ABSTRACT
We performed a systematic review of the literature to determine whether adherence to a gluten-free diet (GFD) leads to improved outcomes for patients with schizophrenia. We searched the AMED (Allied and Complementary Medicine; 1985–June 2016), MEDLINE (1946–June 2016), and Embase (1980–2016 week 24) databases using the terms “wheat” or “glutenin” or “gliadin” or “gluten” AND “schizophrenia.” A total of 9 studies met the inclusion criteria for this review: 1 randomized controlled trial, 7 crossover studies, and 1 open-label pilot study. Six of the included studies demonstrated beneficial effects including improved functioning and decreased symptom severity after the course of a GFD, whereas 3 studies found no benefits. All of the included studies found that a GFD is well tolerated and can be adhered to by patients with schizophrenia. The findings of this systematic review should be interpreted with caution due to limitations inherent to nonrandomized trials, as well as the heterogeneity in the study design and the length of the GFD applied in each study. Publication bias is another potential limitation. Further research is required to examine the biomarkers of gluten sensitivity and inflammation to effectively target those patients with schizophrenia who will benefit most from this dietary intervention.
Keywords: schizophrenia, gluten-free diet, wheat, gluten, gliadin, glutenin, psychosis
Introduction
Schizophrenia is a complex psychiatric illness characterized by heterogeneous symptomatology and multifactorial etiology. Autoimmunity has long been thought to play a role in relation to the etiology (1). A large epidemiologic study that used data from a Danish national registry demonstrated that the prevalence of autoimmune disease was significantly higher in individuals with schizophrenia and their parents than in the unaffected controls (2). Celiac disease is one of the autoimmune illnesses associated with schizophrenia (3). Celiac disease is a multisystem immune-mediated disorder triggered by ingestion of gluten-containing foods in individuals with a genetic predisposition. The disease presents with diarrhea, anemia, weight loss, and, occasionally, a range of neurologic and psychiatric disturbances (4).
Several lines of evidence have identified an intriguing link between ingestion of wheat gluten and symptoms of schizophrenia. For instance, Bender (5) was among the first to notice that children with schizophrenia were more likely to have celiac disease. Several years later, a case report described 5 individuals suffering from both celiac disease and schizophrenia, who had been admitted to the same hospital within the same year (6). A seminal study conducted by Dohan (7) during World War II demonstrated a strong correlation between mean annual wheat consumption and the number of first hospital admissions for schizophrenia in women (r = 0.961, P < 0.01). This ecologic study included data from 5 countries (Finland, Norway, Sweden, Canada, and the United States). The consumption data were based on the reported harvest year production values for each of the countries before and during World War II. These studies collectively suggested that gluten, a component of cereal grains, may contribute to symptoms of schizophrenia.
At the same time, limiting gluten in the diet was suggested to have therapeutic value. In another observational manuscript, Dohan et al. (8) noted that prevalence of schizophrenia was substantially lower in places where wheat consumption was unusual or limited (e.g., in remote regions of Papua New Guinea, Solomon Islands, and an island in Micronesia), whereas “westernization” of the diet in these regions, over time, was accompanied by an increase in schizophrenia prevalence, that approximated European levels. In substantiation of this line of evidence, a number of case reports have shown a dramatic improvement in psychotic symptoms after strict adherence to a gluten-free diet (GFD) (9–11). For instance, a report by Kraft and Westman (9) described a dietary intervention for a 70-y-old female patient with a long-standing diagnosis of schizophrenia and multiple medical comorbidities. The patient's original diet included egg and cheese sandwich, diet soda, water, pimento cheese, barbequed pork, chicken salad, hamburger helper, macaroni and cheese, and potatoes. The new diet consisted of unlimited meats and eggs, 4 ounces (113 g) of hard cheese, 2 cups of salad vegetables, and 1 cup of low-carbohydrate vegetables/d. Although not explicitly described as such in the original report, the low-carbohydrate diet appears to be significantly lower in gluten content. After 1 wk on the low-carbohydrate diet, the patient noticed a decrease in hallucinations and an overall improvement in energy levels. Over the course of 12 mo on the low-carbohydrate diet the patient reported no recurrence of her schizophrenic symptoms.
Another case study discussed a schizophrenic patient with untreated celiac disease who was found to have perfusion abnormalities in the left frontal brain regions on single-photon emission computed tomography imaging (10). After treatment with a GFD, the patient displayed a rapid improvement in her physical and psychiatric symptoms, with a concomitant reversal of the frontal cortex hypoperfusion shown on a single-photon emission computed tomography scan. Remarkably, when the neuroleptic medications were discontinued, the psychiatric symptoms did not recur and, at 1-y follow-up, the patient remained asymptomatic.
Based on these observations, gluten ingestion appears to be an important factor in the symptomatology of schizophrenia. This notion is supported by findings of increased amounts of antibodies against gliadin, an immunogenic component of gluten, in patients with schizophrenia from German (12) and Chinese (13) populations. In another study, individuals with recent-onset psychosis had increased concentrations of gliadin antibodies compared with healthy controls. Interestingly, the individuals with multiepisode schizophrenia not of recent onset also had elevated gliadin antibodies. However, their antibody concentrations were lower than in subjects with recent-onset psychosis (14). Similar to Dohan's observations, these results point to the importance of gluten in the acute phase of psychosis. It appears that individuals with a long-standing diagnosis of schizophrenia, as well as those with recent-onset psychosis, share some of the immunologic features of celiac disease. However, the underlying pathoimmunologic process appears to differ between acute and latent phases of the disease. It would be of interest to determine whether the implementation of a GFD might prevent schizophrenia onset among those who are predisposed and/or with existing autoimmune disorders. However, to our knowledge, research has yet to be conducted to explore this theory.
The importance of removing gluten from the diet of patients with celiac disease was first demonstrated in the 1950s, with studies that document the toxic effects of gluten in the context of the pathogenesis of celiac disease. Over the subsequent years strict adherence to a GFD became the mainstay treatment of celiac disease (15).
Gluten is found in grains such as wheat, barley, and rye. Thus, a GFD subsumes avoidance of common foods such as breads, pasta, pastries, and cereals. Furthermore, gluten-containing derivatives are ubiquitously used as sauce thickeners, fillers, and stabilizers (16). Given the pervasive use of gluten-containing products in the food industry, complete avoidance of gluten is a significant challenge for patients and their caretakers. Nevertheless, studies show that the effects of gluten are dose-dependent (17), and most patients with celiac disease on a GFD notice significant improvements in their symptoms over the course of a few weeks (18).
In light of the potential link between gluten ingestion and schizophrenia, we hypothesize that improvement in symptoms can similarly be expected in patients with schizophrenia after the implementation of a GFD. In this study we performed a systematic review of the literature to determine whether a GFD offers effective symptomatic relief to those suffering from schizophrenia.
Methods
Design and literature search
We performed a systematic review using an a priori protocol. We searched the AMED (Allied and Complementary Medicine; 1985–June 2016), MEDLINE (1946–June 2016), and Embase (1980–2016 week 24) databases using the OVID interface (Supplemental Table 1). The terms “wheat” or “glutenin” or “gliadin” or “gluten” AND “schizophrenia” were used as keywords. In order to capture all relevant articles, limits were not applied to the search. In databases that had a hierarchal structure, the search terms were mapped to subject headings and exploded to include specific subheadings. Duplicate articles were removed.
Article selection
The process of article selection involved multiple stages by 2 reviewers (IM and CT). Titles and abstracts were first scanned for relevance, after which the full texts of selected articles were accessed and reviewed with reference to eligibility criteria. The inclusion and exclusion criteria are summarized in Table 1. The references of the included studies and recent review articles were scanned for possible missed articles.
TABLE 1.
Inclusion and exclusion criteria1
| Inclusion criteria | Exclusion criteria |
|---|---|
| • RCTs, cohort studies, case series | • Editorials, reviews, case studies, commentaries, gray literature |
| • Prospective and retrospective studies | • Non–English language studies |
| • Male or female patients, any age | • In vitro or animal studies |
| • Diagnosis of schizophrenia | |
| • GFD intervention OR gluten challenge after GFD |
1GFD, gluten-free diet; RCT, randomized controlled trial.
Data extraction
The included studies were assessed to extract the study design, sample size, participant age, length of GFD, and any relevant outcome measures. The duration of schizophrenia (i.e., time since diagnosis) was not extracted from the studies, because this information was not consistently reported in the original articles.
Quality assessment of the included studies
A single reviewer (AL) used the Methodological Index for Non-Randomized Studies (MINORS) scale to evaluate the quality of the included studies (19). The MINORS scale consists of a 12-item checklist assessing study parameters such as a clearly stated study aim, inclusion of consecutive patients, prospective collection of data, and unbiased assessment of endpoints, with each item scored between 0 and 2 points (Supplemental Table 2). Of the 12-item checklist, 8 items assess the quality of noncomparative trials, and an additional 4 items assess the quality of comparative trials, allowing for maximum scores of 16 and 24, respectively. Only the first 8 items were used to assess the quality, because the majority of the studies were noncomparative.
Outcome measures
There was substantial heterogeneity in the type of outcome measures used in the included studies, making it difficult to pool data across studies. The results were synthesized by reporting changes in the scores on the various rating scales or the reported symptom burden in the treatment group. These were broadly categorized by their overall effect (e.g., improvement, no change, or worsening of the symptoms) and reported in Table 2.
TABLE 2.
Summary of studies included in the analysis1
| Year | Authors (ref) | Title | Sample size | Length of GFD | Study design | Outcome of GFD |
|---|---|---|---|---|---|---|
| 1969 | Dohan et al. (20) | Relapsed schizophrenics: more rapid improvement on a milk- and cereal-free diet | 102 (102 M) | 175 d | Crossover, randomized | Faster release from locked to open ward with GFD. |
| 1973 | Dohan and Grasberger (21) | Relapsed schizophrenics: earlier discharge from the hospital after cereal free, milk free diet | 115 (115 M) | 175 d | Crossover, randomized | Faster release from locked to open ward with GFD. Gluten is pathogenic to those with schizophrenia. |
| 1976 | Singh and Kay (22) | Wheat gluten as a pathogenic factor in schizophrenia | 14 (3 M, 11 F) | 4 wk | Crossover | Gluten is pathogenic to those with schizophrenia. |
| 1981 | Potkin et al. (23) | Wheat gluten challenge in schizophrenic patients | 8 (5 M, 3 F) | 5 wk | Crossover, randomized | No effect. |
| 1982 | Storms et al. (24) | Effects of gluten on schizophrenics | 26 (26 M) | 10 d | RCT (n = 13 in each group) | No effect. |
| 1982 | Osborne et al. (25) | Lack of effect of a gluten-free diet on neuroleptic blood levels in schizophrenic patients | 5 (n/a) | 36 wk | Crossover | No effect. |
| 1986 | Vlissides et al. (26) | A double-blind gluten-free/gluten-load controlled trial in a secure ward population | 172 (10 M) | 14 wk | Crossover, double blind | Improvement in PIP with GFD. |
| 1990 | Reichelt et al. (27) | The effect of gluten-free diet on urinary peptide excretion and clinical state in schizophrenia | 10 (10 M) | 8 wk3 | Crossover, randomized; with an optional open-label extension | Improvement in NOSIE, CPRS, WIST scores with GFD. |
| 2012 | Jackson et al. (28) | A gluten-free diet in people with schizophrenia and anti-tissue transglutaminase or anti-gliadin antibodies | 2 (1 M, 1 F) | 2 wk | Open-label pilot | Improvement in BPRS scores, SANS, and in extrapyramidal symptoms. |
1BPRS, Brief Psychiatric Rating Scale (29); CPRS, Comprehensive Psychopathological Rating Scale (30); GFD, gluten-free diet; n/a, not available; NOSIE, Nurses’ Observation Scale for Inpatient Evaluation (31); PIP, Psychotic In-Patient Profile (32); RCT, randomized controlled trial; ref, reference; SANS, Scale for the Assessment of Negative Symptoms (33); WIST, Whitaker Index of Schizophrenic Thinking (34).
2A total of 22 ward patients were included, 16 with paranoid schizophrenia, and 1 with undifferentiated schizophrenia.
3In a subgroup of patients, a GFD was maintained for ≤56 wk.
Results
Studies included
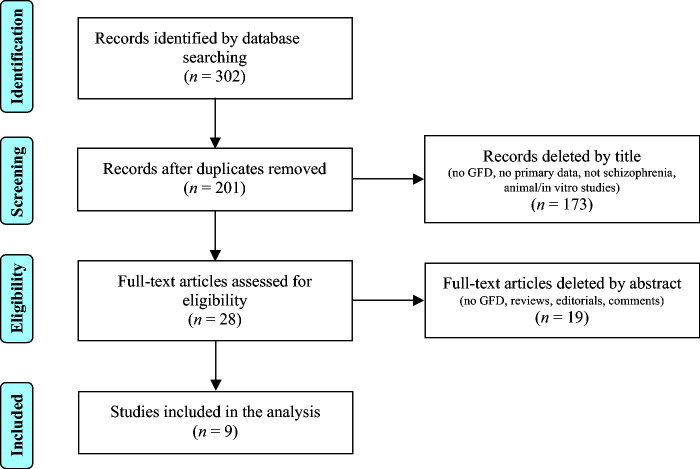
The electronic database search identified 302 articles (Figure 1). The articles were exported to RefWorks (http://www.refworks.com), and duplicate articles were removed, yielding a total of 201 articles. After further screening, we identified 9 eligible studies. Eight of the 9 studies were prospective, and only 1 was a randomized controlled trial (Table 2). The mean MINORS score for the included studies was 11 (SD 2.2; range 8–14; maximum possible score 16). The randomized controlled study received full points on the criteria for comparative studies (Table 3). There was no significant variation in the MINORS score across study types. Table 2 summarizes the key features of the included studies’ sample size, length, design, and main outcomes.
FIGURE 1.
Literature search strategy. The electronic database search identified 302 articles. Duplicate articles were removed, resulting in a total of 201 articles. The titles were further screened by title and abstract, yielding 9 articles for analysis. GFD, gluten-free diet.
TABLE 3.
Calculated MINORS scores for the included articles1
| Authors (reference) | MINORS score2 | Comparative? | Additional criteria score (out of 8)3 |
|---|---|---|---|
| Dohan et al. (20) | 10 | No | — |
| Dohan and Grasberger (21) | 10 | No | — |
| Singh and Kay (22) | 13 | No | — |
| Potkin et al. (23) | 11 | No | — |
| Storms et al. (24) | 14 | Yes | 8 |
| Osborne et al. (25) | 8 | No | — |
| Vlissides et al. (26) | 12 | No | — |
| Reichelt et al. (27) | 13 | No | — |
| Jackson et al. (28) | 8 | No | — |
1MINORS, Methodological Index for Non-Randomized Studies.
2The items are scored 0 (not reported), 1 (reported but inadequate), or 2 (reported and adequate). The global ideal score is 16 for noncomparative studies and 24 for comparative studies.
3For comparative studies.
Dietary gluten and schizophrenia
Dohan et al. (20) performed a dietary study on 102 male inpatients with schizophrenia (median age: 38 y) who had recently experienced an exacerbation of their symptoms. In the study the patients were assigned either a cereal- and milk-free diet (CMFD) or a high-cereal diet (HCD). Dohan et al. used the following rationale for the elimination of milk: “Milk was eliminated from the diet because some patients with celiac disease do not improve unless milk as well as cereal is omitted.” In this study, the authors state that “the high-cereal diet was the usual well-balanced hospital diet (including milk) modified by daily substitution of 1 or 2 wheat products (e.g. spaghetti for potatoes).” Patients were admitted to a “locked” psychiatric ward with restricted privileges and were only released to an “open” ward when their symptoms improved. Over the course of period 1 (175 d), patients admitted to the locked ward were assigned to either the CMFD group (n = 47) or the HCD group (n = 55), which they maintained for the whole period. The study found that 62% of the patients on the CMFD were released into the open ward before the end of day 7 compared with only 36% of patients placed on an HCD (P < 0.01). The mean length of stay on the locked ward was 17.3 d for the CMFD group and 30.6 d for the HCD group. To reduce investigator bias, 19 g of wheat gluten were added daily, unbeknownst to the ward staff, to the CMFD during period 2 (143 d) of the study. Over this time, there was no difference in the number of patients that were released to the open ward between the groups, thus addressing the role of potential confounders, such as the elimination of milk, as well as nonspecific psychological effects in patients or ward staff. Together, these results suggest that ingestion of cereals may play a role in the pathogenesis of schizophrenia by showing that a CMFD may lead to an improvement of acutely exacerbated symptoms.
In 1973, Dohan and Grasberger (21) published a follow-up, retrospective cohort study involving the same subjects, assessing the effect of a GFD on the length of time from admission to discharge from the hospital. The results revealed a significant difference between the CMFD and HCD groups in terms of the cumulative percentage of patients discharged from the hospital. At the end of 90 d, the cumulative proportion of patients discharged was 37.2% for the CMFD group and only 16.1% for the HCD group. Moreover, the mean time until discharge was 62 d shorter for the CMFD group. Among the HCD patients who were later discharged, nearly half returned to the locked ward owing to behavioral difficulties in the open ward before being discharged from the hospital. When 3 of the patients were later assigned to the CMFD, these patients were discharged from the hospital 21, 78, and 87 d later (less than the median discharge time of 102 d for the HCD group). Importantly, when gluten was covertly added to the CMFD, the difference in the rate of discharge between the groups was no longer observed (CMFD + gluten, n = 17; HCD, n = 25).
Interestingly, 1 patient from the 1973 Dohan study was admitted to the hospital twice, each time on a different diet. When this patient was randomly assigned to an HCD, he remained on the locked ward for 17 d and left the hospital without permission on day 65. The same patient was readmitted nearly 1 y later and randomly assigned to the CMFD. On that occasion he spent 7 d on the locked ward, and was discharged entirely on day 22. These findings reflect that schizophrenic patients assigned to the CMFD were discharged more than twice as quickly as those in the HCD group during the first 90 d after admission. Given that the rates became similar after this period, the authors speculated that only a proportion of those diagnosed with schizophrenia respond to a CMFD.
In 1976, a paper published in Science demonstrated that wheat gluten has primary schizophrenia-promoting effects (22). In a double-blind, placebo-controlled experiment, Singh and Kay (22) studied 14 schizophrenic patients in a locked research ward for 14 wk. Unlike previous studies, this study included 11 female patients, and involved a longitudinal research design in which each patient served as his/her own control. Strict dietary controls were implemented, and patients were fed a CMFD. Patients were kept medication-free for the first 2 wk of the study and then received neuroleptic medication for 12 wk. During the medication period, patients consumed a drink that contained soy flour during weeks 3–6 and 11–14, and wheat gluten during weeks 7–10. The other components of the drink remained the same, and included Kool-Aid powder (Kraft Foods, Inc.), acacia, dextrose, and water. Both patients and raters were blinded to the nature of the drinks. Assessments were performed every 2 wk. Each participant was interviewed for 90 min, and independently rated by a psychiatrist and psychologist on the 33 dimensions of a psychopathology rating schedule. Five measures of social avoidance behavior (Supplemental Data 1, Note 1) and a measure of social participation in planned recreational activities (Supplemental Data 1, Note 2) were obtained twice daily, 5 d/wk, by the day and evening ward staff. The data from all forms of observation indicated that wheat gluten exacerbated symptomology of schizophrenia and diminished response to treatment. During the gluten challenge, therapeutic progress stopped or even reversed, only to resume once gluten was removed. Statistical analysis of the data showed that 30 of the 39 measures had worsened after the administration of gluten (P < 0.001). An analysis of the dosage data during the gluten period revealed no variations in neuroleptic dosage. The authors concluded that wheat gluten might be a pathogenic factor in schizophrenia.
Potkin et al. (23) also performed a gluten challenge study in 8 chronic schizophrenic patients maintained on a CMFD. In a double-blind randomized experiment patients were challenged with dietary wheat gluten and placebo. This study included 5 men and 3 women with a mean age of 25 y. All patients had previously responded to neuroleptic medication, but were unable to function independently outside of a halfway house or hospital. During the study period of a minimum of 13 wk, all patients received a CMFD. All meals and snacks were supervised by staff. Each challenge and placebo period was reported to be ≥5 wk, during which the participants received a snack and beverage that contained gluten (30 g powder) or placebo twice daily. The order of treatment was random. The primary outcome measure included daily scores on the modified Brief Psychiatric Rating Scale (BPRS). Patient scores during the last 2 wk of the placebo challenge were compared with their own scores for the last 2 wk of the gluten challenge. No statistically significant difference in BPRS scores was observed between the gluten challenge and placebo periods. Furthermore, serum glycoprotein concentrations did not differ between the groups during the gluten challenge, which indicates the lack of an inflammatory response. The results suggest that these chronic patients may not be sensitive to gluten, which would explain the ineffectiveness of the diet. The authors concluded that patient cohorts that are less responsive to treatment may require a longer gluten-free period for behavioral changes to occur.
In an effort to replicate the findings from the previous studies by Dohan et al. (20, 21) and Singh and Kay (22), and address their limitations, Storms et al. (24) performed a double-blind study using 26 male patients in a locked ward. Patients were randomly assigned between 2 groups and received cookies with either gluten or peanut flour. A strict CMFD was implemented 2 wk before and during the 10-d course of the experiment. The study did not find any significant differences in the total number of inpatient days on the locked ward, or in the amount of antipsychotic medications received by the patients, in either group. Although both groups showed improvement during the intervention period, there was no evidence of deterioration in the group receiving gluten. The authors suggested that a longer time on the diet, or a larger sample size, was required to elucidate the beneficial effects of a GFD.
Osborne et al. (25) studied 5 chronic schizophrenic patients to examine the long-term effects of a GFD on blood concentrations of the neuroleptic butaperazine. Patients in this trial had not responded to neuroleptic treatment, and were placed on a constant dose of butaperazine (80–160 mg daily) for the duration of the trial. The study period was broken down into 46 wk (6 wk pre-GFD, 36 wk on GFD, and 4 wk post-GFD). Plasma concentrations of butaperazine were measured weekly for the duration of the trial. In addition, psychiatric symptoms were assessed weekly with the use of the BPRS. The mean blood concentrations of butaperazine varied between 10 and 680 ng/mL among the 5 patients. There was no significant difference in the blood concentrations of butaperazine or in BPRS scores over the course of the GFD. The authors noted that only a subpopulation of schizophrenic patients may be gluten sensitive, and the notion that gluten might interfere with the absorption of neuroleptics was not supported by the results. In addition, patients with chronic schizophrenia may require months to years on a GFD, whereas younger patients with a shorter duration of illness may experience more immediate benefits. Finally, the small sample size was noted to limit the ability to draw conclusions.
Vlissides et al. (26) performed a double-blind controlled trial comparing a gluten-free with a gluten-containing diet in a secure ward population of 17 patients with schizophrenia. During the 14-wk trial, patients consumed a drink containing Casilan or gluten powder twice daily. Each subject served as his own control. The study was conducted in 3 parts: the first 2 wk marked the baseline with a GFD and Casilan drinks to allow for adjustment; over the next 6 wk the patients continued to receive Casilan in the drinks, and, in the third period of 6 wk, gluten (30 g/d) was added to the drinks. Neither the patients, staff, nor the investigators knew the type of drink that had been prepared. Several observations were made on a regular basis, including: assessment of the patients’ behavior, nursing staff records, and interviews noting any changes in patients’ mental states. Analysis revealed an improvement on 5 parameters of the Psychotic In-Patient Profile with the GFD: hostile belligerence, anxious depression, retardation, psychotic disorganization, and depressive mood (P < 0.05). Interestingly, there were 2 patients who improved significantly during the GFD, but deteriorated during the gluten challenge period. Each of these patients was diagnosed with paranoid schizophrenia. These patients displayed a “striking” improvement in functioning and a decrease in psychotic symptoms while on a GFD, but the same symptoms were greatly exacerbated during the gluten challenge period. The authors acknowledged the heterogeneous nature of their study population, and the short time frame during which the GFD was instituted. Further, the addition of gluten back into the diet did not reverse the improvements, which suggests that a longer period of gluten challenge would be required for clinical relapse to occur. The authors concluded that although some patients with schizophrenia respond to a GFD, it might not always play a role in the pathogenesis of the disease.
Reichelt et al. (27) assessed the urinary profiles of 11 chronic schizophrenic patients on a GFD who had been unresponsive to neuroleptic medication. All patients were male with a mean duration of illness of 4.9 y. After a weeklong adjustment period, the patients were randomly assigned to 2 groups. The first group received the GFD for 8 wk and switched to a regular diet for the remaining 8 wk. The second group began on a normal diet for 8 wk and then switched to a GFD for the remaining 8 wk. After 16 wk, patients returned to their original places of residence, and were permitted to continue the GFD on a volunteer basis. Patient ratings were performed by physicians blinded to the patients’ group assignment. Urine peptide analysis was performed on samples collected at 0, 16, 28, and 56 wk. Samples after 16 wk included patients voluntarily on a GFD, with 5 patients providing samples at 56 wk. All critical behavioral measures showed improvement after a GFD (P < 0.05). Slightly elevated concentrations of antibodies to gluten were detected in 2 patients and to glyc-gliadin in 1. Interestingly, urine analysis revealed that dietary peptide concentrations in individuals on a GFD were significantly higher than in the control group (P < 0.01). The results support the notion that a GFD is beneficial for some patients, and indicate that the abnormally high urinary peptide concentrations found in some patients with schizophrenia can be reversibly altered by a GFD. The investigators noted marked changes in those patients who adhered to the GFD for >2 y (n = 5).
Jackson et al. (28) reported the results of a GFD in individuals with schizophrenia and anti-tissue transglutaminase (tTG) antibodies or anti-gliadin antibodies (AGAs). Given the high prevalence of gluten sensitivity in patients with schizophrenia, the study examined a subpopulation of patients who may be gluten-sensitive. Two patients, of whom 1 was tTG-positive and the other was AGA-positive, were recruited. The study involved a 2-wk inpatient open-label pilot study with one-on-one supervision to ensure compliance to the GFD. Participants were rated at baseline and at the end of the 2-wk GFD based on clinical symptoms, side effects, and adherence to the diet. Both participants improved on the BPRS total score, and on the Scale for the Assessment of Negative Symptoms. Both participants reported good tolerance of the GFD, maintained a stable weight, and displayed improved serum antibody concentrations. Furthermore, both participants noted improvements in extrapyramidal side effects. This study showed that a GFD could be beneficial to individuals with schizophrenia, especially those with anti-tTG antibodies and AGAs.
An overview of the findings of each study is provided in Supplemental Table 3.
Discussion
A total of 9 studies were included in this review (Table 2). Six of the 9 studies demonstrate a strong association between gluten elimination and improved outcomes in schizophrenic patients. These outcomes include fewer patients and shorter times spent on the locked ward (20), faster discharge times from the hospital (21), improvement on various behavioral measures (26–28), and decreased extrapyramidal symptoms (28). Three of the 6 studies that demonstrated an improved outcome with gluten elimination also reflect that the group consuming gluten had slower (20, 21) or reversed (22) therapeutic progress. These 6 studies had a duration between 14 and 175 d. The earliest reported improvements were noted to occur 1 (20) and 2 wk (28) into the GFD, which corresponds to patients with celiac disease who also notice improvements after the first 2 wk of the implementation of a GFD (18). Furthermore, Dohan and Grasberger (21) demonstrated that discharge rates between the CMFD and HCD groups became similar after 3 mo. This finding suggests that patients who were able to benefit from a GFD did so in the first month of the intervention.
Three of the 9 studies did not demonstrate an improvement in functioning with a GFD. One of these studies involved a sample of 8 individuals, and concluded that this group of subjects may have lacked the inflammatory response to gluten, and thus could not have benefitted from the diet (23). The second study involved 26 subjects who were on a CMFD for 2 wk before the 10 d of the intervention phase (24). The authors did not observe any significant functional deterioration during the gluten challenge. However, they did report comparable improvements in the treatment and control groups, which could be related to the CMFD instituted at the beginning of the experiment. The third study involved a small sample size of only 5 patients who may not have been gluten sensitive. The study used plasma drug concentrations and a single psychiatric scale as outcome measurements, which makes it difficult to draw conclusions (25). It is distinctly possible that these 3 studies did not demonstrate a GFD to be beneficial because the small sample of patients lacked the inflammatory response to gluten and thus could not have benefitted from the diet. After all, it was not until the 2000s that serum screening for gluten sensitivity became available (35).
The elimination of milk, in association with a GFD, in several of the reviewed studies presents a possible confounding variable. Nevertheless, the addition of gluten (while maintaining milk-free conditions) led to a cessation of benefits that many patients experienced with the double elimination diet. This suggests that gluten, in and of itself, is pathogenic. A comparison of a GFD and a milk-free diet in relation to schizophrenia symptoms could clarify this relationship and provide information on whether a double elimination diet is superior to a GFD alone.
Inflammation has been proposed as a link between gluten consumption and schizophrenia exacerbation. Inflammatory processes associated with gluten reaction could disrupt the blood-brain barrier and increase its permeability to exogenous peptides. Existing evidence supports this notion in that schizophrenic patients display increased concentrations of haptoglobin-2 (36), an inflammatory marker that has been shown to modulate the permeability of the blood-brain barrier (37). Interestingly, the release of haptoglobin-2, also known as zonulin, is known to be triggered by gluten peptides (38). Furthermore, there is extensive evidence showing that gliadin has direct effects on the brain in patients with celiac disease (39). It is thus possible that gluten triggers inflammatory responses in the brain, increasing its permeability and uptake of peptides that could cause behavioral changes, such as those observed in schizophrenia patients deteriorating on high-cereal diets.
Recently, gluten sensitivity has been considered a syndrome distinct from celiac disease. Patients suffering from the latter are found to have classical histologic findings on intestinal biopsy that include increased lymphocytes, villous atrophy, and crypt hyperplasia. Gluten sensitivity on the other hand is not accompanied by the intestinal mucosal changes, but can show variable changes in gluten-related antibody titers (40). Patients with gluten sensitivity experience symptoms similar to celiac disease, including gastrointestinal disturbances such as bloating, diarrhea, and extraintestinal manifestations (41). Significantly, there appears to be a clear association between gluten sensitivity and neurologic dysfunction. In addition to schizophrenia, other neuropsychiatric disorders commonly seen in patients with gluten sensitivity include autism spectrum disorders, mood disorders (41), ataxia, neuropathy, and white matter abnormalities (42).
Although the symptoms are often similar, gluten sensitivity and celiac disease appear to be distinct in their pathogenesis. In a meta-analysis studying individuals with schizophrenia and nonaffective psychoses, researchers found significant elevations in 5 different biomarkers of gluten sensitivity when compared with the control group. Interestingly, the pattern of the immune response to gluten in these individuals differed from that seen in celiac disease, which is suggestive of a distinct syndrome (43). One study, in particular, found that among the group with neurologic dysfunction of unknown etiology, 57% had positive AGAs, yet the majority of these patients had no histologic evidence of celiac disease (44).
The reviewed literature thus suggests that there is a potential therapeutic benefit to applying a GFD to a subpopulation of patients with schizophrenia who demonstrate sensitivity to gluten, and that a lack of formal diagnosis of celiac disease may not necessarily preclude effective symptom relief with a GFD. Attesting to the latter, a case report by Genuis and Lobo (45) discusses a 23-y-old female with a long-standing history of auditory and visual hallucinations reporting complete symptom resolution with the initiation of a GFD. The patient also noticed a consistent pattern of symptom relapse with inadvertent exposure to gluten. The patient's tTG antibodies were negative while on a regular, gluten-containing diet. The patient declined intestinal biopsy and additional antibody testing, stating that maintaining a GFD kept her well, and that she did not feel a need for additional investigations. Thus, it is unknown whether this patient had a true celiac disease or gluten sensitivity. Further, the authors did not specify which tTG isoform they tested for. Yet, gluten elimination was effective and safe in relieving her psychotic symptoms.
Advances in characterization of inflammatory markers may aid in the selection of patients who may benefit from an intervention, as was attempted by Jackson et al. (28). Based on the available evidence, the psychiatric manifestations of gluten sensitivity may be accompanied by several different biomarker elevations (43). It is important that the selected screening tool is specific for detecting the gluten-associated inflammatory response seen in schizophrenia. For instance, one of the isoforms of tissue transglutaminase, tTG6, is primarily expressed in the brain, which makes it a plausible biomarker for neuroinflammation (46). It has also been shown that antibodies to this particular isoform can be found in ≤22% of schizophrenic patients, who have previously tested positive for tTG2, the primary serologic marker for celiac disease and gluten sensitivity (47). However, these antibodies do not display cross-reactivity between the different isoforms, and therefore require separate testing. Importantly, whereas tTG2 antibodies have been linked with gastrointestinal disease, the antibodies to the tTG6 isoform, which is found in the brain, occur independently of the intestinal involvement (46). Based on these observations, the tTG6 isoform appears to be a more sensitive marker for identification of a subgroup of patients at risk of developing psychiatric symptoms in the context of schizophrenia illness.
Other inflammatory markers associated with gluten sensitivity include anti-gliadin (AGA) IgG, AGA IgA, anti-tTG2 IgG, anti-tTG2 IgA, anti-endomysial, anti-gliadin, anti-wheat, anti-gluten, and anti-deamidated gliadin IgG (43). Future trials of gluten elimination should attempt to stratify patients with schizophrenia based on different biomarkers of gluten sensitivity to establish the predictive values of each.
The findings of this systematic review should be interpreted with caution owing to limitations and bias inherent to nonrandomized trials, and owing to the heterogeneity in the study design and length of GFD applied in each study. Publication bias is another significant limitation. Despite this, it appears that a GFD has minimal risk in patients with schizophrenia, and is a feasible option in terms of adherence. Furthermore, this treatment may provide relief of symptoms in up to one-quarter of patients (antibody-positive) afflicted by this disorder (47). Further research is warranted to examine the biomarkers of gluten sensitivity and inflammation in order to effectively target those patients with schizophrenia who may experience a substantial positive impact from this dietary intervention.
Supplementary Material
Acknowledgments
All authors read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: AL, IM, and CT, no conflicts of interest.
Supplemental Tables 1–3 and Supplemental Data 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used:
- AGA
anti-gliadin antibody
- BPRS
Brief Psychiatric Rating Scale
- CMFD
cereal-free milk-free diet
- GFD
gluten-free diet
- HCD
high-cereal diet
- MINORS
Methodological Index for Non-Randomized Studies
- tTG
tissue transglutaminase
References
- 1. Knight JG. Is schizophrenia an autoimmune disease? A review. Methods Find Exp Clin Pharmacol 1984;6(7):395–403. [PubMed] [Google Scholar]
- 2. Eaton WW, Byrne M, Ewald H, Mors O, Chen C, Agerbo E, Mortensen P. Association of schizophrenia and autoimmune diseases: linkage of Danish national registers. Am J Psychiatry 2006;163(3):521–8. [DOI] [PubMed] [Google Scholar]
- 3. Ludvigsson JF, Osby U, Ekbom A, Montgomery SM. Coeliac disease and risk of schizophrenia and other psychosis: a general population cohort study. Scand J Gastroenterol 2007;42(2):179–85. [DOI] [PubMed] [Google Scholar]
- 4. Lebwohl B, Ludvigsson JF, Green PHR. Celiac disease and gluten sensitivity. BMJ 2015;351:h4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bender L. Childhood schizophrenia. Psychiatr Q 1953;27:663–81. [DOI] [PubMed] [Google Scholar]
- 6. Graff H, Handford A. Celiac syndrome in the case histories of five schizophrenics. Psychiatr Q 1961;35(2):306–13. [DOI] [PubMed] [Google Scholar]
- 7. Dohan FC. Wheat “consumption” and hospital admissions for schizophrenia during World War II. A preliminary report. Am J Clin Nutr 1966;18(1):7–10. [DOI] [PubMed] [Google Scholar]
- 8. Dohan FC, Harper EH, Clark MH. Is schizophrenia rare if grain is rare? Biol Psychiatry 1984;19(3):385–99. [PubMed] [Google Scholar]
- 9. Kraft BD, Westman EC. Schizophrenia, gluten, and low-carbohydrate, ketogenic diets: a case report and review of the literature. Nutr Metab 2009;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Santis A, Addolorato G, Romito A, Caputo S, Giordano A, Gambassi G, Taranto C, Manna R, Gasbarrini G. Schizophrenic symptoms and SPECT abnormalities in a coeliac patient: regression after a gluten-free diet. J Intern Med 1997;242(5):421–3. [DOI] [PubMed] [Google Scholar]
- 11. Jansson N, Kristjánsson E, Nilsson L. Schizophrenic psychosis disappearing after patient is given gluten-free diet. Lakartidningen 1984; 81:448–9. [PubMed] [Google Scholar]
- 12. Okusaga O, Yolken RH, Langenberg P, Sleemi A, Kelly D, Vaswani D, Giegling I, Hartmann A, Konte B, Friedl M et al. . Elevated gliadin antibody levels in individuals with schizophrenia. World J Biol Psychiatry 2013;14(7):509–15. [DOI] [PubMed] [Google Scholar]
- 13. Jin SZ, Wu N, Xu Q, Zhang X, Ju G, Law M, Wei J. A study of circulating gliadin antibodies in schizophrenia among a Chinese population. Schizophr Bull 2012;38(3):514–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, Leister F, Yang S, Krivogorsky B, Alaedini A, Yolken R. Markers of gluten sensitivity and celiac disease in recent-onset psychosis and multi-episode schizophrenia. Biol Psychiatry 2010;68(1):100–4. [DOI] [PubMed] [Google Scholar]
- 15. Kaukinen K, Collin P, Mäki M. Natural history of celiac disease. Pediatr Adolesc Med 2008;12:12–17. [Google Scholar]
- 16. See JA, Kaukinen K, Makharia GK, Gibson PR, Murray JA. Practical insights into gluten-free diets. Nat Rev Gastroenterol Hepatol 2015;12(10):580–91. [DOI] [PubMed] [Google Scholar]
- 17. Jansson UHG, Gudjónsdóttir AH, Ryd W, Kristiansson B. Two different doses of gluten show a dose-dependent response of enteropathy but not of serological markers during gluten challenge in children with coeliac disease. Acta Paediatr Int J Paediatr 2001;90(3):255–9. [DOI] [PubMed] [Google Scholar]
- 18. Pink IJ, Creamer B. Response to a gluten-free diet of patients with the coeliac syndrome. Lancet 1967;1(7485):300–4. [DOI] [PubMed] [Google Scholar]
- 19. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non‐randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg 2003;73(9):712–16. [DOI] [PubMed] [Google Scholar]
- 20. Dohan FC, Grasberger JC, Lowell FM, Johnston HT, Arbegast AW. Relapsed schizophrenics: more rapid improvement on a milk- and cereal-free diet. Br J Psychiatry 1969;115(522):595–6. [DOI] [PubMed] [Google Scholar]
- 21. Dohan FC, Grasberger JC. Relapsed schizophrenics: earlier discharge from the hospital after cereal free, milk free diet. Am J Psychiatry 1973;130(6):685–8. [DOI] [PubMed] [Google Scholar]
- 22. Singh M, Kay SR. Wheat gluten as a pathogenic factor in schizophrenia. Science 1976;191(4225):401–2. [DOI] [PubMed] [Google Scholar]
- 23. Potkin SG, Weinberger D, Kleinman J, Nasrallah H, Luchins D, Bigelow L, Linnoila M, Fischer S, Carman J, Bjornsson T et al. . Wheat gluten challenge in schizophrenic patients. Am J Psychiatry 1981;138(9):1208–11. [DOI] [PubMed] [Google Scholar]
- 24. Storms LH, Clopton JM, Wright C. Effects of gluten on schizophrenics. Arch Gen Psychiatry 1982;39(3):323–7. [DOI] [PubMed] [Google Scholar]
- 25. Osborne M, Crayton JW, Javaid J, Davis JM. Lack of effect of a gluten-free diet on neuroleptic blood levels in schizophrenic patients. Biol Psychiatry 1982;17(5):627–9. [PubMed] [Google Scholar]
- 26. Vlissides DN, Venulet A, Jenner FA. A double-blind gluten-free/gluten-load controlled trial in a secure ward population. Br J Psychiatry 1986;148(4):447–52. [DOI] [PubMed] [Google Scholar]
- 27. Reichelt KL, Sagedal E, Landmark J, Sangvik BT, Eggen O, Scott H. The effect of gluten-free diet on urinary peptide excretion and clinical state in schizophrenia. J Orthomol Med 1990;5(4):223–39. [Google Scholar]
- 28. Jackson J, Eaton W, Cascella N, Fasano A, Warfel D, Feldman S, Richardson C, Vyas G, Linthicum J, Santora D et al. . A gluten-free diet in people with schizophrenia and anti-tissue transglutaminase or anti-gliadin antibodies. Schizophr Res 2012;140(1–3):262–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep 1962;10(3):799–812. [Google Scholar]
- 30. Åsberg M, Montgomery SA, Perris C, Schalling D, Sedvall G. A comprehensive psychopathological rating scale. Acta Psychiatr Scand 1978;57(271 S):5–27. [DOI] [PubMed] [Google Scholar]
- 31. Honigfeld G, Gillis RD, Klett CJ. NOSIE-30: a treatment-sensitive ward behavior scale. Psychol Rep 1966;19:180–2. [DOI] [PubMed] [Google Scholar]
- 32. Lorr M, Vestre ND. The psychotic inpatient profile: a nurse's observation scale. J Clin Psychol 1969;25(2):137–40. [DOI] [PubMed] [Google Scholar]
- 33. Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS). Br J Psychiatry 1989;155(Suppl. 7):53–8. [PubMed] [Google Scholar]
- 34. Leslie BA, Landmark J, Whitaker LC. The Whitaker Index of Schizophrenic Thinking (WIST) and thirteen systems for diagnosing schizophrenia. J Clin Psychol 1984;40(3):636–48. [DOI] [PubMed] [Google Scholar]
- 35. Kalaydjian AE, Eaton W, Cascella N, Fasano A. The gluten connection: the association between schizophrenia and celiac disease. Acta Psychiatr Scand 2006;113(2):82–90. [DOI] [PubMed] [Google Scholar]
- 36. Yang Y, Wan C, Li H, Zhu H, La Y, Xi Z, Chen Y, Jiang L, Feng G, He L. Altered levels of acute phase proteins in the plasma of patients with schizophrenia. Anal Chem 2006;78(11):3571–6. [DOI] [PubMed] [Google Scholar]
- 37. Karyekar CS, Fasano A, Raje S, Lu R, Dowling TC, Eddington ND. Zonula occludens toxin increases the permeability of molecular weight markers and chemotherapeutic agents across the bovine brain microvessel endothelial cells. J Pharm Sci 2003;92(2):414–23. [DOI] [PubMed] [Google Scholar]
- 38. Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev 2011;91(1):151–75. [DOI] [PubMed] [Google Scholar]
- 39. Pruimboom L, de Punder K. The opioid effects of gluten exorphins: asymptomatic celiac disease. J Heal Popul Nutr 2015;33(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sapone A, Lammers KM, Mazzarella G, Mikhailenko I, Cartenì M, Casolaro V, Fasano A. Differential mucosal IL-17 expression in two gliadin-induced disorders: gluten sensitivity and the autoimmune enteropathy celiac disease. Int Arch Allergy Immunol 2010;152(1):75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jackson JR, Eaton WW, Cascella NG, Fasano A, Kelly DL. Neurologic and psychiatric manifestations of celiac disease and gluten sensitivity. Psychiatr Q 2012;83(1):91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hadjivassiliou M, Grünewald RA, Davies-Jones GAB. Gluten sensitivity as a neurological illness. J Neurol Neurosurg Psychiatry 2002;72(5):560–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lachance LR, McKenzie K. Biomarkers of gluten sensitivity in patients with non-affective psychosis: a meta-analysis. Schizophr Res 2014;152(2–3):521–7. [DOI] [PubMed] [Google Scholar]
- 44. Hadjivassiliou M, Gibson A, Davies-Jones GAB, Lobo AJ, Stephenson TJ, Milford-Ward A. Does cryptic gluten sensitivity play a part in neurological illness? Lancet 1996;347(8998):369–71. [DOI] [PubMed] [Google Scholar]
- 45. Genuis SJ, Lobo RA. Gluten sensitivity presenting as a neuropsychiatric disorder. Gastroenterol Res Pract 2014;2014:293206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hadjivassiliou M, Aeschlimann P, Strigun A, Sanders DS, Woodroofe N, Aeschlimann D. Autoantibodies in gluten ataxia recognize a novel neuronal transglutaminase. Ann Neurol 2008;64(3):332–43. [DOI] [PubMed] [Google Scholar]
- 47. Cascella NG, Santora D, Gregory P, Kelly DL, Fasano A, Eaton WW. Increased prevalence of transglutaminase 6 antibodies in sera from schizophrenia patients. Schizophr Bull 2013;39(4):867–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.