beverages | — | Ø | — |
| 239 (60/0/0) | — | ↑ | — | ||||
| 239 (60/0/0) | — | ↑ | — | ||||
| 239 (60/0/0) | — | ↑ | — | ||||
| Kobayashi (48) | R-Cross (1 d) | Breakfast | 689 ± 492 (32/98/16) | Not Reported | Ø | ↑ | Ø |
| — | — | — | |||||
| Nair (49) | NR-Cross (1 d) | Unknown | 300 (0/75/0) | Beverages | — | ↑ | — |
| 300 (0/0/33) | — | ↑ | — | ||||
| 300 (75/0/0) | — | ↑ | — | ||||
| Neumann (29) | R-Cross (8 d) | Skippers | 350 (10/59/8) | Foods | Ø | ↑ | — |
| 350 (30/39/8) | Ø | ↑ | — | ||||
| Reeves (50) | R-Cross (7 d) | Consumers and skippers | Bf: 544 (unknown) | Mixed | Ø | Ø | Ø |
| Steiniger (51) | P (1 d) | Unknown | Bf1: 239 (60/0/0) | Beverages | Ø | ↑ | — |
| Bf2: 480 (120/0/0) | Ø | ↑ | — | ||||
| Welle (52) | R-Cross (1 d) | Unknown | Bf1: 400 (0/100/0 | Beverages | — | ↑ | — |
| Bf2: 400 (0/0/44) | — | ↑ | — | ||||
| Bf3: 400 (100/0/0) | — | ↑ | — | ||||
1Bf, Breakfast, CHO, carbohydrate; NR-Cross, non-randomized crossover trial; P, parallel trial; R-Cross, randomized crossover trial; RCT, randomized controlled trial.
2Mean ± SD.
Compared with skipping breakfast, the addition of breakfast increased postprandial energy expenditure in most (17, 18, 29, 44, 45, 48, 49, 51, 52), but not all (47, 50), studies. When extrapolated across 24 h, the increase in postprandial energy expenditure after breakfast intake ranged from ∼40 to 200 kcal/d and varied according to breakfast composition.
Eight studies examined whether breakfast consumption increased RMR compared with breakfast skipping (17, 18, 29, 44, 45, 48, 50, 51). RMR was measured via 24-h chamber-based indirect calorimetry (48, 51) as well indirect calorimetry across single and multiple time points (17, 29, 44, 50). Regardless, 7 of 8 studies reported no difference between breakfast pattern (17, 29, 44, 45, 48, 50, 51). Clayton et al. (18) was the only study to show increased RMR after breakfast consumption compared with breakfast skipping; however, this measurement only spanned a 2.5-h postbreakfast period.
The most variable component of energy expenditure is physical activity, which includes both exercise and nonexercise activity thermogenesis (NEAT). Our search captured 6 studies that examined the effects of breakfast on these outcomes (17, 18, 44, 46, 48, 50). All studies except for one (44) concluded there were no effects of breakfast on physical activity. The exception, Betts et al. (44), included a combination of HR monitoring and accelerometry rather than only pedometry or accelerometry, which may have increased the measure’s sensitivity. The trial showed greater 24-h physical activity after 6 wk of consuming breakfast (mean ± SD: 1449 ± 666 kcal/d) than after skipping breakfast (1007 ± 370 kcal/d) (P < 0.05). The additional 400 kcal expended was primarily from increased NEAT across morning hours (44). Collectively, the strength of evidence as to whether breakfast improves energy expenditure components is poor and should not be considered a primary mechanism (of breakfast) for weight management.
Finally, whether breakfast meals containing higher quantities of protein result in increased energy expenditure compared with skipping breakfast is relatively unexplored. Our search identified 7 trials (29, 45, 47–49, 51, 52), 6 of which showed greater postprandial energy expenditure after the consumption of a higher-protein breakfast compared with breakfast skipping or compared with a normal-protein breakfast. Interpretation limitations include the composition and type of breakfasts. For example, most of the studies within this section of the review used only protein and were beverage breakfasts. Thus, the current evidence is only supportive of beverage breakfasts composed of protein.
Circadian rhythms
Circadian clocks are a complex system of rhythms within the body that promote synchrony of biological processes (8). Nutrient timing is established as one potent synchronizer of circadian systems, and alterations (i.e., meal-skipping) may affect the regulation of metabolism, ingestive behavior, weight management, and sleep (53–56) and increase chronic disease risk (57, 58). Because breakfast is known to have positive regulatory effects on metabolism, it is plausible that breakfast consumption may positively affect these systems.
Only 4 studies were identified. Qin et al. (59) completed a randomized crossover study in which 7 healthy young adults (age: 21.7 ± 1.3 y; BMI: 22.9 ± 3.0) either maintained 3 meal times or skipped the morning meal and snacked late for 21 d/pattern. Plasma melatonin, a robust circadian marker involved in establishing the sleep-wake cycle, was assessed across the overnight hours. Breakfast consumption led to greater plasma melatonin concentrations across the overnight hours (all, P < 0.01) compared with breakfast skipping. The blunted melatonin response after the breakfast skipping trial resembled that which is observed in individuals with clinically diagnosed sleep disturbances (i.e., nighttime eating syndrome), suggesting a protective effect with breakfast consumption.
Kräuchi et al. (60) examined the effects of consuming breakfast (1600 kcal; protein: 44 g; carbohydrate: 300 g; fat: 25 g) compared with skipping breakfast. Breakfast led to beneficial changes in time to peak core body temperature and HR rhythm–related outcomes compared with skipping breakfast. However, dim-lit melatonin onset, a primary phase indicator of the central circadian pacemaker, was not different between treatments.
Pivik et al. (61) conducted a randomized parallel trial assessing morning resting HR and HR variability after the consumption of a 340-kcal (protein: 14 g; carbohydrate: 57 g; fat: 6 g) breakfast or breakfast skipping. Breakfast consumption increased morning HR but decreased HR variability compared with breakfast skipping (all, P < 0.05).
Last, Yoshizaki et al. (62) assessed whether a standard meal pattern, including breakfast, compared with breakfast skipping and a late-night meal altered HR variability over a 2-wk period. Breakfast consumption led to a phase shift of HR variability compared with breakfast skipping, indicating that meal timing affects the circadian rhythmicity of the autonomic nervous system.
Although limited, the evidence suggests that breakfast (skipping) has the potential to dysregulate circadian rhythms and autonomic balance (63, 64), contributing to reduced weight management (54) and increased risk of chronic diseases. However, in addition to the small number of studies, there is a lack of control for and/or documentation of the quality, size, and type of breakfasts included within the study protocols. Thus, a conclusive understanding of how these factors may affect circadian-related outcomes and downstream eating behaviors remains unclear.
After completion of the current review search, we discovered a recent publication concerning breakfast and peripheral clock genes and felt that a brief discussion of the findings was pertinent. Jakubowicz et al. (65) completed a randomized crossover study assessing the effects of breakfast (572 kcal; protein: 46 g; carbohydrate: 70 g; fat: 12 g) compared with breakfast skipping on peripheral clock genes (i.e., period circadian clock 1, cryptochrome circadian clock 1, and clock circadian regulator) known to influence metabolism (56). Breakfast consumption led to a maintenance of clock gene oscillations, whereas breakfast skipping led to adverse changes (i.e., dysregulation of the normal cyclic upregulation and downregulation). This study provides the first direct evidence, to our knowledge, that breakfast consumption effects circadian clock and clock-controlled gene expression.
Future breakfast research agenda—sleep and ingestive behavior interactions
Over the past few years, there has been a heightened focus in the interactions between unhealthy eating patterns, poor sleep health, and chronic diseases, including obesity and type 2 diabetes (66, 67). Of particular interest is the cross-sectional NHANES analysis by Kant and Graubard (68) in which adults with poor sleep duration (i.e., <6 h/night) skipped breakfast, ate later in the day, and had a greater contribution of daily energy intake from snacking occasions compared with adults with healthy sleep duration (7–8 h/night) (all, P < 0.05). Two other trials (59, 69) similarly showed that shortened sleep was associated with an increase in unhealthy patterns, particularly of skipping breakfast.
Although sleep appears to influence eating behaviors, it is possible that eating behaviors affect sleep health. This potential bidirectional relation is supported by the observations that eating near sleep onset is negatively associated with poor sleep quality and shortened sleep (68, 70, 71). Delayed intake may dysregulate sleep-wake cycles and peripheral circadian processes, thus feeding-forward to induce sleep disturbances. Although untested, it is possible that breakfast consumption may indirectly modulate sleep health because previous findings document a protective effect against increased snacking behavior later in the afternoon/evening when breakfast is consumed or skipped (3).
Thus, we conducted a randomized, crossover, pilot study examining the effects of breakfast compared with skipping breakfast on sleep health in 13 young healthy adults (72). Perceived sleep quality and sleep onset tended to improve after breakfast consumption compared with breakfast skipping (P = 0.06 and P = 0.07, respectively). Furthermore, sleep duration and sleep quality were inversely associated with evening snacking (r = –0.623, P < 0.001, and r = –0.505, P < 0.009, respectively). Future breakfast intervention research in overweight or obese habitual breakfast skippers with documented poor sleep health will allow us to determine whether eating behaviors may be optimized to improve sleep and healthy eating and promote overall health.
Summary and Conclusions
The present review examined the effects of breakfast consumption and composition on appetite and satiety and energy expenditure as underlying mechanisms of weight management. The findings showed modest support for the consumption of breakfast for appetite control and satiety. Further investigation of breakfast consumption utilizing longer-term interventions accounting for study duration, meal composition and size, and habitual breakfast behaviors is required to determine the beneficial effects of breakfast to promote health.
Supplementary Material
Acknowledgments
Both authors were responsible for the design, analyses, and interpretation of the information presented in this review, and read and approved the final manuscript.
Notes
Supported by internal funding and the Kellogg Company.
Author disclosures: JAG and HJL, no conflicts of interest.
Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used:
- GLP-1
glucagon-like peptide 1
- HR
heart rate
- PYY
peptide YY
- RCT
randomized controlled trial
- RMR
resting metabolic rate
- VAS
visual analog scale
References
- 1. Pham J, Pelletier D. Action-oriented population nutrition research: high demand but limited supply. Glob Health Sci Pract 2015;3:287–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Odegaard AO, Jacobs DR, Steffen LM, Van Horn L, Ludwig DS, Pereira MA. Breakfast frequency and development of metabolic risk. Diabetes Care 2013;36:3100–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leidy HJ, Ortinau LC, Douglas SM, Hoertel HA. Beneficial effects of a higher-protein breakfast on the appetitive, hormonal, and neural signals controlling energy intake regulation in overweight/obese, "breakfast-skipping," late-adolescent girls. Am J Clin Nutr 2013;97:677–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown AW, Bohan Brown MM, Allison DB. Belief beyond the evidence: using the proposed effect of breakfast on obesity to show 2 practices that distort scientific evidence. Am J Clin Nutr 2013;98:1298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. NPD Group, Inc. U.S. consumers love their breakfasts and morning snacks whether in- or away-from-home. 2016. Available from: https://www.npd.com/wps/portal/npd/us/news/press-releases/2016/us_consumers_love_their_breakfasts_and_morning_snacks_whether_in_or_away_from/. Accessed February 29, 2018. [Google Scholar]
- 6. Leidy HJ, Gwin JA, Roenfeldt CA, Zino AZ, Shafer RS. Evaluating the intervention-based evidence surrounding the causal role of breakfast on markers of weight management, with specific focus on breakfast composition and size. Adv Nutr 2016;7(Suppl):563S–75S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Timlin MT, Pereira MA. Breakfast frequency and quality in the etiology of adult obesity and chronic diseases. Nutr Rev 2007;65:268–81. [DOI] [PubMed] [Google Scholar]
- 8. Vitaterna MH, Takahashi JS, Turek FW. Overview of circadian rhythms. Alcohol Res Health 2001;25:85–93. [PMC free article] [PubMed] [Google Scholar]
- 9. Pandi-Perumal SR, Smits M, Spence W, Srinivasan V, Cardinali DP, Lowe AD, Kayumov L. Dim light melatonin onset (DLMO): a tool for the analysis of circadian phase in human sleep and chronobiological disorders. Prog Neuro Psychopharmacol Biol Psychiatry 2007;31:1–11. [DOI] [PubMed] [Google Scholar]
- 10. Refinetti R, Menaker M. The circadian rhythm of body temperature. Physiol Behav 1992;51:613–37. [DOI] [PubMed] [Google Scholar]
- 11. Huang W, Ramsey KM, Marcheva B, Bass J. Circadian rhythms, sleep, and metabolism. J Clin Investig 2011;121:2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hofstra WA, de Weerd AW. How to assess circadian rhythm in humans: a review of literature. Epilepsy Behav 2008;13:438–44. [DOI] [PubMed] [Google Scholar]
- 13. Paddon-Jones D, Leidy H. Dietary protein and muscle in older persons. Curr Opin Clin Nutr Metab Care 2014;17:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Allerton DM, Campbell MD, Gonzalez JT, Rumbold PL, West DJ, Stevenson EJ. Co-ingestion of whey protein with a carbohydrate-rich breakfast does not affect glycemia, insulinemia or subjective appetite following a subsequent meal in healthy males. Nutrients 2016;8:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Astbury NM, Taylor MA, Macdonald IA. Breakfast consumption affects appetite, energy intake, and the metabolic and endocrine responses to foods consumed later in the day in male habitual breakfast eaters. J Nutr 2011;141:1381–9. [DOI] [PubMed] [Google Scholar]
- 16. Blom WA, Stafleu A, de Graaf C, Kok FJ, Schaafsma G, Hendriks HF. Ghrelin response to carbohydrate-enriched breakfast is related to insulin. Am J Clin Nutr 2005;81:367–75. [DOI] [PubMed] [Google Scholar]
- 17. Chowdhury EA, Richardson JD, Holman GD, Tsintzas K, Thompson D, Betts JA. The causal role of breakfast in energy balance and health: a randomized controlled trial in obese adults. Am J Clin Nutr 2016;103:747–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clayton DJ, Stensel DJ, James LJ. Effect of breakfast omission on subjective appetite, metabolism, acylated ghrelin and GLP-1 7–36 during rest and exercise. Nutrition 2016;32:179–85. [DOI] [PubMed] [Google Scholar]
- 19. de Graaf C, Hulshof T, Weststrate JA, Jas P. Short-term effects of different amounts of protein, fats, and carbohydrates on satiety. Am J Clin Nutr 1992;55:33–8. [DOI] [PubMed] [Google Scholar]
- 20. Defeyter MA, Russo R. The effect of breakfast cereal consumption on adolescents' cognitive performance and mood. Front Hum Neurosci 2013;7:789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Farshchi HR, Taylor MA, Macdonald IA. Deleterious effects of omitting breakfast on insulin sensitivity and fasting lipid profiles in healthy lean women. Am J Clin Nutr 2005;81:388–96. [DOI] [PubMed] [Google Scholar]
- 22. Gottero C, Bellone S, Rapa A, Van Koetsveld P, Vivenza D, Prodam F, Benso A, Destefanis S, Gauna C, Bellone J. Standard light breakfast inhibits circulating ghrelin level to the same extent of oral glucose load in humans, despite different impact on glucose and insulin levels. J Endocrinol Investig 2003;26:1203–7. [DOI] [PubMed] [Google Scholar]
- 23. Hutchison AT, Piscitelli D, Horowitz M, Jones KL, Clifton PM, Standfield S, Hausken T, Feinle-Bisset C, Luscombe-Marsh ND. Acute load-dependent effects of oral whey protein on gastric emptying, gut hormone release, glycemia, appetite, and energy intake in healthy men. Am J Clin Nutr 2015;102:1574–84. [DOI] [PubMed] [Google Scholar]
- 24. Irvine P, Mouzet JB, Marteau C, Salle A, Genaitay M, Favreau AM, Berrut G, Ritz P. Short-term effect of a protein load on appetite and food intake in diseased mildly undernourished elderly people. Clin Nutr 2004;23:1146–52. [DOI] [PubMed] [Google Scholar]
- 25. Kral TV, Whiteford LM, Heo M, Faith MS. Effects of eating breakfast compared with skipping breakfast on ratings of appetite and intake at subsequent meals in 8- to 10-y-old children. Am J Clin Nutr 2011;93:284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leidy H, Racki E. The addition of a protein-rich breakfast and its effects on acute appetite control and food intake in “breakfast-skipping” adolescents. Int J Obes 2010;34:1125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leidy HJ, Hoertel HA, Douglas SM, Higgins KA, Shafer RS. A high‐protein breakfast prevents body fat gain, through reductions in daily intake and hunger, in “breakfast skipping” adolescents. Obesity 2015;23:1761–4. [DOI] [PubMed] [Google Scholar]
- 28. Levitsky DA, Pacanowski CR. Effect of skipping breakfast on subsequent energy intake. Physiol Behav 2013;119:9–16. [DOI] [PubMed] [Google Scholar]
- 29. Neumann BL, Dunn A, Johnson D, Adams J, Baum JI. Breakfast macronutrient composition influences thermic effect of feeding and fat oxidation in young women who habitually skip breakfast. Nutrients 2016;8:490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rains TM, Leidy HJ, Sanoshy KD, Lawless AL, Maki KC. A randomized, controlled, crossover trial to assess the acute appetitive and metabolic effects of sausage and egg-based convenience breakfast meals in overweight premenopausal women. Nutr J 2015;14:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stewart SL, Black RM, Wolever TM, Anderson GH. The relationship between the glycaemic response to breakfast cereals and subjective appetite and food intake. Nutr Res 1997;17:1249–60. [Google Scholar]
- 32. Thomas EA, Higgins J, Bessesen DH, McNair B, Cornier MA. Usual breakfast eating habits affect response to breakfast skipping in overweight women. Obesity 2015;23:750–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Veasey RC, Haskell-Ramsay CF, Kennedy DO, Tiplady B, Stevenson EJ. The effect of breakfast prior to morning exercise on cognitive performance, mood and appetite later in the day in habitually active women. Nutrients 2015;7:5712–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vozzo R, Wittert G, Cocchiaro C, Tan WC, Mudge J, Fraser R, Chapman I. Similar effects of foods high in protein, carbohydrate and fat on subsequent spontaneous food intake in healthy individuals. Appetite 2003;40:101–7. [DOI] [PubMed] [Google Scholar]
- 35. Leidy HJ, Clifton PM, Astrup A, Wycherley TP, Westerterp-Plantenga MS, Luscombe-Marsh ND, Woods SC, Mattes RD. The role of protein in weight loss and maintenance. Am J Clin Nutr 2015;101:1320–9. [DOI] [PubMed] [Google Scholar]
- 36. Leidy HJ, Clifton PM, Astrup A, Wycherley TP, Westerterp-Plantenga MS, Luscombe-Marsh ND, Woods SC, Mattes RD. The role of protein in weight loss and maintenance. Am J Clin Nutr 2015;101(Suppl):1320S–9S. [DOI] [PubMed] [Google Scholar]
- 37. Heymsfield S, Van Mierlo C, Van der Knaap H, Heo M, Frier H. Weight management using a meal replacement strategy: meta and pooling analysis from six studies. Int J Obes 2003;27:537–49. [DOI] [PubMed] [Google Scholar]
- 38. Leidy HJ, Apolzan JW, Mattes RD, Campbell WW. Food form and portion size affect postprandial appetite sensations and hormonal responses in healthy, nonobese, older adults. Obesity (Silver Spring) 2010;18:293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mourao D, Bressan J, Campbell W, Mattes R. Effects of food form on appetite and energy intake in lean and obese young adults. Int J Obes 2007;31:1688–95. [DOI] [PubMed] [Google Scholar]
- 40. Mattes R. Fluid calories and energy balance: the good, the bad, and the uncertain. Physiol Behav 2006;89:66–70. [DOI] [PubMed] [Google Scholar]
- 41. Cassady BA, Considine RV, Mattes RD. Beverage consumption, appetite, and energy intake: what did you expect? Am J Clin Nutr 2012;95:587–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lombardo M, Bellia A, Padua E, Annino G, Guglielmi V, D'Adamo M, Iellamo F, Sbraccia P. Morning meal more efficient for fat loss in a 3-month lifestyle intervention. J Am Coll Nutr 2014;33:198–205. [DOI] [PubMed] [Google Scholar]
- 43. Schlundt DG, Hill JO, Sbrocco T, Pope-Cordle J, Sharp T. The role of breakfast in the treatment of obesity: a randomized clinical trial. Am J Clin Nutr 1992;55:645–51. [DOI] [PubMed] [Google Scholar]
- 44. Betts JA, Richardson JD, Chowdhury EA, Holman GD, Tsintzas K, Thompson D. The causal role of breakfast in energy balance and health: a randomized controlled trial in lean adults. Am J Clin Nutr 2014;100.2:539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bo S, Fadda M, Castiglione A, Ciccone G, De Francesco A Fedele D, Guggino A, Caprino MP, Ferrara S, Boggio MV. Is the timing of caloric intake associated with variation in diet-induced thermogenesis and in the metabolic pattern? A randomized cross-over study. Int J Obes 2015;39:1689–95. [DOI] [PubMed] [Google Scholar]
- 46. Halsey LG, Huber JW, Low T, Ibeawuchi C, Woodruff P, Reeves S. Does consuming breakfast influence activity levels? An experiment into the effect of breakfast consumption on eating habits and energy expenditure. Public Health Nutr 2012;15:238–45. [DOI] [PubMed] [Google Scholar]
- 47. Karst H, Steiniger J, Noack R, Steglich HD. Diet-induced thermogenesis in man: thermic effects of single proteins, carbohydrates and fats depending on their energy amount. Ann Nutr Metab 1984;28:245–52. [DOI] [PubMed] [Google Scholar]
- 48. Kobayashi F, Ogata H, Omi N, Nagasaka S, Yamaguchi S, Hibi M, Tokuyama K. Effect of breakfast skipping on diurnal variation of energy metabolism and blood glucose. Obes Res Clin Pract 2014;8:e201–298. [DOI] [PubMed] [Google Scholar]
- 49. Nair KS, Halliday D, Garrow JS. Thermic response to isoenergetic protein, carbohydrate or fat meals in lean and obese subjects. Clin Sci (Lond) 1983;65:307–12. [DOI] [PubMed] [Google Scholar]
- 50. Reeves S, Huber J, Halsey LG, Villegas-Montes M, Elgumati J, Smith T. A cross-over experiment to investigate possible mechanisms for lower BMIs in people who habitually eat breakfast. Eur J Clin Nutr 2015;69:632–7. [DOI] [PubMed] [Google Scholar]
- 51. Steiniger J, Karst H, Noack R, Steglich HD. Diet-induced thermogenesis in man: thermic effects of single protein and carbohydrate test meals in lean and obese subjects. Ann Nutr Metab 1987;31:117–25. [DOI] [PubMed] [Google Scholar]
- 52. Welle S, Lilavivat U, Campbell RG. Thermic effect of feeding in man: increased plasma norepinephrine levels following glucose but not protein or fat consumption. Metabolism 1981;30:953–8. [DOI] [PubMed] [Google Scholar]
- 53. Tahara Y, Shibata S. Chrono-biology, chrono-pharmacology, and chrono-nutrition. J Pharmacol Sci 2014;124:320–35. [DOI] [PubMed] [Google Scholar]
- 54. Allison KC, Goel N. Timing of eating in adults across the wei ght spectrum: metabolic factors and potential circadian mechanisms. Physiol Behav 2018;192:158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Oike H, Oishi K, Kobori M. Nutrients, clock genes, and chrononutrition. Curr Nutr Rep 2014;3:204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Asher G, Sassone-Corsi P. Time for food: the intimate interplay between nutrition, metabolism, and the circadian clock. Cell 2015;161:84–92. [DOI] [PubMed] [Google Scholar]
- 57. Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci 2003;4:649. [DOI] [PubMed] [Google Scholar]
- 58. Mayeuf-Louchart A, Zecchin M, Staels B, Duez H. Circadian control of metabolism and pathological consequences of clock perturbations. Biochimie 2017. [DOI] [PubMed] [Google Scholar]
- 59. Qin L-Q, Li J, Wang Y, Wang J, Xu J-Y, Kaneko T. The effects of nocturnal life on endocrine circadian patterns in healthy adults. Life Sci 2003;73:2467–75. [DOI] [PubMed] [Google Scholar]
- 60. Kräuchi K, Cajochen C, Werth E, Wirz-Justice A. Alteration of internal circadian phase relationships after morning versus evening carbohydrate-rich meals in humans. J Biol Rhythms 2002;17:364–76. [DOI] [PubMed] [Google Scholar]
- 61. Pivik R, Dykman R, Tennal K, Gu Y. Skipping breakfast: gender effects on resting heart rate measures in preadolescents. Physiol Behav 2006;89:270–80. [DOI] [PubMed] [Google Scholar]
- 62. Yoshizaki T, Tada Y, Hida A, Sunami A, Yokoyama Y, Yasuda J, Nakai A, Togo F, Kawano Y. Effects of feeding schedule changes on the circadian phase of the cardiac autonomic nervous system and serum lipid levels. Eur J Appl Physiol 2013;113:2603–11. [DOI] [PubMed] [Google Scholar]
- 63. Lindmark S, Lönn L, Wiklund U, Tufvesson M, Olsson T, Eriksson JW. Dysregulation of the autonomic nervous system can be a link between visceral adiposity and insulin resistance. Obesity 2005;13:717–28. [DOI] [PubMed] [Google Scholar]
- 64. Harris KF, Matthews KA. Interactions between autonomic nervous system activity and endothelial function: a model for the development of cardiovascular disease. Psychosom Med 2004;66:153–64. [DOI] [PubMed] [Google Scholar]
- 65. Jakubowicz D, Wainstein J, Landau Z, Raz I, Ahren B, Chapnik N, Ganz T, Menaged M, Barnea M, Bar-Dayan Y. Influences of breakfast on clock gene expression and postprandial glycemia in healthy individuals and individuals with diabetes: a randomized clinical trial. Diabetes Care 2017;40:1573–9. [DOI] [PubMed] [Google Scholar]
- 66. Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obesity 2011;19:1374–81. [DOI] [PubMed] [Google Scholar]
- 67. Al Khatib H, Harding S, Darzi J, Pot G. The effects of partial sleep deprivation on energy balance: a systematic review and meta-analysis. Eur J Clin Nutr 2017;71:614. [DOI] [PubMed] [Google Scholar]
- 68. Kant AK, Graubard BI. Association of self-reported sleep duration with eating behaviors of American adults: NHANES 2005–2010. Am J Clin Nutr 2014;100:938–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kim S, DeRoo LA, Sandler DP. Eating patterns and nutritional characteristics associated with sleep duration. Public Health Nutr 2011;14:889–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Crispim CA, Zimberg IZ, dos Reis BG, Diniz RM, Tufik S, de Mello MT. Relationship between food intake and sleep pattern in healthy individuals. J Clin Sleep Med 2011;7:659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yeh S-SS, Brown RF. Disordered eating partly mediates the relationship between poor sleep quality and high body mass index. Eating Behav 2014;15:291–7. [DOI] [PubMed] [Google Scholar]
- 72. Gwin JA, Leidy HJ. A pilot study assessing whether the consumption of a protein-rich breakfast improves appetite control, eating behavior, and sleep quality compared to skipping breakfast in healthy young professionals. FASEB J 2017;31:443-1. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.