Abstract
Introduction
The Montreal Cognitive Assessment (MoCA), scored from 0 to 30, is used as a screening tool for mild cognitive impairment (MCI). The current cutoff (26) may not be optimal among minorities.
Methods
Data from the National Alzheimer's Coordinating Center Uniform Data Set March 2018 data freeze was used to calculate optimal cutoffs for detection of MCI and dementia by race/ethnic group and education.
Results
Of the 3895 individuals included, 80.7% were non-Hispanic White, 15.0% were non-Hispanic Black, and 4.2% were Hispanic. Optimal cutoffs for detection of MCI were 25 among non-Hispanic Whites, 24 among Hispanics, and 23 among non-Hispanic Blacks. Optimal cutoffs for detection of dementia were 19 among non-Hispanic Whites and 16 for both non-Hispanic Blacks and Hispanics. Lower educational attainment produced lower optimal cutoffs.
Discussion
Our findings suggest cutoffs may need to be stratified by race/ethnicity and education to ensure detecting MCI from normal and MCI from dementia.
Keywords: Alzheimer's disease, Dementia, Montreal Cognitive Assessment, Screening, Race, Ethnicity, Disparities, Education
Highlights
-
•
The current MoCA cutoff of 26 is too global and too high for detection of MCI.
-
•
Optimal MoCA cutoffs vary by race/ethnicity.
-
•
Cutoffs also vary by educational attainment.
-
•
Cutoffs should be stratified by race/ethnicity and education before they are applied.
1. Introduction
Alzheimer's disease (AD), the most common form of dementia, affected approximately 5.5 million Americans in 2017; this number is projected to increase to as high as 16 million by 2050 [1]. Risk factors for AD include nonmodifiable factors, such as older age, family history, and the presence of the apolipoprotein E (APOE)-ε4 gene, and potentially modifiable risk factors, including low educational attainment, low socioeconomic status, hypertension, smoking, diabetes, depression, and low social and cognitive engagement [1], [2], [3]. Mild cognitive impairment (MCI), the stage between healthy cognitive aging and dementia, is defined as greater cognitive impairment than is expected for one's age [4], [5]. MCI can be used for early detection and prevention of progression to dementia. By diagnosing MCI, health care professionals can act to control cardiovascular risk factors, increase exercise, and initiate cognitive training; interventions that may reduce progression from MCI to AD [6].
Racial/ethnic minorities are disproportionately at risk for dementia; African Americans and Hispanics are more likely to develop AD and other dementias than their non-Hispanic White counterparts [1], [7] likely because of differences in underlying risk factors [1], [7], [8], [9]. Measurement bias in the Montreal Cognitive Assessment (MoCA) and other screening tools might inflate rates among minorities [8]. This is a timely topic because the number of racial/ethnic minority group members has increased and will continue to increase in the United States [8].
Among minority populations, educational attainment is a particularly important risk factor for dementia; individuals with fewer years of formal education have a greater risk for developing dementia [10], [11], [12], [13]. Educational attainment is also strongly associated with cognitive reserve [14], [15], [16]. The cognitive reserve theory states that individuals with more educational, occupational, and cognitive engagement are more resilient to damage to their brain, delaying the presentation of symptoms of dementia [14], [15], [16]. However, Manly et al. [17] found that literacy level, rather than years of education, better predicts cognitive decline regardless of race/ethnicity; literacy, and years of education are not concordant.
Estimates are that only half of individuals with AD have been diagnosed, and, of those diagnosed, only 33% are aware of their diagnosis [18]. Yet early detection of AD gives individuals time to express their wishes, build a care team, create advance health directives, make legal and financial arrangements, and enroll in clinical trials before their disease progresses to an advanced stage [18]. Early detection also helps physicians deliver better care and manage comorbid conditions that often occur with greater age [18].
Screening is essential for early detection of MCI and AD. The MoCA was developed as a screening tool to distinguish between normal cognition and MCI [19]. Administered by a trained health professional, the MoCA takes approximately 10 minutes to complete and has items on orientation, attention, verbal memory, language, visuospatial function, and executive function [19]. Scored of 30 points, it has a one-point educational adjustment (addition) for individuals with ≤12 years of education [19]. The MoCA has a high sensitivity for detection of MCI in patients who would score as normal on the Mini-Mental State Examination (MMSE), another widely used tool to assess cognitive impairment; using a cutoff of 26 for MCI the MoCA had a sensitivity of 90% whereas the MMSE had a sensitivity of 18% [19]. Although it is widely accepted that the MoCA is better than the MMSE for detection of MCI [19], [20], [21], some studies suggest that the cutoff of 26 results in a high proportion of normal individuals being classified as cognitively impaired [22], [23].
MoCA performance in minority groups has not been widely studied; most studies examining the MoCA and its subtests were limited to Caucasian samples. Studies that included minority participants found that the originally established cutoff of 26 may result in a high likelihood of classifying normal minority individuals as cognitively impaired [24], [25], [26]. For instance, using the recommended cutoff of 26, Sink et al. [26] found that over 90% of their cohort of African Americans with type 2 diabetes participating in the study of type 2 diabetes and cognitive function screened as “positive” for cognitive impairment. Rossetti et al. [25] found that about 80% of their cohort of community-dwelling African Americans fell below the threshold. These findings suggest a need to re-evaluate MoCA cutoff performance in minority populations.
In these analyses, we aim to identify race-specific optimal cutoff values of the MoCA when stratified by education and age, using clinician diagnosis as the gold standard. We hypothesize that minority groups will require lower cutoffs to distinguish between normal cognition and MCI or dementia, and further, between MCI and dementia. We also hypothesize that cutoffs will need to decrease as educational attainment decreases to ensure optimal MoCA performance.
2. Methods
2.1. Data source and participants
Data for these analyses come from the National Alzheimer's Coordinating Center (NACC), which maintains a database of information from Alzheimer's Disease Centers (ADCs). The data set used in these analyses, the uniform data set, includes subjects enrolled at ADCs since 2005 with a range of cognitive status, measured through neuropsychological tests and clinician assessment. Each ADC recruits subjects according to its own protocol; recruitment methods include clinician referral, self-referral by patients or family members, and/or active recruitment through community organizations [27]. Participants who completed a MoCA at their baseline visit, reported being non-Hispanic White, non-Hispanic Black, or Hispanic, reported their education, and had a baseline uniform data set visit that was conducted from March 2005 to March 2018 were included in these analyses (n = 3895).
2.2. Measures
Race and ethnicity variables were combined to create a three-level race/ethnicity variable categorized as non-Hispanic White, non-Hispanic Black, and Hispanic. The MoCA score was recorded as a continuous variable from 0 to 30. Clinician diagnosis was recorded as normal cognition, impaired, not MCI, MCI, or dementia. Only participants who were diagnosed as having normal cognition, MCI, or dementia were included in these analyses. We dropped those coded as impaired, not MCI, because of uncertainty over their diagnosis. Dementia encompassed multiple etiologic diagnoses including AD, Lewy body disease, frontotemporal lobar degeneration. Therefore, the term dementia will be used herein. Education was categorized as high school or less (≤12 years), college (13–16 years), and more than college (>16 years). Because the suggested educational adjustment has been found to affect the validity of the MoCA and may not be appropriate in all groups [28], we used raw MoCA scores for these analyses. We accounted for differences because of education by stratifying cutoffs by education within each racial/ethnic group. Age was categorized by decade of age <50, 50 to 59, 60 to 69, 70 to 79, and ≥80.
2.3. Data analysis
Using SAS 9.4 software (SAS Institute Inc., Cary, NC, USA), descriptive statistics were calculated for population characteristics including race/ethnicity, age, and education. Test measures including specificity, sensitivity, positive predictive value, and negative predictive value were calculated. The SAS 9.4 Receiver Operating Curve Plot (ROCPLOT) macro was used to calculate ideal cutoffs for the MoCA, using the Youden index, and to calculate corresponding areas under the ROC curve when (1) identifying MCI and (2) distinguishing between MCI and dementia, by race/ethnicity, when stratified by education. The Youden index, equal to the sensitivity plus specificity minus 1, is a valued way to summarize the performance of a diagnostic test [29], [30]. MedCalc was used to calculate confidence intervals for areas under the ROC curves and to generate bootstrapped confidence intervals for cutoffs using 100,000 iterations [31].
3. Results
Of the 3895 participants, 80.7% were non-Hispanic White, 15.0% were non-Hispanic Black, and 4.2% were Hispanic; over half (56.8%) were female with an average age of 69.7 (range, 19–101; median, 70.0; interquartile range, 65.0–76.0) years. Most participants reported 13 to 16 years of education (43.8%) or >16 years of education (40.5%). Relatively few reported ≤12 years of education (15.7%). Over half of participants (62.5%), screened as cognitively impaired, with a MoCA score of <26. Most participants had normal cognition as judged by a clinician (48.4%); the remaining had dementia (27.6%) or MCI (24.0%). Age, sex, years of education, clinician judged cognition, and MoCA score all significantly differed by race/ethnicity (Table 1). MoCA scores were negatively skewed in both impaired and unimpaired groups, potentially driven by the individuals with fewer years of education.
Table 1.
Characteristics of study participants, by race/ethnicity (n = 3895)
| Characteristic | Overall (n = 3895) | Non-Hispanic Whites (n = 3145; 80.7%) | Non-Hispanic Blacks (n = 586; 15.0%) | Hispanics (n = 164; 4.2%) | P value |
|---|---|---|---|---|---|
| Mean age (SD) | 69.7 (9.8) | 69.6 (10.0) | 70.1 (8.7) | 68.9 (9.9) | .2905 |
| Decade of age | |||||
| <50 | 108 (2.8%) | 97 (3.1%) | 5 (0.9%) | 6 (3.7%) | |
| 50–59 | 400 (10.3%) | 330 (10.5%) | 52 (8.9%) | 18 (11.0%) | |
| 60–69 | 1300 (33.4%) | 1017 (32.3%) | 228 (38.9%) | 55 (33.5%) | |
| 70–79 | 1531 (39.3%) | 1257 (40.0%) | 213 (36.4%) | 61 (37.2%) | |
| ≥80 | 556 (14.3%) | 444 (14.1%) | 88 (15.0%) | 24 (14.6%) | .0137 |
| Sex | |||||
| Male | 1683 (43.2%) | 1461 (46.5%) | 163 (27.8%) | 59 (36.0%) | <.0001 |
| Female | 2212 (56.8%) | 1684 (53.6%) | 423 (72.2%) | 105 (64.0%) | |
| Years of education | |||||
| ≤12 | 611 (15.7%) | 404 (12.9%) | 153 (26.1%) | 54 (32.9%) | <.0001 |
| 13–16 | 1706 (43.8%) | 1372 (43.6%) | 263 (44.9%) | 71 (43.3%) | |
| >16 | 1578 (40.5%) | 1369 (43.5%) | 170 (29.0%) | 39 (23.8%) | |
| Cognitive status | |||||
| Normal | 1886 (48.4%) | 1469 (46.7%) | 345 (58.9%) | 72 (43.9%) | |
| MCI | 936 (24.0%) | 733 (23.3%) | 158 (27.0%) | 45 (27.4%) | |
| Dementia | 1073 (27.6%) | 943 (30.0%) | 83 (14.2%) | 47 (28.7%) | <.0001 |
| MoCA score | |||||
| ≥26 | 1461 (37.5%) | 1268 (40.3%) | 157 (26.8%) | 36 (22.0%) | |
| <26 | 2434 (62.5%) | 1877 (59.7%) | 429 (73.3%) | 128 (78.1%) | <.0001 |
Abbreviations: MCI, mild cognitive impairment; MoCA, Montreal Cognitive Assessment; SD, standard deviation.
Using the originally established cutoff score of 26 to detect MCI or dementia, the sensitivity of the MoCA was 89.5% in non-Hispanic Whites, 92.6% in non-Hispanic Blacks, and 94.6% in Hispanics. The specificity of the MoCA with the established cutoff was 74.3% in non-Hispanic Whites, 40.3% in non-Hispanic Blacks, and 43.1% in Hispanics. Given these, the positive predictive value of the MoCA was 79.9% in non-Hispanic Whites, 52.0% in non-Hispanic Blacks, and 68.0% in Hispanics, and the negative predictive value of the MoCA was 86.1% in non-Hispanic Whites, 88.5% in non-Hispanic Blacks, and 86.1% in Hispanics (Table 2). With this cutoff, 25.7% of non-Hispanic Whites, 59.7% of non-Hispanic Blacks, and 56.9% of Hispanics without clinician judged impairment screened as impaired on the MoCA (data not shown).
Table 2.
Test characteristics of the Montreal Cognitive Assessment, using the current cutoff of 26 for detection of MCI or dementia, by race/ethnicity, among study participants
| Race/ethnicity | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| Non-Hispanic White | 89.50 | 74.34 | 79.91 | 86.12 |
| Non-Hispanic Black | 92.53 | 40.29 | 51.98 | 88.54 |
| Hispanic | 94.57 | 43.06 | 67.97 | 86.11 |
| Overall | 90.10 | 66.91 | 74.36 | 86.38 |
Abbreviations: MCI, mild cognitive impairment; NPV, negative predictive value; PPV, positive predictive value.
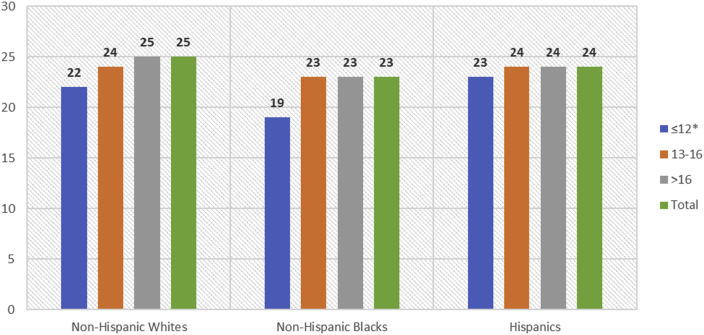
The Youden index was used to identify an optimal cutoff for detecting the presence of either MCI, among individuals who had normal cognition or MCI. Overall, 25 was the optimal cutoff for identifying either MCI (sensitivity = 0.816, specificity = 0.669). When stratified by race/ethnicity, identified cutoffs were 25 among non-Hispanic Whites, 23 among non-Hispanic Blacks, and 24 among Hispanics (Supplementary Fig. S1). Further stratification by education identified a decrease in optimal MoCA cutoffs for detecting either MCI or AD among those with fewer years of education (Table 3). Among non-Hispanic Whites the optimal cutoff was 22 among those with ≤12 years of education, 24 among those with 12 to 16 years of education, and 25 among those with >12 years of education. Among non-Hispanic Blacks the optimal cutoff was 19 among those with ≤12 years of education, 23 among those with 12 to 16 years of education, and 23 among those with >12 years of education. Among Hispanics the optimal cutoff was 23 among those with ≤12 years of education, 24 among those with 12 to 16 years of education, and 24 among those with >12 years of education (Table 3, Fig. 1).
Table 3.
Optimal MoCA cutoffs to distinguish between normal cognition and MCI by race/ethnicity and education level, among study participants; MoCA scores range from 0 to 30 and lower scores signify more impairment (n = 2822)
| Years of education | Number of participants | Cutoff (95% CI)∗ | Sensitivity (%) | Specificity (%) | AUC (95% CI) |
|---|---|---|---|---|---|
| Non-Hispanic Whites | n = 2202; 78.0% | ||||
| Overall | NC = 1469; MCI = 733 | ≤25 [≤24, ≤25] | 79.51 | 74.32 | 0.843 [0.827, 0.858] |
| ≤12† | NC = 116; MCI = 97 | ≤22 [≤21, ≤24] | 71.13 | 83.62 | 0.828 [0.770, 0.876] |
| 13–16 | NC = 623; MCI = 315 | ≤24 [≤23, ≤25] | 72.38 | 83.28 | 0.859 [0.835, 0.880] |
| >16 | NC = 730; MCI = 321 | ≤25 [≤25, ≤26] | 72.50 | 80.96 | 0.832 [0.808, 0.854] |
| Non-Hispanic Blacks | n = 503; 17.8% | ||||
| Overall | NC = 345; MCI = 158 | ≤23 [≤22, ≤24] | 71.52 | 71.59 | 0.769 [0.730, 0.805] |
| ≤12† | NC = 67; MCI = 50 | ≤19 [≤17, ≤20] | 56.00 | 83.58 | 0.721 [0.630, 0.800] |
| 13–16 | NC = 165; MCI = 71 | ≤23 [≤22, ≤23] | 73.24 | 74.55 | 0.765 [0.706, 0.818] |
| >16 | NC = 113; MCI = 37 | ≤23 [≤22, ≤25] | 64.86 | 82.30 | 0.791 [0.717, 0.853] |
| Hispanics | n = 117; 4.2% | ||||
| Overall | NC = 72; MCI = 45 | ≤24 [≤23, ≤26] | 84.44 | 55.56 | 0.727 [0.637, 0.805] |
| ≤12† | NC = 20; MCI = 12 | ≤23 [≤19, ≤28] | 91.67 | 30.00 | 0.569 [0.383, 0.742] |
| 13–16 | NC = 37; MCI = 18 | ≤24 [≤21, ≤26] | 83.33 | 62.16 | 0.773 [0.640, 0.875] |
| >16 | NC = 15; MCI = 15 | ≤24 [≤21, ≤25] | 80.00 | 93.33 | 0.927 [0.770, 0.990] |
Abbreviations: AUC, areas under the ROC curve; CI, confidence interval; MCI, mild cognitive impairment; MoCA, Montreal Cognitive Assessment; NC, normal cognition; SD, standard deviation.
Bootstrap confidence interval (100,000 iterations; random number seed: 978).
MoCA scoring rules adjust for ≤12 years of education, adding one point to the total score of individuals with ≤12 years of education.
Fig. 1.
Optimal MoCA cutoffs to distinguish between normal cognition and MCI by race/ethnicity and education level, among study participants with normal cognition and MCI; MoCA scores range from 0 to 30 and lower scores signify more impairment (n = 2822). ∗MoCA scoring rules adjust for ≤12 years of education, adding one point to the total score of individuals with ≤12 years of education.
Table 3 includes confidence intervals for these calculated cutoffs. A few significant differences should be noted. The overall cutoff for non-Hispanic Whites is significantly different from the overall cutoff for non-Hispanic Blacks. Among non-Hispanic Whites, the cutoff for those with ≤12 years of education is significantly different for the cutoff for individuals with >16 years of education. Among non-Hispanic Blacks, the cutoff for those with ≤12 years of education is significantly different from the cutoff for both individuals with 13 to 16 and >16 years of education. Finally, the cutoff for non-Hispanic Whites with ≤12 years of education is significantly different from the cutoff for non-Hispanic Blacks with ≤12 years of education.
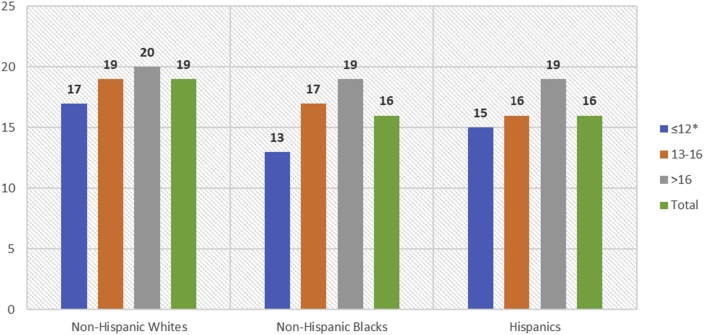
Overall, 19 was the optimal cutoff for further distinguishing between MCI and dementia (sensitivity = 0.727, specificity = 0.799). When stratified by race/ethnicity, identified cutoffs were 19 among non-Hispanic Whites, 16 among non-Hispanic Blacks, and 16 among Hispanics (Supplementary Fig. S2). Further stratification by education identified a decrease in optimal MoCA cutoffs for distinguishing between MCI and dementia among those with fewer years of education (Table 4). Among non-Hispanic Whites the optimal cutoff was 17 among those with ≤12 years of education, 19 among those with 12 to 16 years of education, and 20 among those with >12 years of education. Among non-Hispanic Blacks the optimal cutoff was 13 among those with ≤12 years of education, 17 among those with 12 to 16 years of education, and 19 among those with >12 years of education. Among Hispanics the optimal cutoff was 15 among those with ≤12 years of education, 16 among those with 12 to 16 years of education, and 19 among those with >12 years of education (Table 4, Fig. 2).
Table 4.
Optimal MoCA cutoffs to distinguish between MCI and dementia by race/ethnicity and education level, among study participants with MCI or dementia (DM); MoCA scores range from 0 to 30 and lower scores signify more impairment (n = 2009)
| Years of education | Number of participants | Cutoff (95% CI)∗ | Sensitivity (%) | Specificity (%) | AUC (95% CI) |
|---|---|---|---|---|---|
| Non-Hispanic Whites | n = 1676; 83.4% | ||||
| Overall | MCI = 733; DM = 943 | ≤19 [≤18, ≤20] | 70.31 | 83.06 | 0.844 [0.826, 0.861] |
| ≤12† | MCI = 97; DM = 191 | ≤17 [≤14, ≤18] | 70.16 | 80.41 | 0.840 [0.793, 0.881] |
| 13–16 | MCI = 315; DM = 434 | ≤19 [≤15, ≤20] | 72.12 | 81.27 | 0.844 [0.816, 0.869] |
| >16 | MCI = 321; DM = 318 | ≤20 [≤19, ≤22] | 68.24 | 84.37 | 0.838 [0.807, 0.866] |
| Non-Hispanic Blacks | n = 241; 12.0% | ||||
| Overall | MCI = 158; DM = 83 | ≤16 [≤15, ≤17] | 78.31 | 88.61 | 0.898 [0.853, 0.933] |
| ≤12† | MCI = 50; DM = 36 | ≤13 [≤12, ≤15] | 77.78 | 96.00 | 0.919 [0.839, 0.967] |
| 13–16 | MCI = 71; DM = 27 | ≤17 [≤16, ≤19] | 77.78 | 92.96 | 0.870 [0.787, 0.930] |
| >16 | MCI = 37; DM = 20 | ≤19 [≤15, ≤20] | 90.00 | 83.78 | 0.944 [0.849, 0.987] |
| Hispanics | n = 92; 4.6% | ||||
| Overall | MCI = 45; DM = 47 | ≤16 [≤15, ≤19] | 72.34 | 97.78 | 0.886 [0.803, 0.943] |
| ≤12† | MCI = 12; DM = 22 | ≤15 [≤14, ≤15] | 90.91 | 100.00 | 0.966 [0.839, 0.999] |
| 13–16 | MCI = 18; DM = 16 | ≤16 [≤13, ≤16] | 75.00 | 100.00 | 0.858 [0.695, 0.953] |
| >16 | MCI = 15; DM = 9 | ≤19 [≤12, ≤26] | 77.78 | 73.33 | 0.726 [0.507, 0.886] |
Abbreviations: AUC, areas under the ROC curve; CI, confidence interval; MCI, mild cognitive impairment; MoCA, Montreal Cognitive Assessment.
Bootstrap confidence interval (100,000 iterations; random number seed: 978).
MoCA scoring rules adjust for ≤12 years of education, adding one point to the total score of individuals with ≤12 years of education.
Fig. 2.
Optimal MoCA cutoffs to distinguish between MCI and dementia by race/ethnicity and education level, among study participants with MCI or dementia; MoCA scores range from 0 to 30 and lower scores signify more impairment (n = 2009). ∗MoCA scoring rules adjust for ≤12 years of education, adding one point to the total score of individuals with ≤12 years of education.
Table 4 includes confidence intervals for these calculated cutoffs. A few significant differences should be noted. The overall cutoff for non-Hispanic Whites is significantly different from the overall cutoff for non-Hispanic Blacks. Among non-Hispanic Whites, the cutoff for those with ≤12 years of education is significantly different for the cutoff for individuals with >16 years of education. Among non-Hispanic Blacks, the cutoff for those with ≤12 years of education is significantly different from the cutoff for both individuals with 13 to 16 and >16 years of education. Finally, the cutoff for non-Hispanic Whites with ≤12 years of education is significantly different from the cutoff for non-Hispanic Blacks with ≤12 years of education.
We considered stratifying cutoffs by age, in addition to race/ethnicity and education; however, in the non-Hispanic Black and Hispanic groups the cell counts became too small. Average MoCA scores mostly increased as years of education increased and decreased as age increased in all three groups (Tables S1–S3).
4. Discussion
We found that the MoCA had different optimal cutoffs among different racial/ethnic minorities. These cutoffs were lower than the standardized cutoff and lowered as years of education decreased. Although the original article establishing the cutoff of 26 had almost 300 participants [19], we established these differing cutoffs using a highly regarded national sample of almost 4000 individuals. Uniquely, the NACC database includes clinical diagnosis and MoCA scores for both affected individuals and normal control subjects. This allowed us to calculate cutoffs that maximized sensitivity and specificity to delineate among health aging, MCI and, dementia.
Overall, and consistent with previous findings, we identified 25 as the optimal cutoff for detecting MCI among the total sample. A Cochrane review from 2015 concluded that it is likely that cutoffs <26 would be more useful for correctly identifying MCI or AD [32]. A few studies have found a lower optimal cutoff would be more specific and sensitive. Luis et al. [23] administered the MoCA to community-dwelling older adults in the Southeastern United States; the MoCA had much better sensitivity and specificity (96% and 95%, respectively) to identify cognitive impairment versus normal cognition with a lower cutoff of 23 compared with the recommended cutoff of 26 (97% and 35%, respectively) [19]. Moreover, a systematic review by Carson et al. [22] found that lowering the MoCA cutoff to 23 reduces the false-positive rate. Although we too found a lower cutoff performed better than the recommended 26, our findings can be contrasted with these team's finding because we additionally stratified scores by race/ethnicity and education. This stratification resulted in a need for even lower cutoff values.
When stratifying by race/ethnicity, we found the optimal cutoff value should be lower among minority groups. The optimal cutoff for MCI remained 25 among Whites; however, sensitivity and specificity was maximized when the cutoffs were lowered to 23 among non-Hispanic Blacks and 24 among Hispanics. Previous literature has found that using lower cutoffs of the MoCA would be beneficial in African American populations by improving diagnostic accuracy [24], [25], [26]; however, there is little evidence on what the cutoffs should be. The literature that does include Hispanic populations has found that minorities performed more poorly on the MoCA compared with their White counterparts, and found that Hispanics scored the lowest on the MoCA among all groups measured [33]. These observed differences may be because of a lack of cultural equivalence of test items or because of differential variability in the MoCA score by race/ethnicity. African Americans have previously been found to perform worse on majority-normed cognitive tests compared with their White counterparts, making race-specific data crucial for clinical assessments [34].
When we further stratified by education within each racial/ethnic group, we found different optimal cutoffs for MCI and dementia. Within each racial/ethnic group, the cutoff decreased as years of education decreased. This is to be expected, especially because the MoCA developers recommend an educational adjustment. These patterns were also consistent when distinguishing between MCI and dementia. The observed differences in optimal cutoff when stratifying by race/ethnicity and education level may be attributable to differences in education, socioeconomic status, and comorbid medical conditions, all of which are risk factors for dementia [2], [3], [18] and all of which may be reflected in the education variable. However, it is important to note that although we observed a strong racial difference in optimal cutoffs, non-Hispanic Blacks included in the NACC sample were diagnosed as cognitively normal by a clinician more frequently than their non-Hispanic White and Hispanic counterparts (58.9% vs. 46.7% and 43.9%, respectively).
Furthermore, the MoCA adds a one-point education adjustment for individuals who have ≤12 years of education; however, our results suggest that the one-point educational adjustment may not be adequate when considering differences in test performance because of years of education. We observed that not only do optimal cutoffs differ between those with ≤12 years of education and those with >12 years of education, but also between those with 13 to 16 years of education and >16 years of education. This was particularly evident among non-Hispanic Blacks; when distinguishing between normal cognition and MCI, the cutoffs for the overall group and those with ≤12 years of education significantly differed by four points. The cutoffs for those with ≤12 years of education and those with 12 to 16 years of education also significantly differed. Moreover, again among Non-Hispanic Blacks, when distinguishing between MCI and dementia the cutoffs those with ≤12 years of education and those with 12 to 16 years significantly differed by four points.
Surprisingly, few studies have examined the validity of the educational adjustment; however, studies have examined normative MoCA scores when stratified by education. Rossetti et al. [28] found that overall, the mean MoCA score of their ethnically diverse participants was 24; when stratified by education, the mean MoCA score was 21 among those with <12 years of education, 23 among those with 12 years of education, and 25 among those with >25 years of education. When they reduced their sample to community-dwelling African Americans alone, they found that the mean MoCA score of their cognitively normal participants was 23 [25]. When stratified by education, the mean MoCA score decreased to 20 among those with <12 years of education, 22 among those with 12 years of education, and 24 among those with >25 years of education. Together, our findings suggest that the one-point educational adjustment is not enough given differences in educational attainment. Additional support comes from Malek-Ahmadi et al. [35] who found that younger and more educated individuals performed better on the MoCA than their counterparts. However, their study differs because it only included non-Hispanic Whites [35].
Racial/ethnic differences observed in the performance of the MoCA suggest that the current cutoff is not appropriate for use among minority groups, particularly among non-Hispanic Blacks. Given that the non-Hispanic Blacks included in these analyses are mostly cognitively normal, future research is needed to identify the source of variability in MoCA performance by race/ethnicity.
4.1. Limitations
Some limitations should be considered when interpreting the results of these analyses. Subjects in the NACC database may not represent the United States population, because they are referral-based or volunteer-based participants and tend to be more educated than the general public. Moreover, normal cognition control subjects in ADC samples tend to be highly educated and not representative of the United States population. The potential for selection bias must also be considered; some ADCs require that participants to consent to autopsy before their baseline visit, thus excluding individuals who may not agree to autopsy [36]. This may have resulted in more homogeneity in the sample than expected regardless of race/ethnic differences. There is also potential bias because of the timing of the MoCA; the MoCA is administered before clinician evaluation and may influence clinicians to agree with the MoCA score. This would artificially increase sensitivity and specificity of our calculated MoCA cutoff scores. Nevertheless, clinicians normally see screening results and neurologic testing results when making their diagnosis. In addition, the number of non-Hispanic Black and Hispanic individuals in the NACC database is relatively small compared with White participants. Our distribution of Hispanics is sparse (Supplementary Figs. S1 and S2) potentially underpowering our analysis. This is reflected in the large confidence intervals calculated for our Hispanic group. However, our findings are still novel because many other data sets almost solely focus on Whites. The inclusion of multiple race/ethnicities in the NACC data is unique, especially because data are collected from subjects whose ages fall in a wide age range from <40 to >90 years. Although previous work has found that literacy is a better predictor of cognitive decline [17], our data only captures education. Future work to address the effect of literacy on MoCA scores is warranted. NACC should consider adding a literacy level to the NACC required data capture.
5. Conclusions
Racial/ethnic-specific cutoffs may become increasingly important to correctly identify MCI and dementia in minority populations who are at high risk of developing dementia, given the growing diversity of the United States. More accurate cutoffs for the MoCA should be used among minority populations. By stratifying by race/ethnicity and education level before applying a cutoff value for the MoCA score, we can correctly identify individuals in need of more in-depth screening. Given the importance of early detection and diagnosis of dementia, these new cutoffs might aid health care professionals in early diagnosis and treatment of MCI and dementia.
Research in context.
-
1.
Systematic review: We used traditional sources to review the existing literature on the use of the Montreal Cognitive Assessment (MoCA) among minority populations. However, previous work has suggested that the widely used cutoffs may not constitute optimal thresholds for all racial/ethnic minority groups.
-
2.
Interpretation: Using the Youden index to compare clinician diagnosis and MoCA scores, we found that optimal cutoffs of the MoCA vary by race/ethnicity and by educational attainment. Optimal cutoffs for mild cognitive impairment were 25 among non-Hispanic Whites, 23 among non-Hispanic Blacks, and 24 among Hispanics. Optimal cutoffs for detection of dementia were 19 among non-Hispanic Whites, 16 among non-Hispanic Blacks, and 16 among Hispanics. When further stratified by educational attainment, optimal MoCA cutoffs varied further.
-
3.
Future directions: This article highlights the need to stratify by race/ethnicity and educational attainment before applying cutoffs for the MoCA.
Acknowledgments
This work was supported in part by the 1Florida Alzheimer's Disease Research Center [NIH P50 AG047266], sponsored by the National Institute on Aging (NIA) which governs Alzheimer's Disease Research Centers through the National Alzheimer's Coordinating Center, by the Department of Epidemiology with funding from the College of Medicine and College of Public Health and Health Professions, and by the Graduate School Fellowship with funding from the University of Florida Graduate School. The National Alzheimer's Coordinating Center (NACC) database is funded by National Institute on Aging/National Institutes of Health Grant U01 AG016976. NACC data are contributed by the NIA-funded Alzheimer's Disease Centers: P30 AG019610 (principal investigator [PI] Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI M. Marsel Mesulam, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG005131 (PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), and P50 AG047270 (PI Stephen Strittmatter, MD, PhD).
Footnotes
Conflicts of interest: The authors declare that they have no conflicts of interest.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.dadm.2018.09.003.
Supplementary data
References
- 1.Alzheimer's Association 2017 Alzheimer's disease facts and figures. Alzheimers Dement. 2017;13:325–373. [Google Scholar]
- 2.Richard E., Reitz C., Honig L.H., Schupf N., Tang M.X., Manly J.J. Late-life depression, mild cognitive impairment, and dementia. JAMA Neurol. 2013;70:383–389. doi: 10.1001/jamaneurol.2013.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Flier W.M., Scheltens P. Epidemiology and risk factors of dementia. J Neurol Neurosurg Psychiatry. 2005;76:v2–v7. doi: 10.1136/jnnp.2005.082867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petersen R.C., Caracciolo B., Brayne C., Gauthier S., Jelic V., Fratiglioni L. Mild cognitive impairment: a concept in evolution. J Intern Med. 2014;275:214–228. doi: 10.1111/joim.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts R., Knopman D.S. Classification and epidemiology of MCI. Clin Geriatr Med. 2013;29 doi: 10.1016/j.cger.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langa K.M., Levine D.A. The diagnosis and management of mild cognitive impairment: a clinical review. JAMA. 2014;312:2551–2561. doi: 10.1001/jama.2014.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayeda E.R., Glymour M.M., Quesenberry C.P., Whitmer R.A. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 2016;12:216–224. doi: 10.1016/j.jalz.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chin A.L., Negash S., Hamilton R. Diversity and disparity in dementia: the impact of ethnoracial differences in Alzheimer disease. Alzheimer Dis Assoc Disord. 2011;25:187–195. doi: 10.1097/WAD.0b013e318211c6c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yaffe K., Falvey C., Harris T.B., Newman A., Satterfield S., Koster A. Effect of socioeconomic disparities on incidence of dementia among biracial older adults: prospective study. BMJ. 2013;347:f7051. doi: 10.1136/bmj.f7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beydoun M.A., Beydoun H.A., Gamaldo A.A., Teel A., Zonderman A.B., Wang Y. Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta-analysis. BMC Public Health. 2014;14:643. doi: 10.1186/1471-2458-14-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Contador I., Del Ser T., Llamas S., Villarejo A., Benito-León J., Bermejo-Pareja F. Impact of literacy and years of education on the diagnosis of dementia: A population-based study. J Clin Exp Neuropsychol. 2017;39:112–119. doi: 10.1080/13803395.2016.1204992. [DOI] [PubMed] [Google Scholar]
- 12.Norton S., Matthews F.E., Barnes D.E., Yaffe K., Brayne C. Potential for primary prevention of Alzheimer's disease: an analysis of population-based data. Lancet Neurol. 2014;13:788–794. doi: 10.1016/S1474-4422(14)70136-X. [DOI] [PubMed] [Google Scholar]
- 13.Then F.S., Luck T., Angermeyer M.C., Riedel-Heller S.G. Education as protector against dementia, but what exactly do we mean by education? Age Ageing. 2016;45:523–528. doi: 10.1093/ageing/afw049. [DOI] [PubMed] [Google Scholar]
- 14.Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–460. [PubMed] [Google Scholar]
- 16.Stern Y. Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurol. 2012;11:1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manly J.J., Schupf N., Tang M.-X., Stern Y. Cognitive decline and literacy among ethnically diverse elders. J Geriatr Psychiatry Neurol. 2005;18:213–217. doi: 10.1177/0891988705281868. [DOI] [PubMed] [Google Scholar]
- 18.Alzheimer's Association Early detection and diagnosis of Alzheimer's disease. 2017. https://alzimpact.org/img/Policy_Brief_Early_Detection_and_Diagnosis_Brief_AIM.pdf Available at: Accessed March 26, 2018.
- 19.Nasreddine Z.S., Phillips N.A., Bédirian V., Charbonneau S., Whitehead V., Collin I. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 20.Damian A.M., Jacobson S.A., Hentz J.G., Belden C.M., Shill H.A., Sabbagh M.N. The Montreal Cognitive Assessment and the Mini-Mental State Examination as screening instruments for cognitive impairment: item analyses and threshold scores. Dement Geriatr Cogn Disord. 2011;31:126–131. doi: 10.1159/000323867. [DOI] [PubMed] [Google Scholar]
- 21.Markwick A., Zamboni G., de Jager C.A. Profiles of cognitive subtest impairment in the Montreal Cognitive Assessment (MoCA) in a research cohort with normal Mini-Mental State Examination (MMSE) scores. J Clin Exp Neuropsychol. 2012;34:750–757. doi: 10.1080/13803395.2012.672966. [DOI] [PubMed] [Google Scholar]
- 22.Carson N., Leach L., Murphy K.J. A re-examination of Montreal Cognitive Assessment (MoCA) cutoff scores. Int J Geriatr Psychiatry. 2018;33:379–388. doi: 10.1002/gps.4756. [DOI] [PubMed] [Google Scholar]
- 23.Luis C.A., Keegan A.P., Mullan M. Cross validation of the Montreal Cognitive Assessment in community dwelling older adults residing in the Southeastern US. Int J Geriatr Psychiatry. 2009;24:197–201. doi: 10.1002/gps.2101. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein F.C., Ashley A.V., Miller E., Alexeeva O., Zanders L., King V. Validity of the montreal cognitive assessment as a screen for mild cognitive impairment and dementia in African Americans. J Geriatr Psychiatry Neurol. 2014;27:199–203. doi: 10.1177/0891988714524630. [DOI] [PubMed] [Google Scholar]
- 25.Rossetti H.C., Lacritz L.H., Hynan L.S., Cullum C.M., Van Wright A., Weiner M.F. Montreal Cognitive Assessment performance among community-dwelling African Americans. Arch Clin Neuropsychol. 2017;32:238–244. doi: 10.1093/arclin/acw095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sink K.M., Craft S., Smith S.C., Maldjian J.A., Bowden D.W., Xu J. Montreal Cognitive Assessment and Modified Mini Mental State Examination in African Americans. J Aging Res. 2015;2015:872018. doi: 10.1155/2015/872018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beekly D.L., Ramos E.M., Lee W.W., Deitrich W.D., Jacka M.E., Wu J. The National Alzheimer's Coordinating Center (NACC) database: the Uniform Data Set. Alzheimer Dis Assoc Disord. 2007;21:249–258. doi: 10.1097/WAD.0b013e318142774e. [DOI] [PubMed] [Google Scholar]
- 28.Rossetti H.C., Lacritz L.H., Cullum C.M., Weiner M.F. Normative data for the Montreal Cognitive Assessment (MoCA) in a population-based sample. Neurology. 2011;77:1272–1275. doi: 10.1212/WNL.0b013e318230208a. [DOI] [PubMed] [Google Scholar]
- 29.Perkins N.J., Schisterman E.F. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol. 2006;163:670–675. doi: 10.1093/aje/kwj063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Youden W.J. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 31.Schoonjans F. MedCalc Statistical Software, version 18.10.2. https://www.medcalc.org/manual/howtociteMedCalc.php Available at: Accessed June 2, 2018.
- 32.Davis D.H., Creavin S.T., Yip J.L., Noel-Storr A.H., Brayne C., Cullum S. Montreal Cognitive Assessment for the diagnosis of Alzheimer's disease and other dementias. Cochrane Database Syst Rev. 2015;29:CD010775. doi: 10.1002/14651858.CD010775.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galvin J.E., Tolea M.I., Chrisphonte S., Saravia K. Racial and ethnic disparities in measures of cognitive function: impact of socioeconomic status, physical function, and mood. Alzheimers Dement. 2016;12:P392–P393. [Google Scholar]
- 34.Manly J.J., Jacobs D.M., Sano M., Bell K., Merchant C.A., Small S.A. Cognitive test performance among nondemented elderly African Americans and whites. Neurology. 1998;50:1238–1245. doi: 10.1212/wnl.50.5.1238. [DOI] [PubMed] [Google Scholar]
- 35.Malek-Ahmadi M., Powell J.J., Belden C.M., O'Connor K., Evans L., Coon D.W. Age- and education-adjusted normative data for the Montreal Cognitive Assessment (MoCA) in older adults age 70–99. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2015;22:755–761. doi: 10.1080/13825585.2015.1041449. [DOI] [PubMed] [Google Scholar]
- 36.National Alzheimer's Coordinating Center Researcher home page. https://www.alz.washington.edu/ Available at: Accessed June 15, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.