ABSTRACT
Phytoestrogens might have advantageous effects on diabetes in women. We performed a systematic review and meta-analysis to determine the effect of phytoestrogens on glucose homeostasis and the risk of type 2 diabetes (T2D) among women. Randomized controlled trials (RCTs) and prospective observational studies that assessed associations of phytoestrogens (supplementation, dietary intake, or biomarkers) with fasting glucose or insulin, homeostatic model assessment of insulin resistance (HOMA-IR), or with the risk of T2D were included. We identified 18 RCTs (n = 1687 individuals) investigating the effect of phytoestrogen supplementation on glucose homeostasis and 9 prospective population-based studies (n = 212,796 individuals) examining the association between phytoestrogen intake and the risk of T2D. Compared with placebo, phytoestrogen supplementation resulted in improvements in fasting glucose and HOMA-IR: the pooled mean differences of changes were –0.12 mmol/L (95% CI: –0.20, –0.03 mmol/L) and –0.24 mmol/L (95% CI: –0.45, –0.03 mmol/L), respectively. Although there was no significant decrease in insulin concentrations with overall phytoestrogen supplementation, the pooled mean difference in changes was –0.99 pmol/L (95% CI: –4.65, 2.68 pmol/L). However, the results of RCTs varied by type of phytoestrogens: soy-derived isoflavones and genistein improved glucose homeostasis, whereas isoflavone mix and daidzein had no effect or were associated with an adverse glycemic profile. Higher dietary phytoestrogen intake was associated with a 10% lower risk of developing T2D in observational studies (pooled RR: 0.90; 95% CI: 0.85, 0.96; for the highest compared with the lowest quantiles). Results were similar when the analyses were restricted to only medium- and high-quality studies. Overall, phytoestrogens may have a positive influence on glycemia and could be used for diabetes prevention in women. However, for some individual types of phytoestrogens, such as mixed isoflavones, caution is needed in recommending their use in women, because their use could lead to an adverse glycemic profile in women.
Keywords: phytoestrogens, soy foods, type 2 diabetes, fasting glucose, fasting insulin, insulin resistance, HOMA-IR, women's health
Introduction
Phytoestrogens, nonsteroidal plant compounds with estrogen-like biological activity, have been suggested to improve women's health (1). Many women in Western countries choose to use phytoestrogens as complementary therapy for the treatment of menopausal symptoms (1, 2). Recently, a meta-analysis of clinical trials showed that specific phytoestrogen supplementation led to relief of menopausal symptoms (3), which, due also to the potentially negative health consequences of hormone replacement therapy (4, 5), may motivate women to use these herbal medications. Furthermore, phytoestrogens are becoming progressively common constituents of human diets due to the latest dietary guidelines on substituting animal protein with soy-based foods (6). Therefore, there is an increased interest in the potential health effects of phytoestrogens beyond menopausal symptoms. Due to the structural similarity to estrogen, phytoestrogens bind weakly to estrogen receptor α and more strongly to estrogen receptor β. They may possess organ-specific estrogenic and antiestrogenic effects depending on the circulating estrogen concentration (if the circulating estrogen concentration is high they exert an antiestrogenic effect; when the estrogen concentration is low, their effect becomes more estrogenic) (7, 8). Emerging evidence shows that estradiol signaling can increase the risk of diabetes in postmenopausal women (9), and it has been suggested that phytoestrogens can avoid the estradiol-induced effects on type 2 diabetes (T2D) because of the ability of these compounds to compete with estradiol to bind its receptors, as well as via estrogen-independent pathways (7). In vitro studies have shown that isoflavones, phytoestrogen compounds commonly found in soy, have antidiabetic properties (10, 11); animal studies have indicated that phytoestrogens improve glycemic control and insulin sensitivity (12, 13). However, evidence from studies in humans on the effects of phytoestrogens on glucose homeostasis and T2D risk is inconsistent; some studies showed adverse effects (14), some no association (15), whereas others showed a beneficial effect (16). Previous quantitative reviews are limited by 1) a focus on specific populations (e.g., only Asian women), 2) evaluation of glycemic traits only not the risk of T2D, and 3) including heterogeneous interventions (e.g., phytoestrogen supplementation plus dietary restrictions), making interpretation of results challenging (17–19).
Thus, we performed a systematic review and meta-analysis of intervention studies and prospective population-based studies evaluating the association between phytoestrogen use, glucose homeostasis, and the risk of T2D among women.
Methods
Data sources and search strategy
The Cochrane Handbook for Systematic Reviews of Interventions and PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement were used to guide the conduct and reporting of this review (20, 21). A literature search was conducted using 5 electronic databases (Medline via Ovid www.ovid.com, EMBASE via embase.com, Web of Science Core Collection www.clarivate.com/Web-Of-Science, Cochrane CENTRAL via Wiley www.cochranelibrary.com, and Google Scholar www.scholar.google.com) from inception to 30 June 2017 (date last searched). In addition, reference lists of the included studies and relevant reviews, and studies that cited these articles, were searched with Elsevier's Scopus, the largest abstract and citation database. Details on the search strategy are provided in Supplemental Table 1.
Study selection and eligibility criteria
Studies were included if they met the following inclusion criteria: 1) were randomized controlled trials (RCTs) or prospective observational studies; 2) reported longitudinal associations of phytoestrogen supplementation, dietary phytoestrogens, or phytoestrogens in serum and urine with serum glucose, insulin, HOMA-IR and risk of incident T2D; 3) were performed among women or, when conducted in both men and women, showed results stratified by sex and/or reported that the interaction with sex was not significant (P > 0.05); and 4) were conducted in participants who did not use glucose-lowering medications. Only RCTs comparing an intervention with a placebo were included. Thus, RCTs that compared the intervention group with estrogen, other medications containing phytoestrogens, or an intervention with phytoestrogens in combination with specific diets were excluded. Two reviewers (MG and NK) independently evaluated the titles and abstracts according to the inclusion and exclusion criteria. For each potentially eligible study, 2 reviewers assessed the full text. In cases of disagreement, a decision was made by consensus or, if necessary, a third reviewer was consulted.
Data extraction
A predesigned data extraction form was used to collect relevant information. In case of multiple publications of the same study, the most recent information was extracted. For each study, the most-adjusted estimates were extracted. When studies included both men and women, did not report estimates separately for women, and reported no significant interaction with sex (P > 0.05), we extracted the estimates of the overall population.
Assessing the risk of bias
Bias within each individual study was evaluated by 2 reviewers. To assess the quality of RCTs we used The Cochrane Collaboration's tool for assessing risk of bias (22). Studies are judged to be at low or high risk of bias on the basis of criteria evaluating random-sequence generation, allocation concealment, blinding of participants/personnel, and outcome assessment, as well as incomplete outcome data and selective reporting (22). Studies are considered to have a low risk of bias if allocation concealment, blinding of participants, and outcome assessors were all coded “yes”; if a compliance assessment was done; and if the number of dropouts and reasons for dropout were reported. In case that ≥3 quality criteria were not met, the study was classified as having a high risk of bias; others were classified as having moderate (meeting 2 quality criteria) and low (meeting <2 quality criteria) risk of bias (Supplemental Table 2; 27–35, 37–45). The validated Newcastle-Ottawa scale, a semiquantitative scale designed to evaluate the quality of nonrandomized studies, was used to evaluate bias within each observational study (23). The assessment of the study quality was based on the selection criteria of participants, comparability of cases and controls, and exposure and outcome assessment. Studies that received a score of 9 stars were judged to be of at low risk of bias; studies that scored 7 or 8 stars were considered at medium risk; and studies that scored ≤6 stars were considered at high risk of bias (Supplemental Table 3; 14–16, 46–52).
Data synthesis and analysis
Intervention effects were defined as the differences in outcomes (glucose, insulin, HOMA-IR) between the intervention and placebo groups at the end of the trial. For continuous outcomes (e.g., glucose), summary measures were presented as mean differences; and for dichotomized outcomes (incident diabetes, yes or no), we presented RRs. In case of crossover trials, the data from the first period only were used. To enable a consistent approach to the meta-analysis and to improve interpretation of the findings, units of measurement were converted to common units. For observational studies, we used previously described methods (24) to transform estimates, which were often differentially reported by each study (e.g., comparing quartiles or thirds), to estimates corresponding to comparison of the top with the bottom quintile of the baseline phytoestrogen intake distribution in each study.
Briefly, we transformed the log RR by assuming a normal distribution, with the comparison between extreme quintiles being equivalent to 2.80 times the log risk ratio for 1-SD increases (or, equivalently, as 2.54/2.80 times the log RR for a comparison of extreme quartiles). We calculated SEs of the log RR by using published CIs and standardized them in the same way. Furthermore, when a study reported >1 risk estimate (e.g., for different types of phytoestrogens), the pooled RR (e.g., for any type of phytoestrogen) from the study to be used in meta-analysis was obtained using fixed-effects models. The inverse variance–weighted method was used to combine RRs to produce a pooled RR using random-effects meta-analysis models to allow for between-study heterogeneity. In addition, as a sensitivity analysis, we reported the estimates using fixed-effects models as shown in the forest plots. Heterogeneity was quantified using the I2 statistic, classified as low (I2 ≤ 25%), moderate (I2 > 25% and <75%), or high (I2 ≥ 75%) (25). In addition, a Q-statistic was used to assess the presence of heterogeneity. PQ-statistic ≥ 0.05 was considered to indicate no significant heterogeneity among the included studies. Study characteristics including geographic location, study population, median number of participants, median duration of intervention, median dosage of intervention, difference threshold between the lowest and highest phytoestrogen quintile intake, route of administration, menopausal status, median baseline age and BMI of participants, risk of bias, and RCT/observational design were prespecified as characteristics for the assessment of heterogeneity and were evaluated by using stratified analyses and random-effects meta-regression if ≥10 studies were included in the meta-analysis (26). To assess the influence of each individual study on the overall estimates of the rest of the studies, leave-one-out sensitivity analysis was performed iteratively removing one study at a time to confirm that our findings were not driven by any single study. Publication bias was evaluated through a funnel plot, and asymmetry was assessed using the Egger's test. All tests were 2-tailed and P values ≤0.05 were considered significant. STATA release 14 (StataCorp) was used for all statistical analyses.
Results
The search strategy identified 4724 references. After initial screening on the basis of titles and abstracts, the full texts of 68 articles were retrieved and evaluated further. As shown in Figure 1 after full-text assessment, 40 studies were excluded. The remaining 28 articles (based on 27 unique studies) were included in the review and meta-analysis. Of those, 18 were RCTs and 9 were observational prospective studies.
FIGURE 1.
Flow diagram of studies of included in the current review. RCT, randomized controlled trial; T2D, type 2 diabetes.
Clinical trials
Characteristics of the 18 trials included in this review can be found in Supplemental Table 4 (27–35, 37–45). In total, 1687 women (1006 in intervention group and 681 in the control group) with a baseline age ranging from 18 to 75 y were included. Fifteen RCTs were conducted in postmenopausal women, 2 included women regardless of their menopausal status, and 1 RCT was conducted in women and men but reported no sex interaction. Five RCTs were performed in North America, 5 in Europe, 5 in Asia, 2 in South America, and 1 in Australia. The included RCTs reported data on different types of isoflavones (isoflavone mixture supplementation: 9 RCTs; soy-derived isoflavones: 6 RCTs; isolated genistein supplementation: 4 RCTs; isolated daidzein: 2 RCTs; flaxseed: 1 RCT) and glucose homeostasis (glucose: 17 RCTs; insulin: 15 RCTs; and HOMA-IR: 11 RCTs), but none reported data on the risk of T2D. Thirteen of the RCTs included healthy women, 2 RCTs included women with metabolic syndrome, 2 included women with prediabetes, and 1 clinical trial included women 6 mo after treatment for breast cancer. None of the women included in our meta-analysis used glucose-lowering medications. Details on average changes between baseline and intervention on serum glucose, insulin, and HOMA-IR can be found in Supplemental Table 5 (27–35, 37–45).
Observational prospective studies
Detailed study characteristics of the 10 articles based on prospective observational studies, which included 2 case-cohort (46, 47) and 8 population-based cohort (14–16, 48–52) studies, included in this review can be found in Supplemental Table 6 (14–16, 43–46, 48–50). Of the 9 studies included in meta-analysis, 4 studies were from Asia, 3 from North America, and 2 from Europe. One study included postmenopausal women only (50), whereas the other studies did not specify the menopausal status. Three studies reported estimates for men and women combined; however, they reported that the interaction with sex was not significant. The baseline age of participants included in the 9 studies ranged from 32 to 80 y (median: 53.87 y). The period of follow-up ranged from 4 to 15 y (Supplemental Table 6). All of the included observational studies reported data on phytoestrogens and the risk of T2D, and none reported on prospective glycemic traits. All 9 studies reported on dietary phytoestrogen intake, and some of these reported different types of phytoestrogens (overall isoflavones: 7 studies; genistein: 3 studies; daidzein: 3 studies; soy products: 3 studies; soy protein: 2 studies; flavonoids: 4 studies). In the study by Ko et al. (46), in addition to dietary phytoestrogens, serum concentrations of genistein and daidzein were measured. The overall number of subjects in the 9 observational studies included in our meta-analysis was 212,796 with 9721 incident T2D cases. Details on exposure and outcome assessment can be found in Supplemental Table 7 (14–16, 43–46, 48–50).
Association between phytoestrogens and glucose homeostasis from RCTs
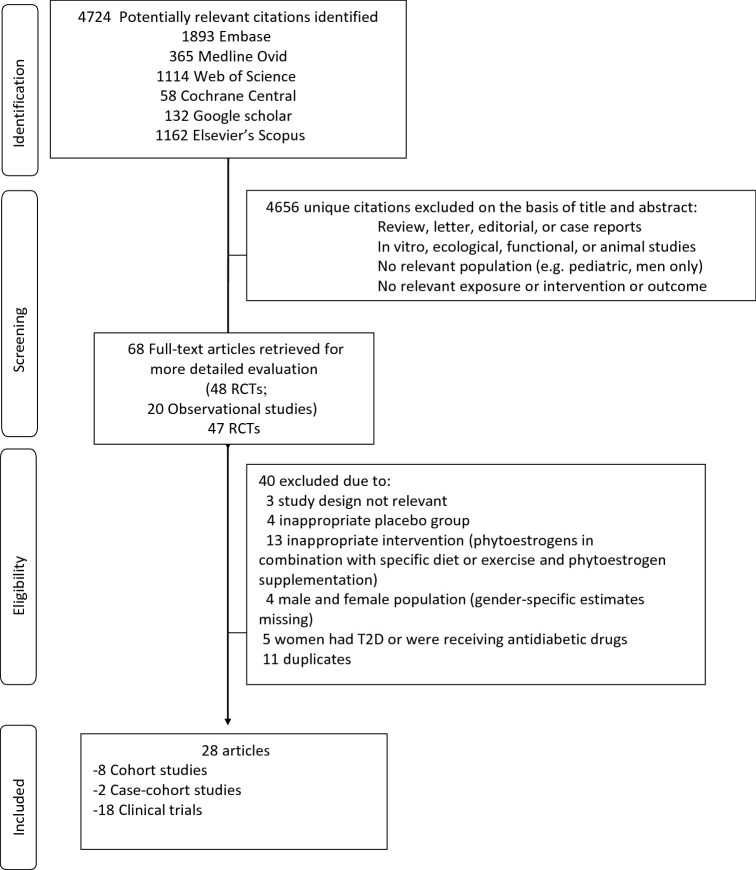
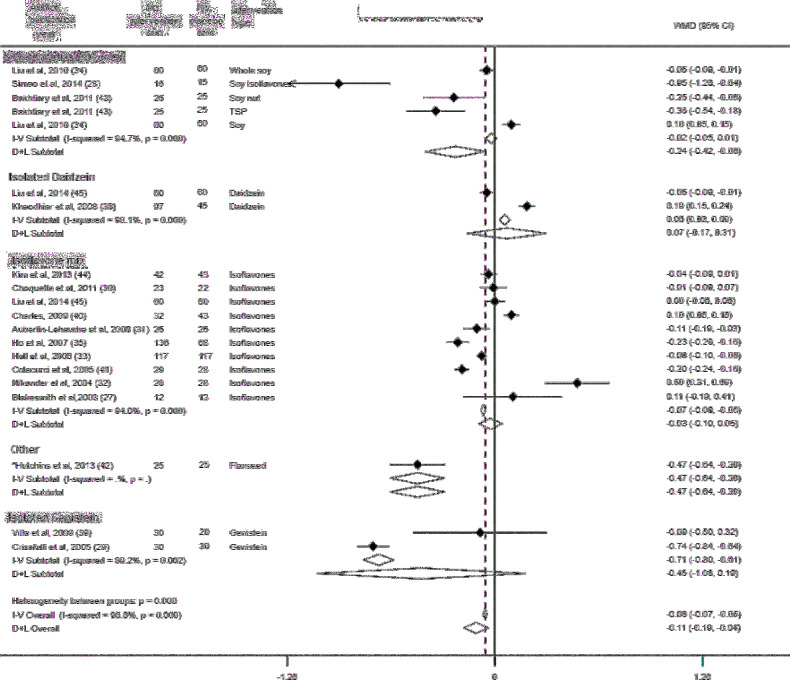
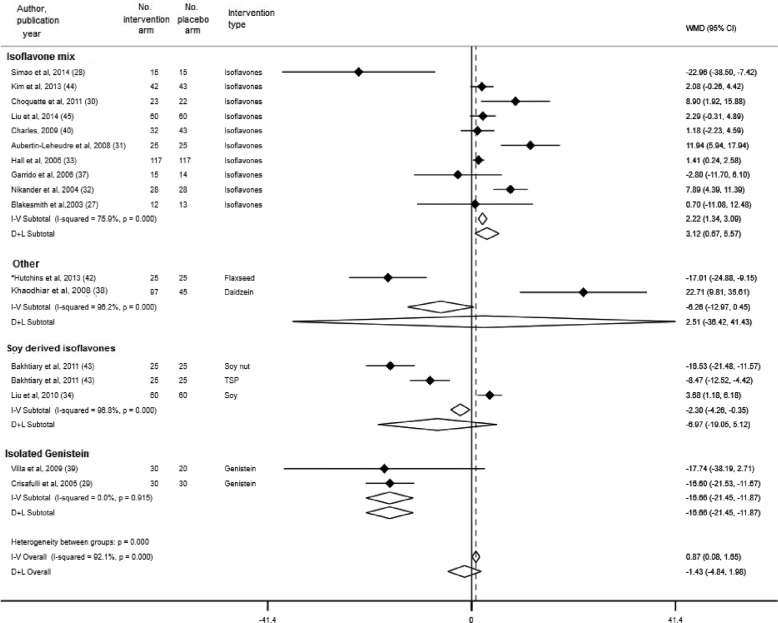
Data from 17 RCTs (including 1658 subjects), 15 RCTs (including 1186 women), and 11 RCTs (including 909 women) contributed to the meta-analysis on the effects of phytoestrogen supplementation on fasting serum glucose concentrations (27–35, 38, 39, 41–45), fasting insulin (27–35, 37–40, 42–44), and HOMA-IR (27–30, 33, 34, 39, 40, 42–44), respectively. Compared with placebo, phytoestrogen supplementation was associated with a reduction in serum glucose concentration (pooled mean difference in changes: –0.12 mmol/L; 95% CI: –0.20, –0.03 mmol/L) (Supplemental Figure 1). In the subgroup analysis by type of phytoestrogen, soy-derived isoflavones were significantly associated with a reduction in serum glucose concentration (pooled mean difference in changes: –0.24 mmol/L; 95% CI: –0.42, –0.06 mmol/L), whereas no significant associations were observed between other subgroups of phytoestrogens (isoflavone mixture, isolated daidzein, and genistein) and changes in glucose concentrations (Figure 2). Compared with placebo, overall phytoestrogen supplementation was not associated with a decrease in insulin concentrations (pooled mean difference in changes: –0.99 pmol/L; 95% CI: –4.65, 2.67 pmol/L) (Supplemental Figure 2). In the subgroup analysis, isolated genistein was associated with a decrease in insulin concentrations (pooled mean difference in changes: –16.66 pmol/L; 95% CI: –21.45, –11.87 pmol/L) (Figure 3), whereas isoflavone mix was associated with an increase in insulin concentrations (pooled mean difference in changes: 3.12 pmol/L; 95% CI: 0.67, 5.57 pmol/L). No association was observed between soy-derived isoflavones and insulin concentrations. Furthermore, we observed a reduction in HOMA-IR in phytoestrogen users as compared with placebo (pooled mean difference in changes: −0.24; 95% CI: −0.45, −0.03) (Supplemental Figure 3). However, in the subgroup analysis, there was an indication for an increased HOMA-IR with the use of isoflavone mix (pooled mean difference in changes: 0.12; 95% CI: 0.02, 0.23), whereas there was significant decrease with isolated genistein as compared to placebo [pooled mean difference of changes: −0.83 (95% CI: −0.94 to −0.73)] (Figure 4).
FIGURE 2.
Subgroup analysis by type of phytoestrogen and changes in serum glucose. Error bars indicate 95% CIs. Solid vertical line represents no effect; the dotted line drawn through the diamond represents the summary measure with its CIs (lateral tips of diamond). Heterogeneity assessment: I2, P values are derived from Q-statistics; in subgroup analysis, we used multiple estimations from the same trial (for different phytoestrogen type), which may result in slight variations in overall estimates in this figure compared with Supplemental Figure 1. *No significant relations between sex, body weight, BMI, or percentage fat mass and changes, or lack of changes, in glucose, insulin, HOMA-IR, and inflammatory or anti-inflammatory biomarkers were found; therefore, all further analyses were conducted based on the intervention. D+L, random-effects model; I-V, fixed-effects model; TSP, texturized soy protein; WMD, weighted mean difference (the mean difference refers to mean difference of changes between treatment groups).
FIGURE 3.
Subgroup analysis by type of phytoestrogen and changes in serum insulin. Error bars indicate 95% CIs. The solid vertical line represents no effect; the dotted line drawn through the diamond represents the summary measure with its CIs (lateral tips of diamond). Heterogeneity assessment: I2, P values are derived from Q-statistics; in subgroup analysis we used multiple estimations from the same trial (for different phytoestrogen type), which may result in slight variations in overall estimates in this figure compared with Supplemental Figure 2. *No significant relations between sex, body weight, BMI, or percentage fat mass and changes, or lack of changes, in glucose, insulin, HOMA-IR, and inflammatory or anti-inflammatory biomarkers were found; therefore, all further analyses were conducted based on the intervention. D+L, random-effects model; I-V, fixed-effects model; TSP, texturized soy protein; WMD, weighted mean difference (the mean difference refers to mean difference of changes between treatment groups).
FIGURE 4.
Subgroup analysis by type of phytoestrogen and changes in HOMA-IR. Error bars indicate 95% CIs. The solid vertical line represents no effect; the dotted line drawn though the diamond represents the summary measure with its CIs (lateral tips of diamond). Heterogeneity assessment: I2, P values are derived from Q-statistics; in subgroup analysis we used multiple estimations from the same trial (for different phytoestrogen type), which may result in slight variations in overall estimates in this figure compared with Supplemental Figure 3. *No significant relations between sex, body weight, BMI, or percentage fat mass and changes, or lack of changes, in glucose, insulin, HOMA-IR, and inflammatory or anti-inflammatory biomarkers were found; therefore, all further analyses were conducted based on the intervention. D+L, random-effects model; I-V, fixed-effects model; TSP, texturized soy protein; WMD, weighted mean difference (the mean difference refers to mean difference of changes between treatment groups).
Association between phytoestrogens and risk of T2D from prospective observational studies
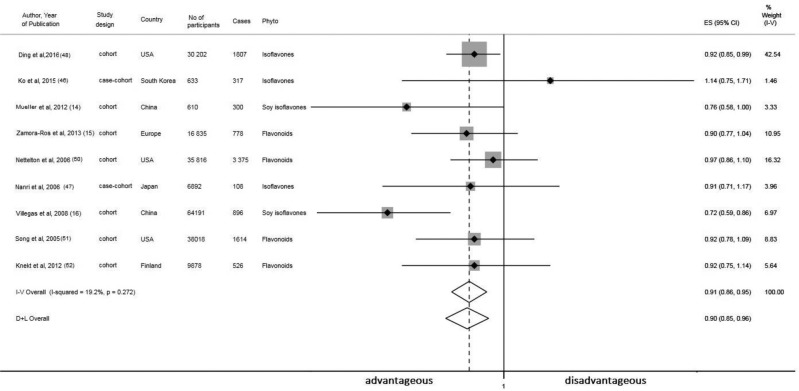
Data from 9 observational studies (14–16, 46–48, 50–52) contributed to the meta-analysis on phytoestrogen intake and the risk of T2D. The meta-analysis of fully adjusted RRs, based on 212,796 subjects and 9721 incident cases of T2D, showed that women who reported high intakes of any type of phytoestrogen had a lower risk of developing T2D (pooled RR: 0.90; 95% CI: 0.85, 0.96; for the highest compared with the lowest quantiles of phytoestrogen intake) (Figure 5). In addition to dietary intake, 2 longitudinal studies also measured phytoestrogen biomarkers in relation to incident T2D. Findings from the Nurses’ Health Study, not included in our meta-analysis, showed that for each SD increase in urinary concentrations of total lignans metabolites, the OR of developing T2D was 0.70 (95% CI: 0.53, 0.92) (49). The study by Ko et al. (46) reported that higher plasma concentrations of genistein were associated with a decreased risk of T2D (comparing extreme quintiles—OR: 0.58; 95% CI: 0.35, 0.95) in women, whereas no association was observed between plasma glycitein or daidzein and the risk of T2D.
FIGURE 5.
Meta-analysis of prospective population-based studies on the associations between dietary phytoestrogen intake and risk of type 2 diabetes in women. Sizes of data markers are proportional to the inverse of the variance of the OR. The solid vertical line represents no effect; the dotted line drawn through the diamond represents the summary measure with its CIs (lateral tips of diamond). Heterogeneity assessment: I2, P values are derived from Q-statistics. D+L, random-effects model; ES, effect size; I-V, fixed-effects model; Phyto, phytoestrogen.
Assessments of bias, heterogeneity, and sensitivity analysis
Four RCTs showed a high risk of bias in 2 domains; however, for most of the RCTs (n = 14) the risk of bias could not be classified in ≥1 domains (Supplemental Table 2). Most included observational studies were considered to be at low risk of bias (n = 8) and one considered to be at medium risk of bias (Supplemental Table 3). The 3 meta-analyses of RCTs showed high between-study heterogeneity, with an I2 estimate >75% and PQ-statistic < 0.05 (Figures 2–4). High heterogeneity observed in our meta-analyses could be explained by differences between studies, including heterogeneous study populations, methods, and effect estimates reported. We attempted to explore sources of heterogeneity contributing to our results, but none of the factors we considered [e.g., disease or menopausal status, route of administration, dosage or duration of the intervention (for RCTs), study location, or design of studies] could explain the heterogeneity. In addition, the heterogeneity was not explained by baseline characteristics of study participants, age, or BMI. However, the quality of RCTs might be an important factor to influence such high heterogeneity observed; RCTs with a low to medium risk of bias that reported insulin and HOMA-IR changes showed lower heterogeneity than RCTs with a high risk of bias (Table 1). Meta-analysis of observational studies showed low heterogeneity, with an I2 estimate of 19.2% and a PQ-statistic = 2.27. Stratification by type of phytoestrogen and difference between phytoestrogen intake in the highest compared with the lowest quantile yielded similar results as observed in the main analysis. In addition, stratification by median BMI and age did not differ significantly from the main observations (Table 2). Separate analysis excluding studies that reported pooled estimates of both sexes, and including only observational studies that investigated the association between phytoestrogen intake and T2D risk in female population, was in line with the main findings (Table 3). In the leave-one-out analysis for glucose, our pooled estimates remained stable, indicating that the pooled results for glucose are not overly influenced by any single study. However, in the pooled analysis of phytoestrogen supplementation, insulin concentrations, and HOMA-IR, the summary effect size did not reach significance in all cases in the leave-one-out analysis, indicating no consistency (Supplemental Figure 4). For the pooled analyses involving ≥5 studies, publication bias was assessed visually by using Begg's funnel plots, which were approximately symmetrical. The Egger's test estimates were nonsignificant for all of these analyses (P values ranging from 0.25 to 0.58) (Supplemental Figures 5 and 6).
TABLE 1.
Subgroup analysis for RCTs included in our review1
| Subgroups by study characteristics | Number of studies | Mean difference (95% CI)2 | I 2 for heterogeneity,3 % | P-heterogeneity4 |
|---|---|---|---|---|
| Phytoestrogen use and mean serum glucose change5 | ||||
| Study population | ||||
| Healthy women | 12 | −0.1 (−0.19, −0.01) | 97.3 | |
| Other women | 5 | −0.2 (−0.54, 0.13) | 96.1 | |
| Median number of participants | ||||
| ≤60 | 8 | −0.14 (−0.3, 0.02) | 93.2 | 0.28 |
| >60 | 9 | −0.1 (0.21, −0.003) | 97.9 | |
| Menopausal status | ||||
| Postmenopausal | 14 | −0.07 (−0.15, 0.01) | 97.2 | 0.17 |
| Other | 3 | −0.44 (−0.94, 0.07) | 91.5 | |
| Age, y | ||||
| ≤55.15 | 9 | −0.06 (−0.18, 0.07) | 96.6 | 0.66 |
| >55.15 | 8 | −0.18 (−0.31, −0.05) | 97.6 | |
| BMI, kg/m2 | ||||
| ≤25.77 | 9 | −0.07 (−0.18, 0.05) | 97.5 | 0.44 |
| >25.77 | 7 | −0.23 (−0.43, −0.02) | 97.1 | |
| Route of administration | ||||
| Tablet/capsule | 14 | −0.07 (−0.15, 0.01) | 97.2 | 0.17 |
| Other | 3 | −0.44 (−0.94, 0.07) | 91.5 | |
| Dosage, mg/d | ||||
| ≤86 | 9 | −0.17 (−0.36, 0.02) | 96.8 | 0.29 |
| >86 | 7 | −0.02 (−0.11, 0.07) | 97.2 | |
| Other | 1 | NA | NA | |
| Intervention duration, wk | ||||
| ≤16 | 7 | −0.09 (−0.24, 0.07) | 96.2 | 0.42 |
| >16 | 10 | −0.15 (−0.24, −0.06) | 96.7 | |
| Location | ||||
| Asia | 6 | −0.07 (−0.15, 0.01) | 90.2 | 0.81 |
| Other | 11 | −0.15 (−0.28, −0.02) | 97.9 | |
| Risk of bias | ||||
| High | 8 | −0.33 (−0.5, −0.16) | 97.1 | 0.62 |
| Low to medium | 9 | 0.03 (−0.06, 0.12) | 96.2 | |
| Design | ||||
| RCT crossover | 4 | 0.01 (−0.31, 0.03) | 94.8 | 0.33 |
| RCT | 13 | −0.15 (−0.26, −0.05) | 97.4 | |
| Phytoestrogen use and mean serum insulin change6 | ||||
| Study population | ||||
| Healthy women | 10 | 1.51 (−2.7, 5.73) | 89.1 | 0.64 |
| Other women | 5 | −6.87 (−16.13, 2.39) | 95.7 | |
| Median number of participants | ||||
| ≤50 | 6 | −4.63 (−11.17, 1.92) | 94.8 | 0.68 |
| >50 | 9 | 1.51 (−44.08, 7.11) | 90.2 | |
| Menopausal status | ||||
| Menopausal | 12 | 0.98 (−2.76, 4.71) | 92.6 | 0.20 |
| Other | 3 | −12.77 (−25.8, 0.25) | 74.3 | |
| Age, y | ||||
| ≤56.61 | 8 | −2.31 (−10.14, 5.51) | 92.1 | 0.33 |
| >56.61 | 7 | −0.28 (−4.85, 4.29) | 93.3 | |
| BMI, kg/m2 | ||||
| ≤26.25 | 8 | 1.51 (−1.95, 4.97) | 90 | 0.13 |
| >26.25 | 7 | −5.36 (−15.71, 4.98) | 91 | |
| Route of administration | ||||
| Tablet/capsule | 9 | 2.51 (−4.15, 9.17) | 91.5 | 0.23 |
| Other | 6 | −5.24 (−10.31, −0.17) | 93.7 | |
| Dosage, mg/d | ||||
| ≤78 | 8 | 0.94 (−6.77, 8.66) | 93.2 | 0.44 |
| >78 | 6 | −1.45 (−5.59, 2.69) | 90.3 | |
| Other | 1 | NA | NA | |
| Intervention duration, wk | ||||
| ≤12 | 8 | −2.32 (−8.82, 4.18) | 93.2 | 0.33 |
| >12 | 7 | 0.42 (−4.7, 5.54) | 91.8 | |
| Location | ||||
| Asia | 4 | −1.85 (−8.69, 4.99) | 94.1 | 0.88 |
| Other | 11 | −0.86 (−6.12, 4.41) | 92.1 | |
| Risk of bias | ||||
| High | 6 | −11.95 (−20.54, −3.35) | 88 | 0.19 |
| Low to medium | 9 | 3.91 (1.54, 6.27) | 76.2 | |
| Design | ||||
| RCT crossover | 4 | −1.02 (−8.2, 6.17) | 91.2 | 0.29 |
| RCT | 11 | −1.13 (−6.43, 4.18) | 92.9 | |
| Phytoestrogen use and mean HOMA-IR change | ||||
| Study population | ||||
| Healthy women | 4 | −0.61 (−1.2, −0.02) | 97.3 | 0.27 |
| Other women | 7 | −0.08 (−0.35, 0.20) | 97.8 | |
| Median number of participants | ||||
| <60 | 6 | −0.49 (−1.05, 0.06) | 96.5 | 0.11 |
| ≥60 | 5 | −0.02 (−0.18, 0.13) | 94.7 | |
| Menopausal status | ||||
| Menopausal | 8 | −0.14 (−0.37, 0.09) | 97.9 | 0.15 |
| Other | 3 | −0.62 (−1.41, 0.17) | 91.5 | |
| Age, y | ||||
| ≤60 | 6 | −0.38 (−0.85, 0.08) | 97.9 | 0.17 |
| >60 | 5 | −0.13 (−0.38, 0.11) | 96.4 | |
| BMI, kg/m2 | ||||
| ≤27.3 | 5 | −0.09 (−0.38, 0.2) | 98.4 | 0.09 |
| >27.3 | 6 | −0.44 (−0.9, 0.02) | 96.1 | |
| Route of administration | ||||
| Tablet/capsule | 5 | −0.16 (−0.371, 0.39) | 98.2 | 0.20 |
| Other | 6 | −0.28 (−0.5, −0.05) | 96 | |
| Dosage, mg/d | ||||
| ≤93 | 5 | −0.51 (−1.1, 0.08) | 98 | 0.35 |
| >93 | 5 | 0.02 (−0.17, 0.22) | 95.4 | |
| Other | 1 | NA | NA | |
| Intervention duration, wk | ||||
| <16 | 5 | −0.43 (−0.82, −0.03) | 96.5 | 0.18 |
| ≥16 | 6 | −0.11 (−0.44, 0.21) | 98.2 | |
| Location | ||||
| Asia | 3 | −0.12 (−0.48, 0.24) | 97.8 | 0.34 |
| Other | 8 | −0.32 (−0.65, 0.02) | 97.1 | |
| Risk of bias | ||||
| High | 6 | −0.62 (−1.07, −0.17) | 91.6 | 0.10 |
| Low to medium | 5 | 0.1 (0.02, 0.18) | 75 | |
| Design | ||||
| RCT crossover | 3 | −0.17 (−0.66, 0.33) | 93.2 | 0.09 |
| RCT | 8 | −0.3 (−0.64, 0.04) | 97.9 | |
1“Healthy women” are considered premenopausal or postmenopausal women included in an RCT; “Other women” are women with metabolic syndrome, glucose intolerance, or unrecognized diabetes (without antidiabetic medications); women treated for breast cancer in the previous 6 mo; and osteopenic and obese women. “Median number of participants” was calculated separately for each outcome. “Menopausal status” indicates postmenopausal women vs. adult women (above the age of 18 y). “Median age” was calculated separately for each outcome. “Median BMI” was calculated separately for each outcome [Liu et at. (45) did not report BMI]. “Route of administration” includes the use of tablets/capsules and other routes of administration (shake, powder, flower). “Dosage” indicates the mean dosage based on all included RCTs was 80 mg/d. “Intervention duration” indicates that the median intervention duration was calculated on the basis of all included RCTs; we compared RCTs with durations of intervention of ≤14 and >14 wk. “Location” refers to study location; studies done in Asia vs. studies done in Europe, America, and Australia. “Risk of bias” means that studies are considered to be low risk of bias if allocation concealment, blinding of participants, and outcome assessors were all coded “yes”; if a compliance assessment was done; and the number of dropouts and reasons for dropout were reported. In the case that ≥3 quality criteria were not met, the study was classified as having a high risk of bias; others were classified as having a moderate risk of bias. “Study design” refers to RCT vs. RCT crossover design. NA, not applicable; RCT, randomized controlled trial.
2Mean difference refers to mean difference of changes between treatment groups in serum glucose, insulin, and HOMA-IR (subjects using phytoestrogens as compared with subjects from control/placebo group).
3 l 2 describes the percentage of variation across studies that is due to heterogeneity rather than chance.
4 P values for heterogeneity were evaluated by using random-effects meta-regression. P values were calculated between 2 or 3 groups that were considered to be source of heterogeneity; the groups are indicated in the table.
5Glucose in mmol/L.
6Insulin in pmol/L.
TABLE 2.
Subgroup analysis for phytoestrogen intake and risk of T2D1
| Subgroups by study characteristics | Number of studies | Participants, n | T2D cases, n | RR (95% CI) | P-heterogeneity2 |
|---|---|---|---|---|---|
| Study population | |||||
| Only women | 7 | 183,919 | 8117 | 0.91 (0.83-0.99) | 0.65 |
| Men and women | 3 | 28,927 | 1604 | 0.88 (0.79, 0.98) | |
| Study design | |||||
| Nested case-control | 2 | 7950 | 425 | 0.97 (0.78, 1.20) | 0.45 |
| Cohort | 7 | 204,896 | 9296 | 0.89 (0.83, 0.96) | |
| Type of phytoestrogen | |||||
| Isoflavones | 5 | 106,006 | 3428 | 0.86 (0.77, 0.98) | 0.30 |
| Flavonoids | 4 | 106,840 | 6293 | 0.93 (0.86, 1.01) | |
| Genistein | 3 | 39,964 | 2232 | 0.87 (0.73, 1.05) | NA |
| Daidzein | 3 | 39,964 | 2232 | 0.89 (0.84, 0.95) | NA |
| Soy products | 3 | 72,997 | 1304 | 0.81 (0.68, 0.97) | NA |
| Location | |||||
| Asia | 4 | 73,997 | 1621 | 0.83 (0.87, 0.98) | 0.05 |
| Other | 5 | 138,849 | 8100 | 0.93 (0.88, 0.98) | |
| Difference between phytoestrogen intake in | |||||
| highest vs. lowest quantile | |||||
| Median or less (5.33-fold) | 5 | 123,123 | 3461 | 0.88 (0.78, 1.00) | 0.49 |
| Higher than median (5.33-fold) | 4 | 89,723 | 6260 | 0.92 (0.87, 0.999) | |
| BMI, kg/m2 | |||||
| Median or less (≤25.89) | 5 | 80,551 | 4146 | 0.91 (0.86, 0.97) | 0.70 |
| Higher than median (>25.89) | 4 | 132,295 | 5575 | 0.88 (0.78, 1.00) | |
| Age | |||||
| Median or less (≤53.87) | 5 | 133,736 | 4131 | 0.88 (0.79, 0.98) | 0.48 |
| Higher than median (>53.87) | 4 | 79,110 | 5590 | 0.92 (0.87, 0.98) | |
1“Study population” indicates studies conducted only in women: investigation performed only among female population, after excluding studies [Muller et al. (14), Zamora-Ros et al. (15) and Knekt et al. (52)] that reported overall results for male and female subjects but stated that they tested the interaction term with sex. “Study design” indicates that only prospective cohort and nested case-control studies were included. “Type of phytoestrogen”: “Soy products” estimates were pooled together for soy beans, soy milk, soy flour, and other soy products. “Location”: “Asia” (South Korea, Japan, and 2 studies from China; “Other”: Europe and 2 studies from the United States). NA, not applicable; T2D, type 2 diabetes.
2 P values for heterogeneity were evaluated by using random-effects meta-regression. P values were calculated between 2 or 3 groups that were considered to be a source of heterogeneity; the groups are indicated in the table (if >5 studies were included).
TABLE 3.
Subgroup analysis for phytoestrogen intake and risk of T2D in studies conducted in women only1
| Subgroups by study characteristics | Number of studies | Participants, n | T2D cases, n | RR (95% CI) |
|---|---|---|---|---|
| Study design | ||||
| Nested case-control | 2 | 7950 | 425 | 0.97 (0.78, 1.20) |
| Cohort | 4 | 186,323 | 8218 | 0.89 (0.81, 0.99) |
| Type of phytoestrogen | ||||
| Isoflavones | 4 | 105,096 | 3128 | 0.88 (0.76, 1.02) |
| Other phytoestrogens | 2 | 89,227 | 5515 | 0.95 (0.86, 1.05) |
| Difference between phytoestrogen intake in | ||||
| highest vs. lowest quantile | ||||
| Median or less (≤5.33-fold) | 3 | 73,037 | 1321 | 0.86 (0.68, 1.10) |
| Higher than median (>5.33-fold) | 3 | 110,832 | 6796 | 0.93 (0.88, 0.99) |
| Location | ||||
| Asia | 3 | 73,087 | 1321 | 0.86 (0.68, 1.10) |
| Other | 3 | 121,236 | 7322 | 0.93 (0.88, 0.99) |
| Age, y | ||||
| Median or less (≤53.35) | 3 | 105,669 | 2827 | 0.87 (0.69, 1.09) |
| Higher than median (>53.35) | 3 | 78,200 | 5290 | 0.93 (0.88, 0.99) |
| BMI, kg/m2 | ||||
| Median or less (≤25.69) | 3 | 39,959 | 2232 | 0.93 (0.86, 0.99) |
| Higher than median (>25.69) | 3 | 143,910 | 5885 | 0.87 (0.74, 1.03) |
1“Study population” indicates studies conducted only in women: investigation performed only among female population, after excluding studies [Muller et al. (14), and Zamora-Ros et al. (15)] that reported overall results for male and female subjects but stated that they tested the interaction term with sex. “Study design”: only prospective cohort and nested case-control studies were included. “Type of phytoestrogen”: “Soy products” estimates were pooled together for soy beans, soy milk, soy flour, and other soy products. “Location”: “Asia” (South Korea, Japan, and 2 studies from China; “Other”: Europe and 2 studies from the United States). T2D, type 2 diabetes.
Discussion
In this systematic review and meta-analysis, we show that overall phytoestrogen supplementation is associated with a reduction in fasting glucose in nondiabetic women. However, the results of clinical trials were not consistent for insulin and HOMA-IR and an indication for increased levels of these traits was observed with some specific types of phytoestrogens, such as the use of an isoflavone mixture and isolated genistein. Findings from observational studies were consistent in showing higher dietary phytoestrogen intake to be associated with a decreased risk of T2D in women.
Our findings of a protective effect of phytoestrogens among women without previous T2D are in line with other studies showing that phytoestrogens can improve glycated hemoglobin concentrations and insulin sensitivity in patients with metabolic syndrome and T2D (36, 53–57). Contrary to previous meta-analyses, which focused on both men and women (17) or on specific women populations (Asian postmenopausal women) (18) included heterogeneous studies (e.g., participants taking glucose-lowering medications) (19), in our study we included the following: 1) studies in women of any ethnicity and menopausal status, 2) studies in women not taking glucose-lowering medications because these can effect glucose/insulin concentrations, and 3) trials comparing any type of phytoestrogen against placebo (RCTs comparing an intervention with a lower dosage of phytoestrogens or estradiol/hormone replacement therapy were not included; all studies that investigated combined interventions—exercise or diet with phytoestrogen supplementation—were also not included). In addition, our review did not restrict the search only to phytoestrogen supplementation, but we also included studies reporting dietary intake of phytoestrogens and phytoestrogens assessed in blood and urine. Therefore, our meta-analysis provides a more detailed assessment of the nature and magnitude of the association between composite and specific phytoestrogens, glucose homeostasis, and T2D in women.
Potential mechanisms linking phytoestrogens and glucose metabolism and their potential role in T2D prevention have been extensively studied (7). Phytoestrogens are thought to affect glucose metabolism via estrogen-dependent and nonestrogen-dependent pathways (7). Phytoestrogens modulate glucose and lipid metabolism directly (lipogenesis, lipolysis, adipogenesis) and indirectly modulate appetite and energy expenditure (7). Phytoestrogens regulate glucose homeostasis–related metabolic processes at cellular levels in intestinal cells, pancreatic islet cells, hepatocytes, and skeletal muscle cells (10, 58, 59). In addition, phytoestrogens increase the expression of genes involved in glucose homeostasis and lipid metabolism (7) and suppress genes that affect gluconeogenesis (60). Another possible protective mechanism in T2D is the antioxidant activity of phytoestrogens (7). Furthermore, clinical trials in humans found that isoflavone-enriched soy products increased antioxidant capacity (7). It is also possible that phytoestrogen's antiobesity features play a significant role in T2D prevention (61). A study in normal-weight postmenopausal women showed that the consumption of isoflavones was associated with lower BMI and fasting insulin concentration (62).
To our knowledge, this is the first systematic review and meta-analysis including >213,000 women to comprehensively address the associations of phytoestrogens with glycemic traits and the risk of developing T2D in women. However, there are several limitations that need to be taken into account. First, our overall findings may have been affected by publication bias. Despite conventional funnel plots and Egger’s test estimates indicating minimal publication bias, these approaches are limited by their qualitative nature. Second, although the quality of observational studies was in general high, the methodologic quality of the RCTs varied considerably, which might have contributed to the heterogeneity we observed in the meta-analyses presented in this study. The factors that may have affected the quality of RCTs may include the composition of supplements or the presence of menopausal symptoms, which is the main reason why women take supplements and which is also linked to adverse cardiometabolic health (63, 64). In addition, there were only 4 RCTs with >100 participants and only 1 RCT with a duration of the intervention of 1 y, which might undermine the precision of the estimates and limit our understanding about long-term effects of phytoestrogen supplementation on glucose homeostasis. Third, pooled estimates for the association between phytoestrogen supplementation and insulin and HOMA-IR levels should be taken with caution, because our leave-one-out sensitivity analysis showed that results were driven by individual studies. Fourth, the ability to meta-analyze studies on insulin and HOMA-IR is largely limited by the nonstandardization of insulin assays. The mean insulin concentrations at baseline across RCTs showed high variability; this was also the case for HOMA-IR, which includes insulin in its calculation. Fifth, a limitation of observational studies included in our meta-analysis is the use of food questionnaires to assess dietary intake of phytoestrogens. This method is prone to measurement error due to recall bias, incomplete inclusion of phytoestrogen-enriched food items in the questionnaire, and incomplete data on phytoestrogen composition of foods from food-composition tables. Sixth, numerous factors may influence phytoestrogen metabolism and its plasma concentrations. However, because the outcome in all observational studies included in this systematic review was assessed prospectively, the subjective measure of dietary phytoestrogen intake would likely lead to nondifferential misclassification with respect to the outcome, and therefore would likely bias our estimates toward the null in our analysis. Considering the limited available evidence, prospective studies using objective biomarkers of phytoestrogen exposure are needed in order to further investigate the potential protective role of phytoestrogens in the prevention of T2D in women. Last, this review underscores a number of gaps in the literature concerning types of phytoestrogens other than isoflavones on their role in diabetes prevention. In light of these observations, the overall results of this study should be interpreted with caution.
This review may have several implications. On the basis of the available evidence, phytoestrogen-based remedies might be a safe choice with regard to T2D in the treatment of menopausal symptoms in women. In addition, our review addresses major literature gaps. It remains unclear if specific phytoestrogen-rich foods are more favorable in the prevention of T2D. Findings from the Singapore Chinese Health Study showed inverse associations between unsweetened soy product consumption and T2D risk; however, in contrast to this, sweetened soybean drink consumption was positively associated with T2D risk (13). In the current review, we did not find differences in the effects of different types of phytoestrogens and the risk of T2D; however, we observed some differences in glucose homeostasis with regard to phytoestrogen type. In our subgroup analysis, soy-derived isoflavones were significantly associated with lower concentrations of glucose, whereas isolated genistein was associated with lower serum insulin and HOMA-IR. Furthermore, an isoflavone mix significantly increased insulin and HOMA-IR levels. Isoflavone mixtures contain genistein, daidzein, and glycitein in various proportions due to variations in isoflavone composition in primary raw material. Therefore, when specific isoflavones (e.g., genistein) are administered alone, they may have different metabolic effects compared with when a mixture of different types of isoflavones is administered. The content of genistein and daidzein is approximately equal, whereas glycitein is present in lower concentration in whole soy beans (19). Genistein has 10-fold more potent estrogenic activity compared with daidzein (65), whereas glycitein has the highest estrogenic potential in vivo (66). Daidzein can be metabolized into equol, which has higher estrogenic potential than daidzein (67), whereas genistein and glycitein can be biodegraded into metabolites with no estrogenic activity (68). However, our findings on subgroup analysis should be interpreted with caution because the limited number of studies precluded our ability to perform comprehensive analysis. Furthermore, it is known that, in Asia, fermented soy products are part of the traditional diet, with isoflavone intakes from 15 to 50 mg/d, with the highest intake in the Southeastern region (69), whereas in the European population, isoflavone intake has been reported to be <2 mg/d (70). The heterogeneity was moderate (I2 = 42.1%, PQ-statistics = 0.16) for the association between phytoestrogens and the risk of T2D in the meta-analysis including countries from Asia, whereas no heterogeneity was observed in the meta-analysis of studies from outside of Asia. Baseline phytoestrogen intake in Asia is higher than in the Western world. In addition, across Asia, phytoestrogen intake varies between different countries (1); thus, there might be more variation and thereby more residual heterogeneity within this group. Furthermore, there might be other factors contributing to higher heterogeneity in Asian studies (2 studies were nested case-control and 2 were prospective cohort studies, whereas all non-Asian studies were prospective cohort or in diverse genetic populations), which merit further investigation.
Emerging evidence shows that soy products may be more effective in maintaining good health in equol-producing individuals (71). The gut microbiome modifies phytoestrogens into metabolites that differ in biological activity from the parent compounds (71). For example, Asian individuals have greater ability than non-Asians to produce equol, which is the metabolite of daidzein (1). Existing trials, although they did not show ethnicity differences on associations of phytoestrogens with glycemic traits, did not properly address this issue. Thus, it is necessary for future trials and observational prospective studies to investigate metabolites that are produced by phytoestrogens, how these metabolites contribute to the relation of phytoestrogens to human health, and whether their levels and effects differ across populations. Furthermore, future studies with adequate sample sizes investigating different types and dosages of phytoestrogens and that examine whether there are dose effects are needed.
In conclusion, the available body of literature suggests that phytoestrogen dietary intake or supplementation might have a beneficial effect in the prevention of insulin resistance and T2D among women. However, the intervention studies conducted up to date are of suboptimal quality and, thus, further rigorous studies with long-term follow-up are needed to determine the role of specific subgroups of phytoestrogens in diabetes prevention.
Supplementary Material
Acknowledgments
All authors read and approved the final manuscript.
Notes
Author disclosures: TM currently works as a pharmaceutical medicine physician at Novo Nordisk, Copenhagen, Denmark; however, this role had no influence on the design or conducting of this study. MG, NK, VG-J, WMB, FA, RC, AHJD, AJMR, TV, and OHF reported no conflicts of interest.
Supplemental Tables 1–7 and Supplemental Figures 1–6 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
References
- 1. Patisaul HB, Jefferson W. The pros and cons of phytoestrogens. Front Neuroendocrinol 2010;31(4):400–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hsu CC, Kuo HC, Chang SY, Wu TC, Huang KE. The assessment of efficacy of Diascorea alata for menopausal symptom treatment in Taiwanese women. Climacteric 2011;14(1):132–9. [DOI] [PubMed] [Google Scholar]
- 3. Franco OH, Chowdhury R, Troup J, Voortman T, Kunutsor S, Kavousi M, Oliver-Williams C, Muka T. Use of plant-based therapies and menopausal symptoms: a systematic review and meta-analysis. JAMA 2016;315(23):2554–63. [DOI] [PubMed] [Google Scholar]
- 4. Beral V, Million Women Study C. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet 2003;362(9382):419–27. [DOI] [PubMed] [Google Scholar]
- 5. Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, Ko M, LaCroix AZ, Margolis KL, Stefanick ML. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 2007;297(13):1465–77. [DOI] [PubMed] [Google Scholar]
- 6. Woclawek-Potocka I, Mannelli C, Boruszewska D, Kowalczyk-Zieba I, Wasniewski T, Skarzynski DJ. Diverse effects of phytoestrogens on the reproductive performance: cow as a model. Int J Endocrinol 2013;2013:650984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Talaei M, Pan A. Role of phytoestrogens in prevention and management of type 2 diabetes. World J Diabetes 2015;6(2):271–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen MN, Lin CC, Liu CF. Efficacy of phytoestrogens for menopausal symptoms: a meta-analysis and systematic review. Climacteric 2015;18(2):260–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Muka T, Nano J, Jaspers L, Meun C, Bramer WM, Hofman A, Dehghan A, Kavousi M, Laven JS, Franco OH. Associations of steroid sex hormones and sex hormone-binding globulin with the risk of type 2 diabetes in women: a population-based cohort study and meta-analysis. Diabetes 2017;66(3):577–86. [DOI] [PubMed] [Google Scholar]
- 10. Vedavanam K, Srijayanta S, O'Reilly J, Raman A, Wiseman H. Antioxidant action and potential antidiabetic properties of an isoflavonoid-containing soyabean phytochemical extract (SPE). Phytother Res 1999;13(7):601–8. [DOI] [PubMed] [Google Scholar]
- 11. Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem 1987;262(12):5592–5. [PubMed] [Google Scholar]
- 12. Ascencio C, Torres N, Isoard-Acosta F, Gomez-Perez FJ, Hernandez-Pando R, Tovar AR. Soy protein affects serum insulin and hepatic SREBP-1 mRNA and reduces fatty liver in rats. J Nutr 2004;134(3):522–9. [DOI] [PubMed] [Google Scholar]
- 13. Lavigne C, Marette A, Jacques H. Cod and soy proteins compared with casein improve glucose tolerance and insulin sensitivity in rats. Am J Physiol Endocrinol Metab 2000;278(3):E491–500. [DOI] [PubMed] [Google Scholar]
- 14. Mueller NT, Odegaard AO, Gross MD, Koh WP, Yu MC, Yuan JM, Pereira MA. Soy intake and risk of type 2 diabetes in Chinese Singaporeans [corrected]. Eur J Nutr 2012;51(8):1033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zamora-Ros R, Forouhi NG, Sharp SJ, González CA, Buijsse B, Guevara M, van der Schouw YT, Amiano P, Boeing H, Bredsdorff L et al. The association between dietary flavonoid and lignan intakes and incident type 2 diabetes in European populations: the EPIC-InterAct study. Diabetes Care 2013;12:3961–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Villegas R, Gao YT, Yang G, Li HL, Elasy TA, Zheng W, Shu XO. Legume and soy food intake and the incidence of type 2 diabetes in the Shanghai Women's Health Study. Am J Clin Nutr 2008;87(1):162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu ZM, Chen YM, Ho SC. Effects of soy intake on glycemic control: a meta-analysis of randomized controlled trials. Am J Clin Nutr 2011;93(5):1092–101. [DOI] [PubMed] [Google Scholar]
- 18. Zhang YB, Chen WH, Guo JJ, Fu ZH, Yi C, Zhang M, Na XL. Soy isoflavone supplementation could reduce body weight and improve glucose metabolism in non-Asian postmenopausal women—a meta-analysis. Nutrition 2013;29(1):8–14. [DOI] [PubMed] [Google Scholar]
- 19. Fang K, Dong H, Wang D, Gong J, Huang W, Lu F. Soy isoflavones and glucose metabolism in menopausal women: A systematic review and meta-analysis of randomized controlled trials. Mol Nutr Food Res 2016;60(7):1602–14. [DOI] [PubMed] [Google Scholar]
- 20. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moher D, Liberati A, Tetzlaff J, Altman DG; Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Higgins JP, Altman DG, Gotzsche PC Juni P Moher D Oxman AD Savovic J Schulz KF Weeks L Sterne JA, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25(9):603–5. [DOI] [PubMed] [Google Scholar]
- 24. Chene G, Thompson SG. Methods for summarizing the risk associations of quantitative variables in epidemiologic studies in a consistent form. Am J Epidemiol 1996;144(6):610–21. [DOI] [PubMed] [Google Scholar]
- 25. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med 1999;18(20):2693–708. [DOI] [PubMed] [Google Scholar]
- 27. Blakesmith SJ, Lyons-Wall PM, George C, Joannou GE, Petocz P, Samman S. Effects of supplementation with purified red clover (Trifolium pratense) isoflavones on plasma lipids and insulin resistance in healthy premenopausal women. Br J Nutr 2003;89(4):467–74. [DOI] [PubMed] [Google Scholar]
- 28. Simao AN, Lozovoy MA, Dichi I. Effect of soy product kinako and fish oil on serum lipids and glucose metabolism in women with metabolic syndrome. Nutrition 2014;30(1):112–5. [DOI] [PubMed] [Google Scholar]
- 29. Crisafulli A, Altavilla D, Marini H, Bitto A, Cucinotta D, Frisina N, Corrado F, D'Anna R, Squadrito G, Adamo EB et al. Effects of the phytoestrogen genistein on cardiovascular risk factors in postmenopausal women. Menopause 2005;12(2):186–92. [DOI] [PubMed] [Google Scholar]
- 30. Choquette S, Riesco E, Cormier E, Dion T, Aubertin-Leheudre M, Dionne IJ. Effects of soya isoflavones and exercise on body composition and clinical risk factors of cardiovascular diseases in overweight postmenopausal women: a 6-month double-blind controlled trial. Br J Nutr 2011;105(8):1199–209. [DOI] [PubMed] [Google Scholar]
- 31. Aubertin-Leheudre M, Lord C, Khalil A, Dionne IJ. Isoflavones and clinical cardiovascular risk factors in obese postmenopausal women: a randomized double-blind placebo-controlled trial. J Womens Health (Larchmt) 2008;17(8):1363–9. [DOI] [PubMed] [Google Scholar]
- 32. Nikander E, Tiitinen A, Laitinen K, Tikkanen M, Ylikorkala O. Effects of isolated isoflavonoids on lipids, lipoproteins, insulin sensitivity, and ghrelin in postmenopausal women. J Clin Endocrinol Metab 2004;89(7):3567–72. [DOI] [PubMed] [Google Scholar]
- 33. Hall WL, Vafeiadou K, Hallund J, Bugel S, Reimann M, Koebnick C, Zunft HJ, Ferrari M, Branca F, Dadd T et al. Soy-isoflavone-enriched foods and markers of lipid and glucose metabolism in postmenopausal women: interactions with genotype and equol production. Am J Clin Nutr 2006;83(3):592–600. [DOI] [PubMed] [Google Scholar]
- 34. Liu ZM, Chen YM, Ho SC, Ho YP, Woo J. Effects of soy protein and isoflavones on glycemic control and insulin sensitivity: a 6-mo double-blind, randomized, placebo-controlled trial in postmenopausal Chinese women with prediabetes or untreated early diabetes. Am J Clin Nutr 2010;91(5):1394–401. [DOI] [PubMed] [Google Scholar]
- 35. Ho SC CY, Ho SS, Woo JL. Soy isoflavone supplementation and fasting serum glucose and lipid profile among postmenopausal Chinese women: a double-blind, randomized, placebo-controlled trial. Menopause 2007;14(5):905–12. [DOI] [PubMed] [Google Scholar]
- 36. Azadbakht L, Kimiagar M, Mehrabi Y, Esmaillzadeh A, Padyab M, Hu FB, Willett WC. Soy inclusion in the diet improves features of the metabolic syndrome: a randomized crossover study in postmenopausal women. Am J Clin Nutr 2007;85(3):735–41. [DOI] [PubMed] [Google Scholar]
- 37. Garrido A, De la Maza MP, Hirsch S, Valladares L. Soy isoflavones affect platelet thromboxane A2 receptor density but not plasma lipids in menopausal women. Maturitas 2006;54(3):270–6. [DOI] [PubMed] [Google Scholar]
- 38. Khaodhiar L, Ricciotti HA, Li L, Pan W, Schickel M, Zhou J, Blackburn GL. Daidzein-rich isoflavone aglycones are potentially effective in reducing hot flashes in menopausal women. Menopause 2008;15(1):125–32. [PMC free article] [PubMed] [Google Scholar]
- 39. Villa P, Costantini B, Suriano R, Perri C, Macri F, Ricciardi L, Panunzi S, Lanzone A. The differential effect of the phytoestrogen genistein on cardiovascular risk factors in postmenopausal women: relationship with the metabolic status. J Clin Endocrinol Metab 2009;94(2):552–8. [DOI] [PubMed] [Google Scholar]
- 40. Charles C, Yuskavage J, Carlson O, John M, Tagalicud AS, Maggio M, Muller DC, Egan J, Basaria S. Effects of high-dose isoflavones on metabolic and inflammatory markers in healthy postmenopausal women. Menopause 2009;16(2):395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Colacurci N, Chiantera A, Fornaro F, de Novellis V, Manzella D, Arciello A, Chiantera V, Improta L, Paolisso G. Effects of soy isoflavones on endothelial function in healthy postmenopausal women. Menopause 2005;12(3):299–307. [DOI] [PubMed] [Google Scholar]
- 42. Hutchins AM, Brown BD, Cunnane SC, Domitrovich SG, Adams ER, Bobowiec CE. Daily flaxseed consumption improves glycemic control in obese men and women with pre-diabetes: a randomized study. Nutr Res 2013;33(5):367–75. [DOI] [PubMed] [Google Scholar]
- 43. Bakhtiary A, Yassin Z, Hanachi P, Rahmat A. Evaluation of the oxidative stress and glycemic control status in response to soy in older women with the metabolic syndrome. Iran Red Crescent Med J 2011;(13):795–804. [Google Scholar]
- 44. Kim J, Lee H, Lee O, Lee KH, Lee YB, Young KD, Jeong YH, Choue R. Isoflavone supplementation influenced levels of triglyceride and luteunizing hormone in Korean postmenopausal women. Arch Pharm Res 2013;36(3):306–13. [DOI] [PubMed] [Google Scholar]
- 45. Liu ZM, Ho SC, Chen YM, Ho S, To K, Tomlinson B, Woo J. Whole soy, but not purified daidzein, had a favorable effect on improvement of cardiovascular risks: a 6-month randomized, double-blind, and placebo-controlled trial in equol-producing postmenopausal women. Mol Nutr Food Res 2014;58(4):709–17. [DOI] [PubMed] [Google Scholar]
- 46. Ko KP, Kim CS, Ahn Y, Park SJ, Kim YJ, Park JK, Lim YK, Yoo KY, Kim SS. Plasma isoflavone concentration is associated with decreased risk of type 2 diabetes in Korean women but not men: results from the Korean Genome and Epidemiology Study. Diabetologia 2015;58(4):726–35. [DOI] [PubMed] [Google Scholar]
- 47. Nanri A, Mizoue T, Takahashi Y, Kirii K, Inoue M, Noda M, Tsugane S. Soy product and isoflavone intakes are associated with a lower risk of type 2 diabetes in overweight Japanese women. J Nutr 2010;140(3):580–6. [DOI] [PubMed] [Google Scholar]
- 48. Ding M, Pan A, Manson JE, Willett WC, Malik V, Rosner B, Giovannucci E, Hu FB, Sun Q. Consumption of soy foods and isoflavones and risk of type 2 diabetes: a pooled analysis of three US cohorts. Eur J Clin Nutr 2016;70(12):1381–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ding M, Franke AA, Rosner BA, Giovannucci E, van Dam RM, Tworoger SS, Hu FB, Sun Q. Urinary isoflavonoids and risk of type 2 diabetes: a prospective investigation in US women. Br J Nutr 2015;114(10):1694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nettleton JA, Harnack LJ, Scrafford CG, Mink PJ, Barraj LM, Jacobs DR Jr. Dietary flavonoids and flavonoid-rich foods are not associated with risk of type 2 diabetes in postmenopausal women. J Nutr 2006;(12):3039–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Song Y, Manson JE, Buring JE, Sesso HD, Liu S. Associations of dietary flavonoids with risk of type 2 diabetes, and markers of insulin resistance and systemic inflammation in women: a prospective study and cross-sectional analysis. J Am Coll Nutr 2005;24(5):376–84. [DOI] [PubMed] [Google Scholar]
- 52. Knekt P, Kumpulainen J, Jarvinen R, Rissanen H, Heliovaara M, Reunanen A, Hakulinen T, Aromaa A. Flavonoid intake and risk of chronic diseases. Am J Clin Nutr 2002;76(3):560–8. [DOI] [PubMed] [Google Scholar]
- 53. Jayagopal V, Albertazzi P, Kilpatrick ES, Howarth EM, Jennings PE, Hepburn DA, Atkin SL. Beneficial effects of soy phytoestrogen intake in postmenopausal women with type 2 diabetes. Diabetes Care 2002;25(10):1709–14. [DOI] [PubMed] [Google Scholar]
- 54. Curtis PJ, Sampson M, Potter J, Dhatariya K, Kroon PA, Cassidy A. Chronic ingestion of flavan-3-ols and isoflavones improves insulin sensitivity and lipoprotein status and attenuates estimated 10-year CVD risk in medicated postmenopausal women with type 2 diabetes: a 1-year, double-blind, randomized, controlled trial. Diabetes Care 2012;35(2):226–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mani UV, Mani I, Biswas M, Kumar SN. An open-label study on the effect of flax seed powder (Linum usitatissimum) supplementation in the management of diabetes mellitus. J Diet Suppl 2011;8(3):257–65. [DOI] [PubMed] [Google Scholar]
- 56. Squadrito F, Marini H, Bitto A, Altavilla D, Polito F, Adamo EB, D'Anna R, Arcoraci V, Burnett BP, Minutoli L et al. Genistein in the metabolic syndrome: results of a randomized clinical trial. J Clin Endocrinol Metab 2013;98(8):3366–74. [DOI] [PubMed] [Google Scholar]
- 57. Wu H, Pan A, Yu Z, Qi Q, Lu L, Zhang G, Yu D, Zong G, Zhou Y, Chen X et al. Lifestyle counseling and supplementation with flaxseed or walnuts influence the management of metabolic syndrome. J Nutr 2010;140(11):1937–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Szkudelska K, Nogowski L, Szkudelski T. Genistein affects lipogenesis and lipolysis in isolated rat adipocytes. J Steroid Biochem Mol Biol 2000;75(4–5):265–71. [DOI] [PubMed] [Google Scholar]
- 59. Mackowiak P, Nogowski L, Nowak KW. Effect of isoflavone genistein on insulin receptors in perfused liver of ovariectomized rats. J Recept Signal Transduct Res 1999;19(1–4):283–92. [DOI] [PubMed] [Google Scholar]
- 60. Quinn PG, Yeagley D. Insulin regulation of PEPCK gene expression: a model for rapid and reversible modulation. Curr Drug Targets Immune Endocr Metabol Disord 2005;5(4):423–37. [DOI] [PubMed] [Google Scholar]
- 61. Bhathena SJ, Velasquez MT. Beneficial role of dietary phytoestrogens in obesity and diabetes. Am J Clin Nutr 2002;76(6):1191–201. [DOI] [PubMed] [Google Scholar]
- 62. Goodman-Gruen D, Kritz-Silverstein D. Usual dietary isoflavone intake is associated with cardiovascular disease risk factors in postmenopausal women. J Nutr 2001;131(4):1202–6. [DOI] [PubMed] [Google Scholar]
- 63. Muka T, Oliver-Williams C, Colpani V, Kunutsor S, Chowdhury S, Chowdhury R, Kavousi M, Franco OH. Association of vasomotor and other menopausal symptoms with risk of cardiovascular disease: a systematic review and meta-analysis. PLoS One 2016;11(6):e0157417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Franco OH, Muka T, Colpani V, Kunutsor S, Chowdhury S, Chowdhury R, Kavousi M. Vasomotor symptoms in women and cardiovascular risk markers: Systematic review and meta-analysis. Maturitas 2015;81(3):353–61. [DOI] [PubMed] [Google Scholar]
- 65. Diel P, Schulz T, Smolnikar K, Strunck E, Vollmer G, Michna H. Ability of xeno- and phytoestrogens to modulate expression of estrogen-sensitive genes in rat uterus: estrogenicity profiles and uterotropic activity. J Steroid Biochem Mol Biol 2000;73(1–2):1–10. [DOI] [PubMed] [Google Scholar]
- 66. Song TT, Hendrich S, Murphy PA. Estrogenic activity of glycitein, a soy isoflavone. J Agric Food Chem 1999;47(4):1607–10. [DOI] [PubMed] [Google Scholar]
- 67. Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr 2002;132(12):3577–84. [DOI] [PubMed] [Google Scholar]
- 68. Simons AL, Renouf M, Hendrich S, Murphy PA. Metabolism of glycitein (7,4'-dihydroxy-6-methoxy-isoflavone) by human gut microflora. J Agric Food Chem 2005;53(22):8519–25. [DOI] [PubMed] [Google Scholar]
- 69. Bingham SA, Atkinson C, Liggins J, Bluck L, Coward A. Phytooestrogens: where are we now?. Br J Nutr 1998;(79):393–406. [DOI] [PubMed] [Google Scholar]
- 70. Rietjens I, Louisse J, Beekmann K. The potential health effects of dietary phytoestrogens. Br J Pharmacol 2017;174(11):1263–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lampe JW. Is equol the key to the efficacy of soy foods? Am J Clin Nutr 2009;89(5):1164S–7S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.