Abstract
Introduction
Dickkopf‐1 (DKK1) is a soluble protein and antagonist of the Wnt/β‐catenin signaling pathway. DKK1 is found elevated in serum from patients affected with various types of cancers and in some instances, it is considered a diagnostic and prognostic biomarker. Elevated serum levels of DKK1 have also been detected in animal models of chronic inflammatory diseases. Previous work from our laboratory has demonstrated upregulation of DKK1 in cells and mouse models of the bone marrow failure (BMF) and cancer‐prone disease Fanconi anemia (FA). The present study aimed to investigate whether DKK1 blood levels in patients are associated with FA or inflammatory responses to acute infections.
Methods
Plasma samples were collected from 58 children admitted to the Centre Mère‐Enfant Soleil du Centre Hospitalier de Québec‐Université Laval with signs of acute infections. Blood plasma specimens were also collected from healthy blood donors at the Héma‐Québec blood donor clinic. Plasmas from patients diagnosed with FA were also included in the study. DKK1 levels in blood plasmas were assessed by standard ELISA.
Results
Patients with acute infections showed dramatically high levels of DKK1 (6072 ± 518 pg/ml) in their blood compared to healthy blood donors (1726 ± 95 pg/ml). No correlations were found between DKK1 levels and C reactive protein (CRP) concentration, platelet numbers, or white blood cell counts. Patients with FA showed higher DKK1 plasma levels (3419 ± 147.5 pg/ml) than healthy blood donors (1726 ± 95 pg/ml) but significantly lower than patients with acute infections.
Conclusion
These findings suggest that blood DKK1 is elevated in response to infections and perhaps to inflammatory responses.
Keywords: Blood plasma, Dickkopf‐1, ELISA, Fanconi anemia, infections
Introduction
Dickkopf‐1 (DKK1) is a secretory protein and antagonist of the Wnt/β‐catenin signal pathway 1, 2. Activation of the Wnt/β‐catenin pathway induces expression of the DKK1 gene. Production of DKK1 acts as a feedback mechanism to limit the Wnt/β‐catenin pathway activation. The ability of DKK1 to block Wnt/β‐catenin activity comes from its capability to interact directly with the Wnt co‐receptor LRP5/6 (low density lipoprotein receptor‐related protein 5 or 6) or indirectly by binding with its receptor Kremen‐1/2 and forming a ternary complex with LRP5/6 2, 3, 4, 5, 6, 7, 8. These interactions prevent the formation of an active Wnt‐Frizzled‐LRP5/6 complex. DKK1 plays fundamental roles in embryogenesis and is required for head induction, eye and limb formation, vertebral and bone development 2, 9, 10, 11, 12, 13. DKK1 expression is high during development but is relatively low in most adult tissues. However, overexpression of DKK1 is associated with several diseases that include various types of cancers 2, 14. Increased expression of DKK1 is found in cancer cells, cancer surrounding tissues and elevated levels of DKK1 in peripheral blood are detectable in patients with cancers 15, 16. In fact, blood levels of DKK1 correlate in some cancers with prognosis 16, 17, 18, 19. Consequently, measurement of DKK1 in plasma or serum is viewed as a diagnostic and prognostic biomarker 20. Moreover, elevated levels of DKK1 in peripheral blood are associated with chronic inflammatory diseases 21.
Interestingly, we previously reported overexpression of DKK1 in cells derived from Fanconi anemia (FA) patients and elevated levels of Dkk1 in blood of FA mutant mice 22. FA is a BMF syndrome associated with congenital malformations and cancer predisposition 23, 24. FA is associated with 22 subtypes (FANC‐A to W) and characterization of the related FA genes has led to the identification of a molecular pathway known as the FA pathway 25, 26. This pathway is a guardian of genome integrity during cellular division 26. In addition, several FA proteins act in other cellular functions including regulation of transcription, response to viral infections and oxidative stress 23. Physiological stresses such as infection‐associated inflammation in FA mutant mice lead to BMF and in part recapitulate the human disease FA 27, 28.
Given that DKK1 is dysregulated in cells and mouse models of FA, that inflammation in FA leads to BMF and that DKK1 is activated in response to inflammation, we hypothesized that DKK1 levels increase in response to infections with or without accompanying inflammation. We thus evaluated DKK1 levels in peripheral blood from children affected by acute infections in comparison to patients with BMF including FA.
Methods
Study design and patients
Children admitted to the Centre Mère‐Enfant Soleil du Centre Hospitalier de Québec‐Université Laval (CHU) with signs of acute infections were recruited and included in the study. Inclusion criteria consisted of patients aged 1 month to 17 years showing signs of infections. Exclusion criteria comprised patients suffering from cancer, anemia, or any other hematological abnormalities. Complete blood counts and CRP levels were analyzed as part of the clinical evaluation. Informed consent was obtained from each patient or parent. The study protocol was approved by the CHU Ethical review board. Blood plasma from healthy donors (Controls) were obtained from the Héma‐Québec blood donor clinics after informed consent according to Héma‐Québec guidelines. Plasma samples previously obtained from patients with BMF that were subsequently diagnosed with FA or excluded from FA (BMF) were collected over several years from Germany patients within the framework of FA diagnostics following informed consent and approval by the Institutional Ethical review boards.
ELISA
Plasma from patients and donors was subjected to an enzyme‐linked immunosorbent assay (Human DKK‐1 Quantikine ELISA kit, DKK100; R&D Systems, Minneapolis, MN) to evaluate DKK1 concentration according to the manufacturer's instructions. Each sample was analyzed in duplicate.
Statistical analyses
Sample size calculation was performed to obtain significant differences in DKK1 levels between populations with a power of >0.8 and a p = 0.05 with a minimum of 50 subjects per group. DKK1 levels were expressed as the means ± standard errors of the mean (SEM). Statistical analyses were performed with GraphPad Prism software (La Jolla, CA, USA) (version 5.0b), and the tests used included linear regression, Pearson's correlation, and two‐tailed Student's t‐tests. Results with a p values less than 0.05 were considered significant.
Results
DKK1 overproduction in children with infectious diseases
Blood plasma was collected from a total of 57 children, 33 males, and 24 females, aged 1 month to 15 years (Table 1). These patients suffered from the different infections listed in Table 2. Plasma DKK1 levels were analyzed from blood samples obtained at the time of admission as part of the clinical evaluation. DKK1 levels in patients with acute infections were found dramatically elevated (mean of 6072 ± 518 pg/ml) compared with 107 healthy blood donors (1771 ± 95 pg/ml; Fig. 1A). No significant correlations were observed between levels of DKK1 and age, gender, levels of CRP, white blood cell counts, neutrophils, platelets, or haemoglobin (Fig. 1B–G). Also, the type of infection did not seem to influence DKK1 production, suggesting that no specific pattern‐recognition receptors critical for the host defence system are involved.
Table 1.
DKK1 levels in blood of patients with infections or hematological disorders
| Variables | Control | Infections | FA | BMF |
|---|---|---|---|---|
| Number of patients | 107 | 57 | 98 | 58 |
| Males | 54 | 33 | 56 | 26 |
| Females | 53 | 24 | 42 | 32 |
| Age at draw (range in years) | 18–45 | 0.08–15 | 0.08–37 a | 0.08–64 |
| DKK1 values (pg/ml) | ||||
| Mean | 1771 | 6072 | 3465 | 4575 |
| Median | 1595 | 5391 | 3212 | 4134 |
| SD | 979 | 3912 | 1888 | 2755 |
SD, standard deviation.
Seven patients with missing age at draw.
Table 2.
DKK1 levels in children with infectious diseases
| DKK1 levels a | ||
|---|---|---|
| Variables | High (>5391 pg/ml) | Low (<5391 pg/ml) |
| No Patients | 29 | 28 |
| Male/female | 16/13 | 17/11 |
| Age groups | ||
| >1 yr | 14 | 9 |
| ≤1 yr | 15 | 19 |
| Types of infections | ||
| Bronchiolitis | 8 | 3 |
| Hyperthermia/fever | 2 | 6 |
| Gastroenteritis | 2 | – |
| Pneumonia | 8 | 7 |
| Viral infection b | 4 | 3 |
| Cellulitis | 1 | 2 |
| Pyelonephritis | – | 3 |
| Sepsis | 1 | 1 |
| Adenitis | 1 | 2 |
| Skin abscess | 1 | – |
| Mononucleosis | 1 | |
| Otitis | – | 1 |
High and low DKK1 levels based on one SD.
Viral infections: undefined viral infections, parotitis, upper respiratory tract infections of unknown origin.
Figure 1.
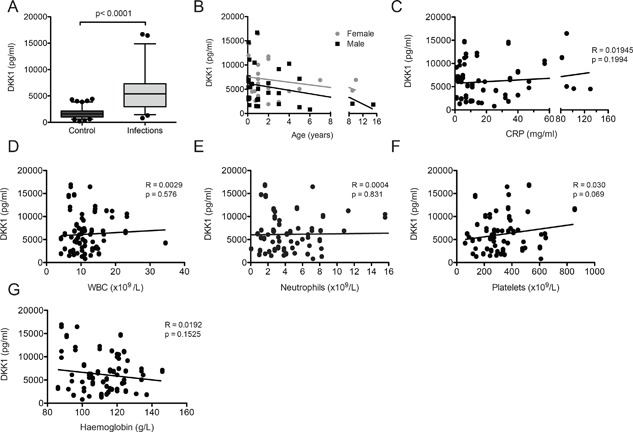
DKK1 levels in blood from children with acute infections. (A) DKK1 plasma levels from children suffering from various infections (n = 57) and from healthy blood donors (Control, n = 107). Graphs represents the average of two separate determinations for each patient's sample. Statistical significance was determined by unpaired Student t‐test. (B‐G) DKK1 levels from patients with infections according to (B) age and gender, (C) levels of the C‐reactive protein (CRP), (D) white blood cell counts (WBC), (E) neutrophils, (F) platelets, and (G) hemoglobin. Statistical significance was determined by linear regression (R 2) and Pearson's correlation.
Patients with Fanconi anemia show elevated levels of DKK1
Peripheral blood plasmas were obtained from a total of 98 patients with FA, both males and females, aged 1 month to 37 years (56 males, 42 females), and 58 patients with BMF (26 males, 32 females; aged 1 month to 64 years) but excluded from FA (Table 1). Patients found positive for FA were assigned to complementation groups A, B, C, D2, G, I, or J. Fifteen patients diagnosed with FA but with undetermined mutations at the time of diagnosis and seven patients with FA with missing age at draw were also included in the study. DKK1 levels found in patients with FA were compared to those found in patients with acute infections and healthy donors. Results show that patients with FA presented with elevated DKK1 levels in their blood (mean value of 3465 ± 190 pg/ml) compared to healthy blood donors (1771 ± 95 pg/ml) but significantly less than patients with acute infections (mean value of 6072 ± 518 pg/ml; Table 1 and Fig. 2). DKK1 levels were similar whether blood was collected onto heparin, EDTA, or sodium citrate (data not shown) as previously reported 29. We also evaluated DKK1 levels in plasma samples obtained from 58 patients admitted on the basis of BMF. Those patients were subsequently excluded from the diagnosis of FA and included 26 males and 32 females aged 1 month to 64 years (Table 1). BMF patients presented with a significant increase in DKK1 protein levels in their blood (4575 ± 362 pg/ml) compared with healthy blood donors. Surprisingly, BMF patients presented significantly more elevated levels of DKK1 than patients with FA but lower than children with acute infections (Table 1 and Fig. 2A). Statistical analysis showed no correlation between DKK1 levels and patient's age or gender in FA and BMF populations (Fig. 2B and C). In addition, no correlations were found between DKK1 levels and the FA gene mutated (Fig. 2D and E). These results suggest that patients with BMF or FA present elevated levels of DKK1 in their blood.
Figure 2.
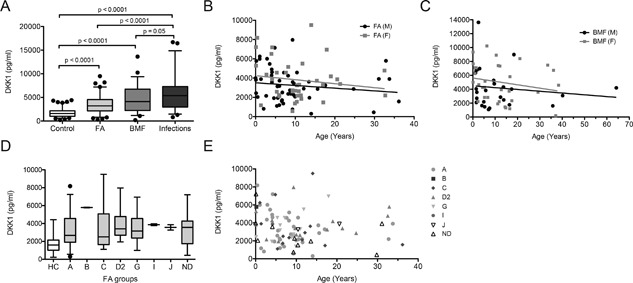
DKK1 levels in blood from patients with hematological disorders. (A) DKK1 plasma levels from patients diagnosed with FA (n = 98) or excluded from FA (BMF, n = 58) and as in Fig. 1 and from children suffering from various infections (n = 57) and from healthy blood donors (Control, n = 107). BMF represent patients with bone marrow failure presented as severe aplastic anemia or myelodysplasia that were excluded from FA at the time of diagnosis. Graphs represents the average of two separate determinations for each patient's sample. (B and C) DKK1 levels from FA (B) and BMF (C) patients according to age and gender. (D and E) DKK1 levels according to the FA gene mutated (in D) and age (in E). HC, healthy controls; ND, not determined. Statistical significance was determined by unpaired Student t‐test, linear regression (R 2) and Pearson's correlation.
Together, our results suggest that the presence of elevated DKK1 levels in peripheral blood is indicative of inflammatory or stress signals such as marrow failure.
Discussion
Identification of disease biomarkers is of importance for early interventions, to monitor disease progression or to evaluate treatment responses. DKK1 has been proposed as a potential biomarker for cancer progression and prognosis. Elevated blood levels of DKK1 have been associated with multiple myeloma and various types of cancers including head and neck, lung, breast, liver, and bone cancers 20. Given that elevated levels of DKK1 were found in blood of FA‐deficient mice and that FA is a cancer prone disease, DKK1 might be of interest for FA. In fact, studies with similar methods of detection show comparable levels of DKK1 between plasma from patients with hepatocellular carcinoma (mean of 3400 pg/ml) 18 to those from patients with FA (mean of 3465 pg/ml; our study) suggesting that increased DKK1 in patients with FA might reflect a propensity for cancer. However, based on the present study showing that acute infections trigger overproduction of DKK1, elevated levels of DKK1 in the blood of patients with FA may reflect the presence of an inflammatory or stress response rather than cancer. This may also be true for patients diagnosed with cancers. Actually, the DKK1 gene was shown to be activated in response to inflammatory and stress signals and the Dkk1 protein was found elevated in blood of animal models of inflammation and radiation‐induced stress 20, 30, 31, 32, 33, 34, 35. These findings support our data and suggest that DKK1 activation and overproduction might be indicative of inflammatory responses in patients rather than malignancies per se.
Surprisingly, but consistent with previous reports, the levels of DKK1 did not correlate with levels of the CRP, which is an acute‐phase marker of inflammation 36, 37. While CRP is produced by hepatocytes in response to cytokines produced during an acute‐phase event 38, the site of DKK1 production remains to be identified. Previous reports have suggested that even though DKK1 is not produced by platelets, it may be stored in platelets and released upon activation 29, 31. In our study, we did not observe any correlation between the number of platelets and DKK1 levels in blood from children with infectious diseases. Unfortunately, we do not have platelet counts from the FA and BMF populations included in this study. Because thrombocytopenia is a feature of FA, we could argue against a role of platelets in DKK1 overproduction at least in these patients.
The strengths of our study reside in the number of samples obtained and the wide range in age at diagnosis for patients with FA or excluded from FA and patients with acute infections. The limitations of our study include differences in age distribution between healthy donors and patients. However, DKK1 levels were not influenced by age nor gender in the different populations. Another limitation is the lack of clinical data in the FA and BMF cohorts and follow‐up of patients with infections. Although the heterogeneity of infections may be interpreted as a limitation of our study, the fact that both high (over one SD) and low (below one SD) DKK1 levels were found within each type of infections indicate that inflammatory responses induce DKK1 overexpression regardless of the type of pathogen.
In summary, the association between DKK1 and acute infections in our study is a novel observation. Based on our data, we advise caution for the use of DKK1 blood levels as an indicator of the course or prognosis of cancer or chronic diseases in patients. However, we propose that DKK1 may serve as an indicator of inflammatory responses that could complement other biomarkers of disease progression. Further testing will be important to determine the actual mechanism leading to increased DKK1 production during infections and whether DKK1 is a marker of chronic or undetected infections secondary to other diseases such as FA.
Conflict of Interest
The authors declare having no conflict of interest.
Acknowledgments
The authors wish to thank the families and children for their contributions as well as donors for providing blood samples. We wish to thank clinical staff for taking care of patients. We also wish to thank Ms Louise Gosselin for coordinating clinical aspect for parts of the project, Ms Jacinthe Julien for her technical expertise, and Ms Marie‐Ève Allard for recruiting healthy blood donors. This work was supported in parts by grants from the Canadian Institutes of Health Research and the Leukemia and Lymphoma Society of Canada (grants to MC).
Current address of Maryse St‐Louis is Institut National d'excellence en santé et en service sociaux, Québec (QC), Canada.
Funding information
This work was supported in parts by grants from the Canadian Institutes of Health Research and the Leukaemia and Lymphoma Society of Canada (grants to MC).
References
- 1. Krupnik, V. E. , Sharp J. D., Jiang C., Robison K., Chickering T. W., Amaravadi L., Brown D. E., Guyot D., Mays G., Leiby K., et al. 1999. Functional and structural diversity of the human Dickkopf gene family. Gene 238:301–313. [DOI] [PubMed] [Google Scholar]
- 2. Niehrs, C. 2006. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene 25:7469–7481. 10.1038/sj.onc.1210054 [DOI] [PubMed] [Google Scholar]
- 3. Bao, J. , Zheng J. J., and Wu D.. 2012. The structural basis of DKK‐mediated inhibition of Wnt/LRP signaling. Sci. Signal. 5:pe22 10.1126/scisignal.2003028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bafico, A. , Liu G., Yaniv A., Gazit A., and Aaronson S. A.. 2001. Novel mechanism of Wnt signalling inhibition mediated by Dickkopf‐1 interaction with LRP6/Arrow. Nat. Cell Biol. 3:683–686. 10.1038/35083081 [DOI] [PubMed] [Google Scholar]
- 5. Bourhis, E. , Tam C., Franke Y., Bazan J. F., Ernst J., Hwang J., Costa M., Cochran A. G., and Hannoush R. N.. 2010. Reconstitution of a frizzled8.Wnt3a.LRP6 signaling complex reveals multiple Wnt and Dkk1 binding sites on LRP6. J. Biol. Chem. 285:9172–9179. 10.1074/jbc.M109.092130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Semenov, M. V. , Tamai K., Brott B. K., Kuhl M., Sokol S., and He X.. 2001. Head inducer Dickkopf‐1 is a ligand for Wnt coreceptor LRP6. Curr. Biol. 11:951–961. [DOI] [PubMed] [Google Scholar]
- 7. Mao, B. , Glinka A., Wu W., Delius H., Monaghan A. P., Blumenstock C., and Niehrs C.. 2002. Kremen proteins are Dickkopf receptors that regulate Wnt/beta‐catenin signalling. Nature 417:664–667. 10.1038/nature756 [DOI] [PubMed] [Google Scholar]
- 8. Mao, B. , Wu W., Li Y., Hoppe D., Stannek P., Glinka A., and Niehrs C.. 2001. LDL‐receptor‐related protein 6 is a receptor for Dickkopf proteins. Nature 411:321–325. 10.1038/35077108 [DOI] [PubMed] [Google Scholar]
- 9. Glinka, A. , Wu W., Delius H., Monaghan A. P., Blumenstock C., and Niehrs C.. 1998. Dickkopf‐1 is a member of a new family of secreted proteins and functions in head induction. Nature 391:357–362. 10.1038/34848 [DOI] [PubMed] [Google Scholar]
- 10. Mukhopadhyay, M. , Shtrom S., Rodriguez‐Esteban C., Chen L., Tsukui T., Gomer L., Dorward D. W., Glinka A., Grinberg A., Huang S. P., et al. 2001. Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev. Cell 1:423–434. [DOI] [PubMed] [Google Scholar]
- 11. Adamska, M. , MacDonald B. T., and Meisler M. H.. 2003. Doubleridge, a mouse mutant with defective compaction of the apical ectodermal ridge and normal dorsal‐ventral patterning of the limb. Dev. Biol. 255:350–362. [DOI] [PubMed] [Google Scholar]
- 12. Lieven, O. , Knobloch J., and Ruther U.. 2010. The regulation of Dkk1 expression during embryonic development. Dev. Biol. 340:256–268. 10.1016/j.ydbio.2010.01.037 [DOI] [PubMed] [Google Scholar]
- 13. Lieven, O. , and Ruther U.. 2011. The Dkk1 dose is critical for eye development. Dev. Biol. 355:124–137. 10.1016/j.ydbio.2011.04.023 [DOI] [PubMed] [Google Scholar]
- 14. Menezes, M. E. , Devine D. J., Shevde L. A., and Samant R. S.. 2011. Dickkopf1: A tumor suppressor or metastasis promoter? Int. J. Cancer 130:1477–1483. 10.1002/ijc.26449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tian, E. , Zhan F., Walker R., Rasmussen E., Ma Y., Barlogie B., and J. D. Shaughnessy, Jr. 2003. The role of the Wnt‐signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N. Engl. J. Med. 349:2483–2494. 10.1056/NEJMoa030847 [DOI] [PubMed] [Google Scholar]
- 16. Yamabuki, T. , Takano A., Hayama S., Ishikawa N., Kato T., Miyamoto M., Ito T., Ito H., Miyagi Y., Nakayama H., et al. 2007. Dikkopf‐1 as a novel serologic and prognostic biomarker for lung and esophageal carcinomas. Cancer Res. 67:2517–2525. 10.1158/0008-5472.CAN-06-3369 [DOI] [PubMed] [Google Scholar]
- 17. Shi, Y. , Gong H. L., Zhou L., Tian J., and Wang Y.. 2014. Dickkopf‐1 is a novel prognostic biomarker for laryngeal squamous cell carcinoma. Acta Otolaryngol. 134:753–759. 10.3109/00016489.2014.894251 [DOI] [PubMed] [Google Scholar]
- 18. Shen, Q. , Fan J., Yang X. R., Tan Y., Zhao W., Xu Y., Wang N., Niu Y., Wu Z., Zhou J., et al. 2012. Serum DKK1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: a large‐scale, multicentre study. Lancet Oncol. 13:817–826. 10.1016/S1470-2045(12)70233-4 [DOI] [PubMed] [Google Scholar]
- 19. Zhou, S. J. , Zhuo S. R., Yang X. Q., Qin C. X., and Wang Z. L.. 2014. Serum Dickkopf‐1 expression level positively correlates with a poor prognosis in breast cancer. Diagn. Pathol. 9:161 10.1186/s13000-014-0161-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mazon, M. , Masi D., and Carreau M.. 2016. Modulating Dickkopf‐1: a strategy to monitor or treat cancer? Cancers (Basel) 8:1–9. 10.3390/cancers8070062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Diarra, D. , Stolina M., Polzer K., Zwerina J., Ominsky M. S., Dwyer D., Korb A., Smolen J., Hoffmann M., Scheinecker C., et al. 2007. Dickkopf‐1 is a master regulator of joint remodeling. Nat. Med. 13:156–163. 10.1038/nm1538 [DOI] [PubMed] [Google Scholar]
- 22. Huard, C. C. , Tremblay C. S., Helsper K., Delisle M. C., Schindler D., Levesque G., and Carreau M.. 2013. Fanconi anemia proteins interact with CtBP1 and modulate the expression of the Wnt antagonist Dickkopf‐1. Blood 121:1729–1739. 10.1182/blood-2012-02-408997 [DOI] [PubMed] [Google Scholar]
- 23. Bagby, G. 2018. Recent advances in understanding hematopoiesis in Fanconi Anemia. F1000Res. 7:105 10.12688/f1000research.13213.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alter, B. P. , Giri N., Savage S. A., and Rosenberg P. S.. 2018. Cancer in the National Cancer Institute inherited bone marrow failure syndrome cohort after fifteen years of follow‐up. Haematologica 103:30–39. 10.3324/haematol.2017.178111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Knies, K. , Inano S., Ramirez M. J., Ishiai M., Surralles J., Takata M., and Schindler D.. 2017. Biallelic mutations in the ubiquitin ligase RFWD3 cause Fanconi anemia. J. Clin. Invest. 127:3013–3027. 10.1172/JCI92069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nalepa, G. , and Clapp D. W.. 2018. Fanconi anaemia and cancer: an intricate relationship. Nat. Rev. Cancer 18:168–185. 10.1038/nrc.2017.116 [DOI] [PubMed] [Google Scholar]
- 27. Walter, D. , Lier A., Geiselhart A., Thalheimer F. B., Huntscha S., Sobotta M. C., Moehrle B., Brocks D., Bayindir I., Kaschutnig P., et al. 2015. Exit from dormancy provokes DNA‐damage‐induced attrition in haematopoietic stem cells. Nature 520:549–552. 10.1038/nature14131 [DOI] [PubMed] [Google Scholar]
- 28. Kaschutnig, P. , Bogeska R., Walter D., Lier A., Huntscha S., and Milsom M. D.. 2015. The Fanconi anemia pathway is required for efficient repair of stress‐induced DNA damage in haematopoietic stem cells. Cell Cycle 14:2734–2742. 10.1080/15384101.2015.1068474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Voorzanger‐Rousselot, N. , Goehrig D., Facon T., Clezardin P., and Garnero P.. 2009. Platelet is a major contributor to circulating levels of Dickkopf‐1: clinical implications in patients with multiple myeloma. Br J. Haematol. 145:264–266. 10.1111/j.1365-2141.2009.07587.x [DOI] [PubMed] [Google Scholar]
- 30. Peng, H. , Li Y., Liu Y., Zhang J., Chen K., Huang A., and Tang H.. 2016. HBx and SP1 upregulate DKK1 expression. Acta Biochim. Pol. 64:35–39. 10.18388/abp.2016_1250 [DOI] [PubMed] [Google Scholar]
- 31. Chae, W. J. , Ehrlich A. K., Chan P. Y., Teixeira A. M., Henegariu O., Hao L., Shin J. H., Park J. H., Tang W. H., Kim S. T., et al. 2016. The Wnt antagonist Dickkopf‐1 promotes pathological type 2 cell‐mediated inflammation. Immunity 44:246–258. 10.1016/j.immuni.2016.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Heiland, G. R. , Zwerina K., Baum W., Kireva T., Distler J. H., Grisanti M., Asuncion F., Li X., Ominsky M., Richards W., et al. 2010. Neutralisation of Dkk‐1 protects from systemic bone loss during inflammation and reduces sclerostin expression. Ann. Rheum. Dis. 69:2152–2159. 10.1136/ard.2010.132852 [DOI] [PubMed] [Google Scholar]
- 33. Schett, G. , and Sieper J.. 2009. Inflammation and repair mechanisms. Clin. Exp. Rheumatol. 27:S33–S35. [PubMed] [Google Scholar]
- 34. Wang, S. Y. , Liu Y. Y., Ye H., Guo J. P., Li R., Liu X., and Li Z. G.. 2011. Circulating Dickkopf‐1 is correlated with bone erosion and inflammation in rheumatoid arthritis. J. Rheumatol. 38:821–827. 10.3899/jrheum.100089 [DOI] [PubMed] [Google Scholar]
- 35. Weng, L. H. , Wang C. J., Ko J. Y., Sun Y. C., Su Y. S., and Wang F. S.. 2009. Inflammation induction of Dickkopf‐1 mediates chondrocyte apoptosis in osteoarthritic joint. Osteoarthr. Cartil. 17:933–943. 10.1016/j.joca.2008.12.008 [DOI] [PubMed] [Google Scholar]
- 36. Garnero, P. , Tabassi N. C., and Voorzanger‐Rousselot N.. 2008. Circulating dickkopf‐1 and radiological progression in patients with early rheumatoid arthritis treated with etanercept. J. Rheumatol. 35:2313–2315. [DOI] [PubMed] [Google Scholar]
- 37. Voorzanger‐Rousselot, N. , Ben‐Tabassi N. C., and Garnero P.. 2009. Opposite relationships between circulating Dkk‐1 and cartilage breakdown in patients with rheumatoid arthritis and knee osteoarthritis. Ann. Rheum. Dis. 68:1513–1514. 10.1136/ard.2008.102350 [DOI] [PubMed] [Google Scholar]
- 38. Vigushin, D. M. , Pepys M. B., and Hawkins P. N.. 1993. Metabolic and scintigraphic studies of radioiodinated human C‐reactive protein in health and disease. J. Clin. Invest. 91:1351–1357. 10.1172/JCI116336 [DOI] [PMC free article] [PubMed] [Google Scholar]


