Abstract
Epidemiological studies in chromate production have established hexavalent chromium as a potent lung carcinogen. Inhalation of chromium(VI) most often occurs in mixtures with other metals as among stainless steel welders, which is the largest occupational group with Cr(VI) exposure. Surprisingly, carcinogenicity of Cr(VI)-containing welding fumes is moderate and not consistently higher than that of Cr-free welding. Here, we investigated interactions between chromate and three other metal ions [Fe(III), Mn(II), Ni(II)] that are typically released from stainless steel welding particles. In human lung epithelial cells with physiological levels of ascorbate and glutathione, Cr(VI) was by far the most cytotoxic metal in single exposures. Coexposure with Fe(III) suppressed cytotoxicity and genotoxicity of Cr(VI), which resulted from a severe inhibition of Cr uptake by cells and required extracellular ascorbate/glutathione. Chemically, detoxification of Cr(VI) occurred via its rapid extracellular reduction by Fe(II) that primarily originated from ascorbate-reduced Fe(III). Glutathione was a significant contributor to reduction of Cr(VI) by Fe only in the presence of ascorbate. We further found that variability in Cr(VI) metabolism among common cell culture media was caused by their different Fe content. Ni(II) and Mn(II) had no detectable effects on metabolism, cellular uptake or cytotoxicity of Cr(VI). The main biological findings were confirmed in three human lung cell lines, including stem cell-like and primary cells. We discovered extracellular detoxification of carcinogenic chromate in coexposures with Fe(III) ions and identified the underlying chemical mechanism. Our findings established an important case when exposure to mixtures causes inactivation of a potent human carcinogen.
Introduction
Chemical compounds containing chromium(VI) are recognized carcinogens in the human respiratory system.1,2 In physiological solutions, Cr(VI) exists as chromate anion (CrO42–) that is readily taken up by human cells leading to its many-fold accumulation over outside concentrations.2 Human lung cancers associated with occupational Cr(VI) exposures are squamous lung carcinomas that exhibited high mutation loads.3,4 Cr(VI) is a genotoxic carcinogen that produces mutagenic Cr-DNA adducts5−7 and other forms of DNA damage.8−10 Induction of DNA damage by Cr(VI) requires its cellular reduction, yielding Cr(III) as the final product.11 A key reducer of Cr(VI) in cells in vivo is ascorbate (Asc) that is responsible for >95% of Cr(VI) metabolism in the lung.12,13 Other reducers of Cr(VI) include small thiols, primarily glutathione (GSH), and to a smaller extent, less abundant cysteine.11 At physiological levels of the reactants, reduction of Cr(VI) by Asc yields Cr(IV) as the only detectable intermediate.14−16 A severe deficiency of cultured cells in Asc leads to their metabolism of Cr(VI) by thiols, which is accompanied by the formation of the pro-oxidant Cr(V). Restoration of physiological levels of Asc in cultured cells blocks Cr(V) formation and suppresses induction of oxidative DNA damage and related stress signaling responses.17,18 Reduction of chromate outside the cells converts it into membrane-impermeable, nontoxic Cr(III). This extracellular detoxification process is important physiologically11 and critical for chemoprotective activity of N-acetylcysteine against Cr(VI) toxicity.19 The presence of high Asc concentrations in the lung lining fluid of rodents (∼10-times higher than in humans) leads to a very rapid detoxification of chromate, explaining their resistance to lung carcinogenicity by soluble Cr(VI) compounds.20
Epidemiological studies among large cohorts of chromate production workers have obtained clear evidence of elevated incidence of lung cancers with linear dose-dependence.21−24 Higher risks of lung cancers were also found in other occupations with Cr(VI) exposures such as in chrome plating.25,26 A risk assessment modeling of cancer incidence data in chromate production estimated that a current permissible exposure limit for Cr(VI), which was lowered 10 times in 2006 to 5 μg/m3, still confers high lifetime risks with up to 45 additional lung cancer deaths per 1000 workers.27,28 Chromate production represents occupational exposure to the single metal, Cr(VI), which is ideal for epidemiological studies. However, inhalation exposures to Cr(VI) typically involve coexposures with other metals. Stainless steel welders are the largest occupational group exposed to Cr(VI), which always occurs in a mixture with other metals. Stainless steel is the iron alloy containing at least 10.5% Cr by weight. Ni and Mn are also frequently added to stainless steel to improve its properties. Ni is a known human lung carcinogen albeit it is less potent than Cr(VI).25 Welding fumes contain oxidized metals that can be solubilized in saline solutions, although the degree of solubility is highly variable and depends on the welding process characteristics such as a type of welding process, its parameters, use of shielding gas and composition of electrode.29−31 Cr(VI) was the most soluble component of welding fumes, reaching 70% solubilization in the saline solutions.29 In contrast to high carcinogenicity of soluble Cr(VI) found among chromate workers,22−24 cancer risks of stainless steel welding were only modestly elevated and did not always differ from risks among mild steel welders (no Cr exposures).32−37 A recent review of welding by the International Agency for Cancer Research (IARC) concluded that both stainless steel and mild steel welding fumes represent group I carcinogens.38 A weak contribution of Cr(VI) to welding-associated lung cancers is puzzling as cancer risks of individual components in mixtures are considered to be at least additive and often suspected as being synergistic.
Using solutions of individual metal salts, we experimentally modeled pairwise toxicological and chemical interactions between chromate [solubilized Cr(VI)], the most carcinogenic and toxic component, and three other metal ions that are typically released from stainless steel welding particles. We found that in the presence of physiological concentrations of Asc and GSH, Fe(III) was converted into Fe(II) that acted as a very rapid extracellular reducer of Cr(VI), which prevented its uptake by cells. Ni(II) and Mn(II) ions showed no detectable impact on cytotoxicity or metabolism of Cr(VI). Overall, our results established a defined chemical mechanism that can explain a weak carcinogenicity of Cr(VI) exposures through inhalation of welding fumes. To our knowledge, this is the first example when coexposures with other toxicants result in the loss of activity by a major human carcinogen.
Experimental Procedures
Materials
l-ascorbic acid (99.9% pure), dehydro-l-(+)-ascorbic acid dimer, potassium chromate (K2CrO4, 99% pure), l-glutathione (>98% pure), l-cysteine (C7352) (>98% pure), ammonium iron(III) citrate (F5879), iron(III) chloride hexahydrate (31232) (>99% pure), ammonium iron(II) sulfate hexahydrate (203505, 99.997% pure), iron(II) chloride tetrahydrate (44939, >99% pure), nickel(II) chloride hexahydrate (223387, ReagentPlus grade), manganese(II) chloride tetrahydrate (203734, 99.99% trace metal purity), 3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazone-4′,4″-disulfonic acid sodium salt (ferrozine) (82950, >97% pure), 1,10-phenanthroline monohydrate (P9375), diethylenetriaminepentaacetic acid (DTPA) (D6518), and nitric acid (>99.999% pure) were all obtained from Sigma-Aldrich. Deferoxamine (1459) was purchased from Cayman Chemical.
Cell Culture
H460 and HBEC3-KT cells were obtained from the American Type Culture Collection (ATCC). H460 cells were grown in RPMI-1640 media (22400089, ThermoFisher) containing 10% (v/v) fetal bovine serum (FBS), and penicillin/streptomycin. HBEC3-KT cells were propagated in Airway Epithelial Cell Basal Medium (PCS-300-030, ATCC) with added Bronchial Epithelial Growth Kit (PCS-300-040, ATCC). Primary human bronchial epithelial cells were obtained from Lonza and propagated in the vendor’s recommended serum-free medium (BEBM, CC-2540) supplemented with growth factors (CC-3170, Lonza). All cell lines were grown in the atmosphere of 95% air/5% CO2. Other cell culture media tested for metals were DMEM (Gibco, 12430-062), F12-K (ATCC, 30-2004), and EMEM (ATCC, 30-2003). Cells were treated with the indicated concentrations of Cr(VI) next day after seeding. Stock solutions of K2CrO4 (in water), NiCl2 (in water), MnCl2 (in water), iron(III) citrate (in water), and iron(III) chloride (in 10 mM HCl) were freshly prepared for each experiment.
Restoration of Cellular Asc
H460 cells were incubated with dehydroascorbic acid (DHA) in Krebs-HEPES buffer [30 mM HEPES (pH 8.0), 130 mM NaCl, 4 mM KH2PO4, 1 mM MgSO4, 1 mM CaCl2] supplemented with 5% FBS and 0.5 mM glucose. DHA stocks were freshly prepared in the same buffer and kept on ice before use. For HBEC3-KT cells, DHA and Asc were added in the growth medium for 2 and 3 h, respectively.
Asc Concentrations in Cells
Cellular Asc was extracted on ice with 50 mM methanesulfonic acid/5 mM DTPA and detected as a fluorescent conjugate with 1,2-diamino-4,5-dimethoxybenzene dihydrochloride.39 Cellular precipitates formed after Asc extraction were dissolved in 1% SDS-50 mM NaOH and used for protein measurements. Final Asc values were normalized per protein content of each sample.
Reduction of Cr(VI)
The source of Cr(VI) was K2CrO4 dissolved in water. Reduction of Cr(VI) was monitored by chromate absorbance at 372 nm. Equal volumes of two 2× concentrated solutions (one containing Cr(VI) and a specified other metal and the second containing reducers and any nonmetal additives) were rapidly mixed in 96-well plates followed by the measurements of the initial A372. Plates were maintained at the specified temperatures (25 or 37 °C) inside the SpectraMax M5 microplate reader.
Cellular Uptake of Cr(VI)
A previously described high-recovery procedure based on extraction of Cr from cells with nitric acid was followed.40 Cells were seeded on 6-well plates and allowed to grow overnight before treatment. Cr(VI)-containing media was removed, cells were washed twice with warm PBS, and then collected by trypsinization. Pellets were washed twice with ice-cold PBS (1100×g, 5 min, 4 °C) and resuspended in cold water. Equal volume of 10% nitric acid was added, vortexed, and stored overnight at −80 °C. Samples were thawed at 50 °C for 60 min and then placed on ice for 30 min. Supernatants were collected and diluted 2.5 times with water to give 2% nitric acid and stored at 4 °C until Cr was measured by graphite furnace atomic absorption spectroscopy (AAnalyst600 Atomic Absorption Spectrometer, PerkinElmer). Pellets were washed twice with cold 5% nitric acid (10,000×g, 5 min, 4 °C) then dissolved in 0.5 M NaOH at 37 °C for 30 min. Solubilized pellets were used for the determination of protein amounts.
Measurements of Metals in Cell Culture Media
Media was acidified with nitric acid to a final concentration of 2%. Iron, copper, nickel, and manganese were measured by graphite furnace atomic absorption spectroscopy (AAnalyst600 Atomic Absorption Spectrometer, PerkinElmer).
Fe(II) Assay
Fe(II) was measured by recording absorbance of its complex with ferrozine.41,42 Ammonium iron(III) citrate was dissolved in water and 1 mM iron(III) chloride hexahydrate stock solution was prepared in 10 mM HCl. Both Fe(III) compounds were added to reactions from water-diluted 10× stocks. Reducers and ferrozine (100 μM final concentration) were prepared in solutions at 1.1× concentrations. Fe(III) was added to the reducer solutions and rapidly mixed in 96-well plate to make all components at a 1× concentration. A562 values were measured every 20 or 30 s. Plates were maintained at 37 °C inside the SpectraMax M5 microplate reader.
Western Blotting
Cells were washed twice with cold PBS and collected from the dishes by scraping in PBS. After pelleting and another wash in cold PBS at 1100×g for 5 min, cells were boiled for 10 min in a lysis buffer containing 2% SDS, 50 mM Tris, pH 6.8, 10% glycerol and protease/phosphate inhibitors (#78425, ThermoFisher Scientific). Insoluble debris was removed by centrifugation at 10000×g for 10 min at room temperature. Samples were analyzed on 12% SDS-PAGE gels and electrotransferred by a semidry procedure onto PVDF membranes (162-0177, Bio-Rad). For the γ-H2AX blots, a standard buffer supplied for the semidry transfer apparatus (PierceG2 Fast Blotter, ThermoScientific) was supplemented with 12% ethanol. Primary antibodies for detection of Ser139-phosphorylated histone H2AX (#2577, 1:1000 dilution) and CHK2 (#3440, 1:1000 dilution) were from Cell Signaling. Antibodies for phospho-Ser4/8-RPA32 (#A300-245A, 1:1000 dilution) were obtained from Bethyl Laboratories.
Cell Viability
The CellTiter-Glo luminescent assay (Promega) was used to measure the cytotoxic effects of Cr(VI) and other metals. Cells were seeded into 96-well plates (2000 cells per well for H460 cells, 1000, and 4000 cells per well for HBEC3-KT cells in 72 and 48 h recovery experiments, respectively) and treated with metals on the next day. Cytotoxicity was determined following 48 h recovery for H460 and 72 h recovery for HBEC3-KT cells.
Statistics
Differences between the groups were evaluated by two-tailed, unpaired t-test.
Results
Cytotoxicity of Metal Ions in Human Lung Epithelial Cells
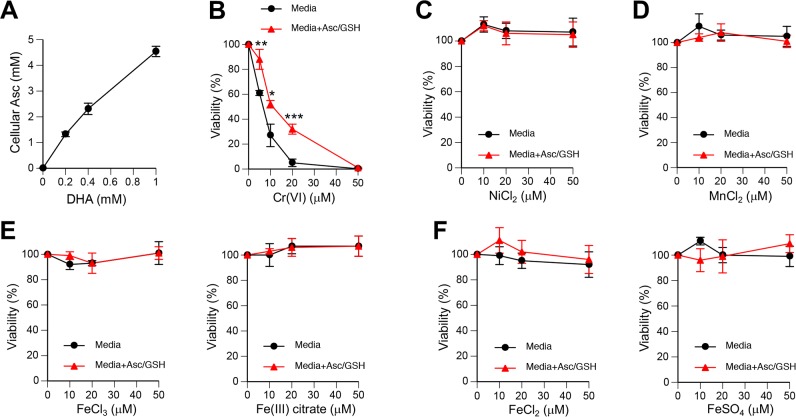
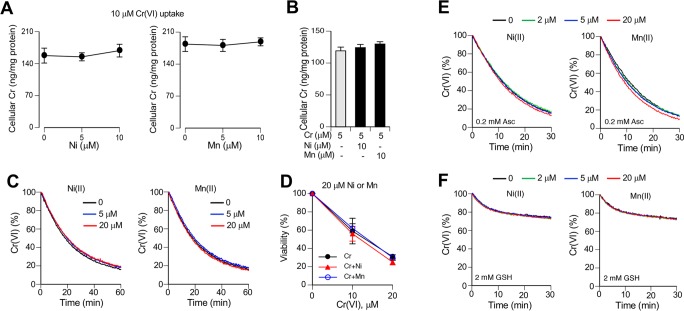
Human exposures to Cr(VI) and welding fumes are both associated with the development of squamous cell lung carcinoma,3,33 a type of cancer that arises from malignant transformation of lung epithelial cells. Thus, it is important to investigate the effects of welding fumes-associated metal ions on human lung epithelial cells. We chose H460 cells as our primary biological model, which we have previously found to exhibit efficient uptake of chromate and other metal ions19,43 and a robust activation of the stress-sensitive transcription factor p53.44,45 H460 cells have also shown normal biological responses to Cr-DNA damage,46 ionizing radiation,47 and proteotoxic stress.48 H460 and all other cultured cells are severely deficient in Asc due to its absence in the synthetic medium formulations and its minimal supply through the addition of 10–15% serum that usually irreversibly lost the majority of ascorbate during handling and storage. To restore physiological levels of Asc, we incubated H460 cells with the oxidized form of vitamin C, DHA, which rapidly enters cells through ubiquitously expressed glucose transporters GLUT1, GLUT3, and GLUT4.49 Addition of 0.2 mM DHA was sufficient to bring Asc levels from barely detectable (∼5 μM) to the physiologically relevant 1–2 mM range (Figure 1A).50 In addition to the restoration of cellular Asc, we also tested the effects of cell culture medium supplementation with 50 μM Asc and 100 μM GSH, which are physiological concentrations of these antioxidants in human lung lining fluid.51 We found that treatments with 10 μM and higher concentrations of Cr(VI) caused a high cytotoxicity (measured following 48 h recovery), which was significantly less severe when the cell culture medium contained Asc/GSH (Figure 1B). This cytoprotective effect reflects extracellular reduction of toxic Cr(VI) by Asc/GSH (primarily Asc) into cell-impermeable Cr(III).20 In contrast to Cr(VI), the same concentrations of Ni(II), Mn(II) and four Fe compounds produced no significant cytotoxicity with or without addition of Asc/GSH to the media (Figure 1C–F). Thus, solubilized Cr(VI) (chromate) was clearly the most cytotoxic metal ion in the human lung epithelial cell model, which was established with physiological levels of Asc inside and outside the cells.
Figure 1.
Cytotoxic effects of metal ions in H460 cells. In all cytotoxicity experiments, H460 cells were preincubated with 0.2 mM DHA to restore physiological concentrations of vitamin C and then treated with metal salts for 3 h in standard or Asc/GSH-supplemented media (50 μM Asc and 100 μM GSH). Cell viability was assayed at 48 h post-treatments. Graphs show means ± SD (n = 3). (A) Concentrations of Asc in H460 cells after incubations with DHA. (B) Viability of cells treated with chromate anions. Statistics: *, p < 0.05, **, p < 0.01, ***, p < 0.001 relative to the corresponding concentrations of Cr(VI) in cell culture medium without reducers. (C–F) Cell viability treated with indicated metal salts.
Cr(VI) Metabolism in Different Cell Culture Media
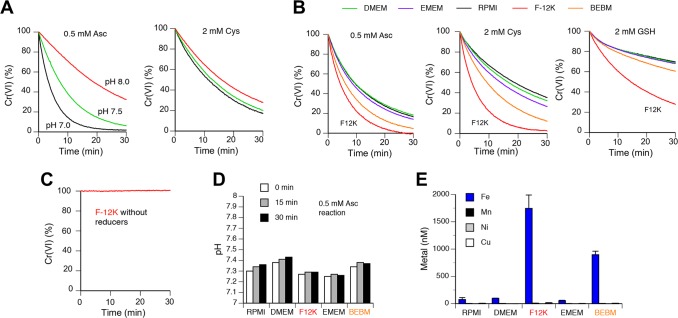
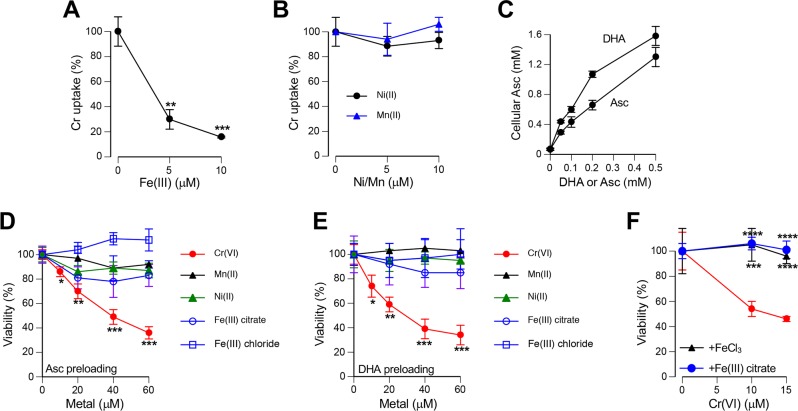
A much greater toxicity of Cr(VI) and its high abundance in the soluble fraction29 all indicate that if they exist at all the most critical toxicological interactions for metals released from stainless steel welding fume particles should involve Cr(VI). A critical aspect of Cr(VI) toxicity is its dependence on reductive metabolism, which converts Cr(VI) into nontoxic Cr(III) outside the cells.2,20 Chemical and biological properties of metal ions can be strongly affected by their binding to specific molecules, which led us to skip the use of simple buffer systems and to focus on the selection of the appropriate biological medium. Our initial tests of several commercial cell culture media for reduction of Cr(VI) produced highly variable results. During investigation of potential causes of this variability, we identified shifts to higher pH in our Cr(VI) reactions occurring due to the loss of bicarbonate. Bicarbonate is the main buffering system in cell culture media, which requires 5–10% ambient CO2 for maintenance of physiological pH. Our examination of pH sensitivity found that even modest alkalization strongly inhibited Cr(VI) reduction by its main biological reducer, Asc (Figure 2A, left panel). Rates of Cr(VI) reduction by Cys were much less sensitive to pH of the reactions (Figure 2A, right panel). To eliminate the effects of pH changes due to bicarbonate losses, we added 50 mM HEPES (pH 7.4) to each of the five tested biological media and then examined kinetics of Cr(VI) reduction by Asc, Cys, or GSH (Figure 2B). For all three reducers, we found the highest rates of Cr(VI) reduction in F-12K followed by BEBM medium (formulated for maintenance of primary lung cells without serum). Three other media (RPMI-1640, DMEM, and EMEM) showed the lowest reduction rates and clustered together. In the absence of exogenously added reducers, F-12K (Figure 2C) or other media (not shown) displayed no metabolism of Cr(VI). F-12K is the only medium that includes a significant amount of Cys (0.4 mM). The lack of Cr(VI) reduction in this medium in the absence of exogenous reducing agents likely reflects a complete oxidation of Cys during storage. The differences among media in Cr(VI) reduction rates were not caused by the remaining variations in pH as only very minor differences were observed for the most pH-sensitive reactions involving Asc (Figure 2D). Because our goal was to examine interactions among metal ions some of which are physiological (Fe, Mn), we next measured metal levels in the same five media that we tested for Cr(VI) metabolism. We found that two media with the highest rates of Cr(VI) reduction by Asc/thiols (F-12K, BEBM) also had the highest concentrations of iron (Figure 2E). Three other tested metals were present at very low (3–8 nM for Mn, 3–15 nM Cu) or undetectable (Ni) levels. Recipes for RPMI-1640, DMEM, and EMEM do not include the addition of iron salts, which is in agreement with our findings on very low Fe amounts in these media. BEBM is a proprietary medium (Lonza) formulated for growth of primary human bronchial epithelial cells in serum-free conditions, which explains its high Fe content due to the need to compensate for the absence of the serum-derived source of iron (transferrin-Fe complex). We attribute a moderately lower Fe concentration found by us in F-12K medium than expected from its formal composition (found 1.7 μM versus expected 2.9 μM) to losses of Fe(III) through precipitation and/or surface adsorption. Experiments with spiked samples showed excellent recovery of Fe in our analyses (96 ± 6% for RPMI-1640 and 106 ± 4% for F-12K medium). On the basis of its low Fe content, similarity in Cr(VI) metabolism to other media with low Fe and use for growth of H460 cells, we selected RPMI-1640 for examination of potential interactions between Cr(VI) and other metals. A strong correlation between Fe levels and the rates of Cr(VI) reduction in different media suggested that Fe ions could exhibit very significant effects on Cr(VI) metabolism.
Figure 2.
Cr(VI) metabolism and metal levels in common cell culture media. Rates of Cr(VI) reduction (50 μM chromate) were measured at 25 °C to avoid excessive losses of bicarbonate from biological media. All kinetics data are means of triplicate measurements (error bars not shown for clarity, SD < 5% of the means). (A) pH dependence of Cr(VI) reduction by Asc or Cys in HEPES buffer (50 mM HEPES, 100 mM NaCl). (B) Rates of Cr(VI) reduction in different cell culture media supplemented with 50 mM HEPES, pH 7.4. (C) Lack of Cr(VI) reduction in F-12K medium (HEPES-supplemented) in the absence of exogenous reducers. (D) pH values in HEPES-supplemented cell culture media during reduction of Cr(VI) with 0.5 mM ascorbate. (E) Concentrations of total Fe, Mn, Ni, and Cu in five cell culture media. Data are means ± SD for duplicate (Mn, Ni, Cu) or quadruplicate measurements (Fe).
Effects of Fe Ions on Cr(VI) Metabolism
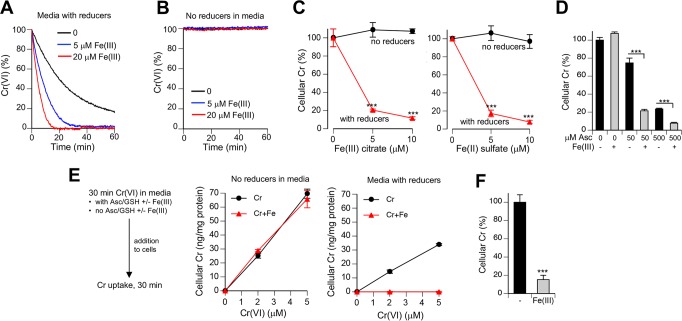
Welding fume particles release iron as Fe(III).29 Because stocks of inorganic Fe(III) salts quickly produce poorly soluble hydroxides upon dissolution in water (especially at neutral or near neutral pH), we first tested Fe(III) citrate which is a soluble form of Fe(III) at physiological pH. We found that addition of Fe(III) citrate strongly accelerated reduction of Cr(VI) by a mixture of Asc/GSH but had no effect when RPMI-1640 medium lacked these reducers (Figure 3A,B). Importantly, speed-up of Cr(VI) reduction was clearly evident even when Fe(III) was present in the substoichiometric amount (5 μM Fe versus 20 μM Cr). Reduction of Cr(VI) outside the cells is a detoxification process generating membrane-impermeable Cr(III).2,20 Thus, acceleration of Cr(VI) reduction in media by Fe(III) would be expected to diminish cellular accumulation of Cr. To examine this prediction, we measured uptake of Cr(VI) by H460 cells incubated in complete medium with or without addition of Fe ions. Two forms of Fe were tested, Fe(III) citrate and Fe(II) sulfate. Consistent with the observed more rapid reduction of Cr(VI) in the presence of Fe(III), we found that the addition of either Fe(III) or Fe(II) salt dramatically suppressed cellular uptake of Cr(VI) in media containing human lung lining fluid-relevant concentrations of Asc and GSH (Figure 3C). In the absence of Asc/GSH in culture medium, both Fe salts produced no significant effects on Cr accumulation by cells. Fe(II) is rapidly oxidized at physiological pH by O2, which means that our Fe(II) sulfate reactions actually tested Fe(III) delivered in a different form. Human and rodent lung lining fluids contain approximately 10-times different concentrations of Asc,20 which is the most rapid biological reducer of Cr(VI). We found that addition of stoichiometric amounts of Fe(III) strongly inhibited uptake of Cr(VI) at both human (50 μM) and rodent lung lining fluid (500 μM)-relevant Asc concentrations (Figure 3D). Remarkably, based on metal uptake data the extent of Cr(VI) detoxification in the medium containing 50 μM Asc and Fe(III) was comparable to the protective effect of the 10 times higher concentration of Asc in the absence of Fe(III). To test a possibility that Fe(III) also suppressed Cr(VI) accumulation by acting on cells, we preincubated Cr(VI) and Fe(III) citrate for 30 min in the presence or absence of physiological concentrations of Asc and GSH and then added this medium to cells for a short, 30 min long Cr uptake (Figure 3E). Consistent with the reduction-based loss of Cr(VI), we found that the addition of Fe(III) completely eliminated cellular accumulation of Cr when media contained Asc/GSH and had no effect in reducers-free media. A dramatic decrease in Cr uptake was also observed when Fe(III) chloride instead of Fe(III) citrate was added to reducers-containing media (Figure 3F). To evaluate the effects of Fe(III) on biological responses to Cr(VI), we examined genotoxic and cytotoxicity of Cr(VI) in Asc-restored H460 cells. As a readout of genotoxicity, we measured Ser139-phophorylation of histone H2AX (known as γ-H2AX), which we have previously validated as a biodosimeter of DNA double-strand breaks in Cr(VI)-treated cells.10,46 ATR kinase is responsible for γ-H2AX formation by Cr-DNA damage.52 In full agreement with uptake studies, we found that the addition of Fe(III) to cell culture media abolished Cr(VI)-induced genotoxicity, as evidenced by the loss of all three forms of γ-H2AX (Figure 4A,B). The disappearance of ubiquitinated species of γ-H2AX is especially important, as they are a more specific biomarker of DNA double-strand breaks than nonubiquitinated γ-H2AX.53 Formation of DNA double-strand breaks in human cells by Cr(VI) is a replication-dependent process.46 The presence of Ser4/8-phosphorylated RPA32 serves as a biochemical marker of replication-associated DNA double-strand breaks.54 We found that addition of Fe(III) to Asc/GSH-containing media eliminated the production of Ser4/8-phospho-RPA32 by Cr(VI) (Figure 4C), confirming our findings with γ-H2AX. Consistent with genotoxicity results, we found that the inclusion of Fe(III) during Cr(VI) treatments in Asc/GSH-containing medium resulted in the complete loss of cytotoxic effects (Figure 4D). Overall, our studies found that Fe(III) was a potent antagonist of Cr(VI) toxicity resulting from a dramatically faster extracellular detoxification of Cr(VI) in the presence of physiological antioxidants Asc and GSH.
Figure 3.
Effects of Fe ions on Cr(VI) metabolism. (A) Rates of Cr(VI) reduction at 37 °C in the presence of Fe(III). Reactions contained RPMI-1640 medium, 50 mM HEPES (pH 7.4), 20 μM Cr(VI), 0–20 μM Fe(III) citrate and a mixture of reducers (100 μM Asc, 200 μM GSH, 40 μM cysteine). Data are means of triplicates measurements. SD values were <5% of the means and not shown for clarity. (B) Lack of Cr(VI) reduction by Fe(III) in the absence of reducers in media (other conditions as in panel A). (C) Reducer-dependent suppression of Cr(VI) uptake by H460 cells in the presence of Fe ions. Cells were incubated with 5 μM Cr(VI) for 3 h in complete medium (RPMI-1640, 50 mM HEPES, pH 7.4, 10% FBS) in the absence or presence of reducers (50 μM Asc, 100 μM GSH). Data are means ± SD (n = 3, ***, p < 0.001 relative to samples without reducers). (D) Cr(VI) uptake by H460 cells in the presence of different Asc concentrations in complete medium [1 h incubation, 10 μM Cr(VI), Fe(III), 10 μM Fe(III) citrate]. Both 50 μM and 500 μM Asc-supplemented media also contained 100 μM GSH. Data are means ± SD (n = 3; ***, p < 0.001). (E) Effects of Cr(VI) preincubation with Fe(III) on Cr accumulation by H460 cells. RPMI-1640 media containing 50 mM HEPES (pH 7.4), 0–5 μM Cr(VI), 0 or 5 μM Fe(III) citrate and reducers (50 μM Asc, 100 μM GSH) or no reducers was incubated for 30 min at 37 °C prior to the addition to cells for 30 min uptake. Data are means ± SD (n = 3). (F) Conditions were as in panel E except that Fe(III) chloride and 2 μM Cr(VI) were incubated in media containing reducers (means ± SD; ***, p < 0.001 relative to the no Fe group).
Figure 4.
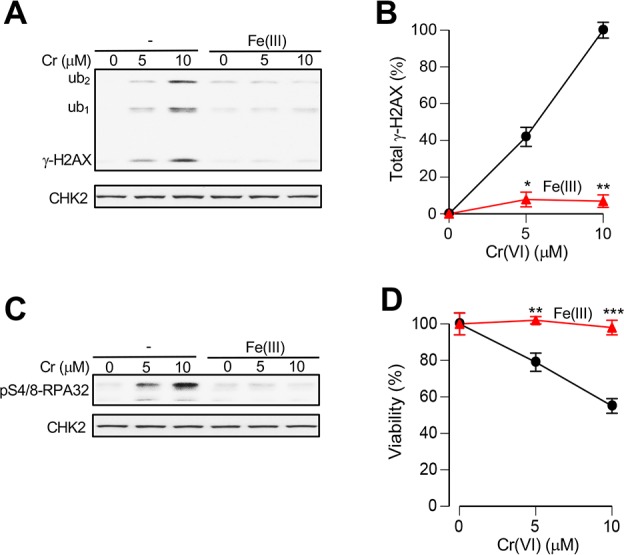
Loss of Cr(VI) genotoxicity and cytotoxicity in the presence of extracellular Fe(III). (A) Formation of γ-H2AX in Asc-restored H460 cells treated with Cr(VI) for 4 h in complete medium containing 50 μM Asc, 100 μM GSH, and 0 or 10 μM Fe(III) chloride (ub1 and ub2, mono- and diubiquitinated γ-H2AX). Total cell lysates were prepared immediately after Cr(VI) treatments. (B) Quantitation of total γ-H2AX (sum of all three forms) in cells treated with Cr(VI) with and without Fe(III) supplementation (means ± SD, n = 2; *, p < 0.05; **, p < 0.01 relative to Cr(VI) alone treatments). ImageJ data from γ-H2AX westerns were background-subtracted and normalized to the 10 μM Cr samples without Fe(III). (C) Loss of RPA32-Ser4/8 phosphorylation in Cr(VI)/Fe(III)-cotreated cells. Treatment conditions were the same as in panel A. (D) Viability of Asc-restored H460 cells treated with Cr(VI) for 4 h in medium containing 50 μM Asc, 100 μM GSH, and 0 or 5 μM Fe(III) citrate. Cell viability was measured at 48 h post-Cr (means ± SD; n = 3; **, p < 0.01; ***, p < 0.001 relative to Cr alone treatments).
Reduction of Fe(III) to Fe(II)
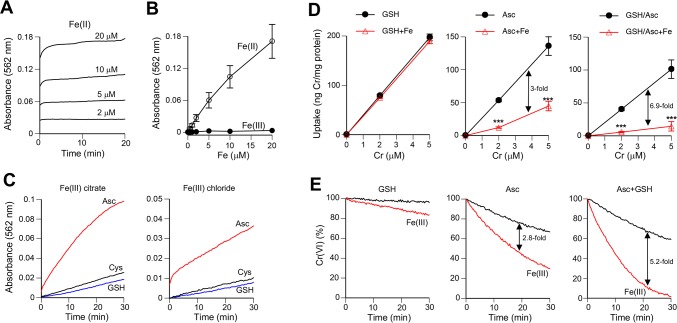
Our observations on a dramatic acceleration of Cr(VI) reduction by Asc/GSH in the presence of Fe(III) indicates the appearance of some kinetically superior Cr(VI)-reducing species. Fe(II) is an attractive candidate for this reducing agent, as it is a well-known reducer of Cr(VI) under acidic pH or anaerobic conditions.55 Addition of Fe(II) failed to reduce Cr(VI) at physiological pH in aerobic solutions,56 which is consistent with our data on the inability of Fe(II) sulfate to inhibit Cr(VI) uptake in the absence of Asc/GSH supplementation (Figure 3C). The loss of Cr(VI)-reducing activity by Fe(II) in aerobic solutions is caused by its very rapid oxidation to Fe(III) by O2. Despite this counter supportive evidence, we decided to investigate the possibility that Fe(II) could still be formed from Fe(III) in the complex biological medium with full oxygenation. We used a well-established ferrozine assay for the specific detection of Fe(II).41,42 The reaction between ferrozine and Fe(II) (stabilized with ascorbic acid) was complete within a few minutes and showed the expected concentration-dependent increases in A562, which was unchanged for Fe(III) (Figure 5A, B). The ability of ferrozine to outcompete O2 for Fe(II) in the biological medium indicated that this assay can detect the appearance (even if transient) of Fe(II) in Fe(III)-containing solutions. To evaluate the production of Fe(II) from Fe(III), we incubated Fe(III) citrate or Fe(III) chloride with 0.1 mM concentrations of Asc, GSH or Cys in RPMI-1640 medium in the presence of ferrozine. We found that all three bioreducers were capable of generating Fe(II), although Asc was dramatically more effective in Fe(III) reduction in comparison to both thiols (Figure 5C). A lower extent of Fe(II) formation from Fe(III) chloride most likely resulted from the formation of poorly soluble polynuclear products and unreactive hydroxides when it was added to the medium with physiological pH. Complexation of Fe(III) with bidentate ligands present in the biological medium (amino acids, phosphate, carbonate) can retain a large portion of Fe(III) in the soluble form permitting its reduction to Fe(II). Thus, Fe(II) was formed at physiologically relevant conditions in the presence of O2, principally through reduction of Fe(III) by Asc. To obtain further support for this conclusion, we investigated cellular uptake of Cr(VI) as a measure of its detoxification via reduction by Fe(II) in the extracellular medium. Cells were incubated in the presence of Asc, GSH and their mixture with and without addition of Fe(III) to cell culture medium. In agreement with Fe(II) measurements, we found that the inclusion of Fe(III) in the GSH-supplemented medium did not significantly decrease Cr(VI) uptake whereas Fe(III) strongly inhibited (3-fold difference in slopes) cellular accumulation of Cr from the Asc-containing medium (Figure 5D). The suppressive effect of Fe(III) on Cr(VI) uptake was even more potent when both GSH and Asc were present in the medium (6.9-fold difference in slopes) (Figure 5D, right panel). Examination of Cr(VI) reduction by the same combination of reducers and Fe(III) found very similar effects. Specifically, Fe(III) only modestly accelerated Cr(VI) reduction by GSH whereas its impact on promotion of Cr(VI) reduction by Asc was much stronger and even more potent for a mixture of Asc with GSH (Figure 5E). Overall, these results revealed the formation of Fe(II) from Fe(III) by physiological concentrations of Asc and GSH, which strongly enhanced rates of Cr(VI) reduction. A rapid disappearance of Cr(VI) from cell culture media led to a severely diminished accumulation of this toxic metal in cells.
Figure 5.
Conversion of Fe(III) to Fe(II) by Asc and thiols. All experiments were performed at 37 °C and used RPMI-1640 medium supplemented with 50 mM HEPES, pH 7.4. (A) Time dependence of ferrozine-Fe(II) complex formation. Reactions contained 100 μM ferrozine and indicated concentrations of Fe(II) ammonium sulfate prepared as 1 mM stock in 10 mM ascorbic acid. (B) Fe(II) specificity of A562 absorbance by ferrozine (10 min incubations). Fe(II)–Fe(II) ammonium sulfate dissolved in ascorbic acid, Fe(III)–Fe(III) ammonium citrate dissolved in water. Data are means ± SD of triplicate measurements. (C) Time-course of Fe(II) formation from Fe(III) in the presence of 0.1 mM concentrations of reducers (ferrozine assay, means of triplicate measurements). The source of Fe(III) was Fe(III) ammonium citrate or Fe(III) chloride hexahydrate (both at 10 μM final concentrations, 1 mM stock solutions in H2O for Fe-citrate and in 10 mM HCl for FeCl3). (D) Uptake of Cr(VI) by H460 cells in the presence of different extracellular reducers with or without 5 μM Fe(III) citrate (1 h uptake). Cell culture media contained 100 μM GSH, 50 μM Asc, or both reducers. Data are means ± SD, n = 3. Statistics: ***, p < 0.001 relative to samples without Fe(III). Fold differences in the slopes of linear fits are indicated. (E) Effects of Fe(III) on reduction of Cr(VI) by GSH, Asc or Asc+GSH (10 μM Cr, 5 μM Fe(III) citrate, 100 μM GSH, 50 μM Asc or both reducers). Plots are based on means of triplicate measurements. Fold differences in the slopes of exponential fits are indicated.
Effects of Fe(II/III) Chelators
Variation in reduction rates for Cr(VI) among different cell culture media closely correlated with their Fe concentrations (Figure 2). F-12K medium had the highest Fe content and displayed the fastest reduction of Cr(VI) by Asc or thiols. To test the Fe-dependence of these effects, we examined the impact of addition of specific chelators of Fe(III) and Fe(II) that block redox cycling of iron. We found that the presence of either a highly specific Fe(III) chelator deferoxamine or the Fe(II) chelator o-phenanthroline in the iron-rich F-12K medium strongly suppressed rates of Cr(VI) reduction by Cys whereas these chelators did not change Cr(VI) reduction in the iron-poor RPMI-1640 medium (Figure 6A, left and middle panels). The addition of the Fe(III)-binding DTPA practically eliminated the differences in Cr(VI) reduction by Cys between F-12K and RPMI media (Figure 6A, right panel). Similar to Cys-driven reactions, the presence of Fe(III) chelators also inhibited Cr(VI) reduction by Asc in F-12K but not in RPMI-1640 medium (Figure 6B). Thus, a high Fe content of F-12K medium was responsible for its fast rates of Cr(VI) metabolism by bioreducers, further highlighting a potent catalytic effect of Fe(II) formation.
Figure 6.
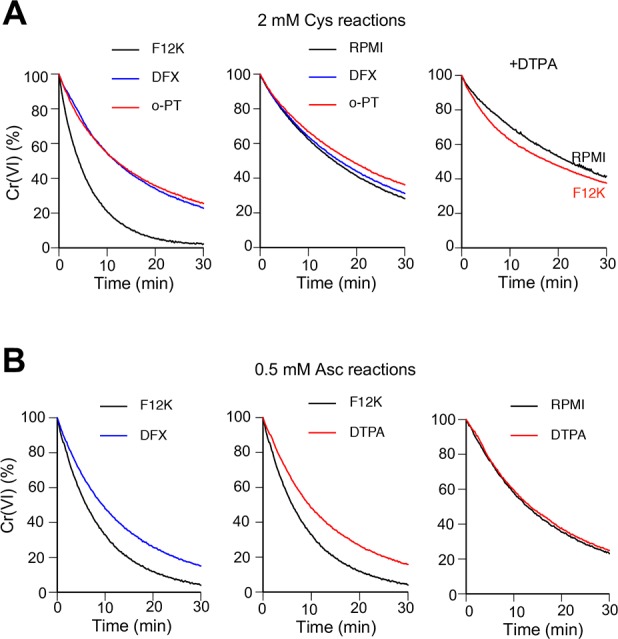
Effects of Fe(II/III) chelators on Cr(VI) reduction in iron-rich and iron-poor cell culture media. Kinetics of Cr(VI) reduction in F-12K (iron-rich) and RPMI-1640 (iron-poor) media were measured as described in Figure 2. Reactions contained 50 μM Cr(VI), indicated reducer and solvent control or one of Fe chelators (200 μM deferoxamine (DFX), 200 μM o-phenanthroline (o-PT) or 5 mM DTPA). (A) Reduction of Cr(VI) in the presence of 2 mM Cys or (B) 0.5 mM Asc. Plots are based on means of triplicate measurements taken every 20 s.
Cr(VI) Metabolism in the Presence of Ni(II) or Mn(II)
In addition to Fe, soluble fractions of stainless steel welding fumes also contain variable amounts of Ni and Mn, which are present in lower concentrations than Cr(VI).29,57 We found that cellular accumulation of Cr was not affected by the addition of Ni(II) or Mn(II) ions at 0.5:1 or 1:1 ratio to Cr(VI) (Figure 7A), which contrasts with the above findings on a dramatic inhibition of Cr uptake in media with the same stoichiometry of Fe to Cr(VI) (Figure 3 and Figure 5). A preincubation of Ni(II) or Mn(II) with cells for 2 h prior to the addition of Cr(VI) also failed to change cellular accumulation of Cr (Figure 7B). Consistent with the uptake results, the presence of Ni(II) or Mn(II) ions had no effects on kinetics of Cr(VI) reduction in the cell culture medium (Figure 7C), indicating that the same concentrations of Cr(VI) were available for cells. The presence of Ni(II) or Mn(II) during Cr(VI) treatments also produced no effects on cell viability (Figure 7D). To exclude a possibility that some cell culture/serum components masked potential interactions of Ni(II) or Mn(II) with Cr(VI), we measured kinetics of Cr(VI) reduction in the presence of these metal ions in HEPES buffer. Again, no impact of either Ni(II) or Mn(II) ions on Asc- or GSH-driven reduction of Cr(VI) was detected (Figure 7E,F). Thus, Ni(II) and Mn(II) did not show detectable chemical or toxicological interactions with Cr(VI) at environmentally relevant ratios of these metals.
Figure 7.
Metabolism and toxicity of Cr(VI) in the presence of Ni(II) and Mn(II) ions. H460 cells were treated with Cr(VI) in the complete cell culture medium (RPMI-1640, 50 mM HEPES, pH 7.4, 10% FBS) additionally containing 50 μM Asc and 100 μM GSH. Reduction kinetics of Cr(VI) was measured at 37 °C. (A) Cellular uptake of Cr(VI) in the presence of Ni(II) or Mn(II). Cells were incubated with 10 μM Cr(VI) for 1 h. Data are means ± SD (n = 3). (B) Accumulation of Cr by cells preincubated with Ni/Mn ions for 2 h prior to the addition of Cr(VI) (1 h uptake). Data are means ± SD (n = 3). (C) Kinetics of Cr(VI) reduction by Asc/GSH in HEPES-supplemented RPMI-1640 medium. Samples contained 100 μM Asc, 200 μM GSH, 20 μM Cr(VI) and 0, 5, or 20 μM Ni(II) or Mn(II). (D) Viability of cells treated with Cr(VI) in the presence of 20 μM Ni(II) or Mn(II) ions. Cells were treated with metals for 3 h followed by 48 h recovery prior to cytotoxicity measurements. Data are means ± SD (n = 3). (E) Kinetics of Cr(VI) reduction in HEPES buffer (100 mM HEPES, pH 7.4, 50 mM NaCl) in the presence of 0.2 mM Asc or (F) 2 mM GSH. Reactions contained 20 μM Cr(VI). Graphs show means of triplicate measurements.
Studies in Stem Cell-like HBEC3-KT and Primary Human Bronchial Epithelial Cells
Multiple lines of evidence indicate that a majority of human cancers originate from tissue-specific stem cells.58,59 Thus, it would be important to confirm our main findings in a stem cell-like model. As primary human lung stem cells are not available, we chose CDK4/telomerase-immortalized HBEC3-KT human bronchial epithelial cells as a biological model of stem cells. HBEC3-KT cells display stem cell-like properties such as the ability to differentiate into different types of lung cells.60 As prolonged exposure to serum triggers their differentiation, HBEC3-KT cells are grown in a serum-free medium that we found to be rich in iron (0.84 ± 0.18 μM). To exclude the confounding influences of the already present iron and potential other medium-specific effects, we treated HBEC3-KT cells with Cr(VI) and other metals in RPMI-1640 medium, which provides conditions for a direct comparison of H460 and HBEC3-KT cells instead of combined effects of a cell line and its medium. As in H460 cells, we found that the addition of Fe(III) to a complete RPMI-1640 medium supplemented with physiological Asc/GSH resulted in a severe inhibition of Cr(VI) uptake by HBEC3-KT cells (Figure 8A). Ni(II) and Mn(II) ions again had no significant effects on the cellular accumulation of Cr(VI) (Figure 8B). Similar to other cultured cells, HBEC3-KT are ascorbate-deficient in the standard tissue culture although they are proficient at transporting both reduced and oxidized forms of vitamin C (Figure 8C). We used this property of HBEC3-KT cells to restore their levels of Asc by preincubation with Asc- or DHA-supplemented media, which was possible only with DHA in H460 cells. We found that irrespective of the Asc restoration approach, only chromate but no other metal ions induced dose-dependent cytotoxic effects in HBEC3-KT cells (Figure 8D,E). Consistent with Cr(VI) uptake findings, addition of Fe(III) in two chemical forms also prevented cytotoxicity of Cr(VI) treatments (Figure 8F). Overall, studies in HBEC3-KT confirmed our main observations in H460 cells, such as the ability of Fe(III) to inhibit cytotoxicity and uptake of Cr(VI), which was by far the most cytotoxic metal among the main metal ions that are typically released from stainless steel welding particles.
Figure 8.
Cr(VI) uptake and metal toxicity in HBEC3-KT cells. (A) Accumulation of Cr in cells after 1 h incubation in RPMI-1640 medium containing 50 mM HEPES (pH 7.4), 10% FBS, 100 μM GSH, 50 μM Asc, 5 μM Cr(VI) and 0–10 μM Fe(III) citrate. Data are means ± SD; n = 3; **, p < 0.01; ***, p < 0.001 relative to 0 μM Fe. (B) Normal cellular uptake of Cr(VI) in the presence Ni(II) or Mn(II) (experimental conditions as in panel A). (C) Cellular levels of Asc after incubations with Asc (3 h) or DHA (2 h) in the complete growth medium. Data are means ± SD, n = 3. (D) Viability of cells preloaded with 0.5 mM Asc and treated with indicated metals for 3 h in RPMI-1640 medium supplemented with 50 mM HEPES (pH 7.4) and growth factors. During 48 h recovery, a standard growth medium for HBEC3-KT cells was used. Data are means ± SD; n = 3; *, p < 0.05; **, p < 0.01; ***, p < 0.001 relative to untreated controls. (E) Viability of cells pretreated with 0.2 mM DHA and then treated with metals as in panel D. (F) Loss of Cr(VI) cytotoxicity during cotreatments with Fe(III). Cells were preincubated with 0.2 mM DHA and then treated for 4 h with Cr(VI) alone or in the presence of 5 μM Fe(III) citrate or 10 μM FeCl3 in RPMI-1640 media containing 50 mM HEPES (pH 7.4), growth factors and 50 μM Asc/100 μM GSH. A standard growth medium for HBEC3-KT cells was used during 72 h recovery. Data are means ± SD; n = 3; ***, p < 0.001; ****, p < 0.0001 relative to Cr(VI) alone.
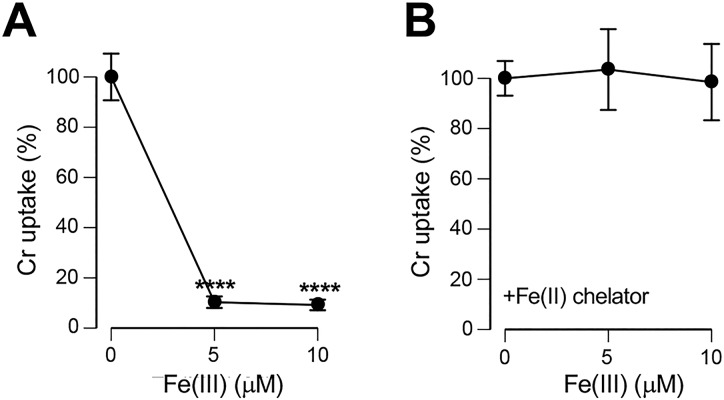
Finally, we measured the effects of Fe(III) ions on Cr(VI) uptake in primary human bronchial epithelial cells. Similar to two immortalized cell models, we found that the addition of Fe(III) ions to the cell culture medium resulted in a severe inhibition (∼10-fold) of Cr(VI) uptake by primary cells (Figure 9A). This antagonistic effect required interactions of Cr(VI) with Fe(II), as the inclusion of a specific Fe(II) chelator o-phenanthroline completely eliminated uptake-suppressing effects of the Fe(III) addition (Figure 9B). These results are fully consistent with the earlier observed loss of catalytic activity of Fe ions on Cr(VI) reduction when o-phenanthroline was added to the Fe-rich cell culture media (Figure 6A). As in other cells, addition of Ni(II) or Mn(II) to the Asc/GSH-supplemented culture media did not significantly change Cr(VI) accumulation by primary cells (1 h uptake, 5 μM Cr ±10 μM Ni or Mn: 100 ± 6.3%, 117.5 ± 13.1%, and 108.3 ± 2.6% Cr uptake, respectively).
Figure 9.
Effects of Fe(III) ions on Cr(VI) uptake in primary human bronchial epithelial cells. Cells were incubated for 1 h with 5 μM Cr(VI) for 1 h in RPMI-1640 medium supplemented with 50 mM HEPES (pH 7.4), growth factors, 50 μM Asc, 100 μM GSH and 0–10 μM Fe(III) citrate. (A) Suppression of Cr(VI) uptake by extracellular Fe(III). Data are means ± SD,; n = 3; ****, p < 0.0001 relative to 0 μM Fe(III). (B) Loss of Fe(III) effects on Cr(VI) uptake in the presence of o-phenanthroline (0.1 mM).
Discussion
Studies of welding fumes composition and release of its constituents into solutions showed that Cr(VI) was the main soluble metal species.29−31,57 Other metals such as Ni, Mn, and Fe were also present in the soluble fraction but generally in significantly lower amounts. Cr(VI) is a firmly established human respiratory carcinogen1,2 with high risks and a linear-dose dependence for lung cancer incidence found in chromate production workers.22−24 Recognition of these risks led OSHA in 2006 to lower permissible exposure limit (PEL) for ambient Cr(VI) 10-fold to 5 μg/m3.28 Even under this new occupational standard, Cr(VI) exposures could cause up to 45 lethal lung cancers per 1000 workers according to the EPA’s estimates.27 PEL values for other metals present in welding fumes are much higher than for Cr(VI) reflecting their lower carcinogenic and other toxic properties (soluble Ni, 50 μg/m3, Mn compounds or fumes, −200 μg/m3, iron oxide fumes, −5 mg/m3). Our data on a dramatically higher toxicity of soluble Cr(VI) in comparison to Ni(II), Mn(II), and Fe(III) ions in human lung epithelial cells are consistent with the PEL values for these metals and earlier studies on cytotoxicity of mild steel (no Cr) and stainless steel welding particles in cultures of lung macrophages without Asc supplementation.57 However, inhalation of welding fumes from Cr(VI)-rich stainless steel welding fumes has not been associated with particularly high risks of lung cancers. A recent IARC evaluation has concluded that both mild steel (no Cr) and stainless steel welding increased lung cancer risks.38 Epidemiological studies, on the basis of which this conclusion was reached, have found that cancer risks for stainless steel welders were either similar or only moderately higher in comparison to mild steel welders.32−37 Considering a potent carcinogenic activity of Cr(VI) as a single metal (in chromate production workers) and generally assumed additive or synergistic effects of carcinogens in mixtures, low cancer risks for stainless steel welders exposed to Cr(VI) in combination with other toxic in excess (Mn, Fe) and carcinogenic (Ni) metals have been surprising.
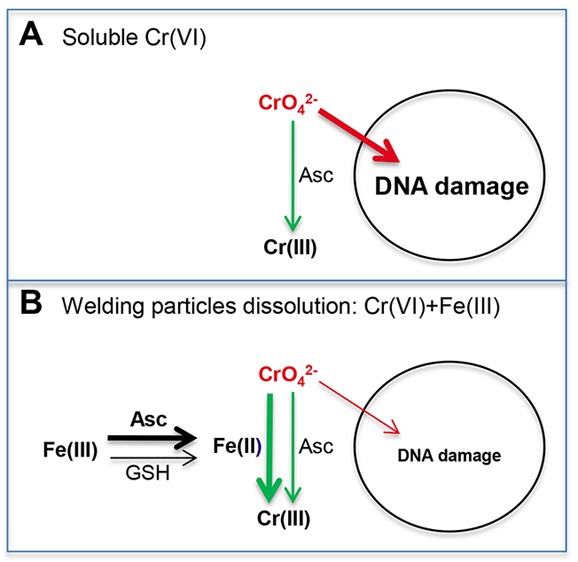
Our findings offer a chemical explanation for the weakened toxicological potency of Cr(VI) when present in the biological solutions in the mixture with other constituents of welding fumes, namely, Fe(III). Collectively, our results provide a strong support for the role of Fe ions in the accelerated detoxification of solubilized Cr(VI) through its rapid extracellular reduction (Figure 10). Although welding fumes contain and release Fe in its high oxidative state +3,29 a mixture of GSH and Asc added to the cell culture medium at concentrations approximating those in human lung lining fluid were capable of a relatively fast reduction of Fe(III) to Fe(II). Fe(II) then rapidly reduced chromate, which led to the several-fold acceleration of Cr(VI) detoxification outside the cells and the resulting dramatic suppression of cellular accumulation of Cr. Importantly, this catalytic activity of Fe ions was readily detectable at much lower concentrations relative to Cr(VI) (1.7 μM Fe versus 50 μM Cr in reactions in F-12K medium, for example). Acceleration of chromate reduction was observed in different biological media with and without serum and using different Fe compounds. Although reduction of Cr(VI) by Fe(II) via one-electron transfer is a well-known chemical reaction,55 it has not yet been considered for the biological fluids at the sites of Cr(VI) exposure. A rapid oxidation of Fe(II) by O2 at neutral pH was thought to limit its involvement in Cr(VI) reduction to acidic or anaerobic abiotic environments. A direct addition of Fe(II) to buffers with physiological pH reduced Cr(VI) only in anaerobic conditions.56 Two physiologically relevant conditions of our experimental setup allowed us to detect extensive Fe(II) formation and the resulting acceleration of Cr(VI) reduction. One is the addition to the cell culture medium of physiological concentrations of Asc and GSH, which catalyzed reduction of Fe(III) to Fe(II). Although it was present at 2 times lower concentration relative to GSH, Asc was responsible for the majority of Fe(II) production and the subsequent acceleration of chromate reduction. The second important characteristics of our conditions is the use of complex biological solutions such as cell culture media. Previous studies have generally used simple buffer systems, which presumably made them more amenable to mechanistic interpretations of the measured parameters. However, buffers with neutral pH promote the formation of poorly soluble and unreactive polynuclear Fe(III) species, which can be avoided through the use of Fe(III) in a complex with a chelating agent. We found that Fe(III) was redox active in cell culture media even when it was added in the form of inorganic salt, which can be attributed to binding of Fe(III) by bidentate physiological anions, such as amino acids, carbonate, or phosphate. Consistent with this suggestion, Fe(III)-binding bidentate carboxylates strongly promoted Cr(VI) reduction by Fe(II) in buffers with mildly acidic pH.61 Fe(II) formed from Fe(III) bound to biological ligands could also be less reactive with O2 than Fe(II)-aqua complex. The concept of a diminished reactivity with O2 after complexation with organic ligands is supported by the stability of Fe(II)-ferrozine complex in the aerobic solutions [as observed in the Fe(II) assay].
Figure 10.
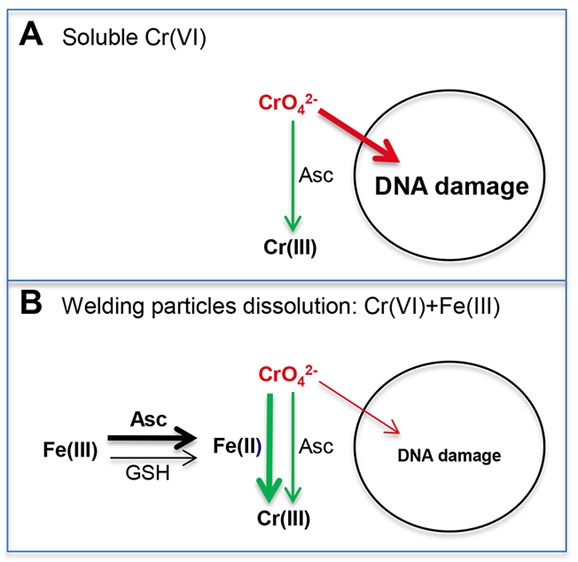
Mechanism for detoxification of solubilized Cr(VI) in the presence of Fe(III) ions. (A) In the cases of exposures to soluble Cr(VI) as a single metal, extracellular Asc offers a moderate protection by reducing a fraction of chromate anions to nontoxic Cr(III). (B) Coexposure to chromate and Fe(III) ions released from welding fumes causes a much faster detoxification of Cr(VI) via its extracellular reduction by Fe(II) produced primarily from Fe(III) by ascorbate. GSH also contributes to the formation of Fe(II) from Fe(III) but to a much smaller extent than ascorbate.
The mechanism of accelerated Cr(VI) reduction via Asc-dependent formation of Fe(II) raises a question why Fe(II) is a much better reducer of Cr(VI) than Asc or GSH despite inhibitory effects of dissolved O2. The rate of Cr(VI) reduction is clearly driven by kinetic not thermodynamic factors, as Asc has a lower reduction potential (E0 = +0.06) than >10-times slower reducer GSH (E0 = −0.23). The reduction potential of Fe(II) is even lower (E0 = +0.77) than that of Asc. For thiols, reduction of Cr(VI) requires establishment of the inner sphere complex (direct bond) between the reducer and Cr center.62,63 The formation of Cr(VI)-thioester complex is known to be a slow and rate-limiting step in reduction of Cr(VI) by GSH.62 The sluggishness of GSH-chromate thioester formation can be attributed to the negative charge of both molecules [Cr(VI) exists as CrO42– anion at physiological conditions] and a weak affinity of Cr6+ center toward the SH group. Although Asc is also anionic species at physiological pH (Asc-O–), its association with CrO42– is facilitated by a strong preference of the Cr6+ center for the negatively charged oxygen. A very rapid Cr(VI) reduction by Fe(II) can be attributed to (i) electrostatic attraction between CrO42– and Fe2+ ions and (ii) a fast electron transfer through binding to Cr–O– (outer sphere complex) and its reduction to Cr–OH bond. Electron transfer to a metal center via the outer sphere mechanism permits much faster reduction reactions.64
GSH was a very slow reducer of Cr(VI) with barely detectable activity at 100 μM (t1/2 = 5.4 h), which is its average concentration in human lung lining fluid. Consequently, it made a marginal impact on the loss of Cr(VI) in physiological mixtures of GSH with Asc. In the presence of Fe(III), GSH showed a more significant contribution to Cr(VI) metabolism, as assessed by higher Cr(VI) reduction rates and inhibition of Cr uptake by cells. A greater effect of GSH on Cr(VI) in Asc/GSH-containing medium in the presence of Fe(III) can be attributed to the following factors:
-
(i)
slower oxidation of Asc (1.3-times slower loss in our analyses);
-
(ii)
Asc-independent generation of Fe(II) from Fe(III);
-
(iii)
expanded participation of GSH in Cr(VI) to Cr(III) reduction, preserving Asc for Fe(III) to Fe(II) reduction. In Fe-free conditions, Cr(VI) reduction is dominated by the two-electron reducer Asc, yielding Cr(IV) as the first intermediate.14−16 In Fe-containing reactions, one-electron reduction of Cr(VI) by Fe(II) produces Cr(V), which can be further reduced by GSH. GSH is known to readily form complexes with Cr(V), which are stable enough to be purified at low temperature.65
Complexity of Welding Fume Effects
Lung tumorigenesis by inhaled particles generated from stainless steel welding probably results from a combined activity of metals present in both soluble and insoluble fractions. Our results showed that solubilized Cr(VI) undergoes a rapid extracellular detoxification in the presence of Fe ions and physiological concentrations of Asc. These findings argue that soluble Cr(VI), a potent human lung carcinogen by itself as found in chromate production and chrome plating,23,24,26−28 released in the biological solutions along with the catalytic amounts of another welding fume component, iron, loses its toxicological potency. Insoluble Cr(VI) is resistant to this reduction-based detoxification20 and therefore, internalization and a subsequent dissolution of stainless-steel welding particles can deliver carcinogenic Cr(VI) into the cells. The amount of the soluble metals varies depending on the specifics of the particular welding process.29−31 Thus, it is possible that the observed variability in lung cancer risks found in different cohorts of stainless steel welders33−37 is linked to the different frequency of specific welding procedures and the resulting differences in the relative amounts of insoluble Cr(VI) escaping extracellular inactivation by Fe/Asc. The extent of Fe solubilization should also affect the carcinogenic potency of welding particles containing soluble Cr(VI). Carcinogenicity of Cr-free mild steel welding38 and significant tumor promotion effects in mouse lungs by welding particles with no66 or a low content of Cr(VI)67 also suggest that a high lung burden of Fe may also be tumorigenic by itself, possibly as a result of elevated oxidative stress and chronic inflammation.
Glossary
Abbreviations
- Asc
ascorbate
- ATCC
American Type Culture Collection
- DTPA
diethylenetriaminepentaacetic acid
- FBS
fetal bovine serum
- GSH
glutathione
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- IARC
International Agency for Cancer Research
- PEL
permissible exposure limit
This work was supported by Grants ES008786 and ES028072 from the National Institute of Environmental Health Sciences.
The authors declare no competing financial interest.
References
- Langard S. (1990) One hundred years of chromium and cancer: a review of epidemiological evidence and selected case reports. Am. J. Ind. Med. 17, 189–214. 10.1002/ajim.4700170205. [DOI] [PubMed] [Google Scholar]
- Salnikow K.; Zhitkovich A. (2008) Genetic and epigenetic mechanisms in metal carcinogenesis and cocarcinogenesis: nickel, arsenic and chromium. Chem. Res. Toxicol. 21, 28–44. 10.1021/tx700198a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh Y.; Ishikawa Y.; Nakagawa K.; Hirano T.; Tsuchiya E. (1997) A follow-up study of progression from dysplasia to squamous cell carcinoma with immunohistochemical examination of p53 protein overexpression in the bronchi of ex-chromate workers. Br. J. Cancer 75, 678–683. 10.1038/bjc.1997.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose T.; Kondo K.; Takahashi Y.; Ishikura H.; Fujino H.; Tsuyuguchi M.; Hashimoto M.; Yokose T.; Mukai K.; Kodama T.; Monden Y. (2002) Frequent microsatellite instability in lung cancer from chromate-exposed workers. Mol. Carcinog. 33, 172–180. 10.1002/mc.10035. [DOI] [PubMed] [Google Scholar]
- Voitkun V.; Zhitkovich A.; Costa M. (1998) Cr(III)-mediated crosslinks of glutathione or amino acids to the DNA phosphate backbone are mutagenic in human cells. Nucleic Acids Res. 26, 2024–2030. 10.1093/nar/26.8.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quievryn G.; Peterson E.; Messer J.; Zhitkovich A. (2003) Genotoxicity and mutagenicity of chromium(VI)/ascorbate-generated DNA adducts in human and bacterial cells. Biochemistry 42, 1062–1070. 10.1021/bi0271547. [DOI] [PubMed] [Google Scholar]
- Reynolds M.; Peterson E.; Quievryn G.; Zhitkovich A. (2004) Human nucleotide excision repair efficiently removes chromium-DNA phosphate adducts and protects cells against chromate toxicity. J. Biol. Chem. 279, 30419–30424. 10.1074/jbc.M402486200. [DOI] [PubMed] [Google Scholar]
- Costa M. (1991) DNA-protein complexes induced by chromate and other carcinogens. Environ. Health Perspect. 92, 45–52. 10.1289/ehp.919245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfie A.; Hagan E.; Zhitkovich A. (2010) Mechanism of DNA-protein cross-linking by chromium. Chem. Res. Toxicol. 23, 341–347. 10.1021/tx9003402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecevic A.; Menard H.; Gurel V.; Hagan E.; DeCaro R.; Zhitkovich A. (2009) WRN helicase promotes repair of DNA double-strand breaks caused by aberrant mismatch repair of chromium-DNA adducts. Cell Cycle 8, 2769–2778. 10.4161/cc.8.17.9410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhitkovich A. (2005) Importance of chromium-DNA adducts in mutagenicity and toxicity of chromium(VI). Chem. Res. Toxicol. 18, 3–11. 10.1021/tx049774+. [DOI] [PubMed] [Google Scholar]
- Standeven A. M.; Wetterhahn K. E. (1991) Ascorbate is the principal reductant of chromium (VI) in rat liver and kidney ultrafiltrates. Carcinogenesis 12, 1733–1737. 10.1093/carcin/12.9.1733. [DOI] [PubMed] [Google Scholar]
- Standeven A. M.; Wetterhahn K. E. (1992) Ascorbate is the principal reductant of chromium(VI) in rat lung ultrafiltrates and cytosols, and mediates chromium-DNA binding in vitro. Carcinogenesis 13, 1319–1324. 10.1093/carcin/13.8.1319. [DOI] [PubMed] [Google Scholar]
- Stearns D. M.; Wetterhahn K. E. (1994) Reaction of Cr(VI) with ascorbate produces chromium(V), chromium(IV), and carbon-based radicals. Chem. Res. Toxicol. 7, 219–230. 10.1021/tx00038a016. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Lay P. A. (1996) EPR studies of the reactions of Cr(VI) with L-ascorbic acid, L-dehydroascorbic acid, and 5,6-O-isopropylidene-L-ascorbic acid in water. Implications for chromium(VI) genotoxicity. J. Am. Chem. Soc. 118, 12624–12637. 10.1021/ja961824c. [DOI] [Google Scholar]
- DeLoughery Z.; Luczak M. W.; Zhitkovich A. (2014) Monitoring Cr intermediates and reactive oxygen species with fluorescent probes during chromate reduction. Chem. Res. Toxicol. 27, 843–851. 10.1021/tx500028x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds M.; Armknecht S.; Johnston T.; Zhitkovich A. (2012) Undetectable role of oxidative DNA damage in cell cycle, cytotoxic and clastogenic effects of Cr(VI) in human lung cells with restored ascorbate levels. Mutagenesis 27, 437–443. 10.1093/mutage/ger095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczak M. W.; Green S. E.; Zhitkovich A. (2016) Different ATM signaling in response to chromium(VI) metabolism via ascorbate and nonascorbate reduction: implications for in vitro models and toxicogenomics. Environ. Health Perspect. 124, 61–66. 10.1289/ehp.1409434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczak M. W.; Zhitkovich A. (2013) Role of direct reactivity with metals in chemoprotection by N-acetylcysteine against chromium(VI), cadmium(II) and cobalt(II). Free Radical Biol. Med. 65, 262–269. 10.1016/j.freeradbiomed.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawic C.; Luczak M. W.; Zhitkovich A. (2017) Variation in extracellular detoxification is a link to different carcinogenicity among chromates in rodent and human lungs. Chem. Res. Toxicol. 30, 1720–1729. 10.1021/acs.chemrestox.7b00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso T. F. (1997) Chromium as an industrial carcinogen: Part I. Am. J. Ind. Med. 31, 129–139. . [DOI] [PubMed] [Google Scholar]
- Gibb H. J.; Lees P. S.; Pinsky P. F.; Rooney B. C. (2000) Lung cancer among workers in chromium chemical production. Am. J. Ind. Med. 38, 115–126. . [DOI] [PubMed] [Google Scholar]
- Luippold R. S.; Mundt K. A.; Austin R. P.; Liebig E.; Panko J.; Crump C.; Crump K.; Proctor D. (2003) Lung cancer mortality among chromate production workers. Occup. Environ. Med. 60, 451–457. 10.1136/oem.60.6.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor D. M.; Suh M.; Mittal L.; Hirsch S.; Valdes Salgado R.; Bartlett C.; Van Landingham C.; Rohr A.; Crump K. (2016) Inhalation cancer risk assessment of hexavalent chromium based on updated mortality for Painesville chromate production workers. J. Exposure Sci. Environ. Epidemiol. 26, 224–231. 10.1038/jes.2015.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC Monograph on the Evaluation of Carcinogenic Risk to Humans. Chromium, Nickel and Welding; IARC: Lyon, 1990; Vol. 48, pp 49–508. [PMC free article] [PubMed] [Google Scholar]
- Sorahan T.; Burges D. C.; Hamilton L.; Harrington J. M. (1998) Lung cancer mortality in nickel/chromium platers, 1946–95. Occup. Environ. Med. 55, 236–242. 10.1136/oem.55.4.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park R. M.; Bena J. F.; Stayner L. T.; Smith R. J.; Gibb H. J.; Lees P. S. (2004) Hexavalent chromium and lung cancer in the chromate industry: a quantitative risk assessment. Risk Anal 24, 1099–1108. 10.1111/j.0272-4332.2004.00512.x. [DOI] [PubMed] [Google Scholar]
- Occupational Safety and Health Administration (OSHA), Department of Labor . Occupational exposure to hexavalent chromium. Final rule. Fed. Regist. 2006, 71, 10099–10385. [PubMed] [Google Scholar]
- Mei N.; Belleville L.; Cha Y.; Olofsson U.; Odnevall Wallinder I.; Persson K. A.; Hedberg Y. S. (2018) Size-separated particle fractions of stainless steel welding fume particles - A multi-analytical characterization focusing on surface oxide speciation and release of hexavalent chromium. J. Hazard. Mater. 342, 527–535. 10.1016/j.jhazmat.2017.08.070. [DOI] [PubMed] [Google Scholar]
- Taylor M. D.; Roberts J. R.; Leonard S. S.; Shi X.; Antonini J. M. (2003) Effects of welding fumes of different composition and solubility on free radical production and acute lung injury and inflammation in rats. Toxicol. Sci. 75, 181–191. 10.1093/toxsci/kfg173. [DOI] [PubMed] [Google Scholar]
- Antonini J. M.; Leonard S. S.; Roberts J. R.; Solano-Lopez C.; Young S. H.; Shi X.; Taylor M. D. (2005) Effects of stainless steel manual arc welding fume on free radical production, DNA damage, and apoptosis induction. Mol. Cell. Biochem. 279, 17–23. 10.1007/s11010-005-8211-6. [DOI] [PubMed] [Google Scholar]
- Steenland K. (2002) Ten-year update on mortality among mild-steel welders. Scand. J. Work, Environ. Health 28, 163–167. 10.5271/sjweh.660. [DOI] [PubMed] [Google Scholar]
- Siew S. S.; Kauppinen T.; Kyyronen P.; Heikkila P.; Pukkala E. (2008) Exposure to iron and welding fumes and the risk of lung cancer. Scand. J. Work, Environ. Health 34, 444–450. 10.5271/sjweh.1296. [DOI] [PubMed] [Google Scholar]
- Sorensen A. R.; Thulstrup A. M.; Hansen J.; Ramlau-Hansen C. H.; Meersohn A.; Skytthe A.; Bonde J. P. (2007) Risk of lung cancer according to mild steel and stainless steel welding. Scand. J. Work, Environ. Health 33, 379–386. 10.5271/sjweh.1157. [DOI] [PubMed] [Google Scholar]
- Mannetje A.; Brennan P.; Zaridze D.; Szeszenia-Dabrowska N.; Rudnai P.; Lissowska J.; Fabiánová E.; Cassidy A.; Mates D.; Bencko V.; Foretova L.; Janout V.; Fevotte J.; Fletcher T.; Boffetta P. (2012) Welding and lung cancer in Central and Eastern Europe and the United Kingdom. Am. J. Epidemiol. 175, 706–714. 10.1093/aje/kwr358. [DOI] [PubMed] [Google Scholar]
- Kendzia B.; Behrens T.; Jöckel K. H.; Siemiatycki J.; Kromhout H.; Vermeulen R.; Peters S.; Van Gelder R.; Olsson A.; Brüske I.; Wichmann H. E.; Stücker I.; Guida F.; Tardón A.; Merletti F.; Mirabelli D.; Richiardi L.; Pohlabeln H.; Ahrens W.; Landi M. T.; Caporaso N.; Consonni D.; Zaridze D.; Szeszenia-Dabrowska N.; Lissowska J.; Gustavsson P.; Marcus M.; Fabianova E.; ’t Mannetje A.; Pearce N.; Tse L. A.; Yu I. T.; Rudnai P.; Bencko V.; Janout V.; Mates D.; Foretova L.; Forastiere F.; McLaughlin J.; Demers P.; Bueno-de-Mesquita B.; Boffetta P.; Schüz J.; Straif K.; Pesch B.; Brüning T. (2013) Welding and lung cancer in a pooled analysis of case-control studies. Am. J. Epidemiol. 178, 1513–1525. 10.1093/aje/kwt201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrat M.; Guida F.; Mattei F.; Cénée S.; Cyr D.; Févotte J.; Sanchez M.; Menvielle G.; Radoï L.; Schmaus A.; Woronoff A. S.; Luce D.; Stücker I. (2016) Welding, a risk factor of lung cancer: the ICARE study. Occup. Environ. Med. 73, 254–261. 10.1136/oemed-2015-102964. [DOI] [PubMed] [Google Scholar]
- Guha N.; Loomis D.; Guyton K. Z.; Grosse Y.; El Ghissassi F.; Bouvard V.; Benbrahim-Tallaa L.; Vilahur N.; Muller K.; Straif K. (2017) Carcinogenicity of welding, molybdenum trioxide, and indium tin oxide. Lancet Oncol. 18, 581–582. 10.1016/S1470-2045(17)30255-3. [DOI] [PubMed] [Google Scholar]
- Reynolds M.; Zhitkovich A. (2007) Cellular vitamin C increases chromate toxicity via a death program requiring mismatch repair but not p53. Carcinogenesis 28, 1613–1620. 10.1093/carcin/bgm031. [DOI] [PubMed] [Google Scholar]
- Messer J.; Reynolds M.; Stoddard L.; Zhitkovich A. (2006) Causes of DNA single-strand breaks during reduction of chromate by glutathione in vitro and in cells. Free Radical Biol. Med. 40, 1981–1992. 10.1016/j.freeradbiomed.2006.01.028. [DOI] [PubMed] [Google Scholar]
- Hirayama T.; Nagasawa H. (2017) Chemical tools for detecting Fe ions. J. Clin. Biochem. Nutr. 60, 39–48. 10.3164/jcbn.16-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P. (1971) Spectrophotometric determination of serum iron at the submicrogram level with a new reagent (ferrozine). Anal. Biochem. 40, 450–458. 10.1016/0003-2697(71)90405-2. [DOI] [PubMed] [Google Scholar]
- Green S. E.; Luczak M. W.; Morse J. L.; DeLoughery Z.; Zhitkovich A. (2013) Uptake, p53 pathway activation and cytotoxic responses for Co(II) and Ni(II) in human lung cells: implications for carcinogenicity. Toxicol. Sci. 136, 467–477. 10.1093/toxsci/kft214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong V. C.; Cash H. L.; Morse J. L.; Lu S.; Zhitkovich A. (2012) S-phase sensing of DNA-protein crosslinks triggers TopBP1-independent ATR activation and p53-mediated cell death by formaldehyde. Cell Cycle 11 (2012), 2526–2537. 10.4161/cc.20905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong V. C.; Morse J. L.; Zhitkovich A. (2013) p53 activation by Ni(II) is a HIF-1α independent response causing caspases 9/3-mediated apoptosis in human lung cells. Toxicol. Appl. Pharmacol. 269, 233–239. 10.1016/j.taap.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds M. F.; Peterson-Roth E. C.; Johnston T.; Gurel V. M.; Menard H. L.; Zhitkovich A. (2009) Rapid DNA double-strand breaks resulting from processing of Cr-DNA crosslinks by both MutS dimers. Cancer Res. 69, 1071–1079. 10.1158/0008-5472.CAN-08-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D.; Zaugg K.; Mak T. W.; Elledge S. J. (2006) A role for the deubiquitinating enzyme USP28 in control of the DNA-damage response. Cell 126, 529–542. 10.1016/j.cell.2006.06.039. [DOI] [PubMed] [Google Scholar]
- Ortega-Atienza S.; Rubis B.; McCarthy C.; Zhitkovich A. (2016) Formaldehyde is a potent proteotoxic stressor causing rapid heat shock transcription factor 1 activation and Lys48-linked polyubiquitination of proteins. Am. J. Pathol. 186 (2016), 2857–2868. 10.1016/j.ajpath.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas C. I.; Zuniga F. A.; Salas-Burgos A.; Mardones L.; Ormazabal V.; Vera J. C. (2008) Vitamin C transporters. J. Physiol. Biochem. 64, 357–375. 10.1007/BF03174092. [DOI] [PubMed] [Google Scholar]
- Slade R.; Stead A. G.; Graham J. A.; Hatch G. E. (1985) Comparison of lung antioxidant levels in humans and laboratory animals. Am. Rev. Respir. Dis. 131, 742–746. [DOI] [PubMed] [Google Scholar]
- van der Vliet A.; O’Neill C. A.; Cross C. E.; Koostra J. M.; Volz W. G.; Halliwell B.; Louie S. (1999) Determination of low-molecular-mass antioxidant concentrations in human respiratory tract lining fluids. Am. J. Physiol. 276, L289–296. 10.1152/ajplung.1999.276.2.L289. [DOI] [PubMed] [Google Scholar]
- DeLoughery Z.; Luczak M. W.; Ortega-Atienza S.; Zhitkovich A. (2015) DNA double-strand breaks by Cr(VI) are targeted to euchromatin and cause ATR-dependent phosphorylation of histone H2AX and its ubiquitination. Toxicol. Sci. 143, 54–63. 10.1093/toxsci/kfu207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luczak M. W.; Zhitkovich A. (2018) Monoubiquitinated γ-H2AX: Abundant product and specific biomarker for non-apoptotic DNA double-strand breaks. Toxicol. Appl. Pharmacol. 355, 238–246. 10.1016/j.taap.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartori A. A.; Lukas C.; Coates J.; Mistrik M.; Fu S.; Bartek J.; Baer R.; Lukas J.; Jackson S. P. (2007) Human CtIP promotes DNA end resection. Nature 450, 509–514. 10.1038/nature06337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espenson J. H. (1970) Oxidation of transition metal complexes by chromium(VI). Acc. Chem. Res. 3, 347–353. 10.1021/ar50034a004. [DOI] [Google Scholar]
- Mikalsen A.; Capellmann M.; Alexander J. (1995) The role of iron chelators and oxygen in the reduced nicotinamide adenine dinucleotide phosphate-cytochrome P450 oxidoreductase-dependent chromium(VI) reduction. Analyst 120, 935–938. 10.1039/an9952000935. [DOI] [PubMed] [Google Scholar]
- Antonini J. M.; Lawryk N. J.; Murthy G. G.; Brain J. D. (1999) Effect of welding fume solubility on lung macrophage viability and function in vitro. J. Toxicol. Environ. Health, Part A 58, 343–363. 10.1080/009841099157205. [DOI] [PubMed] [Google Scholar]
- Visvader J. E. (2011) Cells of origin in cancer. Nature 469, 314–322. 10.1038/nature09781. [DOI] [PubMed] [Google Scholar]
- Sutherland K. D.; Berns A. (2010) Cell of origin of lung cancer. Mol. Oncol. 4, 397–403. 10.1016/j.molonc.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado O.; Kaisani A. A.; Spinola M.; Xie X. J.; Batten K. G.; Minna J. D.; Wright W. E.; Shay J. W. (2011) Multipotent capacity of immortalized human bronchial epithelial cells. PLoS One 6, e22023. 10.1371/journal.pone.0022023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buerge I. J.; Hug S. J. (1998) Influence of organic ligands on chromium(VI) reduction by iron(II). Environ. Sci. Technol. 32, 2092–2099. 10.1021/es970932b. [DOI] [Google Scholar]
- Borges K. M.; Boswell J. S.; Liebross R. H.; Wetterhahn K. E. (1991) Activation of chromium(VI) by thiols results in chromium(V) formation, chromium binding to DNA and altered DNA conformation. Carcinogenesis 12, 551–561. 10.1093/carcin/12.4.551. [DOI] [PubMed] [Google Scholar]
- Lay P. A.; Levina A. (1996) Kinetics and mechanism of chromium(VI) reduction to chromium(III) by L-cysteine in neutral aqueous solutions. Inorg. Chem. 35, 7709–7717. 10.1021/ic960663a. [DOI] [Google Scholar]
- Bansch B.; Martinez P.; Uribe D.; Zuluaga J.; Van Eldik R. (1991) Is the oxidation of L-ascorbic acid by aquated iron(III) ions in acidic aqueous solution substitution- or electron-transfer-controlled? A combined chloride, pH, temperature, and pressure dependence study. Inorg. Chem. 30, 4555–4559. 10.1021/ic00024a018. [DOI] [Google Scholar]
- O’Brien P.; Pratt J.; Swanson F. J.; Thornton P.; Wang G. (1990) The isolation and characterization of a chromium(V) containing complex from the reaction of glutathione with chromate. Inorg. Chim. Acta 169, 265–269. 10.1016/S0020-1693(00)80527-7. [DOI] [Google Scholar]
- Falcone L. M.; Erdely A.; Kodali V.; Salmen R.; Battelli L. A.; Dodd T.; McKinney W.; Stone S.; Donlin M.; Leonard H. D.; Cumpston J. L.; Cumpston J. B.; Andrews R. N.; Kashon M. L.; Antonini J. M.; Zeidler-Erdely P. C. (2018) Inhalation of iron-abundant gas metal arc welding-mild steel fume promotes lung tumors in mice. Toxicology 409, 24–32. 10.1016/j.tox.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidler-Erdely P. C.; Meighan T. G.; Erdely A.; Battelli L. A.; Kashon M. L.; Keane M.; Antonini J. M. (2013) Lung tumor promotion by chromium-containing welding particulate matter in a mouse model. Part. Fibre Toxicol. 10, 45. 10.1186/1743-8977-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]